Abstract
Obesity is a leading public health problem that currently affects over 650 million individuals worldwide. Although interest in the adverse effects of obesity has grown exponentially in recent years, less attention has been given to studying its management in individuals with CKD. This relatively unexplored area should be considered a high priority because of the rapid growth and high prevalence of obesity in the CKD population, its broad impact on health and outcomes, and its modifiable nature. This article begins to lay the groundwork in this field by providing a comprehensive overview that critically evaluates the available evidence related to obesity and kidney disease, identifies important gaps in our knowledge base, and integrates recent insights in the pathophysiology of obesity to help provide a way forward in establishing guidelines as a basis for managing obesity in CKD. Finally, the article includes a kidney-centric algorithm for management of obesity that can be used in clinical practice.
Keywords: obesity, chronic kidney disease, weight loss, kidney transplantation, quality of life, arteriovenous access, diabetes, bariatric surgery, metabolic surgery, GLP-1
Obesity is a leading public health problem that currently affects over 650 million individuals worldwide.1 Although interest in the adverse effects of obesity has grown exponentially in recent years, less attention has been given to studying its management in individuals with CKD. This relatively unexplored area should be considered a high priority in light of obesity’s rapid growth and high prevalence in the CKD population, its broad effect on health and outcomes, and its modifiable nature.
Guidelines for managing obesity exist,2–4 but do not directly address patients with CKD. This gap has led to confusion about treatment options and even the value of treating obesity in people with CKD.5,6 The purpose of this paper is to provide a comprehensive overview that critically evaluates the available evidence related to obesity and kidney disease and integrates recent insights into the pathophysiology of obesity to help provide a way forward in establishing guidelines as a basis for managing obesity in CKD.
Obesity Is Common in CKD
In clinical settings, obesity is usually estimated by simple anthropometric measurements that define excess body fat in a manner that is partly derived from their association with clinical risk in the general population.7 The body mass index (BMI), defined as weight (kilograms)/height (meters2), is the most commonly such used measurement. BMI has well-known limitations, including being influenced by extracellular volume expansion, but offers a reasonably good reflection of total body fat content in individuals with CKD and ESKD.8,9 Although waist circumference more accurately reflects abdominal visceral adiposity, it is a less practical measurement in the clinical setting and is less accurate in patients with BMI>40 kg/m2 due to variations in abdominal pannus distribution. Other anthropometric measurements offer only additional limited value. In fact, just as the size of a tumor may not necessarily correlate with its malignancy (in contrast to its metastases), neither is it certain that extra fat necessarily correlates with extra risk. Therefore, clinicians should be encouraged to look beyond BMI or other indices alone to instead focus on the potential complications of obesity related to the metabolic, mechanical, and psychologic domains. Newer techniques that can link measurement of fat to predictors of clinical risk in people with CKD would be of great benefit.
Granular data on the prevalence of obesity in persons with CKD are limited but consistent throughout the entire spectrum of kidney disease. In the 2011–2014 National Health and Nutrition Examination Survey, 44.1% of patients with CKD in the United States also had obesity (21.9% with class 1 obesity [BMI=30 to 34.9 kg/m2] and 11.1% each with class 2 [BMI=35 to 39.9 kg/m2] and class 3 obesity [BMI≥40 kg/m2]),10 with the overall percentage having increased by 5% over the preceding 12 years.11 These levels are considerably higher than those in the general population during the same time period (36.5%),12 during which time obesity was considered a major public health problem. Similar trends extend to kidney transplant recipients in whom the prevalence of obesity grew by 44% from 1999 to 2009 to include one third of all such patients.13 Individuals initiating dialysis have also had a persistent increase in BMI over time, with the mean rising from 26 to 28 kg/m2 between 1995 and 2002.14 Identifying more recent trends not only in the prevalence of obesity in the CKD population but also in the prevalence of CKD in the greater obesity population would help frame the scope of the problem.15
Obesity Is a Predominating Global Mediator of Kidney Disease
Obesity is estimated to account for approximately 20%–25% of kidney disease worldwide. However, the true number is likely far higher after including intermediate disease states like type 2 diabetes (the most common global cause of CKD) and hypertension (the second most common cause in the United States).16,17 In fact, diabetes and hypertension alone explain most obesity-associated kidney risk.18 The presence of obesity increases the lifetime risk of CKD by 25% compared with individuals with normal weight.19
In a variety of epidemiologic studies, obesity is independently associated with the development of proteinuria, AKI, CKD, and ESKD both in otherwise healthy individuals and in higher-risk groups, like persons with prehypertension.20–28 Two large studies deserve special attention. In a global database of over 5.4 million healthy individuals, the risk of a decline in the eGFR linearly and progressively increased as BMI rose above 25 kg/m2.29 Risk also extends to the pediatric population, where the prevalence of obesity continues to rise.30 In 1.2 million adolescents, overweight or obesity was independently associated with the development of ESKD over an average follow-up of 25 years, with the relationship being especially pronounced for diabetic ESKD (hazard ratio 39.95 [14.5 to 110.0]) in youths in the >95th percentile of BMI).31 Obesity is also an independent risk factor for accelerated CKD in certain monogenic (i.e., polycystic) and glomerular (i.e., IgA nephropathy) kidney diseases whose underlying pathophysiology is seemingly unrelated to weight32–35 and for AKI in critically ill patients or those undergoing cardiac surgery.36,37 However, not all studies of IgA nephropathy or other primary glomerulopathies are in accord.38
The causal link between obesity and kidney disease is biologically plausible and believed to be mediated through direct and indirect mechanisms, with the actual pathways requiring further elucidation.18 Development of obesity leads to increased abdominal pressure and fat infiltration of the kidney,39 intraglomerular hypertension with resultant glomerular shear-related stress,40 podocyte damage and depletion leading to segmental sclerosis,41 activation of the renin-angiotensin-aldosterone42 and sympathetic systems leading to tubular sodium avidity and altered hemodynamics,43 and lipotoxicity of kidney parenchymal cells.44 Adipocyte secretory products, like leptin, contribute to sympathetic activation and may have separate nephrotoxic effects.45 Individuals may also develop obesity-related glomerulopathy (ORG), which is characterized in certain instances by proteinuria and FSGS. Why only a relatively small percentage of people with obesity appear, on the basis of admittedly limited data, to develop ORG or other obesity-related kidney diseases is unknown. Reduced nephron endowment at childbirth with resultant stress on remaining nephrons over time could explain the susceptibility to ORG.46–48 Population-based studies are indicated to better understand the prevalence of ORG. Indirect adverse effects via disease states that can arise from obesity, like diabetic nephropathy and hypertensive nephrosclerosis,18 but also cardiorenal processes (e.g., heart failure, pulmonary hypertension, and obstructive sleep apnea) are major causes of obesity-related CKD.18,49
It should be noted that when estimating GFR in people with obesity, standard creatinine-based equations will for the following reasons offer less accurate results.50–53 The equations themselves were not derived in populations with obesity, so their validation in this subgroup is not as strong. Serum creatinine will fluctuate independent of GFR as weight (and muscle mass) is gained or lost, and therefore, change in weight will throw off the results. The equations also include an adjustment for body surface area due to the observed relationship in mammals that GFR is proportional to body size. This observation is presumably due to the increasing load of metabolic by-products that larger organisms need to excrete.54 Yet, it is lean (and not fat) mass that generates the metabolic waste that is filtered, so adjusting for fat mass introduces systemic bias.55 In general, GFR estimating equations that combine serum creatinine and cystatin C are more accurate in individuals with obesity, although direct GFR measurement would be the ideal strategy.56,57
Obesity Increases the Burden of Disease and Disability in CKD
The independent association between obesity and a variety of chronic physical and mental illnesses, more hospitalization and mortality, and reduced quality of life is well documented in the general population,58,59 but data on these associations among the CKD population are sparse. A recent global survey found a heavy disease burden in persons with obesity and CKD that has grown significantly since 1990.60 For example, in 2015 overweight and obesity in persons with CKD helped account for 24.4% of all disability-adjusted life years and 7.2% of all deaths worldwide. However, controversy surrounds the precise effect of obesity on mortality in patients with CKD. Numerous observational studies have found a protective association between obesity and survival, a phenomenon known as the obesity paradox, that is particularly pronounced in older patients on hemodialysis.61–63 The obesity paradox is controversial, with detractors concerned about multiple residual confounders and biases, whereas supporters point to the consistency of the findings in the literature.64 Regardless of which view is correct, the obesity paradox does not directly address the question of whether patients with CKD and obesity would benefit from weight reduction. That question was recently informed by observations that bariatric surgery, now more commonly referred to as metabolic surgery, was independently associated with a 31% lower all-cause mortality at 5 years compared with usual care (hazard ratio, 0.69; 95% confidence interval, 0.60 to 0.78) in patients with severe obesity on dialysis.65 Similar studies in the pre-ESKD population are currently ongoing.
Obesity Impairs Management of CKD
Independent of any adverse effects on kidney function, the presence of obesity poses an impediment to optimal care of CKD. Placement of permanent dialysis access is one such example. Accesses that fail to work well require great expenditure of resources and expose patients to discomfort, inconvenience, and risk. Obesity can make placement of arteriovenous fistulas (AVFs) more challenging and complex.66 Obesity may also limit the successful placement and function of AVFs particularly in more severe obesity, possibly by physically compressing the AVF and impeding its maturation,67 although not all studies in this area are in agreement.68–71 In patients on peritoneal dialysis, obesity may predispose to more (and more severe) episodes of peritonitis and thus, shorten the life of the dialysis catheter and peritoneal membrane.72–74 More severe obesity may also reduce access to outpatient hemodialysis therapy due to weight limitations that constrain transportation and chair size options and make necessary ambulation difficult.75
Obesity Impedes Access to Kidney Transplantation
Patients with obesity who undergo kidney transplantation will have superior outcomes compared with those remaining on dialysis.76,77 Unfortunately, the presence of obesity impedes access to kidney transplantation. Because of concerns over higher rates of wound complications, longer hospital stay, delayed graft function, graft failure, and even higher mortality, many transplant centers do not offer transplants to individuals who are above certain BMI cutoffs.78–83 Although data on cutoffs are scant, in one survey 29% of respondents stated their cutoff for listing was a BMI>35 kg/m2.84 These restrictions are controversial as some of the concerns may be outdated and are contradicted by other reports.76,78,85,86 Establishing an evidence-based consensus on this important issue will improve transparency and possibly outcomes for donors and recipients.
An Updated Paradigm for Obesity and Implications for Treatment
The traditional understanding of obesity was that it resulted from the passive accumulation of excess calories. Research over recent decades has revised this thinking. Evidence now indicates that obesity is a disorder of energy homeostasis in which the body “defends” against fat loss by returning the subject to a set point of fat after every period of self-induced starvation.87 The mechanisms through which body fat is defended are still unclear and may involve complex interactions between genetic, developmental, and environmental factors that seem to involve adipocyte-gut-neuroendocrine crosstalk.87–89 The revised model helps explain why only about 20% of individuals with obesity are able to achieve sustained significant weight loss using lifestyle changes alone90 and why targeting the hormonal mediators that defend against fat loss using medications or surgery may be a more effective strategy for most individuals. How or even if the uremic milieu contributes to the defense of excess fat requires further research.
Treatment for obesity includes lifestyle interventions, antiobesity medications, and metabolic surgery.58 Lifestyle interventions, which incorporate various combinations of modified diet, physical activity, stress reduction, improved sleep health, and normalization of circadian rhythms, are low cost, have minimal risk, and can easily be integrated into a weight loss program. However, as mentioned above, they are only successful in achieving substantial sustained weight loss in a small minority of patients.90 Renal-specific restrictions on macronutrients (e.g., high protein) or micronutrients (e.g., high potassium/phosphorus/sodium) may further limit dietary obesity treatment options. Medications that induce weight loss and improve obesity by modifying neuroendocrine regulation of body fat hold great potential, although the options for persons with CKD are currently more limited. Four Food and Drug Administration (FDA)–approved antiobesity drugs are available (Table 1). Of these, orlistat has fallen out of favor due to its long-term effectiveness and significant risk of steatorrhea and mild incontinence, as well as a low risk of oxalate-related acute kidney disease and CKD.91,92 Of the remaining three drugs, only the glucagon-like peptide-1 (GLP-1) agonist liraglutide, which lowers mean weight by approximately 8 kg over several years,93 can potentially be safely used in all CKD classes, although dose adjustments may be necessary.
Table 1.
FDA-approved antiobesity medications
| Drug (Brand Name) | Mechanism of Action | Common Adverse Effects | Kidney-Related Precautions | Dosing Adjustments by eGFR, ml/min per 1.73 m2 | |
|---|---|---|---|---|---|
| Stage 3–5 CKD | ESKD | ||||
| Orlistat (Xenical, Alli) | Lipase inhibitor, thus inhibiting absorption | Fecal incontinence, oily spotting, fat-soluble vitamin deficiency | Reports of AKI and chronic kidney injury possibly from oxalate nephropathy | None | None |
| Phentermine (Adipex-P, Lomaira) | Sympathomimetic, anorexic | Hypertension, ischemia, palpitations, dry mouth, constipation | Excreted primarily via the urine | 15–29: maximum 15 g/d | <15: avoid use (not been studied) |
| Phentermine/Topiramate (Qsymia) | Sympathomimetic, anorexic | Tachycardia, paresthesias, dry mouth, constipation, paresthesias, proximal (type 2) renal tubular acidosis, upper respiratory infections | Excreted primarily via the urine | CrCl<50 ml/min: maximum dose 7.5/46 mg once daily | Dialysis: avoid use (not been studied) |
| Buproprion-Naltrexone (Contrave) | Inhibits NE/dodopamine uptake, opioid antagonist | Nausea, constipation, dry mouth, dizziness, transient increase in BP, contraindicated in uncontrolled hypertension | Excreted primarily via the urine | “Moderate or severe impairment”: one tablet (8/90 mg) twice daily | Not recommended |
| Liraglutide (Saxenda) | GLP-1 agonist | Nausea/vomiting, diarrhea, anorexia | None | Use with caution in severe impairment—limited data available | Use with caution due to limited data |
CrCl, creatinine clearance; NE, norepinephrine; GLP-1, glucagon-like peptide-1.
Renoprotective medications not specifically approved by FDA to treat obesity, such as other GLP-1 agonists and the class of sodium-glucose cotransporter 2 (SGLT2) inhibitors, may also have weight-lowering effects in persons with CKD. The GLP-1 agonist dulaglutide lowers weight by 2–3 kg at 52 weeks in patients with CKD stages 3 and 4.94 SGLT2 inhibitors are less effective, with an average weight loss of 0.8 kg in CKD stages 2–4.95 Of note, the American Diabetes Association currently recommends combining SGLT inhibitors and GLP-1 agonists in people with CKD and type 2 diabetes whose glycemic control is not adequate.96 However, benefits of this drug combination on glycemic control, weight reduction, and treatment of CKD are not well established and warrant further attention. Metformin is recommended in patients with type 2 diabetes or prediabetes with an eGFR>30 ml/min per 1.73 m2. Because the variability of weight loss is high with metformin (average of 2%, as high as >10%97,98) and because there are other medications that have may also have a beneficial effect on CKD, metformin should be considered a third- or fourth-line agent in people without type 2 diabetes or prediabetes. Although the weight-lowering effects of antiobesity medications have traditionally been fairly modest, some of the more recently approved agents now average 7%–9% weight loss, including the combination of topiramate and phentermine and the use of liraglutide alone. Newer agents appear to be even more effective. The recently reported results of phase 3 studies of the GLP-1 agonist semaglutide demonstrated an average of 16%–18% weight loss. Phase 2 studies of cagrilinitide, a novel amylin agonist, and terzepatide, a GLP-1/glucose-dependent insulinotropic polypeptide agonist, hold the promise of even greater effectiveness, yielding average weight loss approaching or exceeding 20%.
Metabolic surgery offers the most effective treatment for obesity and encompasses several types of procedures, the most common being sleeve gastrectomy and Roux-en-Y gastric bypass.99 Metabolic surgery has been demonstrated to induce profound and sustained weight loss, including in patients with CKD stages 3 and 4100 and ESKD.17 Drawbacks of metabolic surgery in patients with CKD include initial up-front costs, slightly higher risk of adverse events (reoperation, readmission, and AKI) compared with the general populace, and nephrolithiasis and oxalate nephropathy (on the basis of isolated reports in the literature).65,101–104 Although metabolic benefits of metabolic surgery, such as remission of diabetes, were initially attributed wholly to postsurgical reductions in caloric intake and absorption, there is growing evidence that changes in secretion of gut hormones like GLP-1, gut nutrient–sensing mechanisms, the gut microbiome, and circulating plasma bile acids may also be contributing, particularly to the long-term improvements.105
Mitigation of Kidney Disease through Weight Loss Strategies
Weight loss interventions, particularly metabolic surgery, ameliorate type 2 diabetes and hypertension, the two most common risk factors for CKD in the United States.17 A 5-year lifestyle intervention involving an average 5%–7% weight loss in people with prediabetes lowered the future risk of developing type 2 diabetes by 58% compared with placebo.106 In patients with obesity and preexisting type 2 diabetes, metabolic surgery greatly reduces the need for diabetes medicines and improves glycemic parameters to a much greater degree than intensive medical therapy.107 Metabolic surgery is also nearly seven times more likely to lower the need for antihypertensive medications compared with medical therapy.108 In uncontrolled studies, metabolic surgery improves other risk factors for CKD, including glomerular hyperfiltration, pulmonary hypertension and sleep apnea, and cardiac disease.109–112 There is little information on how weight loss improves metabolic parameters in people with CKD.
Although a growing literature suggests that weight loss interventions offer renoprotection, several important questions remain unanswered. The first is whether benefits are strictly related to weight loss or if weight-independent mechanism(s) play a role. One preclinical study found that metabolic surgery–induced weight loss has a superior antiproteinuric effect compared with weight-matched dietary restriction, supporting the latter possibility.113 A retrospective cohort study of 619 patients found no relationship between changes in BMI and eGFR up to a median of 2.8 years after metabolic surgery.114 However, a pilot study examining possible mechanisms that could explain changes in glomerular hyperfiltration after metabolic surgery found no relationship between circulating GLP-1 and measured GFR nor any changes in GFR immediately postmetabolic surgery before weight loss had occurred. This is in contrast to reports in other populations that describe metabolic improvements at this same time point.115,116
Another question involves the relationship between weight loss and renoprotection. This question has not been directly addressed in the literature, but the studies in Table 2 suggest that as low as 2- to 4-kg weight loss using a variety of interventions may offer some degree of renoprotection. Improvements were observed in a wide variety of parameters, including surrogate markers of glomerular filtration, decline in eGFR, albuminuria/proteinuria, prognostic risk for CKD, development of CKD, and in one study,117 even the need for dialysis. It should be noted that these studies were heterogeneous and varied by intervention (diet, medications, or surgery), population (diabetic versus nondiabetic), baseline eGFR (few with baseline CKD), length (weeks to years), and weight loss achieved (approximately 2–38 kg). Randomized, controlled trials are restricted to one study that found a significantly higher rate of regression of microalbuminuria after metabolic surgery versus best medical care in persons with mild CKD.118 Progress in this area will ultimately require additional randomized, controlled trials in patients with established CKD that are adequately powered to detect surrogate end points of disease progression119 or ideally, hard event rates (i.e., initiation of dialysis or mortality). One such study is currently ongoing (NCT04626323). Comparative effects of weight reduction therapies on kidney-related end points or in patients with CKD are also lacking, as are prospective studies designed to measure metabolic benefits of weight loss in diseases, like diabetic kidney disease. Prioritization should be given to addressing these key clinically oriented questions.
Table 2.
Weight reduction studies in adults with kidney-related outcomes
| Intervention | Design | Baseline eGFRa (% eGFR<60) | Duration/Follow-Up | Weight Lost | Kidney Outcomesb |
|---|---|---|---|---|---|
| Lifestyle/diet based | |||||
| Hypocaloric120 | Prospective proteinuric DKD n=24 | Mean GFR mid-60s (?) | 12 mo | BMI ↓ 7.3 kg/m2 | ↓ Proteinuria (1.3–0.6 g/24 h, P=0.01) |
| ↓ Albuminuria (0.72–0.49 g/24 h, P=0.01) | |||||
| ↑ Measured GFR (63–80 ml/min, P=0.01) | |||||
| Hypocaloric121 | Prospective proteinuric DKD n=22 | Mean CrCl 41 (?) | 4 wk | Mean 6.2 kg | ↓ Proteinuria (3.27–1.5 g/24 h, P<0.001) |
| No change in CrCl | |||||
| Vegan122 | Randomized T2DM n=7 | ? | 12 wk | Mean 7.2 kg | Nonsignificant ↓ in albuminuria (435–155 mg/24 h) |
| Hypocaloric123 | Prospective proteinuric nephropathy n=9 | Mean CrCl 93 | 12 mo | BMI ↓ 4.5 kg/m2 | ↓ Proteinuria (2.9–0.4 g/24 h, P=0.05) |
| Hypocaloric124 | Prospective proteinuric nephropathy n=30 | Mean CrCl 68 (?) | 5 mo | Mean 3.6 kg | ↓ Proteinuria (2.9–1.9 g/24 h, P=0.05) |
| No change in calculated CrCl | |||||
| Hypocaloric and ketogenic125 | Prospective DKD n=6 | 21 (100%) | 12 wk | Median 14.2 kg | ↓ sCr, cystatin C, eGFR |
| Trend toward ↓ albuminuria/proteinuria | |||||
| Intensive lifestyle intervention126 | Randomized stages 3–4 CKD n=83 | Mean eGFR 39 (100%) | 12 mo | Mean 1.8 kg | No change in eGFR |
| Three distinct diets127 | Randomized stages 1–3 CKD n=318 | Stage 2 1–2: mean eGFR 79; stage 3: mean eGFR 53 | 24 mo | 2.9–4.7 kg depending on diet | Stage 3 CKD: ↑ eGFR (7.1% [3.4 to 10.9]) across all diets |
| Stages 1–2 CKD: ↑ eGFR (3.7% [2.1 to 5.4]) across all diets | |||||
| Diet high in fruits/vegetables128 | Randomized stage 3 CKD n=108 | (100%) | 3 yr | Mean 4 kg | In fruits/veggie arm, ↓ albuminuria (318–242 mg/g) |
| In fruits/veggie arm, ↓ eGFR (42–37 ml/min per 1.73 m2) | |||||
| Exercise training129 | Randomized stages 3–4 CKD n=20 | Mean eGFR 40 (100%) | 12 mo | Mean 3.6 kg | Nonsignificant ↑ eGFR (2.3 ml/min per 1.73 m2) versus baseline |
| Intensive lifestyle intervention130 | Randomized stages 3–4 CKD n=111 | Mean eGFR 39–45 (100%) | 4 mo | Mean 2.4 kg (diet/exercise arm) | No change in eGFR |
| Weight management program131 | Prospective n=32 | 52 (100%) | 24 mo | 6.4 kg compared with usual care | Slower eGFR decline (11.5 ml/min per 1.73 m2) compared with usual care |
| Intensive lifestyle intervention132 | Randomized T2DM n=5145 | 90 (5%) | Median 8 yr | 4 kg compared with controls | ↓ Very high-risk CKD (0.69 [0.55 to 0.87]), eGFR<45 (0.79 [0.66 to 0.960]) |
| No improvement in doubling sCr or RRT | |||||
| Antiobesity medicationc | |||||
| Liraglutide133 | RCT secondary analysis n=9340 | Mean 80 (23.1%) | Median 3.7 yr | 2.3 kgd | ↓ New-onset macroalbuminuria |
| (0.74 [0.60 to 0.91]) | |||||
| No improvement in doubling of sCr, RRT, renal death | |||||
| Lorcaserin134 | RCT secondary analysis n=12,000 | Median 76 (19.6%) | Median 3.3 yr | Approximately 4 kgd | ↓ New-onset micro-/macroalbuminuria |
| (0.84 [0.76 to 0.97]) | |||||
| ↓ New/worsening CKD (0.81 [0.72 to 0.93]) | |||||
| No improvement in doubling sCr, composite of ESKD/transplant/renal death, or eGFR≥30%/40% | |||||
| Surgery based | |||||
| BPD135 | Retrospective n=35 | ? (?) | 1 yr | 39.8 kg | ↓ Proteinuria (0.74 to <0.2 g/24 h, P=0.01) |
| RYGB >> SG136 | Retrospective n=985 | 97 (4.7%) | Median 4.4 yr | 34.2 kg | ↓ Risk eGFR decline ≥30% (0.46 [0.36 to 0.60]) |
| ↓ Risk doubling sCr or ESKD (0.49 [0.30 to 0.81]) | |||||
| RYGB, SG100 | Retrospective n=714 | 48 (100%) | Median 3 yr | Approximately 27 kg (RYGB) | Slower eGFR decline compared with controls (9.8 [8.1 to 11.6] ml/min per 1.73 m2) |
| ↑ eGFR in RYBG versus SG groups (6.6 [3.4 to 9.80] ml/min per 1.73 m2) | |||||
| RYGB > LAGB137 | Prospective n=2144 | Median 104 (N/A) | Up to 7 yr | 38.2 kgd | Improvement in CKD prognostic risk |
| ↓ Albuminuria in moderate/high CKD prognostic risk groups | |||||
| VBG > LAGB > RYGB138 | Retrospective n=2010 | Mean 92 (N/A) | Median 18 yr | N/A | ↓ Risk of developing ESKD (= CKD5 + dialysi s+ transplant; 0.46 [0.24 to 0.90]) |
| RYGB > LAGB139 | Prospective T2DM n=737 | Median 94 (12.2%) | Up to 5 yr | Median 25% | ↓ In moderate/severe increase in albuminuria (0.66 [0.48 to 0.90]) |
| Stabilization of prognostic risk for CKD | |||||
| No effect on eGFR | |||||
| RYGB > SG > LAGB140 | Retrospective T2DM n=4024 | Mean 97 (0%) | Median 4.3 yr | N/A | ↓ Risk of developing diabetic nephropathy (0.41 [0.29 to 0.58]) |
| RYGB > SG > LAGB > BPD141 | Retrospective T2DM n=2287 | Median 90 (N/A) | Median 3.9 yr | 20.3 kg compared with controls | ↓ Risk of developing diabetic nephropathy (0.40 [0.31 to 0.52]) |
| Lower eGFR/higher prognostic risk for CKD presurgery associated with lower likelihood of diabetes remission after surgery | |||||
| RYGB117 | Retrospective T2DM n=5321 | Mean 99 (N/A) | Mean 4.7 yr | N/A | ↓ Risk of halving of eGFR (0.58 [0.40 to 0.85]) and development of macroalbuminuria (0.55 [0.47 to 0.65]), AKI (0.57 [0.36 to 0.90]), CKD (0.45 [0.30 to 0.67]), diabetic nephropathy (0.22 [0.10 to 0.47]), or dialysis (0.25 [0.08 to 0.72]) |
| RYGB142 | Retrospective T2DM n=131 | Mean 70 (26%) | Median 12.8 yr | N/A | ↓ Risk of developing CKD compared with patients without bariatric surgery in full (0.53 [0.30 to 0.91]) but not propensity-matched cohort |
| RYGB118 | RCT T2DM n=100 | Median 100 | 2 yr | Relative ↓ in BMI of 7 | 49% improvement in microalbuminuria remission rate compared with best medical treatment |
| N/A143 | Retrospective n=144 | Mean 83 | 9.2 yr | N/A | 1-ml/min per 1.73 m2 slower decline in surgery versus control groups over mean 8–9 yr as confirmed by multiple GFR markers |
| Effects most pronounced in patients with lowest eGFR |
DKD, diabetic kidney disease; DKD, diabetic kidney disease; CrCl, creatinine clearance (in milliliters per minute); T2DM, type 2 diabetes; sCr, serum creatinine; RCT, randomized, controlled trial; BPD, biliopancreatic diversion; RYGB, Roux-en-Y gastric bypass; SG, sleeve gastrectomy; LAGB, laparoscopic adjustable gastric banding; N/A, not available; VGB, vertical banded gastroplasty.
eGFR is in milliliters per minute per 1.73 m2.
May include hazard ratio (95% confidence interval).
Approved by FDA for obesity treatment in 2020.
Published in a separate dataset.
The criteria upon which patients are selected for obesity treatment also require refinement. Selection criteria will depend in part upon the severity of obesity and goals (e.g., metabolic benefits, slowing CKD progression, and disease regression), patient preference and tolerance of risk, and weight eligibility requirements for kidney transplant wait-listing if applicable. Unfortunately, little information is available to help guide recommendations. More advanced CKD has been associated with reduced efficacy of metabolic surgery144 as well as a lower likelihood of diabetes remission139 in retrospective studies that need to be prospectively confirmed. In summary, because of lack of rigorous evidence, management strategies are primarily opinion based at this time.
Figure 1 offers one possible kidney-centric management algorithm. The proposed algorithm balances severity of obesity, the presence of obesity-related uncontrolled modifiable CKD risk factors, and weight-related prohibitions on kidney transplantation in determining obesity management. The longer-term efficacy of any particular intervention is more likely to be reflected in the response to treatment at 3 months. We, therefore, recommend a stepwise approach that starts with lifestyle management, escalating to pharmacotherapy, then surgery, and ultimately, combination approaches. Clinicians can move rapidly from tier to tier if no response is noted within 3 months for each treatment modality. It should be recognized that at present, treatment options are fairly limited, and there is no way to determine which treatment pathway is most suited to a specific patient. If a patient responds to a particular treatment, then their obesity should be aggressively managed to optimize health benefits.
Figure 1.
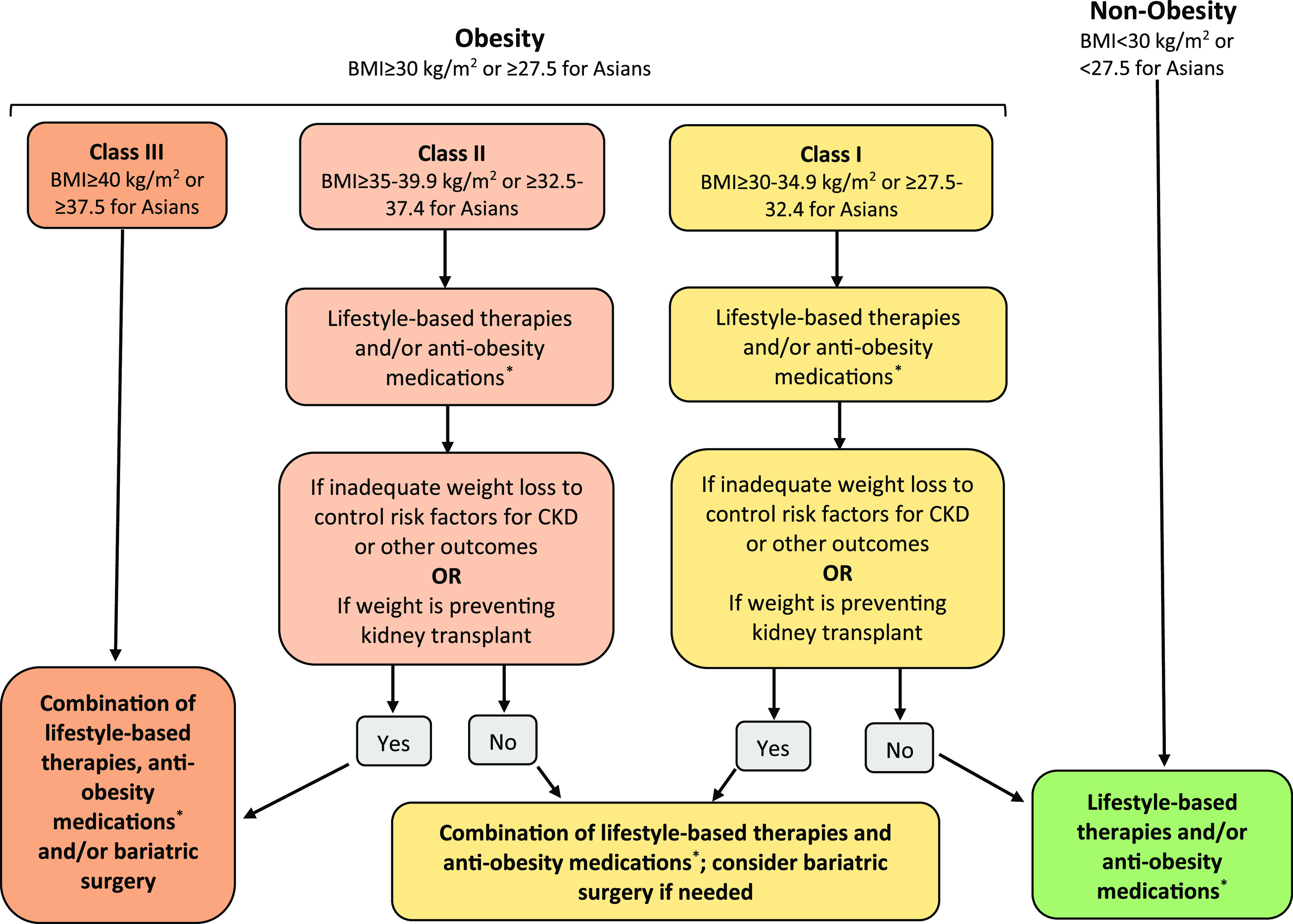
Suggested algorithm for obesity management in persons with CKD. *Opinion-based recommendations for antiobesity medications include choosing from each of the following groups: group 1: orlistat, phentermine/topiramate, buproprion-naltrexone (all of these are dose adjusted as necessary with monitoring for renal side effects); group 2: GLP-1 agonists (dose adjusted as necessary); and group 3: sodium-glucose cotransporter 2 inhibitors (eGFR>30 ml/min per 1.73 m2). Adapted from Rubino et al.,145 with permission.
A Multidisciplinary Approach to CKD Obesity Management
Nephrologists can play an active role in identifying and helping manage obesity in patients with CKD. This should be made somewhat easier by the fact that nephrologists are already comfortable in acting in many ways as primary physicians for their patients. Moreover, the incorporation into nephrology practice of SGTL2 inhibitors, GLP-1 agonists, and perhaps, other new drugs that have renoprotective effects but also induce weight loss will enable nephrologists to more comfortably and confidently participate in obesity management. Educating nephrologists on the importance of obesity as a pivotal risk factor for kidney disease and other major outcomes; the available weight reduction treatments and their efficacy, benefits, and risks; and how to communicate this information effectively to patients will help optimize obesity management. Introducing these concepts into nephrology training curricula will help reinforce these concepts and their importance. Although it is unrealistic given time constraints and other factors to expect that the majority of nephrologists will play a primary role in obesity management, they can help facilitate the process by establishing referral networks with local obesity medicine and metabolic surgery practices that can then take the lead. Active involvement of renal dietitians will also contribute significantly to the success of this endeavor.
A more aggressive push to treat obesity in CKD will also require greater familiarity and comfort on the part of obesity physicians in terms of the goals, benefits, risks, and limitations of weight reduction therapies in the CKD population. As many knowledge gaps remain in these areas, clinical judgement will be required. Possible treatment goals include remission of early CKD; halting progression of later stages of CKD; ameliorating risk factors for CKD, such as type 2 diabetes, systemic and pulmonary hypertension, and kidney inflammation; and improving the likelihood of obtaining a kidney transplant. Regular communication with nephrologists may be necessary, particularly with regard to the safety of treatment strategies and monitoring of kidney function and metabolic profiles.
Developing Clinical Practice Guidelines for Patients with CKD
Management of obesity in individuals with CKD is at an early stage. Despite increasing interest over the past two decades in the pathophysiologic effects and clinical impact of obesity on kidney disease, this topic is given little attention during fellowship training, in day-to-day nephrology care, or in nephrology clinical practice guidelines.146,147 Contributing factors include a lack of understanding about the effect of obesity on kidney disease and its management, uncertainty on how to manage obesity and integrate its management into daily practice, and the important gaps in knowledge, including those listed in Table 3. Although clinical guidelines are not a panacea and have the potential to be harmful if used without application of adequate clinical judgment,148 we think that developing practice guidelines for the management of obesity in CKD could improve quality and consistency of care, promote beneficial interventions, support quality improvement, improve medical education, empower patients, influence public policy, and identify gaps and limitations in the evidence. Ideally, such guidelines should be developed in collaboration with leading professional societies focused on obesity.
Table 3.
Research gaps related to obesity management in CKD
| Topics |
|---|
| Techniques to more accurately measure clinically meaningful excess adiposity |
| Updated obesity trends in the CKD population |
| Understanding the pathophysiologic pathways linking obesity and CKD |
| Explaining the individual predisposition to obesity-related kidney disease and its prevalence |
| Developing consensus on weight-related contraindications to kidney transplantation |
| Determining the effect of obesity on mortality and other nonkidney-related clinical outcomes |
| Understanding the contribution of uremia to the defense of fat |
| Defining the precise relationship between weight loss and renoprotection |
| Comparing renoprotective effects of various weight loss strategies |
| Establishing evidence-based indications for antiobesity therapy |
Despite its centrality as a risk factor for kidney disease, disability, and death and an obstacle for optimal management of CKD, obesity is relatively undermanaged and understudied by nephrologists. Improving obesity management for persons with kidney disease will require a concerted effort to integrate this topic into nephrology training curricula and standard clinical care, establish collaborative relationships with obesity experts, and address the many unanswered research questions to help fill current knowledge gaps. Establishing clinical guidelines in this area can help focus and advance efforts in this field.
Disclosures
A.N. Friedman is a member of the scientific advisory board for GI Dynamics and a consultant for Goldfinch Bio; reports ownership interest in Eli Lilly; is an editorial board member of the Journal of Renal Nutrition and Frontiers in Nephrology; is a council member of the International Society of Renal Nutrition and Metabolism; and is a Data Safety and Monitoring Board member of Watermark Research Partners. L.M. Kaplan is a scientific advisor to Boehringer Ingelheim, Fractyl, Gelesis, GI Dynamics, Johnson & Johnson, Lilly, Novo Nordisk, Pfizer, and Rhythm. C.W. le Roux is a member of the scientific advisory boards for Boehringer Ingelheim, GI Dynamics, Herbalife, Johnson & Johnson, Keyron, Novo Nordisk, and Sanofi; received research funding from the European Foundation for Study of Diabetes, the Health Research Board, the Innovative Medicine Initiative of the European Union, the Irish Research Council, and the Science Foundation Ireland; has ownership interest in Keyron; received honoraria from Boehringer Ingelheim, GI Dynamics, Herbalife, Johnson & Johnson, Keyron, Novo Nordisk, and Sanofi; is an editorial board member of Surgery for Obesity and Related Diseases and Obesity Surgery; and was on speakers bureaus from Boehringer Ingelheim, GI Dynamics, Herbalife, Johnson & Johnson, Keyron, Novo Nordisk, and Sanofi. P.R. Schauer is a member of the scientific advisory boards for GI Dynamics, Keyron, and Persona; has received consulting fees from Ethicon and Medtronic; and has received grant funding from Ethicon, Medtronic, and Pacira. L. Kaplan reports Consultancy Agreements with Eli Lilly & Co., Johnson & Johnson, Novo Nordisk, Pfizer, Rhythm Pharmaceuticals; Ownership Interest in Fractyl, Gelesis, GI Dynamics, Rhythm Pharmaceuticals; Honoraria from Eli Lilly & Co., Johnson & Johnson, Novo Nordisk, Pfizer, Rhythm Pharmaceuticals; Other Interests/Relationships as a Member of Executive Committee of The Obesity Society. P. Schauer reports Consultancy Agreements with GI Dynamics, Keyron, Persona, Mediflix; Ownership Interest in SE Healthcare LLC; Research Funding from Ethicon, Medtronic, Pacira, Persona; Honoraria from Ethicon, Medtronic, BD Surgical, Gore; Scientific Advisor or Membership as SE Healthcare Board of Directors. GI Dynamics Advisory Board, Keyron Advisory Board, Persona Advisory Board, and Mediflix Advisory Board.
Funding
None.
Footnotes
Published online ahead of print. Publication date available at www.jasn.org.
References
- 1.World Health Organization: Obesity and overweight fact sheet, 2020. Available at: https://www.who.int/news-room/fact-sheets/detail/obesity-and-overweight. Accessed November 30, 2020
- 2.Garvey WT, Mechanick JI, Brett EM, Garber AJ, Hurley DL, Jastreboff AM, et al.; Reviewers of the AACE/ACE Obesity Clinical Practice Guidelines: American Association of Clinical Endocrinologists and American College of Endocrinology comprehensive clinical practice guidelines for medical care of patients with obesity. Endocr Pract 22[Suppl 3]: 1–203, 2016 [DOI] [PubMed] [Google Scholar]
- 3.Jensen MD, Ryan DH, Apovian CM, Ard JD, Comuzzie AG, Donato KA, et al.; American College of Cardiology/American Heart Association Task Force on Practice Guidelines; Obesity Society: 2013 AHA/ACC/TOS guideline for the management of overweight and obesity in adults: A report of the American College of Cardiology/American Heart Association task force on practice guidelines and the obesity society [published correction appears in Circulation 129(Suppl 2): S139–S140, 2014]. Circulation 129[Suppl 2]: S102–S138, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Tsigos C, Hainer V, Basdevant A, Finer N, Fried M, Mathus-Vliegen E, et al.; Obesity Management Task Force of the European Association for the Study of Obesity: Management of obesity in adults: European clinical practice guidelines. Obes Facts 1: 106–116, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Suresh A, Robinson L, Milliron BJ, Leonberg K, McAdams-DeMarco M, Earthman C, et al.: Approaches to obesity management in dialysis settings: Renal dietitian perspectives. J Ren Nutr 30: 561–566, 2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lambert K, Beer J, Dumont R, Hewitt K, Manley K, Meade A, et al.: Weight management strategies for those with chronic kidney disease: A consensus report from the Asia Pacific Society of Nephrology and Australia and New Zealand Society of Nephrology 2016 renal dietitians meeting. Nephrology (Carlton) 23: 912–920, 2018 [DOI] [PubMed] [Google Scholar]
- 7.NHLBI Obesity Education Initiative Expert Panel on the Identification, Evaluation, and Treatment of Obesity in Adults (US): Clinical Guidelines on the Identification, Evaluation, and Treatment of Overweight and Obesity in Adults, Bethesda, MD, National Institutes of Health: National Heart, Lung, and Blood Institute, 1998 [Google Scholar]
- 8.Agarwal R, Bills JE, Light RP: Diagnosing obesity by body mass index in chronic kidney disease: An explanation for the “obesity paradox?”. Hypertension 56: 893–900, 2010 [DOI] [PubMed] [Google Scholar]
- 9.Leinig C, Pecoits-Filho R, Nascimento MM, Gonçalves S, Riella MC, Martins C: Association between body mass index and body fat in chronic kidney disease stages 3 to 5, hemodialysis, and peritoneal dialysis patients. J Ren Nutr 18: 424–429, 2008 [DOI] [PubMed] [Google Scholar]
- 10.Chang AR, Grams ME, Navaneethan SD: Bariatric surgery and kidney-related outcomes. Kidney Int Rep 2: 261–270, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Flegal KM, Kruszon-Moran D, Carroll MD, Fryar CD, Ogden CL: Trends in obesity among adults in the United States, 2005 to 2014. JAMA 315: 2284–2291, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ogden CL, Carroll MD, Fryar CD, Flegal KM: Prevalence of obesity among adults and youth: United States, 2011-2014. NCHS Data Brief 219: 1–8, 2015 [PubMed] [Google Scholar]
- 13.Lentine KL, Axelrod D, Abbott KC: Interpreting body composition in kidney transplantation: Weighing candidate selection, prognostication, and interventional strategies to optimize health. Clin J Am Soc Nephrol 6: 1238–1240, 2011 [DOI] [PubMed] [Google Scholar]
- 14.Kramer HJ, Saranathan A, Luke A, Durazo-Arvizu RA, Guichan C, Hou S, et al.: Increasing body mass index and obesity in the incident ESRD population. J Am Soc Nephrol 17: 1453–1459, 2006 [DOI] [PubMed] [Google Scholar]
- 15.Ward ZJ, Bleich SN, Cradock AL, Barrett JL, Giles CM, Flax C, et al.: Projected U.S. state-level prevalence of adult obesity and severe obesity. N Engl J Med 381: 2440–2450, 2019 [DOI] [PubMed] [Google Scholar]
- 16.Wang Y, Chen X, Song Y, Caballero B, Cheskin LJ: Association between obesity and kidney disease: A systematic review and meta-analysis. Kidney Int 73: 19–33, 2008 [DOI] [PubMed] [Google Scholar]
- 17.United States Renal Data System , USRDS 2018 Annual Data Report: Atlas of End-Stage Renal Disease in the United States National Institutes of Health, National Institute of Diabetes and Digestive and Kidney Diseases Bethesda, MD 2018. Available at https://www.usrds.org/annual-data-report/. Accessed January 18, 2021
- 18.Zhu P, Herrington WG, Haynes R, Emberson J, Landray MJ, Sudlow CLM, et al.: Conventional and genetic evidence on the association between adiposity and CKD. J Am Soc Nephrol 32: 127–137, 2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Yarnoff BO, Hoerger TJ, Shrestha SS, Simpson SK, Burrows NR, Anderson AH, et al.; CRIC Study Investigators: Modeling the impact of obesity on the lifetime risk of chronic kidney disease in the United States using updated estimates of GFR progression from the CRIC study. PLoS One 13: e0205530, 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Druml W, Metnitz B, Schaden E, Bauer P, Metnitz PG: Impact of body mass on incidence and prognosis of acute kidney injury requiring renal replacement therapy. Intensive Care Med 36: 1221–1228, 2010 [DOI] [PubMed] [Google Scholar]
- 21.Ryu S, Chang Y, Woo HY, Kim SG, Kim DI, Kim WS, et al.: Changes in body weight predict CKD in healthy men. J Am Soc Nephrol 19: 1798–1805, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Fox CS, Larson MG, Leip EP, Culleton B, Wilson PW, Levy D: Predictors of new-onset kidney disease in a community-based population. JAMA 291: 844–850, 2004 [DOI] [PubMed] [Google Scholar]
- 23.de Boer IH, Katz R, Fried LF, Ix JH, Luchsinger J, Sarnak MJ, et al.: Obesity and change in estimated GFR among older adults. Am J Kidney Dis 54: 1043–1051, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ejerblad E, Fored CM, Lindblad P, Fryzek J, McLaughlin JK, Nyrén O: Obesity and risk for chronic renal failure. J Am Soc Nephrol 17: 1695–1702, 2006 [DOI] [PubMed] [Google Scholar]
- 25.Bello AK, de Zeeuw D, El Nahas M, Brantsma AH, Bakker SJ, de Jong PE, et al.: Impact of weight change on albuminuria in the general population. Nephrol Dial Transplant 22: 1619–1627, 2007 [DOI] [PubMed] [Google Scholar]
- 26.Taylor EN, Stampfer MJ, Curhan GC: Obesity, weight gain, and the risk of kidney stones. JAMA 293: 455–462, 2005 [DOI] [PubMed] [Google Scholar]
- 27.Munkhaugen J, Lydersen S, Widerøe TE, Hallan S: Prehypertension, obesity, and risk of kidney disease: 20-year follow-up of the HUNT I study in Norway. Am J Kidney Dis 54: 638–646, 2009 [DOI] [PubMed] [Google Scholar]
- 28.Hsu CY, McCulloch CE, Iribarren C, Darbinian J, Go AS: Body mass index and risk for end-stage renal disease. Ann Intern Med 144: 21–28, 2006 [DOI] [PubMed] [Google Scholar]
- 29.Chang AR, Grams ME, Ballew SH, Bilo H, Correa A, Evans M, et al.; CKD Prognosis Consortium (CKD-PC): Adiposity and risk of decline in glomerular filtration rate: Meta-analysis of individual participant data in a global consortium. BMJ 364: k5301, 2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Hales CM, Carroll MD, Fryar CD, Ogden CL: Prevalence of obesity among adults and youth: United States, 2015–2016. NCHS Data Brief 288: 1–8, 2017 [PubMed] [Google Scholar]
- 31.Vivante A, Golan E, Tzur D, Leiba A, Tirosh A, Skorecki K, et al.: Body mass index in 1.2 million adolescents and risk for end-stage renal disease. Arch Intern Med 172: 1644–1650, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Nowak KL, You Z, Gitomer B, Brosnahan G, Torres VE, Chapman AB, et al.: Overweight and obesity are predictors of progression in early autosomal dominant polycystic kidney disease. J Am Soc Nephrol 29: 571–578, 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Berthoux F, Mariat C, Maillard N: Overweight/obesity revisited as a predictive risk factor in primary IgA nephropathy. Nephrol Dial Transplant 28[Suppl 4]: iv160–iv166, 2013 [DOI] [PubMed] [Google Scholar]
- 34.Kataoka H, Ohara M, Shibui K, Sato M, Suzuki T, Amemiya N, et al.: Overweight and obesity accelerate the progression of IgA nephropathy: Prognostic utility of a combination of BMI and histopathological parameters. Clin Exp Nephrol 16: 706–712, 2012 [DOI] [PubMed] [Google Scholar]
- 35.Wu C, Wang AY, Li G, Wang L: Association of high body mass index with development of interstitial fibrosis in patients with IgA nephropathy. BMC Nephrol 19: 381, 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Vargo PR, Steffen RJ, Bakaeen FG, Navale S, Soltesz EG: The impact of obesity on cardiac surgery outcomes. J Card Surg 33: 588–594, 2018 [DOI] [PubMed] [Google Scholar]
- 37.Danziger J, Chen KP, Lee J, Feng M, Mark RG, Celi LA, et al.: Obesity, acute kidney injury, and mortality in critical illness. Crit Care Med 44: 328–334, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Elyan BMP, Lees JS, Gillis KA, Mackinnon B, Fox JG, Geddes CC, et al.: Obesity is not associated with progression to end stage renal disease in patients with biopsy-proven glomerular diseases. BMC Nephrol 20: 237, 2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Foster MC, Hwang SJ, Porter SA, Massaro JM, Hoffmann U, Fox CS: Fatty kidney, hypertension, and chronic kidney disease: The Framingham Heart Study. Hypertension 58: 784–790, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Chagnac A, Zingerman B, Rozen-Zvi B, Herman-Edelstein M: Consequences of glomerular hyperfiltration: The role of physical forces in the pathogenesis of chronic kidney disease in diabetes and obesity. Nephron 143: 38–42, 2019 [DOI] [PubMed] [Google Scholar]
- 41.Chen HM, Liu ZH, Zeng CH, Li SJ, Wang QW, Li LS: Podocyte lesions in patients with obesity-related glomerulopathy. Am J Kidney Dis 48: 772–779, 2006 [DOI] [PubMed] [Google Scholar]
- 42.Tuck ML, Sowers J, Dornfeld L, Kledzik G, Maxwell M: The effect of weight reduction on blood pressure, plasma renin activity, and plasma aldosterone levels in obese patients. N Engl J Med 304: 930–933, 1981 [DOI] [PubMed] [Google Scholar]
- 43.Esler M, Straznicky N, Eikelis N, Masuo K, Lambert G, Lambert E: Mechanisms of sympathetic activation in obesity-related hypertension. Hypertension 48: 787–796, 2006 [DOI] [PubMed] [Google Scholar]
- 44.de Vries AP, Ruggenenti P, Ruan XZ, Praga M, Cruzado JM, Bajema IM, et al.; ERA-EDTA Working Group Diabesity: Fatty kidney: Emerging role of ectopic lipid in obesity-related renal disease. Lancet Diabetes Endocrinol 2: 417–426, 2014 [DOI] [PubMed] [Google Scholar]
- 45.Simonds SE, Pryor JT, Ravussin E, Greenway FL, Dileone R, Allen AM, et al.: Leptin mediates the increase in blood pressure associated with obesity. Cell 159: 1404–1416, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.D’Agati VD, Chagnac A, de Vries AP, Levi M, Porrini E, Herman-Edelstein M, et al.: Obesity-related glomerulopathy: Clinical and pathologic characteristics and pathogenesis. Nat Rev Nephrol 12: 453–471, 2016 [DOI] [PubMed] [Google Scholar]
- 47.Abitbol CL, Ingelfinger JR: Nephron mass and cardiovascular and renal disease risks. Semin Nephrol 29: 445–454, 2009 [DOI] [PubMed] [Google Scholar]
- 48.Praga M: Synergy of low nephron number and obesity: A new focus on hyperfiltration nephropathy. Nephrol Dial Transplant 20: 2594–2597, 2005 [DOI] [PubMed] [Google Scholar]
- 49.Schnurr TM, Jakupović H, Carrasquilla GD, Ängquist L, Grarup N, Sørensen TIA, et al.: Obesity, unfavourable lifestyle and genetic risk of type 2 diabetes: A case-cohort study. Diabetologia 63: 1324–1332, 2020 [DOI] [PubMed] [Google Scholar]
- 50.Verhave JC, Fesler P, Ribstein J, du Cailar G, Mimran A: Estimation of renal function in subjects with normal serum creatinine levels: Influence of age and body mass index. Am J Kidney Dis 46: 233–241, 2005 [DOI] [PubMed] [Google Scholar]
- 51.Friedman AN, Strother M, Quinney SK, Hall S, Perkins SM, Brizendine EJ, et al.: Measuring the glomerular filtration rate in obese individuals without overt kidney disease. Nephron Clin Pract 116: c224–c234, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Wuerzner G, Bochud M, Giusti V, Burnier M: Measurement of glomerular filtration rate in obese patients: Pitfalls and potential consequences on drug therapy. Obes Facts 4: 238–243, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Kittiskulnam P, Tiskajornsiri K, Katavetin P, Chaiwatanarat T, Eiam-Ong S, Praditpornsilpa K: The failure of glomerular filtration rate estimating equations among obese population. PLoS One 15: e0242447, 2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Vupputuri S, Fox CS, Coresh J, Woodward M, Muntner P: Differential estimation of CKD using creatinine- versus cystatin C-based estimating equations by category of body mass index. Am J Kidney Dis 53: 993–1001, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Friedman AN: The importance of considering metabolism when indexing the GFR. Am J Kidney Dis 56: 1218, 2010 [DOI] [PubMed] [Google Scholar]
- 56.Friedman AN, Moe S, Fadel WF, Inman M, Mattar SG, Shihabi Z, et al.: Predicting the glomerular filtration rate in bariatric surgery patients. Am J Nephrol 39: 8–15, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Chang A, Greene TH, Wang X, Kendrick C, Kramer H, Wright J, et al.: The effects of weight change on glomerular filtration rate. Nephrol Dial Transplant 30: 1870–1877, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Heymsfield SB, Wadden TA: Mechanisms, pathophysiology, and management of obesity. N Engl J Med 376: 1492, 2017 [DOI] [PubMed] [Google Scholar]
- 59.Schafer MH, Ferraro KF: Long-term obesity and avoidable hospitalization among younger, middle-aged, and older adults. Arch Intern Med 167: 2220–2225, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Afshin A, Forouzanfar MH, Reitsma MB, Sur P, Estep K, Lee A, et al.; GBD 2015 Obesity Collaborators: Health effects of overweight and obesity in 195 countries over 25 years. N Engl J Med 377: 13–27, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Ahmadi SF, Zahmatkesh G, Ahmadi E, Streja E, Rhee CM, Gillen DL, et al.: Association of body mass index with clinical outcomes in non-dialysis-dependent chronic kidney disease: A systematic review and meta-analysis. Cardiorenal Med 6: 37–49, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Ladhani M, Craig JC, Irving M, Clayton PA, Wong G: Obesity and the risk of cardiovascular and all-cause mortality in chronic kidney disease: A systematic review and meta-analysis. Nephrol Dial Transplant 32: 439–449, 2017 [DOI] [PubMed] [Google Scholar]
- 63.Hoogeveen EK, Halbesma N, Rothman KJ, Stijnen T, van Dijk S, Dekker FW, et al.; Netherlands Cooperative Study on the Adequacy of Dialysis-2 (NECOSAD) Study Group: Obesity and mortality risk among younger dialysis patients. Clin J Am Soc Nephrol 7: 280–288, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Kramer H, Dugas L, Shoham D: Obesity as an effect modifier of the risk of death in chronic kidney disease. Nephrol Dial Transplant 28[Suppl 4]: iv65–iv72, 2013 [DOI] [PubMed] [Google Scholar]
- 65.Sheetz KH, Gerhardinger L, Dimick JB, Waits SA: Bariatric surgery and long-term survival in patients with obesity and end-stage kidney disease. JAMA Surg 155: 581–588, 2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Miles Maliska C 3rd, Jennings W, Mallios A: When arteriovenous fistulas are too deep: Options in obese individuals. J Am Coll Surg 221: 1067–1072, 2015 [DOI] [PubMed] [Google Scholar]
- 67.Plumb TJ, Adelson AB, Groggel GC, Johanning JM, Lynch TG, Lund B: Obesity and hemodialysis vascular access failure. Am J Kidney Dis 50: 450–454, 2007 [DOI] [PubMed] [Google Scholar]
- 68.Chan MR, Young HN, Becker YT, Yevzlin AS: Obesity as a predictor of vascular access outcomes: Analysis of the USRDS DMMS Wave II study. Semin Dial 21: 274–279, 2008 [DOI] [PubMed] [Google Scholar]
- 69.Kats M, Hawxby AM, Barker J, Allon M: Impact of obesity on arteriovenous fistula outcomes in dialysis patients. Kidney Int 71: 39–43, 2007 [DOI] [PubMed] [Google Scholar]
- 70.Weyde W, Krajewska M, Letachowicz W, Porazko T, Watorek E, Kusztal M, et al.: Obesity is not an obstacle for successful autogenous arteriovenous fistula creation in haemodialysis. Nephrol Dial Transplant 23: 1318–1322, 2008 [DOI] [PubMed] [Google Scholar]
- 71.Arhuidese IJ, Holscher CM, Elemuo C, Parkerson GR, Johnson BL, Malas MB: Impact of body mass index on outcomes of autogenous fistulas for hemodialysis access. Ann Vasc Surg 68: 192–200, 2020 [DOI] [PubMed] [Google Scholar]
- 72.Obi Y, Streja E, Mehrotra R, Rivara MB, Rhee CM, Soohoo M, et al.: Impact of obesity on modality longevity, residual kidney function, peritonitis, and survival among incident peritoneal dialysis patients. Am J Kidney Dis 71: 802–813, 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Ong LM, Ch’ng CC, Wee HC, Supramaniam P, Zainal H, Goh BL, et al.: Risk of peritoneal dialysis-related peritonitis in a multi-racial asian population. Perit Dial Int 37: 35–43, 2017 [DOI] [PubMed] [Google Scholar]
- 74.McDonald SP, Collins JF, Johnson DW: Obesity is associated with worse peritoneal dialysis outcomes in the Australia and New Zealand patient populations. J Am Soc Nephrol 14: 2894–2901, 2003 [DOI] [PubMed] [Google Scholar]
- 75.Friedman AN, Decker B, Seele L, Hellman RN: Challenges of treating a 466-kilogram man with acute kidney injury. Am J Kidney Dis 52: 140–143, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Krishnan N, Higgins R, Short A, Zehnder D, Pitcher D, Hudson A, et al.: Kidney transplantation significantly improves patient and graft survival irrespective of BMI: A cohort study. Am J Transplant 15: 2378–2386, 2015 [DOI] [PubMed] [Google Scholar]
- 77.Gill JS, Lan J, Dong J, Rose C, Hendren E, Johnston O, et al.: The survival benefit of kidney transplantation in obese patients. Am J Transplant 13: 2083–2090, 2013 [DOI] [PubMed] [Google Scholar]
- 78.Lafranca JA, IJermans JN, Betjes MG, Dor FJ: Body mass index and outcome in renal transplant recipients: A systematic review and meta-analysis [published correction appears in BMC Med 13: 141, 2015 10.1186/s12916-015-0387-3]. BMC Med 13: 111, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Sood A, Hakim DN, Hakim NS: Consequences of recipient obesity on postoperative outcomes in a renal transplant: A systematic review and meta-analysis. Exp Clin Transplant 14: 121–128, 2016 [PubMed] [Google Scholar]
- 80.Kanthawar P, Mei X, Daily MF, Chandarana J, Shah M, Berger J, et al.: Kidney transplant outcomes in the super obese: A national study from the UNOS dataset. World J Surg 40: 2808–2815, 2016 [DOI] [PubMed] [Google Scholar]
- 81.Cannon RM, Jones CM, Hughes MG, Eng M, Marvin MR: The impact of recipient obesity on outcomes after renal transplantation. Ann Surg 257: 978–984, 2013 [DOI] [PubMed] [Google Scholar]
- 82.Kwan JM, Hajjiri Z, Metwally A, Finn PW, Perkins DL: Effect of the obesity epidemic on kidney transplantation: Obesity is independent of diabetes as a risk factor for adverse renal transplant outcomes. PLoS One 11: e0165712, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Naik AS, Sakhuja A, Cibrik DM, Ojo AO, Samaniego-Picota MD, Lentine KL: The impact of obesity on allograft failure after kidney transplantation: A competing risks analysis. Transplantation 100: 1963–1969, 2016 [DOI] [PubMed] [Google Scholar]
- 84.Hossain M, Woywodt A, Augustine T, Sharma V: Obesity and listing for renal transplantation: Weighing the evidence for a growing problem. Clin Kidney J 10: 703–708, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Hill CJ, Courtney AE, Cardwell CR, Maxwell AP, Lucarelli G, Veroux M, et al.: Recipient obesity and outcomes after kidney transplantation: A systematic review and meta-analysis. Nephrol Dial Transplant 30: 1403–1411, 2015 [DOI] [PubMed] [Google Scholar]
- 86.MacLaughlin HL, Campbell KL: Obesity as a barrier to kidney transplantation: Time to eliminate the body weight bias? Semin Dial 32: 219–222, 2019 [DOI] [PubMed] [Google Scholar]
- 87.Schwartz MW, Seeley RJ, Zeltser LM, Drewnowski A, Ravussin E, Redman LM, et al.: Obesity pathogenesis: An endocrine society scientific statement. Endocr Rev 38: 267–296, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Davis RAH, Plaisance EP, Allison DB: Complementary hypotheses on contributors to the obesity epidemic. Obesity (Silver Spring) 26: 17–21, 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Lund J, Lund C, Morville T, Clemmensen C: The unidentified hormonal defense against weight gain. PLoS Biol 18: e3000629, 2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Gregg EW, Jakicic JM, Blackburn G, Bloomquist P, Bray GA, Clark JM, et al.; Look AHEAD Research Group: Association of the magnitude of weight loss and changes in physical fitness with long-term cardiovascular disease outcomes in overweight or obese people with type 2 diabetes: A post-hoc analysis of the Look AHEAD randomised clinical trial. Lancet Diabetes Endocrinol 4: 913–921, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Weir MA, Beyea MM, Gomes T, Juurlink DN, Mamdani M, Blake PG, et al.: Orlistat and acute kidney injury: An analysis of 953 patients. Arch Intern Med 171: 703–704, 2011 [DOI] [PubMed] [Google Scholar]
- 92.Solomon LR, Nixon AC, Ogden L, Nair B: Orlistat-induced oxalate nephropathy: An under-recognised cause of chronic kidney disease. BMJ Case Rep 2017: bcr2016218623, 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Pi-Sunyer X, Astrup A, Fujioka K, Greenway F, Halpern A, Krempf M, et al.; SCALE Obesity and Prediabetes NN8022-1839 Study Group: A randomized, controlled trial of 3.0 mg of liraglutide in weight management. N Engl J Med 373: 11–22, 2015 [DOI] [PubMed] [Google Scholar]
- 94.Tuttle KR, Lakshmanan MC, Rayner B, Busch RS, Zimmermann AG, Woodward DB, et al.: Dulaglutide versus insulin glargine in patients with type 2 diabetes and moderate-to-severe chronic kidney disease (AWARD-7): A multicentre, open-label, randomised trial. Lancet Diabetes Endocrinol 6: 605–617, 2018 [DOI] [PubMed] [Google Scholar]
- 95.Perkovic V, Jardine MJ, Neal B, Bompoint S, Heerspink HJL, Charytan DM, et al.; CREDENCE Trial Investigators: Canagliflozin and renal outcomes in type 2 diabetes and nephropathy. N Engl J Med 380: 2295–2306, 2019 [DOI] [PubMed] [Google Scholar]
- 96.American Diabetes Association: 9. Pharmacologic approaches to glycemic treatment: Standards of medical care in diabetes-2020. Diabetes Care 43[Suppl 1]: S98–S110, 2020 [DOI] [PubMed] [Google Scholar]
- 97.Diabetes Prevention Program Research Group: Long-term safety, tolerability, and weight loss associated with metformin in the Diabetes Prevention Program Outcomes Study. Diabetes Care 35: 731–737, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Desilets AR, Dhakal-Karki S, Dunican KC: Role of metformin for weight management in patients without type 2 diabetes. Ann Pharmacother 42: 817–826, 2008 [DOI] [PubMed] [Google Scholar]
- 99.Angrisani L, Santonicola A, Iovino P, Vitiello A, Higa K, Himpens J, et al.: IFSO worldwide survey 2016: Primary, endoluminal, and revisional procedures. Obes Surg 28: 3783–3794, 2018 [DOI] [PubMed] [Google Scholar]
- 100.Imam TH, Fischer H, Jing B, Burchette R, Henry S, DeRose SF, et al.: Estimated GFR before and after bariatric surgery in CKD. Am J Kidney Dis 69: 380–388, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Doble B, Wordsworth S, Rogers CA, Welbourn R, Byrne J, Blazeby JM; By-Band-Sleeve Trial Management Group: What are the real procedural costs of bariatric surgery? A systematic literature review of published cost analyses [published correction appears in Obes Surg 27: 2193, 2017 10.1007/s11695-017-2768-5]. Obes Surg 27: 2179–2192, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Nasr SH, D’Agati VD, Said SM, Stokes MB, Largoza MV, Radhakrishnan J, et al.: Oxalate nephropathy complicating Roux-en-Y Gastric Bypass: An underrecognized cause of irreversible renal failure. Clin J Am Soc Nephrol 3: 1676–1683, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Cohen JB, Tewksbury CM, Torres Landa S, Williams NN, Dumon KR: National postoperative bariatric surgery outcomes in patients with chronic kidney disease and end-stage kidney disease. Obes Surg 29: 975–982, 2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Nor Hanipah Z, Punchai S, Augustin T, Brethauer SA, Schauer PR, Aminian A: Impact of early postbariatric surgery acute kidney injury on long-term renal function. Obes Surg 28: 3580–3585, 2018 [DOI] [PubMed] [Google Scholar]
- 105.le Roux CW, Heneghan HM: Bariatric surgery for obesity. Med Clin North Am 102: 165–182, 2018 [DOI] [PubMed] [Google Scholar]
- 106.Knowler WC, Barrett-Connor E, Fowler SE, Hamman RF, Lachin JM, Walker EA, et al.; Diabetes Prevention Program Research Group: Reduction in the incidence of type 2 diabetes with lifestyle intervention or metformin. N Engl J Med 346: 393–403, 2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Schauer PR, Bhatt DL, Kirwan JP, Wolski K, Aminian A, Brethauer SA, et al.; STAMPEDE Investigators: Bariatric surgery versus intensive medical therapy for diabetes - 5-year outcomes. N Engl J Med 376: 641–651, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Schiavon CA, Bersch-Ferreira AC, Santucci EV, Oliveira JD, Torreglosa CR, Bueno PT, et al.: Effects of bariatric surgery in obese patients with hypertension: The GATEWAY randomized trial (gastric bypass to treat obese patients with steady hypertension). Circulation 137: 1132–1142, 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Chagnac A, Weinstein T, Herman M, Hirsh J, Gafter U, Ori Y: The effects of weight loss on renal function in patients with severe obesity. J Am Soc Nephrol 14: 1480–1486, 2003 [DOI] [PubMed] [Google Scholar]
- 110.Sheu EG, Channick R, Gee DW: Improvement in severe pulmonary hypertension in obese patients after laparoscopic gastric bypass or sleeve gastrectomy. Surg Endosc 30: 633–637, 2016 [DOI] [PubMed] [Google Scholar]
- 111.Peromaa-Haavisto P, Tuomilehto H, Kössi J, Virtanen J, Luostarinen M, Pihlajamäki J, et al.: Obstructive sleep apnea: The effect of bariatric surgery after 12 months. A prospective multicenter trial. Sleep Med 35: 85–90, 2017 [DOI] [PubMed] [Google Scholar]
- 112.Le Jemtel TH, Samson R, Jaiswal A, Lewine EB, Oparil S: Regression of left ventricular mass after bariatric surgery. Curr Hypertens Rep 19: 68, 2017 [DOI] [PubMed] [Google Scholar]
- 113.Neff KJ, Elliott JA, Corteville C, Abegg K, Boza C, Lutz TA, et al.: Effect of Roux-en-Y gastric bypass and diet-induced weight loss on diabetic kidney disease in the Zucker diabetic fatty rat. Surg Obes Relat Dis 13: 21–27, 2017 [DOI] [PubMed] [Google Scholar]
- 114.Fischer H, Weiss RE, Friedman AN, Imam TH, Coleman KJ: The relationship between kidney function and body mass index before and after bariatric surgery in patients with chronic kidney disease [published online ahead of print November 19, 2020]. Surg Obes Relat Dis 10.1016/j.soard.2020.11.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Friedman AN, Considine RV, Quinney SK: Inquiry into the short- and long-term effects of Roux-en-Y gastric bypass on the glomerular filtration rate. Ren Fail 42: 624–628, 2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Rubino F, Gagner M, Gentileschi P, Kini S, Fukuyama S, Feng J, et al.: The early effect of the Roux-en-Y gastric bypass on hormones involved in body weight regulation and glucose metabolism. Ann Surg 240: 236–242, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Liakopoulos V, Franzén S, Svensson AM, Sattar N, Miftaraj M, Björck S, et al.: Renal and cardiovascular outcomes after weight loss from gastric bypass surgery in type 2 diabetes: Cardiorenal risk reductions exceed atherosclerotic benefits. Diabetes Care 43: 1276–1284, 2020 [DOI] [PubMed] [Google Scholar]
- 118.Cohen RV, Pereira TV, Aboud CM, Petry TBZ, Lopes Correa JL, Schiavon CA, et al.: Effect of gastric bypass vs best medical treatment on early-stage chronic kidney disease in patients with type 2 diabetes and obesity: A randomized clinical trial. JAMA Surg 155: e200420, 2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Levey AS, Gansevoort RT, Coresh J, Inker LA, Heerspink HL, Grams ME, et al.: Change in albuminuria and GFR as end points for clinical trials in early stages of CKD: A scientific workshop sponsored by the National Kidney Foundation in collaboration with the US Food and Drug Administration and European Medicines Agency. Am J Kidney Dis 75: 84–104, 2020 [DOI] [PubMed] [Google Scholar]
- 120.Solerte SB, Fioravanti M, Schifino N, Ferrari E: Effects of diet-therapy on urinary protein excretion albuminuria and renal haemodynamic function in obese diabetic patients with overt nephropathy. Int J Obes 13: 203–211, 1989 [PubMed] [Google Scholar]
- 121.Saiki A, Nagayama D, Ohhira M, Endoh K, Ohtsuka M, Koide N, et al.: Effect of weight loss using formula diet on renal function in obese patients with diabetic nephropathy. Int J Obes 29: 1115–1120, 2005 [DOI] [PubMed] [Google Scholar]
- 122.Nicholson AS, Sklar M, Barnard ND, Gore S, Sullivan R, Browning S: Toward improved management of NIDDM: A randomized, controlled, pilot intervention using a lowfat, vegetarian diet. Prev Med 29: 87–91, 1999 [DOI] [PubMed] [Google Scholar]
- 123.Praga M, Hernández E, Andrés A, León M, Ruilope LM, Rodicio JL: Effects of body-weight loss and captopril treatment on proteinuria associated with obesity. Nephron 70: 35–41, 1995 [DOI] [PubMed] [Google Scholar]
- 124.Morales E, Valero MA, León M, Hernández E, Praga M: Beneficial effects of weight loss in overweight patients with chronic proteinuric nephropathies. Am J Kidney Dis 41: 319–327, 2003 [DOI] [PubMed] [Google Scholar]
- 125.Friedman AN, Chambers M, Kamendulis LM, Temmerman J: Short-term changes after a weight reduction intervention in advanced diabetic nephropathy. Clin J Am Soc Nephrol 8: 1892–1898, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Howden EJ, Leano R, Petchey W, Coombes JS, Isbel NM, Marwick TH: Effects of exercise and lifestyle intervention on cardiovascular function in CKD. Clin J Am Soc Nephrol 8: 1494–1501, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Tirosh A, Golan R, Harman-Boehm I, Henkin Y, Schwarzfuchs D, Rudich A, et al.: Renal function following three distinct weight loss dietary strategies during 2 years of a randomized controlled trial. Diabetes Care 36: 2225–2232, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Goraya N, Simoni J, Jo CH, Wesson DE: Treatment of metabolic acidosis in patients with stage 3 chronic kidney disease with fruits and vegetables or oral bicarbonate reduces urine angiotensinogen and preserves glomerular filtration rate. Kidney Int 86: 1031–1038, 2014 [DOI] [PubMed] [Google Scholar]
- 129.Greenwood SA, Koufaki P, Mercer TH, MacLaughlin HL, Rush R, Lindup H, et al.: Effect of exercise training on estimated GFR, vascular health, and cardiorespiratory fitness in patients with CKD: A pilot randomized controlled trial. Am J Kidney Dis 65: 425–434, 2015 [DOI] [PubMed] [Google Scholar]
- 130.Ikizler TA, Robinson-Cohen C, Ellis C, Headley SAE, Tuttle K, Wood RJ, et al.: Metabolic effects of diet and exercise in patients with moderate to severe CKD: A randomized clinical trial. J Am Soc Nephrol 29: 250–259, 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.MacLaughlin HL, Cook SA, Kariyawasam D, Roseke M, van Niekerk M, Macdougall IC: Nonrandomized trial of weight loss with orlistat, nutrition education, diet, and exercise in obese patients with CKD: 2-year follow-up. Am J Kidney Dis 55: 69–76, 2010 [DOI] [PubMed] [Google Scholar]
- 132.Look AHEAD Research Group: Effect of a long-term behavioural weight loss intervention on nephropathy in overweight or obese adults with type 2 diabetes: A secondary analysis of the Look AHEAD randomised clinical trial. Lancet Diabetes Endocrinol 2: 801–809, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Mann JFE, Ørsted DD, Brown-Frandsen K, Marso SP, Poulter NR, Rasmussen S, et al.; LEADER Steering Committee and Investigators: Liraglutide and renal outcomes in type 2 diabetes. N Engl J Med 377: 839–848, 2017 [DOI] [PubMed] [Google Scholar]
- 134.Scirica BM, Bohula EA, Dwyer JP, Qamar A, Inzucchi SE, McGuire DK, et al.: Lorcaserin and renal outcomes in obese and overweight patients in the CAMELLIA-TIMI 61 trial. Circulation 139: 366–375, 2019 [DOI] [PubMed] [Google Scholar]
- 135.Palomar R, Fernández-Fresnedo G, Domínguez-Diez A, López-Deogracias M, Olmedo F, Martín de Francisco AL, et al.: Effects of weight loss after biliopancreatic diversion on metabolism and cardiovascular profile. Obes Surg 15: 794–798, 2005 [DOI] [PubMed] [Google Scholar]
- 136.Chang AR, Chen Y, Still C, Wood GC, Kirchner HL, Lewis M, et al.: Bariatric surgery is associated with improvement in kidney outcomes. Kidney Int 90: 164–171, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Friedman AN, Wahed AS, Wang J, Courcoulas AP, Dakin G, Hinojosa MW, et al.: Effect of bariatric surgery on CKD risk. J Am Soc Nephrol 29: 1289–1300, 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Shulman A, Peltonen M, Sjöström CD, Andersson-Assarsson JC, Taube M, Sjöholm K, et al.: Incidence of end-stage renal disease following bariatric surgery in the Swedish Obese Subjects Study. Int J Obes 42: 964–973, 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Friedman AN, Wang J, Wahed AS, Docherty NG, Fennern E, Pomp A, et al.: The association between kidney disease and diabetes remission in bariatric surgery patients with type 2 diabetes. Am J Kidney Dis 74: 761–770, 2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.O’Brien R, Johnson E, Haneuse S, Coleman KJ, O’Connor PJ, Fisher DP, et al.: Microvascular outcomes in patients with diabetes after bariatric surgery versus usual care: A matched cohort study. Ann Intern Med 169: 300–310, 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Aminian A, Zajichek A, Arterburn DE, Wolski KE, Brethauer SA, Schauer PR, et al.: Association of metabolic surgery with major adverse cardiovascular outcomes in patients with type 2 diabetes and obesity. JAMA 322: 1271–1282, 2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Alkharaiji M, Anyanwagu U, Donnelly R, Idris I: Effect of bariatric surgery on diagnosed chronic kidney disease and cardiovascular events in patients with insulin-treated type 2 diabetes: A retrospective cohort study from a large UK primary care database. Obes Surg 30: 1685–1695, 2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Chang AR, Wood GC, Chu X, Surapaneni A, Grams ME: Association of bariatric surgery with rates of kidney function decline using multiple filtration markers. JAMA Netw Open 3: e2014670, 2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Hansel B, Arapis K, Kadouch D, Ledoux S, Coupaye M, Msika S, et al.: Severe chronic kidney disease is associated with a lower efficiency of bariatric surgery. Obes Surg 29: 1514–1520, 2019 [DOI] [PubMed] [Google Scholar]
- 145.Rubino F, Nathan DM, Eckel RH, Schauer PR, Alberti KGMM, Zimmet PZ,et al.; Delegates of the 2nd Diabetes Surgery Summit: Metabolic surgery in the treatment algorithm for type 2 diabetes: A joint statement by international diabetes organizations. Diabetes Care 39: 861–877, 2016 [DOI] [PubMed] [Google Scholar]
- 146.Ikizler TA, Burrowes JD, Byham-Gray LD, Campbell KL, Carrero JJ, Chan W, et al.: KDOQI clinical practice guideline for nutrition in CKD: 2020 update. Am J Kidney Dis 76[Suppl 1]: S1–S107, 2020 [DOI] [PubMed] [Google Scholar]
- 147.Kidney Disease: Improving Global Outcomes (KDIGO) Diabetes Work Group, KDIGO 2020. clinical practice guideline for diabetes management in chronic kidney disease. Available at https://kdigo.org/guidelines/diabetes-ckd/. Accessed January 18, 2021
- 148.Woolf SH, Grol R, Hutchinson A, Eccles M, Grimshaw J: Clinical guidelines: Potential benefits, limitations, and harms of clinical guidelines. BMJ 318: 527–530, 1999 [DOI] [PMC free article] [PubMed] [Google Scholar]


