Significance Statement
The effects of AKI on clinical outcomes of severe acute respiratory syndrome (SARS) and coronavirus disease 2109 (COVID-19) are unclear. The authors’ territory-wide, retrospective cohort study showed higher rates of AKI and major adverse clinical outcomes among patients with SARS than in those with COVID-19. Among patients with either of these two coronavirus infections, patients with diabetes mellitus, abnormal liver function, or AKI were significantly more likely to have major adverse clinical outcomes. Diabetes mellitus and hypertension were significant factors that were associated with AKI in patients with either SARS or COVID-19. Among patients with AKI, those with COVID-19 were less likely have major adverse clinical outcomes compared with patients who had SARS. In patients with either SARS or COVID-19, renal function usually recovered within 30 days of an initial AKI event.
Keywords: kidney, kidney disease, kidney dysfunction, renal dysfunction, renal injury, renal function decline, COVID-19
Abstract
Background
Severe acute respiratory syndrome (SARS) and coronavirus disease 2019 (COVID-19) are closely related. The effect of AKI on the clinical outcomes of these two conditions is unclear.
Methods
This retrospective, territory-wide cohort study used an electronic public healthcare database in Hong Kong to identify patients with SARS or COVID-19 by diagnosis codes, virologic results, or both. The primary endpoint was a composite of intensive care unit admission, use of invasive mechanical ventilation, and/or death.
Results
We identified 1670 patients with SARS and 1040 patients with COVID-19 (median ages, 41 versus 35 years, respectively). Among patients with SARS, 26% met the primary endpoint versus 5.3% of those with COVID-19. Diabetes mellitus, abnormal liver function, and AKI were factors significantly associated with the primary endpoint among patients with either SARS or COVID-19. Among patients with SARS, 7.9%, 2.1%, and 3.7% developed stage 1, stage 2, and stage 3 AKI, respectively; among those with COVID-19, 6.6%, 0.4%, and 1.1% developed stage 1, stage 2, and stage 3 AKI, respectively. In both groups, factors significantly associated with AKI included diabetes mellitus and hypertension. Among patients with AKI, those with COVID-19 had a lower rate of major adverse clinical outcomes versus patients with SARS. Renal function recovery usually occurred within 30 days after an initial AKI event.
Conclusions
AKI rates were higher among patients with SARS than those with COVID-19. AKI was associated with major adverse clinical outcomes for both diseases. Patients with diabetes mellitus and abnormal liver function were also at risk of developing severe consequences after SARS and COVID-19 infection.
Coronavirus disease 2019 (COVID-19) was declared a pandemic by the World Health Organization on March 11, 2020, and the disease spread rapidly to more than 200 countries worldwide.1,2 As of July 25, 2020, more than 15 million patients and 635,000 deaths had been reported globally.3 The exponential increment of patients with COVID-19 has had a severe effect on the health care systems globally.4,5
In fact, COVID-19 is not the first human coronavirus disease that has resulted in such global health concern. In 2003, the severe acute respiratory syndrome (SARS) outbreak affected 29 countries and resulted in a total of 8422 patients worldwide.6 Asian countries were mostly affected during the SARS outbreak,7 and Hong Kong is one of the few areas suffering from both SARS and COVID-19.8
COVID-19 generally has a lower mortality rate than SARS; however, it can still impose a severe threat to certain vulnerable patients.9,10 Angiotensin-converting enzyme 2 (ACE2) has been established as a host receptor for both SARS and COVID-19.11,12 As the kidney has an abundant expression of ACE2 receptors, it has been postulated that this organ may be at risk of damage from COVID-19 infection. The degree of kidney injury may also have important implications on the clinical outcomes of COVID-19.
In this study, we utilized a territory-wide cohort to compare the degree of AKI in patients with SARS or COVID-19, and investigate the effect of AKI on major adverse clinical outcomes. We further investigated the factors associated with AKI and described the temporal trend of renal function recovery along the clinical course.
Methods
Study Design and Data Source
We conducted a territory-wide retrospective cohort study using the Clinical Data Analysis and Reporting System (CDARS) under the Hospital Authority in Hong Kong.13 CDARS is an electronic health care database used in all public hospitals and clinics in Hong Kong. Information including patients’ demographics, death, diagnoses, procedures, drug prescription and dispensing history, and laboratory results were all documented in CDARS.14 In Hong Kong, all confirmed patients with SARS or COVID-19 required hospitalization in public hospitals. All data extracted from CDARS were deidentified to ensure confidentiality. The International Classification of Diseases, Ninth Revision, Clinical Modification (ICD-9-CM) coding was used in CDARS. Using the ICD-9-CM codes to identify medical conditions in CDARS was 99% accurate with reference to clinical, laboratory, imaging, and endoscopy results from the electronic medical records.15 This methodology has been used in studies on various infectious diseases previously.16–18 The study protocol was approved by the Joint Chinese University of Hong Kong—New Territories East Cluster Research Ethics Committee.
Study Subjects
Consecutive patients with SARS from March to June 2003 were identified by ICD-9-CM diagnosis codes and/or virologic results. Consecutive laboratory-confirmed patients with COVID-19 from January 23, 2020 to May 1, 2020 were identified by virologic results. Baseline characteristics of all identified patients were reported. For the subsequent analyses on serial changes in renal function, we excluded patients with missing creatinine measurement at the time of diagnosis of SARS or COVID-19, patients who had fewer than two creatinine measurements during follow-up, and patients who were on RRT before the diagnosis of SARS or COVID-19. All patients were followed-up for at least 30 days from the diagnosis of SARS or COVID-19.
Data Collection
The date of SARS or COVID-19 diagnosis was on the basis of the date of ICD-9-CM diagnosis code and/or positive virologic results for patients with SARS, and positive virologic results alone for patients with COVID-19. At baseline, demographic data including date of birth and sex were retrieved. Data on relevant diagnoses and procedures, hematologic parameters, liver and renal biochemistries, and other laboratory parameters were retrieved. Serial changes in creatinine levels were recorded until the end of follow-up. Information on the use of medications including nonsteroid anti-inflammatory medications, ACE inhibitors, angiotensin receptor blockers, diuretics, antihypertensives, antiplatelet, anticoagulants, antibiotics, antivirals, antifungals, corticosteroids, IFN beta, and intravenous Ig during hospitalization were also retrieved.
Primary Endpoint and Definitions of Events
The primary endpoint was a composite endpoint of intensive care unit (ICU) admission, use of invasive mechanical ventilation, and/or death. Information on any ICU admissions was extracted from CDARS directly. Use of invasive mechanical ventilation was defined by the ICD-9-CM procedure codes (96.7). Death and its date were captured from CDARS and the Hong Kong Death Registry. AKI was defined by the creatinine level and the need of RRT according to the Kidney Disease Improving Global Outcomes (KDIGO) criteria.19 Persistence of AKI stage 1 or greater was defined as acute kidney disease (AKD) at day 30, and CKD at day 90, from the initial AKI event.20 Renal recovery was defined as complete recovery from any AKI, AKD, or CKD. eGFR was calculated using the CKD-Epidemiology Collaboration equation for patients aged 18 years or above.21 Data on RRT were retrieved using ICD-9-CM procedure codes (38.95, 39.27, 39.42, 39.43, 39.95, 54.98). Diabetes mellitus (DM) was defined by hemoglobin A1c ≥6.5%, fasting plasma glucose ≥7 mmol/L in two measurements or ≥11.1 mmol/L in one measurement, any use of antidiabetic medications, and/or ICD-9-CM diagnosis codes for DM (250). Hypertension was defined by any use of antihypertensives and/or ICD-9-CM diagnosis codes for hypertension (401–404).
Statistical Analyses
Baseline characteristics of the whole cohort were reported. Continuous variables were expressed in mean±SD or median (interquartile range), as appropriate, whereas categorical variables were presented as number (percentage). We analyzed and compared the peak creatinine levels, the risk, and the degree of AKI between patients who had SARS or COVID-19 infection. We made further comparisons between patients with SARS or COVID-19, among those who met our predefined primary endpoint and those who did not. Qualitative and quantitative differences between subgroups were analyzed by Mann–Whitney U test for continuous parameters, and chi-squared or Fisher’s exact tests for categorical parameters, as appropriate. Univariate and multivariable logistic regression analyses were performed to investigate for factors associated with the primary endpoint. Factors associated with AKI were also analyzed by logistic regression in the whole cohort, and separately in the SARS group and the COVID-19 group. Further logistic regression analysis on the primary endpoint was performed among patients who developed SARS- or COVID-19–related AKI. Odds ratios (ORs) and adjusted ORs (aORs) with 95% confidence intervals (95% CI) were estimated. Hosmer-Lemeshow goodness-of-fit test was used to assess the goodness of fit of the model. All statistical tests were two sided. For patients with AKI, we compared their creatinine levels at day 0, 7, 30, and 90 using a Friedman test; a Bhapkar test was used to compare the AKI stages between day 0 and 7; a generalized estimating equation for logistic regression was used to compare the incidence of AKI, AKD, or CKD at day 7, 30, and 90. P<0.05 was considered statistically significant. All statistical analyses were performed using SPSS version 25.0 (SPSS Inc., Chicago, Illinois), and R software (3.6.3; R Foundation for Statistical Computing, Vienna, Austria).
Results
Demographics
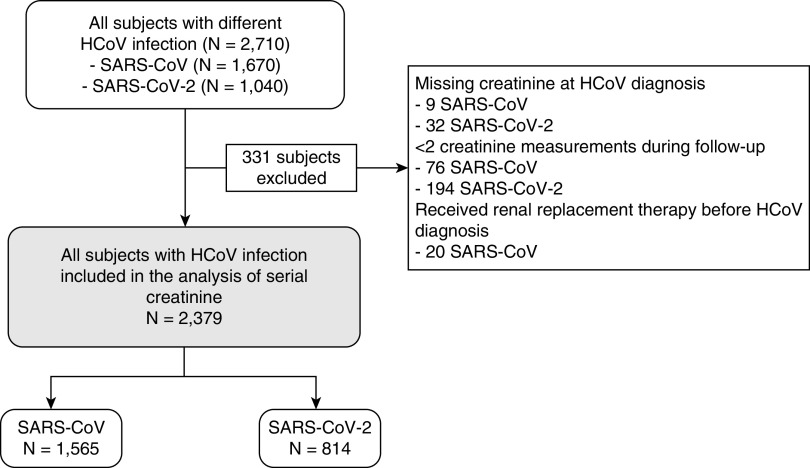
The whole cohort comprised 2710 patients, including 1670 patients with SARS and 1040 patients with COVID-19 (Figure 1). The median age was 41 years for patients with SARS and 35 years for patients with COVID-19 (P<0.001). At baseline, most patients had good renal function as reflected by their creatinine levels (median of 80 μmol/L for patients with SARS and 71 μmol/L for patients with COVID-19, P<0.001) and eGFR (mean of 83.8±25.1 ml/min per 1.73 m2 for patients with SARS and 102.3±19.0 ml/min per 1.73 m2 for patients with COVID-19, P<0.001). In the SARS group, 20 patients (1.3%) had prior RRT in the form of peritoneal dialysis or hemodialysis. More patients in the SARS group had known history of DM (16.6% versus 7.8%, P<0.001) and hypertension (21.3% versus 13.8%, P<0.001) than the COVID-19 group. Antivirals were commonly used in patients with SARS or COVID-19. Antibiotics and corticosteroids were more commonly used in patients with SARS than patients with COVID-19. The demographics are summarized in Table 1.
Figure 1.
Selection of patients with SARS or COVID-19. HCoV, human coronavirus.
Table 1.
Clinical characteristics of patients infected by SARS-CoV and SARS-CoV-2
| Clinical Characteristics | SARS-CoV, n=1670 | SARS-CoV-2, n=1040 | P value |
|---|---|---|---|
| Male sex, n (%) | 734 (44.0) | 560 (53.8) | <0.001 |
| Age, yr, mean±SD | 44±20 | 38±18 | <0.001 |
| Age in yr, median (interquartile range) | 41 (29–56) | 35 (22–52) | <0.001 |
| Creatinine, μmol/L, median (interquartile range) | 80 (67–97) | 71 (60–84) | <0.001 |
| Missing, % | 0.5 | 3.1 | |
| eGFR, ml/min per 1.73 m2, mean±SD | 83.8±25.1 | 102.3±19.0 | <0.001 |
| Missing, %a | 6.8 | 8.3 | |
| RRT, n (%) | 20 (1.3) | 0 (0) | <0.001 |
| Peritoneal dialysis | 13 (0.8) | 0 (0) | 0.003 |
| Hemodialysis | 7 (0.4) | 0 (0) | 0.05 |
| Platelet, ×109/L, mean±SD | 196±84 | 229±75 | <0.001 |
| Platelet <100×109/L, n (%) | 85 (5.1) | 5 (0.5) | <0.001 |
| Missing, % | 0.3 | 3.6 | |
| ALT, U/L, median (interquartile range) | 23 (15–39) | 22 (15–34) | 0.13 |
| Missing, % | 0.6 | 3.5 | |
| AST, U/L, median (interquartile range) | 26 (19–39) | 26 (21–37) | 0.53 |
| Missing, % | 51.5 | 53.8 | |
| Albumin, g/L, mean±SD | 39±5 | 42±5 | <0.001 |
| Missing, % | 0.6 | 3.5 | |
| Total bilirubin, μmol/L, mean±SD | 9±10 | 8±5 | <0.001 |
| Missing, % | 0.6 | 3.5 | |
| INR, mean±SD | 1.1±0.3 | 1.1±0.1 | 0.70 |
| Missing, % | 11.7 | 46.3 | |
| CRP, mg/dl, mean±SD | 4.2±5.8 | 1.4±3.5 | <0.001 |
| Missing, % | 23.1 | 6.7 | |
| ESR, mm/h, mean±SD | 32±31 | 24±23 | <0.001 |
| Missing, % | 45.9 | 49.7 | |
| LDH, U/L, median (interquartile range) | 300 (208–423) | 184 (156–223) | <0.001 |
| Missing, % | 3.9 | 10.3 | |
| WCC, ×109/L, mean±SD | 6.7±3.5 | 5.8±2.0 | <0.001 |
| WCC <3.5×109/L, n (%) | 160 (9.6) | 88 (8.8) | 0.47 |
| Missing, % | 0.3 | 3.6 | |
| Neutrophil, ×109/L, mean±SD | 5.1±3.2 | 3.7±1.8 | <0.001 |
| Missing, % | 0.1 | 3.8 | |
| Lymphocyte, ×109/L, mean±SD | 1.0±0.7 | 1.5±0.7 | <0.001 |
| Lymphocyte <1×109/L, n (%) | 927 (55.6) | 222 (22.2) | <0.001 |
| Missing, % | 0.1 | 3.8 | |
| Diabetes mellitus, n (%) | 277 (16.6) | 81 (7.8) | <0.001 |
| Hypertension, n (%) | 355 (21.3) | 144 (13.8) | <0.001 |
| Use of medications during follow-up, n (%) | |||
| NSAID | 122 (7.3) | 26 (2.5) | <0.001 |
| ACEI | 99 (5.9) | 21 (2.0) | <0.001 |
| ARB | 4 (0.2) | 41 (3.9) | <0.001 |
| Thiazide diuretics | 18 (1.1) | 6 (0.6) | 0.18 |
| Loop diuretics | 161 (9.6) | 31 (3.0) | <0.001 |
| Potassium-sparing diuretics | 22 (1.3) | 5 (0.5) | 0.03 |
| Other antihypertensive drugsb | 230 (13.8) | 113 (10.9) | 0.03 |
| Antiplatelet | 135 (8.1) | 38 (3.7) | <0.001 |
| Anticoagulants | 166 (9.9) | 38 (3.7) | <0.001 |
| Oseltamivir | 145 (8.7) | 66 (6.3) | 0.03 |
| Ribavirin | 1511 (90.5) | 538 (51.7) | <0.001 |
| Lopinavir-ritonavir | 168 (10.1) | 612 (58.8) | <0.001 |
| IFN beta | 0 (0) | 330 (31.7) | <0.001 |
| Antibiotic treatment | 1610 (96.4) | 377 (36.3) | <0.001 |
| Antifungal treatment | 161 (9.6) | 2 (0.2) | <0.001 |
| Corticosteroid at baselinec | 69 (4.1) | 0 (0) | <0.001 |
| Corticosteroid during follow-up | 1427 (85.4) | 59 (5.7) | <0.001 |
| Pulse methylprednisolone ≥250 mg daily, | 1076 (64.4) | 5 (0.5) | <0.001 |
| Peak daily dose, prednisolone equivalent, mg, median (interquartile range) | 625 (625–625) | 37.5 (37.5–50.0) | <0.001 |
| IVIG | 175 (10.5) | 3 (0.3) | <0.001 |
| Clinical outcomes in 30 d, n (%) | |||
| Primary endpoint | 435 (26.0) | 55 (5.3) | <0.001 |
| Death | 180 (10.8) | 4 (0.4) | <0.001 |
| ICU admission | 330 (19.8) | 53 (5.1) | <0.001 |
| Invasive mechanical ventilation | 62 (3.7) | 22 (2.1) | 0.02 |
All concomitant medications were represented as binary parameters. Percentages were computed on the basis of nonmissing values. INR, international normalized ratio; CRP, C-reactive protein; ESR, erythrocyte sedimentation rate; LDH, lactate dehydrogenase; WCC, white cell count; NSAID, nonsteroidal anti-inflammatory drugs; ACEI, angiotensin-converting enzyme inhibitors; ARB, angiotensin receptor blocker; IVIG, intravenous immunoglobulin therapy.
eGFR was estimated using CKD-Epidemiology Collaboration equation for patients aged above 18 yr.
Other antihypertensive drugs included beta blockers and calcium channel blockers.
Corticosteroid use at baseline referred to the use of steroid within 1 mo before the diagnosis of CoV.
Peak Creatinine Levels and AKI after the Diagnosis of SARS and COVID-19
After excluding those with missing creatinine level measurements and those who were on prior RRT, 2379 patients remained for subsequent analyses. The median peak creatinine level was 86 μmol/L in the SARS group and 82 μmol/L in the COVID-19 group (P<0.001). For the SARS group, 7.9%, 2.1%, and 3.7% of the patients developed stage 1, stage 2, and stage 3 AKI, respectively. For the COVID-19 group, 6.6%, 0.4%, and 1.1% of the patients developed stage 1, stage 2, and stage 3 AKI, respectively (Table 2).
Table 2.
Occurrence of AKI in the first 30 d after the diagnosis of SARS-CoV and SARS-CoV-2
| Occurrence of AKI | SARS-CoVa n=1565 | SARS-CoV-2 n=814 | P valueb |
|---|---|---|---|
| Peak creatinine level median (range) | 86 (25–1975) | 82 (21–1137) | <0.001 |
| AKI, n (%) | <0.001 | ||
| No AKI | 1351 (86.3) | 748 (91.9) | |
| AKI stage 1 | 123 (7.9) | 54 (6.6) | |
| AKI stage 2 | 33 (2.1) | 3 (0.4) | |
| AKI stage 3 | 58 (3.7) | 9 (1.1) | |
| Presence of moderate to severe AKI, n (%) | 91 (5.8) | 12 (1.5) | <0.001 |
Baseline creatinine level was on the basis of the creatinine level before or upon hospital admission. Peak creatinine level was presented in median (range). Qualitative and quantitative differences between subgroups were analyzed by chi-squared or Fisher’s exact tests for categorical parameters and Mann–Whitney U test for continuous parameters, as appropriate.
A total of 20 patients who received RRT at diagnosis of SARS-CoV were not included in the analysis.
Mann–Whitney U test for continuous variable, and chi-squared or Fisher’s exact tests for categorical variables were used to compare patients infected by SARS-CoV and SARS-CoV-2.
Primary Endpoint and Individual Study Outcomes
In the SARS group, 26% met the primary endpoint; 10.8% died, 19.8% required ICU admission, and 3.7% required invasive mechanical ventilation. In the COVID-19 group, 5.3% met the primary endpoint; 0.4% died, 5.1% required ICU admission, and 2.1% required invasive mechanical ventilation.
Among the patients who did not meet the primary endpoint, patients with SARS had higher peak creatinine levels and higher stages of AKI than patients with COVID-19. Such difference between the patients with SARS or COVID-19 was consistently demonstrated among those who did not require ICU admission, those who did not require invasive mechanical ventilation, and those who survived. Among the patients who met the primary endpoint, there was no difference in the peak creatinine levels and the stages of AKI between patients with SARS or COVID-19. Similarly, no difference was detected between patients with SARS or COVID-19 among those who required ICU admission, those who required invasive mechanical ventilation, and those who died. These results were, however, limited by the small number of patients and events, particularly in the COVID-19 group. The results are summarized in Table 3.
Table 3.
AKI in the first 30 d among patients infected by SARS-CoV and SARS-CoV-2 with or without the study outcomes
| Occurrence of AKI | Without Primary Endpoint | With Primary Endpoint | ||||
|---|---|---|---|---|---|---|
| SARS-CoV, n=1148 | SARS-CoV-2, n=759 | P value | SARS-CoV, n=417 | SARS-CoV-2, n=55 | P value | |
| Peak creatinine level median (range) | 83 (73–96) | 81 (68–95) | 0.001 | 101 (81–177) | 89 (76–119) | 0.06 |
| No AKI, n (%) | 1083 (94.3) | 707 (93.1) | 0.04 | 268 (64.3) | 41 (74.5) | 0.07 |
| AKI stage 1, n (%) | 55 (4.8) | 49 (6.5) | 68 (16.3) | 5 (9.1) | ||
| AKI stage 2, n (%) | 3 (0.3) | 3 (0.4) | 30 (7.2) | 0 (0) | ||
| AKI stage 3, n (%) | 7 (0.6) | 0 (0) | 51 (12.2) | 9 (16.4) | ||
| Without ICU admission | With ICU admission | |||||
| SARS-CoV, n=1240 | SARS-CoV-2, n=761 | P value | SARS-CoV, n=325 | SARS-CoV-2, n=53 | P value | |
| Peak creatinine level median (range) | 84 (73–99) | 81 (68–95) | <0.001 | 97 (80–144) | 88 (75–118) | 0.12 |
| No AKI, n (%) | 1128 (91.0) | 708 (93.0) | 0.003 | 223 (68.6) | 40 (75.5) | 0.11 |
| AKI stage 1, n (%) | 82 (6.6) | 50 (6.6) | 41 (12.6) | 4 (7.5) | ||
| AKI stage 2, n (%) | 10 (0.8) | 3 (0.4) | 23 (7.1) | 0 (0) | ||
| AKI stage 3, n (%) | 20 (1.6) | 0 (0) | 38 (11.7) | 9 (17.0) | ||
| Without invasive mechanical ventilation | With invasive mechanical ventilation | |||||
| SARS-CoV, n=1504 | SARS-CoV-2, n=792 | P value | SARS-CoV, n=61 | SARS-CoV-2, n=22 | P value | |
| Peak creatinine level median (range) | 86 (74–102) | 81 (68–95) | <0.001 | 109 (87–181) | 88 (77–315) | 0.24 |
| No AKI, n (%) | 1319 (87.7) | 734 (92.7) | <0.001 | 32 (52.5) | 14 (63.6) | 0.41 |
| AKI stage 1, n (%) | 112 (7.4) | 51 (6.4) | 11 (18.0) | 3 (13.6) | ||
| AKI stage 2, n (%) | 26 (1.7) | 3 (0.4) | 7 (11.5) | 0 (0) | ||
| AKI stage 3, n (%) | 47 (3.1) | 4 (0.5) | 11 (18.0) | 5 (22.7) | ||
| Survival | Death | |||||
| SARS-CoV, n=1398 | SARS-CoV-2, n=810 | P value | SARS-CoV, n=167 | SARS-CoV-2, n=4 | P value | |
| Peak creatinine level median (range) | 84 (73–98) | 81 (68–95) | <0.001 | 161 (104–289) | 332 (117–595) | 0.31 |
| No AKI, n (%) | 1290 (92.3) | 747 (92.2) | 0.27 | 61 (36.5) | 1 (25.0) | 0.81 |
| AKI stage 1, n (%) | 77 (5.5) | 53 (6.5) | 46 (27.5) | 1 (25.0) | ||
| AKI stage 2, n (%) | 13 (0.9) | 3 (0.4) | 20 (12.0) | 0 (0) | ||
| AKI stage 3, n (%) | 18 (1.3) | 7 (0.9) | 40 (24.0) | 2 (50.0) | ||
A total of 20 patients who received RRT at diagnosis of SARS-CoV were not included in the analysis.
Primary endpoint was a composite endpoint of ICU admission, use of invasive mechanical ventilation, and/or death. Baseline creatinine level was on the basis of the creatinine level before or upon hospital admission. Peak creatinine level was presented in median (range). Qualitative and quantitative differences between subgroups were analyzed by chi-squared or Fisher’s exact tests for categorical parameters and Mann–Whitney U test for continuous parameters, as appropriate.
Of note, among the 325 patients with SARS who were admitted to ICU or received invasive mechanical ventilation, 105 (32.0%) had AKI; 13 of them (12.4%) had AKI before ICU admission or the use of invasive mechanical ventilation. Among the 53 patients with COVID-19 who were admitted to ICU or received invasive mechanical ventilation, 13 (24.5%) had AKI; one of them (7.7%) had AKI before ICU admission or use of invasive mechanical ventilation.
On multivariable logistic regression analysis for patients with SARS, old age (aOR, 1.02; 95% CI, 1.01 to 1.03, P<0.001), DM (aOR, 2.67; 95% CI, 1.93 to 3.69, P<0.001), hypertension (aOR, 1.65; 95% CI, 1.17 to 2.33, P=0.005), peak alanine aminotransferase (ALT) and/or aspartate aminotransferase (AST) ≥80 U/L (aOR, 2.47; 95% CI, 1.87 to 3.25, P<0.001) and AKI (aOR, 4.96; 95% CI, 3.48 to 7.06, P<0.001) were significant factors associated with the primary endpoint. For patients with COVID-19, DM (aOR, 4.77; 95% CI, 2.32 to 9.84, P<0.001), lymphocyte count of <1×109/L (aOR, 1.93; 95% CI, 1.01 to 3.69, P=0.05), peak ALT and/or AST ≥80 U/L (aOR, 7.64; 95% CI, 3.99 to 14.64, P<0.001), and AKI (aOR, 2.44; 95% CI, 1.06 to 5.58, P=0.04) were significant factors associated with the primary endpoint. The results are summarized in Table 4.
Table 4.
Univariate and multivariable analysis by logistic regression on factors associated with primary endpoint (a composite endpoint of admission to intensive care unit, use of invasive mechanical ventilation, and death) in patients infected by SARS-CoV and SARS-CoV-2
| Parameters | SARS-CoV (n=1565)a | SARS-CoV-2 (n=814) | ||||||
|---|---|---|---|---|---|---|---|---|
| Univariate Analysis | Multivariable Analysisb | Univariate Analysis | Multivariable Analysisc,d | |||||
| OR (95% CI) | P value | aOR (95% CI) | P value | OR (95% CI) | P value | aOR (95% CI) | P value | |
| Age | 1.04 (1.03 to 1.04) | <0.001 | 1.02 (1.01 to 1.03) | <0.001 | 1.05 (1.03 to 1.07) | <0.001 | 1.02 (1.00 to 1.04) | 0.05 |
| Male sex | 1.76 (1.40 to 2.21) | <0.001 | 1.76 (0.99 to 3.12) | 0.06 | ||||
| Diabetes mellitus | 4.89 (3.70 to 6.46) | <0.001 | 2.67 (1.93 to 3.69) | <0.001 | 11.33 (6.21 to 20.66) | <0.001 | 4.77 (2.32 to 9.84) | <0.001 |
| Hypertension | 4.24 (3.27 to 5.49) | <0.001 | 1.65 (1.17 to 2.33) | 0.005 | 6.01 (3.41 to 10.59) | <0.001 | ||
| Lymphocyte <1×109/L | 1.10 (0.87 to 1.38) | 0.43 | 2.90 (1.67 to 5.04) | <0.001 | 1.93 (1.01 to 3.69) | 0.048 | ||
| Platelet <100×109/L | 1.29 (0.80 to 2.09) | 0.29 | 3.49 (0.38 to 31.78) | 0.27 | ||||
| Peak ALT and/or AST ≥80 U/L | 1.72 (1.37 to 2.16) | <0.001 | 2.47 (1.87 to 3.25) | <0.001 | 10.59 (5.76 to 19.49) | <0.001 | 7.64 (3.99 to 14.64) | <0.001 |
| AKI defined by KDIGO | 9.26 (6.72 to 12.76) | <0.001 | 4.96 (3.48 to 7.06) | <0.001 | 4.64 (2.38 to 9.06) | <0.001 | 2.44 (1.06 to 5.58) | 0.04 |
Twenty patients who received RRT at diagnosis of SARS-CoV were not included in the analysis.
P value = 0.20 for Hosmer-Lemeshow goodness-of-fit test, which did not indicate significant poor fit.
P value = 0.77 for Hosmer-Lemeshow goodness-of-fit test, which did not indicate significant poor fit.
Age was retained in the model on the basis of the result of backward stepwise selection by likelihood ratio test with a P value of 0.05.
Factors Associated with AKI
Among the patients with SARS, age (aOR, 1.04; 95% CI, 1.03 to 1.05, P<0.001), DM (aOR, 2.03; 95% CI, 1.41 to 2.92, P<0.001), hypertension (aOR, 1.54; 95% CI, 1.04 to 2.29, P=0.03), and baseline creatinine level (aOR, 1.01; 95% CI, 1.00 to 1.01, P<0.001) were significant factors associated with AKI (Table 5). Among the patients with COVID-19 (Table 5), DM (aOR, 2.23; 95% CI, 1.11 to 4.48, P=0.03), hypertension (aOR, 3.03; 95% CI, 1.63 to 5.62, P<0.001), and the use of antiviral agents (aOR, 11.06; 95% CI, 2.66 to 45.98, P=0.001) were significant factors associated with AKI. For the whole cohort (Supplemental Table 1), on multivariable logistic regression analysis, age (aOR, 1.03; 95% CI, 1.02 to 1.04, P<0.001), DM (aOR, 1.94; 95% CI, 1.41 to 2.67, P<0.001), hypertension (aOR, 1.67; 95% CI, 1.19 to 2.34, P=0.003), the use of antiviral agents (aOR, 1.80; 95% CI, 1.08 to 3.01, P=0.03), and baseline creatinine level (aOR, 1.01; 95% CI, 1.00 to 1.01, P<0.001) were significant factors associated with AKI.
Table 5.
Univariate and multivariable analysis by logistic regression on factors associated with AKI in patients infected by SARS-CoV and SARS-CoV-2 separately
| Parameters | SARS-CoV n=1565 | SARS-CoV-2 n=814 | ||||||
|---|---|---|---|---|---|---|---|---|
| Univariate analysis | Multivariable analysisa | Univariate analysis | Multivariable analysisb | |||||
| OR (95% CI) | P value | aOR (95% CI) | P value | OR (95% CI) | P value | aOR (95% CI) | P value | |
| Age | 1.05 (1.04 to 1.06) | <0.001 | 1.04 (1.03 to 1.05) | <0.001 | 1.03 (1.01 to 1.04) | <0.001 | ||
| Male sex | 1.78 (1.33 to 2.38) | <0.001 | 1.32 (0.79 to 2.20) | 0.29 | ||||
| Diabetes mellitus | 4.43 (3.23 to 6.08) | <0.001 | 2.03 (1.41 to 2.92) | <0.001 | 4.38 (2.40 to 8.01) | <0.001 | 2.23 (1.11 to 4.48) | 0.03 |
| Hypertension | 5.86 (4.31 to 7.95) | <0.001 | 1.54 (1.04 to 2.29) | 0.03 | 3.94 (2.31 to 6.72) | <0.001 | 3.03 (1.63 to 5.62) | <0.001 |
| Use of antiviral agents | 0.49 (0.28 to 0.83) | 0.008 | 11.21 (2.72 to 46.21) | 0.001 | 11.06 (2.66 to 45.98) | 0.001 | ||
| Use of corticosteroid | 0.50 (0.34 to 0.74) | <0.001 | 3.31 (1.66 to 6.62) | 0.001 | ||||
| Baseline creatinine | 1.02 (1.01 to 1.02) | <0.001 | 1.01 (1.00 to 1.01) | <0.001 | 1.02 (1.01 to 1.03) | 0.001 | ||
A total of 20 patients who received RRT at diagnosis of SARS-CoV were not included in the analysis.
P value = 0.77 for Hosmer-Lemeshow goodness-of-fit test, which did not indicate significant poor fit.
P value = 0.19 for Hosmer-Lemeshow goodness-of-fit test, which did not indicate significant poor fit.
Further Analysis on the Primary Endpoint in Patients with AKI
Further analysis was performed in the 280 patients who developed AKI (Supplemental Table 2). On multivariable analysis, COVID-19 (aOR, 0.11; 95% CI, 0.05 to 0.25, P<0.001), male sex (aOR, 2.46; 95% CI, 1.30 to 4.66, P=0.006), DM (aOR, 3.13; 95% CI, 1.63 to 6.01, P=0.001), peak ALT and/or AST ≥80 U/L (aOR, 1.92; 95% CI, 1.03 to 3.58, P=0.05), stage 2 AKI (aOR, 4.99; 95% CI, 1.81 to 13.70, P=0.002), and stage 3 AKI (aOR, 9.66; 95% CI, 3.87 to 24.12, P<0.001) were significant factors associated with the primary endpoint.
Renal Function Recovery in Patients with SARS or COVID-19
Among the patients with SARS and AKI, 32.4% had persistent AKI at day 7, 22.1% progressed to AKD at day 30, and 17.1% progressed to CKD at day 90. Therefore, complete renal recovery was noted in 77.9% of patients at day 30, and 82.9% of patients at day 90. Among the patients with COVID-19 with AKI, 15.4% had persistent AKI at day 7, 12.7% progressed to AKD at day 30, and 7.9% progressed to CKD at day 90. Therefore, complete renal recovery was noted in 87.3% of patients at day 30, and 92.1% of patients at day 90. The results are summarized in Table 6.
Table 6.
The recovery of AKI in 214 patients infected by SARS-CoV and 66 patients infected by SARS-CoV-2 from the date of initial AKI event
| Creatinine Level | SARS-CoV | |||||
|---|---|---|---|---|---|---|
| At d 0 (n=214) | At d 7 (n=139) | At d 30 (n=85) | At d 90 (n=70) | P valuea,b | P valuec | |
| Median (IQR) | 140 (114–196) | 99 (68–191) | 75 (57–108) | 75 (63–103) | <0.001a | |
| No AKI/AKD/CKD, n (%) | 0 (0) | 94 (67.6) | 67 (77.9) | 58 (82.9) | <0.001b | 0.17c |
| AKI stage 1, n (%) | 177 (82.7) | 19 (13.7) | AKD 19 (22.1) | CKD 12 (17.1) | ||
| AKI stage 2, n (%) | 17 (7.9) | 3 (2.2) | ||||
| AKI stage 3, n (%) | 20 (9.3) | 23 (16.5) | ||||
| Creatinine Level | SARS-CoV-2 | |||||
| At d 0 (n=66) | At d 7 (n=65) | At d 30 (n=63) | At d 90 (n=63) | P valuea,b | P valuec | |
| Median (IQR) | 108 (91–129) | 83 (69–103) | 75 (67–97) | 75 (66–94) | <0.001a | |
| No AKI/AKD/CKD, n (%) | 0 (0) | 55 (84.6) | 55 (87.3) | 58 (92.1) | <0.001b | 0.20c |
| AKI stage 1, n (%) | 63 (95.5) | 3 (4.6) | AKD 8 (12.7) | CKD 5 (7.9) | ||
| AKI stage 2, n (%) | 3 (4.5) | 1 (1.5) | ||||
| AKI stage 3, n (%) | 0 (0) | 6 (9.2) | ||||
| P value | 0.009d | 0.005c | ||||
Friedman test was used to compare creatinine level at d 0, 7, 30, and 90.
Bhapkar test was used compare creatinine level and AKI status at d 0 and 7.
Generalized estimating equations was used to compare presence of any AKI/AKD at d 7, 30, and 90.
Categories of AKI/AKD between SARS-CoV and SARS-CoV-2 were compared by chi-squared test or Fisher’s exact test, as appropriate.
The temporal trends of creatinine levels in patients with SARS or COVID-19 are illustrated in Figure 2. Rising creatinine levels and the need of hemodialysis were mostly noted in those who developed the primary endpoint (Figure 2, A and C). Patients requiring hemodialysis had persistently high creatinine levels. Among the six patients with COVID-19 who developed stage 3 AKI at day 7, one died before day 30, and five progressed to AKD at day 30. Of the five patients with AKD at day 30, three progressed to CKD at day 90, and two fully recovered from AKD. Among the 23 patients with SARS who developed stage 3 AKI at day 7, 15 died before day 30, and eight progressed to AKD at day 30. Of the eight patients with AKD at day 30, five progressed to CKD at day 90, and two fully recovered from AKD. Creatinine levels were mostly stable in those who did not develop the primary endpoint (Figure 2, B and D).
Figure 2.
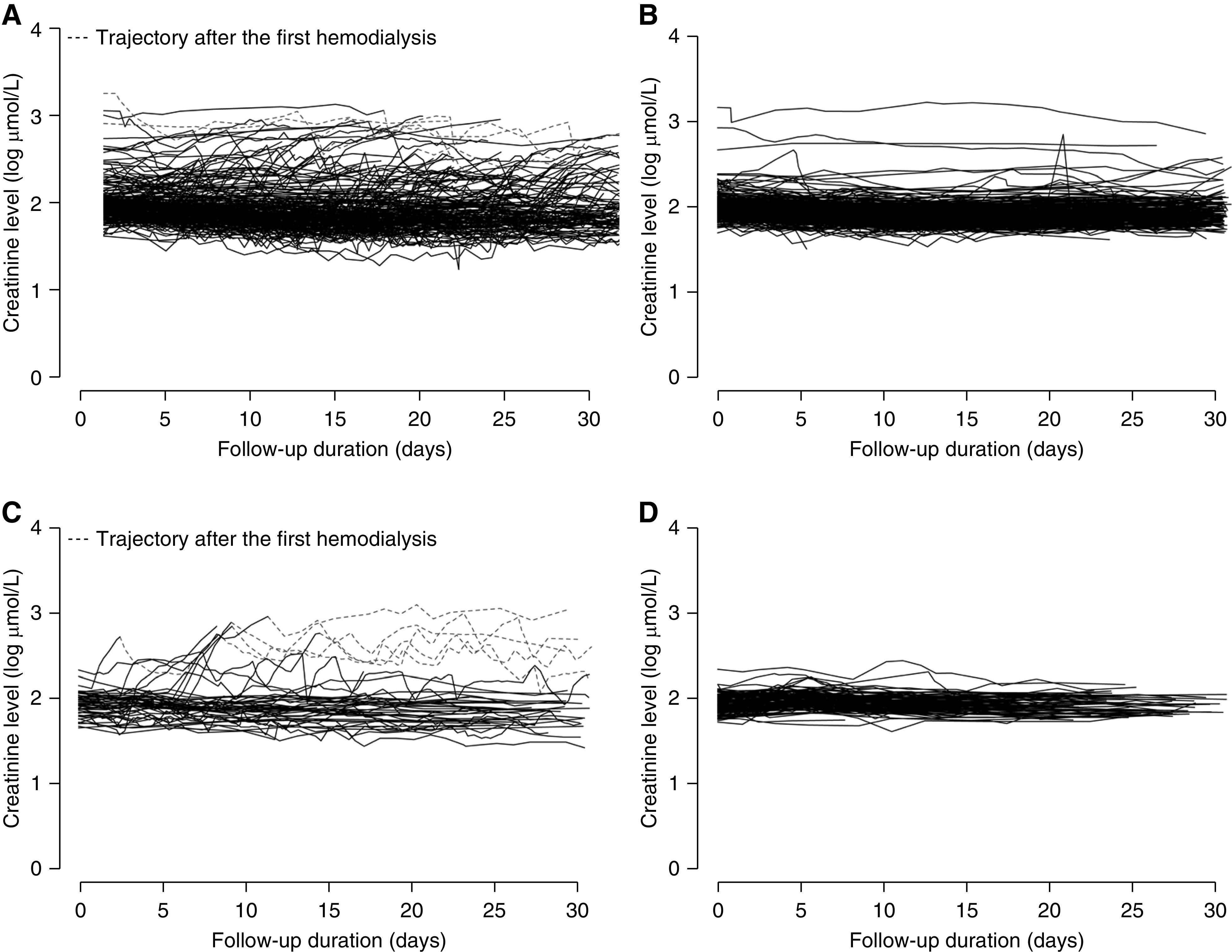
Serial creatinine level of patients with SARS with elevated creatinine (>109 μmol/L for males and >83 μmol/L for females) who (A) developed and (B) did not develop primary endpoint. Serial creatinine level of patients with COVID-19 with elevated creatinine (>109 μmol/L for males and >83 μmol/L for females) who (C) developed and (D) did not develop primary endpoint.
Discussion
Being in an Asian country affected by both SARS and COVID-19, we are able to investigate and compare the effect of AKI on the clinical outcomes of both diseases. The electronic public healthcare database is used in all public hospitals and clinics in Hong Kong; this broad coverage allows identification and data extraction from almost all patients with SARS or COVID-19 in Hong Kong. All patients who were diagnosed with SARS or COVID-19 were hospitalized in Hong Kong, regardless of the severity of their illnesses. Such arrangements were mandatory and reinforced by regulatory authorities, hence the data being captured are very representative for both eras of SARS and COVID-19. In this study, we also analyzed the serial changes in creatinine level along the clinical course of SARS or COVID-19. AKI was defined by the increase of creatinine level from baseline and the need of RRT according to the KDIGO criteria.22 Defining the associations between AKI and clinical outcomes of SARS or COVID-19, and investigating the factors associated with AKI carry important implications in our clinical practice.
ACE2 is a host receptor for SARS-CoV-2.12 Because ACE2 is expressed predominantly in the proximal tubules in kidneys, it is possible that viral infection may occur in these sites. However, such route of infection is complicated; viremia must be present, followed by penetration of the glomerular barrier or the proximal tubule junction.23 There were several types of evidence supporting this hypothesis. First, viral RNA was detected in kidneys of patients with COVID-19 by RT-PCR and/or in situ hybridization.24–26 Second, viral protein was detected in kidneys of patients with COVID-19 by immunocytochemistry.24,27 Third, live virus was recovered from kidney tissue of affected patients.25 Fourth, coronavirus-like structures were identified in the kidney tissue of patients with COVID-19 by electron microscopy.27–29 All these results suggest SARS-CoV-2 can infect the kidney. In addition, targeting ACE2 may also result in angiotensin dysregulation, innate and adaptive immune pathway activation, and hypercoagulation, leading to AKI and multiorgan injury.30
Current literature showed that AKI might be a common presentation after COVID-19 infection.31 Pei et al.32 showed that up to 75.4% of the patients with COVID-19 had abnormal urine dipstick including proteinuria and hematuria, or AKI. In a large series of 5499 patients with COVID-19, AKI developed in 36.6%.33 Among those who developed AKI, 46.5% had stage 1, 22.5% had stage 2, and 31.1% had stage 3 AKI. Our study showed that only 8.1% of the patients with COVID-19 developed AKI; 6.6% had stage 1 AKI and 1.5% had stage 2–3 AKI. Given that all laboratory-confirmed patients with COVID-19 in Hong Kong were hospitalized regardless of their disease severity, we believe our cohort is able to provide a more accurate representation of COVID-19 in terms of its renal manifestations.
Our study showed that only 5.3% of patients with COVID-19 developed the primary endpoint, compared with 26.0% of patients with SARS. Among the patients who did not meet the primary endpoint, patients with SARS had higher peak creatinine levels and higher stages of AKI than patients with COVID-19. Among the patients who met the primary endpoint, although there was no significant difference in the peak creatinine levels and the stages of AKI between patients with SARS or COVID-19, this is limited by the small number of patients and events, particularly in the COVID-19 group.
Our logistic regression analysis showed that DM, deranged liver function, and AKI were common factors in patients with SARS or COVID-19 that were associated with the primary endpoint. These factors can serve as important prognostic factors and patients presenting with these factors may warrant more aggressive treatment earlier on. Recognizing the increasing trend of creatinine level is also important, as reflected by our temporal trend analysis in patients who developed the primary endpoint.
Our study also showed that DM and hypertension were common significant factors that were associated with AKI. We believe patients with DM and hypertension are at risk of CKD, and hence they are prone to developing AKI after SARS or COVID-19 infection. The use of antivirals was also a significant factor that was associated with AKI, particularly in the COVID-19 group. As antivirals were more commonly used in severe infections, this might explain their apparent associations with AKI along the treatment course. In contrast, the use of antivirals might have potential renal toxicities and this should not be overlooked.34 Among the 280 patients with AKI, we found that male sex, DM, deranged liver function, and AKI were significant factors that were associated with the primary endpoint. Our results also showed that SARS was associated with a higher risk of major adverse clinical outcomes than COVID-19 in patients with AKI. We must emphasize that the association between AKI and the primary endpoint does not imply any causality. In fact, most patients developed AKI after they had met the primary endpoint.
There are several limitations in our study. First, this was a retrospective cohort study. Data were extracted from real-life clinical patients, and their clinical management pathways might differ across different centers. Serial monitoring of renal function was on the basis of clinical necessity, and this may induce bias to our analysis. Second, ascertainment bias may affect the reliability of certain diagnosis codes for comorbidities, such as diabetes and hypertension. However, such bias was already minimized by extracting diagnosis-defining data from laboratory measurements and drug prescription and dispensing history. Third, this cohort is on the basis of real-world experience and inevitably there are missing data in our dataset. Patients with missing creatinine levels had to be excluded because imputation of this data may in turn affect the study outcome of interest, that is, AKI. Nevertheless, most of the other missing values (including albumin, total bilirubin, international normalized ratio, C-reactive protein, erythrocyte sedimentation rate, lactate dehydrogenase, white cell count, and neutrophil) were not used in any of the subsequent analyses. Fourth, the baseline characteristics of patients with SARS or COVID-19 were very different, but we did perform various multivariable analyses to adjust for potential confounding factors. Fifth, the event numbers of certain outcomes of interest particularly in the COVID-19 group was small. This may affect the precision of the analysis and the interpretation of the results.
In conclusion, AKI was associated with major adverse clinical outcomes in patients with SARS or COVID-19. Patients with DM, deranged liver function, and AKI were at risk of developing severe consequences after SARS and COVID-19 infection. DM and hypertension were common significant factors that were associated with AKI. Among patients with AKI, COVID-19 was associated with a lower rate of major adverse clinical outcomes when compared with SARS. Renal function recovery was usually observed within 30 days after SARS and COVID-19 infection. We should remain vigilant in the identification of high-risk patients, and more aggressive treatment may be needed early on. Serial renal function monitoring may be helpful in detecting any earlier deterioration of the disease.
Disclosures
C.-C. Szeto reports consultancy agreements with Baxter Healthcare and Gilead Science; research funding from Baxter Healthcare, Fresnius, Fibrogen Inc., and Gilead; honoraria from Baxter Healthcare; has served as an advisory committee member for Baxter Healthcare, Gilead, and Novartis; and reports other interests/relationships with AstraZeneca and Pfizer. C. Ng has served as an advisory committee member for Astellas, Boston Scientific, Ipsen, Janssen, and MSD; speaker for Ferring, Ipsen, Janssen, Olympus, and Takeda; and reports receiving research grants from Ferring, Janssen, and Takeda. D. Hui has served as an advisory committee member for Roche. G. Wong reports consultancy agreements with Gilead Sciences, Janssen, MSD, Sanofi Pasteur, and ViiV; honoraria from Abbott, Abbvie, Ascletis, Bristol-Myers Squibb, Echosens, Gilead Sciences, Janssen, MSD, and Roche; has served as an advisory committee member for Gilead Sciences; as a speaker for Abbott, Abbvie, Bristol-Myers Squibb, Echosens, Furui, Gilead Sciences, Janssen, and Roche; and received research grants from Gilead Sciences, Janssen, MSD, and ViiV; speakers bureau from Abbott, Abbvie, Ascletis, Bristol-Myers Squibb, Echosens, Gilead Sciences, Janssen, and Roche. H. Chan reports having consultancy agreements with AbbVie, Aligos, Arbutus, Gilead, Glaxo-Smith-Kline (GSK), Hepions, Janssen, Merck, Roche, Vaccitech, VenatoRx, and Vir Biotechnology; reports receiving honoraria from AbbVie, Aligos, Arbutus, Gilead, GSK, Hepions, Janssen, Merck, Roche, Vaccitech, VenatoRx, and Vir Biotechnology; reports being a scientific advisor to or member of Aptorum, Shanghai Henlius Biotech Inc.; Speakers Bureau from Gilead, Mylan, Roche; and is an advisor for AbbVie, Aptorum, Arbutus, Gilead, GSK, GRAIL, Hepion, Intellia, Janssen, Medimmune, Merck, Roche, Vaccitech, VenatoRx, and Vir Biotechnology; and a speaker for Gilead, Mylan, and Roche. J. Teoh reports receiving research funding from Ferring and Janssen; reports receiving honoraria from Astellas, Ferring, and Janssen; being a scientific advisor to or member of an advisory committee for Astellas, Ferring, and Janssen; Speakers Bureau for Astellas, Ferring, and Janssen; has served as an advisory committee member and a speaker for Astellas, Ferring, and Janssen; and has received research grants from Ferring and Janssen. G. Lui has served as an advisory committee member for Gilead, GSK, and Merck; a speaker for Gilead and Merck; and reports receiving research grants from Gilead, GSK, and Merck. P. Chow has served as an advisory committee member for Astellas, and speaker for Astellas and Janssen. T. Yip reports consultancy agreements with Gilead Sciences; and has served as an advisory committee member and a speaker for Gilead Sciences. V. Wong has served as an advisory committee member for 3V-BIO, AbbVie, Allergan, Boehringer Ingelheim, Center for Outcomes Research in Liver Diseases, Echosens, Gilead Sciences, Hanmi Pharmaceutical, Intercept, Janssen, Novartis, Novo Nordisk, Perspectum Diagnostics, Pfizer, ProSciento, Sagimet Biosciences, TARGET-NASH, and Terns; speaker for Bristol-Myers Squibb, Echosens, Gilead Sciences, and Merck; reports receiving a research grant from Gilead Sciences; reports receiving honoraria from 3V-BIO, AbbVie, Allergan, Boehringer Ingelheim, Center for Outcomes Research in Liver Diseases, Echosens, Gilead Sciences, Hanmi Pharmaceutical, Intercept, Merck, Novartis, Novo Nordisk, Perspectum Diagnostics, Pfizer, ProSciento, Sagimet Biosciences, TARGET PharmaSolutions, and Terns; reports being a scientific advisor or member of associate editor of Clinical Gastroenterology and Hepatology and editorial board member for the Journal of Hepatology, Hepatology, Alimentary Pharmacology and Therapeutics, Journal of Gastroenterology and Hepatology, JHEP Reports and Hepatology Communications. All remaining authors have nothing to disclose.
Funding
None.
Supplementary Material
Acknowledgments
All authors were responsible for the study concept and design. J. Y.-C. Teoh, T. C.-F. Yip, Y.-K. Tse, C. C. Szeto, and G. L.-H. Wong were responsible for the acquisition and analysis of data, had full access to all of the data in the study, and take responsibility for the integrity of the data and the accuracy of the data analysis. All authors were responsible for the interpretation of data, the drafting, and critical revision of the manuscript for important intellectual content.
Footnotes
Published online ahead of print. Publication date available at www.jasn.org.
Supplemental Material
This article contains the following supplemental material online at http://jasn.asnjournals.org/lookup/suppl/doi:10.1681/ASN.2020071097/-/DCSupplemental.
Supplemental Table 1. Univariate and multivariable analysis by logistic regression on factors associated with AKI in patients infected by SARS-CoV and SARS-CoV-2.
Supplemental Table 2. Univariate and multivariable analysis by logistic regression on factors associated with primary endpoint (a composite endpoint of admission to intensive care unit, use of invasive mechanical ventilation, and death) in 214 and 66 patients infected by SARS-CoV and SARS-CoV-2 with AKI, respectively.
References
- 1.World Health Organization: WHO Director - General’s opening remarks at the media briefing on COVID-19 - March 11, 2020. Available at: https://www.who.int/dg/speeches/detail/who-director-general-s-opening-remarks-at-the-media-briefing-on-covid-19—11-march-2020. Accessed on July 25, 2020
- 2.Worldometer: Countries where COVID-19 has spread. Available at: https://www.worldometers.info/coronavirus/countries-where-coronavirus-has-spread/. Accessed on July 25, 2020
- 3.World Health Organization: Coronavirus disease (COVID-19) Situation Report - 187. Available at: https://www.who.int/docs/default-source/coronaviruse/situation-reports/20200725-covid-19-sitrep-187.pdf?sfvrsn=1ede1410_2. Accessed on July 26, 2020
- 4.Teoh JY, Ong WLK, Gonzalez-Padilla D, Castellani D, Dubin JM, Esperto F, et al.; UroSoMe Working, Group: A global survey on the impact of COVID-19 on urological services. Eur Urol 78: 265–275, 2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ong WLK, Lechmiannandan S, Loeb S, Teoh JY: Urological services in public hospitals suffered a greater detriment than private hospitals during the battle of COVID-19. Urology 144: 269–270, 2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.World Health Organization: Summary table of SARS cases by country, November 1, 2002 - August 7, 2003. Available at: https://www.who.int/csr/sars/country/country2003_08_15.pdf?ua=1. Accessed on July 25, 2020
- 7.Lam WK, Zhong NS, Tan WC: Overview on SARS in Asia and the world. Respirology 8[Suppl 1]: S2–S5, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Law S, Leung AW, Xu C: Severe acute respiratory syndrome (SARS) and coronavirus disease-2019 (COVID-19): From causes to preventions in Hong Kong. Int J Infect Dis 94: 156–163, 2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Guan WJ, Ni ZY, Hu Y, Liang WH, Ou CQ, He JX, et al.; China Medical Treatment Expert Group for Covid-19: Clinical characteristics of coronavirus disease 2019 in China. N Engl J Med 382: 1708–1720, 2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Zhou F, Yu T, Du R, Fan G, Liu Y, Liu Z, et al.: Clinical course and risk factors for mortality of adult inpatients with COVID-19 in Wuhan, China: A retrospective cohort study. Lancet 395: 1054–1062, 2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Li W, Moore MJ, Vasilieva N, Sui J, Wong SK, Berne MA, et al.: Angiotensin-converting enzyme 2 is a functional receptor for the SARS coronavirus. Nature 426: 450–454, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bourgonje AR, Abdulle AE, Timens W, Hillebrands JL, Navis GJ, Gordijn SJ, et al.: Angiotensin-converting enzyme 2 (ACE2), SARS-CoV-2 and the pathophysiology of coronavirus disease 2019 (COVID-19). J Pathol 251: 228–248, 2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Cheng MC, Tong YH, Kwok TC, Cheng IT, Chung AP, Leung JK, et al.: Development Journey of Clinical Data Analysis and Reporting System (CDARS) in hospital authority of Hong Kong. Medinfo: 1468, 2010 [Google Scholar]
- 14.The Hospital Authority: Hospital authority statistical report 2018–2019. Available at: https://www3.ha.org.hk/data/HAStatistics/StatisticalReport/2018-2019. Accessed on April 29, 2020
- 15.Wong JC, Chan HL, Tse YK, Yip TC, Wong VW, Wong GL: Statins reduce the risk of liver decompensation and death in chronic viral hepatitis: A propensity score weighted landmark analysis. Aliment Pharmacol Ther 46: 1001–1010, 2017 [DOI] [PubMed] [Google Scholar]
- 16.Yip TC, Wong VW, Chan HL, Tse YK, Lui GC, Wong GL: Tenofovir is associated with lower risk of hepatocellular carcinoma than entecavir in patients with chronic HBV infection in China. Gastroenterology 158: 215–225.e6, 2020 [DOI] [PubMed] [Google Scholar]
- 17.Lai JC, Wong GL, Yip TC, Tse YK, Lam KL, Lui GC, et al.: Chronic hepatitis B increases liver-related mortality of patients with acute hepatitis E: A territorywide cohort study from 2000 to 2016. Clin Infect Dis 67: 1278–1284, 2018 [DOI] [PubMed] [Google Scholar]
- 18.Lui GCY, Wong NS, Wong RYK, Tse YK, Wong VWS, Leung CC, et al.: Antiviral therapy for hepatitis B prevents liver injury in patients with tuberculosis and hepatitis B coinfection. Clin Infect Dis 70: 660–666, 2020 [DOI] [PubMed] [Google Scholar]
- 19.Kidney Disease Improving Global Outcomes: Acute Kidney Injury (AKI). Available at: https://kdigo.org/guidelines/acute-kidney-injury/#. Accessed July 25, 2020
- 20.Chawla LS, Bellomo R, Bihorac A, Goldstein SL, Siew ED, Bagshaw SM, et al.; Acute Disease Quality Initiative Workgroup 16.: Acute kidney disease and renal recovery: Consensus report of the Acute Disease Quality Initiative (ADQI) 16 Workgroup. Nat Rev Nephrol 13: 241–257, 2017 [DOI] [PubMed] [Google Scholar]
- 21.Levey AS, Stevens LA, Schmid CH, Zhang YL, Castro AF 3rd, Feldman HI, et al.; CKD-EPI (Chronic Kidney Disease Epidemiology Collaboration): A new equation to estimate glomerular filtration rate [published correction appears in Ann Intern Med 155: 408, 2011]. Ann Intern Med 150: 604–612, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Khwaja A: KDIGO clinical practice guidelines for acute kidney injury. Nephron Clin Pract 120: c179–c184, 2012 [DOI] [PubMed] [Google Scholar]
- 23.Khan S, Chen L, Yang CR, Raghuram V, Khundmiri SJ, Knepper MA: Does SARS-CoV-2 infect the kidney? J Am Soc Nephrol 31: 2746–2748, 2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Puelles VG, Lütgehetmann M, Lindenmeyer MT, Sperhake JP, Wong MN, Allweiss L, et al.: Multiorgan and renal tropism of SARS-CoV-2. N Engl J Med 383: 590–592, 2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Braun F, Lütgehetmann M, Pfefferle S, Wong MN, Carsten A, Lindenmeyer MT, et al.: SARS-CoV-2 renal tropism associates with acute kidney injury. Lancet 396: 597–598, 2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hanley B, Naresh KN, Roufosse C, Nicholson AG, Weir J, Cooke GS, et al.: Histopathological findings and viral tropism in UK patients with severe fatal COVID-19: A post-mortem study. Lancet Microbe 1: e245–e253, 2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Su H, Yang M, Wan C, Yi LX, Tang F, Zhu HY, et al.: Renal histopathological analysis of 26 postmortem findings of patients with COVID-19 in China. Kidney Int 98: 219–227, 2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kissling S, Rotman S, Gerber C, Halfon M, Lamoth F, Comte D, et al.: Collapsing glomerulopathy in a COVID-19 patient. Kidney Int 98: 228–231, 2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Farkash EA, Wilson AM, Jentzen JM: Ultrastructural evidence for direct renal infection with SARS-CoV-2. J Am Soc Nephrol 31: 1683–1687, 2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Batlle D, Soler MJ, Sparks MA, Hiremath S, South AM, Welling PA, et al.; COVID-19 and ACE2 in Cardiovascular, Lung, and Kidney Working Group: Acute kidney injury in COVID-19: Emerging evidence of a distinct pathophysiology. J Am Soc Nephrol 31: 1380–1383, 2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Chan VW, Chiu PK, Yee CH, Yuan Y, Ng CF, Teoh JY: A systematic review on COVID-19: Urological manifestations, viral RNA detection and special considerations in urological conditions [published online ahead of print May 27, 2020]. World J Urol 10.1007/s00345-020-03246-42020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Pei G, Zhang Z, Peng J, Liu L, Zhang C, Yu C, et al.: Renal involvement and early prognosis in patients with COVID-19 pneumonia. J Am Soc Nephrol 31: 1157–1165, 2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Hirsch JS, Ng JH, Ross DW, Sharma P, Shah HH, Barnett RL, et al.; Northwell COVID-19 Research Consortium; Northwell Nephrology COVID-19 Research Consortium: Acute kidney injury in patients hospitalized with COVID-19. Kidney Int 98: 209–218, 2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Adamsick ML, Gandhi RG, Bidell MR, Elshaboury RH, Bhattacharyya RP, Kim AY, et al.: Remdesivir in patients with acute or chronic kidney disease and COVID-19. J Am Soc Nephrol 31: 1384–1386, 2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.