Significance Statement
Expression of immediate early response gene X-1 (Iex-1), also known as Ier-3, is increased in venous neointimal hyperplasia (VNH) and stenosis in hemodialysis arteriovenous fistulas (AVF). In a porcine model, 1α,25(OH)2D3, an inhibitor of Ier3, reduced VNH/stenosis formation. The 1α,25(OH)2D3 released in the perivascular AVF space from poly(lactic-co-glycolic acid) nanoparticles embedded in a Pluronic F127 hydrogel (1,25 NP) reduced Ier3 gene and protein expression, MCP-1, CD68, HIF-1α, and VEGF-A immunostaining. Concomitantly, blood flow, lumen area, pulse wave velocity, and Young’s modulus increased, and neointima area, peak systolic velocity, and wall shear stress decreased in 1,25 NP–treated vessels. RNA sequencing analysis identified 242 genes involved in inflammatory and apoptotic pathways that had decreased expression in 1,25 NP vessels.
Keywords: arteriovenous fistula, vascular access, shear stress, restenosis, drug delivery
Visual Abstract
Abstract
Background
Few therapies prevent venous neointimal hyperplasia (VNH) and venous stenosis (VS) formation in arteriovenous fistulas (AVF). Expression of the immediate early response gene X-1 (Iex-1), also known as Ier3, is associated with VNH and stenosis in murine AVFs. The study aimed to determine if local release of Ier3 long-acting inhibitor 1α,25(OH)2D3 from poly(lactic-co-glycolic acid) (PLGA) nanoparticles embedded in a thermosensitive Pluronic F127 hydrogel (1,25 NP) could affect VNH/VS formation in a large animal model.
Methods
Immediately after AVF creation in a porcine model of renal failure, 1,25 NP or vehicle control was injected into the adventitia space of AVF outflow veins. Scanning electron microscopy and dynamic light scattering characterized drug and control nanoparticles. Animals were sacrificed 3 and 28 days later for gene expression, immunohistologic, magnetic resonance imaging and angiography, and ultrasound analyses. Whole transcriptome RNA sequencing with differential gene expression analysis was performed on outflow veins of AVF.
Results
Encapsulation of 1α,25(OH)2D3 in PLGA nanoparticles formed nanoparticles of uniform size that were similar to nanoparticles without 1α,25(OH)2D3. The 1,25 NP–treated AVFs exhibited lower VNH/VS, Ier3 gene expression, and IER-3, MCP-1, CD68, HIF-1α, and VEGF-A immunostaining, fibrosis, and proliferation. Blood flow and lumen area increased significantly, whereas peak systolic velocity and wall shear stress decreased. Treatment increased Young’s modulus and correlated with histologic assessment of fibrosis and with no evidence of vascular calcification. RNA sequencing analysis showed changes in the expression of genes associated with inflammatory, TGFβ1, and apoptotic pathways.
Conclusions
Local release of 1,25 NP improves AVF flow and hemodynamics, and reduces stenosis in association with reduction in inflammation, apoptosis, and fibrosis in a porcine model of arteriovenous fistula.
ESKD is a burgeoning global clinical problem with more than 4 million patients worldwide.1 Patients with ESKD often require hemodialysis (HD) either as a bridge to transplantation or as a destination therapy. A well-functioning vascular access (arteriovenous fistula or AVF) is essential for long-term HD to provide circulatory access necessary for HD. AVFs are preferred for vascular access because of their higher patency rates, reduced risks of infection, and thrombotic complications when compared with AV grafts or tunneled catheters.2 AVF failure frequently occurs due to the development of venous neointimal hyperplasia (VNH) resulting in venous stenosis (VS). The patency of AVFs at 1 and 2 years is approximately 60% and 51%, respectively.3 The improvement in AVF patency would be highly beneficial, because it would reduce the need for subsequent percutaneous angioplasties and surgical thrombectomies.
Currently, there are no effective therapies to prevent VNH in AVFs. Modulating the expression of factors involved in the pathogenesis of VNH might reduce subsequent endovascular and surgical interventions in AVFs. Immediate early response gene X-1 (Iex-1), also known as Ier3, has been implicated in cellular responses to shear stress, which contribute to VNH, causing AVF failure.4 Data implicate Ier3 in the regulation of CCL2 expression, which is an important regulatory chemokine in the pathogenesis of VS and hyperplasia in AVFs.4 Shear stress on endothelial cells and mechanical stretch on smooth muscle cells in AVFs upregulates the expression of the Ier3 gene, with subsequent increase in MCP-1/CCL2 expression, resulting in monocyte/macrophage recruitment and the development of VNH.4 Overexpression of MCP-1/CCL2 causes inflammation, fibrosis, proliferation, and migration of cells, resulting in VNH.5–8 The data suggest that Ier3 regulates vascular responses after creation of an AVF by increasing inflammation, fibrosis, proliferation, and cellular migration. Inhibition of Ier3 has been shown to reduce VNH experimentally using an Ier3 knockout model with AVF.4 Furthermore, 1α,25(OH)2D3, an inhibitor of Ier3 gene expression, when delivered to the periadventitia of the outflow vein of AVF, has been shown to reduce VNH experimentally in mice.4,9
A myriad of approaches has not controlled AVF pathology—interestingly, the bulk of these involve systemic administration of potent compounds and, not unexpectedly, high local drug concentration cannot be obtained in AVFs without off-target complications.10 Local therapies delivering drugs that modulate key biologically relevant pathways at the site of vascular injury may offer an alternative and attractive approach to reducing VNH/VS. The basis of this study is our previous report, published in 2014,4 where we showed that IER3 expression was increased in VS removed from patients with AVF. Adventitial delivery of nanoparticles composed of poly(lactic-co-glycolic acid) (PLGA) with 1α,25(OH)2D3 embedded within thermal responsive Pluronic F127 hydrogel (1,25 NP) significantly reduced Ier3 gene expression and VNH in a mouse model of AVF with CKD.4 The outflow vein from AVF removed from Ier3 knockout mice with CKD had a significant increase in the lumen vessel area and a decrease in the neointima area, VNH/VS.4
Several studies have investigated the role of oral vitamin D metabolites in improving AVF maturation or preventing VS in AVF. There are conflicting data on the role of the prehormone, vitamin D3, in improving AVF access patency and maturation.11–15 Two studies demonstrated a beneficial effect of vitamin D3 on AVF performance. In the first, decreased levels of vitamin D3 were associated with poorer outcomes of AVF.13,15 In the second, vitamin D3 supplements improved AVF outcomes.13 Other studies, however, have not shown a salutary effect of vitamin D3 on fistula maturation.11,12 Of note, the latter studies did not use the biologically active, 1α-hydroxylated vitamin D analog, 1α,25(OH)2D3 or 1α-hydroxylated vitamin D analogs. Recently, a study of patients on HD showed that catheter injection of 1α,25(OH)2D3 to stenotic lesions after balloon angioplasty reduced AVF restenosis.14 Our own study in mice demonstrated a salutary effect of 1α,25(OH)2D3 in preventing AVF stenosis.4
The goal of this study was to gain preclinical data to allow for a phase 1 clinical trial using a drug delivery platform that was tested in a clinically relevant large animal model of AVF with CKD. We determined the effectiveness of adventitial delivery of 1,25 NP in decreasing VNH/VS formation in pigs with AVFs and CKD. Advanced imaging methods including ultrasound to assess the pulse wave velocity (PWV) and calculate Young’s modulus, and magnetic resonance imaging (MRI) with phase contrast (PC) magnetic resonance angiography (MRA) were utilized to determine wall shear stress and assess blood flow. We also performed rheologic studies, pharmacokinetic studies, gene expression, histomorphometric, and immunostaining analyses of the vessels to demonstrate the mechanisms by which 1,25 NPs function in the prevention of VNH. We characterized the hydrogel with NPs using scanning electron microscopy and dynamic scattering light analyses. Whole transcriptomic RNA sequencing (seq) with differential gene expression analysis was performed on outflow veins of AVF. This paper provides the rationale for performing clinical studies using adventitial delivery of 1,25 NPs in preventing VNH in HD AVFs.
Methods
Encapsulation of 1α,25(OH)2D3 in PLGA Nanoparticles and Loaded in a Pluronic F127 Hydrogel
The 1α,25(OH)2D3 was encapsulated into PLGA nanoparticles using the interfacial method and loaded into Pluronic F127 hydrogel as described previously.4 Briefly, 100 mg of PLGA (Sigma-Aldrich, St. Louis, MO) and 0.1 mg of 1α,25(OH)2D3 (Tocris Bioscience, Bristol, UK) were dissolved in 10 ml of acetone. Then, the solution was added dropwise to 500 ml of deionized water with constant stirring. After particle formation, the organic solvent was evaporated, and the PLGA nanoparticles were dried by lyophilization. The dried PLGA nanoparticles were dispersed in an aqueous solution to make a 200 µM solution. For vehicle controls, an equivalent amount of PLGA particles without 1α,25(OH)2D3 was used.
The 1,25 NP Characterization
Scanning Electron Microscopy
Morphology of the NPs was visualized by performing scanning electron microscopy, using a Hitachi S 4700 scanning electron microscope. The samples were coated with a 60% gold and 40% palladium mixture in an Ion Sputter at 20 mA for 90 seconds. Images were captured at 3.0 kV accelerating voltage, 15.3 mm working distance, and at 25.0× magnification power.
Particle Size
NPs were suspended in saline (0.1 mg/ml) and the measurement of mean NP size with distribution and polydispersity index were determined using a dynamic light scattering (DLS) method. We used a Zetasizer Nano-Series (Nano-ZS90; Malvern Instruments, England) at 25°C and a 90° scattering angle. The software DTS-Version 4.1 (Malvern, England) was used to determine the zeta-potential of the PLGA-NPs and 1α,25(OH)2D3-PLGA NPs.
Preparation of NP-Hydrogel Suspension and Storage Modulus Measurement
A 40% aqueous solution of Pluronic F127 (Sigma-Aldrich, St. Louis, MO) was prepared under sterile conditions at 4°C. An equal portion of PLGA particles in water and 40% Pluronic F127 gel was mixed to obtain a final concentration of 100 µM of 1α,25(OH)2D3–PLGA nanoparticles in 20% hydrogel.
The hydrogel with nanoparticle preparations was suspended at 4°C, and as the temperature rose, the gel hardened. The suspension at 4°C is shown (Supplemental Figure 1A) and as a solid gel at 37°C (Supplemental Figure 1B). The change in storage modulus was determined over a temperature range of 5–40°C at 1 Hz frequency and 0.1 strain using a Discovery series Rheometer.
In Vitro 1α,25(OH)2D3 Release
For in vitro 1α,25(OH)2D3 release kinetics, 1α,25(OH)2D3- or 1α,25(OH)2D3-encapsulated nanoparticles were suspended in 20% hydrogel at a final concentration of 100 µM. Next, 250 µl aliquots of 1α,25(OH)2D3 were added to Eppendorf tubes, and allowed to solidify. The gel was then suspended in 250 µl of saline and incubated at 37°C. The supernatant was collected at indicated time points from 0.1 to 168 hours, and the cumulative 1α,25(OH)2D3 levels were determined using mass spectrometry analysis as described.16
Experimental Animals
Approval from the Mayo Clinic Rochester Institutional Animal Care and Use Committee was obtained (A00002813) before performing any procedures. The maintenance and housing of the animals was performed in accordance with the Public Health Service Policy on Humane Care and Use of Laboratory Animal.17
Female (n=12) and castrated juvenile male pigs (n=13) aged 7–8 months old, with an average weight 49.77±0.94 kg (Larson Products, Sargeant, MN) were used in the study. Before all procedures, animals were fasted for 12 hours. For all surgical procedures, animals were initially anesthetized with an intramuscular injection of tiletamine hydrochloride (5 mg/kg), zolazepam hydrochloride (5 mg/kg), xylazine (2 mg/kg), and glycopyrrolate (0.06 mg/kg). During the procedure, animals were intubated and placed on a positive-pressure ventilator delivering oxygen (3–5 ml/kg) and isoflurane (1%–3%). The end-tidal CO2 volume, oxygen saturation, heart rate, electrocardiogram, and BP were monitored throughout the procedure.
Study Design
Renal artery embolization was performed to induce CKD as shown (Supplemental Figure 2A) and described previously.18–20 AVFs were placed 28 days later by creating an anastomosis to connect the end of the left external jugular vein to the side of the left common carotid artery (Supplemental Figure 2B). Animals were randomly assigned to either a 1,25 NP group (n=15) or vehicle group (n=10). In total, 11 animals (1,25 NP group n=6 and vehicle group n=5) were sacrificed at day 3 after AVF creation plus 1,25 NP or vehicle delivery and 14 animals (1,25 NP group n=9 and vehicle group n=5) were sacrificed at day 28 (Supplemental Figure 2C).
Induction of CKD
Renal artery embolization was used to induce CKD as previously described.18–20 Hickman catheters (Bard Medical, Tempe, AZ) were placed via the femoral vein for blood draws and fluid administration, as described previously.20 After a sterile prep, the right or left femoral vein was punctured with single-wall technique using an 18-gauge needle. A single lumen Hickman catheter was placed into femoral vein such that the tip was at the junction of the inferior vena cava and right atrium. A subcutaneous tunnel was created so the catheter could be accessed while the animal was in a standing position. A protective sleeve was placed around the catheter. The catheter was flushed every 48–72 hours with a mixture of heparin 3 U/ml NaCl and 1 mg/ml of vancomycin or cefazolin to prevent infection and maintain patency.
Creation of AVF and 1,25 NP Delivery to the Outflow Vein in AVF
Then 28 days after renal artery embolization, AVFs were created by connecting the end of the left external jugular vein to the side of the left common carotid artery anastomosis, as previously described.6,18 The maximal intraoperative diameter of the outflow vein was measured to determine the amount of drug to be layered on the adventitia of the first 4 cm of the outflow vein distal to the anastomosis and 1 cm of inflow artery proximal to the anastomosis (Supplemental Figure 2B). The amount of drug delivered to outflow vein was based on the surface area of the vessel using the formula: 1.88×2πrl, where r is the radius of the outflow vein and l is the length of the vessel (4 cm). The amount of drug with hydrogel received by each animal, vein diameter, and surface area are shown (Supplemental Table 1).
MRI/PC MRA
MRI was performed of the outflow vein after 1,25 NP or vehicle delivery at 14 and 28 days after AVF creation, as previously described.21,22 We assessed the lumen vessel area, blood flow, peak systolic velocity (PSV), and fistula patency using MRI.
PWV
Ultrasound data using plane wave compounding23 were acquired with a Verasonics research system (Verasonics, Inc., Kirkland, WA) and linear array transducer (L11–4v; Verasonics, Inc.) at 1600 frames/second to measure the pulse wave.24 The motion of the venous walls was extracted for estimation of the PWV. A correlation-based method was used with the flutter-like motion of the wall due to the AVF.25,26 PWV measurements were done on the proximal wall (front wall, FW) and the distal wall (back wall, BW), and the results were pooled as total. These measurements were performed 3–5 times at the outflow vein, 6 cm distal to the AVF anastomosis. The Young’s modulus, E, was calculated from the following equation: E=(2rρ [1−ν2] PWV2)/h where r is the radius of the vein, h is the vein wall thickness, ν is the Poisson’s ratio, 0.5, and ρ is the mass density, 1060 kg/m3.
Postsurgical Blood Collection
Blood was removed from the Hickman catheter before embolization, on the day of AVF creation, and 3, 7, 14, 21, and 28 days after AVF creation.
Tissue Harvesting
At euthanasia, the outflow vein, contralateral vein, and surrounding tissues were harvested from each animal as described previously.27,28 Each vein segment was divided into two parts. The proximal vein closest to the anastomosis was flash frozen and stored in RNAlater solution at −80°C for quantitative RT-PCR (qRT-PCR) analysis. The distal portion was fixed in 10% formalin reagent (Fisher Scientific, Pittsburgh, PA) for histologic studies.
Measurement of Biochemical Parameters
BUN, creatinine, phosphate, calcium, glucose, albumin, total carbon dioxide, sodium, potassium, and chloride were measured using the Abaxis vet scan VS2 machine (Abaxis Inc., Union City, CA), as previously described.29
Measurement of 1α,25(OH)2D3 Concentration in Serum and Outflow Vein
The 1α,25(OH)2D3 was measured in the serum samples on the day of AVF creation and 3, 7, 14, and 28 days after AVF creation by using liquid chromatography-tandem mass spectrometry (LC-MS/MS), as previously described.16 Briefly, 1α,25(OH)2D was purified using a monoclonal anti-1,25(OH)2–vitamin D mouse antibody (Cat KM1100; Immundiagnostik AG, Bensheim, Germany). The 200 µl of each standard, blank, or pig serum was added to 100 µl of antibody resin slurry. Deuterated D6 1α,25-dihydroxyvitamin D2 and carbon labeled 13C3 1α,25-dihydroxyvitamin D3 were added to the mixture internal standards. After a short incubation period, 1α,25(OH)2D was extracted from the resin, derivatized using the dienophile, 4-phenyl-1,2,4,-triazoline-3,5-dione and analyzed by LC-MS/MS using multiple reaction monitoring. The concentration of 1α,25(OH)2D3 in the outflow vein of each animal in both groups was measured at day 3 and 28 after AVF creation. For tissue samples, residual blood was removed before extraction and tissues were homogenized in PBS (1 ml/0.1 g) using a glass homogenizer. The 1α,25(OH)2D3 was extracted from tissue homogenate using a dichloromethane extraction method and concentrations were measured by LC-MS/MS. The sensitivity of the LC-MS/MS method is ≥8 pg of 1α,25(OH)2D3/mg of vein, and concentrations <8 pg/mg tissue are defined as undetectable.
RNA Isolation with qRT-PCR Analysis
Primers for real-time PCR analysis were obtained from Integrated DNA technologies (ITD, San Diego). RNA was isolated using the miRNeasy Kit (Qiagen, Germantown, MD). cDNA was prepared using the iScript c-DNA synthesis kit (Bio-Rad, Hercules, CA, USA), and real-time qRT-PCR was performed using the iTaq universal SYBR green super mix (Bio-Rad), in a C1000 thermal cycler (Bio-Rad) equipped with CFX96 real-time system. Primers used for PCR reactions are listed (Supplemental Table 2). Gene expression data were analyzed using TBP-1 as the reference gene and normalized to respective control veins. The fold change in the gene expression was calculated according to the 2−ΔΔct method.
Histologic Methods and Staining Procedures
Morphometric and Image Analysis
Morphometric analysis was performed on 8–10 4-µm-thick outflow vein segments that had been stained with hematoxylin and eosin, as described previously.28,29 All histology supplies, including buffers, blocking reagents, and secondary antibodies were obtained from Dako Agilent (Santa Clara, CA), unless otherwise stated.
Verhoeff Van Gieson Staining
To examine the elastic fibers of the outflow vein including the different layers of the vessel, Verhoeff Van Gieson staining (Cat 9116A; Newcomer Supply, Middleton, WI) was performed, according to the manufacturer’s protocols.
Immunohistochemistry
Immunohistochemistry was performed as described previously.28 Antibodies, supplies, and dilutions are listed (Supplemental Table 3). Nonspecific IgG was used as a negative control.
Terminal Deoxynucleotidyl Transferase dUTP Nick-End Labeling Staining
The extent of apoptotic cell death in the outflow veins was measured using terminal deoxynucleotidyl transferase dUTP nick-end labeling (TUNEL) staining (Trevigen, Gaithersburg, MD), following the manufacturer’s protocols. Tissue sections stained with TUNEL but without terminal deoxynucleotidyl transferase enzyme treatment served as negative controls.
Picrosirius Red and Masson’s Trichrome Staining
To measure collagen deposition (collagen 1 and 3), tissue sections of AVF outflow veins were stained with Picrosirius red stain (Cat 365548; Sigma-Aldrich, MO), as described previously,28 and Masson’s trichrome stain (Thermo Scientific Waltham, MA), following the manufacturer’s protocols. Image analysis and quantification were performed as previously described.28
Carstairs Staining
Carstairs staining was performed to distinguish between platelets (navy blue), fibrin (bright red), collagen (bright blue), muscle (red), and red blood cells (yellow). Formalin-fixed tissue sections were used. Briefly tissue sections were hydrated, stained in 5% ferric ammonium sulfate (Cat 26381–01; Electron Microscopy Sciences, PA) for 5 minutes, rinsed in tap water, and stained in Mayer’s Hematoxylin (Cat 26381–02; Electron Microscopy Sciences, PA) for 5 minutes. After rinsing in tap water, the sections were stained in Picric Acid–orange G solution (Cat 26381–03; Electron Microscopy Sciences, PA) for 30 minutes, then rinsed once in distilled water. They were then stained in Ponceau Fuchsin solution (Cat 26381–04; Electron Microscopy Sciences, PA) for 5 minutes, rinsed in distilled water, and differentiated with 1% phosphotungstic acid (Cat 26381–05; Electron Microscopy Sciences, PA), then washed in distilled water. Next, they underwent aniline blue solution (Cat 26381–06; Electron Microscopy Sciences, PA) staining for 30 minutes, followed by washing with several changes of distilled water. Finally, slides were dehydrated, cleared in xylene, and placed on a coverslip using synthetic mounting medium. Image analysis was performed as previously described.28
Alizarin Red S Staining
To determine if there was calcium formation after 1,25 NP or vehicle delivery, tissue sections of AVF outflow veins were stained with 2% Alizarin Red S stain, pH 4.1–4.3 (Cat 130–22–3; Spectrum Chemical, CA). Briefly, formalin-fixed tissue sections were deparaffinized, and hydrated to 70% alcohol. After rinsing in distilled water, the sections were stained in Alizarin Red S solution for 30 minutes, and washed twice in distilled water. Next, the sections were dipped in acetone, followed by acetone-xylene, and cleared in xylene. Image analysis was performed, as previously described.28
Image Analysis and Staining Intensity Measurements
Entire tissue-section images were acquired at 10× magnification using a Zeiss Axio Imager-M2, equipped with an Axiocam 503 Color camera (Zeiss, Oberkochen, Germany) and a motorized stage. The color intensity of positive staining was quantified using Zen Pro 2.0 software (Zeiss), as described previously.28 The entire tissue section was selected as the region of interest to measure the total tissue area and area of tissue that stained positive (brown for immunohistochemistry, red for picrosirius red, blue for Masson’s trichrome) was selected. The percent index of brown, red, and blue positive stain in the total tissue area was calculated.
Whole Transcriptome RNA Seq with Differential Gene Expression
Whole transcriptome RNA seq with differential gene expression was performed at the Medical Gnome Facility at the Mayo Clinic on day 28 outflow veins from 1,25 NP and vehicle control groups. Total RNA was isolated as described, and RNA libraries were prepared with 500 ng of total RNA isolated using TruSeq Stranded Total RNA Sample Prep Kits (Illumina, San Diego, CA), following the manufacturer’s instructions.30 The concentration and size distribution of the completed libraries were determined using an Agilent Bioanalyzer DNA 1000 chip (Santa Clara, CA) and Qubit fluorometry (Invitrogen, Carlsbad, CA). Libraries were sequenced at three samples per lane following Illumina’s standard protocol using the Illumina cBot and HiSeq 3000/4000 PE Cluster Kit. The flow cells were sequenced as 100×2 paired-end reads on an Illumina HiSeq 4000, using HiSeq 3000/4000 sequencing kit and HCS version 3.4.038 collection software. Base calling was performed using Illumina’s RTA version 2.7.7. The expression values were normalized as reads per kilobase of transcript per million reads (RPKM) for each gene. The fold change in gene expression was calculated, and the genes were considered differentially upregulated if the fold change was >1.5 or down regulated if less than <0.75 fold with a P<0.05 and an average RPKM >0.01. Panther analysis (http://pantherdb.org/) was performed to identify the most common pathways involved in the differentially regulated genes. The RNA seq data are available at https://www.ncbi.nlm.nih.gov/geo/query/acc.cgi?acc=GSE161554 using the token code: qpgrukoqdnkzxgj. We used qRT-PCR to validate the gene expression of IL29 and EDIL3 (Supplemental Table 2).
Statistical Methods
Data were analyzed using Graph Pad Prism Software version 8 (Graph Pad Software Inc, La Jolla, CA) and are presented as mean±SEM (SEM). Statistical significance was calculated by two-way ANOVA or nonparametric Mann–Whitney test with Bonferroni’s correction. Linear regression analysis was used for the correlation model. The level of significance was set at *P<0.05, **P<0.01, or ***P<0.001.
Results
In vitro Drug-Release Measurements
We assessed 1α,25(OH)2D3 release from two different preparations: a suspension of hydrogel with 1α,25(OH)2D3 and a hydrogel suspension of 1α,25(OH)2D3 encapsulated in PLGA nanoparticles (1,25 NP). We observed a rapid burst of 1α,25(OH)2D3 from hydrogel into the media within the first 24 hours when compared with 1,25 NPs, which showed a slower, steady, and longer release of 1α,25(OH)2D3 (Figure 1D). Because of the slower and longer in vitro release kinetics, the 1,25 NP formulation was used.
Figure 1.
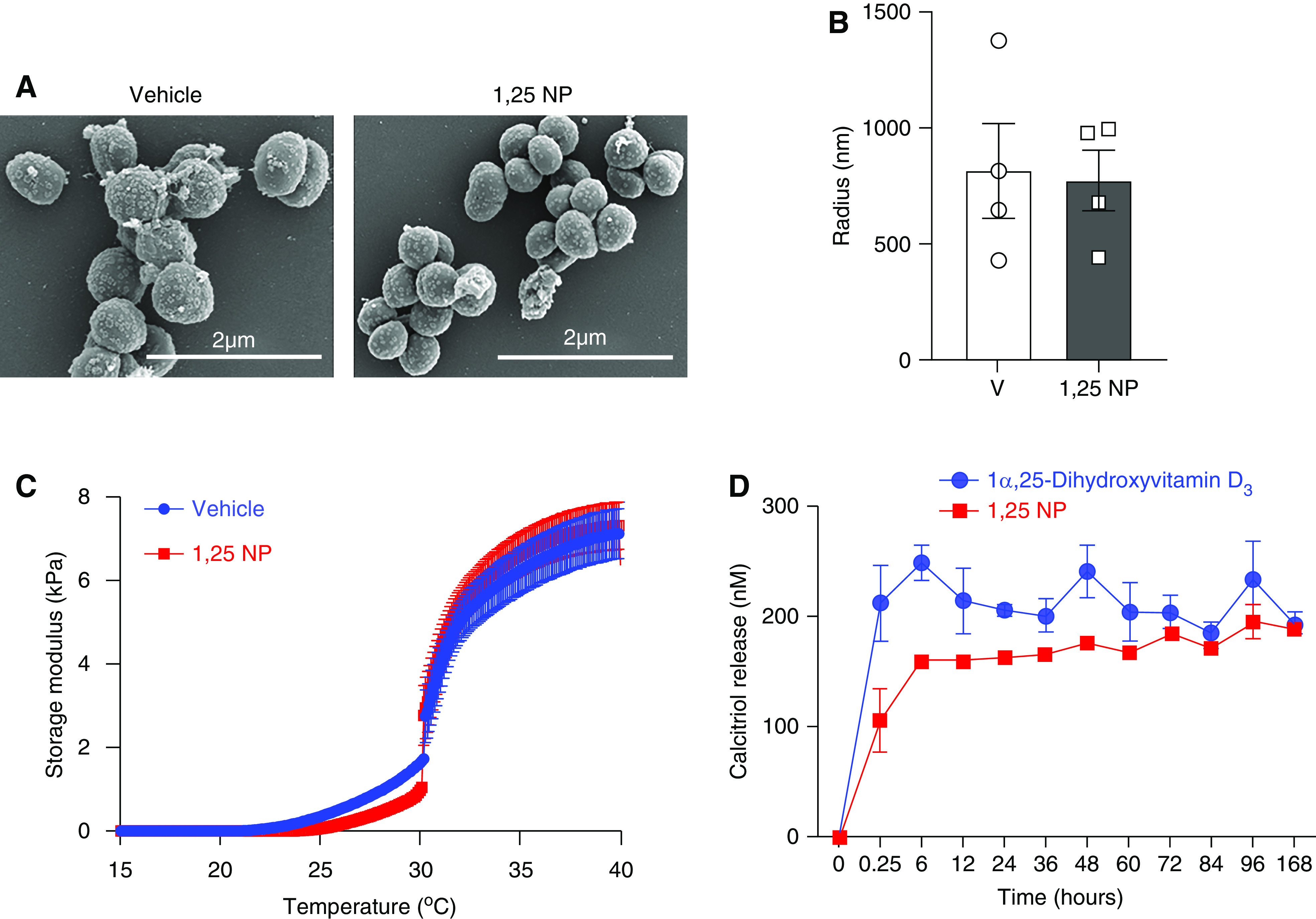
Characterization of PLGA nanoparticles and 1α,25(OH)2D3 release kinetics. (A) Representative scanning electron microscopic images of PLGA nanoparticles (vehicle), with 1α,25(OH)2D3 encapsulated NPs (1,25 NP). (B) DLS was performed to assess the particle size. (C) Storage modulus analysis was performed on 1,25 NP (blue trace) or Hydrogel with PLGA nanoparticles (red trace). (D) In vitro 1α,25(OH)2D3 release studies were performed from Hydrogel (blue) or 1,25 NP (red trace) when suspended in saline at 37°C for the indicated time points. Each data point is the average of three different experiments.
Nanoparticle Characterization
Scanning electron microscopy images showed that there was no difference between the 1,25 NP and vehicle controls with respect to shape and size (Figure 1A). DLS analysis demonstrated no significant difference in size between the 1,25 NP group compared with vehicle controls (1,25 NP: 778.1±132.3 nm; vehicle: 818.5±207 nm; P=0.88, Figure 1B).
Rheologic Assessment
At 4°C, vehicle controls and 1,25 NP were in suspension, and moved with gravity when the microtubes were flipped (Supplemental Figure 1A). After incubation at 37°C for 15 minutes, both mixtures became a gel, and failed to move when subjected to gravity (Supplemental Figure 1B). We assessed the storage modulus of 1,25 NP or vehicle control. The storage modulus increased with temperature and plateaued at 35°C. There was no difference in the storage modulus between 1,25 NP and vehicle controls (Figure 1C).
Biochemical Profile of CKD Pigs with AVFs
No animals died during this study. The BUN, creatinine, calcium, phosphate, glucose, albumin, total carbon dioxide, sodium, potassium, and chloride concentrations were measured at AVF creation, 3, 7, 14, 21, and 28 days after AVF creation in both groups. The average BUN and creatinine levels increased significantly 28 days after renal artery embolization compared with before embolization in both groups, which indicates there was induction of CKD (Supplemental Tables 4 and 5). There was no significant difference in the average BUN and serum creatinine concentrations between groups at any time point. At day 28 after embolization, the average serum BUN level increased by 138.86% in the 1,25 NP group and by 144.36% in the vehicle group compared with before embolization (1,25 NP: baseline, 1.7±0.13 mmol/L, AVF, 2.32±0.31 mmol/L; vehicle: baseline, 2.17±0.24, AVF, 3.14±0.31 mmol/L). At day 28 after embolization, the average serum creatinine level increased in the 1,25 NP and vehicle groups compared with before embolization (1,25 NP: baseline, 113±6.06 µmol/L, AVF, 158.2±014.98 µmol/L, average increase, 140%; vehicle: baseline, 126.62±9.19 µmol/L, AVF, 178.2±9.15 µmol/L, average increase, 141%). At day 3 after AVF placement, the serum calcium concentration increased significantly in the 1,25 NP group compared with the vehicle group (1,25 NP, 11.69±0.20 mg/dl; vehicle, 10.4±0.11 mg/dl; average increase 12%; P=0.003, (Supplemental Table 6). At day 7 after AVF placement, the average phosphate concentration level decreased significantly in the 1,25 NP group compared with the vehicle group (1,25 NP, 7.22±0.46 mg/dl; vehicle, 9.38±0.33 mg/dl; average reduction, 23%; P=0.003, Supplemental Table 7). At day 28 after AVF placement, the average potassium concentration level decreased significantly in the 1,25 NP group compared with the vehicle group (1,25 NP, 5.08±0.10 mmol/L; vehicle, 7.1±0.09 mmol/L; average reduction, 28.34%; P<0.0001, Supplemental Table 8). There was no difference in the other biochemical measurements at any time points (Supplemental Tables 9–13). There was no difference in the average body weight, systolic BP, and diastolic BP between groups at any time point (Supplemental Tables 14–16).
Delivery of 1,25 NP to the Outflow Vein
The surface area of the outflow vein was measured before administering 1,25 NP or vehicle controls. There was no difference in the average diameter of the outflow vein between the 1,25 NP and vehicle groups (1,25 NP, 8.4±0.33 mm; vehicle, 8.4±0.74 mm; Supplemental Figure 3, A and B). There was no difference in the total amount of 1α,25(OH)2D3 delivered between the day 3 and 28 groups (day 3, 27.07±1.30 µg; day 28, 28.93±0.73 µg; Supplemental Figure 3C).
Concentration of 1α,25(OH)2D3 in Serum and Outflow Vein Tissue after 1,25 NP or Vehicle Delivery
The serum 1α,25(OH)2D3 concentration was measured before AVF creation, 3, 7, 14, and 28 days after AVF creation in both groups. At day 3 after AVF, the average serum 1α,25(OH)2D3 concentration decreased significantly in 1,25 NP group compared with the vehicle group (1,25 NP, 56.40±6.48 pg/ml; vehicle, 88.24±11.84 pg/ml; average reduction, 36%; P=0.01, Supplemental Figure 3D) with no significant difference between the two groups at any other time points. The average 1α,25(OH)2D3 concentration in the outflow veins of 1,25 NP–treated animals at day 3 was 19±3.46 pg/mg, whereas in the vehicle group, 1α,25(OH)2D3 was undetectable (Supplemental Figure 3E). At day 28, in both groups, the average 1α,25(OH)2D3 concentrations were undetectable in outflow vein tissue (Supplemental Figure 3E).
In the Outflow Vein of AVF 1,25 NP Reduces Ier3 Gene and Protein Expression
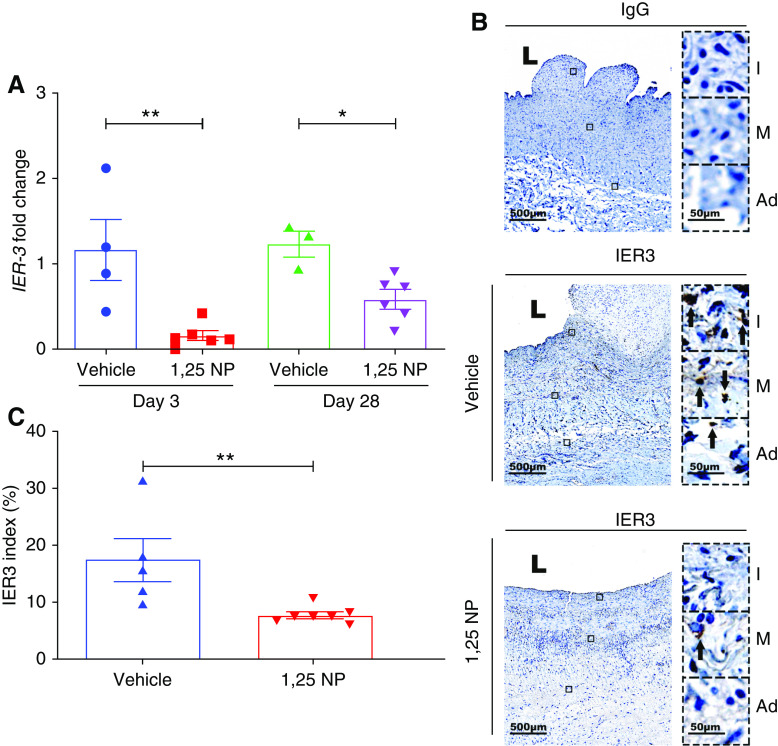
The Ier3 gene expression was significantly decreased at both day 3 and 28 in the outflow veins treated with 1,25 NP compared with vehicle (day 3: 1,25 NP, 0.16±0.05; vehicle, 1.16±0.35; average reduction, 86%; P=0.004; day 28: 1,25 NP, 0.65±0.11; vehicle, 1.06±0.19; average reduction, 38%; P=0.02; Figure 2A). Semiquantitative analysis of tissue immunostained with IER3 antibody demonstrated there was a significant decrease in the average IER3 index at day 28 in the 1,25 NP group compared with the vehicle group (1,25 NP, 7.60±0.56; vehicle, 17.43±3.80; average reduction, 56.4%; P=0.006; Figure 2, B and C). We performed semiquantitative analysis of IER3 staining in the different layers of the AVF outflow vein. We observed a significant decrease in the average IER3 index in the 1,25 NP group compared with the vehicle control in all of the three different layers in the outflow vein of AVF (intima: 1,25 NP, 10.42±0.93; vehicle, 23.05±2.13; average reduction, 58.8%; P=0.003, Supplemental Figure 4A; media: 1,25 NP, 6.29±0.89; vehicle, 12.73±2.48; average reduction, 50.61%; P=0.05 Supplemental Figure 4B; adventitia: 1,25 NP, 6.89±0.69; vehicle, 18.53±3.0; average reduction, 68.83%; P=0.003, Supplemental Figure 4C).
Figure 2.
Expression of IER3 gene and protein in the outflow vein. (A) IER3 gene expression was determined using qRT-PCR analysis in the outflow vein of vehicle and 1,25 NP group after 3 and 28 days after AVF placement. There is a significant decrease in the average IER3 gene expression in the 1,25 NP group, compared with the vehicle group at both time points. (B) Staining for IER3 on day 28 after AVF creation of outflow vein from vehicle and 1,25 NP groups. The first column is the IgG antibody–negative control. IER3-positive cells have brown staining. Images were captured at ×40 magnification. Right panel shows enlarged view of (+) cells across three different layers (×400 magnifications). (C) Semiquantitative analysis shows reduction in IER3 (+) cells in the 1,25 NP group compared with vehicle group. Each bar represents mean±SEM of n≥4. Nonparametric Mann–Whitney test was performed. *P<0.05, **P<0.01. L, lumen; Ad, adventitia; M, media; and I, intima. Scale bar is 500 µm and 50 µm (panel inset).
Vessels Treated with 1,25 NP Have Improved Vascular Remodeling and Reduced VNH
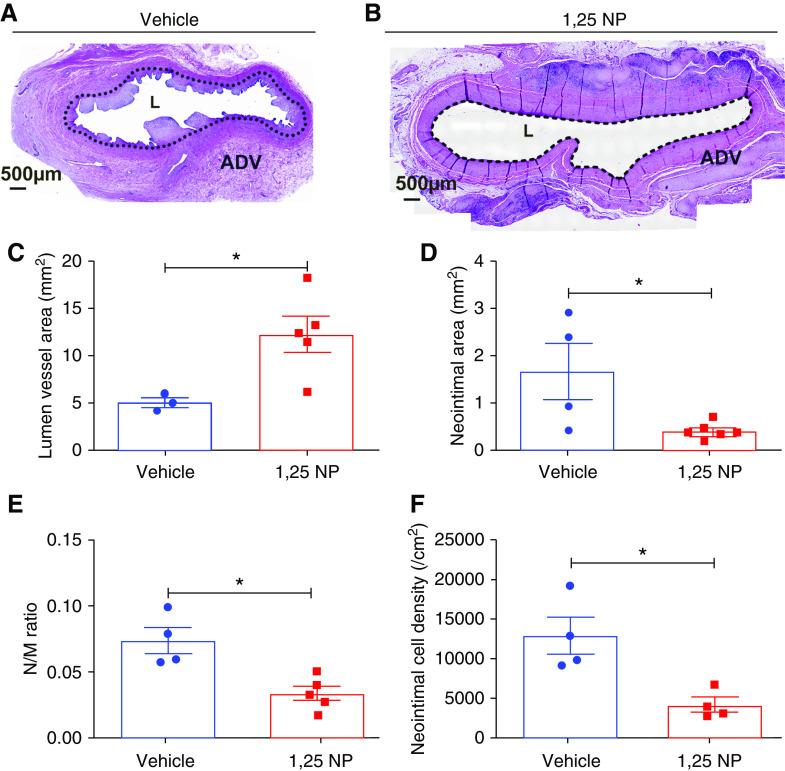
Vascular remodeling of the outflow vein was determined by performing histomorphometric analysis at day 28 after AVF creation. The neointima, media, and adventitia can be differentiated using hematoxylin and eosin staining (Figure 3, A and B). At 28 days after AVF placement, the average lumen vessel area was significantly increased in the outflow vein of the 1,25 NP group compared with the vehicle group (1,25 NP, 12.31±1.93 mm2; vehicle, 5.08±0.54 mm2; average increase, 242%; P=0.02; Figure 3C). There was a significant reduction in the average area of the neointima of the 1,25 NP–treated vessels compared with vehicle group (1,25 NP, 0.41±0.07 mm2; vehicle, 1.65±0.58 mm2; average reduction, 75.2%; P=0.01; Figure 3D), with a significant reduction in the mean ratio of the neointima area to media area in 1,25 NP group compared with the vehicle group (1,25 NP, 0.033±0.005; vehicle, 0.073±0.009; average reduction, 54.24%; P=0.02; Figure 3E). There was a significant reduction in the cell density in the neointima of 1,25 NP group compared with the vehicle group (1,25 NP, 4244.14±922.45 per cm2; vehicle, 13,083±2334.08 per cm2; average reduction, 67.56%; P=0.03; Figure 3F).
Figure 3.
Histomorphometric analysis of the outflow vein. (A and B) Hematoxylin and eosin staining of outflow vein treated with 1,25 NP and vehicle groups at day 28 after AVF creation. The neointima and media are identified with a dotted line in the ×20 magnification images. Neointima is significantly decreased in the 1,25 NP group compared with vehicle group. (C) The vessel lumen area in the 1,25 NP group is significantly increased compared with vehicle group at day 28. (D) The neointima area in 1,25 NP group is significantly decreased compared with vehicle group at day 28. (E) The ratio of neointima/media is decreased in 1,25 NP group compared with vehicle group at day 28. (F) Cell density in the neointima is decreased in 1,25 NP group compared with vehicle group at day 28. Each bar represents mean±SEM of n≥4. Nonparametric Mann–Whitney test was performed. *P<0.05. L, lumen. Scale bar is 500 µm. ADV, adventitia.
Blood Flow, Lumen Vessel Area, Wall Shear Stress, and Systolic Velocity Changes by PC MRI with MRA
Functional analysis of the AVF was performed using PC MRI with MRA of the outflow vein at day 14 and 28 after AVF placement to determine outflow vein lumen vessel area, PSV, and blood flow (Figure 4, A and B). The average lumen vessel area of the outflow vein was significantly higher at day 28 in the 1,25 NP group compared with the vehicle group (1,25 NP, 192.76±11.55 mm2; vehicle, 103.21±9.69 mm2; average increase, 186%; P=0.0001; Figure 4C). We next assessed the PSV because a lower velocity indicates a lack of stenosis.31 The average PSV was significantly lower at day 14 in the 1,25 NP group compared with the vehicle group (1,25 NP, 93.20±10.10 cm/sec; vehicle, 148.03±11.88 cm/sec; average reduction, 37%; P=0.007; Figure 4D). Increased blood flow in AVF means a better function of the fistula.31 The average blood flow was significantly higher at both time points in the 1,25 NP group, compared with vehicle group (day 14: 1,25 NP, 58.97±3.43 ml/sec; vehicle, 39.34±6.45 ml/sec; average increase, 149%; P=0.02; day 28: 1,25 NP, 88.58±4.28 ml/sec; vehicle, 62.93±7.85 ml/sec; average increase, 141%; P=0.01; Figure 4E). Wall shear stress changes have been associated with AVF failure.32 The average wall shear stress was significantly lower at day 14 in the 1,25 NP group compared with the vehicle group (1,25 NP, 0.61±0.11 N/m2; vehicle, 1.14±0.12 N/m2; average reduction, 46%; P=0.05; Figure 4F). There was no difference in the average pulsatility index (Figure 4G). The average resistivity index was significantly lower at day 28 in the 1,25 NP group compared with the vehicle group (1,25 NP, 0.31±0.039; vehicle, 0.51±0.08; average reduction, 38.41%; P=0.03; Figure 4H). We assessed the Reynolds number to determine if the blood flow was turbulent, and at all time points it was <1000 a lack of turbulent flow (Figure 4I).33 There was no difference in the average Reynolds number between both groups at any time point.
Figure 4.
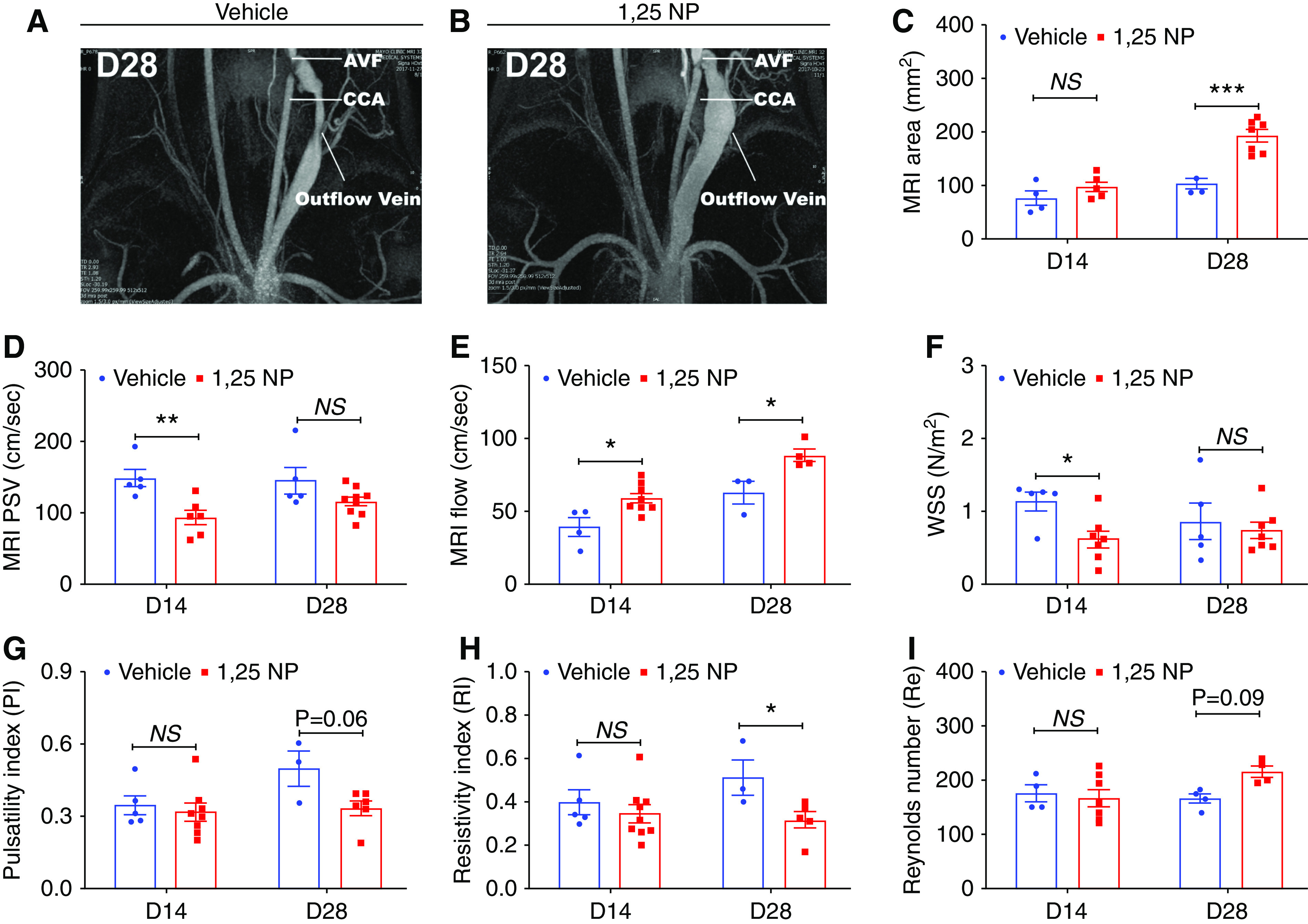
MRI analysis of outflow vein. (A and B) Representative images from the MRI of the outflow vein after vehicle or 1,25 NP delivery at day 28. (C) The average lumen vessel area measured by MRI is significantly increased in 1,25 NP group compared with the vehicle group at day 28. (D) The average PSV measured by MRI is significantly decreased in 1,25 NP group compared with vehicle group at day 14 after AVF creation. (E) The average blood flow measured by MRI is significantly increased in 1,25 NP group compared with vehicle group at both days 14 and days 28 after AVF placement. (F) The average wall shear stress (WSS) is significantly decreased in 1,25 NP group compared with vehicle group at day 14 after AVF creation. (G) The pulsatility index (PI) is not significantly changed in 1,25 NP group compared with vehicle group at any time points after AVF placement. (H) The resistivity index (RI) is significantly decreased in 1,25 NP group compared with vehicle group at day 28 after AVF placement. (I) The Reynolds number (Re) is not significantly changed in 1,25 NP group compared with vehicle group at any time points after AVF placement. Each bar represents mean±SEM of n≥3. Two-way ANOVA was performed. *P<0.05, **P<0.01, ***P<0.001. CCA, common carotid artery.
PWV and Young’s Modulus are Improved in 1,25 NP–Treated AVFs
Ultrasound of the outflow vein was performed to determine the PWV and this was used to calculate the Young’s modulus of the 1,25 NP– (Figure 5, A–C) and vehicle-treated groups (Figure 5, D–F). A representative B-mode image of the external jugular vein treated with 1,25 NP is shown (Figure 5A). We performed ultrasound analysis of the front (anterior) and back (posterior) walls of the jugular vein to determine if there were differences. The FW and BW velocity record over 500 ms is shown (Figure 5B, top row). The truncated time window from the top row (Figure 5B) for wall acceleration of FW and BW was used for correlation analysis for time delay identification is shown (Figure 5B, bottom row). The plot of time delays of pulse waves versus distance to calculate PWV as Δx/Δt (Figure 5C). The reference for t=0 is denoted in the middle of the ultrasound image. Similar results for the vehicle groups are shown (Figure 5, D–F). There was a significant increase in average PWV at day 14 in the 1,25 NP group compared with the vehicle group (1,25 NP, 3.79±0.34 m/s; vehicle, 2.61±0.21 m/s; average increase, 145.3%; P=0.001, Figure 5G). The total average Young’s modulus was significantly higher at day 28 in the 1,25 NP group compared with the vehicle group (1,25 NP, 172.87±34.33 kPa; vehicle, 74.62±8.39 kPa; average increase, 231.6%; P=0.03; Figure 5H). The average Young’s modulus of the FW was significantly higher at day 28 in the 1,25 NP group compared with the vehicle group (1,25 NP, 183.82±48.52 kPa; vehicle, 68.96±15.09 kPa; average increase, 266.5%; P=0.03; Figure 5I). The average Young’s modulus of the BW was also significantly higher at day 28 in the 1,25 NP group compared with the vehicle group (1,25 NP, 191.97±32.97 kPa; vehicle, 85.02±12.97 kPa; average increase, 225.7%; P=0.02; Figure 5J).
Figure 5.
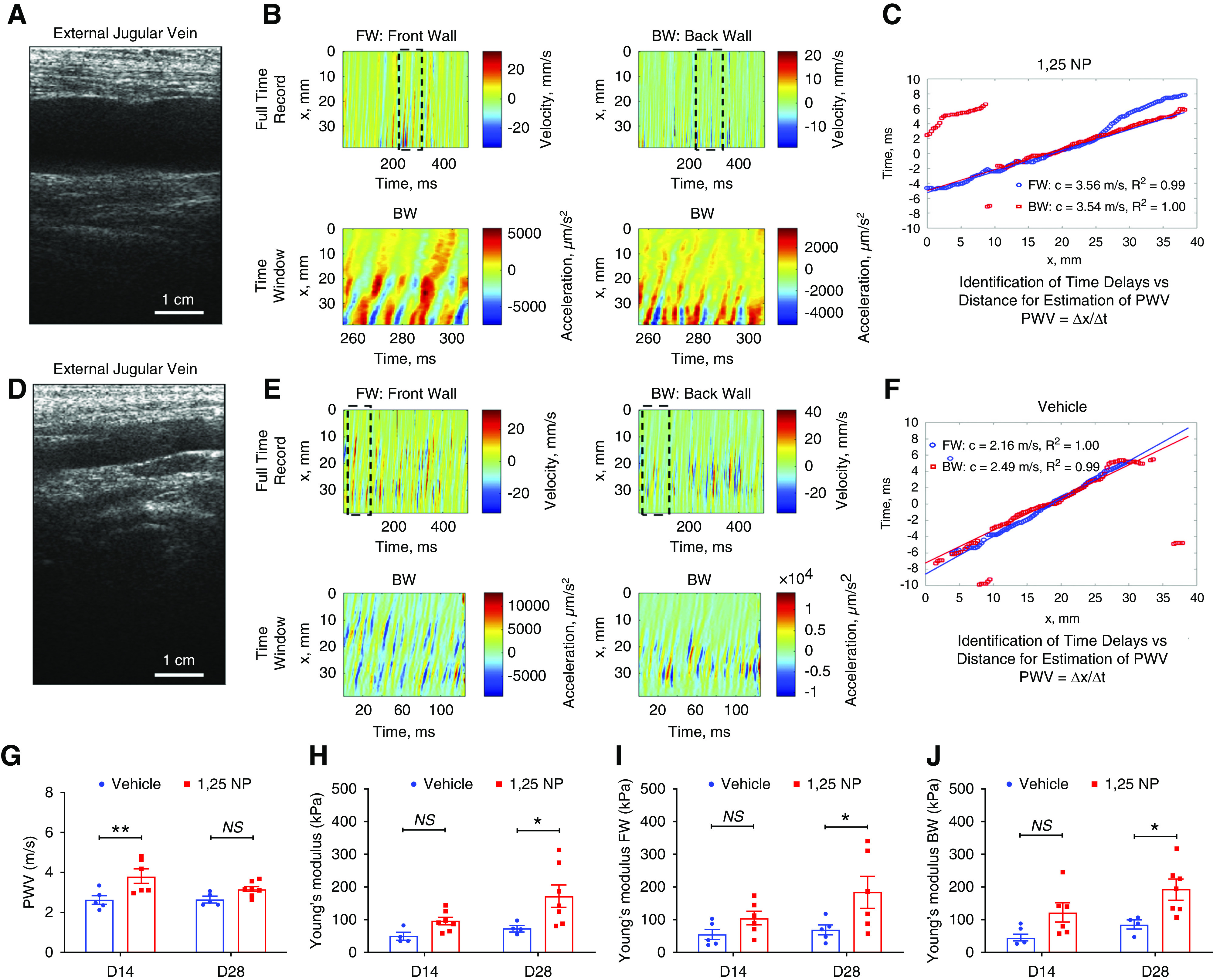
Ultrasound, PWV, and Young’s modulus analysis of outflow vein. (A) B-mode image of outflow vein treated with 1,25 NP. Scale bar is 10 mm. (B) Top row: FW and BW velocity records over 500 ms for veins treated with 1,25 NP. Bottom row: truncated time window from top row for wall acceleration of FW and BW used for correlation analysis for time delay identification in veins treated with 1,25 NP. (C) Plot of time delays of pulse waves versus distance to calculate PWV as Δx/Δt for veins treated with 1,25 NP. The reference for t=0 is denoted in the middle of the ultrasound image. A linear regression was performed to estimate the PWV and PWV velocity values are shown in the legend along with R2 from the regression. (D) B-mode image of outflow vein treated with vehicle. (E) Top row: FW and BW velocity records over 500 ms for veins treated with vehicle. Bottom row: truncated time window from top row for wall acceleration of FW and BW used for correlation analysis for time delay identification in veins treated with vehicle. (F) Plot of time delays of pulse waves versus distance to calculate PWV as Δx/Δt for vein treated with vehicle. (G) The PWV measured by ultrasound is significantly increased in 1,25 NP group compared with vehicle group at day 14. (H) The total Young’s modulus is significantly increased in 1,25 NP group compared with vehicle group at day 28 after AVF creation. (I) The FW Young’s modulus is significantly increased in 1,25 NP group compared with vehicle group at day 28 after AVF creation. (J) The BW Young’s modulus is significantly increased in 1,25 NP group compared with vehicle group at day 28 after AVF creation. Each bar represents mean ± SEM of n≥4. Two-way ANOVA was performed. *P<0.05, **P<0.01.
In aggregate, histomorphometric analysis, ultrasound, and MRI data suggest 1,25 NP treatment significantly reduces VNH in AVF, and is associated with positive vascular remodeling with increase in lumen vessel area, PWV, Young’s modulus, blood flow, and decrease in neointima area, neointima cell density, and wall shear stress.
Adventitial Delivery of 1,25 NP Reduces Fibrosis in the Outflow Vein
In failed AVF, there is medial fibrosis of the outflow vein and excessive synthesis of extracellular matrix by activated smooth muscle cells and myofibroblasts.34 We assessed venous fibrosis using two different histologic stains, Masson’s trichrome and Picrosirius red staining, at day 28 after AVF placement (Figure 6, A and B). We observed a significant decrease in the average Masson’s trichrome index in the 1,25 NP group compared with the vehicle group (1,25 NP, 26.77±4.67; vehicle, 45.08±5.65; average reduction, 41%; P=0.02; Figure 6C). We also observed a significant reduction in average Picrosirius red index in the 1,25 NP group compared with the vehicle group (1,25 NP, 5.76±0.94; vehicle, 12.03±0.82; average reduction, 52%; P=0.03; Figure 6D).
Figure 6.
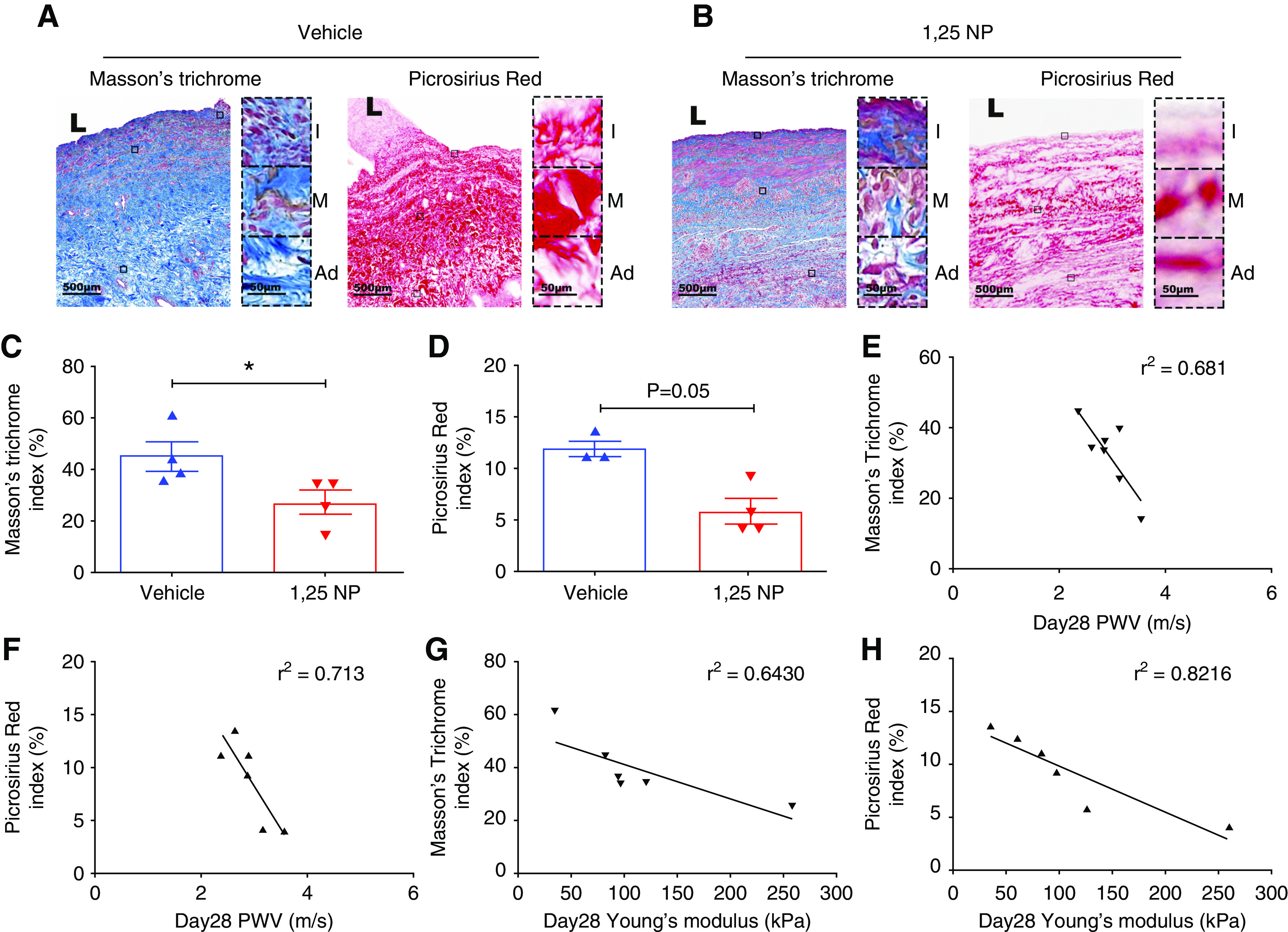
Masson’s trichrome and Picrosirius red staining. (A) Masson’s trichrome staining and Picrosirius red staining on day 28 after AVF creation of outflow vein from vehicle group. Collagen (blue color) is positive for Masson’s trichrome staining, and collagen (dark red color) is positive for Picrosirius red staining. Images were captured at ×40 magnification. Right panel shows enlarged view of positive cells across three different layers (×400 magnification). (B) Masson’s trichrome and Picrosirius red staining on day 28 after AVF creation of outflow vein from 1,25 NP group. (C) Semiquantitative analysis of collagen by Masson’s trichrome staining shows a reduction in the 1,25 NP group compared with the vehicle group. (D) Semiquantitative analysis of collagen by Picrosirius red staining shows a reduction in the 1,25 NP group compared with the vehicle group. (E) Correlation between Masson’s trichrome index and PWV. (F) Correlation between Picrosirius red index and PWV. (G) Correlation between Masson’s trichrome index and Young’s modulus. (H) Correlation between Picrosirius red index and Young’s modulus. Each bar represents mean±SEM of n≥3. Nonparametric Mann–Whitney test was performed. *P<0.05. L, lumen; Ad, adventitia; M, media; and I, intima. Scale bar is 500 µm and 50 µm (panel inset).
We performed semiquantitative analysis of Masson’s trichrome and Picrosirius red staining in adventitia, media, and intima of the outflow vein. In the intima layer of the AVF, there was no significant change in the average Masson’s trichrome and Picrosirius red index in the 1,25 NP group compared with the vehicle group (Supplemental Figure 4, D and G). However, in the media and adventitia, there was a significant decrease in the average Picrosirius red index in the 1,25 NP group compared with the vehicle group in (media, 1,25 NP, 4.13±0.72; vehicle, 12.25±2.57; average reduction, 66.27%; P=0.03, Supplemental Figure 4E; adventitia: 1,25 NP, 9.37±2.0; vehicle, 22.36±1.93; average reduction, 58.03%; P=0.03, Supplemental Figure 4F). We also observed a significant reduction in average Masson’s trichrome index in the 1,25 NP group compared with the vehicle group in the media and adventitia layer of outflow vein of AVF (media: 1,25 NP, 20.95±3.63; vehicle, 39.82±6.77; average reduction, 47.37%; P=0.05, Supplemental Figure 4H; adventitia: 1,25 NP, 32.91±6.14; vehicle, 56.57±4.64; average reduction, 41.82%; P=0.05, Supplemental Figure 4I).
We performed a linear regression analysis to determine the correlation between PWV and Young’s modulus and venous fibrosis as assessed by Masson’s trichrome and Picrosirius staining. We observed good correlation between both histologic stains. A better correlation was found between Picrosirius red index and PWV (r2=0.71; Figure 6F) compared with Masson’s trichrome index and PWV (r2=0.68; Figure 6E). We also observed a better correlation between Picrosirius red index and Young’s modulus (r2=0.82; Figure 6H) compared with Masson’s trichrome index (r2=0.64; Figure 6G). These data indicate that PWV and Young’s modulus correlate with fibrotic changes as assessed using histologic techniques.
Adventitial Delivery of 1,25 NP to the Outflow Vein of AVFs is Associated with Reduction in Hypoxia-Inducible Factor-1α and Vascular Endothelial Growth Factor-A
It was hypothesized that hypoxic injury occurs to the vessel wall after surgical placement of an AVF. This can cause inflammation, angiogenesis, and proliferation leading to VNH.18 Several studies have demonstrated increased expression of hypoxia-inducible factor-1α (HIF-1α) and vascular endothelial growth factor-A (VEGF-A) in animal models of HD graft failure, and in clinical specimens obtained from patients with HD vascular access failure.18,32,35 Therefore, we sought to investigate whether 1,25 NP treatment would have decreased HIF-1α and VEGF-A staining compared with vehicle at 28 days after AVF creation (Figure 7, A and B). There was increased expression of HIF-1α and VEGF-A in the vehicle group compared with 1,25 NP group, which was localized to the neointima and media area of the outflow vein (Figure 7, A and B). Semiquantitative analysis of sections immunostained with HIF-1α antibody demonstrated there was a significant decrease in the average HIF-1α index in the 1,25 NP group compared with the vehicle group (1,25 NP, 32.81±4.79; vehicle, 58.51±4.75; average reduction, 43.92%; P=0.003; Figure 7C). The average VEGF-A index was significantly decreased in the 1,25 NP group compared with the vehicle group (1,25 NP, 10.80±1.41; vehicle, 19.07±1.92; average reduction, 43.71%; P=0.003; Figure 7D).
Figure 7.
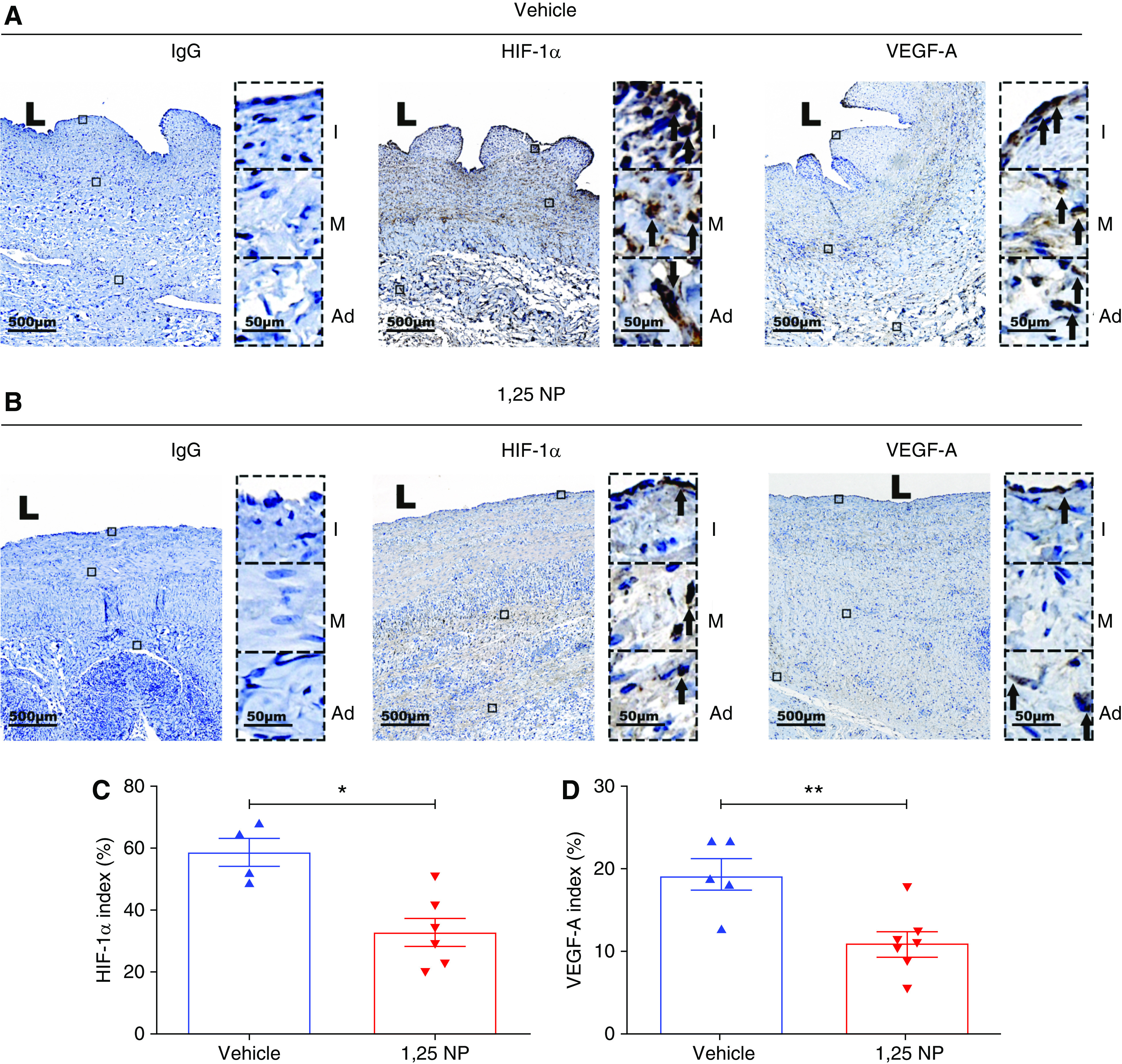
Immunohistochemical staining for HIF-1α and VEGF-A. (A) Staining for HIF-1α and VEGF-A on day 28 after AVF creation of outflow vein from vehicle group. HIF-1α and VEGF-A (+) cells have brown staining (black arrows). Images were captured at ×40 magnification. Right panel shows enlarged view of (+) cells across three different layers (×400 magnifications). (B) Staining for HIF-1α and VEGF-A on day 28 after AVF creation of outflow vein from 1,25 NP group. HIF-1α and VEGF-A (+) cells have brown staining. (C) Semiquantitative analysis shows reduction in HIF-1α (+) cells in 1,25 NP group compared with vehicle group. (D) Semiquantitative analysis shows reduction in VEGF-A (+) cells in 1,25 NP group compared with vehicle group. Each bar represents mean±SEM of n≥4. Nonparametric Mann–Whitney test was performed. *P<0.05, **P<0.01. L, lumen; Ad, adventitia; M, media; and I, intima. Scale bar is 500 µm and 50 µm (panel inset).
Adventitial Delivery of 1,25 NP to the Outflow Vein of AVFs is Associated with a Reduction in α-SMA, Desmin, and FSP-1 Staining
Experimental studies have shown that myofibroblasts (α-SMA [+] cells) and fibroblasts (FSP-1 [+] cells) promote VNH in AVFs.36,37 Hypoxic stress can induce the dedifferentiation of fibroblasts to a α-SMA [+] phenotype.38 Therefore, we sought to investigate whether 1,25 NP–treated veins have decreased α-SMA (+) cells and fibroblasts as compared with vehicle after AVF creation. The expression of α-SMA was localized to the neointima and media area of the outflow vein in the vehicle group (Supplemental Figure 5A) compared with 1,25 NP group (Supplemental Figure 5B). We observed a significant decrease in the average α-SMA index in the 1,25 NP group compared with the vehicle group (1,25 NP, 14.62±1.85; vehicle, 31.29±4.31; average reduction, 53.3%; P=0.0009; Supplemental Figure 5C). The expression of desmin was localized to the neointima and media area of the outflow vein of the vehicle group (Supplemental Figure 5A) compared with 1,25 NP group (Supplemental Figure 5B). There was a significant reduction in the desmin index in the 1,25 NP group compared with the vehicle group (1,25 NP, 5.08±0.84; vehicle, 11.95±1.14; average reduction, 57.4%; P=0.0004; Supplemental Figure 5D). There was no difference in the average vimentin index between the two groups (Supplemental Figure 5E). The average FSP-1 index was significantly decreased in the 1,25 NP group compared with the vehicle group (1,25 NP, 4.54±0.63; vehicle, 8.54±1.05; average reduction, 46.8%; P=0.004; Supplemental Figure 5F).
Adventitial Delivery of 1,25 NP to the Outflow Vein of AVFs is Associated with a Reduction in MCP-1 and CD68 Staining
We have shown a reduction of MCP-1 after treatment with 1,25 NP in murine AVF.4 Therefore, we sought to determine the expression of MCP-1 and CD68 as a measure of inflammation. The average MCP-1 index was significantly decreased in the 1,25 NP group compared with vehicle control (1,25 NP, 8.56±1.36; vehicle, 16.59±0.62; average reduction, 48.4%; P=0.0005; Supplemental Figure 6, A and C). The average CD68 index was significantly decreased in the 1,25 NP group compared with vehicle control (1,25 NP, 5.53±0.29; vehicle, 10.37±1.54; average reduction, 46.63%; P=0.04; Supplemental Figure 6, B and D).
Adventitial Delivery of 1,25 NP to the Outflow Vein of AVFs Reduces Cell Proliferation and Apoptosis in the Outflow Vein of AVF
Because there was a reduction in neointimal cell density, we investigated whether these changes were due to apoptosis and cell proliferation changes in AVFs treated with 1,25 NP or controls. Therefore, we evaluated this using TUNEL staining (Supplemental Figure 7, A and B). We found that the mean TUNEL (+) index was significantly decreased in the 1,25 NP group compared with vehicle group (1,25 NP, 6.91±1.02; vehicle, 12.44±1.28; average reduction, 55.6%; P=0.005; Supplemental Figure 7C). Cell proliferation was evaluated using Ki-67 staining (Supplemental Figure 7, A and B), and it was observed that there were increased Ki-67 (+) cells in the neointima compared with media and adventitia of the outflow vein (Supplemental Figure 7A). There was a significant reduction in average Ki-67 index in the 1,25 NP group compared with the vehicle group (1,25 NP, 8.40±1.01; vehicle, 18.73±2.47; average reduction: 55.1%; P=0.0004; Supplemental Figure 7D). Together, these results suggest outflow veins treated with 1,25 NP had decreased cellular proliferation and apoptosis at day 28 after fistula placement.
Adventitial Delivery of 1,25 NP to the Outflow Vein of AVFs Is Not Associated with Thrombosis and Calcium Deposition
Adventitial delivery of 1,25 NP may cause thrombosis in the outflow vein of AVF. We performed Carstairs staining of the outflow vein of AVF after 28 days of AVF placement in both groups (Supplemental Figure 8, A and B). Carstairs staining was used to distinguish between platelets (navy blue), fibrin (bright red), collagen (bright blue), muscle (red), and red blood cells (yellow). We did not observe any evidence of thrombosis in the 1,25 NP–treated vessel but did observe a decrease in collagen staining.
Since used active vitamin D metabolite, 1α,25(OH)2D3, and local delivery of 1,25 NP may result in calcium deposition in the outflow vein of AVF. To investigate this possibility, we performed Alizarin Red S staining in the outflow vein (Supplemental Figure 8, A and B). Calcium forms an Alizarin Red S–calcium complex, which gives an orange-red color in the staining. We did not observe any deposition of calcium in the outflow vein treated with 1,25 NP.
Transcriptomic Signature of 1,25 NP–Treated Outflow Vein of AVF
We performed whole transcriptome RNA seq with differential gene expression analysis in 1,25 NP– or vehicle-treated outflow veins at 28 days after AVF placement. A heat map of the differential gene expression in 1,25 NP or vehicle controls at an average fold change >1.5 (upregulated) or <0.75 (downregulated), with a RPKM >0.01 and a P<0.05 is shown (Figure 8A). There were 53 genes that were upregulated (Supplemental Table 17), and 468 genes that were downregulated in 1,25 NP–treated outflow vein compared with vehicle (Supplemental Table 18). Panther analysis identified that one of the most affected signaling pathways associated with the upregulated genes included TGFβ1 signaling (Figure 8B), and for the downregulated genes, included apoptosis (Figure 8C) and inflammation. Because we had demonstrated histologically that inflammation is decreased, we selected IL29, and confirmed the RNA seq data by performing qRT-PCR.39 The average IL29 gene expression was significantly decreased in 1,25 NP–treated vessels compared with vehicle controls (IL-29: 1,25 NP, 0.40±0.11; vehicle, 1.00±0.04; average reduction, 60%; P=0.003, Supplemental Figure 9A). Because we observed changes in epithelial to mesenchymal transition (EMT) with reduction in FSP-1 (+) and α-SMA (+) cells, we confirmed the gene expression of EGF-like repeats and discoidin domains 3 (EDIL3), which is involved in EMT.40 We observed that it was significantly upregulated in 1,25 NP–treated vessels compared with vehicle controls. The gene expression was confirmed using qRT-PCR, and there was a significant increase in the average EDIL3 in 1,25 NP–treated vessels compared with control vehicles (1,25 NP, 19.4±5.9; vehicle, 1.13±0.23; average increase, 1721%; P=0.002; Supplemental Figure 9B).
Figure 8.
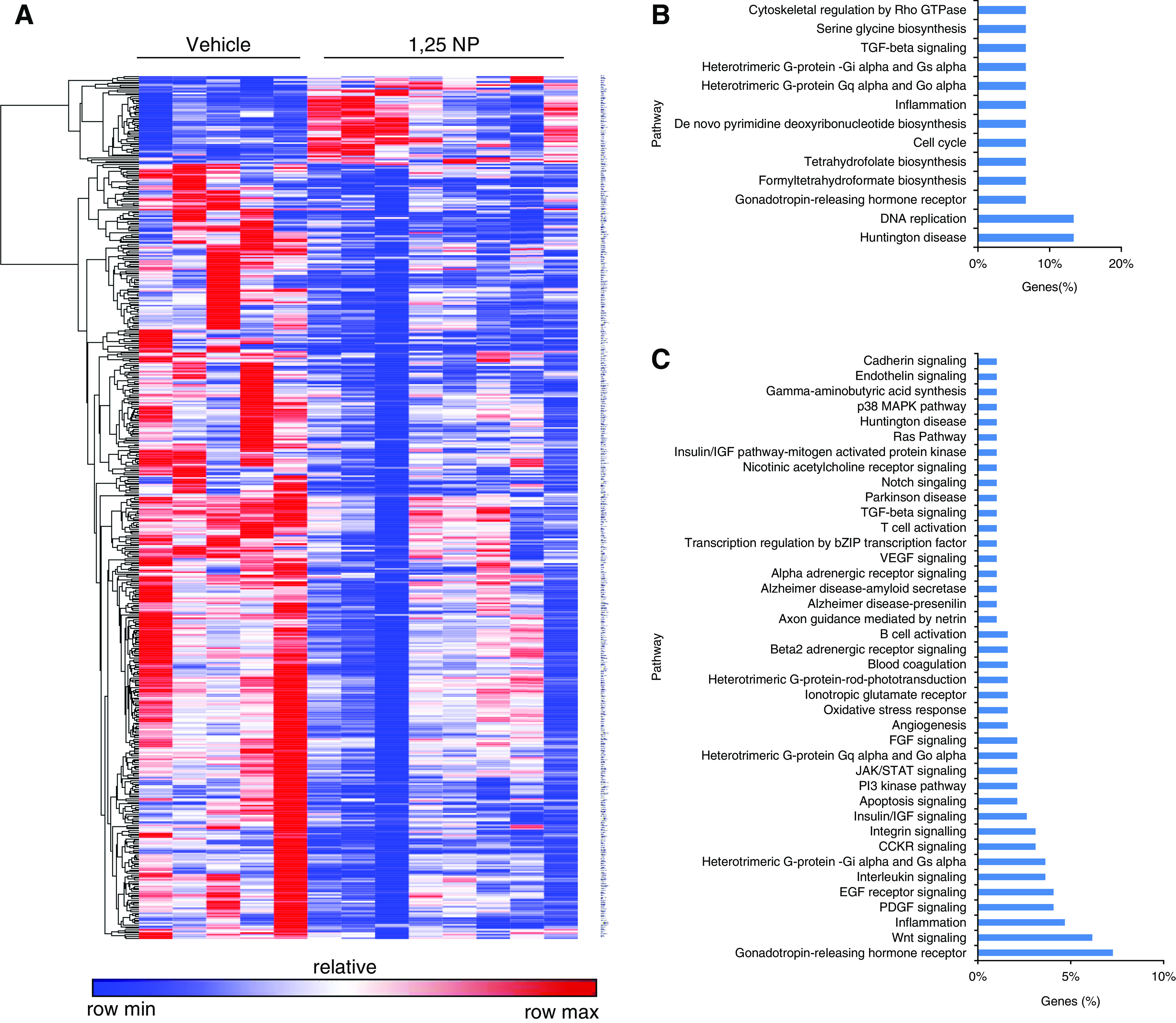
Whole transcriptome analysis using RNA seq with differential gene expression and Panther analyses at day 28. Unbiased whole transcriptome analysis total RNA from AVF outflow veins was performed as described in methods. (A) Heat map depicting all of the genes that are 1.5-fold up (red) or 0.75-fold down (blue) in outflow veins treated with 1,25 NPs (n=5) compared with vehicle controls (n=8) at day 28. (B) Panther analysis of upregulated genes. (C) Panther analysis of downregulated genes.
Discussion
The strength and novelty of this study was that we demonstrated a salutary effect of 1,25 NP on AVF VNH and stenosis by multiple methods, including histomorphometry, ultrasonography, and magnetic resonance imaging. PWV, Young’s modulus, PSV, blood flow, and lumen vessel area were measured using ultrasonography and magnetic resonance imaging. There was good correlation between the Young’s modulus and PWV to fibrosis. We found a significant reduction in fibrosis in the outflow vein of animals treated with 1,25 NP compared with vehicle group by Masson’s trichrome and Picrosirius red staining. All methods assessing vascular flow and vessel wall diameter showed a remarkable positive effect of 1,25 NP administration on the patency of AVFs. We characterized the PLGA nanoparticles using scanning electron microscopy and DLS analysis, and found the two different NPs were similar in shape and size, which indicates that encapsulation of 1α,25(OH)2D3 in PLGA nanoparticles did not alter the particle size and shape of nanoparticles. We performed whole transcriptomic analysis with differential gene expression analysis of outflow veins treated with 1,25 NP to assess the changes in gene expression, and found that 53 genes were upregulated and 468 genes were downregulated in 1,25 NP–treated vein compared with controls. The majority of the differentially expressed genes were associated with calcitriol, TGF-β1, apoptosis, and inflammatory pathways. We confirmed the gene-expression changes of the RNA seq analysis by performing qRT-PCR analysis of IL29 and EDIL3.
Disturbances in vitamin D metabolism are common in ESKD patients and may contribute to vascular dysfunction. Several studies have investigated the role of orally administered vitamin D metabolites in improving AVF maturation, or preventing VS in AVFs. There are conflicting data on the role of the prehormone, vitamin D3, in improving AVF access patency and maturation.11–15 Two studies demonstrated a beneficial effect of vitamin D3 on AVF performance. In the first, decreased levels of vitamin D3 were associated with poorer outcomes of AVFs.13,15 In the second, vitamin D3 and 1α-hydroxylated vitamin D analogs improved AVF outcomes.13 Rosenberg et al. noted that patients receiving any vitamin D replacement therapy had a lower incidence of access failure compared with those receiving no therapy. Those receiving vitamin D3 therapy with or without paricalcitol or calcitriol had an adjusted hazard ratio of 0.18 (95% confidence interval, 0.06 to 0.54) compared with those receiving no vitamin D therapy. Other studies, however, have not shown a salutary effect of vitamin D3 on fistula maturation.11,12 Of note, none of these studies used the biologically active, 1α-hydroxylated vitamin D analog, 1α,25(OH)2D3 or 1α-hydroxylated vitamin D analogs. Recently, a study of patients on HD showed that catheter injection of 1α,25(OH)2D3 into stenotic lesions after balloon angioplasty reduced AVF restenosis.14 Our own study in mice demonstrated a salutary effect of 1α,25(OH)2D3 in preventing AVF stenosis.4
Previously we reported that IER3 regulates vascular responses after creation of an AVF by decreasing inflammation, fibrosis, proliferation, and migration, and mice lacking the Ier3 gene are at decreased risk for developing VNH.4 The 1,25-dihydroxyvitamin D3 decreases the expression of IER3 gene via specific sequences present within the Ier3 gene.9 Our data show the adventitial delivery of 1,25 NP to the outflow vein of AVF reduces VS and VNH formation, by reducing gene and protein expression of IER3 at day 3 and 28 after AVF creation. It is likely that IEX-1/IER3 alters the expression of various cytokines in the vessel wall, thereby altering the infiltration of cells. The data suggest adventitial treatment of the outflow vein of AVFs with 1,25 NP safely reduces VNH formation, leading to positive vascular remodeling.
Prior studies have reported increased cell proliferation in AVF failure.41–43 VS in AVFs is hypothesized to be caused by many factors, including inflammation, oxidative stress, fibrosis, changes in wall shear stress, and hypoxic injury resulting in increased cellular proliferation and migration of adventitial cells accompanied by angiogenesis.32 Hypoxic injury to the vessel wall of the outflow vein at the time of AVF creation and arterial bypass grafts can result in VNH.5,44 Cytokines such as VEGF-A and HIF-1α have been shown to propagate inflammation, which can subsequently induce cell proliferation. These and other cytokines have been linked to activation of MCP-1.32 In this study, we demonstrated a reduction in HIF-1α, VEGF-A, and MCP-1 expression after treatment with 1,25 NP, compared with vehicle controls. Differentiation of adventitial fibroblasts to myofibroblasts and their proliferation and invasion into the intimal space has been previously shown to cause HD vascular access dysfunction.28,29 In our study, we found a significant reduction in α-SMA (+) cells, desmin (+), and FSP-1 (+) cells in the outflow veins treated with 1,25 NP. The reduction in the numbers of cells infiltrating the neointima was due to a reduction in cell division and could not be accounted for by changes in apoptosis.
Excess 1α,25(OH)2D3 can lead to vascular calcification,45 raising the possibility of vascular calcification of the outflow veins of AVFs with 1,25 NP delivery. An ideal therapy to reduce VS formation would be one that can be delivered selectively to the adventitia of the vessel wall. In this study, we did not observe calcium deposition in the outflow vein of 1,25 NP–treated animals as assessed by Alizarin Red S staining. We assessed serum calcium and phosphate levels, and observed a transient elevation of serum calcium concentration 3 days after delivery of 1,25 NP compared with vehicle control, with no difference at any other time points. At day 7 after AVF placement, phosphate levels increased in the 1,25 NP group compared with control.
In vitro experiments in this study indicate that 1α,25(OH)2D3 release from 1,25 NP with hydrogel is slow, and a steady process as opposed to a burst release of 1α,25(OH)2D3 from hydrogel alone. We choose our drug platform for this reason to limit the potential for hypercalcemia and potential calcium deposition to the vessel wall after delivery. This suggests the slow and optimal release of the 1α,25(OH)2D3 might be one plausible reason for the lack of vascular calcification after 1,25 NP delivery. Moreover, rheologic experiments suggest the temperature-sensitive biodegradable hydrogel becomes hard at physiologic temperature, and this would allow for local controlled drug release to the vessel wall. These results indicate that adventitial delivery of 1,25 NP is safe with respect to the development of hypercalcemia.
To simulate the clinical scenario, we used a porcine model with CKD. This model had several advantages. One advantage is that it simulates the clinically relevant features of uremia, with an average 2.5 fold increase in the serum creatinine and BUN.20 Another advantage of this model is that the vessels of the pig have the same dimensions as those of humans (the carotid artery is 4 mm and jugular vein is 6 mm), and it allows for evaluation of hemodynamics using MRI and VS. One final advantage of this model is that it allowed for the evaluation of local drug concentration after periadventitial delivery to the veins and in the blood temporally, so that potential complications of hypercalcemia, hyperphosphatemia, and calcification of the vessel can be monitored.
MRI was used to measure lumen vessel area, PSV, and blood flow. This demonstrated increased lumen vessel area and blood flow in 1,25 NP–treated vessels. We next estimated the Reynolds number and wall shear using the MRI data. Abnormal and high wall shear stress has been implicated as a potential mechanism for VNH and VS.19,21,22,32,46,47 The average shear stress was reduced in 1,25 NP–treated vessels compared with control vessels. Finally, we utilized VS to determine PWV and estimate Young’s modulus, and found good correlation between PWV and Young’s modulus to histologic evidence of fibrosis. We observed that at day 14, there was an increase in PWV in the 1,25 NP–treated vessels, which decreased by day 28. This observation may be due to arterialization of the outflow vein after the creation of AVF at day 14 in 1,25 NP–treated vessels.32
To identify the mechanisms by which 1,25 NP improves vascular remodeling, we used whole transcriptome RNA seq with differential gene expression analysis that identified 521 differentially regulated genes, including 53 genes that were upregulated and 468 genes that were downregulated. Panther analysis of the upregulated genes identified that TGFβ1 pathway is involved, whereas apoptosis and inflammatory pathways are involved with downregulated genes. IER3 can alter the rate of cell growth and apoptosis in various cells.9,48–57 We observed a reduction in apoptosis as assessed using TUNEL staining in 1,25 NP–treated vessels compared with controls. There was also a reduction in inflammation with a decrease in CD68 and MCP-1 staining, along with a decrease in fibrosis (TGFB1) pathway. Finally, we performed qRT-PCR to validate the RNA seq data. We observed that IL29, which was downregulated in the RNA seq data, was also significantly decreased by qRT-PCR analysis in the 1,25 NP–treated vessels compared with vehicle controls. IL29 has been shown to be involved with inflammation by activating TIMP-1, an inhibitor of MMP-9, in macrophages.39 Changes in MMP-9 expression has been shown to be associated with HD AVF failure.19,35,58 We also confirmed the expression of EDIL3, which was upregulated in the RNA seq data. It was found to be significantly increased in 1,25 NP–treated vessels compared with controls. EDIL3 has been shown to be involved with EMT.40 EMT through hypoxic differentiation of fibroblasts to smooth muscle cell differentiation has been shown to be responsible for VNH formation.32
There are several limitations are associated with the study. Clinically, AVFs are placed in patients with advanced CKD stage 5 and patients with ESKD with multiple different comorbidities, including hypertension and diabetes. Thus, the conditions of this animal model may still not simulate the clinical condition. We assessed the development of AVF after 28 days of placement, which may be an early time point, compared with human AVF creation. Longer time points to assess treatment benefits may need to be performed in future studies. VNH is historically associated with stenosis and early failure of vascular accesses based on histologic observations, but some clinical studies have shown this is not the most important feature in determining AVF maturation failure and stenosis.59,60
In conclusion, we demonstrate that adventitial delivery of 1,25 NP to the outflow vein is associated with a decrease in the gene and protein expression of IER3. This is accompanied by a reduction of CD68, α-SMA, and FSP-1 (+) cells. These changes result in a decrease in cell proliferation and decrease in collagen deposition, and effectively prevent VNH/VS formation in AVFs, leading to positive vascular remodeling with increased fistula patency. These findings suggest adventitial delivery of 1,25 NP can be used as a translational therapy for reducing VNH in patients with AVF among the ESKD population.
Disclosures
A US patent (WO2016011397A1) has been issued for the use of 1,25 NP. E.R. Edelman reports consultancy agreements with Abbvie, Abiomed, Alucent, Autus, Biodevek, CBSET, Edwards Life Sciences, Panther, Peregrine, and Tekla; reports having an ownership interest in Autus, BioDevek, and Panther; reports receiving research funding from Abiomed and Edwards Life Sciences; reports having patents and inventions with the Massachusetts Institute of Technology; and reports being a scientific advisor to or member of American Association for the Advancement of Science and the Food and Drug Administration. H. Kong reports being a scientific advisor to or membership of Biomaterials Journal, Biofabrication Journal, and Biomaterials Research. M. Urban reports consultancy agreements with Michigan State University; reports receiving research funding from Computerized Imaging Reference Systems, Incorporated and the National Institutes of Health (NIH) grants R01DK092255, R01HL145268; and reports having patents and inventions with General Electric Healthcare. R. Kumar reports consultancy agreements with Orfan; reports having an ownership interest in Orfan; reports receiving research funding from the Andersen Foundation and NIH; reports receiving honoraria from Bridgebio Pharma and Orfan; and reports being a scientific advisor to or membership of Bridgebio Pharma. S. Misra reports receiving research funding from NIH grants.
Funding
This work has been funded by NIH grants HL098967 and DK107870 (to S. Misra). E.R. Edelman was funded in part by NIH grant R01 49039.
Supplementary Material
Acknowledgments
The authors thank Boston Scientific (Natick, MA) and BD (Franklin Lakes, NJ) for supplying endovascular supplies needed to perform the animal experiments.
A.K. Singh, C. Cai, C. Zhao, S. Kilari, M. W. Urban, T. Macedo, R.J. Singh, and E. Takahashi performed the experiments and analyzed the data. E.R. Edelman and H. Kong designed the rheologic experiments and interpreted the data. R. Kumar interpreted the data and assisted in writing the paper. R. Kumar and S. Misra designed the research studies and interpreted the data, A.K. Singh, C. Cai, S. Kilari, R. Kumar, and S. Misra wrote the manuscript.
Footnotes
Published online ahead of print. Publication date available at www.jasn.org.
Supplemental Material
This article contains supplemental material online at http://jasn.asnjournals.org/lookup/suppl/doi:10.1681/ASN.2020060832/-/DCSupplemental.
Supplemental Figure 1. In vitro characterization of hydrogel.
Supplemental Figure 2. Schematic Representation of Pig AVF Model and Study Design.
Supplemental Figure 3. Drug delivery and Measurement of 1,25(OH)2D3 Concentration in Serum and Outflow vein after Delivery of Drug.
Supplemental Figure 4. Immunohistochemical staining of IER3, Masson’s trichrome, and picrosirus red staining.
Supplemental Figure 5. Immunohistochemical Staining for α-SMA, Desmin, Vimentin and FSP-1.
Supplemental Figure 6. Immunohistochemical Staining for MCP-1 and CD68.
Supplemental Figure 7. Assessment of Cell Apoptosis and Proliferation in the AVF outflow vein.
Supplemental Figure 8. Carstairs and Alizarin-Red S Staining.
Supplemental Figure 9. qRT-PCR analysis of IL29 and EDIL3.
Supplemental Table 1. Amount of Drug Delivery.
Supplemental Table 2. List of primers used for gene expression analysis.
Supplemental Table 3. List of antibodies used for IHC in the present study.
Supplemental Table 4. Serum Blood Urea Nitrogen (BUN) analysis of animals.
Supplemental Table 5. Serum Creatinine analysis of animals.
Supplemental Table 6. Serum Calcium analysis of animals.
Supplemental Table 7. Serum Phosphate analysis of animals.
Supplemental Table 8. Serum Potassium analysis of animals.
Supplemental Table 9. Serum Glucose analysis of animals.
Supplemental Table 10. Serum Albumin analysis of animals.
Supplemental Table 11. Serum Total Carbon Dioxide analysis of animals.
Supplemental Table 12. Serum Sodium analysis of animals.
Supplemental Table 13. Serum Chloride analysis of animals.
Supplemental Table 14. Weight of animals.
Supplemental Table 15. Systolic Blood Pressure of animals.
Supplemental Table 16. Diastolic Blood Pressure of animals.
Supplemental Table 17. List of up-regulated genes.
Supplemental Table 18. List of down-regulated genes.
References
- 1.Jager KJ, Kovesdy C, Langham R, Rosenberg M, Jha V, Zoccali C: A single number for advocacy and communication-worldwide more than 850 million individuals have kidney diseases. Kidney Int 96: 1048–1050, 2019 [DOI] [PubMed] [Google Scholar]
- 2.Santoro D, Benedetto F, Mondello P, Pipitò N, Barillà D, Spinelli F, et al.: Vascular access for hemodialysis: Current perspectives. Int J Nephrol Renovasc Dis 7: 281–294, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Al-Jaishi AA, Oliver MJ, Thomas SM, Lok CE, Zhang JC, Garg AX, et al.: Patency rates of the arteriovenous fistula for hemodialysis: A systematic review and meta-analysis. Am J Kidney Dis 63: 464–478, 2014 [DOI] [PubMed] [Google Scholar]
- 4.Brahmbhatt A, NievesTorres E, Yang B, Edwards WD, Roy Chaudhury P, Lee MK, et al.: The role of Iex-1 in the pathogenesis of venous neointimal hyperplasia associated with hemodialysis arteriovenous fistula. PLoS One 9: e102542, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lee ES, Bauer GE, Caldwell MP, Santilli SM: Association of artery wall hypoxia and cellular proliferation at a vascular anastomosis. J Surg Res 91: 32–37, 2000 [DOI] [PubMed] [Google Scholar]
- 6.Misra S, Fu AA, Rajan DK, Juncos LA, McKusick MA, Bjarnason H, et al.: Expression of hypoxia inducible factor-1 alpha, macrophage migration inhibition factor, matrix metalloproteinase-2 and -9, and their inhibitors in hemodialysis grafts and arteriovenous fistulas. J Vasc Interv Radiol 19: 252–259, 2008 [DOI] [PubMed] [Google Scholar]
- 7.Bruchfeld A, Carrero JJ, Qureshi AR, Lindholm B, Barany P, Heimburger O, et al.: Elevated serum macrophage migration inhibitory factor (MIF) concentrations in chronic kidney disease (CKD) are associated with markers of oxidative stress and endothelial activation. Mol Med 15: 70–75, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Weiss MF, Scivittaro V, Anderson JM: Oxidative stress and increased expression of growth factors in lesions of failed hemodialysis access. Am J Kidney Dis 37: 970–980, 2001 [DOI] [PubMed] [Google Scholar]
- 9.Im HJ, Craig TA, Pittelkow MR, Kumar R: Characterization of a novel hexameric repeat DNA sequence in the promoter of the immediate early gene, IEX-1, that mediates 1alpha,25-dihydroxyvitamin D(3)-associated IEX-1 gene repression. Oncogene 21: 3706–3714, 2002 [DOI] [PubMed] [Google Scholar]
- 10.Shiu YT, Rotmans JI, Geelhoed WJ, Pike DB, Lee T: Arteriovenous conduits for hemodialysis: How to better modulate the pathophysiological vascular response to optimize vascular access durability. Am J Physiol Renal Physiol 316: F794–F806, 2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kubiak RW, Zelnick LR, Hoofnagle AN, Alpers CE, Terry CM, Shiu YT, et al.; Hemodialysis Fistula Maturation Study Group: Mineral metabolism disturbances and arteriovenous fistula maturation. Eur J Vasc Endovasc Surg 57: 719–728, 2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wasse H, Huang R, Long Q, Zhao Y, Singapuri S, McKinnon W, et al.: Very high-dose cholecalciferol and arteriovenous fistula maturation in ESRD: A randomized, double-blind, placebo-controlled pilot study. J Vasc Access 15: 88–94, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Rosenberg JE, Astor BC, Deluca HF, Yevzlin AS: The association of mineral metabolism with vascular access patency. J Vasc Access 17: 392–396, 2016 [DOI] [PubMed] [Google Scholar]
- 14.Sato T, Iwasaki Y, Kikkawa Y, Fukagawa M: An efficacy of intensive vitamin D delivery to neointimal hyperplasia in recurrent vascular access stenosis. J Vasc Access 17: 72–77, 2016 [DOI] [PubMed] [Google Scholar]
- 15.Walker JP, Hiramoto JS, Gasper WJ, Auyang P, Conte MS, Rapp JH, et al.: Vitamin D deficiency is associated with mortality and adverse vascular access outcomes in patients with end-stage renal disease. J Vasc Surg 60: 176–183, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Strathmann FG, Laha TJ, Hoofnagle AN: Quantification of 1α,25-dihydroxy vitamin D by immunoextraction and liquid chromatography-tandem mass spectrometry. Clin Chem 57: 1279–1285, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.National Research Council (US) Committee for the Update of the Guide for the Care and Use of Laboratory Animals : Guide for the Care and Use of Laboratory Animals, 8th Ed., Washington (DC), National Academies Press (US), 2011 [PubMed] [Google Scholar]
- 18.Misra S, Fu AA, Puggioni A, Glockner JF, Rajan DK, McKusick MA, et al.: Increased expression of hypoxia-inducible factor-1 alpha in venous stenosis of arteriovenous polytetrafluoroethylene grafts in a chronic renal insufficiency porcine model. J Vasc Interv Radiol 19: 260–265, 2008 [DOI] [PubMed] [Google Scholar]
- 19.Misra S, Fu AA, Puggioni A, Karimi KM, Mandrekar JN, Glockner JF, et al.: Increased shear stress with upregulation of VEGF-A and its receptors and MMP-2, MMP-9, and TIMP-1 in venous stenosis of hemodialysis grafts. Am J Physiol Heart Circ Physiol 294: H2219–H2230, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Misra S, Gordon JD, Fu AA, Glockner JF, Chade AR, Mandrekar J, et al.: The porcine remnant kidney model of chronic renal insufficiency. J Surg Res 135: 370–379, 2006 [DOI] [PubMed] [Google Scholar]
- 21.Misra S, Fu AA, Misra KD, Glockner JF, Mukhopadhyay D: Wall shear stress measurement using phase contrast magnetic resonance imaging with phase contrast magnetic resonance angiography in arteriovenous polytetrafluoroethylene grafts. Angiology 60: 441–447, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Misra S, Woodrum DA, Homburger J, Elkouri S, Mandrekar JN, Barocas V, et al.: Assessment of wall shear stress changes in arteries and veins of arteriovenous polytetrafluoroethylene grafts using magnetic resonance imaging. Cardiovasc Intervent Radiol 29: 624–629, 2006 [DOI] [PubMed] [Google Scholar]
- 23.Montaldo G, Tanter M, Bercoff J, Benech N, Fink M: Coherent plane-wave compounding for very high frame rate ultrasonography and transient elastography. IEEE Trans Ultrason Ferroelectr Freq Control 56: 489–506, 2009 [DOI] [PubMed] [Google Scholar]
- 24.Pernot M, Fujikura K, Fung-Kee-Fung SD, Konofagou EE: ECG-gated, mechanical and electromechanical wave imaging of cardiovascular tissues in vivo. Ultrasound Med Biol 33: 1075–1085, 2007 [DOI] [PubMed] [Google Scholar]
- 25.Kolumam Parameswaran P, Dai D, Ding YH, Urban MW, Manlove L, Sathish V, et al.: Downstream vascular changes after flow-diverting device deployment in a rabbit model. J Neurointerv Surg 11: 523–527, 2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kasai C, Namekawa K, Koyano A, Omoto R: Real-time two-dimensional blood flow imaging using an autocorrelation technique. IEEE Trans Sonics Ultrason 32: 458–464, 1985 [Google Scholar]
- 27.Nieves Torres EC, Yang B, Roy B, Janardhanan R, Brahmbhatt A, Leof E, et al.: Adventitial delivery of lentivirus-shRNA-ADAMTS-1 reduces venous stenosis formation in arteriovenous fistula [published correction appears in PLoS One 9: e113312, 2014]. PLoS One 9: e94510, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Yang B, Janardhanan R, Vohra P, Greene EL, Bhattacharya S, Withers S, et al.: Adventitial transduction of lentivirus-shRNA-VEGF-A in arteriovenous fistula reduces venous stenosis formation. Kidney Int 85: 289–306, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Janardhanan R, Yang B, Vohra P, Roy B, Withers S, Bhattacharya S, et al.: Simvastatin reduces venous stenosis formation in a murine hemodialysis vascular access model. Kidney Int 84: 338–352, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Cai C, Kilari S, Zhao C, Simeon ML, Misra A, Li Y, et al.: Therapeutic effect of adipose derived mesenchymal stem cell transplantation in reducing restenosis in a murine angioplasty model. J Am Soc Nephrol 31: 1781–1795, 2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Robbin ML, Greene T, Cheung AK, Allon M, Berceli SA, Kaufman JS, et al.; Hemodialysis Fistula Maturation Study Group: Arteriovenous fistula development in the first 6 Weeks after creation. Radiology 279: 620–629, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Brahmbhatt A, Remuzzi A, Franzoni M, Misra S: The molecular mechanisms of hemodialysis vascular access failure. Kidney Int 89: 303–316, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Yellin EL: Laminar-turbulent transition process in pulsatile flow. Circ Res 19: 791–804, 1966 [DOI] [PubMed] [Google Scholar]
- 34.Martinez L, Duque JC, Tabbara M, Paez A, Selman G, Hernandez DR, et al.: Fibrotic venous remodeling and nonmaturation of arteriovenous fistulas. J Am Soc Nephrol 29: 1030–1040, 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Misra S, Shergill U, Yang B, Janardhanan R, Misra KD: Increased expression of HIF-1alpha, VEGF-A and its receptors, MMP-2, TIMP-1, and ADAMTS-1 at the venous stenosis of arteriovenous fistula in a mouse model with renal insufficiency. J Vasc Interv Radiol 21: 1255–1261, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Misra S, Doherty MG, Woodrum D, Homburger J, Mandrekar JN, Elkouri S, et al.: Adventitial remodeling with increased matrix metalloproteinase-2 activity in a porcine arteriovenous polytetrafluoroethylene grafts. Kidney Int 68: 2890–2900, 2005 [DOI] [PubMed] [Google Scholar]
- 37.Wang Y, Krishnamoorthy M, Banerjee R, Zhang J, Rudich S, Holland C, et al.: Venous stenosis in a pig arteriovenous fistula model--anatomy, mechanisms and cellular phenotypes. Nephrol Dial Transplant 23: 525–533, 2008 [DOI] [PubMed] [Google Scholar]
- 38.Misra S, Fu AA, Misra KD, Shergill UM, Leof EB, Mukhopadhyay D: Hypoxia-induced phenotypic switch of fibroblasts to myofibroblasts through a matrix metalloproteinase 2/tissue inhibitor of metalloproteinase-mediated pathway: Implications for venous neointimal hyperplasia in hemodialysis access. J Vasc Interv Radiol 21: 896–902, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Zhang H, Song B, He S: Interleukin 29 activates expression of tissue inhibitor of metalloproteinase 1 in macrophages via toll-like receptor 2. Mol Med Rep 17: 8363–8368, 2018 [DOI] [PubMed] [Google Scholar]
- 40.Gasca J, Flores ML, Jiménez-Guerrero R, Sáez ME, Barragán I, Ruíz-Borrego M, et al.: EDIL3 promotes epithelial-mesenchymal transition and paclitaxel resistance through its interaction with integrin αVβ3 in cancer cells. Cell Death Discov 6: 86, 2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Rekhter M, Nicholls S, Ferguson M, Gordon D: Cell proliferation in human arteriovenous fistulas used for hemodialysis. Arterioscler Thromb 13: 609–617, 1993 [DOI] [PubMed] [Google Scholar]
- 42.Swedberg SH, Brown BG, Sigley R, Wight TN, Gordon D, Nicholls SC: Intimal fibromuscular hyperplasia at the venous anastomosis of PTFE grafts in hemodialysis patients. Clinical, immunocytochemical, light and electron microscopic assessment. Circulation 80: 1726–1736, 1989 [DOI] [PubMed] [Google Scholar]
- 43.Roy-Chaudhury P, Kelly BS, Miller MA, Reaves A, Armstrong J, Nanayakkara N, et al.: Venous neointimal hyperplasia in polytetrafluoroethylene dialysis grafts. Kidney Int 59: 2325–2334, 2001 [DOI] [PubMed] [Google Scholar]
- 44.Lata C, Green D, Wan J, Roy S, Santilli SM: The role of short-term oxygen administration in the prevention of intimal hyperplasia. J Vasc Surg 58: 452–459, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Rodriguez M, Martinez-Moreno JM, Rodríguez-Ortiz ME, Muñoz-Castañeda JR, Almaden Y: Vitamin D and vascular calcification in chronic kidney disease. Kidney Blood Press Res 34: 261–268, 2011 [DOI] [PubMed] [Google Scholar]
- 46.Krishnamoorthy MK, Banerjee RK, Wang Y, Zhang J, Sinha Roy A, Khoury SF, et al.: Hemodynamic wall shear stress profiles influence the magnitude and pattern of stenosis in a pig AV fistula. Kidney Int 74: 1410–1419, 2008 [DOI] [PubMed] [Google Scholar]
- 47.Rajabi-Jagahrgh E, Krishnamoorthy MK, Wang Y, Choe A, Roy-Chaudhury P, Banerjee RK: Influence of temporal variation in wall shear stress on intima-media thickening in arteriovenous fistulae. Semin Dial 26: 511–519, 2013 [DOI] [PubMed] [Google Scholar]
- 48.de Laval B, Pawlikowska P, Barbieri D, Besnard-Guerin C, Cico A, Kumar R, et al.: Thrombopoietin promotes NHEJ DNA repair in hematopoietic stem cells through specific activation of Erk and NF-κB pathways and their target, IEX-1. Blood 123: 509–519, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Gurlek A, Pittelkow MR, Kumar R: Modulation of growth factor/cytokine synthesis and signaling by 1alpha,25-dihydroxyvitamin D(3): Implications in cell growth and differentiation. Endocr Rev 23: 763–786, 2002 [DOI] [PubMed] [Google Scholar]
- 50.Haugen JD, Pittelkow MR, Zinsmeister AR, Kumar R: 1 alpha,25-dihydroxyvitamin D3 inhibits normal human keratinocyte growth by increasing transforming growth factor beta 2 release. Biochem Biophys Res Commun 229: 618–623, 1996 [DOI] [PubMed] [Google Scholar]
- 51.Im HJ, Pittelkow MR, Kumar R: Divergent regulation of the growth-promoting gene IEX-1 by the p53 tumor suppressor and Sp1. J Biol Chem 277: 14612–14621, 2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Itin PH, Pittelkow MR, Kumar R: Effects of vitamin D metabolites on proliferation and differentiation of cultured human epidermal keratinocytes grown in serum-free or defined culture medium. Endocrinology 135: 1793–1798, 1994 [DOI] [PubMed] [Google Scholar]
- 53.Kobayashi T, Pittelkow MR, Warner GM, Squillace KA, Kumar R: Regulation of a novel immediate early response gene, IEX-1, in keratinocytes by 1alpha,25-dihydroxyvitamin D3. Biochem Biophys Res Commun 251: 868–873, 1998 [DOI] [PubMed] [Google Scholar]
- 54.Kumar R, Kobayashi T, Warner GM, Wu Y, Salisbury JL, Lingle W, et al.: A novel immediate early response gene, IEX-1, is induced by ultraviolet radiation in human keratinocytes. Biochem Biophys Res Commun 253: 336–341, 1998 [DOI] [PubMed] [Google Scholar]
- 55.Kumar R, Pittelkow MR, Salisbury JL, Grande JP, Im HJ, Feldmann KA, et al.: A novel vitamin D-regulated immediate-early gene, IEX-1, alters cellular growth and apoptosis. Recent Results Cancer Res 164: 123–134, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Schilling D, Pittelkow MR, Kumar R: IEX-1, an immediate early gene, increases the rate of apoptosis in keratinocytes. Oncogene 20: 7992–7997, 2001 [DOI] [PubMed] [Google Scholar]
- 57.Veenstra TD, Pittelkow MR, Kumar R: Regulation of cellular growth by 1,25-dihydroxyvitamin D(3)-mediated growth factor expression. News Physiol Sci 14: 37–40, 1999 [DOI] [PubMed] [Google Scholar]
- 58.Misra S, Fu A, Anderson J, Glockner J, McKusick M, Bjarnason H, et al.: The rat femoral arteriovenous fistula model: Increased expression of matrix metalloproteinase-2 and -9 at the venous stenosis. J Vasc Interv Radiol 19: 587–594, 2008 [DOI] [PubMed] [Google Scholar]
- 59.Duque JC, Tabbara M, Martinez L, Paez A, Selman G, Salman LH, et al.: Similar degree of intimal hyperplasia in surgically detected stenotic and nonstenotic arteriovenous fistula segments: A preliminary report. Surgery 163: 866–869, 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Duque JC, Tabbara M, Martinez L, Cardona J, Vazquez-Padron RI, Salman LH: Dialysis arteriovenous fistula failure and angioplasty: Intimal Hyperplasia and other causes of access failure. Am J Kidney Dis 69: 147–151, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.