Significance Statement
A cyclic regimen of corticosteroid and cyclophosphamide is the first-line therapy for membranous nephropathy. Rituximab is superior to conservative treatment and noninferior to cyclosporine in inducing remission; it also may have a more favorable safety profile compared with cyclic therapy, but a head-to-head comparison of rituximab versus cyclic therapy is lacking. Using a multisite design, the authors designed a pilot randomized trial to obtain estimates of the effects of the two therapies and to assess the recruitment potential of a noninferiority trial. They found rituximab and cyclophosphamide may have comparable effects on disease remission and a similar short-term safety profile. These data suggest that, although rituximab may be a valid alternative to cyclic therapy for patients with membranous nephropathy, a head-to-head pragmatic comparison would require a large, global, noninferiority trial.
Keywords: membranous nephropathy, glomerulonephritis, nephrotic syndrome
Visual Abstract
Abstract
Background
A cyclic corticosteroid-cyclophosphamide regimen is the first-line therapy for membranous nephropathy. Compared with this regimen, rituximab therapy might have a more favorable safety profile, but a head-to-head comparison is lacking.
Methods
We randomly assigned 74 adults with membranous nephropathy and proteinuria >3.5 g/d to rituximab (1 g) on days 1 and 15, or a 6-month cyclic regimen with corticosteroids alternated with cyclophosphamide every other month. The primary outcome was complete remission of proteinuria at 12 months. Other outcomes included determination of complete or partial remission at 24 months and occurrence of adverse events.
Results
At 12 months, six of 37 patients (16%) randomized to rituximab and 12 of 37 patients (32%) randomized to the cyclic regimen experienced complete remission (odds ratio [OR], 0.4; 95% CI, 0.13 to 1.23); 23 of 37 (62%) receiving rituximab and 27 of 37 (73%) receiving the cyclic regimen had complete or partial remission (OR, 0.61; 95% CI, 0.23 to 1.63). At 24 months, the probabilities of complete and of complete or partial remission with rituximab were 0.42 (95% CI, 0.26 to 0.62) and 0.83 (95% CI, 0.65 to 0.95), respectively, and 0.43 (95% CI, 0.28 to 0.61) and 0.82 (95% CI, 0.68 to 0.93), respectively, with the cyclic regimen. Serious adverse events occurred in 19% of patients receiving rituximab and in 14% receiving the cyclic regimen.
Conclusions
This pilot trial found no signal of more benefit or less harm associated with rituximab versus a cyclic corticosteroid-cyclophosphamide regimen in the treatment of membranous nephropathy. A head-to-head, pragmatic comparison of the cyclic regimen versus rituximab may require a global noninferiority trial.
Clinical Trial registry name and registration number:
Rituximab versus Steroids and Cyclophosphamide in the Treatment of Idiopathic Membranous Nephropathy (RI-CYCLO), NCT03018535
Primary membranous nephropathy (MN) is a common cause of nephrotic syndrome in adults. In 70% of patients, the target antigen is the M-type phospholipase A2 receptor (PLA2R)1, whereas the thrombospondin type-1 domain-containing 7A is the target in 1%–5% of patients2. A subset of MN is associated with accumulation of exostosin 1 and 2 or neural EGF-like 1 protein in the glomerular basement membrane.3,4
Long-term follow-up studies have shown that 35%–40% of patients with MN and heavy proteinuria who are not treated may die or progress to ESKD.5,6 In patients who are nephrotic, guidelines recommend a cyclic corticosteroid-cyclophosphamide regimen (referred to hereafter as “cyclic regimen”) as first-line intervention.7 Although the cyclic regimen induces a partial or complete disease remission in 60%–80% of patients at 1 year,8–10 cyclophosphamide may cause bone-marrow suppression, gonadal toxicity, and oncogenic effects.
Rituximab, a selective B cell–depleting agent with a relatively more favorable safety profile, was recently found to be noninferior to cyclosporine, a second-line agent, in inducing long-term remission of MN.11 It is not known how rituximab compares with the cyclic regimen, and a trial may be difficult to conduct for many reasons. First, available evidence indicates lower remission rates with rituximab (60% at 1 year)11 than with the cyclic regimen (70% at 1 year).5,6 A superiority trial would be difficult to justify. Second, testing noninferiority while comparing safety will likely require a large trial. Data on recruitment rates and adherence to therapies are needed to ensure the feasibility of such a trial.
We designed this pilot, randomized controlled study to obtain estimates of the effects of rituximab relative to the cyclic regimen in people with MN and assess the recruitment potential using a multisite design.
Methods
Trial Design and Oversight
We conducted this open-label, pilot, two-parallel-arm, randomized controlled trial (Supplemental Figure 1) in 11 centers (ten in Italy, one in Switzerland). This investigator-initiated trial12 was approved by the institutional ethics committee at each site (ethics committee of Brescia, NP 1063, December 10, 2011).
Participants
All patients with incident MN and proteinuria >3.5 g/d referred to each participating institution were screened for eligibility. Participants had to be ≥18 years and have a biopsy sample–proven diagnosis of MN within 24 months before enrollment, proteinuria >3.5 g/d on three 24-hour urine collections (once a week for 3 weeks, after the 3-month run-in phase), and an eGFR of ≥30 ml/min per 1.73 m2.13 The complete list of eligibility criteria is provided in Supplemental Appendix 1.
Randomization
Participants were randomized 1:1 to the intervention or active comparator arm. An analyst from a distant site, with no clinical involvement in the trial, generated the randomization lists and kept them concealed. A local study coordinator, responsible for recruitment, assigned a unique participant study number and requested participant assignment, after obtaining signed consent, from the institution responsible for randomization.
Interventions
Patients assigned to the intervention arm received two courses of rituximab at a dose of 1 g on days 1 and 15.12 Patients who were assigned to the active-comparator arm received the cyclic regimen, consisting of three consecutive cycles lasting for 2 months each (for a total of 6 months), where steroids were alternated with cyclophosphamide every other month10; the cumulative dose of cyclophosphamide per patient was 180 mg/kg. A complete blood count was performed monthly in all study participants; a weekly blood count was taken during treatment with cyclophosphamide in the cyclic-therapy arm to allow for dose changes, as per guideline recommendations.7 A complete description of the study treatments is provided in the Supplemental Appendix 1.
Outcomes and Follow-Up
The primary outcome measure was the probability of complete remission (proteinuria to ≤0.3 g/d) at 1 year. Secondary outcome measures at 6, 12, 18, and 24 months included the change in proteinuria from baseline, the probability of complete or partial remission (proteinuria at least 50% lower than the baseline and ≤3.5 g/d), and estimated eGFR and serum creatinine levels. We planned to assess the levels of anti-PLA2R autoantibodies and their relation to therapy and proteinuria. We summarized data on serious adverse events, including death, life-threatening events, and disability.
Study visits were completed at baseline and after 6, 12, 18, 24, and 36 months, and follow-up continued until complications or relapses occurred. We assessed the levels of anti-PLA2R autoantibodies at baseline and at 6, 12, 18, 24, and 36 months after treatment, using a standardized commercial ELISA (EUROIMMUN, Lubeck, Germany).14 Patients were considered positive for anti-PLA2R autoantibodies when baseline serum levels were >20 RU/ml; the same cutoff was used for defining the immunologic response. More detailed descriptions of outcomes and follow-up are provided in Supplemental Appendix 1.
Statistical Methods
We planned to include at least 35 participants per arm. Analyses were mainly descriptive and focused on confidence-interval estimation. We used standard statistical methods to summarize the sample characteristics overall and by arm assignment, using statistics for quantitative (mean and SD or median, when appropriate) and qualitative (frequencies) data as appropriate. Acknowledging the nature and purpose of this pilot study, we performed comparative analyses, including logistic regression to compare binary outcomes and obtain probabilities, and methods for continuous or survival data in order to obtain time-to-event analyses. In the main analyses, we used an intention-to-treat approach. A description of the statistical methods is provided in the Supplemental Appendix 1.
Results
Study Participants
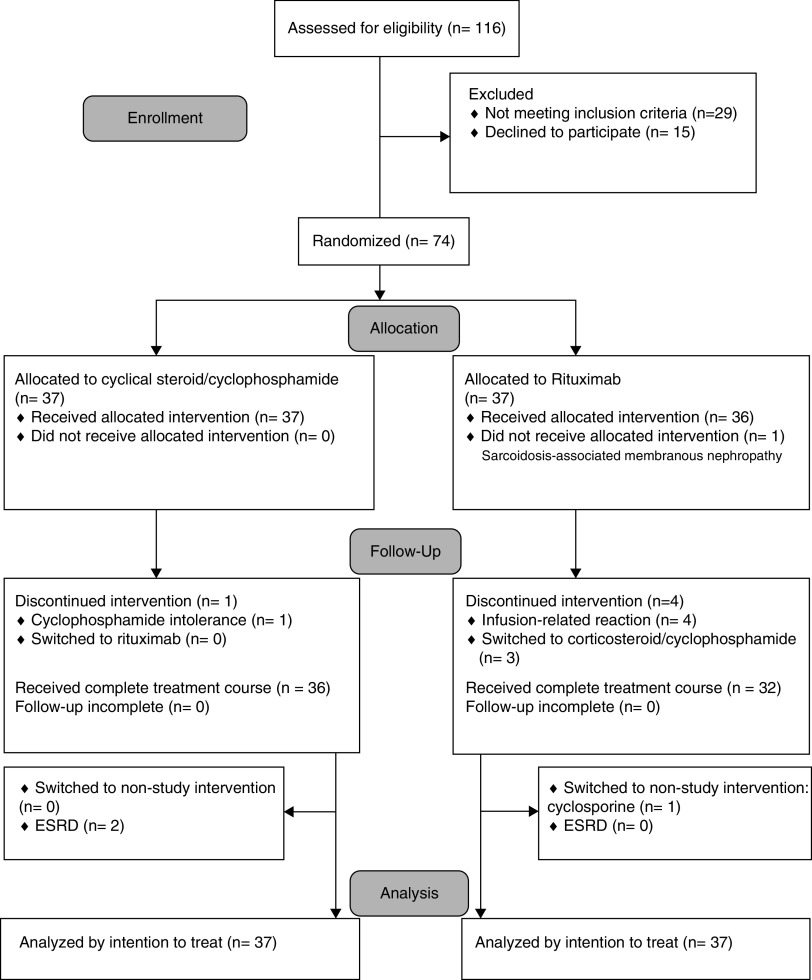
From January 2012 through December 2018, 116 potential participants were screened and 74 were enrolled. A total of 37 participants were randomly assigned to the cyclic regimen arm, and 37 to the rituximab arm (Figure 1). Baseline characteristics were comparable between arms (Table 1). The mean age of the patients was 55 years; 72% were men. Average (SD) eGFR was 84 (24) ml/min per 1.73 m2, median (interquartile range [IQR]) proteinuria was 6 (4–10) g/d, and mean (SD) serum albumin was 2 (1) g/dl. Most participants had stage I–II disease (71%); 46% had signs of interstitial fibrosis and tubular atrophy. One participant assigned to rituximab was diagnosed with sarcoidosis-associated MN after randomization and before treatment. Four patients withdrew from rituximab treatment because of an infusion reaction: three patients (8%) experienced a severe infusion reaction (dyspnea, itchy throat, and urticaria), discontinued the treatment intervention, and received the cyclic regimen; one patient had a mild reaction (cough, itchy throat) and refused completion of rituximab treatment, remaining on symptomatic treatment only. All four of these patients remained in the study. One patient in the cyclic-regimen arm discontinued the study intervention due to cyclophosphamide intolerance (severe nausea and vomiting). The remaining patients (36 in the cyclic-regimen arm and 32 in the rituximab arm) received the complete intervention. At 12 months, one patient in the rituximab arm received a nonstudy intervention (cyclosporine) due to treatment failure. Two patients assigned to the cyclic regimen reached kidney failure requiring renal replacement, at 7 and 24 months, respectively. The baseline eGFR of these patients was 69 ml/min and 41 ml/min, respectively. Complete data at 12 months were available for 36 participants assigned to the rituximab arm and for all 37 participants assigned to the cyclic regimen arm. At 24 and 36 months, 26 (70%) and 20 (54%) participants remained in the study in the rituximab arm and 31 (84%) and 22 (59%) remained in the cyclic-regimen arm. Details on follow-up throughout the study are provided in the Supplemental Appendix 2 (patient follow-up section).
Figure 1.
CONSORT diagram. Patients were randomly assigned, in a 1:1 ratio, to receive cyclic regimen or rituximab. One patient who was assigned to the rituximab group was diagnosed with sarcoidosis-associated MN after randomization. Premature discontinuation occurred in four patients in the rituximab arm, and in one patient in the cyclic-regimen arm. The remaining patients received the complete intervention: 32 in the rituximab arm and 36 in the cyclic-regimen arm. During the follow-up of patients in the rituximab arm, one was switched to a nonstudy intervention (cyclosporine) because of no response, another patient reached ESKD.
Table 1.
Baseline characteristics in the RI-CYCLO study according to treatment group
| Characteristic | Total | Cyclic Regimen | Rituximab | P Value |
|---|---|---|---|---|
| N | 74 | 37 | 37 | |
| Age at randomization (yr), mean (SD) | 55 (15) | 55 (17) | 54 (14) | 0.97 |
| Age at biopsy (yr), mean (SD) | 54 (15) | 54 (17) | 54 (14) | 0.89 |
| Male sex, n (%) | 53 (72) | 25 (68) | 28 (76) | 0.44 |
| History of hypertension, n (%) | 48 (65) | 22 (59) | 26 (70) | 0.33 |
| Interstitial fibrosis, tubular atrophy, n (%) | 34 (46) | 15 (41) | 19 (51) | 0.35 |
| Disease stage, n (%) | 0.31 | |||
| I | 12 (16) | 8 (22) | 4 (11) | |
| II | 41 (55) | 16 (43) | 25 (68) | |
| III | 15 (20) | 8 (22) | 7 (19) | |
| IV | 3 (4) | 2 (5) | 1 (3) | |
| Missing | 3 (4) | 3 (8) | 0 (0) | |
| Body wt (kg), mean (SD) | 76 (13) | 76 (15) | 75 (11) | 0.71 |
| Height (m), mean (SD) | 2 (0) | 2 (0) | 2 (0) | 0.76 |
| Body mass index (kg/m2), mean (SD) | 26 (4) | 26 (5) | 26 (3) | 0.53 |
| Systolic BP (mm Hg), mean (SD) | 127 (13) | 129 (13) | 126 (13) | 0.23 |
| Diastolic BP (mm Hg), mean (SD) | 76 (9) | 76 (10) | 77 (8) | 0.79 |
| Serum creatinine (mg/dl), mean (SD) | 1 (0) | 1 (0) | 1 (0) | 0.30 |
| eGFR (ml/min per 1.73 m2), mean (SD)a | 84 (24) | 86 (25) | 83 (24) | 0.59 |
| Proteinuria (g/d), median (IQR) | 6 (4–10) | 6 (5–9) | 6 (4–10) | 0.71 |
| Serum albumin (g/dl), mean (SD) | 2 (1) | 2 (1) | 2 (1) | 0.56 |
| Total cholesterol (mg/dl), mean (SD) | 278 (87) | 283 (96) | 273 (77) | 0.62 |
| LDL cholesterol (mg/dl), mean (SD) | 185 (70) | 185 (77) | 186 (64) | 0.97 |
| Patients positive for anti-PLA2R, n (%)b | 41 (66) | 19 (59) | 22 (73) | 0.25 |
| Anti-PLA2R levels (RU/ml), median (IQR) | 58 (43–86) | 58 (40–81) | 63 (52–87) | 0.50 |
Data are presented as mean (SD) or median (IQR) for continuous measures, and n (%) for categoric measures.
eGFR was calculated according to the Chronic Kidney Disease Epidemiology Collaboration equation.
Anti-PLA2R positivity defined by a value >20 RU/ml. Anti-PLA2R status was assessed in 62 patients, 32 treated with Cyclical Regimen and 30 with Rituximab
Primary Outcome: Complete Remission at 1 Year
Fewer participants in the rituximab arm (n=6; 16%) than in the cyclic-regimen arm (n=12; 32%) achieved complete remission at 12 months (odds ratio [OR], 0.40; 95% CI, 0.13 to 1.23; Table 2). According to per-protocol analysis, complete remission at 12 months was achieved in four participants (13%) in the rituximab arm and 13 participants (34%) in the cyclic-regimen arm (OR, 0.28; 95% CI, 0.08 to 0.95; Supplemental Table 1).
Table 2.
Complete remission or composite (complete or partial remission) at 6–12 months by intention-to-treat analysis
| Study Time Points | No. of Patients with Remission/Total No. (%) | OR (95% CI) | |
|---|---|---|---|
| Rituximab | Cyclic Regimen | ||
| Complete remission | |||
| 6 mo | 3/37 (8) | 2/37 (5) | 1.54 (0.24 to 9.8) |
| 12 mo | 6/37 (16) | 12/37 (32) | 0.40 (0.13 to 1.23) |
| 18 mo | 10/32 (31) | 7/34 (21) | 1.75 (0.57 to 5.36) |
| 24 mo | 11/26 (42) | 11/31 (35) | 1.33 (0.46 to 3.89) |
| 36 mo | 6/20 (30) | 7/22 (32) | 0.92 (0.25 to 3.41) |
| Complete or partial remission | |||
| 6 mo | 19/37 (51) | 24/37 (65) | 0.57 (0.22 to 1.45) |
| 12 mo | 23/37 (62) | 27/37 (73) | 0.61 (0.23 to 1.63) |
| 18 mo | 21/32 (66) | 27/34 (79) | 0.49 (0.16 to 1.49) |
| 24 mo | 22/26 (85) | 25/31 (81) | 1.32 (0.33 to 5.29) |
| 36 mo | 17/20 (85) | 16/22 (73) | 2.12 (0.45 to 9.96) |
The intention-to-treat population included all of the patients who underwent randomization. The primary outcome was complete remission at 12 mo.
Secondary Outcomes
In the intention-to treat analysis, 23 of 37 participants (62%) receiving rituximab and 27 of 37 (73%) receiving the cyclic regimen had a complete or partial remission (OR, 0.61; 95% CI, 0.23 to 1.63) at 12 months. Moreover, in both the intention-to-treat and per-protocol analyses, the numbers of participants who achieved remission at months 6–12 was comparable by treatment arm (Supplemental Table 1). In post hoc analyses, we found a trend for lower complete remission rates at 12 months in the rituximab arm for men, people <55 years old, and for those with more severe proteinuria or lower serum albumin; however, statistical tests for interaction were all nonsignificant (Supplemental Figure 2; nonsignificant interactions). The probabilities of achieving remission during a longer follow-up were also similar by study arm (Figure 2, Supplemental Tables 2 and 3). The probabilities of complete remission at 18 and 24 months were 0.29 (95% CI, 0.17 to 0.48) and 0.42 (95% CI, 0.26 to 0.62), respectively, in the rituximab arm, and 0.33 (95% CI, 0.2 to 0.51) and 0.43 (95% CI, 0.28 to 0.61), respectively, in the cyclic-regimen arm. Probabilities of complete or partial remission at 18 and 24 months were 0.71 (95% CI, 0.55 to 0.85) and 0.83 (95% CI, 0.65 to 0.95), respectively, in the rituximab arm, and 0.82 (95% CI, 0.68 to 0.93) and 0.82 (95% CI, 0.68 to 0.93), respectively, in the cyclic-regimen arm.
Figure 2.

Kaplan–Meier estimates of complete or composite remission. Kaplan–Meier estimates of (A) complete remission and (B) composite remission (complete or partial) in the cyclic-regimen and rituximab arms.
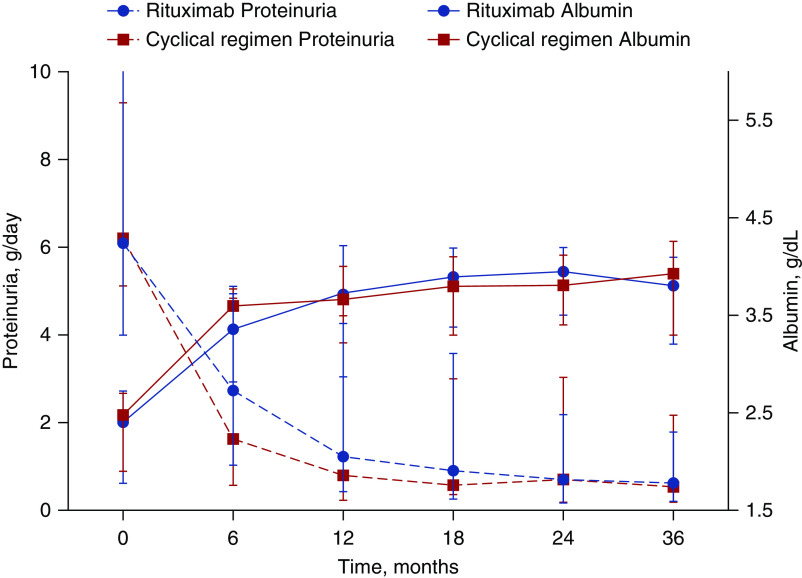
Proteinuria decreased from 6.1 g/d (IQR, 4.0–10.1) at baseline to 0.7 g/d (IQR, 0.2–2.2) at 24 months in the rituximab arm, and from 6.2 g/d (IQR, 5.1–9.3) at baseline to 0.7 g/d (IQR, 0.2–3.0) at 24 months in the cyclic-regimen arm (Figure 3, Supplemental Table 2). Albumin increased from 2.4 g/dl (IQR, 1.8–2.7) at baseline to 4.0 g/dl (IQR, 3.5–4.2) at 24 months in the rituximab arm, and from 2.5 g/dl (IQR, 1.9–2.7) at baseline to 3.8 g/dl (IQR, 3.4–4.1) at 24 months in the cyclic-regimen arm (Supplemental Table 2). Levels of serum creatinine and eGFR remained stable over time in both arms (Supplemental Figure 3, Supplemental Table 3).
Figure 3.
Proteinuria and serum albumin over time. Data are presented as median (IQR) over time, by assigned treatment.
A total of 62 patients were tested for circulating anti-PLA2R1 antibodies. Of these patients, 41 (66%) were positive at baseline; 22 were in the rituximab arm and 19 were in the cyclic-regimen arm (Table 1). Immunologic response was achieved in most patients (Supplemental Table 4). Patients with lower anti-PLA2R1 antibody levels tended to respond better to either treatment (Supplemental Table 5). Anti-PLA2R levels decreased in both arms during follow-up, more rapidly in the rituximab arm (Supplemental Figure 4). The reduction of anti-PLA2R1 serum levels was accompanied by a decrease in proteinuria in both groups (Supplemental Figure 4).
Nine participants relapsed after they had achieved remission. In the rituximab arm, disease relapse occurred in three out of the 23 participants (13%) who had achieved complete or partial remission at 12 months; in the cyclic regimen, relapse occurred in six participants out of the 27 (22%) who had achieved complete or partial remission at 6 months (four patients) or 12 months (two patients). Proteinuria and anti-PLA2R titer throughout follow-up, in patients who experienced a relapse are shown in Supplemental Table 6.
Adverse Events
Adverse events occurred in 32 participants during the study, evenly distributed by arm (43%); serious adverse events occurred in seven patients (19%) receiving rituximab and in five (14%) receiving the cyclic regimen (Table 3). Four participants had an infusion-related reaction with rituximab, which determined therapy cessation. Leukopenia (≤4000/cmm) and pneumonia, requiring reduction or temporary cessation of cyclophosphamide, were more frequent in the cyclic-regimen arm. Three patients positive for anti-PLA2R1 antibodies developed cancer: two were in the rituximab arm (lung and breast carcinoma, detected at month 24 and 18, respectively), and one was in the cyclic-regimen arm (prostate carcinoma, detected at month 12). At cancer diagnosis, the two patients in the rituximab arm were in partial remission; the patient in the cyclic-regimen arm had a substantial reduction in proteinuria without fulfilling partial remission criteria. The patient with lung cancer died during follow-up.
Table 3.
Adverse events according to treatment group
| Event | Cyclic Regimen (n=37) | Rituximab (n=37) | P Valuea | ||
|---|---|---|---|---|---|
| Patients, n (%) | Events, No. of Events (rate per 100 patient-yr) | Patients, n (%) | Events, No. of Events (rate per 100 patient-yr) | ||
| Any adverse event | 16 (43) | 30 (54) | 16 (43) | 25 (47) | >0.99 |
| Serious adverse event | 5 (14) | 6 (7) | 7 (19) | 8 (11) | 0.75 |
| Fatal | 0 (0) | 0 (0) | 1 (3) | 1 (1) | >0.99 |
| Nonfatal | 5 (14) | 6 (7) | 7 (19) | 7 (9) | 0.75 |
| Nonserious adverse events | 13 (35) | 24 (40) | 11 (30) | 19 (30) | 0.80 |
| Adverse events in at least 5% of patients or any serious adverse event | |||||
| Hyperglycemia | 2 (5) | 2 (2) | 1 (3) | 1 (1) | >0.99 |
| Leukopenia | 3 (8) | 6 (8) | 0 (0) | 0 (0) | 0.24 |
| Pneumoniae | 3 (8) | 7 (8) | 0 (0) | 0 (0) | 0.24 |
| Other infectious events | 6 (16) | 8 (10) | 5 (14) | 6 (8) | >0.99 |
| Acute coronary syndrome | 1 (3) | 1 (1) | 1 (3) | 1 (1) | >0.99 |
| Stroke | 0 (0) | 0 (0) | 1 (3) | 1 (1) | >0.99 |
| Drug infusion reaction/drug intolerance | 1 (3) | 1 (1) | 9 (24) | 10 (13) | 0.01 |
| Cancer | >0.99 | ||||
| Lung | 0 (0) | 0 (0) | 1 (3) | 1 (1) | |
| Prostate | 1 (3) | 1 (1) | 0 (0) | 0 (0) | |
| Breast | 0 (0) | 0 (0) | 1 (3) | 1 (1) | |
| Other | 3 (8) | 4 (5) | 3 (8) | 4 (5) | >0.99 |
P values are for the difference in proportions of patients having a specific type of event.
Discussion
This pilot, randomized trial comparing rituximab versus cyclic regimen in people with MN and severe proteinuria showed that the rationale for a superiority design is weak, and slow recruitment may make it difficult to complete a sufficiently large, noninferiority trial testing whether rituximab is safer than the cyclic regimen. This trial failed to detect any difference in benefits or harms between the two treatments. Although nonsignificantly different according to the intention-to treat analysis, the 1-year probability of complete remission was lower in the rituximab arm in per-protocol analysis. When considering complete and partial responses combined, we found no difference between arms throughout the follow-up. Although our data suggest the cyclic regimen may induce remission earlier, a larger and longer trial is required to test evidence of long-term differences between treatments. The rates of combined complete and partial responses in our study were higher than those previously reported,11,15,16 reaching 80% at 24 months, and no differences in rates of short-term adverse events between treatments were detected. Infusion reactions were more common in the rituximab arm. Leukopenia and infectious complications were more common in the cyclic-regimen arm, although less frequent when compared with historical data, possibly due to the intensive blood-test monitoring that was performed during the cyclophosphamide month.
The comparable effects of cyclic regimen and rituximab on disease remission may be due to their ability to halt the production of anti-PLA2R1 or other pathogenetic autoantibodies. In our cohort, 66% of the population included in the antibody study tested positive for anti-PLA2R1; the low median antibody levels in this subgroup suggest a relatively benign prognosis in a fraction of study participants.17 A significant reduction of anti-PLA2R1 levels occurred quickly in both arms, and preceded the decrease of proteinuria, supporting a pathogenic role for these antibodies.18–21 A similar decrease in proteinuria was observed in patients negative for anti-PLA2R1 antibodies, suggesting both regimens were equally effective in cases of MN that were potentially mediated by other pathogenic autoantibodies. Of note, immunologic remission rates were similar in the two arms and were in keeping with the rates reported in recent randomized controlled trials.11,15 Lower immunologic response rate in the cyclic-regimen arm of this study as compared with retrospective studies22 may be explained by differences in cumulative cyclophosphamide doses, which were lower in our trial.
The ideal rituximab dose for MN is still a matter of controversy and debate.11,16 In the GEMRITUX and MENTOR trials, rituximab was given at a dose of 375 mg/m2 at days 1–8 and 1 g at days 0–15, respectively.11,15 In MENTOR, if proteinuria was reduced from baseline by at least 25% at 6 months, a second course of rituximab was administered, regardless of the level of CD19+ B cells. Complete and partial remission rates at 6 months were 35% in both trials. In GEMRITUX, after a median of 17 months from enrollment, the proportion of responders increased to 65%. Similar response rates were recorded in MENTOR (at 12 and 18 months, 60% and 62%, respectively). In our study, response rates at 6, 12, and 18 months were 51%, 62%, and 66%, respectively. Despite lower doses of rituximab in the RI-CYCLO versus MENTOR trial, the 12-month rates of complete response were similar (14% and 16%, respectively). Efficacy and safety of different treatment schemes will need to be tested in future prospective trials.
Although our findings are consistent with those of two recent controlled trials,11,15 comparisons should also acknowledge the differences in inclusion criteria. In our study, as in GEMRITUX and MENTOR, baseline characteristics were in keeping with a full-blown nephrotic syndrome. For example, mean (SD) or median (IQR) serum albumin levels were 2 (1) g/dl, 2.2 (1.9–2.6) g/dl, and 2.5 (2.1–2.9) g/dl in RI-CYCLO, GEMRITUX, and MENTOR, respectively. MENTOR had a higher proteinuria cutoff for inclusion, possibly in keeping with the higher titer of anti-PLA2R1 antibodies at baseline; of note the latter were assessed with a different method compared with that used in our study and in GEMRITUX.11,15
Our study is the only existing trial providing a head-to-head comparison of rituximab monotherapy versus cyclic regimen. Its multicenter design and rigorous methodology are additional strengths. To date, only a retrospective study compared effectiveness and safety of rituximab versus a cyclic regimen, showing a similar incidence of complete remission but adverse events were significantly higher in the cyclic regimen.23 In that study, cyclophosphamide was given with steroids and administered for a longer period (6–12 months). The signal of a trend toward increased effectiveness with the cyclic regimen in patients with more severe nephrotic syndrome, along with its shorter time to induce complete remission, is intriguing; this hypothesis may be tested in future studies.
Our study has also limitations. Given the complex treatment regimens, patients and healthcare providers were aware of the treatment assignments. However, whether this had an effect on remission outcomes is questionable, considering the definitions were based on objective laboratory values. Another limitation is that the primary end point is a surrogate end point. However, remission of proteinuria at 1 year is widely accepted as a surrogate end point in clinical studies.24 Moreover, our findings may not apply to patients with a higher titer of anti-PLA2R1 antibodies, and the study duration may have been insufficient to capture the long-term toxicity potentially attributable to the treatment used.
Findings from this study have implications for research. The direction of the effects we found are inconsistent with the hypothesis of superior benefits of rituximab versus the cyclic regimen, suggesting a superiority trial may be difficult to justify unless it used alternative protocol designs, particularly including different dosing regimens and inclusion criteria. Given the high remission rates with either treatment found in our and other studies, a large, noninferiority trial comparing standard rituximab doses to cyclophosphamide might be unfeasible. For example, considering the most frequent outcome (complete or partial remission), if there is a true difference in favor of the cyclic regimen of 5% (70% versus 65%), then 1500 participants per arm are required to be 90% sure that the upper limit of a one-sided 95% CI (or, equivalently, a 90% two-sided CI) will exclude a difference in favor of the cyclic regimen of >10%.25 Considering the sample size will need to be increased to account for protocol violations, an adequately powered trial can only be completed with a properly funded, global effort.
Our pilot study provides potentially useful information for clinicians and patients. Considering the comparable benefits and short-term safety profiles of these interventions, rituximab may represent an alternative treatment option to the cyclic regimen. Although long-term benefits on the risk of kidney failure and death are only available for the cyclic regimen,5 the long-term toxicity of cyclophosphamide remains a matter of concern. Because no safe threshold for a cumulative amount of cyclophosphamide associated with carcinogenic effect and gonadal toxicity has been determined, the use of cyclophosphamide has been proposed for patients at high risk of declining kidney function.26 Our post hoc analysis data are consistent with this hypothesis. Given the similar efficacy of the two interventions, and the potentially better long-term safety profile of rituximab, rituximab may be considered as a first-line therapy for MN or for repeat treatments,26 considering MN tends to relapse.1 However, rituximab may not be the best option for all patients. In our trial and in the MENTOR study,11 20%–40% of patients did not respond to rituximab. A variable proportion of patients are intolerant to rituximab and can respond to the cyclic regimen.27 Identification of patients potentially resistant to rituximab and/or with worse prognosis remains challenging. The use of clinical and serologic markers, including proteinuria, kidney function, titer of anti-PLA2R antibodies, anti-PLA2R epitope spreading,28–30 and, in the future, genetic markers,31 might allow for more accurate risk stratification of patients with MN to inform treatment decisions.
In conclusion, a slow recruitment rate may make a sufficiently large noninferiority trial difficult to complete. In this pilot trial, there was no signal of superiority of rituximab versus the cyclic regimen in people with MN and severe proteinuria. Further research is required to establish a more personalized therapy for patients with MN.32
Disclosures
F. Alberici reports having consultancy agreements with Baxter. S. Feriozzi reports having consultancy agreements with, receiving honoraria from, and being on a speakers bureau for Amicus, Sanofi, and Takeda; and receiving research funding from Takeda. L. Gesualdo reports receiving honoraria from AstraZeneca, Estor, GlaxoSmithKline, Mundipharma, Retrophin, and Sanofi Genzyme; being on the board of directors for European Renal Association–European Dialysis and Transplant Association (ERA-EDTA), Renal Pathology Society (RPS), and Italian Society of Nephrology (SIN); being a scientific advisor for, or member of, Journal of Nephrology and Nephrology Dialysis Transplantation; having consultancy agreements with Medtronic and Retrophin; and receiving research funding from Sanofi Genzyme and philanthropic sources. R. Magistroni reports having consultancy agreements with and being a scientific advisor for, or member of, Otsuka Pharmaceutical. A. Pani reports being a scientific advisor for, or member of, BMC Nephrology and Journal of Nephrology; having other interests in/relationships with ERA-EDTA and the Italian Society of Nephrology; and being the president of the Italian Renal Federation. D. Santoro reports receiving honoraria from Alexion, Astellas, AstraZeneca, and Mundipharma. M. Santostefano reports receiving honoraria from Amicus and Takeda; and being a scientific advisor for, or member of, the Italian Rare Diseases Register, and being a member of the Italian Society of Amyloidosis (SIA). All remaining authors have nothing to disclose.
Funding
None.
Supplementary Material
Acknowledgments
The authors thank the safety and monitoring board at the Spedali Civili di Brescia Hospital for study monitoring. The authors thank Dr. Francesca Brognoli of the Clinical Research Design and Phase I Studies unit of the ASST Spedali Civili of Brescia for the support in reviewing the protocol. We thank the Immunopathology Working Group of the Italian Society of Nephrology for contributing to center identification.
The interpretation and conclusions contained herein are those of the researchers. All authors affirm that this manuscript is an honest, accurate, and transparent account of the study being reported; that no important aspects of the study have been omitted; and that any discrepancies from the study as planned have been explained.
Prof. Francesco Scolari, Prof. Domenico Santoro, Prof. Loreto Gesualdo, Prof. Antonello Pani, Dr. Sandro Feriozzi, Dr. Giuliano Boscutti, Dr. Marco Quaglia, Dr. Claudio Ponticelli, Dr. Gian Marco Ghiggeri, and Prof. Pietro Ravani were involved in conception and trial design; Prof. Francesco Scolari, Dr. Elisa Delbarba, Prof. Pietro Ravani, Dr. Gian Marco Ghiggeri, and Dr. Claudio Ponticelli drafted the article; Prof. Pietro Ravani, Prof. Federico Alberici, and Dr. Elisa Delbarba analyzed the data; Dr. Elisa Delbarba made the figures. All authors revised and approved the final version of the manuscript.
Footnotes
Published online ahead of print. Publication date available at www.jasn.org.
Contributor Information
Collaborators: RI-CYCLO Investigators, Francesco Scolari, Elisa Delbarba, Domenico Santoro, Loreto Gesualdo, Paolo Protopapa, Lucia Argentiero, Antonello Pani, Andrea Angioi, Nicola Lepore, Nadia Dallera, Laila-Yasmin Mani, Bruno Vogts, Marisa Santostefano, Sandro Feriozzi, Marco Quaglia, Giuliano Boscutti, Angelo Ferrantelli, Carmelita Marcantoni, Patrizia Passerini, Riccardo Magistroni, Federico Alberici, Gian Marco Ghiggeri, Claudio Ponticelli, and Pietro Ravani
Supplemental Material
This article contains the following supplemental material online at http://jasn.asnjournals.org/lookup/suppl/doi:10.1681/ASN.2020071091/-/DCSupplemental.
Supplemental Summary 1. Investigators and committee members.
Supplemental Appendix 1. Supplemental methods.
Supplemental Appendix 2. Supplemental results.
Supplemental Table 1. Complete remission or composite (complete or partial remission) at 6 to 12 months by per-protocol analysis.
Supplemental Table 2. Proteinuria and serum albumin by arm and study time-points.
Supplemental Table 3. Serum creatinine by arm and study time-points.
Supplemental Table 4. Evolution of anti-PLA2R and percentage of immunological remission according to treatment group, in anti-PLA2R positive patients.
Supplemental Table 5. Clinical response in anti-PLA2R positive patients, by anti-PLA2R levels at baseline.
Supplemental Table 6. Proteinuria and anti-PLA2R titer throughout follow-up, in patients who experienced a relapse.
Supplemental Figure 1. Design of the study.
Supplemental Figure 2. Pre-specified subgroup analysis of the composite outcome (complete or partial remission) at 12-month follow-up.
Supplemental Figure 3. Serum creatinine and eGFR over time.
Supplemental Figure 4. Anti-PLA2R levels and proteinuria by treatment group and time in anti-PLA2R positive patients (A). Proteinuria by group and time in anti-PLA2R negative patients (B).
References
- 1.Beck LH Jr, Bonegio RG, Lambeau G, Beck DM, Powell DW, Cummins TD, et al.: M-type phospholipase A2 receptor as target antigen in idiopathic membranous nephropathy. N Engl J Med 361: 11–21, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Tomas NM, Beck LH Jr, Meyer-Schwesinger C, Seitz-Polski B, Ma H, Zahner G, et al.: Thrombospondin type-1 domain-containing 7A in idiopathic membranous nephropathy. N Engl J Med 371: 2277–2287, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Sethi S, Madden BJ, Debiec H, Charlesworth MC, Gross L, Ravindran A, et al.: Exostosin 1/exostosin 2-associated membranous nephropathy. J Am Soc Nephrol 30: 1123–1136, 2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Sethi S, Debiec H, Madden B, Charlesworth MC, Morelle J, Gross L, et al.: Neural epidermal growth factor-like 1 protein (NELL-1) associated membranous nephropathy. Kidney Int 97: 163–174, 2020 [DOI] [PubMed] [Google Scholar]
- 5.Ponticelli C, Zucchelli P, Passerini P, Cesana B, Locatelli F, Pasquali S, et al.: A 10-year follow-up of a randomized study with methylprednisolone and chlorambucil in membranous nephropathy. Kidney Int 48: 1600–1604, 1995 [DOI] [PubMed] [Google Scholar]
- 6.Jha V, Ganguli A, Saha TK, Kohli HS, Sud K, Gupta KL, et al.: A randomized, controlled trial of steroids and cyclophosphamide in adults with nephrotic syndrome caused by idiopathic membranous nephropathy. J Am Soc Nephrol 18: 1899–1904, 2007 [DOI] [PubMed] [Google Scholar]
- 7.Kidney Disease: Improving Global Outcomes (KDIGO) Glomerulonephritis Work Group., KDIGO clinical practice guidelines for glomerulonephritis 2012. Available at: https://kdigo.org/guidelines/gd/. Accessed August 16, 2020
- 8.Ponticelli C, Zucchelli P, Imbasciati E, Cagnoli L, Pozzi C, Passerini P, et al.: Controlled trial of methylprednisolone and chlorambucil in idiopathic membranous nephropathy. N Engl J Med 310: 946–950, 1984 [DOI] [PubMed] [Google Scholar]
- 9.Ponticelli C, Zucchelli P, Passerini P, Cagnoli L, Cesana B, Pozzi C, et al.: A randomized trial of methylprednisolone and chlorambucil in idiopathic membranous nephropathy. N Engl J Med 320: 8–13, 1989 [DOI] [PubMed] [Google Scholar]
- 10.Ponticelli C, Altieri P, Scolari F, Passerini P, Roccatello D, Cesana B, et al.: A randomized study comparing methylprednisolone plus chlorambucil versus methylprednisolone plus cyclophosphamide in idiopathic membranous nephropathy. J Am Soc Nephrol 9: 444–450, 1998 [DOI] [PubMed] [Google Scholar]
- 11.Fervenza FC, Appel GB, Barbour SJ, Rovin BH, Lafayette RA, Aslam N, et al.; MENTOR Investigators: Rituximab or cyclosporine in the treatment of membranous nephropathy. N Engl J Med 381: 36–46, 2019 [DOI] [PubMed] [Google Scholar]
- 12.Scolari F, Dallera N, Gesualdo L, Santoro D, Pani A, Santostefano M, et al.: Rituximab versus steroids and cyclophosphamide for the treatment of primary membranous nephropathy: Protocol of a pilot randomised controlled trial. BMJ Open 9: e029232, 2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Levey AS, Stevens LA, Schmid CH, Zhang YL, Castro AF III, Feldman HI, et al.; CKD-EPI (Chronic Kidney Disease Epidemiology Collaboration): A new equation to estimate glomerular filtration rate [published correction appears in Ann Intern Med 155: 408, 2011]. Ann Intern Med 150: 604–612, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Dähnrich C, Komorowski L, Probst C, Seitz-Polski B, Esnault V, Wetzels JF, et al.: Development of a standardized ELISA for the determination of autoantibodies against human M-type phospholipase A2 receptor in primary membranous nephropathy. Clin Chim Acta 421: 213–218, 2013 [DOI] [PubMed] [Google Scholar]
- 15.Dahan K, Debiec H, Plaisier E, Cachanado M, Rousseau A, Wakselman L, et al.; GEMRITUX Study Group: Rituximab for severe membranous nephropathy: A 6-month trial with extended follow-up. J Am Soc Nephrol 28: 348–358, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ruggenenti P, Cravedi P, Chianca A, Perna A, Ruggiero B, Gaspari F, et al.: Rituximab in idiopathic membranous nephropathy. J Am Soc Nephrol 23: 1416–1425, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ruggenenti P, Debiec H, Ruggiero B, Chianca A, Pellé T, Gaspari F, et al.: Anti-phospholipase A2 receptor antibody titer predicts post-rituximab outcome of membranous nephropathy. J Am Soc Nephrol 26: 2545–2558, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Qin W, Beck LH Jr, Zeng C, Chen Z, Li S, Zuo K, et al.: Anti-phospholipase A2 receptor antibody in membranous nephropathy. J Am Soc Nephrol 22: 1137–1143, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kanigicherla D, Gummadova J, McKenzie EA, Roberts SA, Harris S, Nikam M, et al.: Anti-PLA2R antibodies measured by ELISA predict long-term outcome in a prevalent population of patients with idiopathic membranous nephropathy. Kidney Int 83: 940–948, 2013 [DOI] [PubMed] [Google Scholar]
- 20.Hoxha E, Thiele I, Zahner G, Panzer U, Harendza S, Stahl RA: Phospholipase A2 receptor autoantibodies and clinical outcome in patients with primary membranous nephropathy. J Am Soc Nephrol 25: 1357–1366, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Bech AP, Hofstra JM, Brenchley PE, Wetzels JF: Association of anti-PLA2R antibodies with outcomes after immunosuppressive therapy in idiopathic membranous nephropathy. Clin J Am Soc Nephrol 9: 1386–1392, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.van de Logt AE, Dahan K, Rousseau A, van der Molen R, Debiec H, Ronco P, et al.: Immunological remission in PLA2R-antibody-associated membranous nephropathy: Cyclophosphamide versus rituximab. Kidney Int 93: 1016–1017, 2018 [DOI] [PubMed] [Google Scholar]
- 23.van den Brand JAJG, Ruggenenti P, Chianca A, Hofstra JM, Perna A, Ruggiero B, et al.: Safety of rituximab compared with steroids and cyclophosphamide for idiopathic membranous nephropathy. J Am Soc Nephrol 28: 2729–2737, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Thompson A, Cattran DC, Blank M, Nachman PH: Complete and partial remission as surrogate end points in membranous nephropathy. J Am Soc Nephrol 26: 2930–2937, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Sealed Envelope Ltd: Power calculator for binary outcome non-inferiority trial, 2012. Available at: https://www.sealedenvelope.com/power/binary-noninferior/. Accessed June 9, 2020
- 26.Howman A, Chapman TL, Langdon MM, Ferguson C, Adu D, Feehally J, et al.: Immunosuppression for progressive membranous nephropathy: A UK randomised controlled trial. Lancet 381: 744–751, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Bonanni A, Calatroni M, D’Alessandro M, Signa S, Bertelli E, Cioni M, et al.: Adverse events linked with the use of chimeric and humanized anti-CD20 antibodies in children with idiopathic nephrotic syndrome. Br J Clin Pharmacol 84: 1238–1249, 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Beck LH Jr, Fervenza FC, Beck DM, Bonegio RG, Malik FA, Erickson SB, et al.: Rituximab-induced depletion of anti-PLA2R autoantibodies predicts response in membranous nephropathy. J Am Soc Nephrol 22: 1543–1550, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.De Vriese AS, Glassock RJ, Nath KA, Sethi S, Fervenza FC: A proposal for a serology-based approach to membranous nephropathy. J Am Soc Nephrol 28: 421–430, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Seitz-Polski B, Debiec A, Rousseau A, Dahan K, Zaghrini C, Payré C, et al.: Phospholipase A2 receptor 1 epitope spreading at baseline predicts reduced likelihood of remission of membranous nephropathy. J Am Soc Nephrol 29: 401–408, 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Xie J, Liu L, Mladkova N, Li Y, Ren H, Wang W, et al.: The genetic architecture of membranous nephropathy and its potential to improve non-invasive diagnosis. Nat Commun 11: 1600, 2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Glassock RJ: Antiphospholipase A2 receptor autoantibody guided diagnosis and treatment of membranous nephropathy: A new personalized medical approach. Clin J Am Soc Nephrol 9: 1341–1343, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.