Abstract
Severe Acute Respiratory Syndrome Coronavirus 2 (SARS CoV2) disease (COVID-19) is a novel threat that hampers life expectancy especially in obese individuals. Though this association is clinically relevant, the underlying mechanisms are not fully elucidated. SARS CoV2 enters host cells via the Angiotensin Converting Enzyme 2 receptor, that is also expressed in adipose tissue. Moreover, adipose tissue is also a source of many proinflammatory mediators and adipokines that might enhance the characteristic COVID-19 cytokine storm due to a chronic low-grade inflammatory preconditioning. Further obesity-dependent thoracic mechanical constraints may also incise negatively into the prognosis of obese subjects with COVID-19. This review summarizes the current body of knowledge on the obesity-dependent circumstances triggering an increased risk for COVID-19 severity, and their clinical relevance.
Keywords: Obesity, SARS-CoV-2, COVID-19, Cytokine storm, Adipokines, Leptin, Adiponectin, ARDS, Mortality, Diabetes mellitus, Bariatric surgery, Hypertension
1. Introduction
Obesity is a chronic disease with complex pathophysiology characterized by excessive hypertrophy and hyperplasia of adipose tissue that presents a risk to health, caused by a chronic imbalanced energy state [1]. Obesity is a fast growing, non-communicable disease (NCD) of pandemic proportions for already several decades with a current global prevalence of 39% according to the World Health Organisation (WHO). Besides being a storage site, adipose tissue is also responsible for secretion of various adipokines that, in the context of obesity, are misregulated [2]. Adipocyte dysfunction along with altered adipokines release results in various metabolic disturbances, mainly insulin resistance/type 2 diabetes mellitus, hypertension, cardiovascular diseases and cancer [3,4].
Severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), responsible for Coronavirus Disease 2019 (COVID-19) has so far infected over 85 millions and killed over 1.8 million people worldwide. The increased hospitalization and mortality linked to COVID-19 are associated with older age and a number of underlying conditions such as obesity [[5], [6], [7], [8]] and related comorbidities along with secondary medical complications [hypertension [5,6,9], diabetes [6,[9], [10], [11]], and history of cardiovascular diseases [6,9,11] among others]. Moreover, a strong correlation of obesity with influenza and other respiratory viral infections had already been established [12,13]. Severe cases of COVID-19 are associated with high circulating levels of inflammatory cytokines akin to a cytokine release syndrome that frequently results in respiratory failure, multiorgan failure and death [14]. Histologically, pneumonitis and morphological features of acute respiratory distress syndrome (ARDS) have been described as the pathological substrate of the most severe cases [15]. It is noteworthy that obesity is associated with increased risk of severity especially in younger patients [16,17].
1.1. Obesity as a risk factor for infections
Despite being a NCD, obesity has gained a preponderant impact on communicable diseases since it increases the risk for incident and severe infections [[18], [19], [20]]. As a consequence of the 2009 H1N1 influenza A virus pandemic, obesity was identified as a risk factor for increased disease severity and mortality in infected individuals [19]. A multicentre study evaluated 4778 participants with influenza and influenza-like infections. Moser et al. [13] found that adults with excessive adiposity and influenza had an increased Odds Ratio (OR) for hospital admission compared to normal-weight adults, that was OR: 3.2 for obesity and OR: 18.4 for severe obesity. In the same report, an increased risk for hospital admission (OR: 2.8) in adults living with severe obesity and with influenza-like infections (including coronavirus) was also reported [13]. Systemic alterations to antiviral immunity, including both the innate and adaptive responses, baseline alterations in the obese lung environment and limitations for the spontaneous and mechanical ventilation explain, at least in part, the poorer outcomes. In addition to excess adiposity itself, most people with obesity are also sedentary. Clinical and translational studies on humans have demonstrated that regular bouts of short-lasting (i.e., 45–60 min), moderate-intensity exercise (50–70% VO2max), performed at least 3 times a week is beneficial for the host immune defense, particularly in older adults and people with chronic diseases [21].
1.2. Obesity and SARS-CoV-2
SARS-CoV-2 is a betacoronavirus that uses the angiotensin-converting enzyme–related carboxypeptidase (ACE2) receptor to gain entry into cells mediated by the transmembrane spike glycoprotein. Following receptor binding, the virus must gain access to the host cytosol, which is accomplished by acid-dependent proteolytic cleavage of the spike protein by cellular transmembrane protease serine 2 (TMPRSS2) [22]. The infection by SARS-CoV-2 likely eliminates the ACE2/ANG(1–7)/MASR regulatory axis leading to sudden exaggeration of the ACE/ANGII/AT1R activity that directly facilitates ARDS, and that is further enhanced by MHC-dependent antigen presentation and an aggressive inflammatory infiltrate [22]. SARS-CoV2 also infects and activates monocytes, macrophages, and dendritic cells. Cytokine release then instigates an amplification cascade that results in cis signalling with TH17 differentiation, among other lymphocytic changes, and trans signalling in many cell types, such as endothelial cells. The resulting increased systemic cytokine production contributes to the pathophysiology of severe COVID-19, including hypotension and ARDS [23].
The level of ACE2 expression in adipose tissue is higher than that in lung tissue, a major target tissue affected by COVID‐19. This is an important finding because adipose tissue might also be vulnerable to COVID‐19, and weight loss is among the most prevalent symptoms of the disease. It should be noted, however, that there was no difference in the expression of ACE2 protein by adipocytes between lean and obese individuals [24]. However, individuals with obesity have more adipose tissue, which ultimately translates into an increased number of ACE2‐expressing cells and, consequently, a larger amount of ACE2. Adipose tissue constitutes a reservoir for SARS-CoV-2 increasing the integral viral load.
1.3. Obesity and COVID-19
1.3.1. Proinflammatory state
Obesity promotes a chronic low-grade inflammatory state that develops following activation of resident macrophages in adipose tissue, promoting the recruitment of M1-polarized macrophages, which display a proinflammatory phenotype [3,20], increasing the local and systemic production of proinflammatory cytokines (TNFα, IL-6, and others). A type 1 immune response (Th1), which is usually activated acutely in response to infection, characterizes this adipose pro-inflammatory state. In the case of obesity, this immune response in adipose tissue is chronic and involves effector T cells, B cells, and natural killer (NK) cells that produce cytokines orchestrating the accumulation and activation of proinflammatory M1 macrophages. A chronic proinflammatory state via IL-6 and other factors [25] may skew the immune system to enable SARS-CoV-2 to unleash the cytokine storm and the deadly inflammatory complications of COVID-19 [20,26]. Critically ill COVID-19 patients experiencing a cytokine storm have a worse prognosis and an increased fatality rate. In COVID-19 patients, the cytokine storm is reportedly important to the pathogenesis of several severe manifestations: ARDS, thromboembolic diseases (acute ischemic strokes caused by large vessel occlusion, myocardial infarction) encephalitis, acute kidney injury, and vasculitis (Kawasaki-like syndrome in children and renal vasculitis in adults) [27].
Many of the aforementioned complications are associated to vascular disease. In this context, the potential role of specific dysfunctional depots merits consideration. Perivascular adipose tissue (PVAT) surrounds most blood vessels except the cerebral vasculature, and is elevated in obesity. Noteworthy, PVAT is capable of interacting with inflammatory cells, the nervous system, and vascular cells to promote or modulate vascular disease [28]. In physiological conditions, PVAT plays a fundamental role in vasodilation via production of adipokines, including adiponectin. Under inflammatory states, such as obesity, vasodilatory adipokine release is diminished from inflamed, dysfunctional PVAT, thus promoting vasoconstriction [2,28] (Fig. 1 ).
Fig. 1.
Severe COVID-19 obesity tetrad. Obesity entails an increased proinflammatory, prothrombotic state associated with a hormonal rearrangement in a context of decreased cardio-respiratory fitness and limited respiratory capacity. IL6: interleukin 6; IL8: interleukin 8; TNFα: tumor necrosis factor α; Ang II: angiotensin II; vWF: von Willebrand Factor; FVIII: coagulation-cascade factor VIII; NET: Neutrophil extracellular trap; TF: tissue factor.
1.3.2. Hormonal rearrangement
Leptin released from adipocytes can stimulate macrophages to produce proinflammatory cytokines [28]. Immune cells infiltrating PVAT in obesity can also promote vasoconstriction and endothelial dysfunction via cross talk with vascular cells, suggesting that the crosstalk between immune cells and adipocytes may be an important feature driving a proinflammatory enviroment. It may be worthwhile to establish the potential relevance of PVAT in the COVID-19 vascular complications.
Leptin is an essential hormone secreted in a pulsatile fashion to the bloodstream involved in maintaining energy homeostasis besides its role in regulation of neuroendocrine functions. The typical modern-diet induced obesity is characterized by hyperleptinemia and resistance to the weight control effects of leptin, but not to its proinflammatory effects. Leptin has structural similarities with IL-6, and the leptin receptor, as the receptor for IL-6, is a class I cytokine receptor family member [29]. Leptin acts as a potent inflammatory cytokine and stimulates innate immune responses by promoting the activation of monocytes/macrophages, which are a mainstay of COVID-19. Leptin also stimulates the production of a myriad of proinflammatory chemokines (IL-6, CCL11/eotaxin, CCL2/MCP-1, CXCL8/IL-8, and CXCL10/ IP-10) [30] that may promote the cytokine storm associated to COVID-19. Notably, in patients with pneumonia unrelated to COVID-19, plasma leptin levels were found to correlate positively with subsequent mortality due to ARDS, suggesting that the association between obesity and elevated risk of pulmonary infection may be driven, at least in part, by hyperleptinemia [31].
Adiponectin is also secreted almost exclusively by adipocytes, and decreases in most persons living with obesity [2]. Both leptin and adiponectin have been related to cardiometabolic risk factors. The adiponectin/leptin ratio has been proposed as a marker of adipose tissue dysfunction. Moreover, the adiponectin/leptin ratio is negatively correlated with markers of low-grade chronic inflammation, such as C-reactive protein [2,32] (Fig. 1).
1.3.3. Prothrombotic state
COVID-19 has also a direct effect on endothelial cells that ease the thrombus formation with a bidirectional enhancement of the proinflammatory state and hormonal rearrangement [22,23]. Direct infection of the endothelial cells through the ACE2 receptor also leads to endothelial activation and dysfunction, expression of tissue factor, and platelet activation and increased levels of von Willebrand Factor and Factor VIII, all of which contribute to thrombin generation and fibrin clot formation [33]. This effect may be potentiated by the local effect of PVAT, especially under inflammatory states (i.e. excessive dysfunctional adiposity). Thrombin, in turn, causes inflammation through its effect on platelets, which promote neutrophil extracellular trap formation. It also activates endothelium through the PAR receptor, which leads to release of C5A that further activates monocytes [33,34]. Venous thromboembolism is the most common thrombotic manifestation of COVID-19, about 25% [35], and thus thromboprophylaxis should always be considered. In comparison to venous thrombosis, the incidence of arterial thrombosis in COVID-19 appears to be less in prevalence [36], but due to their risk of major long-term sequels, myocardial infarction, ischemic stroke and microvascular thrombosis [15,36] prevention requires further studies (Fig. 1).
1.3.4. Mechanical complications due to obesity
The respiratory physiology of people living with obesity might also contribute to the increased incidence of ARDS criteria in these patients. Anatomic and physiological alterations are observed in patients with obesity, affecting the face, neck, pharynx, chest wall and lungs. Excess abdominal fat may increase abdominal pressure. The displacement of the diaphragm upwards, added to the increased chest wall weight, may raise baseline pleural pressure. While total lung capacity and spirometric values usually remain normal, there is a decrease in functional residual capacity. Reduced functional residual capacity can trigger the closure of peripheral dependent airways during tidal ventilation, and decreased lung compliance due to tidal ventilation below the lower inflection point of the inspiratory pressure–volume curve. These changes result in atelectasis and ventilation–perfusion mismatch together with hypoxemia, which are increased in the supine position (Fig. 1).
1.3.5. Non-intubated obese patient with acute lung injury
Our and other COVID-19 protocols [37] encourage patients to prone themselves for variable periods, 2 to 3 times per day. In our experience, persons living with obesity tolerate proning worse than lean ones do, albeit tolerance-dependent repeated periods being better than nothing. Prone positioning of a subject with obesity, even without mechanical ventilator support, might have ergonomic challenges for the patient if unable to prone himself/herself and/or keep the prone position. Anyway, obesity alone should not discourage prone positioning, as long as the patient is able to tolerate the position and is monitored at appropriate intervals.
Prone positioning in patients with ARDS helps improve pulmonary physiology and oxygenation through different mechanisms, regardless of being intubated or not [38]. Proning helps alveolar recruitment in the dorsal lung by increasing the transpulmonary pressure (TPP) gradient, due to the local drop in pleural pressure. Once recruited, alveolar patency can be maintained even with minimal positive end espiratory pressure (PEEP), hence, the benefits may persist even after the patient returns to the supine position. Proning also reduces over distension of the ventral lung due to the drop in the trans pulmonary pressure. These physiological changes make ventilation more homogeneous throughout the lung. On the other hand, the blood flow pattern is relatively unaffected with change from supine to prone position [38]. In the setting of acute hypoxemic injury, in persons living with obesity, ventral to dorsal lung TPP gradients are exaggerated, leading to easier decruitment of alveoli, as compared to nonobese individuals. Thus, homogenization of ventilation and caudal movement of abdominal contents might have even more benefits in patients with obesity. However, for a patient in obvious ARDS, a prone position assay should never delay intubation.
1.3.6. Intubated obese patient with acute lung injury
There are some specific issues regarding ARDS in people living with obesity. Despite a similar pulmonary injury, persons with obesity might be more prone to hypoxemia because of a greater incidence of atelectasis. Alveolar recruitment might be more frequently needed, when compared with a lean patient. In addition, the increased prevalence of gastroesophageal reflux disease and difficult intubation can also increase aspiration incidence during intubation, and the underlying insult of ARDS. On the other hand, transthoracic pressure is higher in patients with obesity than in non-obese patients. Finally, to counteract the onset of atelectasis in the dependent region of the lungs, prone positioning may be advised in patients living with obesity and ARDS by a trained team [39]. A study by Pelosi et al [38] supported that the prone position in persons with obesity improves pulmonary function and increases functional residual capacity, lung compliance, and oxygenation [38]. However, in people with obesity and ARDS, the prone position may worsen visceral hypertension in the abdomen, and lead to subsequent renal and hepatic dysfunction. Thus, it is reasonable to monitor intraabdominal pressure while the patient is in a prone position and consider using an air mattress or a suspended abdomen if abdominal pressures become excessive [40]. It is also relevant that, as in non-intubated ones, prone positioning of an intubated patient with obesity has ergonomic challenges for the patient and requires numerous staff to perform it safely. Studies have reported increased vomiting and decreased tolerance of high-volume enteral feeding while in a prone position, that worsens the high prevalence of gastroesophageal reflux in these patients.
1.3.7. Clinical repercussion
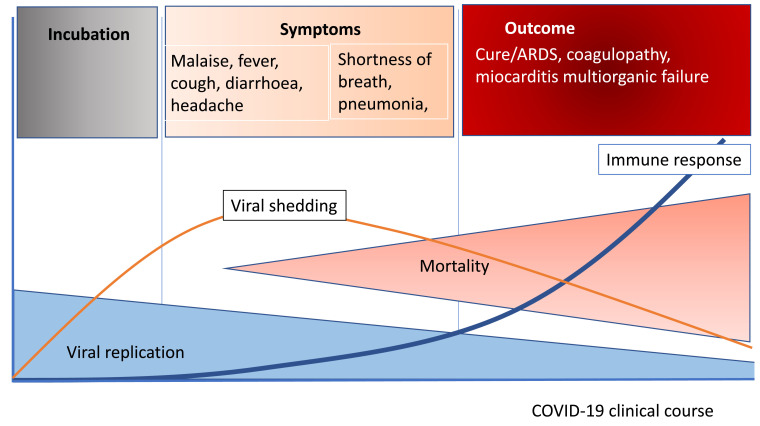
Clinically, most patients infected with SARS-CoV-2 have mild symptoms including sore throat, anosmia, dysgeusia, diarrhoea and others. Only a small proportion develop severe disease with progressively severe respiratory failure requiring mechanical ventilation and with a high mortality rate (Fig. 2 ).
Fig. 2.
COVID-19 clinical course. ARDS: acute respiratory distress syndrome.
The influence of obesity in fatal SARS-CoV-2 infection is clearly documented [5,16,[41], [42], [43]] and reinforces the hypothesis that excess adiposity, at least in part due to chronic low-grade inflammation, may be central to COVID-19 evolution facilitating the cytokine storm. In a study involving more than 3000 COVID-19 patients, with 307 obese subjects younger than 60 years, individuals with a BMI between 30 to 34 kg/m2 were 2 times more likely to be admitted to acute care and 1.8 to be admitted to critical care for ARDS compared with those with a BMI below 30 kg/m2. Likewise, younger patients with a BMI of 35 kg/m2 or higher were 2.2 times more likely to be admitted to acute and 3.6 to be admitted to critical care than patients in the same age category who had a BMI below 30 kg/m2[16]. Consistently, a study evaluating 781,000 young adults aged 18 to 34 years hospitalized with COVID-19 found a substantial rate of adverse outcomes: 21% required intensive care, 10% required mechanical ventilation, and 2.7% died. This in-hospital mortality rate is lower than that reported for older adults with COVID-19, but approximately doubled that of young adults with acute myocardial infarction. Severe obesity, hypertension, and diabetes were common and associated with greater risks of adverse events. Young adults with more than one of these conditions faced risks comparable with those observed in middle-aged adults without them [17]. Similar results are available in an older population with a mean age of 60 years [5], were 47% of subjects admitted to the ICU had a BMI above 30 kg/m2; whilst 28% of them were above 35 kg/m2. The proportion of patients who required invasive mechanical ventilation increased with BMI categories and it was greatest in patients with a BMI above 35 kg/m2 [5].
Whether the mechanical derangement due to the fat mass amount or the grade of dysfunctional adiposity is more relevant in terms of risk of death, although both factors are likely to be critical requires further investigation. Remarkably Asian-ethnic subjects with obesity also suffer a higher mortality compared with lean controls [44,45]. The Asian threshold for obesity is usually placed in 27 kg/m2. This means that Asiatic ethnicity has a lesser degree of mechanical limitations with similar lipotoxicity. In a Chinese cohort of 383 COVID-19 patients, those who were overweight (BMI 24.0–27.9 kg/m2) exhibited a 1.84-fold OR, while individuals with obesity presented a 3.40-fold OR of developing respiratory failure due to COVID19, compared with normal-weight patients (BMI 18.5–23.9 kg/m2) in the age, sex, and comorbidities adjusted model [44]. In another Chinese study involving 150 patients with COVID-19, obesity was associated with a threefold increased risk of disease severity in the logistic regression analyses and in the multivariate adjusted model. A nearly linear relationship between higher BMI and severe illness was shown with each 1-unit increase in BMI associated with a 12% increase in the risk of severe COVID-19 [45].
In addition to obesity itself, its major complications, namely diabetes [6,[9], [10], [11],46] and hypertension [5,6,9,46], are also closely associated to a worse prognosis in COVID-19. Pre-existing diabetes is associated with an approximate twofold higher risk of having severe/critical COVID-19 illness and circa threefold increased risk of in-hospital mortality [47,48]. Furthermore, the association between obesity, diabetes and severe COVID-19 is bidirectional [49]. In a population-based cohort study of people with previously diagnosed type 1 or type 2 diabetes, deaths rose sharply during the initial COVID-19 pandemic. Increased COVID-19-related mortality was associated not only with cardiovascular and renal complications of diabetes but, independently, also with glycaemic control and BMI [49]. Hypertension also entails a smaller but significant excess of risk for severe COVID-19 [5,6,9,46].
1.3.8. Bariatric surgery and COVID-19
There are only a few reports on whether bariatric surgery modifies the prognosis of people living with obesity in the current COVID-19 pandemic or not [43,[50], [51], [52]]. In a survey-based study of 738 patients that had undergone bariatric surgery, the authors assessed the prevalence of symptom-based likelihood of COVID-19 and its risk factors, based on a retrospective comparison of characteristics at baseline, and one-year post surgery [43]. It is remarkable that pre-surgery, no differences between patients who developed COVID-19 and those who did not could be found. Symptoms suggesting COVID-19 occurred in 62 (8.4%) patients among whom 4 (6.4%) suffered a severe form requiring hospitalization and one (1.6%) died. At the time of the survey, a global estimation was performed in the greater Paris region (Ile de France), area of residence of the majority of the patients, and reported an estimated prevalence of COVID-19 cases of 9.9% [53]. The COVID-19 likely group had a higher proportion of persistent type 2 diabetes in the last follow-up and a lower BMI with higher percent weight loss [43]. Consistent data have been reported among patients operated in Italy [51]. A case-control study has also shown that post-bariatric patients with subsequent weight loss and improvement of metabolic abnormalities developed lower rates of hospital and ICU admission, compared to patients living with severe obesity [52].
These observations add additional arguments on the benefit of bariatric surgery in the management of obesity and reinforces, as aforementioned, the relevance of diabetes itself in association with COVID-19 severity. Additionally, specifically during the COVID-19 pandemic, many candidates for bariatric or metabolic surgery are at highest risk of morbidity and mortality. For these patients, access to surgical treatment should be prioritised on the basis of the severity of the disease, rather than primarily on BMI, to mitigate harm from delaying surgery [54]. Disease-oriented, medically meaningful strategies to triage patients seeking metabolic surgery after the COVID-19 crisis should help prioritise patients in more urgent need, both now and long into the future [54].
1.3.9. Summary and perspectives
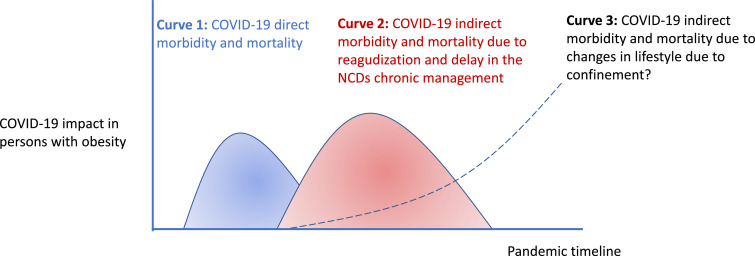
In summary, COVID-19 is causing massive health problems and suffering around the world in which obesity might take the worst part in the two sides of the coin, as the European Association for the Study of Obesity recently stated [55]. On one side, obesity is a disease that propels COVID-19 patients into a downhill course. A low grade chronic inflammatory state in obesity can enable the cytokine storm in COVID-19 patients. The globally increased amount of ACE 2 receptors in obesity, with a more aggressive distribution and changes in breathing physiology may be another reason why obese individuals are prone to severe COVID-19. On the other side, the health emergency caused by the COVID-19 outbreak diverts attention (and funding) from the prevention and care of NCDs to communicable diseases. Home-confinement has forced a decrease in physical activity [56] that is at high risk to entail long lasting changes in lifestyle of patients with obesity, for whom physical activity is a mainstay of their treatment but also a daily challenge (Fig. 3 ). Paying special attention to promote the physical reactivation and healthy nutrition of the patients, particularly for those living with obesity, is a major responsibility for attending physicians.
Fig. 3.
Putative COVID-19 impact on health of people living with obesity.
1.3.10. Review criteria
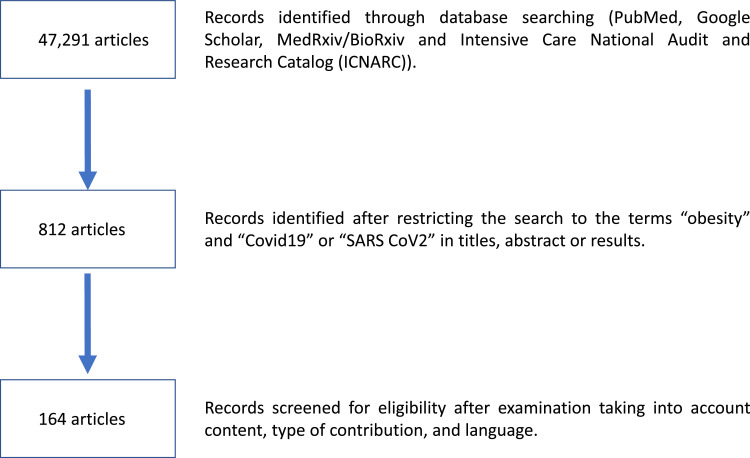
A search for original articles and reviews published focusing on the association between COVID-19 and obesity was performed in PubMed and MEDLINE using the following search terms (or combination of terms): “COVID-19,” “obesity,” “adiposity,” “epicardial fat,” “visceral fat,” “dysfunctional fat,” “comorbidity or comorbidities,” “outcome,” “mortality,” “adipokines,” “ARDS,” “prone ventilation,” “diabetes,” “hypertension.” Only English-language, full-text articles were included. Additional articles that were identified from the bibliographies of the retrieved articles were also used. Fig. 4 shows the Flow-diagram of the search strategy.
Fig. 4.
Flow diagram of the search strategy.
Funding sources
None.
CRediT authorship contribution statement
MF Landecho: Conceptualization, Writing - original draft. M Marin-Oto: Resources, Visualization. B Recalde-Zamacona: Investigation. I Bilbao: Writing - review & editing. Gema Frühbeck: Conceptualization, Supervision, Writing - review & editing.
Declaration of Competing Interest
None.
Acknowledgments
The authors gratefully acknowledge the valuable collaboration of all the patients as well as the members of the Multidisciplinary Obesity and COVID-19 Team, Clínica Universidad de Navarra, Pamplona, Spain. This work was supported by Fondo de Investigación Sanitaria-FEDER (FIS PI19/00785) from the Spanish Instituto de Salud Carlos III. CIBER de Fisiopatología de la Obesidad y Nutrición (CIBERobn) is an initiative of the Instituto de Salud Carlos III, Spain.
References
- 1.Frühbeck G., Busetto L., Dicker D., Yumuk V., Goossens G.H., Hebebrand J., et al. The ABCD of obesity: an EASO position statement on a diagnostic term with clinical and scientific implications. Obes Facts. 2019;12(2):131–136. doi: 10.1159/000497124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Landecho M.F., Tuero C., Valentí V., Bilbao I., de la Higuera M., Frühbeck G. Vol. 11. NLM (Medline); 2019. Relevance of leptin and other adipokines in obesity-associated cardiovascular risk. (Nutrients). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Unamuno X., Gómez-Ambrosi J., Rodríguez A., Becerril S., Frühbeck G., Catalán V. Adipokine dysregulation and adipose tissue inflammation in human obesity. Eur J Clin Invest [Internet] 2018;48(9) doi: 10.1111/eci.12997. http://doi.wiley.com/10.1111/eci.12997 Sep 1 [cited 2018 Nov 8]e12997. Available from. [DOI] [PubMed] [Google Scholar]
- 4.Pérez-Hernández A.I., Catalán V., Gómez-Ambrosi J., Rodríguez A., Frühbeck G. Vol. 5. Frontiers Research Foundation; 2014. Mechanisms linking excess adiposity and carcinogenesis promotion [Internet]. p. 65.www.frontiersin.org (Frontiers in Endocrinology). [cited 2021 Jan 5]Available from. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Simonnet A., Chetboun M., Poissy J., Raverdy V., Noulette J., Duhamel A., et al. High prevalence of obesity in severe acute respiratory syndrome coronavirus-2 (SARS-CoV-2) requiring invasive mechanical ventilation. Obesity. 2020;28(7):1195–1199. doi: 10.1002/oby.22831. Jul 1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Cdc. Certain medical conditions and risk for severe COVID-19 illness | CDC [Internet]. [cited 2020 Aug 31]. Available from: https://www.cdc.gov/coronavirus/2019-ncov/need-extra-precautions/people-with-medical-conditions.html.
- 7.Hussain A., Mahawar K., Xia Z., Yang W., EL-Hasani S. Vol. 14. Elsevier Ltd; 2020. Obesity and mortality of COVID-19. Meta-analysis [Internet] pp. 295–300.https://pubmed.ncbi.nlm.nih.gov/32660813/ (Obesity research and clinical practice). [cited 2020 Sep 7]. Available from. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 8.Frühbeck G., Baker J.L., Busetto L., Dicker D., Goossens G.H., Halford J.C.G., et al. European association for the study of obesity position statement on the global COVID-19 pandemic. Obes Facts [Internet] 2020;13(2):292–296. doi: 10.1159/000508082. https://www.karger.com/Article/FullText/508082 May 1 [cited 2020 Sep 10]. Available from. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ssentongo P., Ssentongo A.E., Heilbrunn E.S., Ba D.M., Chinchilli V.M. Association of cardiovascular disease and 10 other pre-existing comorbidities with COVID-19 mortality: a systematic review and meta-analysis. Hirst J.A., editor. Association of cardiovascular disease and 10 other pre-existing comorbidities with COVID-19 mortality: A systematic review and meta-analysis.PLoS One [Internet] 2020;15(8) doi: 10.1371/journal.pone.0238215. https://dx.plos.org/10.1371/journal.pone.0238215 Aug 26 [cited 2020 Sep 8]e0238215. Available from. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Li J., Huang D., Zou B., Yang H., Hui W.Z., Rui F., et al. Epidemiology of COVID-19: a systematic review and meta-analysis of clinical characteristics, risk factors and outcomes. J Med Virol [Internet] 2020 doi: 10.1002/jmv.26424. https://pubmed.ncbi.nlm.nih.gov/32790106/ [cited 2020 Aug 31]. Available from. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Del Sole F., Farcomeni A., Loffredo L., Carnevale R., Menichelli D., Vicario T., et al. Features of severe COVID-19: a systematic review and meta-analysis. Eur J Clin Invest [Internet] 2020 doi: 10.1111/eci.13378. https://pubmed.ncbi.nlm.nih.gov/32860457/ Aug 9 [cited 2020 Aug 31]. Available from. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Honce R., Schultz-Cherry S. Vol. 10. Frontiers in Immunology. Frontiers Media S.A.; 2019. p. 1071. (Impact of obesity on influenza A virus pathogenesis, immune response, and evolution [Internet].). [cited 2020 Aug 31]. Available from: /pmc/articles/PMC6523028/?report=abstract. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Moser J.A.S., Galindo-Fraga A., Ortiz-Hernández A.A., Gu W., Hunsberger S., Galán-Herrera J.F., et al. Underweight, overweight, and obesity as independent risk factors for hospitalization in adults and children from influenza and other respiratory viruses. Influenza Other Respi Viruses [Internet] 2019;13(1):3–9. doi: 10.1111/irv.12618. https://pubmed.ncbi.nlm.nih.gov/30515985/ Jan 1 [cited 2020 Aug 31]. Available from. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Mehta P., McAuley D.F., Brown M., Sanchez E., Tattersall R.S., Manson JJ. Vol. 395. The Lancet. Lancet Publishing Group; 2020. pp. 1033–1034. (COVID-19: consider cytokine storm syndromes and immunosuppression.). Vol. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Recalde B., García-Tobar L., Argueta A., Álvarez L., De Andrea C.E., Fernández Alonso M., et al. Histopathological findings in fatal COVID-19 severe acute respiratory syndrome: preliminary experience from a series of 10 Spanish patients. Thorax [Internet] 2020 doi: 10.1136/thoraxjnl-2020-215577. http://www.ncbi.nlm.nih.gov/pubmed/32839288 Aug 24 [cited 2020 Aug 28]. Available from. [DOI] [PubMed] [Google Scholar]
- 16.Lighter J., Phillips M., Hochman S., Sterling S., Johnson D., Francois F., et al. Obesity in patients younger than 60 years is a risk factor for COVID-19 hospital admission. Clin Infect Dis [Internet] 2020;71(15):896–897. doi: 10.1093/cid/ciaa415. https://academic.oup.com/cid/article/71/15/896/5818333 Jul 28 [cited 2020 Aug 31]. Available from. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Cunningham J.W., Vaduganathan M., Claggett B.L., Jering K.S., Bhatt A.S., Rosenthal N., et al. Clinical outcomes in young US adults hospitalized with COVID-19. JAMA Intern Med [Internet] 2020 doi: 10.1001/jamainternmed.2020.5313. http://www.ncbi.nlm.nih.gov/pubmed/32902580 Sep 9 [cited 2020 Sep 11]. Available from. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Falagas M.E., Kompoti M. Obesity and infection. Lancet Infect Dis. 2006;6:438–446. doi: 10.1016/S1473-3099(06)70523-0. [DOI] [PubMed] [Google Scholar]
- 19.Sun Y., Wang Q., Yang G., Lin C., Zhang Y., Yang P. Weight and prognosis for influenza A(H1N1)pdm09 infection during the pandemic period between 2009 and 2011: a systematic review of observational studies with meta-analysis. Infect Dis (Auckl) [Internet] 2016 doi: 10.1080/23744235.2016.1201721. Dec 1 [cited 2020 Sep 1];48(11–12):813–22. Available from: https://www.tandfonline.com/doi/full/10.1080/23744235.2016.1201721. [DOI] [PubMed] [Google Scholar]
- 20.Goossens G.H., Dicker D., Farpour-Lambert N.J., Frühbeck G., Mullerova D., Woodward E., et al. Obesity and COVID-19: a perspective from the European association for the study of obesity on immunological perturbations, therapeutic challenges, and opportunities in obesity. Obes Facts. 2020;13:1–14. doi: 10.1159/000510719. Aug. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Leandro C.G., Ferreira E., Silva W.T., Lima-Silva A.E. Covid-19 and Exercise-Induced Immunomodulation [Internet] NeuroImmunoModulation. S. Karger AG. 2020:1. doi: 10.1159/000508951. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC7316658/ [cited 2020 Sep 1]. Available from. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Gkogkou E., Barnasas G., Vougas K., Trougakos IP. Vol. 36. Redox Biol [Internet]; 2020. https://pubmed.ncbi.nlm.nih.gov/32863223/ (Expression profiling meta-analysis of ACE2 and TMPRSS2, the putative anti-inflammatory receptor and priming protease of SARS-CoV-2 in human cells, and identification of putative modulators). Sep 1 [cited 2020 Sep 1]. Available from. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Moore J.B., June C.H. Vol. 368. Science. American Association for the Advancement of Science; 2020. pp. 473–474.https://pubmed.ncbi.nlm.nih.gov/32303591/ (Cytokine release syndrome in severe COVID-19 [Internet].). Vol.[cited 2020 Sep 1]. Available from. [DOI] [PubMed] [Google Scholar]
- 24.Pinheiro T de A., Barcala-Jorge A.S., Andrade J.M.O., Pinheiro T de A., Ferreira E.C.N., Crespo T.S., et al. Obesity and malnutrition similarly alters the renin–angiotensin system and inflammation in mice and human adipose. J Nutr Biochem [Internet] 2017;48:74–82. doi: 10.1016/j.jnutbio.2017.06.008. https://pubmed.ncbi.nlm.nih.gov/28779634/ Oct 1 [cited 2020 Sep 2]. Available from. [DOI] [PubMed] [Google Scholar]
- 25.Fortuño A., Bidegain J., José G.S., Robador P.A., Landecho M.F., Beloqui O., et al. Insulin resistance determines phagocytic nicotinamide adenine dinucleotide phosphate oxidase overactivation in metabolic syndrome patients. J Hypertens [Internet] 2009;27(7):1420–1430. doi: 10.1097/HJH.0b013e32832b1e8f. http://www.ncbi.nlm.nih.gov/pubmed/19357530 Jul [cited 2016 Oct 17]. Available from. [DOI] [PubMed] [Google Scholar]
- 26.Mauvais-Jarvis F. Aging, male sex, obesity, and metabolic inflammation create the perfect storm for COVID-19. Diabetes. 2020 doi: 10.2337/dbi19-0023. Jul 15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Bhaskar S., Sinha A., Banach M., Mittoo S., Weissert R., Kass J.S., et al. Cytokine storm in COVID-19—immunopathological mechanisms, clinical considerations, and therapeutic approaches: the REPROGRAM consortium position paper. Front Immunol [Internet] 2020;11 doi: 10.3389/fimmu.2020.01648. www.frontiersin.org Jul 10 [cited 2020 Sep 3]:1648. Available from. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Won Kim H., Shi H., Winkler M.A., Lee R., Weintraub N.L. Perivascular adipose tissue and vascular perturbation/atherosclerosis. Arterioscler Thromb Vasc Biol [Internet] 2020 doi: 10.1161/ATVBAHA.120.312470. http://www.ncbi.nlm.nih.gov/pubmed/32878476 Sep 3 [cited 2020 Sep 4];ATVBAHA120312470. Available from. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Frühbeck G. Intracellular signalling pathways activated by leptin [Internet]. Biochem J. 2006;393:7–20. doi: 10.1042/BJ20051578. [cited 2020 Sep 2]. Available from: /pmc/articles/PMC1383660/?report=abstract. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Watanabe K., Suzukawa M., Arakawa S., Kobayashi K., Igarashi S., Tashimo H., et al. Leptin enhances cytokine/chemokine production by normal lung fibroblasts by binding to leptin receptor. Allergol Int [Internet] 2019;68:S3–S8. doi: 10.1016/j.alit.2019.04.002. https://pubmed.ncbi.nlm.nih.gov/31029506/ Sep 1 [cited 2020 Sep 2]. Available from. [DOI] [PubMed] [Google Scholar]
- 31.Ubags N.D.J., Stapleton R.D., Vernooy J.H.J., Burg E., Bement J., Hayes C.M., et al. Hyperleptinemia is associated with impaired pulmonary host defense. JCI Insight [Internet] 2016;1(8) doi: 10.1172/jci.insight.82101. https://pubmed.ncbi.nlm.nih.gov/27347561/ Jun 2 [cited 2020 Sep 2]. Available from. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Frühbeck G., Catalán V., Rodríguez A., Ramírez B., Becerril S., Salvador J., et al. Adiponectin-leptin ratio is a functional biomarker of adipose tissue inflammation. Nutrients [Internet] 2019;11(2) doi: 10.3390/nu11020454. Feb 1 [cited 2020 Sep 3]. Available from: /pmc/articles/PMC6412349/?report=abstract. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Abou-Ismail M.Y., Diamond A., Kapoor S., Arafah Y., Nayak L. The hypercoagulable state in COVID-19: incidence, pathophysiology, and management [Internet]. Thrombosis Res. 2020;194:101–115. doi: 10.1016/j.thromres.2020.06.029. [cited 2020 Sep 10]. Available from: /pmc/articles/PMC7305763/?report=abstract. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Foley J.H., Conway EM. Vol. 118. Lippincott Williams and Wilkins; 2016. Cross talk pathways between coagulation and inflammation [Internet]. pp. 1392–1408.http://circres.ahajournals.org (Circul Res). [cited 2020 Sep 10]. Available from. [DOI] [PubMed] [Google Scholar]
- 35.Cui S., Chen S., Li X., Liu S., Wang F. Prevalence of venous thromboembolism in patients with severe novel coronavirus pneumonia. J Thromb Haemost [Internet] 2020;18(6):1421–1424. doi: 10.1111/jth.14830. https://pubmed.ncbi.nlm.nih.gov/32271988/ Jun 1 [cited 2020 Sep 11]. Available from. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Lodigiani C., Iapichino G., Carenzo L., Cecconi M., Ferrazzi P., Sebastian T., et al. Venous and arterial thromboembolic complications in COVID-19 patients admitted to an academic hospital in Milan, Italy. Thromb Res [Internet] 2020;191:9–14. doi: 10.1016/j.thromres.2020.04.024. https://pubmed.ncbi.nlm.nih.gov/32353746/ Jul 1 [cited 2020 Sep 11]. Available from. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Massachusetts General Hospital Prone Positioning for Non-Intubated Patients Guideline - Buscar con Google [Internet]. [cited 2020 Sep 3]. Available from: https://www.google.com/search?q=Massachusetts+General+Hospital+Prone+Positioning+for+Non-Intubated+Patients+Guideline&rlz=1C1GCEU_esES821ES821&oq=Massachusetts+General+Hospital+Prone+Positioning+for+Non-Intubated+Patients+Guideline&aqs=chrome..69i57.614j0j7&sourceid=chrome&ie=UTF-8.
- 38.Paul V., Patel S., Royse M., Odish M., Malhotra A., Koenig S. Proning in non-intubated (PINI) in times of COVID-19: case aeries and a review. J Intensive Care Med [Internet] 2020;35(8):818–824. doi: 10.1177/0885066620934801. Aug 1 [cited 2020 Sep 3]. Available from: /pmc/articles/PMC7394050/?report=abstract. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.De Jong A., Verzilli D., Jaber S. ARDS in obese patients: specificities and management [Internet]. Crit Care. 2019;23 doi: 10.1186/s13054-019-2374-0. [cited 2020 Sep 3]. Available from: /pmc/articles/PMC6408839/?report=abstract. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Scholten E.L., Beitler J.R., Prisk G.K., Malhotra A. Treatment of ARDS with prone positioning [Internet]. Chest. 2017;151:215–224. doi: 10.1016/j.chest.2016.06.032. https://pubmed.ncbi.nlm.nih.gov/27400909/ [cited 2020 Sep 3]. Available from. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Tamara A., Tahapary D.L. Obesity as a predictor for a poor prognosis of COVID-19: A systematic review. Diabetes Metab Syndr Clin Res Rev [Internet] 2020;14(4):655–659. doi: 10.1016/j.dsx.2020.05.020. Jul 1 [cited 2020 Sep 1]. Available from: /pmc/articles/PMC7217103/?report=abstract. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Wu J., Li W., Shi X., Chen Z., Jiang B., Liu J., et al. Early antiviral treatment contributes to alleviate the severity and improve the prognosis of patients with novel coronavirus disease (COVID-19) J Intern Med [Internet] 2020;288(1):128–138. doi: 10.1111/joim.13063. https://onlinelibrary.wiley.com/doi/abs/10.1111/joim.13063 Jul 20 [cited 2020 Sep 7]. Available from. [DOI] [PubMed] [Google Scholar]
- 43.Bel Lassen P., Poitou C., Genser L., Marchelli F., Aron-Wisnewsky J., Ciangura C., et al. COVID-19 and its severity in bariatric surgery operated patients. Obesity [Internet] 2020 doi: 10.1002/oby.23026. https://onlinelibrary.wiley.com/doi/abs/10.1002/oby.23026 Sep [cited 2020 Sep 7];oby.23026. Available from. [DOI] [PubMed] [Google Scholar]
- 44.Cai Q., Chen F., Wang T., Luo F., Liu X., Wu Q., et al. Obesity and COVID-19 severity in a designated hospital in Shenzhen, China. Diabetes Care [Internet] 2020;43(7):1392–1398. doi: 10.2337/dc20-0576. Jul 1 [cited 2020 Sep 7]. Available from. [DOI] [PubMed] [Google Scholar]
- 45.Gao F., Zheng K.I., Wang X.B., Sun Q.F., Pan K.H., Wang T.Y., et al. Obesity is a risk factor for greater COVID-19 severity [Internet]. Diabetes Care. 2020;43:E72–E74. doi: 10.2337/dc20-0682. [cited 2020 Sep 7]. Available from: [DOI] [PubMed] [Google Scholar]
- 46.Xu L., Mao Y., Chen G. Risk factors for 2019 novel coronavirus disease (COVID-19) patients progressing to critical illness: a systematic review and meta-analysis [Internet]. Aging Impact J LLC. 2020;12:12410–12421. doi: 10.18632/aging.103383. [cited 2020 Sep 8]. Available from: /pmc/articles/PMC7343456/?report=abstract. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Kumar A., Arora A., Sharma P., Anikhindi S.A., Bansal N., Singla V., et al. Is diabetes mellitus associated with mortality and severity of COVID-19? A meta-analysis. Diabetes Metab Syndr Clin Res Rev [Internet] 2020;14(4):535–545. doi: 10.1016/j.dsx.2020.04.044. https://pubmed.ncbi.nlm.nih.gov/32408118/ Jul 1 [cited 2020 Sep 8]. Available from. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Mantovani A., Byrne C.D., Zheng M.H., Targher G. Diabetes as a risk factor for greater COVID-19 severity and in-hospital death: a meta-analysis of observational studies. Nutr Metab Cardiovasc Dis [Internet] 2020;30(8):1236–1248. doi: 10.1016/j.numecd.2020.05.014. https://pubmed.ncbi.nlm.nih.gov/32571616/ Jul 24 [cited 2020 Sep 8]. Available from. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Holman N., Knighton P., Kar P., O'Keefe J., Curley M., Weaver A., et al. Risk factors for COVID-19-related mortality in people with type 1 and type 2 diabetes in England: a population-based cohort study. Lancet Diabetes Endocrinol [Internet] 2020 doi: 10.1016/S2213-8587(20)30271-0. https://linkinghub.elsevier.com/retrieve/pii/S2213858720302710 Aug [cited 2020 Sep 1]. Available from. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Uccelli M., Cesana G.C., Ciccarese F., Oldani A., Zanoni A.A.G., De Carli S.M., et al. COVID-19 and obesity: postoperative risk in patients who have undergone bariatric surgery. Preliminary report from high volume center in Italy (Lombardy)” [Internet]. Obesity Surg. 2020 doi: 10.1007/s11695-020-04792-x. https://pubmed.ncbi.nlm.nih.gov/32592016/ [cited 2020 Sep 7]. Available from. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Uccelli M., Cesana G.C., De Carli S.M., Ciccarese F., Oldani A., Zanoni A.A.G., et al. COVID-19 and obesity: is bariatric surgery protective? Retrospective analysis on 2145 patients undergone bariatric-metabolic surgery from high volume center in Italy (Lombardy) Obes Surg [Internet] 2020 doi: 10.1007/s11695-020-05085-z. https://pubmed.ncbi.nlm.nih.gov/33128218/ [cited 2020 Dec 18]. Available from. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Aminian A., Fathalizadeh A., Tu C., Butsch W.S., Pantalone K.M., Griebeler M.L., et al. Association of prior metabolic and bariatric surgery with severity of coronavirus disease 2019 (COVID-19) in patients with obesity. Surg Obes Relat Dis. 2020 doi: 10.1016/j.soard.2020.10.026. Nov. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Salje H., Kiem C.T., Lefrancq N., Courtejoie N., Bosetti P., Paireau J., et al. Estimating the burden of SARS-CoV-2 in France. Science (80-) [Internet] 2020;369(6500):208–211. doi: 10.1126/science.abc3517. https://pubmed.ncbi.nlm.nih.gov/32404476/ Jul 10 [cited 2020 Sep 7]. Available from. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Rubino F., Cohen R.V., Mingrone G., le Roux C.W., Mechanick J.I., Arterburn D.E., et al. Bariatric and metabolic surgery during and after the COVID-19 pandemic: DSS recommendations for management of surgical candidates and postoperative patients and prioritisation of access to surgery. Lancet Diabetes Endocrinol. 2020;8:640–648. doi: 10.1016/S2213-8587(20)30157-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Dicker D., Bettini S., Farpour-Lambert N., Frühbeck G., Golan R., Goossens G., et al. Obesity and COVID-19: the two sides of the coin. Obes Facts. 2020 doi: 10.1159/000510005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Castañeda-Babarro A., Coca A., Arbillaga-Etxarri A., Gutiérrez-Santamaría B. Physical activity change during COVID-19 confinement. Int J Environ Res Public Health [Internet] 2020;17(18):1–10. doi: 10.3390/ijerph17186878. https://pubmed.ncbi.nlm.nih.gov/32967091/ Sep 1 [cited 2020 Dec 31]. Available from. [DOI] [PMC free article] [PubMed] [Google Scholar]