To the Editor:
Trimethoprim–sulfamethoxazole (TMP-SMX) is an antibiotic associated with rare respiratory adverse drug reactions (ADRs) that are typically mild and self-limited (1). Here we describe 14 previously healthy children and young adults exposed to TMP-SMX before the development of fulminant respiratory failure, potentially reflecting a previously unappreciated, life-threatening ADR to TMP-SMX. Some of the results of this study have been previously reported (2–5).
Cases were identified following national news coverage of an adolescent patient requiring extracorporeal membrane oxygenation (ECMO) support owing to unexplained acute respiratory failure (4). Inclusion criteria were as follows: 1) no previous history of pulmonary disease, 2) ≥7 days exposure to TMP-SMX with documentation of prescribing date and dose, 3) medical records available for review by the authors, and 4) no alternative explanation for acute respiratory failure revealed by medical evaluation and testing. The Naranjo causality assessment tool for adverse drug reactions was completed on review of each case (6). Adult patients provided signed authorization for medical records from outside facilities, whereas a family member provided authorization for medical records for deceased patients and children. The institutional review board deemed this case series exempt from review. An interfacility data use agreement was completed between Children’s Mercy Hospital and Massachusetts General Hospital to share case-specific clinical information.
Lung tissue from biopsy and/or autopsy was sent to Massachusetts General Hospital for central pathology review. Histology was performed using routine clinical techniques with slides stained with hematoxylin and eosin; immunohistochemistry for keratins and CD68 was performed as described previously (5). All available specimens were assessed by three board-certified pathologists (A.R.S., M.M.-K., and M.S.T.).
We report clinical and pathological findings from 14 previously healthy children and young adults (median, 16.5 yr; range, 10–37 yr) with TMP-SMX–associated fulminant respiratory failure occurring between 1997 and 2018 (Table 1). Seven cases were previously published, and seven are newly reported (4, 5). Median TMP-SMX exposure duration was 21 days (range, 7–28 d) for nonsystemic illnesses including urinary tract infection, folliculitis, and acne vulgaris. No clear racial or geographic trend was present (Table 1). Our patients had no history of inhalation or smoking exposure except for a single patient who had one incident of electronic cigarette use 2 months before illness. There was no unifying TMP-SMX manufacturing company link across cases.
Table 1.
Clinical Characteristics of 14 Patients with TMP-SMX–associated Respiratory Failure
| Characteristic | Value |
|---|---|
| Median age (range), yr | 16.5 (10–37) |
| Sex, F, n (%) | 9 (64) |
| Race, n (%) | |
| White | 11 (79) |
| Mixed race | 1 (7) |
| Asian | 2 (14) |
| Geographical region, n (%) | |
| Midwest | 6 (43) |
| West | 2 (14) |
| Northeast | 2 (14) |
| Southeast | 4 (29) |
| TMP-SMX exposure | |
| Median duration (range), d | 21 (7–28) |
| Indication for TMP-SMX, n (%) | |
| Acne | 8 (57) |
| Skin or soft tissue infection | 5 (36) |
| Urinary tract infection | 1 (7) |
| Symptoms at hospital admission, n (%) | |
| Any respiratory symptoms | 14 (100) |
| Shortness of breath | 14 (100) |
| Chest pain | 8 (57) |
| Cough | 9 (64) |
| Hypoxemia | 13 (93) |
| Any constitutional symptoms | 11 (79) |
| Fever | 8 (57) |
| Pharyngitis | 4 (29) |
| Malaise | 4 (29) |
| Vital signs at hospital admission, n (%) | |
| Temperature ≥38°C | 8 (57) |
| Heart rate >100 beats/min | 13 (93) |
| Respiratory rate >20 breaths/min | 13 (93) |
| Oxygen saturation on room air | |
| ≥90% | 6 (43) |
| 80–89% | 4 (29) |
| ≤79% | 4 (29) |
| Laboratory results at hospital admission, n (%) | |
| White blood cell count >11,000 × 103/μl | 0 (0) |
| White blood cell count <4,500 × 103/μl | 1 (7) |
| White blood cell count >80% neutrophils | 6 (43) |
| Absolute eosinophil count >500/μl | 0 (0) |
| Creatinine mg/dl >2 × upper limit of normal | 0 (0) |
| Alanine aminotransferase U/L >2 × upper limit of normal | 1 (7) |
| Absolute eosinophil count >500/μl during hospitalization, n/total n (%) | 9/10 (90) |
| Air leak syndrome prior to intubation, n (%) | |
| Pneumomediastinum | 8 (57) |
| Pneumothorax | 5 (36) |
| Median hospital length of stay (range), d | 102 (16–459) |
| Median duration of advanced airway (range), d | 122 (12–455) |
| Tracheostomy, n (%) | 13/14 (93) |
| ECMO, n/total n (%) | 12/14 (86) |
| VV, n/total n (%) | 7/12 (58) |
| VA, n/total n (%) | 1/12 (8) |
| VA to VV or VV to VA, n/total n (%) | 4/12 (33) |
| Median ECMO duration (range), d | 71 (9–193) |
| Ambulating on ECMO, n/total n (%) | 6/12 (50) |
| Treatment, n (%) | |
| Broad-spectrum antimicrobials | 14 (100) |
| Systemic glucocorticoids | 14 (100) |
| Intravenous immunoglobulin | 4 (29) |
| Plasmapheresis | 4 (29) |
| Hydroxychloroquine | 1 (7) |
| Azathioprine | 1 (7) |
| Mycophenolate | 1 (7) |
| Rituximab | 1 (7) |
| Lung pathology, n/total n (%) | 9/14 (64) |
| Biopsy or autopsy consistent with DAIDE | 7/9 (78)* |
| Organ transplant, n/total n (%) | 3/14 (21) |
| Lung | 2/3 (67) |
| Heart/lung | 1/3 (33) |
| Death, n (%) | 5 (36) |
Definition of abbreviations: DAIDE = diffuse alveolar injury with delayed epithelialization; ECMO = extracorporeal membrane oxygenation; TMP-SMX = trimethoprim–sulfamethoxazole; VA = venoarterial; VV = venovenous.
In two specimens in which DAIDE morphology was not observed, one biopsy was on Day 18 of hospitalization; the sample was a small fragment of peripheral lung demonstrating alveolar hemorrhage consistent with the clinical history of hemothorax. The second was explanted lung tissue from a 16-year-old female who received a bilateral lung transplant on Day 98 of hospitalization; this showed advanced-stage fibrosis, lung architecture remodeling, and bronchiolarization consistent with a history of DAIDE but not diagnostic (6).
All patients presented with shortness of breath and required hospital admission and mechanical ventilation. Initial laboratory studies were unremarkable. Chest computed tomographic scans showed extensive lung involvement with ground-glass opacities and consolidation. Pneumomediastinum and/or pneumothorax was present in eight patients (57%) before intubation. Twelve patients (86%) required ECMO support for a median duration of 71 days (range, 9–193 d). All patients were empirically treated with corticosteroids and broad-spectrum antibiotics. Comprehensive medical evaluation revealed no alternate explanation for acute respiratory failure. All cases scored “probable” for TMP-SMX causality using the Naranjo assessment tool (6). Working and discharge diagnoses included eosinophilic pneumonia, acute interstitial pneumonia (Hamman-Rich syndrome), autoimmune disease, or a nonspecified etiology.
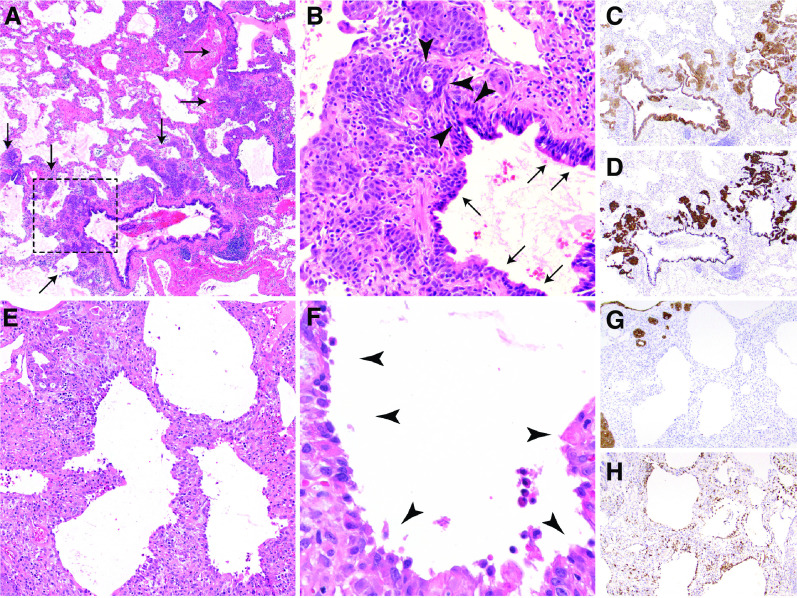
Seven adequate lung tissue samples were available (five biopsies and two autopsy specimens). Four biopsies were obtained within the first week of hospital admission and one was obtained on Day 23. Death occurred on Day 16 and 45 for the two specimens obtained at autopsy. All revealed a pattern of injury consistent with a recently identified pattern termed diffuse alveolar injury with delayed epithelialization (DAIDE; Figure 1) (5, 7), which is characterized by early organizing diffuse alveolar damage with a paucity of hyaline membranes, diffuse alveolar denudation, and macrophages lining denuded alveolar walls (Figure 1). The diffuse alveolar denudation and lack of significant bronchiolocentric injury in DAIDE is unique and distinct from ECMO-associated changes and the pathology in vaping-related lung injury, influenza, or coronavirus disease (COVID-19) (8–10). Cases initially diagnosed as eosinophilic pneumonia did not meet criteria for the diagnosis (11). Explant pathology was available for the three patients who underwent organ transplantation as described below. The mean date of transplant was 107 days (range, 98–119 d) from hospital admission. Histopathology of explanted lungs (one of which was reported previously [5]) no longer shows diffuse alveolar injury and instead shows reepithelization of alveolar walls as well as advanced fibrosis and architectural remodeling characteristic of a late phase of diffuse alveolar injury along with prominent bronchiolarization. Notably, bronchiolarization has been shown to arise from peribronchiolar basaloid pods (Figure 1) and is an evolutionarily conserved response to acute lung injury that is particularly prominent in all late-stage DAIDE cases yet identified (5, 12).
Figure 1.
Histopathology of trimethoprim–sulfamethoxazole–associated fulminant respiratory failure: diffuse alveolar injury with delayed epithelialization. (A) Low magnification (×20) shows diffuse alveolar denudation and peribronchiolar basaloid pods (PBPs, arrows), with thickened alveolar walls. (B) High-power magnification (×200) of the rectangular area in A shows an absence of pneumocytes, relative sparing of bronchioles, and prominent PBPs (previously “squamous metaplasia”), which are proliferating regenerative basaloid/squamoid cells adjacent to terminal bronchioles that are focally contiguous (arrowheads) with ciliated bronchiolar epithelium (arrows). (C and D) Pancytokeratin (C) and keratin 5/6 (D) immunohistochemical stains highlight the diffuse lack of alveolar epithelium, relatively spared bronchiolar epithelium, and basaloid nature of PBPs. (E and F) Thickened alveolar walls lack hyaline membranes or pneumocytes (E) and instead are lined by macrophages (arrowheads) (F). (G and H) Pancytokeratin stain (G) confirms the lack of alveolar pneumocytes, which are replaced by CD68-positive macrophages (H).
Five patients (36%) died, and three patients (21%) underwent transplantation (two lung and one heart/lung). Of the nine survivors, eight are known to have been weaned to room air over time. One was lost to follow-up 1 year after illness but was on nasal cannula oxygen at that time. Of the seven nontransplant survivors, three available pulmonary function tests all showed mild restrictive disease at 1 year after illness. All survivors are highly functional and include two nurses, one physician, one physical therapist, and four who returned to being full-time students/employees.
Limitations of this study include potential biases associated with identification of cases following media coverage and associated patient self-reporting, retrospective review of cases, the lack of blinding by the examining pathologists, and a relatively small number of cases. Identified cases likely underrepresent the scope of those affected; however, this series reveals an effective way to identify rare ADRs among subjects exposed to a relatively common drug.
The severity of these cases and resultant mortality is remarkable. Although causation cannot be proved, these cases have striking clinical and pathological findings, absence of an identified alternative etiology, and shared TMP-SMX exposure before respiratory symptom onset. All seven adequate lung samples demonstrated a novel pathologic finding, DAIDE, which has not yet been described other than in these TMP-SMX–exposed patients.
In summary, our results identify a novel, rare ADR to TMP-SMX with a distinct pathological pattern (DAIDE). Several important questions remain. The pathology shows a near-complete lack of alveolar epithelium and apparent macrophage barrier formation. It is unclear why TMP-SMX is selectively toxic or destructive to alveolar epithelium. The diffuse denudation and lack of hyaline membranes appears to be distinct from other forms of diffuse alveolar damage associated with more common causes of acute respiratory distress syndrome (ARDS). Risk factors for developing this rare but severe ADR are also unknown. Although these cases occurred in children and young adults, we do not know whether younger age is a risk factor. We speculate that children and young adults are less likely to have comorbidities, making this diagnosis of exclusion more easily recognized in younger patients; however, further work is needed to determine risk. Appropriate management is unclear. The patients’ lack of response to TMP-SMX discontinuation in combination with immunosuppression suggests the process rapidly causes irreversible destruction. Transplant has been curative, and survivors have not had evidence of disease recurrence. Early consideration of lung biopsy in ARDS is controversial but in similar cases may allow for earlier diagnosis of TMP-SMX–associated ARDS and consideration of lung transplant (13).
Supplementary Material
Acknowledgments
Acknowledgment
The authors thank the patients and family members who participated in this research. They also thank Raghu Chivukula and B. Taylor Thompson for helpful discussion.
Footnotes
Supported by NIH grants T32CA009216 (M.S.T.) and R01GM129783 (J.L.G).
Author Contributions: All designated authors met all four criteria, including 1) substantial contributions to the conception or design of the work or to the acquisition, analysis, or interpretation of data for the work; 2) drafting the work or revising it critically for important intellectual content; 3) final approval of the version to be published; and 4) agreement to be accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Originally Published in Press as DOI: 10.1164/rccm.202009-3421LE on January 29, 2021
Author disclosures are available with the text of this letter at www.atsjournals.org.
References
- 1.Yuzurio S, Horita N, Shiota Y, Kanehiro A, Tanimoto M. Interstitial lung disease during trimethoprim/sulfamethoxazole administration. Acta Med Okayama. 2010;64:181–187. doi: 10.18926/AMO/40010. [DOI] [PubMed] [Google Scholar]
- 2.Miller JO, Mino-Kenudson M, Shih AR, Taylor MS, Goldman JL. Expand your differential: trimethoprim-sulfamethoxazole (TMP-SMX) associated severe respiratory failure as a cause for prolonged ECMO. ASAIO J. 2000;66:6. [Google Scholar]
- 3.Shih AR, Taylor MS, Miller JO, Goldman JL, Mino-Kenudson M.Diffuse Alveolar Injury with Delayed Epithelialization (DAIDE) in previously healthy patients with idiopathic acute respiratory distress syndrome after trimethoprim-sulfamethoxazole exposure Mod Pathol 2020332097–2136.32139837 [Google Scholar]
- 4.Miller JO, Taylor J, Goldman JL. Severe acute respiratory failure in healthy adolescents exposed to trimethoprim-sulfamethoxazole. Pediatrics. 2019;143:e20183242. doi: 10.1542/peds.2018-3242. [DOI] [PubMed] [Google Scholar]
- 5.Taylor MS, Chivukula RR, Myers LC, Jeck WR, Waghray A, Tata PR, et al. A conserved distal lung regenerative pathway in acute lung injury. Am J Pathol. 2018;188:1149–1160. doi: 10.1016/j.ajpath.2018.01.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Naranjo CA, Busto U, Sellers EM, Sandor P, Ruiz I, Roberts EA, et al. A method for estimating the probability of adverse drug reactions. Clin Pharmacol Ther. 1981;30:239–245. doi: 10.1038/clpt.1981.154. [DOI] [PubMed] [Google Scholar]
- 7.Taylor MS, Chivukula RR, Myers LC, Jeck WR, Tata PR, O’Donnell WJ, et al. Delayed alveolar epithelialization: a distinct pathology in diffuse acute lung injury. Am J Respir Crit Care Med. 2018;197:522–524. doi: 10.1164/rccm.201706-1094LE. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ackermann M, Verleden SE, Kuehnel M, Haverich A, Welte T, Laenger F, et al. Pulmonary vascular endothelialitis, thrombosis, and angiogenesis in Covid-19. N Engl J Med. 2020;383:120–128. doi: 10.1056/NEJMoa2015432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Butt YM, Smith ML, Tazelaar HD, Vaszar LT, Swanson KL, Cecchini MJ, et al. Pathology of vaping-associated lung injury. N Engl J Med. 2019;381:1780–1781. doi: 10.1056/NEJMc1913069. [DOI] [PubMed] [Google Scholar]
- 10.Layden JE, Ghinai I, Pray I, Kimball A, Layer M, Tenforde MW, et al. Pulmonary illness related to E-cigarette use in Illinois and Wisconsin - final report. N Engl J Med. 2020;382:903–916. doi: 10.1056/NEJMoa1911614. [DOI] [PubMed] [Google Scholar]
- 11.Tazelaar HD, Linz LJ, Colby TV, Myers JL, Limper AH. Acute eosinophilic pneumonia: histopathologic findings in nine patients. Am J Respir Crit Care Med. 1997;155:296–302. doi: 10.1164/ajrccm.155.1.9001328. [DOI] [PubMed] [Google Scholar]
- 12.Kanegai CM, Xi Y, Donne ML, Gotts JE, Driver IH, Amidzic G, et al. Persistent pathology in influenza-infected mouse lungs. Am J Respir Cell Mol Biol. 2016;55:613–615. doi: 10.1165/rcmb.2015-0387LE. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Patel SR, Karmpaliotis D, Ayas NT, Mark EJ, Wain J, Thompson BT, et al. The role of open-lung biopsy in ARDS. Chest. 2004;125:197–202. doi: 10.1378/chest.125.1.197. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.