Abstract
Rationale: It is unclear why select patients with moderate-to-severe asthma continue to lose lung function despite therapy. We hypothesized that participants with the smallest responses to parenteral corticosteroids have the greatest risk of undergoing a severe decline in lung function.
Objectives: To evaluate corticosteroid-response phenotypes as longitudinal predictors of lung decline.
Methods: Adults within the NHLBI SARP III (Severe Asthma Research Program III) who had undergone a course of intramuscular triamcinolone at baseline and at ≥2 annual follow-up visits were evaluated. Longitudinal slopes were calculated for each participant’s post-bronchodilator FEV1% predicted. Categories of participant FEV1 slope were defined: severe decline, >2% loss/yr; mild decline, >0.5–2.0% loss/yr; no change, 0.5% loss/yr to <1% gain/yr; and improvement, ≥1% gain/yr. Regression models were used to develop predictors of severe decline.
Measurements and Main Results: Of 396 participants, 78 had severe decline, 91 had mild decline, 114 had no change, and 113 showed improvement. The triamcinolone-induced difference in the post-bronchodilator FEV1% predicted (derived by baseline subtraction) was related to the 4-year change in lung function or slope category in univariable models (P < 0.001). For each 5% decrement in the triamcinolone-induced difference the FEV1% predicted, there was a 50% increase in the odds of being in the severe decline group (odds ratio, 1.5; 95% confidence interval, 1.3–1.8), when adjusted for baseline FEV1, exacerbation history, blood eosinophils and body mass index.
Conclusions: Failure to improve the post-bronchodilator FEV1 after a challenge with parenteral corticosteroids is an evoked biomarker for patients at risk for a severe decline in lung function.
Keywords: severe asthma, corticosteroid sensitivity, longitudinal, lung function, exacerbations
At a Glance Commentary
Scientific Knowledge on the Subject
Numerous factors related to loss of lung function are known at the population level for patients with asthma. These include age, sex, genetic factors, duration of asthma, allergen sensitization, tobacco exposure, body mass index, baseline airflow limitation, bronchial hyperresponsiveness, blood and sputum eosinophils, maintenance therapy, and exacerbation history. In patients with moderate-to-severe asthma on stable medical therapy, the responsiveness to parenteral corticosteroids has not been evaluated as a predictor of lung function decline in a cohort with more than 1 year of follow-up.
What This Study Adds to the Field
Fewer than half of adults with moderate or severe persistent asthma continue to lose lung function while on maintenance therapy not including a biologic. The absolute change from baseline in the post-bronchodilator FEV1% predicted 2–3 weeks after an intramuscular injection with triamcinolone acetonide (post–triamcinolone acetonide value − pre–triamcinolone acetonide value) is a useful measure to help predict the lung function trajectory over the next 4 years.
Loss of lung function is one of the four risk criteria composing the definition of severe asthma, and numerous studies have documented predictors for this process. Early studies quantified this loss as a height-adjusted average rate of approximately 50 ml/yr, with variability relating to bronchial hyperresponsiveness and baseline airflow limitation (1–4), which can be modified by the use of inhaled corticosteroids (5–7). Additional risk factors for lung function decline in patients with mild-to-moderate asthma include exacerbations, second-hand tobacco exposure, female sex, allergen sensitization, blood eosinophils, exhaled nitric oxide, body mass index (BMI), and genetic determinants (8–20). Many of these studies involved patients with newly diagnosed asthma on low concentrations of therapy. By contrast, cohort studies of patients with severe asthma on high-dose inhaled corticosteroids and other controllers have been more limited. Matsunaga and colleagues followed 54 adults with severe asthma for 10 years and showed that the rate of decline for FVC exceeded the rate for the FEV1, with predictors including age, low baseline FVC, exacerbations, and maintenance oral corticosteroid use (21), which could relate to compressive air trapping during forced expiratory maneuvers (22). Newby and colleagues showed that 26% of 430 adults with severe asthma had evidence of progressive loss of lung function, possibly related to highly variable sputum eosinophils (23). Finally, Calhoun and colleagues studied 2,429 participants with severe or difficult-to-treat asthma of 6 years of age and above; compared with patients with good control, patients with exacerbations during the study period experienced a difference of −2.0% ± 0.4% predicted per year in terms of their FEV1 (24). Although many of these studies identified heterogeneity in the risk of lung function decline, there remains a gap for clinicians caring for patients with severe asthma in terms of using a baseline evaluation to identify those at risk for an accelerated decline in lung function.
The mechanisms underlying loss of lung function in asthma have been suggested but remain unclear. Years of chronic airway inflammation can lead to thickening of the basement membrane and oxidized mucus plugs (25, 26), loss of bronchodilator responsiveness (27), and a physiological transition from airflow limitation to the development of baseline air trapping (28, 29). These mechanisms of lung function loss are likely to be relatively resistant to corticosteroid therapy. Whether measured responses to a systemic corticosteroid challenge serve as evoked biomarkers for such processes is unclear. Of the few studies evaluating resistance to systemic corticosteroids, the follow-up period has been significantly less than a year (30–33).
The NHLBI SARP III (Severe Asthma Research Program III) established a longitudinal cohort of 183 adolescents and 526 adults with moderate-to-severe asthma. Baseline characterization included survey data, blood biomarkers, fractional exhaled nitric oxide (FeNO) and lung function measurements, and analysis of induced sputum samples (34). A single intramuscular injection of triamcinolone acetonide (40 mg) was administered at the baseline visit, followed by repeated phenotyping 18 ± 3 days later to assess the change in lung function, symptoms, and potential resolution of measures of airway inflammation (35). Whereas our approach was to find the best clinically available predictors of lung function decline in adults, we also hypothesized that the smallest measurements of response to parenteral corticosteroids at baseline would enable identification of patients with the largest loss in lung function after longitudinal follow-up. We evaluated known risk factors of severe decline that can be measured by clinicians, using methods suited for individual patient predictions. A portion of this work has been reported previously as a poster abstract (36).
Methods
Cohort and Clinical Measurements
Institutional review, recruitment methods, informed consent, severe asthma definition, and assessments have been described for this longitudinal cohort at baseline (34, 35). Enrollment occurred from November 2012 through January 2015, with a baseline evaluation, an injection of 40 mg of triamcinolone acetonide, and repeat assessments 18 ± 3 days later. Triamcinolone-induced differences from baseline in measures of lung function, Juniper Asthma Control Questionnaire 6 scores, blood and sputum eosinophils, and FeNO have been described previously and were derived by simple subtraction (post–triamcinolone acetonide values minus the pre–triamcinolone acetonide values) (35); these variables were renamed as triamcinolone-induced differences (e.g., the triamcinolone-induced difference in the FEV1% predicted [tdFEV1]) for this manuscript. Medication updates, clinical outcomes, adverse events, and asthma-control measures were collected by phone interviews 6 months after the baseline visits, as well as 6 months after each of the annual study visits and at the annual study visits using a 6-month recall period. The Asthma Severity Scoring System (ASSESS) asthma-severity measure was calculated as described (37). To avoid complexity associated with adolescent lung growth, adult participants with lung function data from the baseline and at least two follow-up visits were included in this analysis. Use of biologic therapy during this time period was an exclusion; however, if the baseline data and at least two more data points were collected before the start of biologic therapy, the data collected after biologic initiation were censored. The data set for this analysis was accessed on January 15, 2019.
Lung Function Assessment
Spirometry and maximum bronchodilator reversibility were performed annually according to the American Thoracic Society Guidelines and as described previously (34). Normative comparisons were assessed using the Global Lung Initiative data set (38). Post-bronchodilator measurements (four puffs of albuterol) were chosen as the primary lung function measurement to reduce intrasubject variability from year to year and to increase the likelihood of identifying changes that reflected airway remodeling; therefore, all reported data are the post-bronchodilator values unless otherwise specified. Similar to the methods in the SARP III physiology manuscript (28), we partitioned each participant’s spirometric data to determine patterns of change related to a dominance of airflow limitation, airway closure, or a mixture of both. See online supplement for details.
Statistical Analysis
The annualized rate of change (slope) for post-bronchodilator FEV1% predicted was estimated separately for each participant using standard least-squares linear regression models. Participants were classified into four slope categories, namely severe decline (>2.0% loss per year), mild decline (>0.5–2.0% loss per year), no change (0.5% loss to <1.0% gain per year), and improvement (≥1.0% gain per year). Baseline and triamcinolone-induced-difference variables are summarized as the mean ± SD for symmetric variables, the median with the first and third quartiles for asymmetric variables, and percentages for categorical variables. Differences among groups were tested using ANOVA or the Kruskal-Wallis test for continuous variables and Pearson or Fisher exact chi-square tests for categorical variables. The 4-year change in FEV1% predicted was derived by multiplying each participant’s slope by four. Least-squares linear regression models were derived for the 4-year change in FEV1% predicted. The stability of these linear models was assessed by removing highly influential points. Univariable and multivariable ordinal logistic models were derived for predictors of the slope categories using proportional odds assumptions. Predictors for these models were restricted to variables that could be assessed by clinicians currently. A sensitivity analysis of the multivariable ordinal logistic model was performed by adding back excluded data from participants on biologic therapy. In addition, linear mixed-effect models of repeated measures of FEV1 were also performed using data from all participants to assure that minor effects from other predictors were addressed. See online supplement for additional details regarding the models.
Statistical analysis and figures were generated using SAS 9.4 and SAS/JMP software (SAS Institute, Inc.). All statistical tests were two‐sided, with P < 0.05 indicating statistical significance without adjustment for multiple comparisons.
Results
Change Associated with Severe Decline in Lung Function Noted in Only a Subset of Participants
Despite enrichment for severe asthma in this cohort, the distribution of post-bronchodilator lung function measures (mean FEV1% predicted, FVC% predicted and FEV1%/FVC% predicted) did not change over time (Figures 1A–1C). However, given that a minority of patients with severe asthma can have loss of lung function at a rate of 2% predicted per year or more (24), we calculated a rate of change for individual participants on the basis of simple linear regression models, using at least three data points for each participant. There were 396 adults in SARP III, with at least one baseline and two additional annual measurements of lung function preceding the use of biologic therapies; for a Consolidated Standards of Reporting Trials diagram, see Figure E1 in the online supplement. Fifty-nine (11%) of the original 526 adult participants dropped out of the study before completing a second follow-up visit or for other reasons. Compared with the study population, the early-discontinuation group was younger; had worse Asthma Control Test (ACT) scores at baseline, with a trend toward lower medication-compliance scores; and had a smaller triamcinolone-induced decline in blood eosinophils (Table E1). In addition, 71 participants received a biologic therapy before completing a second follow-up visit. Relative to the study population, those receiving biologics were older, had lower baseline lung function, and had worse ACT scores and more exacerbations, resulting in a greater proportion of participants with a severe asthma classification. The number of years of follow-up per participant in the study population was 4.1 ± 0.8 years, with a total of 1,914 participant-years for this analysis. The annualized change in lung function over time was expressed as a slope (percent predicted per year), calculated for each participant. Inspection of the distribution of these slopes for the population suggests that neither baseline severity nor participant sex are significant modifiers (Figure 1D).
Figure 1.

Longitudinal lung function of adult participants in SARP III (Severe Asthma Research Program III) not using biologic therapies. Data shown were collected after the participants were given four puffs of albuterol and are represented as box-and-whisker plots. The sample sizes are shown in A, with ongoing accrual contributing to the lower numbers at Years 4 and 5. (A–C) The FEV1% predicted (Pred) (A), FVC% Pred (B), and FEV1/FVC% Pred (C). (D) The distribution of longitudinal slopes for individual participants shown as a violin plot of the frequency distribution, with stratification by sex and baseline severity.
Consistent with the results of Calhoun and colleagues (24), we defined a severe loss of lung function as a slope less than −2% predicted/yr (Figure 2A). Additional boundaries were created to define participants with mild decline, no change, or an increase in lung function over time (Figure 2A); these boundaries are close to quartile intervals for this SARP III subset but were defined numerically to increase generalizability. The group mean FEV1% predicted values at each annual follow-up are shown in Figure 2B and are stratified by the slope categories. The baseline post-bronchodilator FEV1 measures were not different across the slope categories (P = 0.372 Figure 2B). The mean slopes are −134.4 ± 104.5, −52.5 ± 20.4, −15.3 ± 16.8, and 61.3 ± 63.0 ml/yr in the severe-decline, mild-decline, no-change, and improvement groups, respectively.
Figure 2.
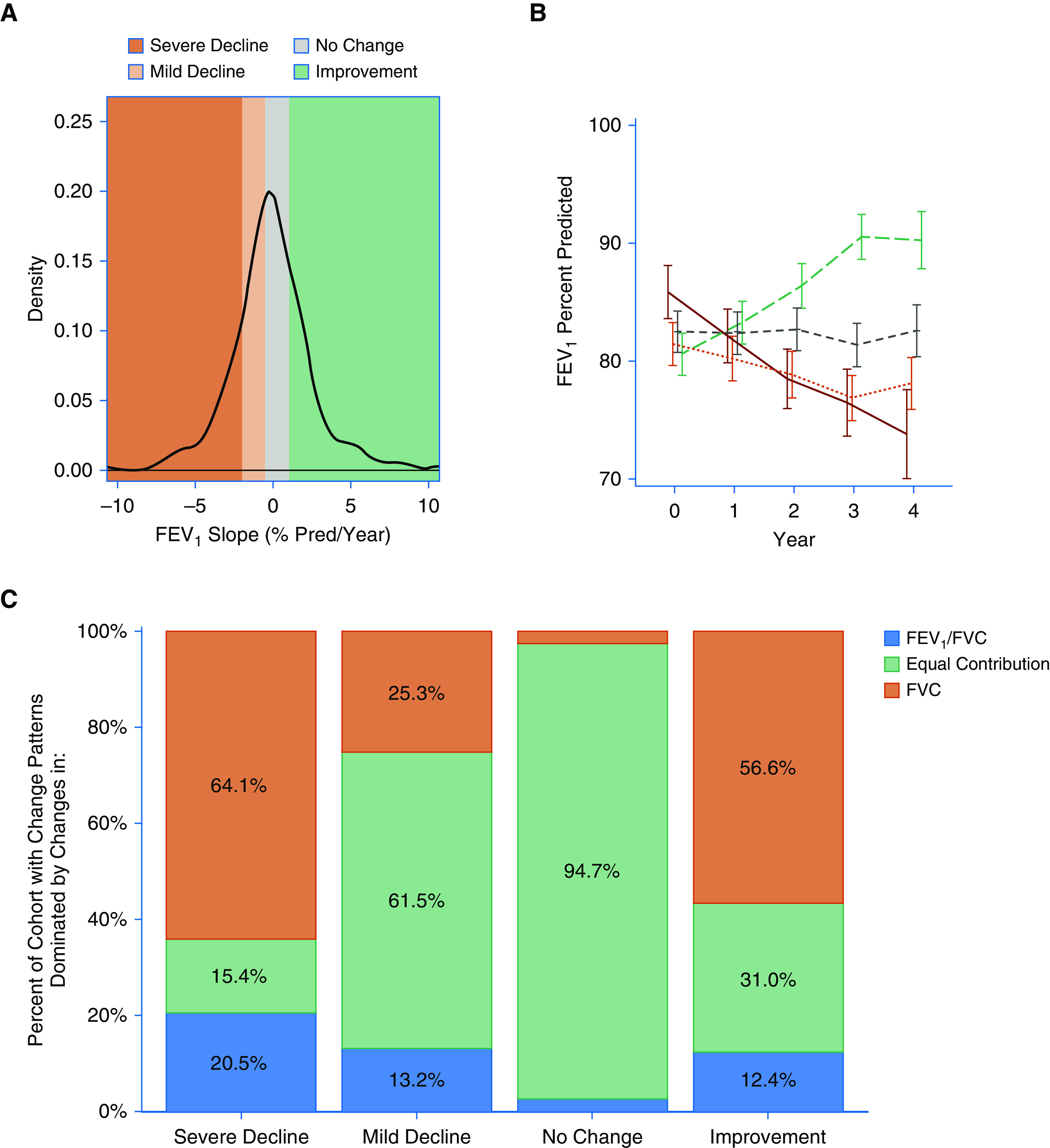
Lung function trajectories of adult participants of SARP III (Severe Asthma Research Program III) not using biologic therapies. (A) Histogram of the FEV1 slope for each participant. As stated in the Methods, participants were classified into four slope categories, namely severe decline (>2% loss/yr), mild decline (2.0 to <0.5% loss/yr), no change (0.5% loss/yr to <1% gain/yr), and improvement (≥1% gain/yr), respectively, denoted by colors in this figure. (B) The annual FEV1% Pred group mean data stratified by these categorical trajectories. The solid line represents the severe decline group, and the dotted line represents the mild decline group. The two dashed lines are the no change (gray) and improvement (green) groups, respectively. Partition analysis of each participant’s pattern of change allows for classification regarding change dominated by airflow limitation, airway closure, or a mixture of both. (C) Dominance pattern for each lung function category showing change over time. Pred = predicted.
To examine the physiologic characteristics of these slope categories, we investigated the distribution of airflow limitation and air-trapping phenotypes (28) across these groups. As expected from correlative studies using plethysmography (22), less than 20% of the cohort had lung function trajectories dominated by changes in airflow limitation (Figure 2C). Conversely, over half of the participants in the severe-decline or improvement groups showed effects that were dominated by changes in the FVC% predicted (Figure 2C). These observations are consistent with changes related to the development or relief of airway closure and/or air trapping.
Identification of the Triamcinolone-induced Change in Lung Function as a Predictor of Longitudinal Trajectory
Table 1 shows categorical analyses of the baseline clinical variables stratified by the longitudinal lung-function-change classifiers. The number of exacerbations in the 12 months before enrollment was different across these groups, with the highest number being in the severe-decline and improvement categories. Compared with participants in the other groups, those with severe decline were using a greater median number of controller therapies at baseline. No other differences were notable in demographic or asthma-severity features across these groups (Table 1), which included differences in the ASSESS score, a comprehensive measure of asthma severity that is more dynamic than the categorical consensus definition (37). The corticosteroid-response variables of interest are shown in Table 2, with a complete listing of the spirometric variables before and after triamcinolone administration shown in Table E2. The triamcinolone-induced differences from baseline FEV1% predicted (tdFEV1; difference in the post-bronchodilator values [post-triamcinolone minus pre-triamcinolone]) ranged from −24.3% to 37.6% predicted and were not correlated with the visit-to-visit differences noted for each participant during screening before the triamcinolone injection (R = −0.093; P = 0.080, with the trend susceptible to influence from a single data point). Of the 388 participants with complete data for the post-bronchodilator tdFEV1, 168 (43%) had a tdFEV1 value less than 0, and 47 of these participants (12% of the total) had a value less than −5. The tdFEV1 was different across these slope categories (P < 0.001); the average value was slightly negative in the participants with severe decline, with a progressive numerical increase across these categories (Table 2). No differences were noted across these categories for the triamcinolone-induced change in baseline Juniper Asthma Control Questionnaire 6 scores, FeNO, blood eosinophils, or sputum granulocytes (Table 2).
Table 1.
Baseline Characteristics of Adult Participants in SARP III Stratified by Longitudinal Category of Change in Lung Function
| Characteristic | Severe Decline | Mild Decline | No Change | Improvement | P Value |
|---|---|---|---|---|---|
| Baseline visit, n | 78 | 91 | 114 | 113 | — |
| Age at baseline, yr | 50.0 ± 12.7 | 47.5 ± 14.2 | 46.9 ± 14.5 | 46.9 ± 14.5 | 0.415 |
| Duration of asthma, yr | 26.3 ± 16.3 | 28.8 ± 16.7 | 28.8 ± 15.3 | 26.7 ± 14.4 | 0.538 |
| Sex | |||||
| M, n (%) | 26 (33.3) | 29 (31.9) | 41 (36) | 35 (31) | 0.869 |
| F, n (%) | 52 (66.7) | 62 (68.1) | 73 (64) | 78 (69) | |
| Race | |||||
| White, n (%) | 53 (67.9) | 59 (64.8) | 79 (69.3) | 69 (61.1) | 0.872 |
| Black, n (%) | 17 (21.%) | 24 (26.4) | 25 (21.9) | 30 (26.5) | |
| Other, n (%) | 8 (10.3) | 8 (8.8) | 10 (8.%) | 14 (12.4) | |
| Hispanic, n (%) | 4 (5.1) | 3 (3.3) | 3 (2.6) | 5 (4.4) | 0.804 |
| BMI, kg/m2 | 32.1 ± 7.7 | 32.3 ± 9.1 | 31.5 ± 7.8 | 32.8 ± 8.8 | 0.727 |
| Severe asthma at baseline, n (%) | 49 (62.8) | 43 (47.3) | 57 (50) | 61 (54) | 0.197 |
| ICS dose | |||||
| None, n (%) | 4 (5.1) | 11 (12.1) | 17 (14.9) | 15 (13.3) | 0.112 |
| Low, n (%) | 13 (16.7) | 13 (14.3) | 11 (9.6) | 17 (15) | |
| Medium, n (%) | 9 (11.5) | 22 (24.2) | 22 (19.3) | 14 (12.4) | |
| High, n (%) | 52 (66.7) | 45 (49.5) | 64 (56.1) | 67 (59.3) | |
| On maintenance OCS, n (%) | 9 (11.5) | 9 (9.9) | 9 (7.9) | 12 (10.6) | 0.845 |
| Dose of OCS, mg/d | 10 (5–15) | 10 (5–21) | 10 (5–10) | 8 (5–15) | 0.630 |
| Number of controller therapies | 3 (2–3) | 2 (2–3) | 2 (2–3) | 2 (2–3) | 0.012 |
| Baseline exacerbations in prior 12 mo | 1 (0–2); min, max: 0, 12 | 0 (0–2), min, max: 0, 9 | 0 (0–1), min, max: 0, 12 | 1 (0–2), min, max: 0, 12 | 0.012 |
| Baseline ACT score | 16.8 ± 4.3 | 17.5 ± 4.6 | 18.1 ± 4.5 | 17.2 ± 4.7 | 0.260 |
| Baseline MARS score | 22.3 ± 2.6 | 22.4 ± 2.9 | 22.3 ± 2.9 | 21.7 ± 3.7 | 0.326 |
| Baseline ASSESS score | 9.1 ± 3.7 (n = 78) | 8.3 ± 3.7 (n = 90) | 7.9 ± 3.7 (n = 114) | 8.7 ± 4.2 (n = 113) | 0.153 |
| PreBD FEV1% predicted | 75.6 ± 20.8 | 73.1 ± 17.6 | 74.5 ± 19.7 | 72.2 ± 21.0 | 0.669 |
| Maximum albuterol reversibility, postBD − preBD | 12.4 ± 7.4 | 10.2 ± 8.2 | 10.1 ± 6.7 | 11.5 ± 7.6 | 0.104 |
| PC20, mg/ml | 2.6 ± 2.7 (n = 27) | 3.1 ± 3.9 (n = 48) | 3.1 ± 3.7 (n = 68) | 3.1 ± 3.7 (n = 56) | 0.957 |
Definition of abbreviations: ACT = Asthma Control Test; ASSESS = Asthma Severity Scoring System; BMI = body mass index; ICS = inhaled corticosteroid; MARS = Medication Adherence Report Scale; max = maximum; min = minimum; OCS = oral corticosteroid; PC20 = provocative concentration of methacholine, causing a 20% fall in FEV1; postBD = post-bronchodilator value; preBD = pre-bronchodilator value; SARP III = Severe Asthma Research Program III.
Continuous data with normal distributions are presented as means and SDs, with testing conducted by ANOVA. Categorical variables are shown as the number of participants and percentage within each lung-function-change category, with significance testing conducted by using the Fisher exact test. The sample size is included for those characteristics with notable amounts of missing data for comparison with the totals in each category.
Table 2.
Corticosteroid-evoked Phenotypes of Adult Participants in SARP III Stratified by Longitudinal Category of Change in Lung Function
| Characteristic | Study Population (N = 396; tdX n = 388) | Decline Group |
||||
|---|---|---|---|---|---|---|
| Severe Decline (n = 78; tdX n = 76) | Mild Decline (n = 91; tdX n = 89) | No Change (n = 114; tdX n = 114) | Improvement (n = 113; tdX n = 109) | P Value | ||
| FEV1 Post4BD, % predicted | ||||||
| BL | 82.2 ± 18.9 | 85.6 ± 19.8 | 81.3 ± 17.3 | 82.5 ± 18.7 | 80.3 ± 19.6 | <0.001 |
| V3 | 84.1 ± 18.5 | 84.5 ± 18.9 | 82.1 ± 17.0 | 83.5 ± 19.1 | 86.1 ± 18.7 | |
| tdX | 1.8 ± 7.2 | −0.8 ± 6.7 | 0.5 ± 4.8 | 1.0 ± 6.0 | 5.7 ± 8.8 | |
| FeNO | ||||||
| BL | 23.0 (13.5 to 38.5) | 24.0 (14.0 to 43.0) | 23.0 (14.0 to 33.0) | 20.5 (14.0 to 36.0) | 23.0 (13.0 to 45.0) | 0.850 |
| V3 | 19.0 (13.0 to 28.0) | 22.0 (13.0 to 35.0) | 17.0 (13.0 to 24.0) | 18.0 (12.0 to 26.0) | 20.0 (13.0 to 30.0) | |
| tdX | −4.0 (−12.0 to 1.0) | −4.0 (−13.0 to 1.0) | −4.0 (−12.0 to 1.0) | −4.0 (−10.0 to 1.0) | −4.5 (−14.0 to 0.0) | |
| ACQ6 score | ||||||
| BL | 1.5 ± 1.0 | 1.6 ± 1.2 | 1.3 ± 1.0 | 1.4 ± 0.9 | 1.6 ± 1.1 | 0.773 |
| V3 | 1.3 ± 1.0 | 1.4 ± 1.1 | 1.1 ± 0.9 | 1.2 ± 0.9 | 1.3 ± 1.1 | |
| tdX | −0.2 ± 0.8 | −0.2 ± 0.8 | −0.3 ± 0.8 | −0.2 ± 0.7 | −0.3 ± 0.9 | |
| Sputum | ||||||
| n | 311 | 57 | 65 | 91 | 98 | — |
| tdX n | 266 | 51 | 56 | 79 | 80 | — |
| Eosinophils, % | ||||||
| BL | 0.7 (0.1 to 2.9) | 1.3 (0.0 to 5.2) | 0.3 (0.0 to 1.9) | 0.5 (0.2 to 1.5) | 1.0 (0.2 to 3.5) | 0.768 |
| V3 | 0.4 (0.0 to 1.5) | 0.8 (0.0 to 2.1) | 0.2 (0.0 to 0.9) | 0.4 (0.0 to 1.1) | 0.4 (0.0 to 1.8) | |
| tdX | −0.2 (−1.7 to 0.2) | −0.4 (−1.6 to 0.8) | −0.2 (−1.7 to 0.0) | −0.2 (−0.8 to 0.2) | −0.2 (−2.2 to 0.2) | |
| Neutrophils, % | ||||||
| BL | 54.0 ± 25.4 | 55.0 ± 24.3 | 56.0 ± 25.0 | 54.0 ± 27.5 | 52.1 ± 24.5 | 0.401 |
| V3 | 56.1 ± 25.3 | 50.8 ± 26.9 | 57.3 ± 26.9 | 57.3 ± 23.5 | 57.7 ± 24.7 | |
| tdX | 3.0 ± 22.8 | −1.7 ± 20.3 | 4.2 ± 22.4 | 3.3 ± 24.4 | 5.0 ± 22.9 | |
| Blood eosinophils, count | ||||||
| n | 395 | 78 | 91 | 114 | 112 | — |
| tdX n | 195 | 48 | 43 | 55 | 49 | — |
| BL | 207 (118 to 374) | 180 (126 to 427) | 217 (108 to 391) | 195 (123 to 320) | 242 (110 to 393) | 0.815 |
| V3 | 147 (90 to 265) | 166 (86 to 328) | 180 (100 to 289) | 130 (82 to 216) | 146 (96 to 236) | |
| tdX | −60 (−170 to 0) | −62 (−155 to −25) | −77 (−226 to 0) | −51 (−158 to 0) | −39 (−167 to 5) | |
Definition of abbreviations: ACQ6 = Juniper Asthma Control Questionnaire 6; BL = baseline; FeNO = fractional exhaled nitric oxide; Post4BD = after four-puff bronchodilator therapy; SARP III = Severe Asthma Research Program III; tdX = triamcinolone-induced difference for the variable in that row’s group, wherein the baseline measures are subtracted from the post-triamcinolone values; V3 = visit 3.
Continuous data with normal distributions are presented as the means and SDs, with testing conducted by ANOVA. Eosinophil data are shown as medians with the boundaries of the first and third quartiles, before log transformation for significance testing. Categorical variables are shown as the number of participants and percentage within each lung-function-change category, with significance testing conducted by using the Fisher exact test. The sample size is included for those characteristics with notable amounts of missing data for comparison with the totals in each category.
From these variables, we selected the tdFEV1 and historic exacerbations for further study. The tdFEV1, measured 18 ± 3 days after the baseline visit, has a linear relationship to the 4-year trajectory in FEV1% predicted (univariable model shown in Figure 3A). Stability of the model was verified with removal of highly influential data points at both extremes of tdFEV1. The tdFEV1 model of the 4-year trajectory was not influenced by adjustment for the baseline FEV1, nor was there interaction between these predictors. Regarding differences in individual participant slopes, the larger the triamcinolone-induced increase in lung function, the greater the 4-year improvement; similarly, the larger the decrease in tdFEV1, the greater the 4-year decline. Figure 3B shows the effects of the baseline exacerbation history on the 4-year change in FEV1. Although the slope of this model is in the predicted direction based on several studies (19, 24), the effect size in this cohort is small and not significant.
Figure 3.
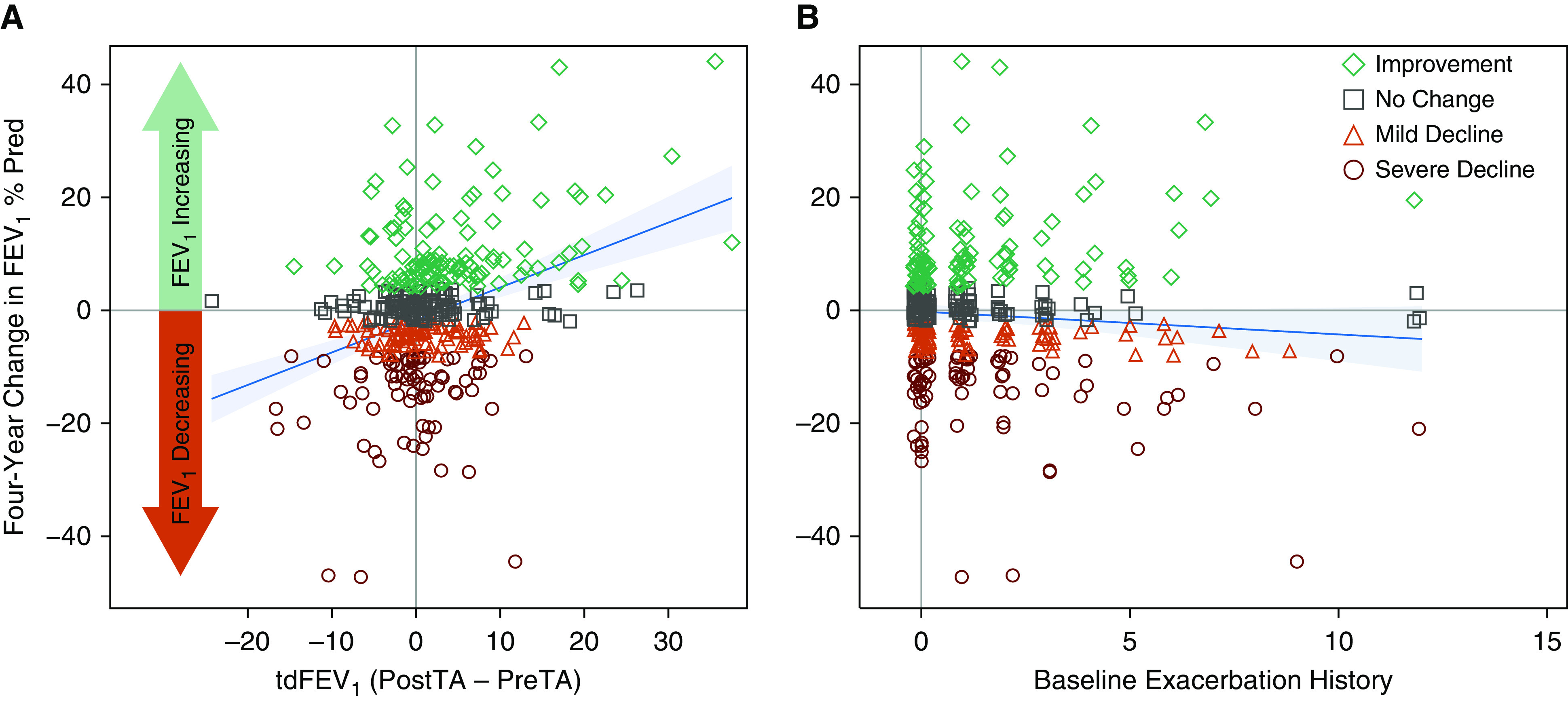
Linear models of the change in FEV1% Pred over 4 years. The symbols represent data from individual participants, with colors denoting the lung-function-change category. (A) Univariable model for tdFEV1 (P < 0.001). (B) Univariable model for the history of exacerbations in the 12 months before enrollment (P = 0.134). For both panels, the solid blue line and surrounding shading represents the model-fitted values and their 95% confidence intervals. PostTA = post–triamcinolone acetonide value; Pred = predicted; PreTA = pre–triamcinolone acetonide value; tdFEV1 = triamcinolone-induced difference in the FEV1 % Pred.
Insensitivity to Triamcinolone as a Multivariable Risk for Severe Decline in Lung Function
Categorical results from Tables 1 and 2, as well as information from the literature, guided our subsequent models of lung function slope category, with a focus on predictors that can be assessed by clinicians. We first fit an ordinal logistic regression model with the tdFEV1 variable as the only predictor. Similar to the linear models, the tdFEV1 model has a strong inverse relationship with the categorical lung function trajectory (P < 0.001). This probability of assignment to individual categories is shown in Figure 4A, with 95% confidence intervals for each fitted line. Participants with a negative tdFEV1 value 18 ± 3 days after the baseline visit have increasing probability of severe decline longitudinally, and those with a positive tdFEV1 value have increasing probability of improvement (Figure 4A). Table E3 shows the predicted probability of group assignment for varying measures of the baseline FEV1 and the tdFEV1. Age, sex, duration of asthma symptoms, baseline severity, or medication adherence were not significant in univariable models of lung function trajectory (P > 0.1).
Figure 4.
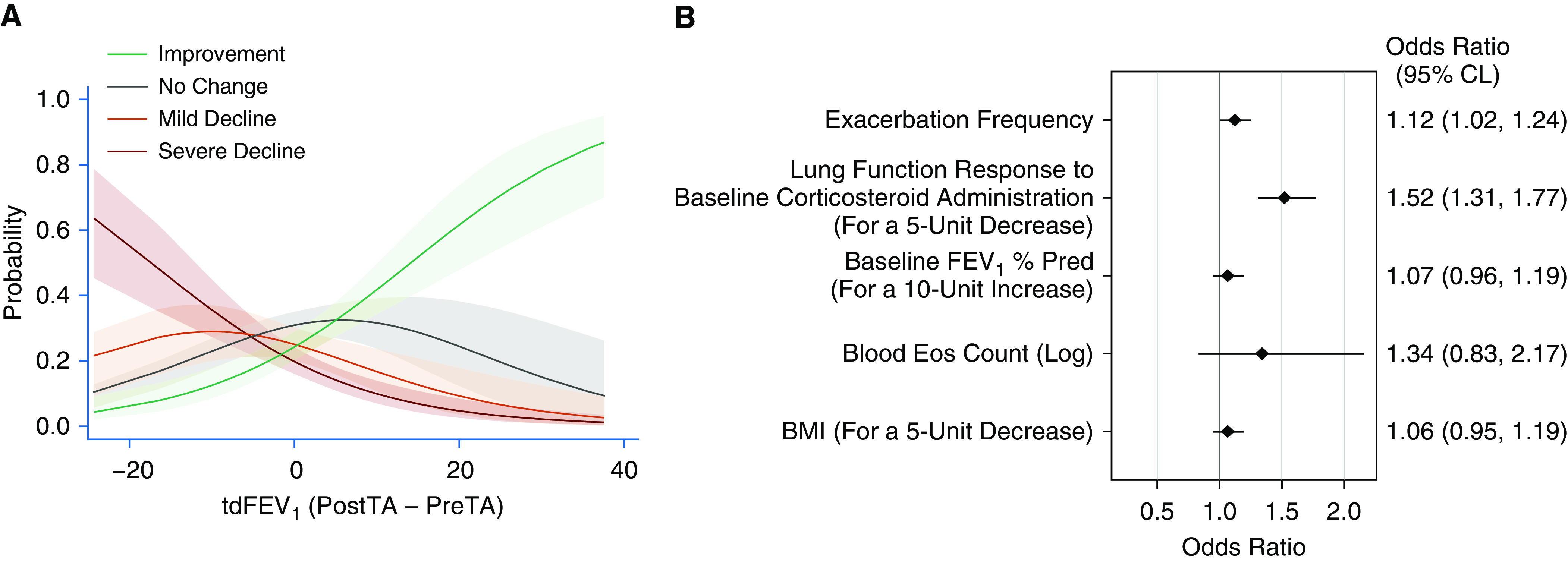
Ordinal logistic models of the categorical lung function trajectory. (A) The probability of assignment to individual categories, with 95% confidence intervals included with the fit lines, derived through subtraction from the base univariable model for the tdFEV1 (P < 0.001). (B) Multivariable ordinal logistic regression model of baseline predictors associated with the risk of severe decline. Baseline predictors are listed at the left of the figure, with the units of change shown in parentheses for continuous variables. The point-estimate limits and 95% CLs for the odds ratios in this model are shown graphically and numerically. BMI = body mass index; CL = confidence limit; Eos = eosinophils; PostTA = post–triamcinolone acetonide value; Pred = predicted; PreTA = pre–triamcinolone acetonide value; tdFEV1 = triamcinolone-induced difference in the FEV1 % Pred.
In a multivariable ordinal logistic model, the tdFEV1 continues as the most significant baseline predictor of longitudinal categories of lung function change (Figure 4B). The odds of assignment to the severe decline category versus other categories are 1.5 times greater (odds ratio, 1.5; 95% confidence interval, 1.3–1.8; P < 0.001) for each 5% decrement in the tdFEV1. The odds of assignment to the severe-decline group are 1.1 times greater for each exacerbation at baseline, with a 95% confidence boundary slightly greater than 1.0 in the multivariable model. These relationships persist after adjustment for baseline measures of FEV1, blood eosinophils, and BMI. Using a sensitivity analysis, these results were not affected by adding back the data collected from visits while participants were on biologic therapy (Figure E2). With data from all participants, we also confirmed that the tdFEV1 is the dominant predictor of lung function in a linear mixed-effect model of the FEV1% predicted, with a highly significant interaction with the years of follow-up (P < 0.001; Table E4). In this model, there was also an interaction of the baseline blood eosinophils and the years of follow-up (P = 0.035). Given the known influence of asthma medications and the potential effect of participant weight on the FVC, we added time-dependent changes in controller medications, biologic therapy, and BMI to this mixed-effect model with data from all participants. The tdFEV1 remained a highly significant predictor of longitudinal lung function even after adjustment for time-dependent variables (Table E4).
Longitudinal Outcomes of Participants in the Lung Function Categories
To assess the impact of slope classification, we evaluated the changes in medication adherence, asthma control, exacerbations, healthcare use, and asthma severity after 3 years of follow-up, the time point at which the remaining participants in the SARP III cohort had completed information (Table 3). There were no differences in medication adherence across the slope categories. Relative to the other groups, participants with severe decline in lung function had worse ACT scores and more exacerbations (Table 3). There was a trend for the severe-decline group to have more unscheduled health visits, although emergency department visits and hospitalizations were not different across slope categories. The mean difference from baseline in the ASSESS score was negative in all slope categories, reflecting an improvement in asthma severity. However, the severe-decline group had the smallest amount of improvement in this metric, and there was a progressive increase in the amount of asthma-severity improvement across the remaining slope categories (P < 0.001). Finally, the changes in the ASSESS scores were driven primarily by the control, lung function, and exacerbation domains, without differences across the groups at Year 3 in the medication components of this composite measure of asthma severity (Table E5).
Table 3.
Longitudinal SARP III Survey Scores and Outcomes Stratified by Lung Function Slope Categories
| Characteristic | Severe Decline | Mild Decline | No Change | Improvement | P Value |
|---|---|---|---|---|---|
| Year 3 follow-up visit, N | 70 of 78 | 90 of 91 | 106 of 114 | 101 of 113 | — |
| Difference in MARS score, Year 3 − baseline | −0.5 ± 2.7 | −0.6 ± 3.0 | −0.3 ± 3.3 (n = 105) | −0.4 ± 3.3 (n = 100) | 0.941 |
| Difference in ACT score, Year 3 − baseline | −0.1 ± 3.8 | 1.3 ± 4.2 | 0.7 ± 4.6 | 1.9 ± 3.7 | 0.017 |
| Cumulative exacerbations, Years 1 through 3 | 2 (0–6); min, max: 0, 21 | 1 (0–2) (n = 89); min, max: 0, 13 | 1 (0–3); min, max: 0,15 | 1 (0–3) (n = 100); min, max: 0, 16 | 0.012 |
| Years with unscheduled health visits, Years 1 through 3 | 1.0 ± 1.1 (n = 68) | 0.6 ± 0.9 (n = 89) | 0.8 ± 1.0 (n = 104) | 0.7 ± 0.9 (n = 97) | 0.075 |
| Years with emergency department visits, Years 1 through 3 | 0.4 ± 0.8 (n = 68) | 0.3 ± 0.7 (n = 89) | 0.3 ± 0.7 (n = 104) | 0.3 ± 0.6 (n = 97) | 0.431 |
| Cumulative asthma-specific hospitalizations, Years 1 through 3 | 0.2 ± 1.1 (n = 68) | 0.2 ± 2.0 (n = 89) | 0.2 ± 0.7 (n = 104) | 0.1 ± 0.7 (n = 97) | 0.925 |
| Cumulative asthma-specific ICU admissions, Years 1 through 3 | 0.1 ± 0.6 (n = 68) | 0.1 ± 0.9 (n = 89) | 0.0 ± 0.2 (n = 104) | 0.0 ± 0.1 (n = 97) | 0.573 |
| Difference in ASSESS score Year 3 − baseline | −0.3 ± 2.4 (n = 70) | −0.9 ± 2.4 (n = 89) | −1.1 ± 2.5 (n = 106) | −2.2 ± 2.9 (n = 100) | <0.001 |
Definition of abbreviations: ACT = Asthma Control Test; ASSESS = Asthma Severity Scoring System; MARS = Medication Adherence Report Scale; max = maximum; min = minimum; SARP III = Severe Asthma Research Program III.
Survey scores were assessed at the baseline and follow-up visits with the differences shown between Year 3 and baseline. The cumulative totals represent events that occurred prospectively after enrollment. Significance testing was conducted by using Kruskal-Wallace, one-way ANOVA, or Fisher exact methods as appropriate.
Discussion
The extensive phenotyping of the SARP III cohort provides a unique opportunity to evaluate the impact of clinical features and biomarkers on the longitudinal trajectories of severe asthma. Within this cohort, only 20% of biologic-naive participants have a 4-year decline in post-bronchodilator FEV1 that is greater than 8% predicted, a result that is consistent with data from Newby and colleagues (23). However, whether using the consensus definition of severity or the ASSESS score (37), baseline severity did not influence the likelihood of severe decline, which is a notable difference from the literature. We also did not observe differences in baseline lung function, airway hyperreactivity, or bronchodilator responsiveness across these groups. Nevertheless, the impact of lung function loss over time was notable, as participants in the severe-decline group had progressively deteriorating asthma control, more exacerbations, and a trend toward more unscheduled healthcare visits at the third year of follow-up (Table 3). These factors in combination influence the ASSESS score, which improved the least for participants in the severe-decline group.
Of the 282 participants who had a longitudinal change in their lung function, only 59 (21%) showed a pattern that was dominated by changes in airflow limitation (FEV1/FVC), whereas 164 (58%) exhibited changes primarily in air trapping (FVC; Figure 2C). Air trapping is a manifestation of peripheral airway instability and closure and has been recognized in asthma as a correlate with severity, symptoms, and poor control in adults and children (22, 28, 29, 39–41). Small airways close when the radial forces of lung elastic recoil decrease as lungs deflate, reaching a critical point of luminal narrowing forced by surface tension and smooth-muscle tone. Increased luminal exudate, airway wall-thickening, and increased smooth-muscle tone in asthmatic airways not only increase airflow resistance but also increase the amount of radial force required to prevent closure. This results in heterogeneous airway closure and air trapping, as the most affected airways close at higher lung volumes (40). Asthma-associated air trapping may be attenuated acutely by reducing smooth-muscle tone with bronchodilators (22, 29, 39), and in this study, the subgroup that improved post-bronchodilator FEV1 longitudinally also exhibited an increase in post-bronchodilator FVC, suggesting that their peripheral airway instability may have reflected attenuation of baseline inflammatory processes. In contrast, the severe-decline subgroup in this study exhibited progressive declines in post-bronchodilator FEV1 that were associated with reductions in FVC, consistent with worsening air trapping. Loss of lung elastic recoil is one mechanism that could contribute to both airflow limitation and air trapping (42), and it has been reported as a contributing mechanism in asthma with persistent airflow limitation (43). Older individuals with asthma commonly have a compressive component of air trapping during forced expiration in addition to their lung volume–dependent airway closure (22). The compressive air trapping is more resistant to bronchodilators (22) and may be associated with accelerated loss of lung elastic recoil with age.
We hypothesized that insensitivity to parenteral corticosteroids is a risk factor for accelerated lung function loss and evaluated several evoked phenotypes; namely, the triamcinolone-induced differences from baseline in blood eosinophils, FeNO, Juniper Asthma Control Questionnaire scores, and FEV1% predicted. Of these, the tdFEV1 was the only measure significantly associated with lung function trajectories in univariable models and had the largest effect size in the multivariable model, using both ordinal logistic and mixed-effect approaches. Although several studies have shown that exacerbations are related to lung function loss, our observations suggest that baseline historical exacerbation data had a small effect on these trajectories. Persistent eosinophilic inflammation has been shown to be related to lower lung function (13); however, our data suggest that baseline measures of this type 2 inflammatory biomarker have little predictive value in this cohort using multiple maintenance therapies, excluding biologics. Both exacerbations and measures of type 2 inflammation may have greater impact on models accommodating time-dependent predictors.
It is interesting to speculate how these results might be useful to clinicians after a baseline evaluation of a new patient. First, it is possible that SARP III volunteers with large values of tdFEV1 had incomplete medication adherence at baseline, allowing the parenteral injection to improve lung function 18 ± 3 days later. In this regard, participation in a cohort study may have passively increased their use of maintenance therapies and blunted any apparent decline in FEV1. For a patient with a baseline FEV1 of 80% predicted and a tdFEV1 of 10%, these data suggest that the probability of improvement in lung function over the next 4 years is 0.45 and that the combined probability of avoiding mild- or severe-decline trajectories is 0.76 (Figure 4A and Table E3). This type of result could be instructive to clinicians in terms of refocusing efforts on patient education and adherence. Alternatively, it is possible that the lack of lung function improvement after a parenteral corticosteroid challenge identifies SARP III participants that have ongoing events related to airway remodeling that contribute to progressive reduction in expiratory airflow and/or VC. A similar patient with a baseline FEV1 of 80% predicted who instead has a tdFEV1 of −10% has a combined probability of further decline (mild or severe) of 0.62 and an individual probability of severe decline of 0.33 (Figure 4A and Table E3). This type of scenario could prompt more frequent assessment of clinical status and lung function. If physiologic decline is confirmed in the short term, these results could serve as an independent justification for change in the patient’s therapy, promoting agents that are not corticosteroids.
The observation that some patients lose lung function after parenteral corticosteroids is relatively unique, and several mechanisms can be postulated from these data. In the short term during the evaluation of tdFEV1, use of parenteral corticosteroids in some participants could contribute to increased concentrations of serum amyloid A and IL-8 and decreased resolution of granulocytic inflammation (44–48), although this was not observed in the subset of participants who contributed a sputum sample 18 ± 3 days after receiving parenteral triamcinolone (Table 2). There are also data to suggest that some patients with severe asthma have increased respiratory muscle weakness after receiving parenteral corticosteroids, which could contribute to a negative tdFEV1 (49, 50). In addition, there are several mechanisms of airway remodeling that are relatively resistant to corticosteroids. Airway myofibroblasts are more common in patients with severe asthma (51) and contribute to excessive production of collagen and other extracellular matrix proteins that would likely not be metabolically cleared after parenteral-steroid injection. Mast cells are also more common in severe asthma, are relatively resistant to corticosteroids, and produce several mediators with bronchoconstrictive and/or remodeling functions (52). Interestingly, our subset analysis showed that participants with severe decline had the highest percentages of sputum eosinophils. Eosinophilic inflammation is a central component of the recently described mucus-plug phenotype (26), and it is possible that these mucus plugs are also relatively resistant to corticosteroids. Hastie and colleagues showed that a phenotype of high sputum neutrophils and eosinophils is associated with worse asthma outcomes (53). In this case, the increased representation of neutrophils may define a group in whom corticosteroid signaling may be proinflammatory (54), thus causing this group to be subject to a greater reduction in FEV1 over time. In both the severe-decline group and the improvement group, the changes in FEV1 were strongly associated with changes in the FVC, suggesting peripheral airway dysfunction with premature airway closure as the component driving the changes.
A limitation of the current study is that use of FEV1-slope categories introduces the possibility of misclassification error and reduces statistical power. Our use of more than two categories reduces the magnitude of any misclassification error. Simulation models suggest that the worst case is a 35% error (not shown) but that only 5% of participants have assignments to noncontiguous groups (e.g., severe decline during observed classification but no change in the simulated assignment). That the results of a multivariable linear mixed-effect model are similar to the ordinal-regression model (Table E4 and Figure 4B, respectively) suggests that misclassification is not a major confounder and that we have not overlooked major contributions from other clinically available predictors.
In summary, in a large cohort of participants with moderate-to-severe asthma, we have shown that most adults with established asthma on standard maintenance therapies do not continue to have accelerated lung function loss, even in the absence of biologics. However, in the 20% of patients who do have a decline, that group has worse asthma control, more exacerbations, and a worse ASSESS score (Table 3). Finally, using a unique biomarker, airway response to parenteral corticosteroids, we were able to identify individuals most likely to experience a decline in airway function. The ability to identify that group prospectively will allow us to examine the pathobiology of progressive loss of airway function and perhaps identify interventions to prevent permanent disability.
Supplementary Material
Acknowledgments
Acknowledgment
The authors thank the study participants, the SARP III clinical research coordinators, and the data coordinating center. Spirometers were provided for SARP III by nSpire Health, Inc.
Footnotes
Supported by the following NHLBI grants to the SARP III (Severe Asthma Research Program III) principal investigators, clinical centers, and data coordinating center: U10 HL109164 (E.R.B., W.C.M., and D.A.M.), U10 HL109257 (M.C.), U10 HL109250 (S.C.E.), U10 HL109146 (J.V.F.), U10 HL109250 (B.G.), U10 HL109172 (B.D.L. and E.I.), U10 HL109168 (N.N.J.), U10 HL109250 (W.G.T.), U10 HL109152 (S.E.W.), and U10 HL109086-04 (D.T.M.). In addition, this program is supported through the following NIH National Center for Advancing Translational Sciences awards: UL1 TR001420 (Wake Forest University), UL1 TR000427 (University of Wisconsin), UL1 TR001102 (Harvard University), and UL1 TR000454 (Emory University). Support for visits occurring beyond the third year of follow-up came from the following partnerships. AstraZeneca supported visits at the Brigham and Women’s Hospital, Boston Children’s Hospital, University of Wisconsin, University of Virginia, and Case Western Reserve University–Rainbow Babies and Children’s Hospital. Boehringer-Ingelheim supported visits at the University of Pittsburgh and University of California, San Francisco. Genentech supported visits at the Wake Forest School of Medicine. GlaxoSmithKline supported core activities occurring at Pennsylvania State University and the Wake Forest School of Medicine. Sanofi–Genzyme–Regeneron supported visits at Washington University and Emory University. The Cleveland Clinic did not participate in these industry partnerships and used internal funds for visits after the third year. Finally, this work was supported in part by funds provided by the William W. and Judith H. Busse Endowed Professorship in Allergy and Asthma Research (L.C.D.).
Author Contributions: Design: L.C.D., B.R.P., R.L.S., E.R.B., M.C., M.D.D., A.M.F., A.T.H., J.M.G., W.C.M., M.C.P., S.P.P., W.P., N.N.J., D.T.M., and E.I. Data acquisition: L.C.D., B.R.P., R.L.S., E.R.B., M.C., M.D.D., A.M.F., A.T.H., J.M.G., W.C.M., M.C.P., S.P.P., W.P., J.C.C., S.C.E., J.V.F., M.L.F., B.G., B.D.L., D.A.M., K.R., W.G.T., S.E.W., P.G.W., J.Z., N.N.J., D.T.M., and E.I. Analysis: L.C.D., B.R.P., R.L.S., D.T.M., and E.I. Interpretation: L.C.D., B.R.P., R.L.S., E.R.B., M.C., M.D.D., A.M.F., A.T.H., J.M.G., W.C.M., M.C.P., S.P.P., W.P., J.C.C., S.C.E., J.V.F., M.L.F., B.G., B.D.L., D.A.M., K.R., W.G.T., S.E.W., P.G.W., J.Z., N.N.J., D.T.M., and E.I.
This article has an online supplement, which is accessible from this issue’s table of contents at www.atsjournals.org.
Originally Published in Press as DOI: 10.1164/rccm.202002-0454OC on December 8, 2020
Author disclosures are available with the text of this article at www.atsjournals.org.
Contributor Information
Collaborators: for the NHLBI SARP III Investigators
References
- 1.Peat JK, Woolcock AJ, Cullen K. Rate of decline of lung function in subjects with asthma. Eur J Respir Dis. 1987;70:171–179. [PubMed] [Google Scholar]
- 2.Van Schayck CP, Dompeling E, Van Herwaarden CL, Wever AM, Van Weel C. Interacting effects of atopy and bronchial hyperresponsiveness on the annual decline in lung function and the exacerbation rate in asthma. Am Rev Respir Dis. 1991;144:1297–1301. doi: 10.1164/ajrccm/144.6.1297. [DOI] [PubMed] [Google Scholar]
- 3.Vonk JM, Jongepier H, Panhuysen CI, Schouten JP, Bleecker ER, Postma DS. Risk factors associated with the presence of irreversible airflow limitation and reduced transfer coefficient in patients with asthma after 26 years of follow up. Thorax. 2003;58:322–327. doi: 10.1136/thorax.58.4.322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Lange P, Scharling H, Ulrik CS, Vestbo J. Inhaled corticosteroids and decline of lung function in community residents with asthma. Thorax. 2006;61:100–104. doi: 10.1136/thx.2004.037978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Haahtela T, Järvinen M, Kava T, Kiviranta K, Koskinen S, Lehtonen K, et al. Comparison of a beta 2-agonist, terbutaline, with an inhaled corticosteroid, budesonide, in newly detected asthma. N Engl J Med. 1991;325:388–392. doi: 10.1056/NEJM199108083250603. [DOI] [PubMed] [Google Scholar]
- 6.Pauwels RA, Pedersen S, Busse WW, Tan WC, Chen YZ, Ohlsson SV, et al. START Investigators Group. Early intervention with budesonide in mild persistent asthma: a randomised, double-blind trial. Lancet. 2003;361:1071–1076. doi: 10.1016/S0140-6736(03)12891-7. [DOI] [PubMed] [Google Scholar]
- 7.Laitinen LA, Laitinen A, Haahtela T. A comparative study of the effects of an inhaled corticosteroid, budesonide, and a beta 2-agonist, terbutaline, on airway inflammation in newly diagnosed asthma: a randomized, double-blind, parallel-group controlled trial. J Allergy Clin Immunol. 1992;90:32–42. doi: 10.1016/s0091-6749(06)80008-4. [DOI] [PubMed] [Google Scholar]
- 8.Grol MH, Gerritsen J, Vonk JM, Schouten JP, Koëter GH, Rijcken B, et al. Risk factors for growth and decline of lung function in asthmatic individuals up to age 42 years: a 30-year follow-up study. Am J Respir Crit Care Med. 1999;160:1830–1837. doi: 10.1164/ajrccm.160.6.9812100. [DOI] [PubMed] [Google Scholar]
- 9.Bisgaard H, Jensen SM, Bønnelykke K. Interaction between asthma and lung function growth in early life. Am J Respir Crit Care Med. 2012;185:1183–1189. doi: 10.1164/rccm.201110-1922OC. [DOI] [PubMed] [Google Scholar]
- 10.Sears MR, Greene JM, Willan AR, Wiecek EM, Taylor DR, Flannery EM, et al. A longitudinal, population-based, cohort study of childhood asthma followed to adulthood. N Engl J Med. 2003;349:1414–1422. doi: 10.1056/NEJMoa022363. [DOI] [PubMed] [Google Scholar]
- 11.Stern DA, Morgan WJ, Halonen M, Wright AL, Martinez FD. Wheezing and bronchial hyper-responsiveness in early childhood as predictors of newly diagnosed asthma in early adulthood: a longitudinal birth-cohort study. Lancet. 2008;372:1058–1064. doi: 10.1016/S0140-6736(08)61447-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Tan DJ, Walters EH, Perret JL, Burgess JA, Johns DP, Lowe AJ, et al. Clinical and functional differences between early-onset and late-onset adult asthma: a population-based Tasmanian Longitudinal Health Study. Thorax. 2016;71:981–987. doi: 10.1136/thoraxjnl-2015-208183. [DOI] [PubMed] [Google Scholar]
- 13.Hancox RJ, Pavord ID, Sears MR. Associations between blood eosinophils and decline in lung function among adults with and without asthma. Eur Respir J. 2018;51:1702536. doi: 10.1183/13993003.02536-2017. [DOI] [PubMed] [Google Scholar]
- 14.McGeachie MJ, Yates KP, Zhou X, Guo F, Sternberg AL, Van Natta ML, et al. Patterns of growth and decline in lung function in persistent childhood asthma. N Engl J Med. 2016;374:1842–1852. doi: 10.1056/NEJMoa1513737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Li X, Hawkins GA, Ampleford EJ, Moore WC, Li H, Hastie AT, et al. Genome-wide association study identifies TH1 pathway genes associated with lung function in asthmatic patients. J Allergy Clin Immunol. 2013;132:313–320, e15. doi: 10.1016/j.jaci.2013.01.051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ulrik CS, Lange P. Decline of lung function in adults with bronchial asthma. Am J Respir Crit Care Med. 1994;150:629–634. doi: 10.1164/ajrccm.150.3.8087330. [DOI] [PubMed] [Google Scholar]
- 17.Bai TR, Vonk JM, Postma DS, Boezen HM. Severe exacerbations predict excess lung function decline in asthma. Eur Respir J. 2007;30:452–456. doi: 10.1183/09031936.00165106. [DOI] [PubMed] [Google Scholar]
- 18.O’Byrne PM, Pedersen S, Busse WW, Tan WC, Chen YZ, Ohlsson SV, et al. START Investigators Group. Effects of early intervention with inhaled budesonide on lung function in newly diagnosed asthma. Chest. 2006;129:1478–1485. doi: 10.1378/chest.129.6.1478. [DOI] [PubMed] [Google Scholar]
- 19.O’Byrne PM, Pedersen S, Lamm CJ, Tan WC, Busse WW START Investigators Group. Severe exacerbations and decline in lung function in asthma. Am J Respir Crit Care Med. 2009;179:19–24. doi: 10.1164/rccm.200807-1126OC. [DOI] [PubMed] [Google Scholar]
- 20.Coumou H, Westerhof GA, de Nijs SB, Zwinderman AH, Bel EH. Predictors of accelerated decline in lung function in adult-onset asthma. Eur Respir J. 2018;51:1701785. doi: 10.1183/13993003.01785-2017. [DOI] [PubMed] [Google Scholar]
- 21.Matsunaga K, Akamatsu K, Miyatake A, Ichinose M. Natural history and risk factors of obstructive changes over a 10-year period in severe asthma. Respir Med. 2013;107:355–360. doi: 10.1016/j.rmed.2012.11.014. [DOI] [PubMed] [Google Scholar]
- 22.Sorkness RL, Kienert C, O’Brien MJ, Fain SB, Jarjour NN. Compressive air trapping in asthma: effects of age, sex, and severity. J Appl Physiol (1985) 2019;126:1265–1271. doi: 10.1152/japplphysiol.00924.2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Newby C, Agbetile J, Hargadon B, Monteiro W, Green R, Pavord I, et al. Lung function decline and variable airway inflammatory pattern: longitudinal analysis of severe asthma. J Allergy Clin Immunol. 2014;134:287–294. doi: 10.1016/j.jaci.2014.04.005. [DOI] [PubMed] [Google Scholar]
- 24.Calhoun WJ, Haselkorn T, Miller DP, Omachi TA. Asthma exacerbations and lung function in patients with severe or difficult-to-treat asthma. J Allergy Clin Immunol. 2015;136:1125–1127, e4. doi: 10.1016/j.jaci.2015.05.014. [DOI] [PubMed] [Google Scholar]
- 25.Aysola RS, Hoffman EA, Gierada D, Wenzel S, Cook-Granroth J, Tarsi J, et al. Airway remodeling measured by multidetector CT is increased in severe asthma and correlates with pathology. Chest. 2008;134:1183–1191. doi: 10.1378/chest.07-2779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Dunican EM, Elicker BM, Gierada DS, Nagle SK, Schiebler ML, Newell JD, et al. NHLBI Severe Asthma Research Program. Mucus plugs in patients with asthma linked to eosinophilia and airflow obstruction. J Clin Invest. 2018;128:997–1009. doi: 10.1172/JCI95693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Goleva E, Hauk PJ, Boguniewicz J, Martin RJ, Leung DY. Airway remodeling and lack of bronchodilator response in steroid-resistant asthma. J Allergy Clin Immunol. 2007;120:1065–1072. doi: 10.1016/j.jaci.2007.07.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Sorkness RL, Bleecker ER, Busse WW, Calhoun WJ, Castro M, Chung KF, et al. NHLBI Severe Asthma Research Program. Lung function in adults with stable but severe asthma: air trapping and incomplete reversal of obstruction with bronchodilation. J Appl Physiol (1985) 2008;104:394–403. doi: 10.1152/japplphysiol.00329.2007. [DOI] [PubMed] [Google Scholar]
- 29.Sorkness RL, Teague WG, Penugonda M, Fitzpatrick AM NIH, NHLBI Severe Asthma Research Program. Sex dependence of airflow limitation and air trapping in children with severe asthma. J Allergy Clin Immunol. 2011;127:1073–1074. doi: 10.1016/j.jaci.2010.12.1079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Schwartz HJ, Lowell FC, Melby JC. Steroid resistance in bronchial asthma. Ann Intern Med. 1968;69:493–499. doi: 10.7326/0003-4819-69-3-493. [DOI] [PubMed] [Google Scholar]
- 31.ten Brinke A, Zwinderman AH, Sterk PJ, Rabe KF, Bel EH. “Refractory” eosinophilic airway inflammation in severe asthma: effect of parenteral corticosteroids. Am J Respir Crit Care Med. 2004;170:601–605. doi: 10.1164/rccm.200404-440OC. [DOI] [PubMed] [Google Scholar]
- 32.Carmichael J, Paterson IC, Diaz P, Crompton GK, Kay AB, Grant IW. Corticosteroid resistance in chronic asthma. Br Med J (Clin Res Ed) 1981;282:1419–1422. doi: 10.1136/bmj.282.6274.1419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Leung DY, Martin RJ, Szefler SJ, Sher ER, Ying S, Kay AB, et al. Dysregulation of interleukin 4, interleukin 5, and interferon gamma gene expression in steroid-resistant asthma. J Exp Med. 1995;181:33–40. doi: 10.1084/jem.181.1.33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Teague WG, Phillips BR, Fahy JV, Wenzel SE, Fitzpatrick AM, Moore WC, et al. Baseline features of the Severe Asthma Research Program (SARP III) cohort: differences with age. J Allergy Clin Immunol Pract. 2018;6:545–554, e4. doi: 10.1016/j.jaip.2017.05.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Phipatanakul W, Mauger DT, Sorkness RL, Gaffin JM, Holguin F, Woodruff PG, et al. Severe Asthma Research Program Effects of age and disease severity on systemic corticosteroid responses in asthma Am J Respir Crit Care Med 20171951439–1448.[Published erratum appears in Am J Respir Crit Care Med 197:970–971.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Denlinger LC, Phillips BR, Sorkness RL, Bleecker ER, Castro M, Fitzpatrick AM, et al. Insensitivity of corticosteroids predicts longitudinal loss of lung function in the NHLBI Severe Asthma Research Program (SARP III) [abstract] Am J Respir Crit Care Med. 2020;201:A2680. [Google Scholar]
- 37.Fitzpatrick AM, Szefler SJ, Mauger DT, Phillips BR, Denlinger LC, Moore WC, et al. Development and initial validation of the Asthma Severity Scoring System (ASSESS) J Allergy Clin Immunol. 2020;145:127–139. doi: 10.1016/j.jaci.2019.09.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Quanjer PH, Stanojevic S, Cole TJ, Baur X, Hall GL, Culver BH, et al. ERS Global Lung Function Initiative. Multi-ethnic reference values for spirometry for the 3–95-yr age range: the global lung function 2012 equations. Eur Respir J. 2012;40:1324–1343. doi: 10.1183/09031936.00080312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Sorkness RL, Zoratti EM, Kattan M, Gergen PJ, Evans MD, Visness CM, et al. Obstruction phenotype as a predictor of asthma severity and instability in children. J Allergy Clin Immunol. 2018;142:1090–1099, e4. doi: 10.1016/j.jaci.2017.09.047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Kelly VJ, Sands SA, Harris RS, Venegas JG, Brown NJ, Stuart-Andrews CR, et al. Respiratory system reactance is an independent determinant of asthma control. J Appl Physiol (1985) 2013;115:1360–1369. doi: 10.1152/japplphysiol.00093.2013. [DOI] [PubMed] [Google Scholar]
- 41.Hartley RA, Barker BL, Newby C, Pakkal M, Baldi S, Kajekar R, et al. Relationship between lung function and quantitative computed tomographic parameters of airway remodeling, air trapping, and emphysema in patients with asthma and chronic obstructive pulmonary disease: a single-center study. J Allergy Clin Immunol. 2016;137:1413–1422, e12. doi: 10.1016/j.jaci.2016.02.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Mead J, Turner JM, Macklem PT, Little JB. Significance of the relationship between lung recoil and maximum expiratory flow. J Appl Physiol. 1967;22:95–108. doi: 10.1152/jappl.1967.22.1.95. [DOI] [PubMed] [Google Scholar]
- 43.Gelb AF, Yamamoto A, Verbeken EK, Schein MJ, Moridzadeh R, Tran D, et al. Further studies of unsuspected emphysema in nonsmoking patients with asthma with persistent expiratory airflow obstruction. Chest. 2018;153:618–629. doi: 10.1016/j.chest.2017.11.016. [DOI] [PubMed] [Google Scholar]
- 44.Jensen LE, Whitehead AS. Regulation of serum amyloid A protein expression during the acute-phase response. Biochem J. 1998;334:489–503. doi: 10.1042/bj3340489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Su Q, Weindl G. Glucocorticoids and Toll-like receptor 2 cooperatively induce acute-phase serum amyloid A. Pharmacol Res. 2018;128:145–152. doi: 10.1016/j.phrs.2017.09.012. [DOI] [PubMed] [Google Scholar]
- 46.Lu H, Lin XS, Yao DM, Zhuang YY, Wen GF, Shi J, et al. Increased serum amyloid A in nasal polyps is associated with systemic corticosteroid insensitivity in patients with chronic rhinosinusitis with nasal polyps: a pilot study. Eur Arch Otorhinolaryngol. 2018;275:401–408. doi: 10.1007/s00405-017-4809-z. [DOI] [PubMed] [Google Scholar]
- 47.Ather JL, Fortner KA, Budd RC, Anathy V, Poynter ME. Serum amyloid A inhibits dendritic cell apoptosis to induce glucocorticoid resistance in CD4+ T cells. Cell Death Dis. 2013;4:e786. doi: 10.1038/cddis.2013.327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Ricklefs I, Barkas I, Duvall MG, Cernadas M, Grossman NL, Israel E, et al. NHLBI Severe Asthma Research Program-3 Investigators. ALX receptor ligands define a biochemical endotype for severe asthma. JCI Insight. 2017;2:e93534. doi: 10.1172/jci.insight.93534. [Published erratum appears in JCI Insight 3:e120932.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Bowyer SL, LaMothe MP, Hollister JR. Steroid myopathy: incidence and detection in a population with asthma. J Allergy Clin Immunol. 1985;76:234–242. doi: 10.1016/0091-6749(85)90708-0. [DOI] [PubMed] [Google Scholar]
- 50.Picado C, Fiz JA, Montserrat JM, Grau JM, Fernandez-Sola J, Luengo MT, et al. Respiratory and skeletal muscle function in steroid-dependent bronchial asthma. Am Rev Respir Dis. 1990;141:14–20. doi: 10.1164/ajrccm/141.1.14. [DOI] [PubMed] [Google Scholar]
- 51.Benayoun L, Druilhe A, Dombret MC, Aubier M, Pretolani M. Airway structural alterations selectively associated with severe asthma. Am J Respir Crit Care Med. 2003;167:1360–1368. doi: 10.1164/rccm.200209-1030OC. [DOI] [PubMed] [Google Scholar]
- 52.Balzar S, Fajt ML, Comhair SA, Erzurum SC, Bleecker E, Busse WW, et al. Mast cell phenotype, location, and activation in severe asthma: data from the Severe Asthma Research Program. Am J Respir Crit Care Med. 2011;183:299–309. doi: 10.1164/rccm.201002-0295OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Hastie AT, Moore WC, Meyers DA, Vestal PL, Li H, Peters SP, et al. National Heart, Lung, and Blood Institute Severe Asthma Research Program. Analyses of asthma severity phenotypes and inflammatory proteins in subjects stratified by sputum granulocytes. J Allergy Clin Immunol. 2010;125:1028–1036, e13. doi: 10.1016/j.jaci.2010.02.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Lachowicz-Scroggins ME, Dunican EM, Charbit AR, Raymond W, Looney MR, Peters MC, et al. Extracellular DNA, neutrophil extracellular traps, and inflammasome activation in severe asthma. Am J Respir Crit Care Med. 2019;199:1076–1085. doi: 10.1164/rccm.201810-1869OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


