Abstract
Rationale: Rhinovirus (RV) C can cause asymptomatic infection and respiratory illnesses ranging from the common cold to severe wheezing.
Objectives: To identify how age and other individual-level factors are associated with susceptibility to RV-C illnesses.
Methods: Longitudinal data from the COAST (Childhood Origins of Asthma) birth cohort study were analyzed to determine relationships between age and RV-C infections. Neutralizing antibodies specific for RV-A and RV-C (three types each) were determined using a novel PCR-based assay. Data were pooled from 14 study cohorts in the United States, Finland, and Australia, and mixed-effects logistic regression was used to identify factors related to the proportion of RV-C versus RV-A detection.
Measurements and Main Results: In COAST, RV-A and RV-C infections were similarly common in infancy, whereas RV-C was detected much less often than RV-A during both respiratory illnesses and scheduled surveillance visits (P < 0.001, χ2) in older children. The prevalence of neutralizing antibodies to RV-A or RV-C types was low (5–27%) at the age of 2 years, but by the age of 16 years, RV-C seropositivity was more prevalent (78% vs. 18% for RV-A; P < 0.0001). In the pooled analysis, the RV-C to RV-A detection ratio during illnesses was significantly related to age (P < 0.0001), CDHR3 genotype (P < 0.05), and wheezing illnesses (P < 0.05). Furthermore, certain RV types (e.g., C2, C11, A78, and A12) were consistently more virulent and prevalent over time.
Conclusions: Knowledge of prevalent RV types, antibody responses, and populations at risk based on age and genetics may guide the development of vaccines or other novel therapies against this important respiratory pathogen.
Keywords: rhinovirus, genetics, wheezing, CDHR3, epidemiology
At a Glance Commentary
Scientific Knowledge on the Subject
There are more than 160 rhinovirus types in three species, and a greater understanding of at-risk populations and the relative importance of rhinovirus types and species in causing illness would help to direct the development of specific treatments such as vaccines.
What This Study Adds to the Field
Young children with genetic predisposition are at high risk for rhinovirus C illnesses, which are closely associated with wheezing. Rhinovirus C is very immunogenic, and the identification of types that are both common and virulent should help to facilitate development of a vaccine for this clinically important respiratory pathogen.
Rhinoviruses (RVs) are common causes of upper respiratory tract illnesses and exacerbations of chronic respiratory diseases, such as asthma and chronic obstructive pulmonary disease (1). In young children, RV infections also cause lower respiratory illnesses that can require hospitalization and even intensive care (2). There are three species of RVs (A, B, and C) based on genetic homology that are further subdivided into more than 160 types (3). Any RV can cause mild or asymptomatic respiratory illness; however, RV-B is less likely to cause severe illnesses or exacerbations of asthma (4, 5). Infections with RV-A and RV-C are common during childhood, but the relative contribution of these viruses to severe respiratory illnesses is controversial. Some studies have shown that RV-C infections are more likely than RV-A infections to cause wheezing illnesses and asthma exacerbations in children (2, 6–8), but other studies have found no species-specific associations (5, 9–11).
Several factors modify susceptibility to RV-C infections. A coding polymorphism (rs6967330) in the gene for CDHR3, which serves as the RV-C receptor, is associated with greater expression of this protein on the surface of cells and increased susceptibility to both RV-C illnesses and childhood asthma (12, 13). In addition, there is evidence that age might also affect the relative frequency of RV-A versus RV-C infections and illnesses. For example, RV-C was detected more often than RV-A in some studies of young children with respiratory illnesses (14–16), whereas some studies in adults have reported the opposite (17–19). The reasons for these observations are not fully understood but could be related to a greater naturally acquired immunity to RV-C over time. Infections with RV induce type-specific antibody responses that reduce the risk of reinfections. Natural infections with RV-A and RV-B induce neutralizing antibody (nAb) responses that protect against reinfection and persist for at least 2–4 years (20–24). NAb responses to RV-C have not previously been analyzed because of the lack of a suitable in vitro system to assay infectivity.
The primary goals of this study were to test the hypothesis that increasing age is associated with reduced frequency and severity of RV-C infections and illnesses and to determine whether age-related reductions in RV-C illness frequency correspond with increased nAb responses to RV-C infections. We also sought to identify other personal factors that are associated with RV species susceptibility and to describe relationships among RV species, type, and illness. To accomplish these objectives, we analyzed longitudinal sets of nasal and plasma samples from children from birth to age 18–19 years in the COAST (Childhood Origins of Asthma) birth cohort, which included high-risk children born to parents with allergy and/or asthma (25). In addition, we conducted a multicenter study by pooling data from 14 different studies across numerous cities located in the United States, Finland, and Australia. Some of the results of these studies have been previously reported in the form of an abstract (26).
Methods
Study Subjects and Study Design
COAST birth cohort study
A total of 289 children from the Madison, Wisconsin, area were enrolled in the COAST study at birth, 259 were followed prospectively to age 6 years, 210 were followed to age 18–19 years, and additional children with asthma were enrolled more recently (25, 27). Families were asked to contact the study center each time the child had respiratory symptoms to enable the collection of a nasal mucus sample for viral diagnostics. Nasal samples were also collected at scheduled study visits (2, 4, 6, 9, 12, 18, and 24 mo and then annually), and respiratory illness symptoms were recorded if present. Plasma samples were collected during these annual visits.
Studies included in the pooled analysis
Investigators from 14 different cohort studies that had collected nasal mucus specimens from children during periods of illness and/or health (see Table E1 in the online supplement) contributed data to the pooled analysis. Five of these cohorts are participating in the ECHO (Environmental influences on Child Health Outcomes) consortium. For each of these cohorts, real-time PCR and partial sequencing was used to identify RV species and type as previously described (28, 29). Each of these studies was approved by the local human research ethics committees, and participants provided informed consent. In addition to the viral diagnostics, 11 additional variables were included in the analysis. All cohorts had data on age, sex, race, and season of collection; nine cohorts provided data on aeroallergen sensitization (skin test or specific IgE measurement), asthma (parent report of asthma diagnosed by a healthcare provider), number of older siblings, history of breastfeeding, exposure to daycare, and illness type; and 6 of the 14 cohorts provided genotypes for the CDHR3 variant rs6967330. These variables were harmonized for the pooled analysis (see online supplement).
RV Neutralization Assay
RV-A16, RV-A36, RV-C2, RV-C15 and RV-C41 were clinical isolates cloned in plasmid vectors and produced by reverse genetics methods in WisL cells (human embryonic lung fibroblasts) (30–32); RV-A7 was isolated from nasal secretions and propagated in HeLa cells (31). HeLa-E8–adapted variants of RV-C isolates (33), possessing the K41 mutation in 3A protein, were used for optimal replication in this cell line.
Neutralizing antibodies specific for RV-A and RV-C types were measured using a novel quantitative PCR (qPCR)–based assay. Briefly, RV isolates were preincubated with serial twofold dilutions of plasma samples, and this mixture was used to inoculate HeLa-E8 cells (34) that were engineered to express CDHR3 (RV-C receptor). Viral replication (progeny yields at 72 h after infection) was measured by qPCR. Neutralizing antibody titers (half-maximal inhibitory concentration [IC50]) were calculated by sigmoidal dose–response nonlinear fit analysis of virus replication curves. For the qualitative assay to identify the presence or absence of nAbs, the same infection procedure was used except that the number of plasma serial dilutions was limited to three (twofold to eightfold) (see online supplement for additional details).
Statistical Analyses
Mixed-effects logistic regression was used to estimate the odds of infection with RV-C alone to infection with RV-A as a function of age (modeled using a natural cubic spline with 2 degrees of freedom). The model included subject as a random effect and age, race, and cohort as fixed effects.
Mixed-effects logistic regression models were used to assess the effects of other covariates (asthma history, allergy history, daycare exposure, number of older siblings, breastfeeding history, race, season of collection, CDHR3 rs6967330 asthma risk genotype, and illness type [Table E2]) on the RV-C to RV-A ratio. Additional details are provided in the online supplement.
Results
RV-A versus RV-C Infections and Illnesses in the COAST Study
In the COAST birth cohort study, we detected and typed RV clinical isolates in surveillance nasal samples collected during scheduled clinic visits (with or without respiratory symptoms) and in samples obtained during times of acute respiratory illnesses (symptom score of ≥5 [35]) during the following four age intervals: ages 0–3 years, 4–8 years, 9–13 years, and 14–18 years. Partial sequence analysis confirmed detection of approximately 94% and 98% of known RV-A and RV-C types, respectively. Although RV-A and RV-C infections and illnesses were similarly common in the first 3 years of life, in older children, RV-C was detected significantly less often than RV-A during both respiratory illnesses and well visits (P < 0.001, χ2; Figure 1).
Figure 1.
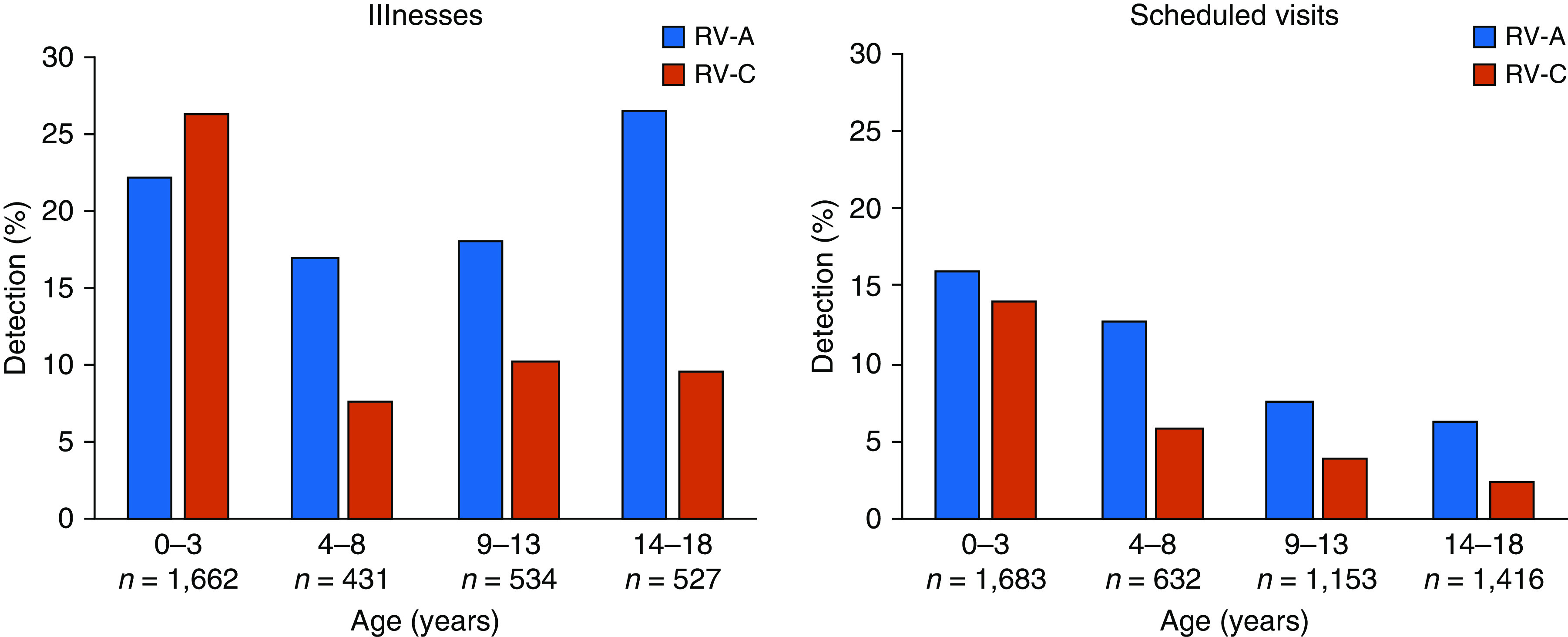
Relationship of age to frequency of rhinovirus (RV) C and RV-A detection during illnesses in the COAST (Childhood Origins of Asthma) birth cohort. RV types and species (A, B, and C) were determined in nasal samples by partial sequencing. Detection rates (%) of RV-A and RV-C at different ages are shown as bar plots. Odds ratios (ORs) refer to the odds of RV-C relative to RV-A at older ages compared with younger ages. Groups were compared using Fisher’s exact test. P values of <0.05 were considered to indicate statistical significance. Illnesses: OR = 0.31; 95% confidence interval (CI), 0.21–0.45); P < 0.0001. Scheduled visits: OR = 0.31; 95% CI, 0.14–0.64; P = 0.0006. N represents the number of samples analyzed for each age category.
RV-Neutralizing Antibody Responses
Both RV-A and RV-C isolates can infect transduced HeLa-E8 cells, which naturally express ICAM-1 and are engineered to express CDHR3 (33). Because of the absence of cytopathic effects (CPEs) after infection with “wild-type” RV-C clinical isolates in vitro, we developed a novel qPCR-based RV neutralization assay in HeLa-E8 cells. We then validated the assay by testing preinfection and postinfection plasma samples from an RV-A16 experimental inoculation study (36). The nAb titers to RV-A16 determined by the assay correlated well (rs = 0.83; P = 0.006) with those obtained by the traditional infectivity assay (endpoint dilution) that is based on CPEs in WI-38 cells (Figure E1).
We next analyzed nAb responses to RV-A and RV-C types in plasma samples from three age groups (2, 10, and 16 yr, same subjects) of COAST study participants (n = 20) by qualitative nAb assay. We selected three clinical isolates representing RV-A and RV-C species to use in the neutralization assay. Two isolates from each species were phylogenetically related (A7 and A36; C15 and C41), and the remaining ones (A16 and C2) were more distant from the first two isolates (Figure E2). The results demonstrated marked species-specific differences in the development of nAb responses (Figure 2). Although nAbs to these RV-A and RV-C types were uncommon at the age of 2 years (5–27%), by the ages of 10 and 16 years, RV-C seropositivity was 70% and 78% compared with only 25% and 18% for RV-A, respectively (P < 0.0001). RV-C–specific nAbs were significantly more prevalent compared with nAbs for RV-A in each of the tested age groups (odds ratio [OR], 3.9; 95% confidence interval [CI], 1.6–9.5 at age 2; OR, 4.0; 95% CI, 2.0–7.7 at age 10; and OR, 7.1; 95% CI, 3.8–13.3 at age 16). There was no correlation between the detection frequency of the selected RV-A or RV-C types and the total number of positive nAb responses to them (Figure E3).
Figure 2.
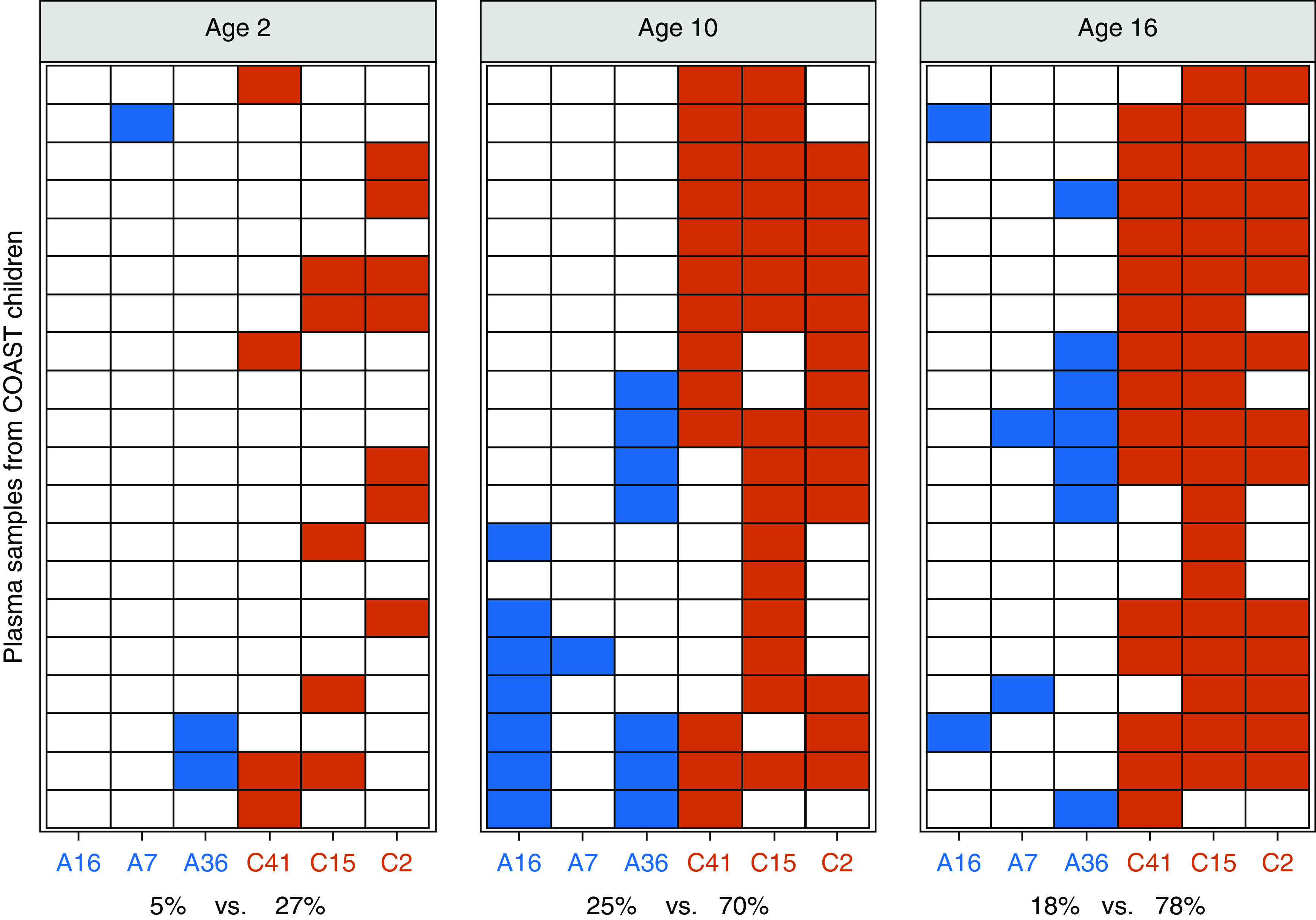
Neutralizing antibody (nAb) responses to selected rhinovirus (RV) A and RV-C types in the COAST (Childhood Origins of Asthma) study children. Heatmaps show the presence of nAbs to indicated RV-A (blue) and RV-C (red) types in plasma (ages 2, 10, and 16 yr; each row represents serial sampling from the same subjects) from COAST study participants (n = 20). Odds ratios (ORs) refer to odds of finding nAbs to RV-C relative to RV-A at different ages. Age 2: OR = 3.9; 95% confidence interval (CI), 1.6–9.5; age 10: OR = 4.0; 95% CI, 2.0–7.7; and age 16: OR = 7.1; 95% CI, 3.8–13.3. The differences in nAb responses to RV-A versus RV-C were highly significant at all ages tested. (P < 0.0001, generalized estimating equation logistic regression). A7 = RV-A7; A16 = RV-A16; A36 = RV-A36; C2 = RV-C2; C15 = RV-C15; C41 = RV-C41.
NAb detected at one time point was very likely to persist from one age to the next (Figure 2; P < 10−7, generalized estimating equation logistic regression model). For RV-A types, positive antibodies persisted from the age of 2 years to the age of 10 years in 67% (2/3) of children and from the age of 10 years to the age of 16 years in 40% (6/15) children. For RV-C types, the corresponding numbers were 94% (15/16) and 90% (38/42), respectively. RV species was not significantly associated with persistence of antibody (P = 0.58, Wald test for interaction), although this analysis was underpowered because of the low number of positive results for RV-A types.
Multicenter Analysis of RV-A and RV-C Infections
Results of viral diagnostics
The 14 study cohorts collected 17,664 samples of nasal mucus from study participants and detected at least one RV in 10,185 samples (Table 1). Of the 10,185 pooled samples, 6,643 were collected during illnesses, and 3,542 were collected during periods of health (e.g., at well-child or routine study visits). Similar numbers of specimens tested positive for RV-A and RV-C. RV-B was detected more often in samples from asymptomatic children compared with samples obtained during illness (857/3,542 [24%] vs. 589/6,643 [9%]; P < 0.001, two-proportion z-test).
Table 1.
Viruses Detected in RV-Positive Samples
| Sample Type |
|||
|---|---|---|---|
| Virus | All | Sick | Well |
| Solo RV-A | 4,379 | 2,890 | 1,489 |
| Solo RV-B | 1,197 | 453 | 744 |
| Solo RV-C | 4,235 | 3,067 | 1,168 |
| Mixed RV | 374 | 233 | 141 |
| Total | 10,185 | 6,643 | 3,542 |
Definition of abbreviation: RV = rhinovirus.
We detected 178 types, including several provisionally assigned types (designated “pat”), in the pooled analysis. The median detection rate for any given type was 0.45% (range, 0.0–2.7%). The most frequently detected RV types in illness samples were A78, C02, C11, A12, and A101 (in descending order), whereas 33 of the RV types were detected in <0.1% (6 or fewer) of illness samples (Table E3). The rate of detection for the frequently detected types was consistently high over the 22-year sampling period (Figure E4). In samples from asymptomatic children, B17, B6, B103, A21, and B91 were detected most frequently (in descending order) (Table E3). We also compared the frequency of types detected during illnesses with that of types detected during healthy periods (Figure 3). Twenty-two types (4 A and 18 C) were significantly more likely to be detected during illnesses, whereas 23 types (4 A, 18 B, and 1 C) were more often detected in healthy children.
Figure 3.
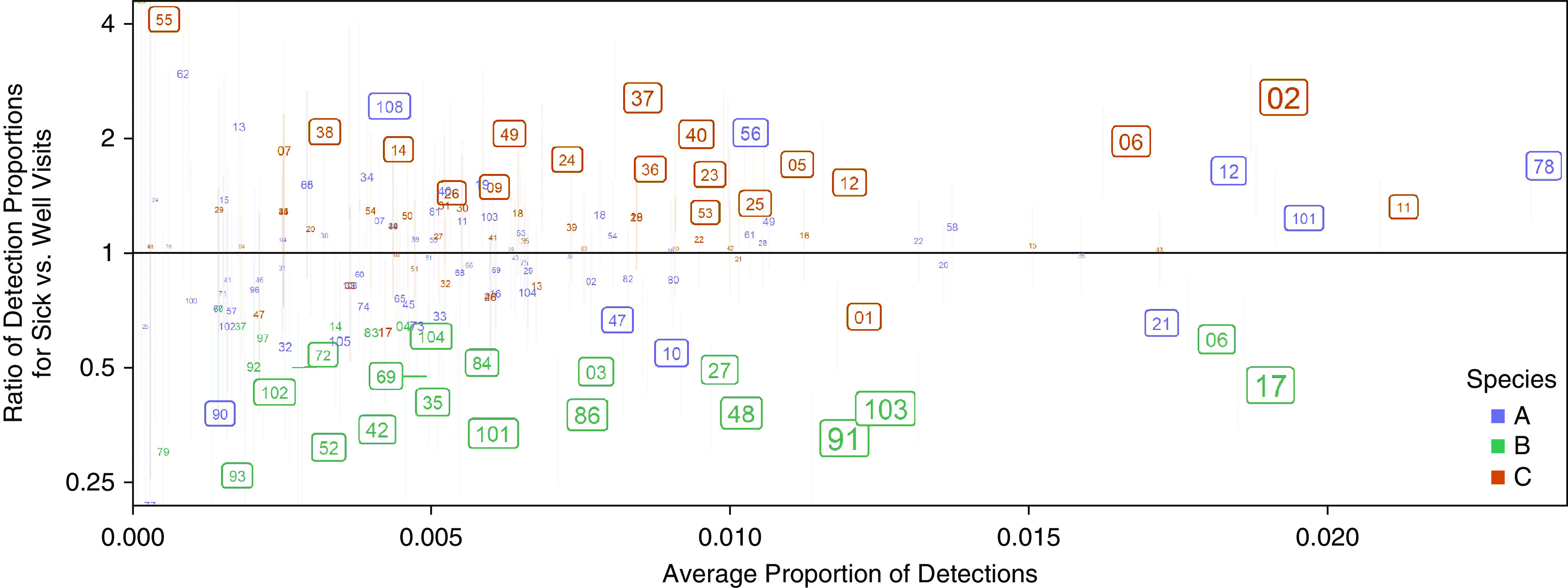
Frequency of detection for each rhinovirus type and ratio of detection proportions for sick versus well visits in the pooled dataset. The species is color coded, and individual types are numbered within each species. The size of the numbers is proportional to the negative logarithm of the P value, and numbers in boxes are significant at the 5% level (unadjusted for multiple comparisons). Error bars (vertical lines) represent 95% confidence intervals for the ratio of detection proportions. Horizontal lines point at error bars for numbers that were scattered from them in congested areas to avoid overlapping.
Analysis of factors related to RV-A and RV-C infections
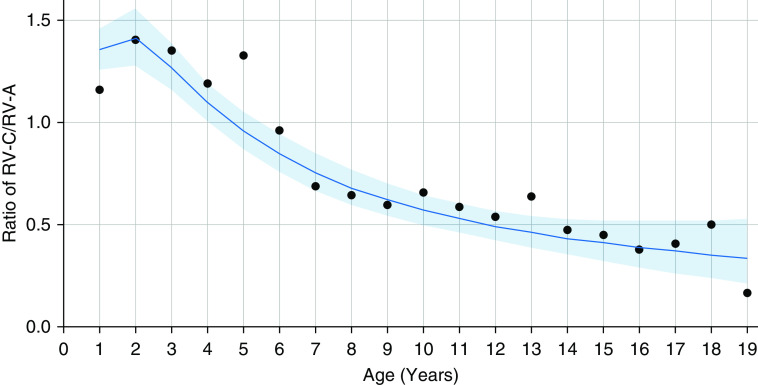
Consistent with findings in COAST, increasing age was strongly correlated with the ratio of RV-C to RV-A infections in the pooled data (Figures 4 and E5). RV-C infections were more prevalent in young children up until the age of approximately 5 years, followed by a steady decline in the ratio such that RV-A infections predominated in adolescents. To evaluate other potential predictors of the RV-C to RV-A ratio, we performed logistic regression on each of the covariates separately while adjusting for age and participant (some studies included multiple samples per participant). Because there was significant variety among the cohort studies in age and covariates measured, the analyses were performed in discrete age intervals (Figure 5). Two covariates were significant predictors of increased RV-C infections compared with RV-A infections. The CDHR3 rs6967330 asthma risk allele (A) significantly increased the risk for RV-C illness (Figure 6A). In addition, the RV-C to RV-A ratio was related to the presence of lower respiratory illness (LRI) with wheezing but not to LRI without wheezing (Figures 6B and 6C).
Figure 4.
Ratio of rhinovirus (RV) C to RV-A in illness samples from the pooled dataset with respect to age. Individual points represent the RV-C to RV-A detection ratio for each year of age. The line and shaded area represent the logistic regression model fitted to the sample-level data and the associated 95% confidence intervals, respectively.
Figure 5.

Age distribution of 14 study cohorts (see Table E1 in the online supplement) included in pooled sample analysis. Symbols represent samples included in this analysis by cohort and the age of sample acquisition. Age windows were used in the multivariable analyses, and start points and endpoints were assigned based on ages at which a study or studies started contributing samples or stopped contributing samples to the overall pool.
Figure 6.
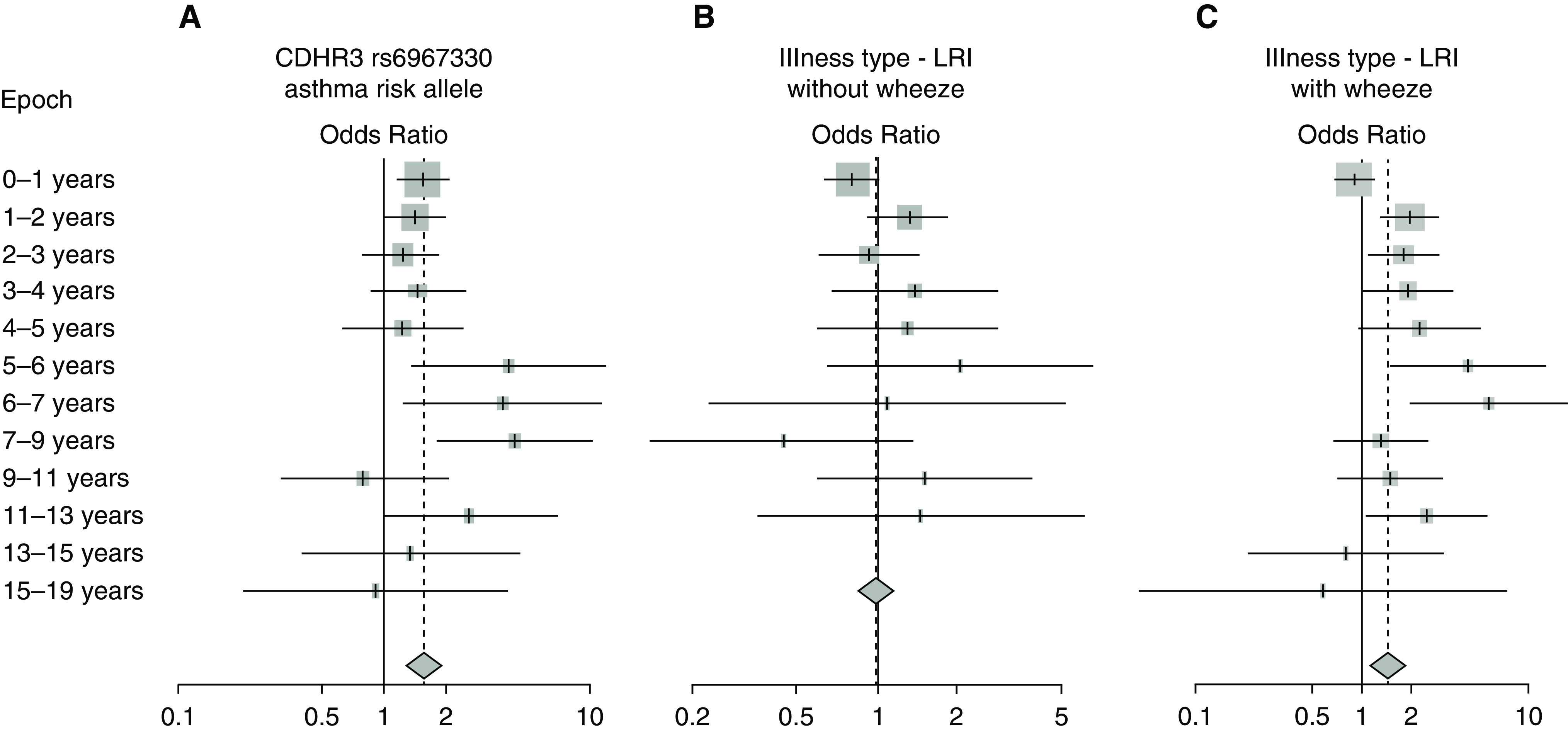
Analysis of covariates related to the rhinovirus (RV) C to RV-A ratio. Odds ratios (logistic regression) were calculated to compare the RV-C/RV-A ratio to the CDHR3 asthma risk allele (A) (rs6967330G→A, 1 or 2 risk alleles compared with 0) and to illness type (B and C) (B, lower respiratory illness [LRI] vs. upper respiratory illness [URI]; C, wheezing LRI vs. URI). Odds ratios for each age interval were calculated together with an overall odds ratio including all ages. Whisker bars represent 95% confidence intervals.
The season of collection was also related to the RV-C to RV-A ratio; children were more likely to be infected with RV-C versus RV-A in fall and winter compared with spring for 5–6 of the 12 age windows (Figure E6). Other personal factors, including race, aeroallergen sensitization, asthma history, history of breastfeeding, exposure to daycare, and number of older siblings, were not consistently related to the RV-C to RV-A ratio (Figure E7 and data not shown).
Discussion
RV infections are important causes of LRI and wheezing during infancy and in children and adults with asthma and other chronic respiratory diseases, and understanding the contributions of different viruses is needed to guide efforts to develop specific treatments. Although there is consensus that RV-B is less likely to cause severe respiratory illnesses, the relative roles of RV-A and RV-C have been uncertain. In the COAST birth cohort study, RV-A and RV-C infections and illnesses were both common in the first 3 years of life, but RV-C was less often detected than RV-A in older children during both respiratory illnesses and scheduled surveillance visits. Analysis of nAb responses to selected RV-A and RV-C types in the COAST cohort demonstrated progressively greater frequency of protective responses to RV-C over time, suggesting that species-specific differences in the development of nAb responses may contribute to the frequency of illnesses with RV-C versus RV-A. Pooling data from 14 cohorts confirmed that RV-C illnesses are more prevalent in preschoolers. These data also provide evidence that RV-C (compared with RV-A) is specifically associated with wheezing LRI and confirm that an SNP (rs6967330) in the CDHR3 gene promotes RV-C infections and illnesses throughout childhood.
Previous studies have demonstrated that RV infection rates peak during infancy and gradually decline with age (37). In parallel, nAb responses to RV-A and RV-B types generally increase during childhood and early adult years and plateau with nAb to approximately 40% of RV types after 35–40 years. RV infections continue at a lower rate throughout adult life, suggesting that protective immunity is either not fully developed to each virus type (low nAb titers) or is not maintained (38). Our new data provide definitive evidence that, in comparison with RV-A, young children have similar rates of RV-C infections but proportionally higher rates of RV-C illnesses and that increasing age is associated with steady reductions in RV-C detection rates during sickness or health. Although some previous studies have detected this trend, this pooled data analysis estimated the odds of detecting RV-C compared with RV-A over a broad age range and in a large and diverse study population with more than 10,000 nasal samples typed.
The significant age-related decline in the RV-C to RV-A ratio during RV infections was associated with marked increases in titers of nAbs specific for RV-C types. Notably, this was true for RV-C types that were commonly detected (RV-C2 and RV-C15) and for RV-C types that were less commonly detected (RV-C41). Although this finding needs to be confirmed by testing for nAb responses to other RV-C types, this raises the possibility that RV-C infections induce antibody responses that can cross-neutralize other RV-C types.
Prior studies of antibody responses to RV-C measured IgG antibody binding to synthetic viral peptides by ELISA. Interestingly, antibody responses specific for RV-A and RV-C peptides were found to be highly cross-reactive (39–41). In addition, serum IgG1 antibody titers to RV-C were significantly lower than those to RV-A and RV-B, suggesting that humoral immune response to RV-C may be muted (40, 41). In contrast, a recent study using an RV peptide array that included representative capsid protein sequences from all three species reported higher antibody concentrations to RV-A and RV-C compared with RV-B in both healthy children and children with asthma (42). The authors also reported that asthma was associated with higher antibody concentrations that did not seem to confer enhanced protection. Antibody responses to viral capsid proteins (VP1) of RV-A and RV-C were also associated with severity of symptoms of previous respiratory illnesses (39, 43).
Previous studies of antibody responses to RV-C did not include measurement of nAbs (40–42). Infectivity assays in HeLa-H1 cells or lung fibroblasts, which were used for RV-A and RV-B neutralization (44), are not suitable for RV-C because these cells lack the CDHR3 expression required for viral binding and replication (34, 45, 46). We developed a novel qPCR-based neutralization assay for both RV-A and RV-C to analyze nAbs in the absence of visible cytopathic effects; the results of the new assay correlated closely with those of a standard CPE-based assay (36). Our findings demonstrate that natural humoral immunity to RV-C develops at an accelerated rate compared with that to RV-A.
These findings suggest that RV-C types are more immunogenic compared with RV-A or that they are more likely to induce cross-neutralizing antibodies. Neutralizing antigenic sites in RV-A and RV-B are located at the highest points of the capsid surface in hypervariable regions of VP1, VP2 and VP3 (47–50). Little is known about neutralizing sites in RV-C, which has unique structural features and receptor interactions (51, 52). The first extracellular domain (EC1) of CDHR3 binds to residues located in a shallow groove formed primarily by VP2 and VP3, with some contribution from VP1 (52). The accessibility of the binding site suggests that certain epitopes in this region could elicit nAbs that block receptor binding. In addition, our data confirm that many RV-C types are more virulent compared with other RV types, and previous studies have demonstrated that viremia (detection of viral RNA in serum specimens) is much more common with RV-C infections compared with infections with other RV species (53, 54). Virulence of RV-C types could also be enhanced by a muted T helper-1 response to RV-C versus RV-A, which could impair antiviral responses (55). Because symptom severity and peak viral titer during acute infections are positively related to the induction of RV-specific nAb responses (26, 56), the increased virulence of RV-C could contribute to the greater prevalence and perhaps duration of nAb responses.
The pooled analysis provided descriptive information on more than 10,000 samples that tested positive for RV. Several RV types (RV-C2, RV-A78, RV-C11, and RV-A12) were frequently detected and found at high rates throughout most of the years during the two-decade period of analysis. Similarly, a recent meta-analysis found that RV-A12, RV-A78, RV-C15, and RV-C2 were reported in more published studies than any other RV-A or RV-C types (57). The consistency of these findings suggests that there are certain RV types, such as RV-C2 and RV-A78, that are either more virulent or more readily transmitted. Notably, the affinity of CDHR3 binding for four RV-C types was recently estimated by measuring how multiple single mutations in a soluble EC1 domain affected its ability to inhibit viral replication (52). The results indicate that CDHR3 binding is tightest for C02 and progressively less for C15, C41, and C45. This estimate of binding affinity corresponded with the frequency with which these viruses were detected in our pooled analysis of illness samples, suggesting that the strength of viral binding to CDHR3 could contribute to virulence and/or transmission. Direct measurements of binding of RV-C types to CDHR3 are needed to test this hypothesis.
This study has several strengths and some limitations to consider when interpreting the results. Longitudinal sampling in COAST from birth to adolescence allowed assessments of relative contributions of RV types across childhood within the same children. The pooled sample analysis provided a large sample and included studies that were diverse in terms of geography, climates, years of sampling, ethnicity, and home and community environments. Several studies collected samples from children with and without illness, which enabled analysis across illness and health. Viral typing was performed by partial sequencing using the same methods. Limitations included the need to harmonize variables such as illness type that were defined differently by each study cohort, which could have led to reduced precision. Several of the studies collected samples and information over a relatively short age range, which necessitated analyzing risk factors for RV-C and RV-A infections in age windows so that age would not be confounded with study site. Finally, we measured neutralizing antibody responses to six RV types, and data for additional RV types would be helpful to confirm our findings and to identify viral characteristics apart from species that can influence the development of neutralizing antibody responses.
In conclusion, this study and others identify young children and those who are genetically predisposed as populations at high risk for RV-C wheezing illnesses, who could potentially benefit from preventive approaches such as vaccination. Obstacles to the development of RV-specific vaccines have included the large number of RV types and a paucity of information about RV-C epidemiology and protective serologic responses. To overcome these limitations, we have identified key subsets of RV-C types that are more prevalent and more virulent, and demonstrated that RV-C infections elicit potent and durable nAb responses. Therefore, building on these data, it may now be more feasible to develop an RV-C vaccine for use early in life to prevent RV-provoked wheezing illnesses and reduce a major health burden in high-risk children.
Supplementary Material
Acknowledgments
Acknowledgment
The authors thank their colleagues, the medical, nursing, and program staff as well as the children and families participating in the cohorts. They also acknowledge the contribution of the ECHO Coordinating Center at Duke Clinical Research Institute (P.B. Smith, K.L. Newby, and D.K. Benjamin) and the Marshfield Clinic Research Foundation (C. Bendixsen).
Footnotes
Supported by the Environmental Influences on Child Health Outcomes program, Office of The Director, NIH, under award numbers U2COD023375 (Coordinating Center), U24OD023382 (Data Analysis Center), U24OD023319 (Person-Reported Outcome [PRO] Core), UG3/UH3 OD023282, and UG3/UH3 OD-023253; NIH grants R01AI148707, P01 HL070831, UM1 AI114271, R01 AI-114552, R01 AI-127507, U19 AI095227, UL1 RR024975, and R01-AI097172; the Sigrid Juselius Foundation, Helsinki, Finland; National Health and Medical Research Council grants 211912, 458513; and by grants APP1045760, APP1087700, APP1129996, APP1147630. The content is solely the responsibility of the authors and does not necessarily represent the official views of the NIH and other funding agencies.
Author Contributions: T.C. and M.D. drafted the manuscript. M.D. and K.G. performed laboratory analyses. T.C., M.D.E., R. Gangnon, and K.E.L. performed the statistical analysis. J.E.G. and Y.A.B. designed the study, analyzed data, and edited the manuscript. Other authors contributed samples and data and reviewed the manuscript. All authors approved the final manuscript.
This article has an online supplement, which is accessible from this issue’s table of contents at www.atsjournals.org.
Originally Published in Press as DOI: 10.1164/rccm.202010-3753OC on December 24, 2020
Author disclosures are available with the text of this article at www.atsjournals.org.
Contributor Information
Collaborators: on behalf of the program collaborators for Environmental influences on Child Health Outcomes
References
- 1.Jartti T, Gern JE. Role of viral infections in the development and exacerbation of asthma in children. J Allergy Clin Immunol. 2017;140:895–906. doi: 10.1016/j.jaci.2017.08.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Cox DW, Khoo SK, Zhang G, Lindsay K, Keil AD, Knight G, et al. Rhinovirus is the most common virus and rhinovirus-C is the most common species in paediatric intensive care respiratory admissions. Eur Respir J. 2018;52:1800207. doi: 10.1183/13993003.00207-2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.McIntyre CL, Knowles NJ, Simmonds P. Proposals for the classification of human rhinovirus species A, B and C into genotypically assigned types. J Gen Virol. 2013;94:1791–1806. doi: 10.1099/vir.0.053686-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Lee WM, Lemanske RF, Jr, Evans MD, Vang F, Pappas T, Gangnon R, et al. Human rhinovirus species and season of infection determine illness severity. Am J Respir Crit Care Med. 2012;186:886–891. doi: 10.1164/rccm.201202-0330OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bashir H, Grindle K, Vrtis R, Vang F, Kang T, Salazar L, et al. Association of rhinovirus species with common cold and asthma symptoms and bacterial pathogens. J Allergy Clin Immunol. 2018;141:822–824, e9. doi: 10.1016/j.jaci.2017.09.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Cox DW, Bizzintino J, Ferrari G, Khoo SK, Zhang G, Whelan S, et al. Human rhinovirus species C infection in young children with acute wheeze is associated with increased acute respiratory hospital admissions. Am J Respir Crit Care Med. 2013;188:1358–1364. doi: 10.1164/rccm.201303-0498OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bizzintino J, Lee WM, Laing IA, Vang F, Pappas T, Zhang G, et al. Association between human rhinovirus C and severity of acute asthma in children. Eur Respir J. 2011;37:1037–1042. doi: 10.1183/09031936.00092410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Zhao M, Zhu WJ, Qian Y, Sun Y, Zhu RN, Deng J, et al. Association of different human rhinovirus species with asthma in children: a preliminary study. Chin Med J (Engl) 2016;129:1513–1518. doi: 10.4103/0366-6999.184463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Iwane MK, Prill MM, Lu X, Miller EK, Edwards KM, Hall CB, et al. Human rhinovirus species associated with hospitalizations for acute respiratory illness in young US children. J Infect Dis. 2011;204:1702–1710. doi: 10.1093/infdis/jir634. [DOI] [PubMed] [Google Scholar]
- 10.Wildenbeest JG, van der Schee MP, Hashimoto S, Benschop KS, Minnaar RP, Sprikkelman AB, et al. Prevalence of rhinoviruses in young children of an unselected birth cohort from the Netherlands. Clin Microbiol Infect. 2016;22:736.e9–736.e15. doi: 10.1016/j.cmi.2016.05.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Martin ET, Kuypers J, Chu HY, Foote S, Hashikawa A, Fairchok MP, et al. Heterotypic infection and spread of rhinovirus A, B, and C among childcare attendees. J Infect Dis. 2018;218:848–855. doi: 10.1093/infdis/jiy232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bønnelykke K, Coleman AT, Evans MD, Thorsen J, Waage J, Vissing NH, et al. Cadherin-related family member 3 genetics and rhinovirus C respiratory illnesses. Am J Respir Crit Care Med. 2018;197:589–594. doi: 10.1164/rccm.201705-1021OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bønnelykke K, Sleiman P, Nielsen K, Kreiner-Møller E, Mercader JM, Belgrave D, et al. A genome-wide association study identifies CDHR3 as a susceptibility locus for early childhood asthma with severe exacerbations. Nat Genet. 2014;46:51–55. doi: 10.1038/ng.2830. [DOI] [PubMed] [Google Scholar]
- 14.Principi N, Zampiero A, Gambino M, Scala A, Senatore L, Lelii M, et al. Prospective evaluation of rhinovirus infection in healthy young children. J Clin Virol. 2015;66:83–89. doi: 10.1016/j.jcv.2015.03.013. [DOI] [PubMed] [Google Scholar]
- 15.Turunen R, Jartti T, Bochkov YA, Gern JE, Vuorinen T. Rhinovirus species and clinical characteristics in the first wheezing episode in children. J Med Virol. 2016;88:2059–2068. doi: 10.1002/jmv.24587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Erkkola R, Turunen R, Räisänen K, Waris M, Vuorinen T, Laine M, et al. Rhinovirus C is associated with severe wheezing and febrile respiratory illness in young children. Pediatr Infect Dis J. 2020;39:283–286. doi: 10.1097/INF.0000000000002570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Chen WJ, Arnold JC, Fairchok MP, Danaher PJ, McDonough EA, Blair PJ, et al. Epidemiologic, clinical, and virologic characteristics of human rhinovirus infection among otherwise healthy children and adults: rhinovirus among adults and children. J Clin Virol. 2015;64:74–82. doi: 10.1016/j.jcv.2015.01.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Prill MM, Dahl RM, Midgley CM, Chern SW, Lu X, Feikin DR, et al. Severe respiratory illness associated with rhinovirus during the enterovirus D68 outbreak in the United States, August 2014-November 2014. Clin Infect Dis. 2018;66:1528–1534. doi: 10.1093/cid/cix1034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Linster M, Donato C, Mah MG, Grau ML, Low JG, Ooi EE, et al. Genetic diversity of respiratory enteroviruses and rhinoviruses in febrile adults, Singapore, 2007-2013. Influenza Other Respir Viruses. 2020;14:67–71. doi: 10.1111/irv.12662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Cate TR, Couch RB, Johnson KM. Studies with rhinoviruses in volunteers: production of illness, effect of naturally acquired antibody, and demonstration of a protective effect not associated with serum antibody. J Clin Invest. 1964;43:56–67. doi: 10.1172/JCI104894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Cate TR, Rossen RD, Douglas RG, Jr, Butler WT, Couch RB. The role of nasal secretion and serum antibody in the rhinovirus common cold. Am J Epidemiol. 1966;84:352–363. doi: 10.1093/oxfordjournals.aje.a120648. [DOI] [PubMed] [Google Scholar]
- 22.Douglas RG, Jr, Fleet WF, Cate TR, Couch RB. Antibody to rhinovirus in human sera: I. Standardization of a neutralization test. Proc Soc Exp Biol Med. 1968;127:497–502. doi: 10.3181/00379727-127-32724. [DOI] [PubMed] [Google Scholar]
- 23.Alper CM, Doyle WJ, Skoner DP, Buchman CA, Seroky JT, Gwaltney JM, et al. Prechallenge antibodies: moderators of infection rate, signs, and symptoms in adults experimentally challenged with rhinovirus type 39. Laryngoscope. 1996;106:1298–1305. doi: 10.1097/00005537-199610000-00025. [DOI] [PubMed] [Google Scholar]
- 24.Taylor-Robinson D. Laboratory and volunteer studies on some viruses isolated from common colds (rhinoviruses) Am Rev Respir Dis. 1963;88(Suppl):262–268. doi: 10.1164/arrd.1963.88.3P2.262. [DOI] [PubMed] [Google Scholar]
- 25.Lemanske RF., Jr The childhood origins of asthma (COAST) study. Pediatr Allergy Immunol. 2002;13:38–43. doi: 10.1034/j.1399-3038.13.s.15.8.x. [DOI] [PubMed] [Google Scholar]
- 26.Choi T, Lee KE, Grindle K, Laing IA, LeSouef PN, Holt PG, et al. Age is differentially associated with rhinovirus A and C species infections in children. Am J Respir Crit Care Med. 2020;201:A6331. [Google Scholar]
- 27.Rubner FJ, Jackson DJ, Evans MD, Gangnon RE, Tisler CJ, Pappas TE, et al. Early life rhinovirus wheezing, allergic sensitization, and asthma risk at adolescence. J Allergy Clin Immunol. 2017;139:501–507. doi: 10.1016/j.jaci.2016.03.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Bochkov YA, Grindle K, Vang F, Evans MD, Gern JE. Improved molecular typing assay for rhinovirus species A, B, and C. J Clin Microbiol. 2014;52:2461–2471. doi: 10.1128/JCM.00075-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Annamalay AA, Khoo SK, Jacoby P, Bizzintino J, Zhang G, Chidlow G, et al. Kalgoorlie Otitis Media Research Project Team. Prevalence of and risk factors for human rhinovirus infection in healthy aboriginal and non-aboriginal Western Australian children. Pediatr Infect Dis J. 2012;31:673–679. doi: 10.1097/INF.0b013e318256ffc6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Bochkov YA, Palmenberg AC, Lee WM, Rathe JA, Amineva SP, Sun X, et al. Molecular modeling, organ culture and reverse genetics for a newly identified human rhinovirus C. Nat Med. 2011;17:627–632. doi: 10.1038/nm.2358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Nakagome K, Bochkov YA, Ashraf S, Brockman-Schneider RA, Evans MD, Pasic TR, et al. Effects of rhinovirus species on viral replication and cytokine production. J Allergy Clin Immunol. 2014;134:332–341. doi: 10.1016/j.jaci.2014.01.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Griggs TF, Bochkov YA, Nakagome K, Palmenberg AC, Gern JE. Production, purification, and capsid stability of rhinovirus C types. J Virol Methods. 2015;217:18–23. doi: 10.1016/j.jviromet.2015.02.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Bochkov YA, Watters K, Basnet S, Sijapati S, Hill M, Palmenberg AC, et al. Mutations in VP1 and 3A proteins improve binding and replication of rhinovirus C15 in HeLa-E8 cells. Virology. 2016;499:350–360. doi: 10.1016/j.virol.2016.09.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Bochkov YA, Watters K, Ashraf S, Griggs TF, Devries MK, Jackson DJ, et al. Cadherin-related family member 3, a childhood asthma susceptibility gene product, mediates rhinovirus C binding and replication. Proc Natl Acad Sci USA. 2015;112:5485–5490. doi: 10.1073/pnas.1421178112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Lemanske RF, Jr, Jackson DJ, Gangnon RE, Evans MD, Li Z, Shult PA, et al. Rhinovirus illnesses during infancy predict subsequent childhood wheezing. J Allergy Clin Immunol. 2005;116:571–577. doi: 10.1016/j.jaci.2005.06.024. [DOI] [PubMed] [Google Scholar]
- 36.Gern JE, Lee WM, Swenson CA, Nakagome K, Lee I, Wolff M, et al. Development of a rhinovirus inoculum using a reverse genetics approach. J Infect Dis. 2019;220:187–194. doi: 10.1093/infdis/jiy629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Hamparian VV, Conant RM, Thomas DC. Rhinovirus reference laboratory, annual contract report to the national institute of allergy and infectious diseases. Bethesda, MD: National Institute of Health; 1970. [Google Scholar]
- 38.Glanville N, Johnston SL. Challenges in developing a cross-serotype rhinovirus vaccine. Curr Opin Virol. 2015;11:83–88. doi: 10.1016/j.coviro.2015.03.004. [DOI] [PubMed] [Google Scholar]
- 39.Niespodziana K, Cabauatan CR, Jackson DJ, Gallerano D, Trujillo-Torralbo B, Del Rosario A, et al. Rhinovirus-induced VP1-specific antibodies are group-specific and associated with severity of respiratory symptoms. EBioMedicine. 2014;2:64–70. doi: 10.1016/j.ebiom.2014.11.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Iwasaki J, Smith WA, Khoo SK, Bizzintino J, Zhang G, Cox DW, et al. Comparison of rhinovirus antibody titers in children with asthma exacerbations and species-specific rhinovirus infection. J Allergy Clin Immunol. 2014;134:25–32. doi: 10.1016/j.jaci.2014.03.014. [DOI] [PubMed] [Google Scholar]
- 41.Iwasaki J, Smith WA, Stone SR, Thomas WR, Hales BJ. Species-specific and cross-reactive IgG1 antibody binding to viral capsid protein 1 (VP1) antigens of human rhinovirus species A, B and C. PLoS One. 2013;8:e70552. doi: 10.1371/journal.pone.0070552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Megremis S, Niespodziana K, Cabauatan C, Xepapadaki P, Kowalski ML, Jartti T, et al. Rhinovirus species-specific antibodies differentially reflect clinical Outcomes in health and asthma. Am J Respir Crit Care Med. 2018;198:1490–1499. doi: 10.1164/rccm.201803-0575OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Stenberg-Hammar K, Niespodziana K, Söderhäll C, James A, Cabauatan CR, Konradsen JR, et al. Rhinovirus-specific antibody responses in preschool children with acute wheeze reflect severity of respiratory symptoms. Allergy. 2016;71:1728–1735. doi: 10.1111/all.12991. [DOI] [PubMed] [Google Scholar]
- 44.Lee WM, Chen Y, Wang W, Mosser A. Infectivity assays of human rhinovirus-A and -B serotypes. Methods Mol Biol. 2015;1221:71–81. doi: 10.1007/978-1-4939-1571-2_7. [DOI] [PubMed] [Google Scholar]
- 45.Watters K, Palmenberg AC. CDHR3 extracellular domains EC1-3 mediate rhinovirus C interaction with cells and as recombinant derivatives, are inhibitory to virus infection. PLoS Pathog. 2018;14:e1007477. doi: 10.1371/journal.ppat.1007477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Everman JL, Sajuthi S, Saef B, Rios C, Stoner AM, Numata M, et al. Functional genomics of CDHR3 confirms its role in HRV-C infection and childhood asthma exacerbations. J Allergy Clin Immunol. 2019;144:962–971. doi: 10.1016/j.jaci.2019.01.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Sherry B, Mosser AG, Colonno RJ, Rueckert RR. Use of monoclonal antibodies to identify four neutralization immunogens on a common cold picornavirus, human rhinovirus 14. J Virol. 1986;57:246–257. doi: 10.1128/jvi.57.1.246-257.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Appleyard G, Russell SM, Clarke BE, Speller SA, Trowbridge M, Vadolas J. Neutralization epitopes of human rhinovirus type 2. J Gen Virol. 1990;71:1275–1282. doi: 10.1099/0022-1317-71-6-1275. [DOI] [PubMed] [Google Scholar]
- 49.Skern T, Neubauer C, Frasel L, Gründler P, Sommergruber W, Zorn M, et al. A neutralizing epitope on human rhinovirus type 2 includes amino acid residues between 153 and 164 of virus capsid protein VP2. J Gen Virol. 1987;68:315–323. doi: 10.1099/0022-1317-68-2-315. [DOI] [PubMed] [Google Scholar]
- 50.Verdaguer N, Blaas D, Fita I. Structure of human rhinovirus serotype 2 (HRV2) J Mol Biol. 2000;300:1179–1194. doi: 10.1006/jmbi.2000.3943. [DOI] [PubMed] [Google Scholar]
- 51.Liu Y, Hill MG, Klose T, Chen Z, Watters K, Bochkov YA, et al. Atomic structure of a rhinovirus C, a virus species linked to severe childhood asthma. Proc Natl Acad Sci USA. 2016;113:8997–9002. doi: 10.1073/pnas.1606595113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Sun Y, Watters K, Hill MG, Fang Q, Liu Y, Kuhn RJ, et al. Cryo-EM structure of rhinovirus C15a bound to its cadherin-related protein 3 receptor. Proc Natl Acad Sci USA. 2020;117:6784–6791. doi: 10.1073/pnas.1921640117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Esposito S, Daleno C, Scala A, Castellazzi L, Terranova L, Sferrazza Papa S, et al. Impact of rhinovirus nasopharyngeal viral load and viremia on severity of respiratory infections in children. Eur J Clin Microbiol Infect Dis. 2014;33:41–48. doi: 10.1007/s10096-013-1926-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Lu X, Schneider E, Jain S, Bramley AM, Hymas W, Stockmann C, et al. Rhinovirus viremia in patients hospitalized with community-acquired pneumonia. J Infect Dis. 2017;216:1104–1111. doi: 10.1093/infdis/jix455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Anderson D, Jones AC, Gaido CM, Carter KW, Laing IA, Bosco A, et al. Differential gene expression of lymphocytes stimulated with rhinovirus A and C in children with asthma. Am J Respir Crit Care Med. 2020;202:202–209. doi: 10.1164/rccm.201908-1670OC. [DOI] [PubMed] [Google Scholar]
- 56.Gern JE, Calhoun W, Swenson C, Shen G, Busse WW. Rhinovirus infection preferentially increases lower airway responsiveness in allergic subjects. Am J Respir Crit Care Med. 1997;155:1872–1876. doi: 10.1164/ajrccm.155.6.9196088. [DOI] [PubMed] [Google Scholar]
- 57.Esneau C, Croft S, Loo S, Ghildyal R.Rhinovirus diversity and virulence factors Bartlett NW, Wark P, Knight D.editors. Rhinovirus infections: rethinking the impact on human health and disease. London, UK: Academic Press; 201925–59. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.