Abstract
Rationale: GLP-1R (glucagon-like peptide-1 receptor) agonists are approved to treat type 2 diabetes mellitus and obesity. GLP-1R agonists reduce airway inflammation and hyperresponsiveness in preclinical models.
Objectives: To compare rates of asthma exacerbations and symptoms between adults with type 2 diabetes and asthma prescribed GLP-1R agonists and those prescribed SGLT-2 (sodium–glucose cotransporter-2) inhibitors, DPP-4 (dipeptidyl peptidase-4) inhibitors, sulfonylureas, or basal insulin for diabetes treatment intensification.
Methods: This study was an electronic health records–based new-user, active-comparator, retrospective cohort study of patients with type 2 diabetes and asthma newly prescribed GLP-1R agonists or comparator drugs at an academic healthcare system from January 2000 to March 2018. The primary outcome was asthma exacerbations; the secondary outcome was encounters for asthma symptoms. Propensity scores were calculated for GLP-1R agonist and non–GLP-1R agonist use. Zero-inflated Poisson regression models included adjustment for multiple covariates.
Measurements and Main Results: Patients initiating GLP-1R agonists (n = 448), SGLT-2 inhibitors (n = 112), DPP-4 inhibitors (n = 435), sulfonylureas (n = 2,253), or basal insulin (n = 2,692) were identified. At 6 months, asthma exacerbation counts were lower in persons initiating GLP-1R agonists (reference) compared with SGLT-2 inhibitors (incidence rate ratio [IRR], 2.98; 95% confidence interval [CI], 1.30–6.80), DPP-4 inhibitors (IRR, 2.45; 95% CI, 1.54–3.89), sulfonylureas (IRR, 1.83; 95% CI, 1.20–2.77), and basal insulin (IRR, 2.58; 95% CI, 1.72–3.88). Healthcare encounters for asthma symptoms were also lower among GLP-1R agonist users.
Conclusions: Adult patients with asthma prescribed GLP-1R agonists for type 2 diabetes had lower counts of asthma exacerbations compared with other drugs initiated for treatment intensification. GLP-1R agonists may represent a novel treatment for asthma associated with metabolic dysfunction.
Keywords: antiasthmatic agents, diabetes mellitus type 2, electronic health records
At a Glance Commentary
Scientific Knowledge on the Subject
GLP-1R (glucagon-like peptide-1 receptor) agonists are approved to treat type 2 diabetes mellitus and obesity. GLP-1R agonists reduce airway inflammation and hyperresponsiveness in preclinical models.
What This Study Adds to the Field
In this study of patients with asthma and type 2 diabetes, use of GLP-1R agonists for diabetes therapy was associated with fewer asthma exacerbations. These clinical, observational data build on preclinical data supporting a role for the GLP-1 metabolic pathway in asthma.
Metabolic dysfunction represents a common and challenging comorbid asthma condition (1). Insulin resistance and metabolic syndrome are associated with asthma development (2, 3) and exacerbation risk (4). Among patients with asthma, those with higher body mass index (BMI) (5) or obesity (6) have higher medication and healthcare use and poor symptom control (7), suggesting that metabolic dysfunction contributes to asthma severity (8). Prior studies have shown that some medical therapies that improve insulin resistance (metformin and sulfonylureas) improve asthma control (9, 10). Limited observational data from patients with type 2 diabetes mellitus (DM) without respiratory disease suggest that GLP-1R (glucagon-like peptide-1 receptor) agonists in combination with metformin may improve baseline pulmonary function (11). However, the use of other diabetes therapies, such as insulin and DPP-4 (dipeptidyl peptidase-4) inhibitors, do not impact incident asthma risk (12, 13). Therefore, therapies targeting metabolic pathways may be key to achieving asthma control for a significant proportion of individuals with asthma (14).
GLP-1 is a hormone stimulated by the ingestion of carbohydrates, fats, and proteins and is secreted by the intestine and the central nervous system, thereby regulating metabolic, cardiovascular, and neuroprotective activities (15, 16). GLP-1R agonists are U.S. Food and Drug Administration (FDA) approved as part of a stepwise approach to treatment intensification beyond metformin for type 2 DM (17). As a class, the GLP-1R agonists potentiate insulin and suppress glucagon secretion in individuals with type 2 diabetes with low risk of hypoglycemia (18), decreased cardiovascular and renal risk (19), and lower all-cause mortality (20). GLP-1R signaling also promotes weight loss through delayed gastric emptying and increased satiety (18), leading to FDA approval for weight management in patients with euglycemia (17).
The GLP-1R is found in lung epithelial and endothelial cells (11), underscoring a possible role for GLP-1 signaling in pulmonary disease (21). The administration of GLP-1R agonists in preclinical murine (22) and ex vivo models (11) significantly inhibits allergic and viral airway inflammation, decreasing airway eosinophilia, mucus production, and hyperresponsiveness (23, 24). However, the impact of GLP-1R agonist use on asthma exacerbations and asthma control (symptoms) in humans has not been assessed.
We analyzed real-world data from the electronic health records (EHR) of a large academic U.S. health system to determine whether the initiation of GLP-1R agonist therapy was associated with decreased asthma exacerbations and asthma symptoms compared with the initiation of other therapeutics used for treatment intensification of type 2 DM among patients with asthma. Some of the results of these studies have been previously reported in the form of an abstract (25).
Methods
Patients, Settings, and Data Source
The study used patient data between January 1, 2000, and March 1, 2018, from the Partners Healthcare Research and Patient Data Repository, which includes academic and community hospitals in the greater Boston, Massachusetts, area. The Research and Patient Data Repository is a central data warehouse that stores clinical data across Partners Healthcare (26). Detailed medical record data elements extracted in this study included demographics, diagnoses, laboratory tests, health maintenance, medications, problem lists, weight, height, and BMI.
Adult patients (≥18 yr) meeting both asthma and type 2 DM definitions were included in the study (Figure 1). Asthma was defined as at least two separate encounters with a qualifying International Classification of Diseases (ICD) code or one asthma encounter with both a diagnosis code and an asthma medication prescription. Type 2 DM was defined as at least one encounter for type 2 DM or a HbA1c value ≥6.5 and a type 2 DM medication prescription. From this cohort, we excluded patients with ICD diagnosis codes for diseases commonly treated with systemic steroids, chronic congestive heart failure, vocal cord dysfunction, and other respiratory diseases except for chronic obstructive pulmonary disease (COPD), which we examined in our analysis. We also excluded patients with more than two distinct encounters with type 1 DM codes. Inclusion and exclusion ICD code criteria can be found in Table E1 in the online supplement. Similar algorithms for inclusion and exclusion criteria have been previously validated at multiple institutions (27–30). Patients with a medication prescription and an ICD-coded encounter diagnosis have a high rate of EHR data completeness in this dataset (31).
Figure 1.
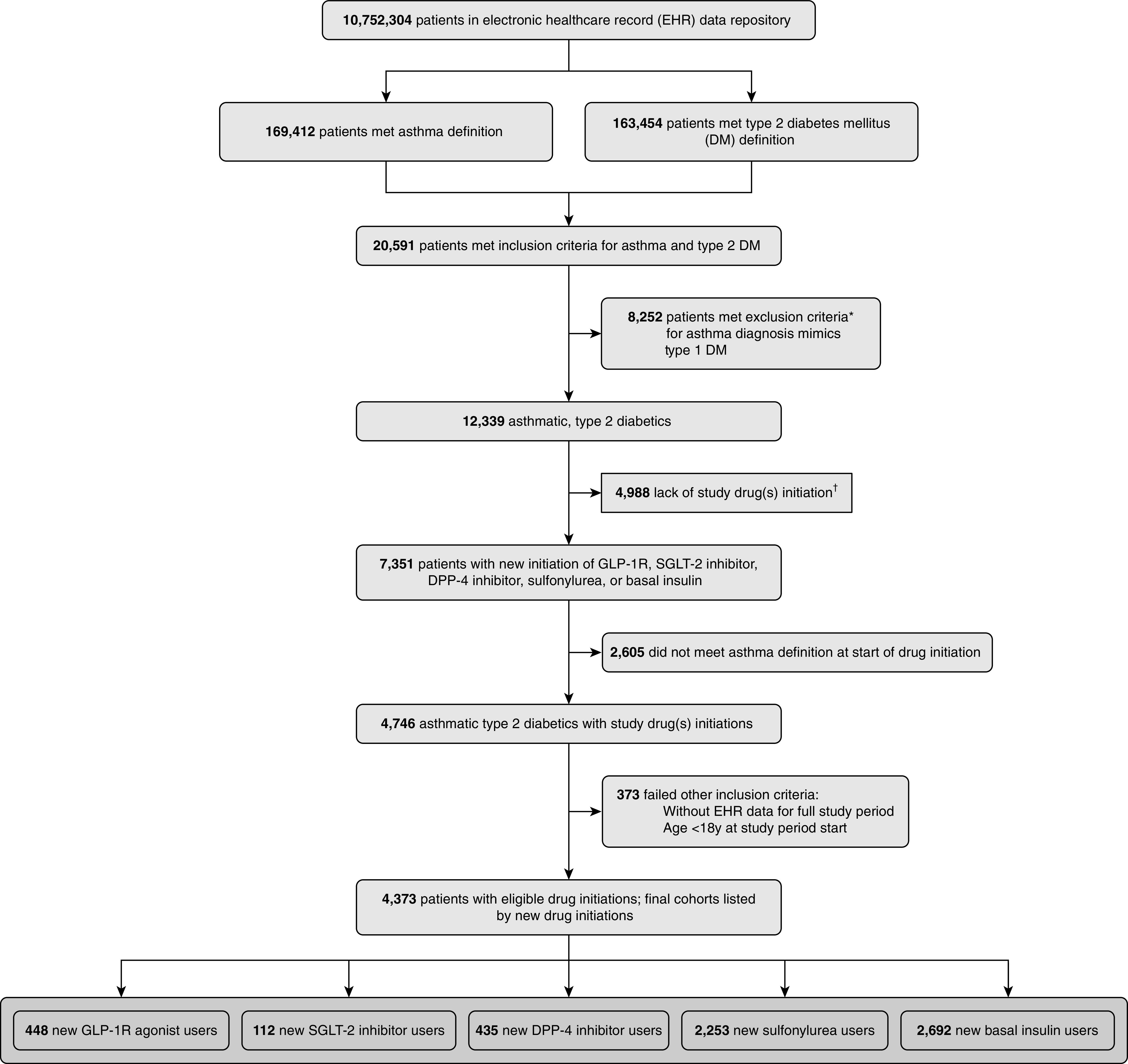
Selection of patients from electronic health records. Diagram depicts patient selection by electronic health records data as of March 2018. Some patients met more than one exclusion criteria. Final comparator groups included DPP-4 (dipeptidyl peptidase-4), GLP-1R (glucagon-like peptide-1 receptor), and SGLT-2 (sodium–glucose cotransporter-2). *Some patients met more than one exclusion criteria, as detailed in Table E1. †List of study drugs detailed in Table E2.
Study Design
We conducted a retrospective cohort study of routinely collected clinical data using a new-user, active-comparator design (32) to determine the association between GLP-1R agonist initiation and asthma outcomes. Active comparators included SGLT-2 (sodium–glucose cotransporter-2) and DPP-4 inhibitors, sulfonylureas, and basal insulin (see Table E2 for a complete listing of drug names). Comparator drug classes were selected based on the American Diabetes Association guidelines for treatment intensification of type 2 DM beyond first-line therapy with metformin, diet, and exercise (17). This stepwise approach is tailored to achieve glycemic targets and prevent diabetes complications.
Patients with diabetes achieving glycemic control on first-line metformin monotherapy are clinically distinct from those requiring second-line or combination therapy by American Diabetes Association guidelines; therefore, metformin monotherapy was not included as an active comparator. Concurrent diabetes medications, including metformin, were allowed. Given the potential effects of metformin on asthma control (10), metformin was included as a covariate in the analysis.
Exposure to any active comparator was defined as one prescription for the medication. Individuals were followed for 6 months from the date of drug initiation as depicted in Figure E1. Duration of follow-up was informed by medication adherence patterns in real-world clinical practice (33–35). We required all patients to have met both asthma criteria and type 2 DM criteria before drug initiation and to be alive at the end of follow-up.
The primary outcome was a count of asthma exacerbations defined as a systemic (oral or i.v.) corticosteroid prescription (36). Further prescriptions within 7 days of the initial prescription were considered a single exacerbation event (37). The secondary outcome was a count of ICD-coded emergency department, inpatient, and outpatient encounters for the following cardinal asthma symptoms: dyspnea, shortness of breath, wheeze, and cough (see Table E3) (36, 38). Exploratory outcomes included counts of prescriptions for short-acting β-agonists (SABAs) in the same period and counts of routine encounters for asthma.
We calculated a propensity score representing the estimated probability of initiating a GLP-1R agonist versus an active comparator for each participant (39). Baseline covariates included in the propensity score were age, sex, race/ethnicity, year of drug initiation to account for drug availability, month of drug initiation categorized by regional seasons (December–February [winter], March–May [spring], June–August [summer], and September–November [fall]) to account for seasonality of exacerbations and symptoms, insurance type, annual income (estimated from zip code), concurrent metformin use, Elixhauser chronic disease comorbidities (40), comorbid COPD, and moderate or severe chronic kidney disease (stage 3–5, defined as estimated glomerular filtration rate < 60) (17). Postbaseline covariates (e.g., post–drug initiation BMI and HbA1c) were not included (41). Propensity scores were then included as a covariate in zero-inflated Poisson (ZIP) regression models. The relationships between covariates, exposure, and outcome are modeled in a directed acyclic graph in Figure E2.
For each participant, the 6-month follow-up period began at new type 2 DM drug initiation. We expected a low event rate of asthma exacerbations on the basis of CDC data (42). ZIP models are selected when the occurrence of the outcome is rare, employing a regression procedure specifically designed to account for positively skewed integer-valued distributions with a high incidence of zeros. The ZIP model first calculates a dichotomous outcome: the odds of a subject belonging to a class that always scores 0 versus 1 on the outcome (e.g., any asthma exacerbation using logistic regression; “zero model”). The model then estimates the frequency of events (e.g., asthma exacerbation count) among subjects in class 1 using a Poisson distribution, providing a count estimate from which a rate ratio can be calculated and treatment conditions can be compared (“count model”). ZIP models were constructed with covariate adjustment for multiple confounders, including season of drug initiation, baseline asthma severity in the 12 months before type 2 DM drug initiation (categorized as mild [controlled without medications or with SABAs or leukotriene receptor antagonists] vs. moderate/severe [requiring inhaled corticosteroid alone or in combination with any other controller agent] on the basis of prescription drug classes adapted from the 2018 Global Initiative for Asthma guidelines) (43), smoking status, concurrent metformin prescription, and COPD (defined as ≥2 encounters at or within the 12 months before drug initiation). Examining the presence of comorbid COPD coding within this asthma cohort reflects the recognition that in clinical practice, patients with asthma may have features compatible with COPD, as in asthma–COPD overlap (ACO) (44), and accounts for cocoding in EHR data (45). Patients with COPD only were excluded from this cohort by the asthma phenotype definition.
To test the robustness of the association between GLP-1R agonist use and asthma outcomes, we added baseline BMI and HbA1c in the propensity score. Change in BMI and change in HbA1c over the follow-up period were included as covariates in the ZIP model as a sensitivity analysis.
In an additional sensitivity analysis, we examined those patients with more than one prescription for the initiated drug during the study period. We examined the effect of prescriptions for any DM drugs in the year preceding the 6-month study period as covariates to minimize confounding by longitudinal metabolic changes or order of treatment intensification. Patients with missing covariates were excluded from the sensitivity analyses. Each drug class has variable effects on weight and HbA1c, both of which may be associated with asthma, and therefore imputed data were not used in any analysis.
In a prespecified exploratory analysis, we examined prescriptions for SABAs and routine nonexacerbation encounters for asthma in the follow-up period, applying the same model constraints and covariates. We also tested whether the findings in our primary analysis were robust for the moderate/severe asthma subgroup and never-smokers.
Statistical significance was accepted at a two-sided P value ≤ 0.05. Statistical analyses were performed in SAS version 14.3. The study was approved by the Partners Healthcare institutional review board (protocol 2017P001730) before data collection.
Results
After inclusion and exclusion criteria were applied, our cohort included 448 new initiations of GLP-1R agonists, 112 of SGLT-2 inhibitors, 435 of DPP-4 inhibitors, 2,253 of sulfonylureas, and 2,692 of basal insulin for a total of 5,940 new initiations among 4,373 patients (Figure 1). Initiation did not differ by season across groups. Within the GLP-1R agonist class, liraglutide (53.1%) and exenatide (35.9%) were the most common drugs initiated. Table 1 shows the baseline characteristics of patients by drug initiation. New users of GLP-1R agonists were more likely to be younger and female. Baseline asthma severity was similar across the groups, supporting a therapeutic balance with regard to baseline asthma status before treatment intensification with GLP-1R agonists or comparators. Seventeen percent of patients with asthma also had a prior encounter coded for COPD.
Table 1.
Baseline Characteristics of the Study Cohort
| Patient Baseline Characteristics* | GLP-1R Agonist Users (n = 448) | SGLT-2 Inhibitor Users (n = 112) | DPP-4 Inhibitor Users (n = 435) | Sulfonylurea Users (n = 2,253) | Insulin Users (n = 2,692) | P Value |
|---|---|---|---|---|---|---|
| Age, yr, mean (SD) | 54 (12.5) | 60 (11.4) | 63.5 (12.2) | 59.5 (14.1) | 58.4 (15) | <0.001 |
| Sex, n (%) | <0.001 | |||||
| F | 323 (72.1) | 66 (58.9) | 286 (65.7) | 1387 (61.6) | 1,751 (65) | |
| M | 125 (27.9) | 46 (41.1) | 149 (34.3) | 866 (38.4) | 941 (35) | |
| Race/ethnicity, n (%) | <0.001 | |||||
| White | 298 (66.5) | 87 (77.7) | 300 (69) | 1,434 (63.6) | 1,714 (63.7) | |
| Black/African American | 58 (12.9) | 13 (11.6) | 47 (10.8) | 299 (13.3) | 382 (14.2) | |
| Hispanic/Latino | 46 (10.3) | 5 (4.5) | 43 (9.9) | 259 (11.5) | 332 (12.3) | |
| Asian | 3 (0.7) | 2 (1.8) | 14 (3.2) | 76 (3.4) | 36 (1.3) | |
| Other/unknown | 43 (9.6) | 5 (4.5) | 31 (7.1) | 185 (8.2) | 228 (8.5) | |
| Insurance type, n (%) | <0.001 | |||||
| Medicaid | 46 (10.3) | 7 (6.3) | 27 (6.2) | 204 (9.1) | 263 (9.8) | |
| Medicare | 172 (38.4) | 38 (33.9) | 196 (45.1) | 913 (40.5) | 1,000 (37.1) | |
| Self | 16 (3.6) | 4 (3.6) | 27 (6.2) | 308 (13.7) | 398 (14.8) | |
| Commercial | 210 (46.9) | 63 (56.3) | 178 (40.9) | 809 (35.9) | 1,011 (37.6) | |
| Other | 4 (0.9) | 0 (0) | 7 (1.6) | 19 (0.8) | 20 (0.7) | |
| Annual income, dollars, n (%)† | 0.01 | |||||
| <30,000 | 10 (2.3) | 2 (1.8) | 13 (3.1) | 88 (4.0) | 104 (4.0) | |
| 30,000–100,000 | 379 (85.6) | 92 (82.1) | 359 (84.5) | 1,939 (87.1) | 2,307 (87.0) | |
| >100,000 | 54 (12.2) | 18 (16.1) | 53 (12.5) | 198 (8.9) | 240 (9.1) | |
| Year of drug initiation, average (SD)‡ | 2013.6 (2.9) | 2015.7 (1.2) | 2013.1 (2.8) | 2010.3 (4.4) | 2011 (4.2) | <0.001 |
| Season of drug initiation, n (%)§ | 0.21 | |||||
| Winter | 95 (21.21) | 16 (14.29) | 107 (24.60) | 517 (22.95) | 658 (24.44) | |
| Spring | 129 (28.79) | 42 (37.50) | 114 (26.21) | 644 (28.58) | 786 (29.20) | |
| Summer | 109 (24.33) | 32 (28.57) | 110 (25.29) | 529 (23.48) | 621 (23.07) | |
| Fall | 115 (25.67) | 22 (19.64) | 104 (23.91) | 563 (24.99) | 627 (23.29) | |
| Smoking status, n (%) | <0.001 | |||||
| Current | 45 (10) | 9 (8) | 40 (9.2) | 353 (15.7) | 402 (14.9) | |
| Never | 208 (46.4) | 69 (61.6) | 205 (47.1) | 889 (39.5) | 964 (35.8) | |
| Prior | 168 (37.5) | 34 (30.4) | 148 (34) | 645 (28.6) | 791 (29.4) | |
| Unknown | 27 (6) | 0 (0) | 42 (9.7) | 366 (16.2) | 535 (19.9) | |
| Chronic kidney disease (eGFR <60), n (%)‖ | <0.001 | |||||
| No | 434 (96.9) | 109 (97.3) | 398 (91.5) | 2,163 (96) | 2,514 (93.4) | |
| Yes | 14 (3.1) | 3 (2.7) | 37 (8.5) | 90 (4) | 178 (6.6) | |
| COPD, n (%)¶ | <0.001 | |||||
| No | 404 (90.2) | 107 (95.5) | 369 (84.8) | 1,907 (84.6) | 2,139 (79.5) | |
| Yes | 44 (9.8) | 5 (4.5) | 66 (15.2) | 346 (15.4) | 553 (20.5) | |
| Asthma severity, n (%)** | 0.13 | |||||
| Mild (without recent medications) | 160 (35.7) | 46 (41.1) | 153 (35.2) | 912 (40.5) | 1,040 (38.6) | |
| Mild (with recent medications) | 77 (17.2) | 18 (16.1) | 63 (14.5) | 289 (12.8) | 354 (13.2) | |
| Moderate/severe | 211 (47.1) | 48 (42.9) | 219 (50.3) | 1,052 (46.7) | 1,298 (48.2) | |
| Metformin use††, n (%) | <0.001 | |||||
| No | 202 (45.1) | 57 (50.9) | 185 (42.5) | 936 (41.5) | 1,504 (55.9) | |
| Yes | 246 (54.9) | 55 (49.1) | 250 (57.5) | 1,317 (58.5) | 1,188 (44.1) | |
| Elixhauser score, mean (SD) | 3.3 (2.2) | 3.1 (2.4) | 3 (2.5) | 2.9 (2.2) | 3.4 (2.5) | <0.001 |
| BMI, mean (SD)‡‡ | 39.5 (8.6) | 34.7 (7.2) | 34.4 (8.0) | 35.4 (8.5) | 35.4 (10.5) | <0.001 |
| HbA1c, mean (SD)§§ | 8.4 (1.9) | 8.2 (1.5) | 8.1(1.6) | 8.1 (1.8) | 8.5 (2.2) | <0.001 |
| Change in BMI, mean (SD)‖‖ | −0.8 (2.7) | −1 (2.3) | −0.2 (8.7) | −0.2 (3.0) | −0.5 (7.6) | 0.14 |
| Change in HbA1c, mean (SD)¶¶ | −0.7 (1.6) | −0.6 (1.1) | −0.6 (1.4) | −0.8 (1.8) | −1.0 (2.1) | 0.002 |
Definition of abbreviations: BMI = body mass index; COPD = chronic obstructive pulmonary disease; DPP-4 = dipeptidyl peptidase-4; eGFR = estimated glomerular filtration rate; GLP-1R = glucagon-like peptide-1 receptor; SGLT-2 = sodium–glucose cotransporter-2
For continuous variables, the P value was calculated by one-way ANOVA; for categorial variables, the P value was calculated by χ2 test.
As of study drug initiation unless otherwise specified. Drugs in each class are detailed in the Table E2.
Based on five-digit zip code.
Initial FDA approval for the newer diabetes classes include the following: GLP-1R agonists (2005), SGLT-2 inhibitors (2013), DPP-4 inhibitors (2006). Basal insulin and sulfonylurea classes were FDA approved before 2000.
December–February (winter), March–May (spring), June–August (summer), and September–November (fall).
Defined as two or more International Classification of Diseases codes for end-stage renal disease, chronic renal failure, or chronic kidney disease stage 3–5 (eGFR <60) at or within 12 months before the initial prescription of study drugs.
Defined as two or more encounters within 12 months before study drug initiation.
Defined by asthma medications prescribed within 12 months before (“recent”) study drug initiation, as follows: mild patient meets asthma definition but no asthma medication prescriptions in the 12 months before study drug initiation or only short-acting β agonists or only leukotriene receptor antagonists; moderate/severe patients receive an inhaled corticosteroid prescription alone or in combination with other controllers or biologics.
Defined as a prescription within 90 days before study drug initiation.
Defined as a measurement closest to initial drug prescription; range included within 365 days of study drug initiation up until 14 days after initiation.
Defined as a measurement closest to drug initiation; range included within 365 days of study drug initiation up until 14 days after initiation.
Defined as the difference between baseline and end values. End BMI values included the measurement closest to the study end; range included 80 days from study drug initiation until 365 days after period end.
Defined as difference between baseline and end values. End HbA1c values included the measurement closest to the study end; range included 80 days from study drug initiation until 365 days after study period end.
Analysis of Primary and Secondary Outcomes
For the primary outcome (asthma exacerbations within 6 months of drug initiation), GLP-1R agonist users had significantly fewer exacerbations compared with patients in all comparator groups in the multivariable count model (Table 2). As shown in Figure 2A, compared with GLP-1R agonist users, adjusted exacerbation rates were higher among SGLT-2 inhibitor, DPP-4 inhibitor, sulfonylurea, and basal insulin users. GLP-1R agonist users also had fewer encounters for asthma symptoms than DPP4-inhibitor, basal insulin, and sulfonylurea groups (Table 2 and Figure 2B). Unadjusted counts in each treatment group and unadjusted rates are presented in Table E4. There were similarly higher symptom counts among 112 SGLT-2 inhibitor users that did not reach statistical significance. As metformin use was a predictor of fewer asthma exacerbations across groups, we included metformin as a covariate in the analysis; the effect of GLP-1R agonists on asthma exacerbations was independent of metformin. Although GLP-1R agonist use was associated with decreased counts of exacerbations after initiation across all comparator groups, the odds of having zero exacerbations was increased by GLP-1R agonist use compared with DDP-4 inhibitor and insulin use, but not compared with sulfonylurea use or SGLT-2 inhibitor use (Table E5). Odds of having zero encounters for asthma symptoms was not increased by GLP-1R agonist use across all groups.
Table 2.
Primary and Secondary Asthma Outcomes by Type 2 Diabetes Treatment Groups
| Treatment Groups* | Asthma Exacerbations |
Asthma Symptoms |
||||
|---|---|---|---|---|---|---|
| Incidence Rate Ratio | 95% CI | P Value | Incidence Rate Ratio | 95% CI | P Value | |
| GLP-1R (n = 448) | ref | — | — | ref | — | — |
| SGLT-2 inhibitor (n = 112) | 2.98 | 1.30–6.80 | 0.01 | 1.44 | 0.72–2.88 | 0.30 |
| DPP-4 inhibitor (n = 435) | 2.45 | 1.54–3.89 | <0.001 | 1.71 | 1.14–2.57 | 0.009 |
| Sulfonylurea (n = 2,253) | 1.83 | 1.20–2.77 | 0.005 | 1.73 | 1.21–2.47 | 0.003 |
| Basal insulin (n = 2,692) | 2.58 | 1.72–3.88 | <0.001 | 1.89 | 1.35–2.65 | <0.001 |
Definition of abbreviations: CI = confidence interval; DPP-4 = dipeptidyl peptidase-4; GLP-1R = glucagon-like peptide-1 receptor; ref = reference; SGLT-2 = sodium–glucose cotransporter-2.
Zero-inflated Poisson regression model and count model (Poisson); both primary and secondary models reflect adjustment for significantly associated covariates including asthma severity, comorbid chronic obstructive pulmonary disease, and propensity score. The primary outcome model also reflects adjustment for metformin use, which was negatively associated with exacerbations but not symptoms.
Drugs in each class are detailed in Table E2.
Figure 2.

Incidence rate ratios and 95% confidence intervals (CIs) for asthma exacerbations and asthma symptom encounters among patients with type 2 diabetes. Users of SGLT-2 (sodium–glucose cotransporter-2) inhibitors, DPP-4 (dipeptidyl peptidase-4) inhibitors, sulfonylureas, and basal insulins were compared with GLP-1R (glucagon-like peptide-1 receptor) agonist users (reference group). (A) GLP-1R agonist users have fewer asthma exacerbations compared with all comparator cohorts. Asthma exacerbations incidence rate ratios and 95% CIs are derived from zero-inflated Poisson regression models. (B) GLP-1R agonist users have fewer asthma symptoms compared with DPP-4 inhibitor, sulfonylurea, and basal insulin groups. Asthma symptom encounter incidence rate ratios and 95% CIs are derived from zero-inflated Poisson regression models. ref = reference.
Sensitivity, Subgroup, and Exploratory Analyses
In the propensity score–adjusted analysis inclusive of pre– and post–study period HbA1c and BMI values, GLP-1R agonist users had statistically significant lower asthma exacerbation counts compared with all comparator groups (Table 3). The odds of having no exacerbations were not increased by GLP-1R agonist initiation compared with DPP-4 inhibitor, sulfonylurea, and insulin initiation; SLGT-2 inhibitor users had increased odds of zero exacerbations (Table E6). Missing covariate data were evenly distributed across the newer diabetes drug classes, including GLP-1R agonist, SGLT-2 inhibitors, and DPP-4 inhibitors. Comparatively, basal insulin and sulfonylurea classes had more missing BMI and HbA1c data. Patients missing any of these four values were excluded in this analysis.
Table 3.
Sensitivity Analysis for Asthma Exacerbations Outcome, Inclusive of Baseline and Change in HbA1c and BMI
| Treatment Groups* | Asthma Exacerbations |
||
|---|---|---|---|
| Incidence Rate Ratio | 95% CI | P Value | |
| GLP-1R (n = 271) | ref | — | — |
| SGLT-2 inhibitor (n = 74) | 2.95 | 1.19–7.31 | 0.02 |
| DPP-4 inhibitor (n = 224) | 2.11 | 1.14–3.91 | 0.02 |
| Sulfonylurea (n = 1,007) | 1.97 | 1.14–3.41 | 0.02 |
| Basal insulin (n = 1,015) | 2.44 | 1.42–4.19 | 0.001 |
Definition of abbreviations: BMI = body mass index; CI = confidence interval; DPP-4 = dipeptidyl peptidase-4; GLP-1R = glucagon-like peptide-1 receptor; ref = reference; SGLT-2 = sodium–glucose cotransporter-2.
Zero-inflated Poisson regression model, count model; reflects adjustment for significantly associated covariates including comorbid chronic obstructive pulmonary disease, metformin use, baseline HbA1c, change in BMI, and propensity score.
Drugs in each class are detailed in Table E2.
Among users with more than two prescriptions for a given study drug during the 6-month study period (n = 1,548), GLP-1R agonist users still had fewer asthma exacerbations compared with DPP-4 inhibitor (incidence rate ratio [IRR], 2.31; 95% confidence interval [CI], 1.08–4.91; P = 0.03) and basal insulin (IRR, 2.88; 95% CI, 1.63–20.85; P = 0.002) groups, with trends observed in sulfonylurea (IRR, 1.81; 95% CI, 0.92–3.56; P = 0.09) and SGLT-2 inhibitor (IRR, 1.75; 95% CI, 0.25–12.23; P = 0.57) groups (Table E7).
The effect of GLP-1R agonists on asthma exacerbations was also independent of prior diabetes drug exposures across study groups. GLP-1R agonist users had fewer exacerbations compared with patients in all comparator groups in the count model, including DPP-4 inhibitor (IRR, 2.51; 95% CI, 1.58–3.98; P = <0.001), sulfonylurea (IRR, 1.84; 95% CI, 1.21–2.78; P = 0.005), SGLT-2 inhibitor (IRR, 2.90; 95% CI, 1.26–6.66; P = 0.01), and basal insulin (IRR, 2.64; 95% CI, 1.76–3.98; P = <0.001) users.
The association between GLP-1R agonist use and decreased asthma exacerbations was robust in the moderate/severe asthma subgroup (Table E8) despite a smaller sample size (n = 2,828). Among never-smokers (n = 2,335), GLP-1R agonist users had fewer exacerbations compared with DPP-4 inhibitor, sulfonylurea, and basal insulin users but not with the SGLT-2 inhibitor group, which was limited by small sample size (n = 69) (Table E9).
We tested for the possibility of asthma medication noncompliance or degree of routine asthma healthcare use as drivers of asthma exacerbations. In exploratory analyses, we found that there were no differences across groups compared with GLP-1R agonist users for routine asthma encounters or SABA prescriptions during the study period (Table E10).
Discussion
In this EHR-based retrospective cohort study of patients with asthma and type 2 diabetes requiring intensified type 2 DM therapy, those initiating GLP-1R agonists had fewer asthma exacerbations than those initiating alternate agents. There were no differences in baseline asthma severity across the groups and no differences in routine asthma care encounters during the study period. The propensity score–adjusted analysis accounted for important demographic confounders, including sex and race/ethnicity, as well as clinical confounders, such as concurrent metformin use, seasonality, and smoking status. There were no differences in rate of drug initiation by individual month or by regional season. This was robust when also accounting for changes in HbA1c and in BMI, suggesting that the observed association of GLP-1R agonists with decreased exacerbations is independent of improved glycemic control and weight loss.
Our secondary outcome analysis suggests that GLP-1R agonist initiators also have reduced asthma symptoms. We excluded diagnostic mimics of asthma, strengthening the specificity of these symptoms even in the context of the metabolic syndrome (46). Studies have shown that a history of asthma, as established in our study cohort, significantly increases the positive predictive value of asthma symptoms, as indicative of poor asthma control in EHR (47), even in the setting of obesity (48). Standardized patient-reported asthma control questionnaires, such as the Asthma Control Test and Asthma Control Questionnaire, use symptoms of “shortness of breath” and “wheeze” to measure symptom control (49, 50). Patients on GLP-1R agonists had fewer associated encounters coded with these symptoms compared with basal insulin and sulfonylurea users. This is distinct from routine encounters for asthma, which do not necessarily indicate any symptomatic state. No difference in symptom burden was seen compared with the SGLT-2 inhibitor group; however, these users had greater systemic steroid exposures because of asthma exacerbations during the same period, which may have improved symptom control. Although we excluded other respiratory diseases from our patient sample, we cannot exclude the possibility that these symptoms may be confounded by another disease process, such as atherosclerotic cardiovascular disease, also ameliorated by GLP-1R agonist use (19).
Our data further suggest that GLP-1R agonists are associated with decreased exacerbation counts among patients with exacerbations, particularly among the moderate/severe asthma subgroup, as GLP-1R agonist use was not consistently associated with increased odds of having zero exacerbations. This supports the observed clinical benefit of GLP-1R agonists among those with a more severe asthma phenotype. Because our sample included patients with a range of asthma severity, we expected a high proportion of 0 counts and low mean counts of exacerbations. This resulted in a modest absolute reduction in exacerbation rates, which has implications for study design and sample size calculations for future prospective studies. Although a minority of patients with asthma in the study were cocoded for COPD, they were predominantly classified as having moderate/severe asthma in line with prior estimates (44). The presence of patients with COPD in the moderate/severe asthma group may reflect the clinical use of ICD codes to capture ACO, which lacks specific diagnostic ICD codes. Clinical studies have traditionally excluded these patients (44), and future research is needed to ascertain the phenotyping of these patients in the EHR in addition to the underlying pathobiology to examine the potential role of metabolic pathways and therapeutic targets (14). Early research suggests that metformin, a first-line diabetes therapy, may be beneficial for ACO, highlighting the potential relevance of metabolic pathways in this group and warranting further investigation in this field (51). As this study’s inclusion criteria only phenotyped patients with asthma, excluding patients with COPD only, future research is needed to assess the impact GLP-1R agonist use on COPD-specific exposures and outcomes.
Strengths of this EHR analysis are the inclusion of BMI and HbA1c data, which are clinically relevant covariates that may not be available in administrative datasets. The relationship of metabolic dysfunction and asthma represents an area of active investigation (8, 52). Other studies using administrative data have found that antidiabetic therapies variably impact asthma in the setting of metabolic dysfunction, with metformin attenuating asthma exacerbation risk (9, 10), consistent with our findings, and basal insulin being associated with incident asthma (12). However, these studies were limited in their ability to address the impact of clinical covariates on their primary outcomes. These analyses point to a therapeutic association of GLP-1R agonists independent of metformin use, baseline and change in BMI, and glycemic control. Future areas of research include examining synergistic effects of antidiabetes therapy, such as metformin and GLP-1R agonists, on pulmonary outcomes.
Limitations
This study relies on EHR data. EHR data are considered real-world data representing routine clinical care (39) that may be used to support supplementary indications for FDA-approved medications (53). However, there is considerable heterogeneity in approaches to disease phenotyping of EHR data (28, 54–57) for asthma and DM, and we chose stringent phenotypes to address this limitation. There is potential for unmeasured confounding in this analysis. To minimize this, we also excluded patients who might routinely receive steroids and did not include prescriptions for inhaled corticosteroids in our outcome definition, consistent with core outcome measures for clinical asthma trials. Despite these steps, we could not exclude systemic steroids prescribed for an indication other than asthma exacerbation.
Similarly, data from the EHR reflect care sought by patients at sites within our health system. Although our system is the largest in the region and patients in this study met a high level of data completeness (31), it is not a closed system, and there is no mechanism in place to flag outcomes that occur elsewhere but are missing in our EHR. Therefore, our outcome events may be underestimated across all drug exposures, as patients may have sought care, particularly for acute episodes such as asthma exacerbations, at unaffiliated sites.
Finally, we took several steps to address confounders stemming from possible mechanistic links between metabolic and pulmonary dysfunction. We required a complete clinical data set for a sensitivity analysis that included baseline HbA1c, baseline BMI, and change in both parameters. The association for lower counts of asthma exacerbations among GLP-1R agonist users was robust across all the comparator groups, as previously discussed. Clinical data missingness was proportionally higher for basal insulin and sulfonylureas than the other three classes, and we did not impute missing data. Missingness across years may be due to changes in EHR platforms or coding practice over time (39). When weight and height were not both available at the same encounter, we carried forward height from prior visits to calculate BMI, yet missing data for this important characteristic remains a weakness of this study. Insulin can be weight promoting, whereas GLP-1R agonists have a favorable weight-loss profile. As obesity is associated with more poorly controlled asthma (6, 7), we cannot rule out residual confounding from weight in the basal insulin–exposed population.
Conclusions
Among patients with asthma and type 2 diabetes requiring intensified type 2 DM therapy, those initiating GLP-1R agonists had lower counts of asthma exacerbations and asthma symptoms within 6 months of drug initiation compared with patients initiating other DM medications. Prospective human studies to validate these findings and to understand the mechanism(s) of the GLP-1R in the airway are needed to support the clinical selection of GLP-1R agonists for patients with asthma with and without comorbid metabolic dysfunction.
Supplementary Material
Acknowledgments
Acknowledgment
The Strengthening the Reporting of Observational studies in Epidemiology guidelines were used in the reporting of this observational study (58).
Footnotes
Supported by an internal grant from the Department of Medicine of Brigham and Women’s Hospital (D.F., K.N.C., and D.W.B.) and by NIH grants P30-AR070253, U01-HG00868, and P30-AR072577 (E.W.K.); T32-AI007306 (D.F.); and K23-AI118804 (K.N.C.).
Author Contributions: All authors have contributed significantly to this work and meet criteria for authorship by the International Committee of Medical Journal Editors recommendations. Conception and design: All authors. Acquisition of data: D.F., P.E.B., and J.C. Analysis and interpretation of data: All authors. Drafting the manuscript for important intellectual content: D.F., P.E.B., E.W.K., and K.N.C. Revising the manuscript for important intellectual content: J.C. and D.W.B. Final approval of the version to be published: All authors.
This article has an online supplement, which is accessible from this issue’s table of contents at www.atsjournals.org.
Originally Published in Press as DOI: 10.1164/rccm.202004-0993OC on October 14, 2020
Author disclosures are available with the text of this article at www.atsjournals.org.
References
- 1.Peters MC, Mauger D, Ross KR, Phillips B, Gaston B, Cardet JC, et al. Evidence for exacerbation-prone asthma and predictive biomarkers of exacerbation frequency. Am J Respir Crit Care Med. 2020;202:973–982. doi: 10.1164/rccm.201909-1813OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Cardet JC, Ash S, Kusa T, Camargo CA, Jr, Israel E. Insulin resistance modifies the association between obesity and current asthma in adults. Eur Respir J. 2016;48:403–410. doi: 10.1183/13993003.00246-2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Peters MC, McGrath KW, Hawkins GA, Hastie AT, Levy BD, Israel E, et al. National Heart, Lung, and Blood Institute Severe Asthma Research Program. Plasma interleukin-6 concentrations, metabolic dysfunction, and asthma severity: a cross-sectional analysis of two cohorts. Lancet Respir Med. 2016;4:574–584. doi: 10.1016/S2213-2600(16)30048-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Wu TD, Brigham EP, Keet CA, Brown TT, Hansel NN, McCormack MC. Association between prediabetes/diabetes and asthma exacerbations in a claims-based obese asthma cohort. J Allergy Clin Immunol Pract. 2019;7:1868–1873, e5. doi: 10.1016/j.jaip.2019.02.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Denlinger LC, Phillips BR, Ramratnam S, Ross K, Bhakta NR, Cardet JC, et al. National Heart, Lung, and Blood Institute’s Severe Asthma Research Program-3 Investigators. Inflammatory and comorbid features of patients with severe asthma and frequent exacerbations. Am J Respir Crit Care Med. 2017;195:302–313. doi: 10.1164/rccm.201602-0419OC. [Published erratum appears in Am J Respir Crit Care Med 197:971.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Schatz M, Hsu JW, Zeiger RS, Chen W, Dorenbaum A, Chipps BE, et al. Phenotypes determined by cluster analysis in severe or difficult-to-treat asthma. J Allergy Clin Immunol. 2014;133:1549–1556. doi: 10.1016/j.jaci.2013.10.006. [DOI] [PubMed] [Google Scholar]
- 7.Holguin F, Bleecker ER, Busse WW, Calhoun WJ, Castro M, Erzurum SC, et al. Obesity and asthma: an association modified by age of asthma onset. J Allergy Clin Immunol. 2011;127:1486–1493, e2. doi: 10.1016/j.jaci.2011.03.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Suratt BT, Ubags NDJ, Rastogi D, Tantisira KG, Marsland BJ, Petrache I, et al. Allergy, Immunology, and Inflammation Assembly. An official American Thoracic Society workshop report: obesity and metabolism. An emerging frontier in lung health and disease. Ann Am Thorac Soc. 2017;14:1050–1059. doi: 10.1513/AnnalsATS.201703-263WS. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Li CY, Erickson SR, Wu CH. Metformin use and asthma outcomes among patients with concurrent asthma and diabetes. Respirology. 2016;21:1210–1218. doi: 10.1111/resp.12818. [DOI] [PubMed] [Google Scholar]
- 10.Wu TD, Keet CA, Fawzy A, Segal JB, Brigham EP, McCormack MC. Association of metformin initiation and risk of asthma exacerbation: a claims-based cohort study. Ann Am Thorac Soc. 2019;16:1527–1533. doi: 10.1513/AnnalsATS.201812-897OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Rogliani P, Calzetta L, Capuani B, Facciolo F, Cazzola M, Lauro D, et al. Glucagon-like peptide 1 receptor: a novel pharmacological target for treating human bronchial hyperresponsiveness. Am J Respir Cell Mol Biol. 2016;55:804–814. doi: 10.1165/rcmb.2015-0311OC. [DOI] [PubMed] [Google Scholar]
- 12.Rayner LH, Mcgovern A, Sherlock J, Gatenby P, Correa A, Creagh-Brown B, et al. The impact of therapy on the risk of asthma in type 2 diabetes. Clin Respir J. 2019;13:299–305. doi: 10.1111/crj.13011. [DOI] [PubMed] [Google Scholar]
- 13.Colice G, Price D, Gerhardsson de Verdier M, Rabon-Stith K, Ambrose C, Cappell K, et al. The effect of DPP-4 inhibitors on asthma control: an administrative database study to evaluate a potential pathophysiological relationship. Pragmat Obs Res. 2017;8:231–240. doi: 10.2147/POR.S144018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Siddiqui S, Denlinger LC, Fowler SJ, Akuthota P, Shaw DE, Heaney LG, et al. Bridging Clinical and Patient Perspectives. Unmet needs in severe asthma subtyping and precision medicine trials. Am J Respir Crit Care Med. 2019;199:823–829. doi: 10.1164/rccm.201809-1817PP. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Scrocchi LA, Marshall BA, Cook SM, Brubaker PL, Drucker DJ. Identification of glucagon-like peptide 1 (GLP-1) actions essential for glucose homeostasis in mice with disruption of GLP-1 receptor signaling. Diabetes. 1998;47:632–639. doi: 10.2337/diabetes.47.4.632. [DOI] [PubMed] [Google Scholar]
- 16.Diakogiannaki E, Gribble FM, Reimann F. Nutrient detection by incretin hormone secreting cells. Physiol Behav. 2012;106:387–393. doi: 10.1016/j.physbeh.2011.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.American Diabetes Association. Standards of medical care in diabetes. Diabetes Care. 2020;43 [Google Scholar]
- 18.Campbell JE, Drucker DJ. Pharmacology, physiology, and mechanisms of incretin hormone action. Cell Metab. 2013;17:819–837. doi: 10.1016/j.cmet.2013.04.008. [DOI] [PubMed] [Google Scholar]
- 19.Bethel MA, Patel RA, Merrill P, Lokhnygina Y, Buse JB, Mentz RJ, et al. EXSCEL Study Group. Cardiovascular outcomes with glucagon-like peptide-1 receptor agonists in patients with type 2 diabetes: a meta-analysis. Lancet Diabetes Endocrinol. 2018;6:105–113. doi: 10.1016/S2213-8587(17)30412-6. [DOI] [PubMed] [Google Scholar]
- 20.Zheng SL, Roddick AJ, Aghar-Jaffar R, Shun-Shin MJ, Francis D, Oliver N, et al. Association between use of sodium-glucose cotransporter 2 inhibitors, glucagon-like peptide 1 agonists, and dipeptidyl peptidase 4 inhibitors with all-cause mortality in patients with type 2 diabetes: a systematic review and meta-analysis. JAMA. 2018;319:1580–1591. doi: 10.1001/jama.2018.3024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Baggio LL, Drucker DJ. Biology of incretins: GLP-1 and GIP. Gastroenterology. 2007;132:2131–2157. doi: 10.1053/j.gastro.2007.03.054. [DOI] [PubMed] [Google Scholar]
- 22.Viby NE, Isidor MS, Buggeskov KB, Poulsen SS, Hansen JB, Kissow H. Glucagon-like peptide-1 (GLP-1) reduces mortality and improves lung function in a model of experimental obstructive lung disease in female mice. Endocrinology. 2013;154:4503–4511. doi: 10.1210/en.2013-1666. [DOI] [PubMed] [Google Scholar]
- 23.Bloodworth MH, Rusznak M, Pfister CC, Zhang J, Bastarache L, Calvillo SA, et al. Glucagon-like peptide 1 receptor signaling attenuates respiratory syncytial virus-induced type 2 responses and immunopathology. J Allergy Clin Immunol. 2018;142:683–687, e12. doi: 10.1016/j.jaci.2018.01.053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Toki S, Goleniewska K, Reiss S, Zhang J, Bloodworth MH, Stier MT, et al. Glucagon-like peptide 1 signaling inhibits allergen-induced lung IL-33 release and reduces group 2 innate lymphoid cell cytokine production in vivo. J Allergy Clin Immunol. 2018;142:1515–1528, e8. doi: 10.1016/j.jaci.2017.11.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Foer D, Beeler PE, Cui J, Karlson E, Bates DW, Cahill KN. Glucagon-like peptide-1 receptor agonist use and asthma exacerbations among type 2 diabetics with asthma [abstract] Am J Respir Crit Care Med. 2020;201:A2736. [Google Scholar]
- 26.Murphy SN, Weber G, Mendis M, Gainer V, Chueh HC, Churchill S, et al. Serving the enterprise and beyond with informatics for integrating biology and the bedside (i2b2) J Am Med Inform Assoc. 2010;17:124–130. doi: 10.1136/jamia.2009.000893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Pacheco JA, Avila PC, Thompson JA, Law M, Quraishi JA, Greiman AK, et al. A highly specific algorithm for identifying asthma cases and controls for genome-wide association studies. AMIA Annu Symp Proc. 2009;2009:497–501. [PMC free article] [PubMed] [Google Scholar]
- 28.Spratt SE, Pereira K, Granger BB, Batch BC, Phelan M, Pencina M, et al. DDC Phenotype Group. Assessing electronic health record phenotypes against gold-standard diagnostic criteria for diabetes mellitus. J Am Med Inform Assoc. 2017;24:e121–e128. doi: 10.1093/jamia/ocw123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Richesson RL, Rusincovitch SA, Wixted D, Batch BC, Feinglos MN, Miranda ML, et al. A comparison of phenotype definitions for diabetes mellitus. J Am Med Inform Assoc. 2013;20:e319–e326. doi: 10.1136/amiajnl-2013-001952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Afshar M, Press VG, Robison RG, Kho AN, Bandi S, Biswas A, et al. A computable phenotype for asthma case identification in adult and pediatric patients: external validation in the Chicago Area Patient-Outcomes Research Network (CAPriCORN) J Asthma. 2018;55:1035–1042. doi: 10.1080/02770903.2017.1389952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Weber GM, Adams WG, Bernstam EV, Bickel JP, Fox KP, Marsolo K, et al. Biases introduced by filtering electronic health records for patients with “complete data”. J Am Med Inform Assoc. 2017;24:1134–1141. doi: 10.1093/jamia/ocx071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Franklin JM, Glynn RJ, Martin D, Schneeweiss S. Evaluating the use of nonrandomized real-world data analyses for regulatory decision making. Clin Pharmacol Ther. 2019;105:867–877. doi: 10.1002/cpt.1351. [DOI] [PubMed] [Google Scholar]
- 33.World Health Organization. Adherence to long-term therapies: evidence for action. Geneva, Switzerland: World Health Organization; 2003 [accessed 2020 Jul 15]. Available from: https://www.who.int/chp/knowledge/publications/adherence_Section1.pdf.
- 34.Divino V, DeKoven M, Hallinan S, Varol N, Wirta SB, Lee WC, et al. Glucagon-like peptide-1 receptor agonist treatment patterns among type 2 diabetes patients in six European countries. Diabetes Ther. 2014;5:499–520. doi: 10.1007/s13300-014-0087-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Johnston SS, Nguyen H, Felber E, Cappell K, Nelson JK, Chu BC, et al. Retrospective study of adherence to glucagon-like peptide-1 receptor agonist therapy in patients with type 2 diabetes mellitus in the United States. Adv Ther. 2014;31:1119–1133. doi: 10.1007/s12325-014-0166-0. [DOI] [PubMed] [Google Scholar]
- 36.Fuhlbrigge A, Peden D, Apter AJ, Boushey HA, Camargo CA, Jr, Gern J, et al. Asthma outcomes: exacerbations. J Allergy Clin Immunol. 2012;129(3) Suppl:S34–S48. doi: 10.1016/j.jaci.2011.12.983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Zeiger RS, Schatz M, Li Q, Chen W, Khatry DB, Gossage D, et al. High blood eosinophil count is a risk factor for future asthma exacerbations in adult persistent asthma. J Allergy Clin Immunol Pract. 2014;2:741–750. doi: 10.1016/j.jaip.2014.06.005. [DOI] [PubMed] [Google Scholar]
- 38.Lane SJ, Petersen H, Seltzer JM, Blanchette CM, Navaratnam P, Allen-Ramey F, et al. Moderate symptom-based exacerbations as predictors of severe claims-based exacerbations in asthma. J Asthma. 2013;50:642–648. doi: 10.3109/02770903.2013.787624. [DOI] [PubMed] [Google Scholar]
- 39.Schneeweiss S, Avorn J. A review of uses of health care utilization databases for epidemiologic research on therapeutics. J Clin Epidemiol. 2005;58:323–337. doi: 10.1016/j.jclinepi.2004.10.012. [DOI] [PubMed] [Google Scholar]
- 40.Quan H, Sundararajan V, Halfon P, Fong A, Burnand B, Luthi JC, et al. Coding algorithms for defining comorbidities in ICD-9-CM and ICD-10 administrative data. Med Care. 2005;43:1130–1139. doi: 10.1097/01.mlr.0000182534.19832.83. [DOI] [PubMed] [Google Scholar]
- 41.Anderson B, Taylor S, Harding A, Meaklim A, O’Keefe C. Thunderstorm asthma management during a code brown in a hospital with broad implementation of electronic prescribing and clinical documentation. Emerg Med Australas. 2018;30:33. [Google Scholar]
- 42.Centers for Disease Control and Prevention. Atlanta, GA: Centers for Disease Control and Prevention; 2018. Asthma data statistics and surveillance. [accessed 2019 Dec 12]. Available from: https://www.cdc.gov/asthma/asthmadata.htm. [Google Scholar]
- 43.Global Initiative for Asthma. Global strategy for asthma management and prevention. 2018 [accessed 2019 Dec 21]. Global Initiative for Asthma. Available from: https://ginasthma.org/
- 44.Woodruff PG, van den Berge M, Boucher RC, Brightling C, Burchard EG, Christenson SA, et al. American Thoracic Society/National Heart, Lung, and Blood Institute asthma-chronic obstructive pulmonary disease overlap workshop report. Am J Respir Crit Care Med. 2017;196:375–381. doi: 10.1164/rccm.201705-0973WS. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Himes BE, Dai Y, Kohane IS, Weiss ST, Ramoni MF. Prediction of chronic obstructive pulmonary disease (COPD) in asthma patients using electronic medical records. J Am Med Inform Assoc. 2009;16:371–379. doi: 10.1197/jamia.M2846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Lee EJ, In KH, Ha ES, Lee KJ, Hur GY, Kang EH, et al. Asthma-like symptoms are increased in the metabolic syndrome. J Asthma. 2009;46:339–342. doi: 10.1080/02770900802660931. [DOI] [PubMed] [Google Scholar]
- 47.Sanders DL, Gregg W, Aronsky D. Identifying asthma exacerbations in a pediatric emergency department: a feasibility study. Int J Med Inform. 2007;76:557–564. doi: 10.1016/j.ijmedinf.2006.03.003. [DOI] [PubMed] [Google Scholar]
- 48.Thomson CC, Clark S, Camargo CA, Jr MARC Investigators. Body mass index and asthma severity among adults presenting to the emergency department. Chest. 2003;124:795–802. doi: 10.1378/chest.124.3.795. [DOI] [PubMed] [Google Scholar]
- 49.Nathan RA, Sorkness CA, Kosinski M, Schatz M, Li JT, Marcus P, et al. Development of the asthma control test: a survey for assessing asthma control. J Allergy Clin Immunol. 2004;113:59–65. doi: 10.1016/j.jaci.2003.09.008. [DOI] [PubMed] [Google Scholar]
- 50.Juniper EF, O’Byrne PM, Guyatt GH, Ferrie PJ, King DR. Development and validation of a questionnaire to measure asthma control. Eur Respir J. 1999;14:902–907. doi: 10.1034/j.1399-3003.1999.14d29.x. [DOI] [PubMed] [Google Scholar]
- 51.Wu TD, Fawzy A, Kinney GL, Bon JM, Neupane M, Hansel NN, et al. Lower rate of COPD exacerbations among metformin users with asthma-COPD overlap [abstract] Am J Respir Crit Care Med. 2020;201:A6286. [Google Scholar]
- 52.Baffi CW, Wood L, Winnica D, Strollo PJ, Jr, Gladwin MT, Que LG, et al. Metabolic syndrome and the lung. Chest. 2016;149:1525–1534. doi: 10.1016/j.chest.2015.12.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.U.S. Food and Drug Administration. Silver Spring, MD: Food and Drug Administration; 2018. Framework for FDA’s Real-World Evidence Program. [accessed 2020 Feb 27]. Available from: www.fda.gov/media/120060/download. [Google Scholar]
- 54.Wei WQ, Denny JC. Extracting research-quality phenotypes from electronic health records to support precision medicine. Genome Med. 2015;7:41. doi: 10.1186/s13073-015-0166-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Shivade C, Raghavan P, Fosler-Lussier E, Embi PJ, Elhadad N, Johnson SB, et al. A review of approaches to identifying patient phenotype cohorts using electronic health records. J Am Med Inform Assoc. 2014;21:221–230. doi: 10.1136/amiajnl-2013-001935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Nissen F, Quint JK, Wilkinson S, Muellerova H, Smeeth L, Douglas IJ. Validation of asthma recording in electronic health records: a systematic review. Clin Epidemiol. 2017;9:643–656. doi: 10.2147/CLEP.S143718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Al Sallakh MA, Vasileiou E, Rodgers SE, Lyons RA, Sheikh A, Davies GA. Defining asthma and assessing asthma outcomes using electronic health record data: a systematic scoping review. Eur Respir J. 2017;49:1700204. doi: 10.1183/13993003.00204-2017. [DOI] [PubMed] [Google Scholar]
- 58.von Elm E, Altman DG, Egger M, Pocock SJ, Gøtzsche PC, Vandenbroucke JP STROBE Initiative. The Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) statement: guidelines for reporting observational studies. Lancet. 2007;370:1453–1457. doi: 10.1016/S0140-6736(07)61602-X. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


