Abstract
Introduction:
Long-term opioid therapy increases the risk of opioid overdose death. Government agencies and medical societies including the Center for Disease Control and Prevention and American Society for Clinical Oncology emphasized risk mitigation strategies including urine drug testing, in published guidelines. Urine drug testing rates, time trends, and covariates among long-term opioid therapy users were examined to gauge guideline adherence.
Methods:
Using Optum’s De-identified Clinformatics DataMart, an incidence cohort (n=28,790) and prevalence cohort (n=621,449) were created to measure baseline and annual urine drug testing respectively, from 2012 to 2018. Urine drug testing time trends were evaluated by demographics, pain conditions, and Elixhauser comorbidity index. A multivariable generalized estimating model was developed in 2020 to examine factors associated with urine drug testing.
Results:
Annual urine drug testing rates doubled from 25.6% in 2012 to 52.2% in 2018, whereas baseline urine drug testing also increased, from 3.75% to 11.1%. Annual urine drug testing increased within each age group over time; however, older patients (OR=0.21, 95% CI=0.21, 0.22, >79 years) and cancer patients (OR=0.82, 95% CI=0.80, 0.84) were less likely to receive urine drug testing. Patients residing in the South (OR=1.99, 95% CI=1.96, 2.01), with back pain (OR=2.04, 95% CI=2.02, 2.06) or other chronic pain (OR=1.64, 95% CI=1.62, 1.66) were significantly more likely to be tested. Independent predictors of baseline urine drug testing were similar to predictors of annual urine drug testing.
Conclusions:
Despite increasing urine drug testing trends from 2012 to 2018, annual and baseline urine drug testing remained low in 2018, relative to numerous guideline recommendations. Findings suggest a need for research on better guideline implementation strategies and effectiveness of urine drug testing on patient outcomes.
INTRODUCTION
Long-term opioid therapy (LTOT; >90 days) presents serious risks, including development of substance use disorder and overdose death.1 Numerous agencies and medical societies, including the Centers for Disease Control and Prevention and the American Society of Clinical Oncology, have published guidelines recommending risk-assessment strategies, drug monitoring, and urine drug testing (UDT) to assist providers in safe opioid prescribing.1–8
Although evidence on improved patient outcomes resulting from UDT is lacking, UDT is important for monitoring compliance and detecting non-prescribed or illicit drug use.1,6,9–12 UDT should include screening and definitive urine testing to effectively identify compliance or misuse13; failure to confirm results, whether positive or negative, has been considered poor practice,7,14 though may be costly. Guidelines generally suggest baseline UDT of chronic pain patients (including cancer) before initiating LTOT, and at least annually thereafter. Others suggest increasing the frequency of UDT based on patient risk assessments.1,6–8,15,16
Rates of UDT vary substantially, from 2% to 50%, with most studies being cross-sectional, limited in sample size, or from regional samples.17–22 This study uses insurance claims to examine national trends and characteristics associated with UDT among LTOT users from 2012 to 2018. This population-based study informs policymakers on potential changes needed to improve guideline-recommended UDT adherence by prescribers.
METHODS
This study used Optum’s De-identified Clinformatics DataMart,23 a large national commercial insurance database. The UDT prevalence cohort included LTOT users with chronic pain conditions between 2012 and 2018. Prevalent LTOT users could be counted for multiple years if they had ≥90 days of opioid use in each calendar year. UDT incidence included LTOT users without opioid use in the year prior to initiation of LTOT, with their first opioid prescription >28 days. Exclusion criteria for each cohort are in Appendix Table 2. Calendar year was used for the prevalence cohort in order to report annual UDT rates, whereas rolling year was used for continuous enrollment in the incidence cohort to capture the time window specific for opioid initiation.
The study outcomes were annual UDT, defined as receiving any UDT within 1 year in the prevalence cohort, and baseline UDT defined as receiving any UDT in the 7 days before or after LTOT initiation in the incidence cohort. Any UDT refers to either presumptive urine screening or definitive testing (Appendix Table 2).
Patient characteristics included age at LTOT initiation, sex, U.S. Census region, Elixhauser comorbidity score, and pain condition. Additionally, opioid morphine milligram equivalents per day, number of opioid prescriptions, and UDTs were included in the prevalence cohort. Elixhauser comorbidities were summed together for score after removing alcohol abuse, drug abuse, psychoses, and depression, which are major risk factors associated with LTOT.24,25 Chronic pain conditions included back pain, joint pain, nerve pain or neuropathy, cancer, musculoskeletal pain, or other chronic pain identified by ICD codes (Appendix Table 2).26
Descriptive statistics were generated for each cohort. Annual and baseline UDT rates were calculated by year and stratified by age and region. To assess the association between the listed variables and UDT, multivariable generalized estimating models with a binomial distribution and AR1 covariance matrix were used in each cohort. All analyses were performed in 2020 using SAS, version 9.4.
RESULTS
The prevalence cohort included 1,228,044 person years, with mean age of 63.4 (SD=13.1) years, while the incidence cohort included 29,202 person years, with mean age 65.5 (SD=14.2) years (Table 1). Patients were mostly female (56%–58.2%), residing in the South (45.5%–48.9%), with prevalent back pain (38.5%–51.4%) or joint pain (30.2%–30.6%).
Table 1.
Patient Characteristics Among LTOT Users in Prevalence and Incidence Cohorts, Describing Age, Sex, Region, Comorbidity Score, and Pain Conditions
| Variable | Prevalent opioid use cohorta | Incident opioid use cohortb |
|---|---|---|
| n (%) | n (%) | |
| Sex | ||
| Female | 714,255 (58.2) | 16,366 (56.0) |
| Male | 513,789 (41.8) | 12,836 (44.0) |
| Age, years | 63.4 (13.1) | 65.5 (14.2) |
| <50 | 176,309 (14.4) | 3,983 (13.6) |
| 50–59 | 286,999 (23.4) | 5,090 (17.4) |
| 60–69 | 343,784 (28.0) | 7,522 (25.8) |
| 70–79 | 274,003 (22.3) | 7,340 (25.1) |
| >79 | 146,949 (12.0) | 5,267 (18.0) |
| Region | ||
| Midwest | 240,448 (19.6) | 5,150 (17.6) |
| Northeast | 83,135 (6.8) | 2,123 (7.3) |
| South | 600,193 (48.9) | 13,294 (45.5) |
| West | 304,268 (24.8) | 8,635 (29.6) |
| Year | ||
| 2012 | 151,664 (12.4) | 3,890 (13.3) |
| 2013 | 167,578 (13.6) | 4,542 (15.6) |
| 2014 | 157,284 (12.8) | 3,660 (12.5) |
| 2015 | 157,516 (12.8) | 5,446 (18.6) |
| 2016 | 169,991 (13.8) | 3,957 (13.6) |
| 2017 | 209,419 (17.1) | 4,668 (16.0) |
| 2018 | 214,592 (17.5) | 3,039 (10.4) |
| Elixhauser comorbidity score,c mean (SD) | 3.4 (2.8) | 2.8 (2.6) |
| Elixhauser category | ||
| 0 | 144,195 (11.7) | 6,324 (21.7) |
| 1 | 200,981 (16.4) | 4,859 (16.6) |
| 2 | 212,918 (17.3) | 4,813 (16.5) |
| 3 | 183,107 (14.9) | 3,965 (13.6) |
| 4 | 142,699 (11.6) | 2,886 (9.9) |
| 5+ | 344,144 (28.0) | 6,355 (21.8) |
| Alcohol abuse | 33,448 (2.7) | 610 (2.1) |
| Drug abuse | 147,414 (12.0) | 1,541 (5.3) |
| Psychoses | 34,720 (2.8) | 723 (2.5) |
| Depression | 375,798 (30.6) | 6,097 (20.9) |
| Back paind | 631,728 (51.4) | 11,237 (38.5) |
| Joint pain | 370,338 (30.2) | 8,948 (30.6) |
| Nerve pain | 108,651 (8.8) | 2,174 (7.4) |
| Other chronic pain | 284,115 (23.1) | 4,173 (14.3) |
| Cancer | 62,740 (5.1) | 1,435 (4.9) |
| Musculoskeletal pain | 194,478 (15.8) | 3,782 (13.0) |
| MME/Day, mean (SD) | 54.4 (81.2) | |
| ≥50 MME/Day | 356,376 (29.0) | |
| ≥90 MME/Day | 184,400 (15.0) | |
| Number of opioid prescriptions, mean (SD) | 14.3 (7.8) | |
| Number of UDT, mean (SD) | 2.9 (2.9) |
Notes: Additionally, information on opioid MME/Day, use of ≥50 or ≥90 MME/day use, number of opioid prescriptions and the number of UDT is presented for the prevalence cohort.
Prevalence cohort included 1,228,004 person-years for 621,449 patients in 2012–2018.
Incidence cohort included 29,202 person-years for 28,790 patients in 2012–2018.
Elixhauser comorbidity scores were calculated after removing alcohol abuse, drug abuse, psychoses, and depression. Elixhauser comorbidity and pain conditions were assessed in the previous calendar year (prevalence cohort) or in the year prior to LTOT initiation (incidence cohort).
In cases when multiple chronic pain conditions were present on the same day, each condition was noted.
MME, morphine milligram equivalent; LTOT, long term opioid therapy.
Annual UDT rates increased from 25.6% in 2012 to 52.2% in 2018 (Figure 1), and generally decreased with age (62.9% in people aged <50 years, 29.1% in those aged >79 years, in 2018) (Appendix Table 3). Baseline UDT rates were significantly lower (3.75%–11.1%). By region, relative increases in UDT of both cohorts were highest in the South and Midwest (Appendix Table 4).
Figure 1.
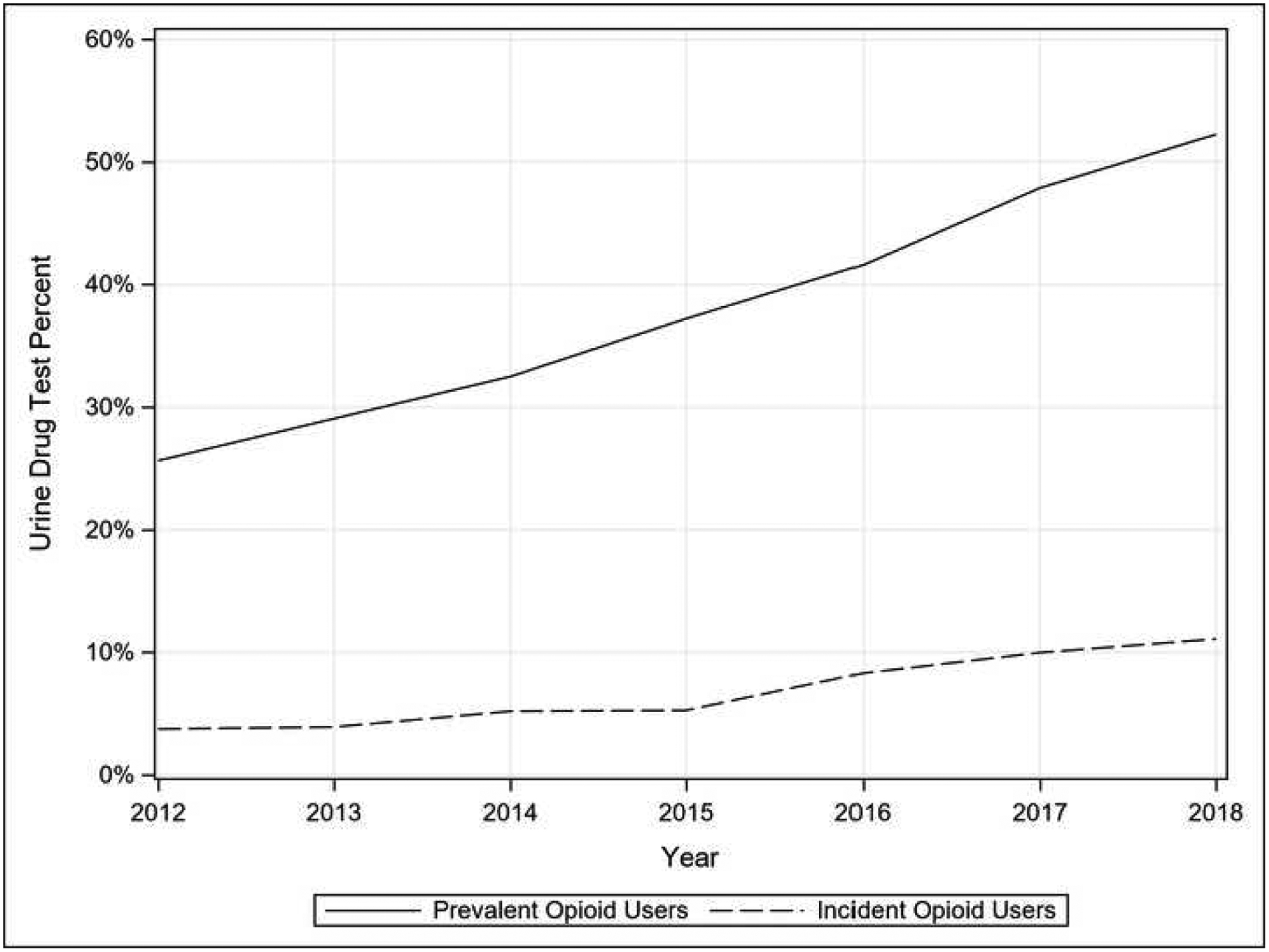
Prevalence and incidence of urine drug testing (UDT) among long-term opioid users, 2012–2018.
Notes: Time trends of UDT represent “any UDT” received by patients with incident or prevalent long-term opioid use. The prevalence cohort represents annual UDT, while the incidence cohort represents baseline UDT.
Patients were less likely to receive annual UDT if they were older (OR=0.21, 95% CI=0.21, 0.22, >79 years) or had cancer (OR=0.82, 95% CI=0.80, 0.84), and more likely if they resided in the South (OR=1.99, 95% CI=1.96, 2.01), had back pain (OR=2.04, 95% CI=2.02, 2.06), or other chronic pain (OR=1.64, 95% CI=1.62, 1.66) (Table 2). Associations with baseline UDT were similar, though cancer patients were even less likely to receive baseline UDT (0.46, 95% CI=0.28, 0.75).
Table 2.
Annual and Baseline UDT Among Long-term Opioid Users of Prevalence and Incidence Cohorts Respectively, Stratified by Age, Sex, Year, Region, Elixhauser Score, and Pain Conditions
| Prevalent long-term opioid users (annual UDT) | Incident long-term opioid users (baseline UDT) | |||
|---|---|---|---|---|
| Parameter | Any UDT, % | Any UDTaOR (95% CI) | Any UDT, % | Any UDTaOR (95% CI) |
| Age, years | ||||
| <50 | 49.94 | ref | 12.70 | ref |
| 50–59 | 48.23 | 0.85 (0.84, 0.86) | 10.06 | 0.75 (0.65, 0.87) |
| 60–69 | 42.01 | 0.62 (0.61, 0.63) | 7.40 | 0.59 (0.51, 0.69) |
| 70–79 | 31.00 | 0.40 (0.40, 0.41) | 3.84 | 0.36 (0.30, 0.42) |
| >79 | 16.73 | 0.21 (0.21, 0.22) | 1.44 | 0.17 (0.13, 0.22) |
| Sex | ||||
| Female | 38.26 | ref | 5.75 | ref |
| Male | 40.32 | 1.02 (1.01, 1.03) | 7.73 | 1.18 (1.07, 1.31) |
| Year | ||||
| 2012 | 25.64 | ref | 3.75 | ref |
| 2013 | 29.07 | 1.21 (1.19, 1.22) | 3.92 | 1.14 (0.90, 1.44) |
| 2014 | 32.49 | 1.49 (1.46, 1.51) | 5.19 | 1.37 (1.09, 1.73) |
| 2015 | 37.22 | 1.82 (1.79, 1.84) | 5.27 | 1.61 (1.30, 2.00) |
| 2016 | 41.61 | 2.14 (2.10, 2.17) | 8.31 | 2.00 (1.62, 2.47) |
| 2017 | 47.88 | 2.66 (2.62, 2.70) | 9.98 | 2.12 (1.73, 2.61) |
| 2018 | 52.23 | 3.28 (3.23, 3.33) | 11.09 | 2.48 (1.99, 3.09) |
| Region | ||||
| Midwest | 31.41 | ref | 4.95 | ref |
| Northeast | 30.45 | 1.00 (0.98, 1.02) | 3.53 | 0.86 (0.65, 1.14) |
| South | 48.25 | 1.99 (1.96, 2.01) | 9.61 | 1.99 (1.71, 2.32) |
| West | 29.57 | 0.98 (0.97, 1.00) | 3.76 | 0.90 (0.75, 1.08) |
| Elixhauser category | ||||
| 0 | 38.13 | ref | 6.12 | ref |
| 1 | 39.26 | 1.05 (1.03, 1.06) | 8.17 | 1.06 (0.90, 1.25) |
| 2 | 38.61 | 1.09 (1.07, 1.10) | 6.79 | 1.01 (0.85, 1.20) |
| 3 | 39.11 | 1.15 (1.14, 1.17) | 6.66 | 1.09 (0.90, 1.31) |
| 4 | 39.37 | 1.18 (1.16, 1.20) | 7.14 | 1.26 (1.03, 1.54) |
| 5+ | 39.67 | 1.23 (1.21, 1.25) | 5.54 | 1.08 (0.90, 1.29) |
| Alcohol abuse | 49.30 | 1.07 (1.05, 1.10) | 10.00 | 0.84 (0.61, 1.17) |
| Depression | 45.65 | 1.16 (1.15, 1.17) | 8.35 | 0.97 (0.86, 1.10) |
| Drug abuse | 66.44 | 1.87 (1.85, 1.89) | 22.71 | 2.76 (2.35, 3.24) |
| Psychoses | 42.62 | 1.03 (1.00, 1.05) | 6.92 | 0.99 (0.71, 1.39) |
| Back pain | 51.47 | 2.04 (2.02, 2.06) | 12.91 | 4.10 (3.65, 4.59) |
| Cancer | 21.05 | 0.82 (0.80, 0.84) | 1.18 | 0.46 (0.28, 0.75) |
| Other chronic pain | 54.78 | 1.64 (1.62, 1.66) | 18.69 | 3.66 (3.25, 4.13) |
| Joint pain | 30.54 | 1.10 (1.09, 1.11) | 4.29 | 1.12 (0.98, 1.28) |
| Musculoskeletal pain | 37.07 | 0.97 (0.96, 0.98) | 7.32 | 0.84 (0.71, 0.99) |
| Nerve pain | 45.32 | 1.23 (1.21, 1.24) | 8.88 | 1.25 (1.05, 1.49) |
Any UDT indicated either receiving of screening test or definitive test. Screening test is usually performed by immunoassay to detect a drug or drug class. Definitive test identifies specific drugs and metabolites, by gas or liquid chromatography and mass spectrometry, or high-performance liquid chromatography.
UDT, urine drug testing.
DISCUSSION
The UDT rates increased from 2012 to 2018; however, 48% of prevalent LTOT users and 89% of incident users remained untested in 2018. Between 2009 and 2016, many opioid-prescribing guidelines were released, most of which included UDT recommendations for LTOT patients.1–5,15,27 In 2015, a total of 37 states (50 states by 2017) issued guidelines ranging from advisory to required by law, though UDT requirements varied.28
Rates of UDT were lower in older patients and those with cancer, and higher among patients in the South with back or other chronic pain. Higher rates of opioid prescribing in the South may contribute to the corresponding increase in UDT. The findings on cancer patients and those with higher Elixhauser scores were consistent with previous literature.29,30 However, older patients have a higher risk of LTOT use,31,32 so lower UDT rates in this population were unexpected. It may be that UDT was used to detect misuse, which is higher in younger adults.33
Use of UDT is recommended for risk mitigation among LTOT patients, where aberrant toxicology may lead to opioid discontinuation.34 Adherence to guideline-recommended UDT and other mitigation strategies is linked to improved patient adherence; however, evidence is lacking on effectiveness of UDT on patient outcomes.1,21,35 This study concluded low rates of adherence to UDT recommendations, specifically in baseline testing. Early UDT rates may reflect disagreement on the importance of UDT, inexperience in UDT interpretation, insurance and resource limitations, and lack of standardized opioid prescribing. Some of these factors may still affect current practice and explain low UDT rates in recent years.
Providers tend to assess individuals as low risk, though UDT results show otherwise.41 Self-reported behavior may not accurately predict misuse, and risk assessments alone may miss an opportunity to prevent substance use disorder.1,37 Universal UDT has been suggested to eliminate subjectivity of providers and reduce opioid misuse; however, it is expensive, may result in fraudulent overuse of testing, and potentially harm the patient–provider relationship if misinterpretation of UDT occurs.12,38
Important strengths of this study include the large, diverse population, specificity to identify use of the UDT and chronic pain, and the ability to control for LTOT risk factors (alcohol abuse, drug use, psychoses, and depression).24
Limitations
Results from commercial insurance data cannot be generalized to the entire U.S. population. The continuous enrollment requirement further limits generalizability to those with short coverage. LTOT was measured by prescriptions claims, rather than consumption. UDT by private labs or the Department of Veteran’s Affairs were not captured. Race and SES were not available, though African Americans on LTOT are tested at a higher rate than White patients.34
CONCLUSIONS
Among LTOT patients with chronic pain, UDT rates increased from 2012 to 2018, indicating there has been some response to opioid prescribing guidelines. However, UDT rates were still disproportionately low by 2018, specifically baseline UDT. Low guideline adherence may be a lost opportunity to identify patients at high risk of substance use disorder and opioid-related toxicity. Research on the effect of UDT on patient outcomes and analysis of cost benefits may provide evidence for a better approach to drug monitoring and guideline adherence.
ACKNOWLEDGMENTS
This work was supported by grant R01-DA039192 from the NIH. The funder had no role in the design or conduct of the study; collection, management, analysis, or interpretation of the data; preparation, review, or approval of the manuscript; or decision to submit the manuscript for publication.
No financial disclosures were reported by the authors of this paper.
Appendix Table 1.
Cohort Flow Descriptions
| Step | Description | Number of patients | Number of person-years |
|---|---|---|---|
| Prevalence cohort | |||
| 1 | All opioid users | 12,930,228 | |
| 2 | At least 90 days opioid use (opioid use periods)a | 1,298,048 | 2,570,004 |
| 3 | Continuous eligibility for entire calendar year and previous yeara | 727,693 | 1,580,955 |
| 4 | At least 18 years old at time of first opioid use, had known gender, region | 721,843 | 1,570,054 |
| 5 | Had no acute pain conditions for entire calendar year | 621,449 | 1,228,044 |
| Incidence cohort | |||
| 1 | All opioid users | 12,930,228 | |
| 2 | At least 90 days opioid use (opioid use periods)a | 1,298,048 | 2,570,004 |
| 3 | Continuous eligibility for 1 year prior to start of long-term opioid use | 665,377 | 1,311,672 |
| 4 | No prior opioid use in 1 year prior to start of long-term opioid use | 49,893 | 50,426 |
| 5 | At least 18 years old at time of first opioid use, had known gender, region | 49,508 | 50,040 |
| 6 | First opioid prescription for at least 28 days | 35,682 | 36,194 |
| 7 | Had no acute pain conditions in prior to first opioid use | 28,790 | 29,202 |
LTOT users could be counted for multiple years if they had at least 90 days of opioid use in each calendar year. For users with only 1 episode of ≥90 days use which spanned across 2 calendar years, the earlier year was used as the year of use, if it included at least 30 days of use. Otherwise, the later year was selected. For example, an LTOT user with 90 days’ use starting December 1, 2012 and ending February 28, 2013 would count for 2012 because the episode had 31 days’ use in 2012. An LTOT user with 90 days use starting December 3, 2012 and ending March 2 would count for 2013 because the episode included only 29 days of use in 2012.
LTOT, long term opioid therapy.
Appendix Table 2.
ICD, CPT and HCPCS Codes for Urine Drug Testing and Chronic Pain Conditions
| Category | Code |
|---|---|
| Presumptive UDT codes | G0477, G0478, G0479, 0007U, G0430, G0431, G0434, H0003, 80100, 80101, 80104, 80300, 80301, 80302, 80304, 80303, 80305, 80306, 80307 |
| Definitive UDT codes | 83925, 80364, 80102, 80152, 80154, 80160, 80166, 80323, 80324, 80325, 80326, 80329, 80330, 80331, 80332, 80333, 80334, 80335, 80336, 80364, 80102, 80152, 80154, 80160, 80166, 80323, 80324, 80325, 80326, 80329, 80330, 80331, 80332, 80333, 80334, 80335, 80336, 80337, 80338, 80339, 80340, 80341, 80342, 80344, 80345, 80346, 80347, 80348, 80349, 80350, 80351, 80352, 80353, 80354, 80355, 80356, 80357, 80358, 80359, 80360, 80361, 80362, 80363, 80365, 80366, 80367, 80368, 80369, 80370, 80371, 80372, 80373, 80374, 80375, 80376, 80377, 82101, 82520, 82646, 82649, 82742, 83925, 83992, G0481, G0482, G0483, G6037, 0006U, G0480, G0659, G6031, G6032, G6034, G6036, G6037, G6041, G6042, G6043, G6044, G6045, G6048, G6050, G6053, G6056 |
| Back pain | ICD-9: 720.1, 720.2, 720.81, 720.89, 720.9, 721.0, 721.1, 721.2, 721.3, 721.41, 721.42, 721.5, 722.0, 722.10, 722.11, 722.2, 722.30, 722.31, 722.6, 722.83, 723.0, 723.1, 723.2, 723.3, 723.5, 723.6, 724.00, 724.01, 724.02, 724.03, 724.09, 724.1, 724.8, 756.10, 756.11, 756.12, 756.13, 756.14, 756.15 ICD-10: M43.27, M43.28, M43.6, M43.8X9, M46.00, M46.1, M46.40, M46.45, M46.47, M46.80, M46.90, M47.10, M47.12, M47.14, M47.16, M47.812, M47.814, M47.817, M47.819, M48.00, M48.02, M48.04, M48.06, M48.06, M48.08, M48.10, M48.20, M48.30, M48.9, M49.80, M50.00, M50.20, M50.30, M50.80, M50.90, M51.04, M51.05, M51.06, M51.24, M51.25, M51.26, M51.27, M51.34, M51.34, M51.35, M51.35, M51.36, M51.36, M51.37, M51.37, M51.44, M51.45, M51.46, M51.47, M51.84, M51.85, M51.86, M51.87, M51.9, M51.9, M51.9, M51.9, M51.9, M53.0, M53.1, M53.2X7, M53.2X8, M53.3, M53.3, M53.3, M53.82, M53.9, M54.02, M54.08, M54.14, M54.15, M54.16, M54.17, M54.2, M54.30, M54.5, M54.6, M54.89, M54.9, M67.88, M96.1, M96.1, M96.1, M96.1, Q76.0, Q76.1, Q76.2, Q76.2, Q76.419, Q76.49, Q76.49, Q76.49, Q76.49, Q76.49 |
| Joint pain | ICD-9: 710.0, 710.1, 710.2, 710.3, 710.4, 710.8, 710.9, 711.00, 711.01, 711.02, 711.03, 711.04, 711.05, 711.06, 711.24, 711.25, 711.26, 711.27, 711.28, 711.29, 711.30, 711.49, 711.50, 711.51, 711.52, 711.53, 711.54, 711.55, 711.74, 711.75, 711.76, 711.77, 711.78, 711.79, 711.80, 711.99, 713.0, 713.1, 713.2, 713.3, 713.4, 713.5, 713.6, 714.0, 714.1, 714.2, 714.30, 714.31, 714.32, 714.33, 715.00, 715.04, 715.09, 715.10, 715.11, 715.12, 715.13, 715.33, 715.34, 715.35, 715.36, 715.37, 715.38, 715.80, 716.00, 716.01, 716.02, 716.03, 716.04, 716.05, 716.24, 716.25, 716.26, 716.27, 716.28, 716.29, 716.49, 716.50, 716.51, 716.52, 716.53, 716.54, 716.85, 716.86, 716.87, 716.88, 716.89, 716.90, 717.0, 717.1, 717.2, 717.3, 717.40, 717.41, 718.00, 718.01, 718.02, 718.03, 718.04, 718.05, 718.26, 718.27, 718.28, 718.29, 718.30, 718.31, 718.65, 718.70, 718.71, 718.72, 718.73, 718.74, 718.94, 718.95, 718.97, 718.98, 718.99, 719.00, 719.01, 719.02, 719.03, 719.04, 719.05, 719.24, 719.25, 719.26, 719.27, 719.28, 719.29, 719.40, 719.41, 719.45, 719.46, 719.47, 719.49, 719.50, 719.51, 719.52, 719.53, 719.54, 719.78, 719.79, 719.80, 719.81, 719.82, 719.83, 720.0, 726.10, 726.5, 729.5, 733.90 ICD-10: M00.039, M00.049, M00.059, M00.069, M00.079, M00.08, M00.09, M00.139, M00.149, M00.159, M00.169, M00.179, M00.18, M00.19, M00.239, M00.249, M00.259, M00.269, M00.279, M00.28, M00.29, M00.839, M00.849, M00.859, M00.869, M00.879, M00.88, M00.89, M00.9, M01.X0, M01.X0, M01.X0, M01.X0, M01.X0, M01.X19, M01.X19, M01.X19, M01.X19, M01.X19, M01.X29, M01.X29, M01.X29, M01.X29, M01.X29, M01.X29, M01.X39, M01.X39, M01.X39, M01.X39, M01.X39, M01.X39, M01.X49, M01.X49, M01.X49, M01.X49, M01.X49, M01.X59, M01.X59, M01.X59, M01.X59, M01.X59, M01.X69, M01.X69, M01.X69, M01.X69, M01.X69, M01.X69, M01.X79, M01.X79, M01.X79, M01.X79, M01.X79, M01.X79, M01.X8, M01.X8, M01.X8, M01.X8, M01.X8, M01.X8, M01.X9, M01.X9, M01.X9, M01.X9, M02.10, M02.119, M02.129, M02.139, M02.149, M02.159, M02.169, M02.179, M02.18, M02.19, M02.20, M02.30, M02.319, M02.329, M02.339, M02.349, M02.359, M02.369, M02.379, M02.38, M02.39, M02.9, M05.10, M05.30, M05.60, M06.1, M06.4, M06.4, M06.9, M08.00, M08.3, M08.40, M08.40, M12.00, M12.129, M12.139, M12.149, M12.159, M12.169, M12.179, M12.18, M12.19, M12.20, M12.219, M12.229, M12.239, M12.269, M12.279, M12.28, M12.29, M12.30, M12.319, M12.329, M12.339, M12.349, M12.359, M12.369, M12.379, M12.38, M12.39, M12.40, M12.419, M12.429, M12.439, M12.449, M12.459, M12.469, M12.479, M12.48, M12.49, M12.50, M12.519, M12.529, M12.539, M12.549, M12.559, M12.569, M12.579, M12.58, M12.59, M12.80, M12.80, M12.80, M12.80, M12.819, M12.819, M12.829, M12.829, M12.839, M12.839, M12.849, M12.849, M12.859, M12.869, M12.879, M12.879, M12.88, M12.88, M12.89, M12.9, M12.9, M12.9, M12.9, M12.9, M12.9, M12.9, M12.9, M12.9, M12.9, M13.0, M13.0, M13.0, M13.0, M13.0, M13.0, M13.0, M13.0, M13.0, M13.10, M13.10, M13.119, M13.129, M13.139, M13.149, M13.159, M13.169, M13.179, M13.80, M13.80, M13.819, M13.819, M13.829, M13.829, M13.839, M13.839, M13.849, M13.859, M13.869, M13.869, M13.879, M13.879, M13.88, M13.88, M13.89, M13.89, M14.60, M14.80, M15.0, M15.3, M15.8, M15.8, M15.9, M16.10, M16.7, M16.9, M16.9, M17.10, M17.5, M17.9, M17.9, M18.9, M19.019, M19.029, M19.039, M19.049, M19.079, M19.219, M19.229, M19.239, M19.249, M19.279, M19.90, M19.90, M19.90, M19.90, M19.90, M19.90, M19.90, M19.90, M19.90, M19.90, M19.90, M19.91, M19.91, M19.93, M19.93, M22.40, M23.009, M23.202, M23.229, M23.239, M23.249, M23.259, M23.269, M23.305, M23.329, M23.339, M23.349, M23.359, M23.369, M23.40, M23.50, M23.50, M23.50, M23.50, M23.50, M23.50, M23.8X9, M23.8X9, M23.90, M24.00, M24.00, M24.019, M24.029, M24.039, M24.049, M24.059, M24.073, M24.076, M24.08, M24.10, M24.10, M24.129, M24.139, M24.149, M24.159, M24.173, M24.176, M24.30, M24.30, M24.30, M24.319, M24.329, M24.339, M24.349, M24.359, M24.40, M24.419, M24.429, M24.439, M24.443, M24.446, M24.459, M24.469, M24.473, M24.476, M24.60, M24.60, M24.60, M24.619, M24.629, M24.639, M24.649, M24.659, M24.669, M24.673, M24.676, M24.80, M24.80, M24.80, M24.80, M24.80, M24.80, M24.819, M24.819, M24.829, M24.829, M24.839, M24.839, M24.849, M24.849, M24.859, M24.859, M24.873, M24.873, M24.876, M24.876, M24.9, M24.9, M24.9, M24.9, M24.9, M24.9, M24.9, M25.00, M25.00, M25.019, M25.029, M25.039, M25.049, M25.059, M25.069, M25.073, M25.076, M25.08, M25.10, M25.10, M25.119, M25.129, M25.139, M25.149, M25.159, M25.169, M25.173, M25.176, M25.18, M25.40, M25.429, M25.439, M25.449, M25.459, M25.469, M25.473, M25.476, M25.48, M25.50, M25.50, M25.519, M25.529, M25.539, M25.541, M25.542, M25.549, M25.559, M25.569, M25.579, M25.60, M25.60, M25.619, M25.629, M25.639, M25.649, M25.659, M25.669, M25.673, M25.676, M25.80, M25.80, M25.819, M25.829, M25.839, M25.849, M25.859, M25.869, M25.879, M25.9, M25.9, M25.9, M25.9, M25.9, M25.9, M25.9, M25.9, M25.9, M25.9, M33.20, M33.90, M35.2, M35.2, M35.2, M35.2, M35.2, M35.2, M35.2, M35.5, M35.9, M36.2, M36.3, M36.4, M43.4, M43.5X9, M43.5X9, M70.60, M70.70, M75.100, M75.50, M76.10, M76.20, M79.609, M79.646, M85.9, M89.9, M94.9, R29.4, R29.898, R29.898, R29.898, R29.898, R29.898, R29.898, R29.898, R29.898, R29.898, V13.4 |
| Nerve pain | ICD-9: 337.0, 337.1, 353.0, 353.1, 353.2, 353.3, 353.4, 353.5, 353.6, 353.8, 353.9, 354.0, 354.1, 354.2, 354.3, 354.4, 354.5, 354.8, 354.9, 355.0, 355.1, 355.2, 355.3, 355.4, 355.5, 355.6, 355.7, 355.71, 356.0, 356.1, 356.2, 356.3, 356.4, 356.8, 356.9, 357.0, 357.1, 357.2, 357.4, 357.5, 357.6, 357.7, 357.8, 357.81, 377.33, 377.34, 377.41, 531.3, 723.4, 724.3, 727.2, 729.2 ICD-10: B02.23, B26.84, E08.42, E09.42, E10.42, E11.42, E13.42, G54.0, G54.1, G54.2, G54.3, G54.4, G54.5, G54.6, G54.7, G54.8, G54.9, G56.00, G56.10, G56.20, G56.30, G56.40, G56.80, G56.90, G57.00, G57.10, G57.20, G57.30, G57.40, G57.50, G57.60, G57.70, G57.80, G57.90, G58.7, G58.9, G60.0, G60.0, G60.0, G60.1, G60.3, G60.8, G60.9, G61.0, G61.81, G61.82, G61.89, G61.9, G62.0, G62.1, G62.2, G62.81, G63., G63., G99.0, H46.2, H46.3, H47.019, M54.10, M54.12, M54.13, M54.30, M79.2 |
| Other chronic pain | ICD-9: 338.29, 338.4, 729.1 ICD-10: G89.29, G89.4, M60.9, M79.1, M79.7 |
| Cancer | ICD-9: 140.x–172.x, 174.x–202.x, 203.0. 238.6 ICD-10: C01-C85, C88, C96, C97, C90.0, C90.2 |
| Musculoskeletal pain | ICD-9: 725.x, 726.0, 727.00, 728.11, 729.0, 781.99, 830.0, 831.00, 832.00, 833.00, 834.00, 835.00, 836.0, 863.80 ICD-10: M25.729, M35.3, M60.10, M60.9, M61.00, M61.10, M62.10, M62.89, M62.9, M65.00, M65.30, M65.4, M65.80, M65.849, M65.879, M65.9, M66.10, M66.239, M66.249, M66.259, M66.269, M66.339, M66.349, M66.369, M66.829, M66.879, M66.88, M66.9, M67.80, M67.88, M67.90, M70.039, M70.10, M70.20, M70.30, M70.30, M70.40, M70.40, M70.50, M70.60, M70.70, M70.98, M71.00, M71.20, M71.30, M71.50, M71.80, M71.9, M72.6, M72.9, M75.00, M75.100, M75.120, M75.20, M75.30, M75.30, M75.40, M75.50, M75.80, M75.80, M76.10, M76.20, M76.40, M76.50, M76.60, M76.829, M76.899, M76.899, M76.899, M77.00, M77.10, M77.20, M77.9, M79.0, M79.1, M79.609, M79.7, M79.81, M79.A19, M79.A29, M79.A3, M79.A9, R29.818, R29.898, R29.898, R29.90, R29.91, R68.89, S01.409A, S03.00XA, S03.00XA, S03.00XS, S03.01XA, S03.01XS, S03.02XA, S03.02XS, S03.03XA, S03.1XXA, S03.40XA, S03.41XA, S03.42XA, S03.43XA, S03.8XXA, S03.9XXA, S03.9XXS, S11.90XA, S11.90XA, S11.90XA, S11.90XA, S11.90XA, S11.90XA, S11.90XA, S11.90XA, S11.90XA, S13.101A, S13.101A, S13.101A, S13.101A, S13.111A, S13.111A, S13.121A, S13.121A, S13.131A, S13.131A, S13.141A, S13.141A, S13.151A, S13.151A, S13.161A, S13.161A, S13.171A, S13.171A, S13.181A, S13.181A, S13.20XS, S13.4XXA, S13.5XXA, S13.8XXA, S13.9XXS, S21.109A, S21.209A, S23.101A, S23.101A, S23.20XA, S23.20XA, S23.20XS, S23.3XXA, S23.41XA, S23.420A, S23.421A, S23.428A, S23.429A, S23.8XXA, S23.9XXA, S23.9XXS, S29.019A, S31.000A, S31.000A, S31.000A, S31.000A, S31.000A, S31.000A, S33.101A, S33.101A, S33.2XXA, S33.2XXA, S33.2XXA, S33.2XXA, S33.30XS, S33.39XA, S33.39XA, S33.39XA, S33.39XA, S33.5XXA, S33.6XXA, S33.8XXA, S33.8XXA, S33.8XXA, S33.8XXA, S33.8XXA, S33.8XXA, S33.8XXA, S33.9XXA, S33.9XXS, S39.011A, S41.009A, S41.009A, S41.009A, S41.109A, S41.109A, S41.109A, S43.006A, S43.006A, S43.016A, S43.016A, S43.026A, S43.026A, S43.036A, S43.036A, S43.086A, S43.086A, S43.109A, S43.109A, S43.206A, S43.206A, S43.306S, S43.409A, S43.419A, S43.429A, S43.439A, S43.499A, S43.50XA, S43.80XA, S43.80XA, S43.80XA, S43.80XA, S43.90XS, S46.019A, S46.119A, S46.819A, S46.919A, S46.919S, S51.009A, S51.009A, S51.009A, S51.009A, S51.009A, S51.009A, S53.006A, S53.006A, S53.016A, S53.016A, S53.026A, S53.026A, S53.033A, S53.096A, S53.096A, S53.106A, S53.106A, S53.116A, S53.116A, S53.136A, S53.136A, S53.146A, S53.146A, S53.196A, S53.196A, S53.409A, S53.419A, S53.429A, S53.439A, S53.449A, S53.499A, S53.499S, S56.919A, S56.919S, S61.009A, S61.009A, S61.209A, S61.209A, S61.409A, S61.509A, S61.509A, S61.509A, S61.509A, S61.509A, S61.509A, S61.509A, S63.006A, S63.006A, S63.006S, S63.016A, S63.016A, S63.026A, S63.026A, S63.036A, S63.036A, S63.046A, S63.056A, S63.056A, S63.066A, S63.066A, S63.076A, S63.076A, S63.096A, S63.096A, S63.106A, S63.106A, S63.116A, S63.116A, S63.126A, S63.126A, S63.259A, S63.259A, S63.269A, S63.269A, S63.279A, S63.279A, S63.289A, S63.299A, S63.329A, S63.509A, S63.519A, S63.529A, S63.599A, S63.629A, S63.639A, S63.649A, S63.659A, S63.8X9A, S63.8X9A, S63.90XA, S63.90XS, S66.919A, S66.919A, S66.919S, S71.009A, S71.009A, S71.009A, S71.009A, S73.006A, S73.006A, S73.006S, S73.016A, S73.016A, S73.026A, S73.026A, S73.036A, S73.036A, S73.109A, S73.109S, S73.119A, S73.129A, S73.199A, S76.919A, S76.919S, S81.009A, S81.009A, S81.009A, S81.009A, S81.009A, S81.009A, S81.009A, S83.006A, S83.006A, S83.006S, S83.106A, S83.106A, S83.106S, S83.116A, S83.116A, S83.126A, S83.126A, S83.136A, S83.136A, S83.146A, S83.146A, S83.196A, S83.196A, S83.209A, S83.209S, S83.219A, S83.289A, S83.30XA, S83.419A, S83.429A, S83.509A, S83.60XA, S83.8X9A, S83.90XA, S83.90XS, S86.019A, S86.819A, S86.919A, S91.009A, S91.109A, S91.109A, S91.309A, S91.309A, S91.309A, S91.309A, S91.309A, S91.309A, S93.06XA, S93.06XA, S93.06XS, S93.106A, S93.119A, S93.119A, S93.129A, S93.129A, S93.306A, S93.306A, S93.306S, S93.316A, S93.316A, S93.316A, S93.316A, S93.326A, S93.326A, S93.336A, S93.336A, S93.336A, S93.336A, S93.409A, S93.419A, S93.429A, S93.439A, S93.499A, S93.519A, S93.529A, S93.609A, S93.609S, S93.629A, S93.699A, S96.919A, S96.919A, S96.919S, T14.90, T14.90, T14.90, Z89.019, Z89.029, Z89.119, Z89.129, Z89.209, Z89.219, Z89.229, Z89.239, Z89.419, Z89.429, Z89.439, Z89.449, Z89.519, Z89.619, Z89.629, Z89.9, Z96.60, Z96.619, Z96.629, Z96.639, Z96.649, Z96.659, Z96.669, Z96.698, Z97.10 |
CPT, current procedural terminology; HCPCS, healthcare common procedure coding system ; UDT, urine drug testing.
Appendix Table 3.
Urine Drug Testing (UDT) by Age and Year
| Prevalent long-term opioid users | Incident long-term opioid users | |||||
|---|---|---|---|---|---|---|
| Age/Year | Denominator | Any UDT | Any UDT, % | Denominator | Any UDT | Any UDT, % |
| <50 years | ||||||
| 2012 | 29,036 | 11,213 | 38.62 | 687 | 53 | 7.71 |
| 2013 | 29,107 | 12,283 | 42.20 | 649 | 46 | 7.09 |
| 2014 | 25,338 | 11,662 | 46.03 | 544 | 61 | 11.21 |
| 2015 | 23,674 | 12,128 | 51.23 | 657 | 93 | 14.16 |
| 2016 | 23,438 | 12,838 | 54.77 | 563 | 96 | 17.05 |
| 2017 | 24,205 | 14,389 | 59.45 | 529 | 92 | 17.39 |
| 2018 | 21,511 | 13,527 | 62.88 | 354 | 65 | 18.36 |
| 50–59 years | ||||||
| 2012 | 38,954 | 13,015 | 33.41 | 710 | 42 | 5.92 |
| 2013 | 42,730 | 16,211 | 37.94 | 838 | 53 | 6.32 |
| 2014 | 39,423 | 16,759 | 42.51 | 687 | 53 | 7.71 |
| 2015 | 38,424 | 18,390 | 47.86 | 923 | 73 | 7.91 |
| 2016 | 38,968 | 20,279 | 52.04 | 686 | 96 | 13.99 |
| 2017 | 45,529 | 26,724 | 58.70 | 801 | 118 | 14.73 |
| 2018 | 42,971 | 27,033 | 62.91 | 445 | 77 | 17.30 |
| 60–69 years | ||||||
| 2012 | 37,518 | 9,355 | 24.93 | 964 | 37 | 3.84 |
| 2013 | 43,625 | 12,712 | 29.14 | 1,155 | 48 | 4.16 |
| 2014 | 41,735 | 13,822 | 33.12 | 940 | 53 | 5.64 |
| 2015 | 43,457 | 16,730 | 38.50 | 1,384 | 72 | 5.20 |
| 2016 | 48,166 | 21,323 | 44.27 | 1,002 | 82 | 8.18 |
| 2017 | 62,862 | 32,657 | 51.95 | 1,300 | 154 | 11.85 |
| 2018 | 66,421 | 37,818 | 56.94 | 777 | 111 | 14.29 |
| 70–79 years | ||||||
| 2012 | 28,414 | 4,177 | 14.70 | 816 | 10 | 1.23 |
| 2013 | 32,924 | 5,907 | 17.94 | 1,049 | 22 | 2.10 |
| 2014 | 31,983 | 6,926 | 21.66 | 771 | 19 | 2.46 |
| 2015 | 33,132 | 8,855 | 26.73 | 1,615 | 44 | 2.72 |
| 2016 | 38,181 | 12,433 | 32.56 | 1,001 | 43 | 4.30 |
| 2017 | 52,174 | 20,661 | 39.60 | 1,229 | 79 | 6.43 |
| 2018 | 57,195 | 25,995 | 45.45 | 859 | 65 | 7.57 |
| >79 years | ||||||
| 2012 | 17,742 | 1,122 | 6.32 | 713 | 4 | 0.56 |
| 2013 | 19,192 | 1,607 | 8.37 | 851 | 9 | 1.06 |
| 2014 | 18,805 | 1,927 | 10.25 | 718 | 4 | 0.56 |
| 2015 | 18,829 | 2,524 | 13.40 | 867 | 5 | 0.58 |
| 2016 | 21,238 | 3,854 | 18.15 | 705 | 12 | 1.70 |
| 2017 | 24,649 | 5,833 | 23.66 | 809 | 23 | 2.84 |
| 2018 | 26,494 | 7,713 | 29.11 | 604 | 19 | 3.15 |
Appendix Table 4.
Urine Drug Testing (UDT) by Region and Year
| Prevalent long-term opioid users | Incident long-term opioid users | |||||
|---|---|---|---|---|---|---|
| Region/Year | Denominator | Any UDT | Any UDT, % | Denominator | Any UDT | Any UDT, % |
| Midwest | ||||||
| 2012 | 31,443 | 5,979 | 19.02 | 773 | 23 | 2.98 |
| 2013 | 34,178 | 7,673 | 22.45 | 792 | 24 | 3.03 |
| 2014 | 33,478 | 8,875 | 26.51 | 728 | 28 | 3.85 |
| 2015 | 30,823 | 9,732 | 31.57 | 670 | 40 | 5.97 |
| 2016 | 33,657 | 11,561 | 34.35 | 743 | 43 | 5.79 |
| 2017 | 39,391 | 15,831 | 40.19 | 995 | 65 | 6.53 |
| 2018 | 37,478 | 15,885 | 42.38 | 449 | 32 | 7.13 |
| Northeast | ||||||
| 2012 | 9,856 | 1,994 | 20.23 | 305 | 6 | 1.97 |
| 2013 | 12,522 | 3,062 | 24.45 | 361 | 11 | 3.05 |
| 2014 | 11,639 | 3,087 | 26.52 | 344 | 6 | 1.74 |
| 2015 | 11,876 | 3,451 | 29.06 | 320 | 17 | 5.31 |
| 2016 | 11,695 | 3,968 | 33.93 | 289 | 16 | 5.54 |
| 2017 | 12,502 | 4,574 | 36.59 | 291 | 12 | 4.12 |
| 2018 | 13,045 | 5,178 | 39.69 | 213 | 7 | 3.29 |
| South | ||||||
| 2012 | 72,584 | 24,658 | 33.97 | 1,782 | 102 | 5.72 |
| 2013 | 78,666 | 29,751 | 37.82 | 1,881 | 109 | 5.79 |
| 2014 | 68,638 | 29,061 | 42.34 | 1,536 | 121 | 7.88 |
| 2015 | 71,839 | 33,717 | 46.93 | 2,231 | 188 | 8.43 |
| 2016 | 80,390 | 40,484 | 50.36 | 1,928 | 212 | 11.00 |
| 2017 | 111,185 | 62,084 | 55.84 | 2,302 | 302 | 13.12 |
| 2018 | 116,891 | 69,830 | 59.74 | 1,634 | 244 | 14.93 |
| West | ||||||
| 2012 | 37,781 | 6,251 | 16.55 | 1,030 | 15 | 1.46 |
| 2013 | 42,212 | 8,234 | 19.51 | 1,508 | 34 | 2.25 |
| 2014 | 43,529 | 10,073 | 23.14 | 1,052 | 35 | 3.33 |
| 2015 | 42,978 | 11,727 | 27.29 | 2,225 | 42 | 1.89 |
| 2016 | 44,249 | 14,714 | 33.25 | 997 | 58 | 5.82 |
| 2017 | 46,341 | 17,775 | 38.36 | 1,080 | 87 | 8.06 |
| 2018 | 47,178 | 21,193 | 44.92 | 743 | 54 | 7.27 |
REFERENCES
- 1.Dowell D, Haegerich TM, Chou R. CDC guideline for prescribing opioids for chronic pain--United States, 2016. JAMA. 2016;315(15):1624–1645. 10.1001/jama.2016.1464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Jeffery MM, Hooten WM, Hess EP, et al. Opioid prescribing for opioid-naive patients in emergency departments and other settings: characteristics of prescriptions and association with long-term use. Ann Emerg Med. 2018;71(3):326–336.e19. 10.1016/j.annemergmed.2017.08.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Manchikanti L, Abdi S, Atluri S, et al. American Society of Interventional Pain Physicians (ASIPP) guidelines for responsible opioid prescribing in chronic non-cancer pain: Part I--evidence assessment. Pain Physician. 2012;15(3 suppl):S1–S65. [PubMed] [Google Scholar]
- 4.Nuckols TK, Anderson L, Popescu I, et al. Opioid prescribing: a systematic review and critical appraisal of guidelines for chronic pain. Ann Intern Med. 2014;160(1):38–47. 10.7326/0003-4819-160-1-201401070-00732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Chou R, Fanciullo GJ, Fine PG, et al. Clinical guidelines for the use of chronic opioid therapy in chronic noncancer pain. J Pain. 2009;10(2):113–130. 10.1016/j.jpain.2008.10.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Jannetto PJ, Bratanow NC, Clark WA, et al. Executive summary: American Association of Clinical Chemistry Laboratory Medicine Practice Guideline—using clinical laboratory tests to monitor drug therapy in pain management patients. J Appl Lab Med. 2018;2(4):489–526. 10.1373/jalm.2017.023341. [DOI] [PubMed] [Google Scholar]
- 7.Owen GT, Burton AW, Schade CM, Passik S. Urine drug testing: current recommendations and best practices. Pain Physician. 2012;15(3 suppl):ES119–ES133. [PubMed] [Google Scholar]
- 8.Rolfs RT, Johnson E, Williams NJ, Sundwall DN. Utah clinical guidelines on prescribing opioids for treatment of pain. J Pain Palliat Care Pharmacother. 2010;24(3):219–235. 10.3109/15360288.2010.503265. [DOI] [PubMed] [Google Scholar]
- 9.Christo PJ, Manchikanti L, Ruan X, et al. Urine drug testing in chronic pain. Pain Physician. 2011;14(2):123–143. [PubMed] [Google Scholar]
- 10.Manchikanti L, Manchukonda R, Pampati V, et al. Does random urine drug testing reduce illicit drug use in chronic pain patients receiving opioids? Pain Physician. 2006;9(2):123–129. [PubMed] [Google Scholar]
- 11.Matteliano D, Chang YP. Describing prescription opioid adherence among individuals with chronic pain using urine drug testing. Pain Manag Nurs. 2015;16(1):51–59. 10.1016/j.pmn.2014.04.001. [DOI] [PubMed] [Google Scholar]
- 12.Wiseman LK, Lynch ME. The utility of universal urinary drug screening in chronic pain management. Can J Pain. 2018;2(1):37–47. 10.1080/24740527.2018.1425980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Pergolizzi J, Pappagallo M, Stauffer J, et al. The role of urine drug testing for patients on opioid therapy. Pain Pract. 2010;10(6):497–507. 10.1111/j.1533-2500.2010.00375.x. [DOI] [PubMed] [Google Scholar]
- 14.Mahajan G Role of urine drug testing in the current opioid epidemic. Anesth Analg. 2017;125(6):2094–2104. 10.1213/ane.0000000000002565. [DOI] [PubMed] [Google Scholar]
- 15.Paice JA, Portenoy R, Lacchetti C et al. Management of chronic pain in survivors of adult cancers: American Society of Clinical Oncology Clinical practice guideline. J Clin Oncol. 2016;34(27):3325–3345. 10.1200/jco.2016.68.5206. [DOI] [PubMed] [Google Scholar]
- 16.Argoff CE, Alford DP, Fudin J, et al. Rational urine drug monitoring in patients receiving opioids for chronic pain: consensus recommendations. Pain Med. 2018;19(1):97–117. 10.1093/pm/pnx285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Adams NJ, Plane MB, Fleming MF, Mundt MP, Saunders LA, Stauffacher EA. Opioids and the treatment of chronic pain in a primary care sample. J Pain Symptom Manage. 2001;22(3):791–796. 10.1016/s0885-3924(01)00320-7. [DOI] [PubMed] [Google Scholar]
- 18.Bhamb B, Brown D, Hariharan J, Anderson J, Balousek S, Fleming MF. Survey of select practice behaviors by primary care physicians on the use of opioids for chronic pain. Curr Med Res Opin. 2006;22(9):1859–1865. . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Boulanger A, Clark AJ, Squire P, Cui E, Horbay GL. Chronic pain in Canada: have we improved our management of chronic noncancer pain? Pain Res Manag. 2007;12(1):39–47. 10.1155/2007/762180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Starrels JL, Becker WC, Weiner MG, Li X, Heo M, Turner BJ. Low use of opioid risk reduction strategies in primary care even for high risk patients with chronic pain. J Gen Intern Med. 2011;26(9):958–964. 10.1007/s11606-011-1648-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Turner JA, Saunders K, Shortreed SM, et al. Chronic opioid therapy urine drug testing in primary care: prevalence and predictors of aberrant results. J Gen Intern Med. 2014;29(12):1663–1671. 10.1007/s11606-014-3010-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Zgierska AE, Vidaver RM, Smith P, et al. Enhancing system-wide implementation of opioid prescribing guidelines in primary care: protocol for a stepped-wedge quality improvement project. BMC Health Serv Res. 2018;18:415. 10.1186/s12913-018-3227-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Libucha S Optum Clinformatics Data Mart (CDM) data dictionary [dataset]. Optum; 2016. [Google Scholar]
- 24.Amari E, Rehm J, Goldner E, Fischer B. Nonmedical prescription opioid use and mental health and pain comorbidities: a narrative review. Can J Psychiatry. 2011;56(8):495–502. 10.1177/070674371105600808. [DOI] [PubMed] [Google Scholar]
- 25.Elixhauser A, Steiner C, Harris DR, Coffey RM. Comorbidity measures for use with administrative data. Med Care. 1998;36(1):8–27. 10.1097/00005650-199801000-00004. [DOI] [PubMed] [Google Scholar]
- 26.Seal KH, Shi Y, Cohen G, et al. Association of mental health disorders with prescription opioids and high-risk opioid use in US veterans of Iraq and Afghanistan [published correction appears in JAMA 2012;307(23):2489]. JAMA. 2012;307(9):940–947. 10.1001/jama.2012.234. [DOI] [PubMed] [Google Scholar]
- 27.Common Elements in Guidelines for Prescribing Opioids for Chronic Pain. Centers for Disease Control and Prevention. https://www.cdc.gov/drugoverdose/pdf/common_elements_in_guidelines_for_prescribing_opioids-a.pdf. Published 2014. Accessed April 2, 2020.
- 28.Davis C State-by-State Summary of Opioid Prescribing Regulations and Guidelines. The Network for Public Health Law. https://www.azdhs.gov/documents/prevention/womens-childrens-health/injury-prevention/opioid-prevention/appendix-b-state-by-state-summary.pdf. Published 2020. Accessed April 30, 2020.
- 29.Arthur JA. Urine drug testing in cancer pain management. Oncologist. 2020;25(2):99–104. 10.1634/theoncologist.2019-0525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Johnson SP, Chung KC, Zhong L, et al. Risk of prolonged opioid use among opioid-naïve patients following common hand surgery procedures. J Hand Surg Am. 2016;41(10):947–957.e3. 10.1016/j.jhsa.2016.07.113. [DOI] [PubMed] [Google Scholar]
- 31.Campbell CI, Weisner C, Leresche L, et al. Age and gender trends in long-term opioid analgesic use for noncancer pain. Am J Public Health. 2010;100(12):2541–2547. 10.2105/ajph.2009.180646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Chau DL, Walker V, Pai L, Cho LM. Opiates and elderly: use and side effects. Clin Interv Aging. 2008;3(2):273–278. 10.2147/cia.s1847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Abuse of Prescription (Rx) Drugs Affects Young Adults Most. National Institute on Drug Abuse, NIH; HHS. https://www.drugabuse.gov/sites/default/files/abuseprescription2016.pdf Published 2016. Accessed May 1, 2020.
- 34.Gaither JR, Gordon K, Crystal S, et al. Racial disparities in discontinuation of long-term opioid therapy following illicit drug use among black and white patients. Drug Alcohol Depend. 2018;192:371–376. 10.1016/j.drugalcdep.2018.05.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Yee DA, Hughes MM, Guo AY, et al. Observation of improved adherence with frequent urine drug testing in patients with pain. J Opioid Manag. 2014;10(2):111–118. 10.5055/jom.2014.0200. [DOI] [PubMed] [Google Scholar]
- 36.Setnik B, Roland CL, Pixton GC, Sommerville KW. Prescription opioid abuse and misuse: gap between primary-care investigator assessment and actual extent of these ehaviors among patients with chronic pain. Postgrad Med. 2017;129(1):5–11. 10.1080/00325481.2017.1245585. [DOI] [PubMed] [Google Scholar]
- 37.Heit HA, Gourlay DL. Urine drug testing in pain medicine. J Pain Symptom Manage. 2004;27(3):260–267. 10.1016/j.jpainsymman.2003.07.008. [DOI] [PubMed] [Google Scholar]
- 38.Centers for Medicare and Medicaid Services. Laboratory registry. https://www.cms.gov/Regulations-and-Guidance/Legislation/CLIA/Laboratory_Registry.html. Published March 31, 2016. Accessed June 26, 2016.


