Abstract
Background
The inflammation indexes in blood routine play an essential role in evaluating the prognosis of patients with hepatocellular carcinoma, but the effect on early recurrence has not been clarified. The study aimed to investigate the risk factors of early recurrence (within 2 years) and recurrence-free survival after curative hepatectomy and explore the role of inflammatory indexes in predicting early recurrence.
Methods
The baseline data of 161 patients with hepatocellular carcinoma were analyzed retrospectively. The optimal cut-off value of the inflammatory index was determined according to the Youden index. Its predictive performance was compared by the area under the receiver operating characteristic curve. Logistic and Cox regression analyses were used to determine the risk factors of early recurrence and recurrence-free survival.
Results
The area under the curve of monocyte to lymphocyte ratio (MLR) for predicting early recurrence was 0.700, which was better than systemic inflammatory response index (SIRI), neutrophil to lymphocyte ratio (NLR), platelet to lymphocyte ratio (PLR) and systemic immune-inflammatory index (SII). MLR, tumour size, tumour differentiation and BCLC stage are all risk factors for early recurrence and recurrence-free survival of HCC. Combining the above four risk factors to construct a joint index, the area under the curve for predicting early recurrence was 0.829, which was better than single MLR, tumour size, tumour differentiation and BCLC stage. Furthermore, with the increase of risk factors, the recurrence-free survival of patients is worse.
Conclusion
The combination of MLR and clinical risk factors is helpful for clinicians to identify high-risk patients with early recurrence and carry out active postoperative adjuvant therapy to improve the prognosis of patients.
Keywords: Hepatocellular carcinoma, Inflammation, Recurrence, Hepatectomy, Prognosis
Background
Among the primary malignant tumours, liver cancer is the sixth most common, and the cancer fatality rate is the fourth. Among them, 75–85% of the pathological types are hepatocellular carcinoma (HCC) [1]. The new incidence of HCC in China accounts for about 50% of the world’s, and most HCC patients are complicated with hepatitis B [2]. Hepatectomy is one of the main treatments for HCC patients to achieve long-term survival. Even so, the postoperative cumulative recurrence rate within 5 years is about 70% [3], which has become the main cause of cancer-related death. According to the postoperative recurrence mechanism in patients with HCC, it can be divided into the early recurrence of intrahepatic metastasis and late recurrence of multicenter. Among them, early recurrence is more aggressive and the prognosis is worse [4, 5].
Hepatitis virus infection [6, 7], nonalcoholic fatty liver disease [8], alcohol [9], aflatoxin B1 [10] and other factors increase the risk of HCC. Although the mechanism of inducing tumourigenesis is different, inflammation is a common key link [11]. As an inflammatory-driven tumour, the occurrence, proliferation and metastasis of HCC are closely related to the inflammatory environment [12]. Therefore, the establishment of inflammatory indexes based on inflammatory cells in peripheral blood can reflect the relationship between tumour inflammatory microenvironment and the prognosis of HCC patients. At present, more and more evidence supports that systemic immune-inflammation index (SII) [13], neutrophil to lymphocyte ratio (NLR) [14], platelet to lymphocyte ratio (PLR) [15], monocyte to lymphocyte ratio (MLR) [16] and systemic inflammatory response index (SIRI) [17] are essential factors in predicting the survival of patients with HCC. However, there are still few reports about the role of the above inflammatory markers in predicting the early recurrence of HCC. Therefore, the purpose of our study is to explore the value of inflammatory indexes in blood routine in predicting early recurrence in patients with HCC and contrast their predictive performance.
Materials and methods
The baseline data of patients undergoing curative hepatectomy in Lishui Municipal Central Hospital from January 2011 to May 2018 were collected and analyzed retrospectively. Inclusion criteria: (1) Postoperative pathology confirmed HCC; (2) Liver reserve function was Child–Pugh A or B grade; (3) Radical hepatectomy for the first time; (4) No distant metastasis was found in preoperative imaging examination and intraoperative metastasis. Exclusion criteria: (1) Lack of clinical data; (2) Suffer from infection or systemic inflammatory disease before the operation; (3) Complicated with other malignant tumours, immune or hematological diseases; (4) No regular follow-up or less than 2 years follow-up; (5) Preoperative treatment with immunosuppressants; (6) Rupture of liver cancer. Patients with HCC were closely followed up after the operation, and a total of 161 patients were enrolled in this study by May 2020 (Fig. 1). This study was in line with the Helsinki Declaration, it was reviewed and approved by the Ethics Committee of Lishui Municipal Central Hospital (Approval No. 2020-249).
Fig. 1.
Flow chart for screening patients
Follow-up
In the first 2 years after the operation, the patients were followed up every 3 months to the outpatient clinic. After 2 years, they were followed up every 6 months in the outpatient clinic until they relapsed or lost follow-up. The examination items included physical examination, serum alpha-fetoprotein test, and an abdominal imaging scan during the follow-up period. Besides, a chest computed tomography scan should be performed at least once a year. When abdominal ultrasound suspected recurrence, abdominal contrast-enhanced computed tomography scan or magnetic resonance imaging should be performed to confirm the diagnosis, while chest computed tomography enhanced scan should be performed to rule out pulmonary metastasis or positron emission tomography to rule out other site metastasis.
Statistical analysis
The MedCalc11.0 version was used to draw the receiver operating characteristic curve. The optimal cut-off values of MLR, SIRI, NLR, PLR and SII were selected according to the Youden index. Meanwhile, the prediction performance is evaluated by comparing the area under the curve. The SPSS 22.0 version statistically analyzed the baseline data. The Pearson Chi-square test analyzed the counting data and logistic multivariate analysis model was used to obtain the independent risk factors of early recurrence after radical resection of HCC. Cox proportional hazards model was used to analyze the prognostic factors of recurrence-free survival (RFS). According to the number of risk factors, patients were divided into three groups by X-Tile software: low-risk group, medium-risk group and high-risk group. The survival curve was drawn by GraphPadPrism software, and the Log-Rank test compared the survival rate between groups. P < 0.05 considered that the difference was statistically significant.
Data processing
Blood routine and blood biochemical results were collected within 1 week before the operation and calculated the inflammation index according to the following formula. SII = platelet × neutrophil/lymphocyte [13], NLR = neutrophil/lymphocyte [14], PLR = platelet/lymphocyte [15], MLR = monocyte/lymphocyte [16], SIRI = monocyte × neutrophil/lymphocyte [17], albumin–bilirubin grade (ALBI) = 0.66 × log10 [bilirubin (μmol/L)] − 0.085 × [albumin (g/L)] [18].
Results
Baseline data
A total of 161 patients were eligible for inclusion, including 141 males (87.6%) and 20 females (12.4%), with an average age of 56.24 ± 11.44 years. According to the definition of Child–Pugh grade, there were 157 patients with grade A and 4 patients with grade B. The average tumour size was 4.76 ± 3.15 cm. Since 21 patients had undergone transcatheter arterial chemoembolization and 3 patients had received radiofrequency ablation combined with transcatheter arterial chemoembolization before the operation, these patients need at least 1 month to consider surgical treatment. 149 patients (92.55%) were complicated with hepatitis B before the operation, and no hepatitis C patients were found. By the end of follow-up, a total of 96 patients (59.63%) relapsed, of which the 1 year, 2 years, 3 years and 5-year cumulative recurrence rates were 29.81%, 50.31%, 53.42%, 58.39%, respectively, and the median recurrence-free survival time was 23.9 months.
The cut-off value and area under the curve of the inflammation index
The receiver operating characteristic curves were drawn according to the preoperative MLR, SIRI, NLR, PLR and SII. The results showed that MLR = 0.25, SIRI = 1.03, NLR = 2.74, PLR = 88 and SII = 330 were the optimal cut-off values (Table 1). According to the cut-off value of the inflammation index, the patients who were less than or equal to the cut-off value were divided into the low group, and those who were greater than the cut-off value were divided into the high group. The area under the curve (AUC) of MLR, SIRI, NLR, PLR and SII were 0.700, 0.618, 0.587, 0.564 and 0.520, respectively. Based on the optimal cut-off value, the sensitivity of MLR, SIRI, NLR, PLR and SII was 81.5%, 48.1%, 30.9%, 49.4% and 38.3%, respectively, and the specificity was 61.2%, 75.0%, 88.7%, 67.5% and 71.2%, respectively. By comparing AUC (Fig. 2), it was found that MLR was superior to SIRI, NLR, PLR and SII in predicting early recurrence in patients with HCC.
Table 1.
The cut-off value and area under the curve of the inflammation index
| Factors | Cut-off value | AUC | Sensitivity (%) | Specificity (%) | Yoden index | 95% CI of AUC |
|---|---|---|---|---|---|---|
| MLR | 0.25 | 0.700 | 81.5 | 61.2 | 0.427 | 0.623–0.770 |
| PLR | 88 | 0.564 | 49.4 | 67.5 | 0.169 | 0.484–0.642 |
| SII | 330 | 0.520 | 38.3 | 71.2 | 0.095 | 0.440–0.599 |
| NLR | 2.74 | 0.587 | 30.9 | 88.7 | 0.196 | 0.507–0.664 |
| SIRI | 1.03 | 0.618 | 48.1 | 75.0 | 0.231 | 0.539–0.694 |
AUC area under curve, CI confidence interval, MLR monocyte to lymphocyte ratio, PLR platelet-to-lymphocyte ratio, SII systemic immune-inflammation index, NLR neutrophil to lymphocyte ratio, SIRI systemic inflammatory response index
Fig. 2.
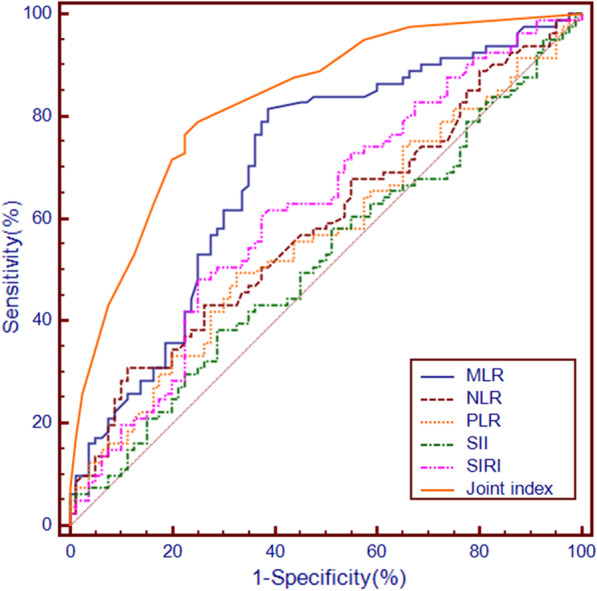
Comparison of the inflammatory index and joint index in predicting early recurrence
Univariate analysis of early postoperative recurrence
According to the recurrence within 2 years after the operation, the patients were divided into early recurrence group (81 cases) and non-early recurrence group (80 cases). The early recurrence group and non-early recurrence group’s clinicopathological characteristics were compared by univariate analysis of 22 factors (Table 2). The study found tumour size (P < 0.001), tumour number (P = 0.007), vascular invasion (P < 0.001), degree of differentiation (P < 0.001), MLR (P < 0.001), NLR (P = 0.001), PLR (P = 0.029), SIRI (P = 0.002), preoperative AFP (P = 0.023) and BCLC B–C stage (P < 0.001) were related to the early recurrence of HCC patients.
Table 2.
Baseline clinicopathological characteristics of patients in early recurrence group and non-early recurrence group
| Factors | Non-early recurrence group | Early recurrence group | X2 | P value |
|---|---|---|---|---|
| n = 80 | n = 81 | |||
| Sex | ||||
| Male | 68 | 73 | 0.971 | 0.324 |
| Female | 12 | 8 | ||
| Age (years) | ||||
| ≤ 55 | 39 | 42 | 0.155 | 0.694 |
| > 55 | 41 | 39 | ||
| AST (U/L) | ||||
| ≤ 40 | 60 | 58 | 0.237 | 0.626 |
| > 40 | 20 | 23 | ||
| ALT (U/L) | ||||
| ≤ 40 | 49 | 51 | 0.050 | 0.823 |
| > 40 | 31 | 30 | ||
| Total bilirubin (umol/L) | ||||
| < 17.1 | 60 | 60 | 0.018 | 0.893 |
| ≥ 17.1 | 20 | 21 | ||
| Albumin (g/L) | ||||
| < 35 | 7 | 15 | 3.256 | 0.071 |
| ≥ 35 | 73 | 66 | ||
| Tumour size (cm) | ||||
| ≤ 5 | 63 | 40 | 15.061 | < 0.001 |
| > 5 | 17 | 41 | ||
| Tumour number | ||||
| Solitary | 73 | 61 | 7.328 | 0.007 |
| Multiple | 7 | 20 | ||
| HBV infection | ||||
| No | 6 | 6 | 0.001 | 0.982 |
| Yes | 74 | 75 | ||
| Liver cirrhosis | ||||
| No | 21 | 20 | 0.052 | 0.820 |
| Yes | 59 | 61 | ||
| Vascular invasion | ||||
| No | 75 | 57 | 14.897 | < 0.001 |
| Yes | 5 | 24 | ||
| Differentiation | ||||
| Moderate and well | 61 | 37 | 15.793 | < 0.001 |
| Poor | 19 | 44 | ||
| Blood transfusion | ||||
| No | 48 | 46 | 0.171 | 0.680 |
| Yes | 32 | 35 | ||
| Hepatic hilar occlusion | ||||
| No | 41 | 40 | 0.056 | 0.813 |
| Yes | 39 | 41 | ||
| SII | ||||
| ≤ 330 | 57 | 51 | 1.252 | 0.263 |
| > 330 | 23 | 30 | ||
| MLR | ||||
| ≤ 0.25 | 49 | 16 | 28.790 | < 0.001 |
| > 0.25 | 31 | 65 | ||
| NLR | ||||
| ≤ 2.74 | 71 | 55 | 10.283 | 0.001 |
| > 2.74 | 9 | 26 | ||
| PLR | ||||
| ≤ 88 | 54 | 41 | 4.743 | 0.029 |
| > 88 | 26 | 40 | ||
| SIRI | ||||
| ≤ 1.03 | 60 | 42 | 9.289 | 0.002 |
| > 1.03 | 20 | 39 | ||
| AFP (ng/mL) | ||||
| < 400 | 65 | 53 | 5.145 | 0.023 |
| ≥ 400 | 15 | 28 | ||
| ALBI grade | ||||
| 1 | 47 | 40 | 1.422 | 0.233 |
| 2 | 33 | 41 | ||
| BCLC stage | ||||
| 0–A | 68 | 47 | 14.351 | < 0.001 |
| B–C | 12 | 34 | ||
AST aspartate aminotransferase, ALT alanine aminotransferase, HBV hepatitis B virus, AFP alpha fetoprotein, ALBI albumin–bilirubin, BCLC Barcelona Clinic Liver Cancer
Multivariate analysis of early recurrence
The statistical significance factors in univariate analysis were included in the Logistic multivariate regression model (Table 3). The results showed that high MLR, tumour size > 5 cm, poor differentiation and BCLC stage were independent risk factors for early recurrence after radical resection of HCC (P < 0.05). The corresponding AUC was 0.700, 0.647, 0.653 and 0.635, respectively (Table 4).
Table 3.
Logistic multivariate analysis of early recurrence
| Factors | β coefficient | OR | 95% CI | P value |
|---|---|---|---|---|
| Tumour size > 5 cm | 1.036 | 2.819 | 1.189–6.679 | 0.019 |
| Multiple tumours | 0.688 | 1.989 | 0.589–6.714 | 0.268 |
| Vascular invasion | 0.931 | 2.537 | 0.719–8.955 | 0.148 |
| Poor differentiation | 1.104 | 3.017 | 1.257–7.242 | 0.013 |
| High MLR | 1.442 | 4.227 | 1.573–11.357 | 0.004 |
| High NLR | 0.655 | 1.926 | 0.620–5.980 | 0.257 |
| High PLR | − 0.021 | 0.979 | 0.407–2.355 | 0.962 |
| High SIRI | 0.017 | 1.017 | 0.346–2.994 | 0.975 |
| AFP ≥ 400 | 0.304 | 1.355 | 0.475–3.868 | 0.570 |
| BCLC B–C stage | 1.107 | 3.026 | 1.139–8.039 | 0.026 |
OR odds ratio
Table 4.
Area under the curve of risk factors for early recurrence
| Factors | AUC | Sensitivity (%) | Specificity (%) | Youden index | 95% CI of AUC |
|---|---|---|---|---|---|
| Differentiation | 0.653 | 54.3 | 76.2 | 0.305 | 0.574–0.726 |
| MLR | 0.700 | 81.5 | 61.2 | 0.427 | 0.623–0.770 |
| Tumour size | 0.647 | 50.6 | 78.7 | 0.293 | 0.568–0.720 |
| BCLC stage | 0.635 | 42.0 | 85.0 | 0.270 | 0.555–0.709 |
| Joint index | 0.829 | 76.5 | 77.5 | 0.540 | 0.761–0.883 |
Univariate and multivariate analysis of recurrence-free survival
In univariate analysis (Table 5), the study found that tumour size (P < 0.001), tumour number (P < 0.001), vascular invasion (P < 0.001), degree of differentiation (P < 0.001), MLR (P < 0.001), NLR (P < 0.001), SIRI (P < 0.001), preoperative AFP (P = 0.035) and BCLC stage (P < 0.001) were factors affecting recurrence-free survival (RFS).
Table 5.
Univariate and multivariate analysis of recurrence-free survival
| Factors | Univariate analysis | Multivariate analysis | ||||
|---|---|---|---|---|---|---|
| HR | 95% CI | P value | HR | 95% CI | P value | |
| Sex (male/female) | 1.436 | 0.722–2.855 | 0.302 | – | – | – |
| Age (≤ 55/> 55 years) | 0.882 | 0.591–1.317 | 0.540 | – | – | – |
| AST (≤ 40/> 40 U/L) | 1.316 | 0.854–2.027 | 0.213 | – | – | – |
| ALT (≤ 40/> 40 U/L) | 1.018 | 0.674–1.538 | 0.933 | – | – | – |
| Total bilirubin (< 17.1/≥ 17.1 umol/L) | 1.032 | 0.649–1.642 | 0.893 | – | – | – |
| Albumin (> 35/≥ 35 g/L) | 0.678 | 0.393–1.167 | 0.160 | – | – | – |
| Tumour size (≤ 5/> 5 cm) | 2.283 | 1.523–3.422 | < 0.001 | 1.768 | 1.144–2.732 | 0.010 |
| Tumour number (solitary/multiple) | 2.421 | 1.495–3.919 | < 0.001 | 2.289 | 1.295–4.047 | 0.004 |
| HBV infection (no/yes) | 0.904 | 0.438–1.865 | 0.785 | – | – | – |
| Liver cirrhosis (no/yes) | 1.095 | 0.694–1.728 | 0.696 | – | – | – |
| Vascular invasion (no/yes) | 2.924 | 1.845–4.635 | < 0.001 | 1.908 | 1.162–3.132 | 0.011 |
| Differentiation (moderate and well/poor) | 2.490 | 1.662–3.732 | < 0.001 | 2.411 | 1.521–3.824 | < 0.001 |
| Blood transfusion (no/yes) | 1.184 | 0.790–1.773 | 0.413 | – | – | – |
| Hepatic hilar occlusion (no/yes) | 1.130 | 0.757–1.687 | 0.551 | – | – | – |
| SII (≤ 330/ > 330) | 1.382 | 0.911–2.098 | 0.129 | – | – | – |
| MLR (≤ 0.25/> 0.25) | 3.801 | 2.341–6.171 | < 0.001 | 2.037 | 1.154–3.597 | 0.014 |
| NLR (≤ 2.74/> 2.74) | 2.513 | 1.599–3.950 | < 0.001 | 2.070 | 1.212–3.537 | 0.008 |
| PLR (≤ 88/> 88) | 1.404 | 0.938–2.100 | 0.099 | – | – | – |
| SIRI (≤ 1.03/> 1.03) | 2.248 | 1.496–3.378 | < 0.001 | 1.320 | 0.773–2.255 | 0.309 |
| AFP (< 400/≥ 400 ng/mL) | 1.587 | 1.034–2.437 | 0.035 | 0.914 | 0.557–1.499 | 0.721 |
| ALBI grade (1/2) | 1.180 | 0.787–1.768 | 0.423 | – | – | – |
| BCLC stage (0–A/B–C) | 2.467 | 1.628–3.740 | < 0.001 | 1.969 | 1.216–3.187 | 0.006 |
HR hazard ratio
Significant factors in COX univariate analysis were included in COX multivariate regression model. The results showed that tumour size > 5 cm (HR 1.768, 95% CI [1.144–2.732]), multiple tumours (HR 2.289, 95% CI [1.295–4.047]), vascular invasion (HR 1.908, 95% CI [1.162–3.132]), poor differentiation (HR 2.411, 95% CI [1.521–3.824]), high MLR (HR 2.037, 95% CI [1.540–3.597]), high NLR (HR 2.070, 95% CI [1.212–3.537]) and BCLC B–C stage (HR 1.969, 95% CI [1.216–3.187]) were independent risk factors for RFS in HCC patients (P < 0.05).
Recurrence-free survival curve of inflammatory markers in blood routine
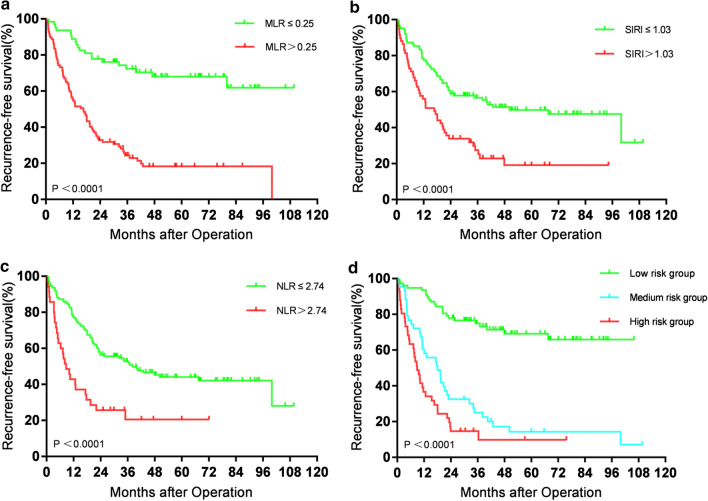
The survival curves of the two groups were drawn by Kaplan–Meier. The results showed that the recurrence-free survival rate of the high MLR group, high SIRI group, and high NLR group were all significantly lower than that of the low MLR group, low SIRI group, and low NLR group (P < 0.0001) (Fig. 3a–c). The high SII and high PLR group was compared separately with the low SII and low PLR group. The high group’s recurrence-free survival rate was not significantly different from those in the low group (P = 0.127, P = 0.098).
Fig. 3.
a Recurrence-free survival curves of patients with high MLR and low MLR. b Recurrence-free survival curve of patients with high SIRI and low SIRI. c Recurrence-free survival curve of patients with high NLR and low NLR. d Recurrence-free survival curves of low-risk, intermediate-risk and high-risk patients
Combined risk factors to predict early recurrence and recurrence-free survival
The results showed that MLR, tumour size, differentiation and BCLC stage were independent risk factors for early recurrence and recurrence-free survival. The β coefficient is taken as the weight of the four risk factors to construct a joint index. Joint index = 1.036 * tumour size + 1.104 * differentiation + 1.107 * BCLC stage + 1.442 * MLR, MLR (low group MLR assignment is 0, high group MLR assignment is 1), tumour size (tumour size ≤ 5 cm assignment is 0, tumour size > 5 cm assignment is 1), differentiation degree (high-middle differentiation assignment is 0, low differentiation assignment is 1). The AUC, sensitivity and specificity of the joint index for predicting early recurrence were 0.829, 76.5% and 77.5%, respectively. The predictive performance was better than that of single MLR, tumour size differentiation, and BCLC stage (Table 4).
Similarly, in the recurrence-free survival, according to the four risk factors of MLR, tumour size, differentiation and BCLC stage, the patients were divided into the low-risk group when they had 0–1 risk factors, medium risk group when they had 2 risk factors and high-risk group when they had more than 3 risk factors. The results showed that there was a significant difference in recurrence-free survival rate between the high-risk group and middle-risk group (P < 0.049) and between the high-risk group and low-risk group (P < 0.0001). Similarly, there was a significant difference in the recurrence-free survival rate between the middle-risk and low-risk groups (P < 0.0001). The recurrence-free survival rate was the best in the low-risk group, the second in the middle-risk group, and the worst in the high-risk group (Fig. 3d).
Discussion
Since tumour-associated inflammation has become one of the top ten characteristics of tumours [19], the value of inflammatory indexes in evaluating patients’ prognosis with HCC has been affirmed. However, there are few studies on the relationship between inflammatory indexes and early recurrence. At present, there is no consensus on time cut-off point of early recurrence, and the definition of early recurrence varies from 6 months to 2 years in most studies [5, 20, 21]. Recurrence accounted for more than 70% of HCC recurrence within 2 years after hepatectomy [22], and the follow-up of this study found that the peak of HCC recurrence was mainly within 2 years, and the cumulative recurrence rate of 2 years was 50.31%. Then the recurrence rate tended to be flat, so the cut-off point of early recurrence was set within 2 years in this study.
However, at present, it is generally accepted that early recurrence may initially originate from the occult micrometastasis of the tumour, which is usually related to the characteristics of the invasive tumour, such as multiple tumours, large tumour size, macrovascular and microvascular invasion, poorly differentiated tumour and satellite nodules [23–26]. In this study, multivariate analysis showed that multiple tumours and vascular invasion were not risk factors for early recurrence. It may be due to tumour necrosis caused by preoperative interventional therapy such as radiofrequency ablation and transarterial chemoembolization [27], affecting the postoperative pathological evaluation of the tumour. However, in the RFS of patients with HCC, the results confirmed that high MLR, high NLR, vascular invasion, multiple tumours, tumour size > 5 cm, poor differentiation and BCLC B–C stage were independent risk factors.
There have been few reports about the predictive value of inflammatory markers in early recurrence in the past. This study found that the predictive value of MLR for early recurrence is better than NLR, SIRI, PLR, and SII. Meanwhile MLR is a risk factor for early recurrence and RFS, reflecting the enhancement of inflammatory response to promote tumour cell proliferation and metastasis and the weakening of immune response to induce tumour cell apoptosis and inhibit tumour cell invasion. Considering that a single MLR does not have an ideal predictive performance, this study combines inflammatory index with clinical factors to evaluate tumour invasiveness more comprehensively from different angles, which can improve the accuracy of predicting early recurrence and RFS of patients with liver cancer, identify high-risk patients, adopt active and effective preventive and therapeutic measures, and strengthen follow-up observation, to prolong the survival time of patients.
At present, the mechanism of inflammation and tumour is not precise. However, neutrophils, platelets and monocytes are representative inflammatory cells in blood routine, and some studies have proposed the relationship between neutrophil, platelet and tumour. In the initial stage of the tumour, tumour-associated macrophages formed by neutrophils and monocytes will release reactive oxygen species, destroying gene stability and causing gene mutation, leading to tumorigenesis [28, 29]. Subsequently, in the stage of tumour proliferation and metastasis, neutrophils, platelets and tumour-associated macrophages secrete a variety of proteins, enzymes and factors, which promote tumour angiogenesis, induce tumour cell epithelial–mesenchymal transformation and participate in extracellular matrix remodeling. It not only provides favourable conditions for tumour growth but also enhances the migration and invasion ability of tumour cells [28–30]. Besides, platelets form a protective film on tumour cells’ surface to protect them from blood flow shear stress while escaping natural killer cells’ immunity [30] and the release of external neutrophil trapping network, which promotes tumour metastasis [29]. Finally, neutrophils and tumour-associated macrophages mediate the suppression of anti-tumour immunity by inhibiting the immune function of natural killer cells and T cells, resulting in malignant progression [28, 29].
In this study, an interesting phenomenon was that the predictive performance of inflammatory markers (PLR and SII) containing platelets was lower than that of SIRI, NLR and MLR. Because HCC patients often experience a chronic liver disease process in which the liver parenchyma is continuously destroyed and then replaced by fibrous tissue under the induction of the hepatitis virus, non-alcoholic fatty liver, alcohol and other causes. Thrombopoietin is an essential factor in regulating megakaryocyte maturation and platelet production [31]. Hepatocytes are the primary source of thrombopoietin production [32]. In the process of chronic liver disease, the number of functional hepatocytes decreases, thus reducing the secretion of thrombopoietin. In addition, cirrhosis and portal hypertension can cause hypersplenism, trapping platelets in the spleen [33] and destroys platelets through macrophages, resulting in thrombocytopenia in the peripheral blood [34]. Therefore, the platelet count can often reflect the severity of chronic liver disease and portal hypertension [35]. Moreover, some of the patients included in this study were complicated with hypersplenism, while others had undergone splenectomy. The above factors will affect the platelet level, thus interfere with the accuracy of PLR and SII in predicting early recurrence. It can be seen that compared with other inflammatory indicators, inflammatory indicators containing platelets, such as PLR and SII, are not effective and have more limitations in patients with HCC.
The study has some shortcomings because this is a single-centre retrospective cohort study, the sample size is small, it can not effectively control confounding factors, there will inevitably be deviations, affecting the quality of the study. Secondly, the aetiology and race of patients with HCC are diverse, which leads to the inconsistent selection of the optimal cut-off value of inflammatory indicators in different studies, which interferes with the results to a certain extent. Therefore, prospective verification is needed in large-scale multicenter studies.
Conclusions
To sum up, as a simple, cheap and convenient inflammatory index, MLR can predict early recurrence and RFS in patients with HCC and will have a more excellent application prospect when combined with other clinical risk factors.
Acknowledgements
All authors thank their colleagues, Dr. Zhou Qingyun, Dr. Zhang Kun, Dr. Tan Wei and others for their hard work in routine follow-up.
Abbreviations
- HCC
Hepatocellular carcinoma
- MLR
Monocyte to lymphocyte ratio
- SIRI
Systemic inflammatory response index
- NLR
Neutrophil to lymphocyte ratio
- PLR
Platelet to lymphocyte ratio
- SII
Systemic immune-inflammatory index
- RFS
Recurrence-free survival
- AUC
Area under the curve
Authors’ contributions
CXS and CYT worked together to conceive the content of the study. YFW and CYT work together to collect and collate clinical data and then conduct statistical analysis. YFW wrote a manuscript of the article. CXS and CYT modify the manuscript. All authors read and approved the final manuscript.
Funding
The study was supported by the Projects of Lishui Key Research and Development Plan in Zhejiang Province, China, Grant/Award Number: 2017ZDYF12. And the study was supported by the Projects of Lishui Key Research and Development Plan in Zhejiang Province, China, Grant/Award Number: 2016zdyf01. Funding was mainly used for data collection, follow-up, statistical analysis, expert communications, and native English editing.
Availability of data and materials
The datasets generated and/or analyzed during the current study are not publicly available due to patient privacy and security of electronic medical information but are (anonymized) available from the corresponding author on reasonable request.
Declarations
Ethics approval and consent to participate
The study was approved by the Ethics Committee of Lishui Municipal Central Hospital (Approval number: 2020-249). Need for written informed consent was waived owing to the retrospective nature of the study.
Consent for publication
Not applicable as there is no identifiable material in this manuscript.
Competing interests
The authors declare that they have no competing interests.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Bray F, Ferlay J, Soerjomataram I, Siegel RL, Torre LA, Jemal A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2018;68:394–424. doi: 10.3322/caac.21492. [DOI] [PubMed] [Google Scholar]
- 2.Chen W, Zheng R, Baade PD, Zhang S, Zeng H, Bray F, et al. Cancer statistics in China, 2015. CA Cancer J Clin. 2016;66:115–132. doi: 10.3322/caac.21338. [DOI] [PubMed] [Google Scholar]
- 3.European Association for the Study of the Liver EASL clinical practice guidelines: management of hepatocellular carcinoma. J Hepatol. 2018;69:182–236. doi: 10.1016/j.jhep.2018.03.019. [DOI] [PubMed] [Google Scholar]
- 4.Portolani N, Coniglio A, Ghidoni S, Giovanelli M, Benetti A, Tiberio GA, et al. Early and late recurrence after liver resection for hepatocellular carcinoma: prognostic and therapeutic implications. Ann Surg. 2006;243:229–235. doi: 10.1097/01.sla.0000197706.21803.a1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Poon RT, Fan ST, Ng IO, Lo CM, Liu CL, Wong J. Different risk factors and prognosis for early and late intrahepatic recurrence after resection of hepatocellular carcinoma. Cancer. 2000;89:500–507. doi: 10.1002/1097-0142(20000801)89:3<500::AID-CNCR4>3.0.CO;2-O. [DOI] [PubMed] [Google Scholar]
- 6.Thiele M, Gluud LL, Fialla AD, Dahl EK, Krag A. Large variations in risk of hepatocellular carcinoma and mortality in treatment naïve hepatitis B patients: systematic review with meta-analyses. PLoS ONE. 2014;9:e107177. doi: 10.1371/journal.pone.0107177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Maki A, Kono H, Gupta M, Asakawa M, Suzuki T, Matsuda M, et al. Predictive power of biomarkers of oxidative stress and inflammation in patients with hepatitis C virus-associated hepatocellular carcinoma. Ann Surg Oncol. 2007;14:1182–1190. doi: 10.1245/s10434-006-9049-1. [DOI] [PubMed] [Google Scholar]
- 8.Lade A, Noon LA, Friedman SL. Contributions of metabolic dysregulation and inflammation to nonalcoholic steatohepatitis, hepatic fibrosis, and cancer. Curr Opin Oncol. 2014;26:100–107. doi: 10.1097/CCO.0000000000000042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Cornellà H, Alsinet C, Villanueva A. Molecular pathogenesis of hepatocellular carcinoma. Alcohol Clin Exp Res. 2011;35:821–825. doi: 10.1111/j.1530-0277.2010.01406.x. [DOI] [PubMed] [Google Scholar]
- 10.Chu YJ, Yang HI, Wu HC, Liu J, Wang LY, Lu SN, et al. Aflatoxin B1 exposure increases the risk of cirrhosis and hepatocellular carcinoma in chronic hepatitis B virus carriers. Int J Cancer. 2017;141:711–720. doi: 10.1002/ijc.30782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Sanghera C, Teh JJ, Pinato DJ. The systemic inflammatory response as a source of biomarkers and therapeutic targets in hepatocellular carcinoma. Liver Int. 2019;39:2008–2023. doi: 10.1111/liv.14220. [DOI] [PubMed] [Google Scholar]
- 12.Sun B, Karin M. Obesity, inflammation, and liver cancer. J Hepatol. 2012;56:704–713. doi: 10.1016/j.jhep.2011.09.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hu B, Yang XR, Xu Y, Sun YF, Sun C, Guo W, et al. Systemic immune-inflammation index predicts prognosis of patients after curative resection for hepatocellular carcinoma. Clin Cancer Res. 2014;20:6212–6222. doi: 10.1158/1078-0432.CCR-14-0442. [DOI] [PubMed] [Google Scholar]
- 14.Mano Y, Shirabe K, Yamashita Y, Harimoto N, Tsujita E, Takeishi K, et al. Preoperative neutrophil-to-lymphocyte ratio is a predictor of survival after hepatectomy for hepatocellular carcinoma: a retrospective analysis. Ann Surg. 2013;258:301–305. doi: 10.1097/SLA.0b013e318297ad6b. [DOI] [PubMed] [Google Scholar]
- 15.Ma W, Zhang P, Qi J, Gu L, Zang M, Yao H, et al. Prognostic value of platelet to lymphocyte ratio in hepatocellular carcinoma: a meta-analysis. Sci Rep. 2016;6:35378. doi: 10.1038/srep35378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Song W, Tian C, Wang K, Zhang RJ, Zou SB. The pretreatment lymphocyte to monocyte ratio predicts clinical outcome for patients with hepatocellular carcinoma: a meta-analysis. Sci Rep. 2017;7:46601. doi: 10.1038/srep46601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Xu L, Yu S, Zhuang L, Wang P, Shen Y, Lin J, et al. Systemic inflammation response index (SIRI) predicts prognosis in hepatocellular carcinoma patients. Oncotarget. 2017;8:34954–34960. doi: 10.18632/oncotarget.16865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Johnson PJ, Berhane S, Kagebayashi C, Satomura S, Teng M, Reeves HL, et al. Assessment of liver function in patients with hepatocellular carcinoma: a new evidence-based approach-the ALBI grade. J Clin Oncol. 2015;33:550–558. doi: 10.1200/JCO.2014.57.9151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hanahan D, Weinberg RA. Hallmarks of cancer: the next generation. Cell. 2011;144:646–674. doi: 10.1016/j.cell.2011.02.013. [DOI] [PubMed] [Google Scholar]
- 20.Shah SA, Greig PD, Gallinger S, Cattral MS, Dixon E, Kim RD, et al. Factors associated with early recurrence after resection for hepatocellular carcinoma and outcomes. J Am Coll Surg. 2006;202:275–283. doi: 10.1016/j.jamcollsurg.2005.10.005. [DOI] [PubMed] [Google Scholar]
- 21.Ibrahim S, Roychowdhury A, Hean TK. Risk factors for intrahepatic recurrence after hepatectomy for hepatocellular carcinoma. Am J Surg. 2007;194:17–22. doi: 10.1016/j.amjsurg.2006.06.051. [DOI] [PubMed] [Google Scholar]
- 22.Tabrizian P, Jibara G, Shrager B, Schwartz M, Roayaie S. Recurrence of hepatocellular cancer after resection: patterns, treatments, and prognosis. Ann Surg. 2015;261:947–955. doi: 10.1097/SLA.0000000000000710. [DOI] [PubMed] [Google Scholar]
- 23.Chan AWH, Zhong J, Berhane S, Toyoda H, Cucchetti A, Shi K, et al. Development of pre and post-operative models to predict early recurrence of hepatocellular carcinoma after surgical resection. J Hepatol. 2018;69:1284–1293. doi: 10.1016/j.jhep.2018.08.027. [DOI] [PubMed] [Google Scholar]
- 24.Imamura H, Matsuyama Y, Tanaka E, Ohkubo T, Hasegawa K, Miyagawa S, et al. Risk factors contributing to early and late phase intrahepatic recurrence of hepatocellular carcinoma after hepatectomy. J Hepatol. 2003;38:200–207. doi: 10.1016/S0168-8278(02)00360-4. [DOI] [PubMed] [Google Scholar]
- 25.Cucchetti A, Piscaglia F, Caturelli E, Benvegnù L, Vivarelli M, Ercolani G, et al. Comparison of recurrence of hepatocellular carcinoma after resection in patients with cirrhosis to its occurrence in a surveilled cirrhotic population. Ann Surg Oncol. 2009;16:413–422. doi: 10.1245/s10434-008-0232-4. [DOI] [PubMed] [Google Scholar]
- 26.Nagasue N, Uchida M, Makino Y, Takemoto Y, Yamanoi A, Hayashi T, et al. Incidence and factors associated with intrahepatic recurrence following resection of hepatocellular carcinoma. Gastroenterology. 1993;105:488–494. doi: 10.1016/0016-5085(93)90724-Q. [DOI] [PubMed] [Google Scholar]
- 27.Ni JY, Liu SS, Xu LF, Sun HL, Chen YT. Meta-analysis of radiofrequency ablation in combination with transarterial chemoembolization for hepatocellular carcinoma. World J Gastroenterol. 2013;19:3872–3882. doi: 10.3748/wjg.v19.i24.3872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Zhang X, Zhang W, Yuan X, Fu M, Qian H, Xu W. Neutrophils in cancer development and progression: roles, mechanisms, and implications (review) Int J Oncol. 2016;49:857–867. doi: 10.3892/ijo.2016.3616. [DOI] [PubMed] [Google Scholar]
- 29.Salmaninejad A, Valilou SF, Soltani A, Ahmadi S, Abarghan YJ, Rosengren RJ, et al. Tumor-associated macrophages: role in cancer development and therapeutic implications. Cell Oncol (Dordr) 2019;42:591–608. doi: 10.1007/s13402-019-00453-z. [DOI] [PubMed] [Google Scholar]
- 30.Li N. Platelets in cancer metastasis: to help the "villain" to do evil. Int J Cancer. 2016;138:2078–2087. doi: 10.1002/ijc.29847. [DOI] [PubMed] [Google Scholar]
- 31.Wolber EM, Jelkmann W. Thrombopoietin: the novel hepatic hormone. News Physiol Sci. 2002;17:6–10. doi: 10.1152/physiologyonline.2002.17.1.6. [DOI] [PubMed] [Google Scholar]
- 32.Sungaran R, Markovic B, Chong BH. Localization and regulation of thrombopoietin mRNa expression in human kidney, liver, bone marrow, and spleen using in situ hybridization. Blood. 1997;89:101–107. doi: 10.1182/blood.V89.1.101. [DOI] [PubMed] [Google Scholar]
- 33.Aster RH. Pooling of platelets in the spleen: role in the pathogenesis of "hypersplenic" thrombocytopenia. J Clin Invest. 1966;45:645–657. doi: 10.1172/JCI105380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Yongxiang W, Zongfang L, Guowei L, Zongzheng J, Xi C, Tao W. Effects of splenomegaly and splenic macrophage activity in hypersplenism due to cirrhosis. Am J Med. 2002;113:428–431. doi: 10.1016/S0002-9343(02)01210-X. [DOI] [PubMed] [Google Scholar]
- 35.Giannini EG. Thrombocytopenia in patients with chronic liver disease: what's in a name? Dig Dis Sci. 2013;58:299–301. doi: 10.1007/s10620-012-2450-7. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The datasets generated and/or analyzed during the current study are not publicly available due to patient privacy and security of electronic medical information but are (anonymized) available from the corresponding author on reasonable request.