Significance
Strategies to suppress memory T cell responses are needed. Th2-polarized cells lacking the transcription factor T-bet strongly suppress not only naive but also memory T cell responses via IL-10 and protect mice against the development of virus-induced type 1 diabetes. We shed light on a population of immune cells with substantial immunomodulatory capacity.
Keywords: autoimmunity, T-bet, IL-10, CD8+ T cells, memory responses
Abstract
Exacerbated immune responses and loss of self-tolerance lead to the development of autoimmunity and immunopathology. Novel therapies to target autoreactive T cells are still needed. Here, we report that Th2-polarized T cells lacking the transcription factor T-bet harbor strong immunomodulatory potential and suppress antigen-specific CD8+ T cells via IL-10. Tbx21−/− Th2 cells protected mice against virus-induced type 1 diabetes development and suppressed not only naive but also memory CD8+ T cell responses. IL-10–producing, but not IL-10–deficient Tbx21−/− Th2 cells down-regulated costimulatory molecules on dendritic cells and reduced their IL-12 production after lymphocytic choriomeningitis virus infection. Impaired dendritic cell activation hindered effector and cytotoxic CD8+ T cell development after infection. These findings indicate that Tbx21−/− Th2 cells strongly suppress proinflammatory responses of naive and memory T cells via IL-10. Thus, in vivo IL-10–secreting Th2 cells could harbor a therapeutic potential for the treatment of T cell-mediated inflammatory disorders.
Autoimmune diseases are common and one of the leading causes of disability and chronic morbidity worldwide (1, 2). The main task of the immune system is to eliminate pathogens; however, it also has to maintain tolerance to self-antigens. Several mechanisms exist to prevent the generation of autoimmune responses such as clonal deletion of developing T cells as well as the inactivation of immune cells in the periphery (3). Modulation of T cell responses via regulatory T (Treg) cells is an important mechanism mediating immunoregulation to prevent graft vs. host disease (4, 5), autoimmune diseases (6–8), and delay graft rejection (9). Studies have reported the suppressive functions of FoxP3+ Treg cells on particularly naive T cell responses to foreign antigens as well as to self-antigens (10). Furthermore, several groups have investigated the regulatory capacity of additional cell subsets such as tolerogenic dendritic cells (11), myeloid-derived suppressor cells (12), regulatory B cells (13), regulatory macrophages (14), and regulatory CD8+ T cells (15). Regulatory mechanisms might result from either cell–cell contact or via soluble mediators, e.g., antiinflammatory cytokines (16, 17). The breakdown of these mechanisms, a susceptible genetic background, and the presence of other concomitant etiological factors can lead to the disruption of immunotolerance and to the development of autoimmunity (8). Strategies aimed at restoring the loss of tolerance mechanisms such as increasing the number and potency of Treg cells (18, 19), autologous hematopoietic stem cell transplantation (20), induction of tolerogenic dendritic cells (21), and treatment with immunomodulatory molecules and cytokines (22) constitute a major focus of research. Adoptive transfer of CD25+CD4+ Treg cells was shown to be a promising approach for the treatment of graft vs. host disease (23, 24) and has also been explored in the treatment of autoimmune disorders in experimental models (25, 26). In addition, other studies have demonstrated the relative resistance of memory T cells to suppression by Treg cells (27, 28). Resistance of memory T cells to immunosuppression, in part due to their fully differentiated phenotype and their relative lack of reliance on costimulation, represents a challenge to the development of immunotherapies (29). Efforts in this field have shown that memory CD8+ T cell responses can be inactivated when antigen is genetically targeted to steady-state dendritic cells (30).
Another proposed strategy to modulate immune responses was to deviate the cytokine balance from a T helper 1 (Th1) toward a Th2 response (31). For decades, Th1 and Th2 cell programs were thought to be mutually exclusive and steady (32, 33). However, the stability of differentiated populations of T cells in vivo has been challenged. We have previously demonstrated that antigen-specific Th2 cells adopt a “Th2+1” phenotype with the capacity to coproduce Th2 and Th1 cytokines upon infection in vivo (34, 35). The Th1 lineage-specifying transcription factor T-bet (Tbx21) was essential for Th2 cell reprogramming because T-bet–deficient (Tbx21−/−) Th2 cells remained stably committed Th2 cells in vivo despite Th1 cell-promoting lymphocytic choriomeningitis virus (LCMV) infection. These T-bet–deficient Th2 cells induced a strong immunosuppression of the endogenous LCMV-specific CD8+ T cell response postinfection (p.i.) in vivo. Therefore, we investigated the mechanism underlying CD8+ T cell immunosuppression and its functional relevance by using the LCMV infection model. Here, we report that Tbx21−/− Th2 but not wild-type (WT) Th2 cells mediate immunosuppression of proinflammatory T cell responses via IL-10. IL-10–producing Tbx21−/− Th2 cells impaired dendritic cell (DC) activation leading to dysfunctional CD8+ T cell activation. Functionally, adoptively transferred virus-specific Tbx21−/− Th2 but not WT Th2 cells protected mice from the development of virus-induced type 1 diabetes. Importantly, Tbx21−/− Th2 cells were able to suppress not only naive but also memory CD8+ T cell responses to LCMV in vivo.
Thus, our findings shed light on a population of immune cells with a remarkably strong immunosuppressive capacity. A better understanding of novel immunomodulatory cell subsets is crucial for developing effective therapies for autoimmune diseases as well as for organ transplantation.
Results
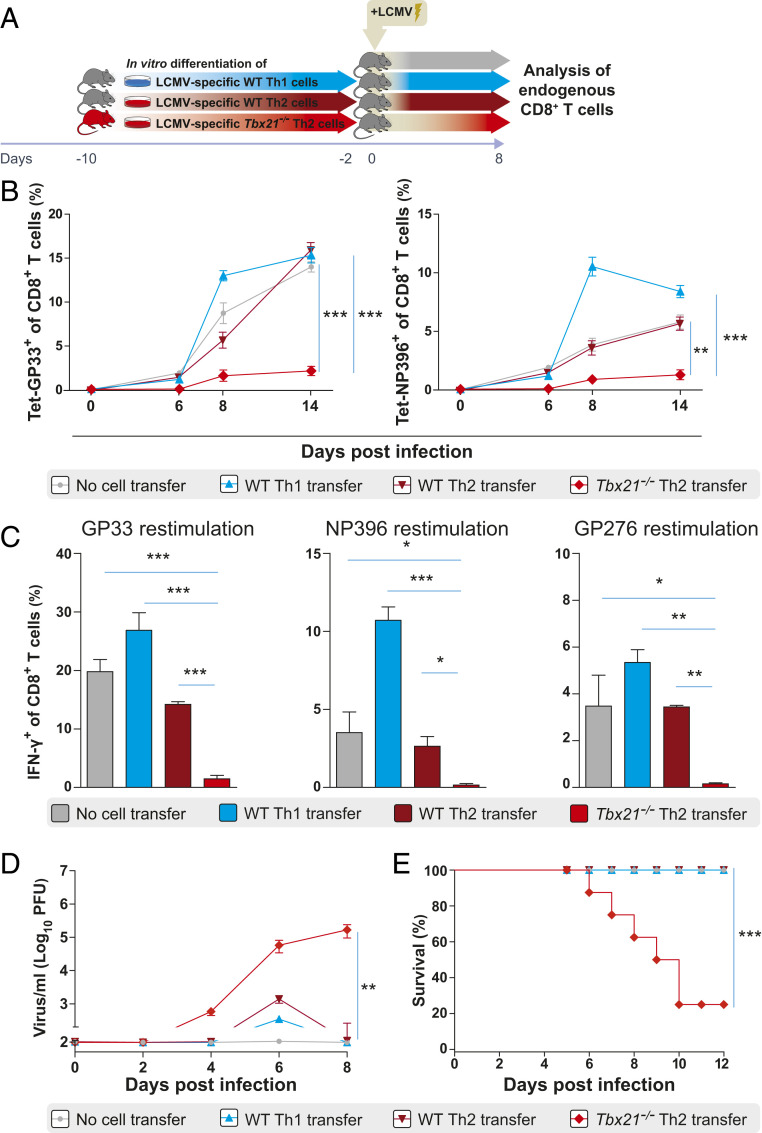
Tbx21−/− Th2 Cells but Not WT Th2 Cells Suppress Endogenous Antigen-Specific CD8+ T Cell Responses.
In order to investigate the immunomodulatory properties of Th2 cells lacking T-bet expression on T cell responses, LCMV-specific WT Th1, WT Th2, or Tbx21−/− Th2 cells were adoptively transferred into naive C57BL/6 mice that were subsequently infected with LCMV (Fig. 1A). Both LCMV-specific WT Th1 and WT Th2 cell recipients as well as control mice without cell transfer displayed similar frequencies of endogenous CD8+ T cells specific to the dominant LCMV glycoprotein GP33 and nucleoprotein NP396 in the blood between days 6 and 14 postinfection (Fig. 1B). In contrast, Tbx21−/− Th2 cell recipients showed markedly reduced frequencies of GP33- and NP396-tetramer+ CD8+ T cells from day 8 postinfection onwards (Fig. 1B). In addition, the frequency of IFN-γ–producing CD8+ T cells of Tbx21−/− Th2 cell recipients was markedly reduced compared to that of WT Th2 and WT Th1 cell recipients as well as nontransferred control mice after GP33, NP396, and GP276 peptide restimulation ex vivo at day 8 postinfection (Fig. 1C). In line with the suppression of virus-specific CD8+ T cells, Tbx21−/− Th2 cell recipients were unable to control the infection. In contrast to WT Th2 and WT Th1 recipients and nontransferred control mice, Tbx21−/− Th2 cell recipients exhibited significantly higher viremia from day 4 postinfection onwards (Fig. 1D). Furthermore, several Tbx21−/− Th2 cell recipients developed a wasting syndrome, with constant weight loss and reduced survival compared to WT Th2 and WT Th1 cell recipient mice and nontransferred controls (Fig. 1E). We then characterized the phenotype of the few GP33- and NP396-tetramer+ CD8+ T cells generated in Tbx21−/− Th2 cell recipients at the peak of infection. LCMV-specific CD8+ T cells in Tbx21−/− Th2 cell recipients displayed reduced expression of CD44 but increased CD62L and CD127 expression compared to the LCMV-specific CD8+ T cells in WT Th2 and WT Th1 cell recipients and nontransferred control mice (SI Appendix, Fig. S1). Taken together, these data indicate that adoptively transferred Tbx21−/− Th2 cells but not WT Th2 cells strongly suppressed the activation and expansion of virus-specific CD8+ T cells and impaired the antiviral cytokine production at the peak of infection. To determine whether the suppressive capacity of adoptively transferred Tbx21−/− Th2 cells could be attributed to the acquisition of a regulatory phenotype in vivo, we assessed the expression of FoxP3 and RORγt in the transferred cells recovered from infected recipient mice. FoxP3 and RORγt expression were similar between LCMV-specific WT Th1 and Tbx21−/− Th2 cells (SI Appendix, Fig. S2). As expected, T-bet expression was absent and GATA-3 expression was significantly higher in Tbx21−/− Th2 cells compared to that in WT Th1 cells ex vivo (SI Appendix, Fig. S2). Thus, adoptively transferred Tbx21−/− Th2 cells suppress virus-specific T cells independently of FoxP3 expression.
Fig. 1.
Tbx21−/− Th2 cells but not WT Th2 cells suppress endogenous antigen-specific CD8+ T cell responses. (A) Schematic experimental layout to analyze endogenous antigen-specific CD8+ T cell responses in WT C57BL/6 recipient mice after adoptive transfer of LCMV-specific SMARTA1 TCR-tg Tbx21−/− Th2, WT Th2, and WT Th1 cells and in control mice without cell transfer at day 8 post LCMV infection. (B) Frequencies of endogenous LCMV-specific CD8+ T cells were assessed by MHC class I tetramer (H-2DbGP33 and H-2DbNP396) staining in peripheral blood of LCMV-specific Tbx21−/− Th2 (n = 3), WT Th2 (n = 3), and WT Th1 (n = 3) recipients and nontransferred control mice (n = 3) on days 0, 6, 8, and 14 after LCMV infection. P values are from day 14 postinfection. (C) Frequencies of IFN-γ–producing CD8+ T cells in spleens of WT Th1, WT Th2, and Tbx21−/− Th2 cell recipients and nontransferred control mice after GP33, NP396, and GP276 peptide restimulation ex vivo at day 8 postinfection and after exclusion of Thy1.1+ donor cells in fluorescence-activated cell sorting analysis. (D) Virus titers in blood of Tbx21−/− Th2, WT Th2, and WT Th1 cell recipients and nontransferred control mice were measured by plaque assay on days 0, 2, 4, 6, and 8 after LCMV infection. P values are from day 8 postinfection. (E) Survival plots of Tbx21−/− Th2 (n = 9), WT Th2 (n = 9), and WT Th1 (n = 9) cell recipients and nontransferred control mice (n = 9) until day 12 postinfection. The log-rank test was used for statistical evaluation of survival (Kaplan–Meier survival curve). All experiments were performed at least twice, and each experimental group included n ≥ 3. Data are representative and expressed as mean ± SEM. Asterisks indicate statistically significant differences as analyzed by one-way ANOVA with Bonferroni’s post test (*P < 0.05, **P < 0.01, ***P < 0.001).
We then examined the in vivo killing capacity of endogenous CD8+ T cells in the various groups of recipient mice. For this, GP33-pulsed and unpulsed target cells were transferred into previously infected WT Th1 or Tbx21−/− Th2 cell recipient mice or nontransferred control mice. WT Th1 cell recipients and infected control mice without cell transfer displayed ∼4- and 10-fold reduction in the frequency of GP33-pulsed target cells compared to that of the unpulsed target cells which translates into a killing capacity of ∼75% and 90%, respectively (SI Appendix, Fig. S3). In contrast, virtually no killing of target cells was observed in Tbx21−/− Th2 cell recipient mice indicated by very similar frequencies of both GP33-pulsed and unpulsed target cells (SI Appendix, Fig. S3). Moreover, we assessed the expression of T-bet, the Th1 key transcription factor, in activated endogenous (CD44+CD62L−) CD8+ and CD4+ T cells in WT Th1 and Tbx21−/− Th2 cell recipients and nontransferred control mice at day 8 postinfection. Activated CD8+ and CD4+ T cells exhibited significantly lower T-bet but higher Eomes expression in the spleen of Tbx21−/− Th2 cell recipients compared to infected control mice without cell transfer (SI Appendix, Fig. S4). These findings collectively suggest that Tbx21−/− Th2 cells suppress the effector differentiation of virus-specific CD8+ T cells. This suppression results in a rather memory cell-like phenotype during the effector phase of LCMV infection combined with impaired killing capacity in vivo.
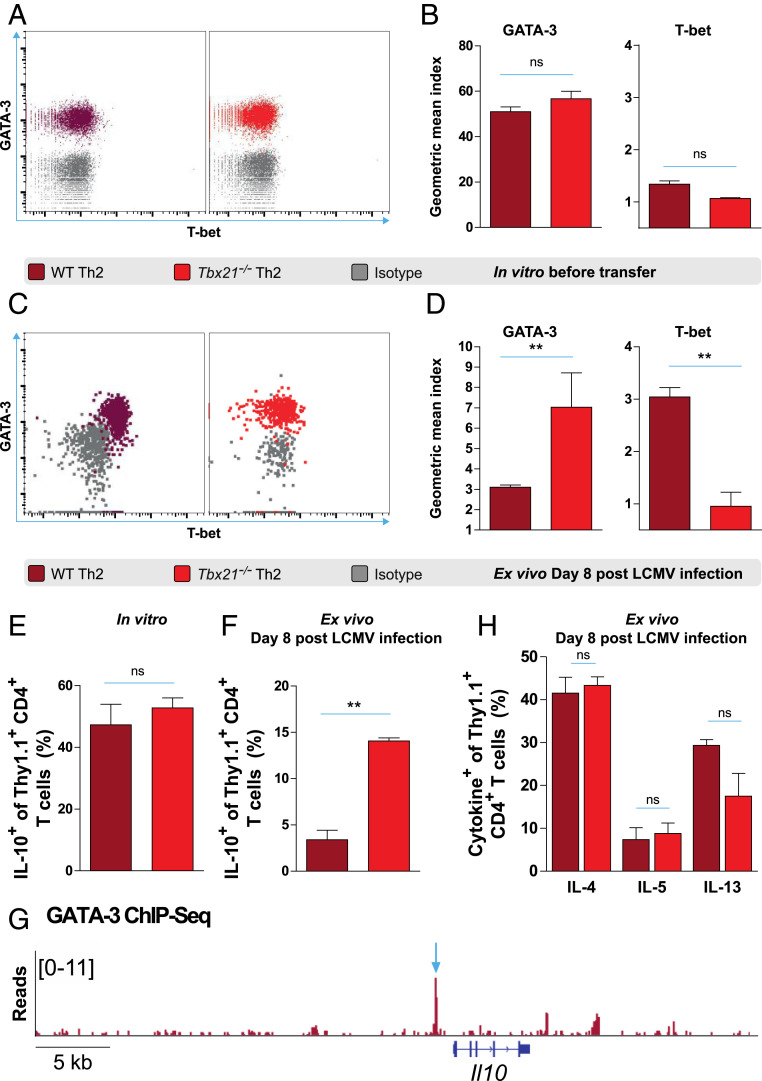
Tbx21−/− Th2 Cells but Not WT Th2 Cells Stably Maintain IL-10 Production In Vivo after LCMV Infection.
Since Tbx21−/− Th2 but not WT Th2 cells strongly suppress virus-specific CD8+ T cells, we characterized their transcription factor expression and cytokine production both after in vitro differentiation and ex vivo at the peak of LCMV infection. After 10 d of in vitro differentiation, all Tbx21−/− Th2 and WT Th2 cells homogenously expressed GATA-3, but virtually no T-bet expression was observed (Fig. 2A). Accordingly, WT Th2 and Tbx21−/− Th2 cells displayed similar GATA-3 and T-bet geometric mean indices in vitro (Fig. 2B). In contrast, upon LCMV infection, WT Th2 cells homogeneously adopted a GATA-3+T-bet+ phenotype ex vivo, whereas Tbx21−/− Th2 cells exclusively maintained high GATA-3 expression (Fig. 2C). Consistently, Tbx21−/− Th2 cells displayed higher GATA-3 but lower T-bet geometric mean indices ex vivo compared to that of WT Th2 cells after LCMV infection (Fig. 2D). In addition, prior to adoptive transfer the frequencies of IL-10–producing WT Th2 cells were similar to that of in vitro differentiated Tbx21−/− Th2 cells (Fig. 2E). Importantly, upon LCMV infection, adoptively transferred WT Th2 cells largely lost their ability to produce IL-10 (Fig. 2F). In contrast, Tbx21−/− Th2 cells continued to produce IL-10 after LCMV infection (Fig. 2F). Furthermore, we analyzed chromatin immunoprecipitation sequencing (ChIP-Seq) tracks of in vitro differentiated murine WT Th2 cells and observed a prominent GATA-3 binding peak within the promoter region of the Il10 gene (Fig. 2G). Notably, other Th2 cytokines such as IL-4, IL-5, and IL-13 were similarly produced by both WT Th2 and Tbx21−/− Th2 cells ex vivo after LCMV infection (Fig. 2H). In addition, WT Th1 cells expressed T-bet but not GATA-3 after in vitro differentiation (SI Appendix, Fig. S5A). However, Tbx21−/− Th1 cells expressed neither T-bet nor GATA-3 as expected (SI Appendix, Fig. S5A). Contrary to WT Th2 and Tbx21−/− Th2 cells, both WT Th1 and Tbx21−/− Th1 cells produced very little IL-10 after in vitro differentiation (SI Appendix, Fig. S5B). Collectively, our results demonstrate that Tbx21−/− Th2 but not WT Th2 cells stably maintain high GATA-3 expression and IL-10 production ex vivo upon LCMV infection.
Fig. 2.
Tbx21−/− Th2 cells but not WT Th2 cells stably maintain IL-10 production in vivo after LCMV infection. (A) Representative fluorescence-activated cell sorting (FACS) plots and (B) geometric mean indices of GATA-3 and T-bet vs. isotype control stainings of LCMV-specific WT Th2 and Tbx21−/− Th2 cells after 10 d of in vitro differentiation. (C) Representative FACS plots and (D) geometric mean indices of GATA-3 and T-bet vs. isotype control stainings of adoptively transferred LCMV-specific WT Th2 and Tbx21−/− Th2 cells ex vivo on day 8 after LCMV infection. (E) Frequencies of IL-10–producing cells in WT Th2 and Tbx21−/− Th2 cells after 10 d of in vitro differentiation and (F) in adoptively transferred WT Th2 and Tbx21−/− Th2 cells ex vivo on day 8 after LCMV infection. (G) GATA-3 occupancy at the Il10 locus. The arrow indicates prominent GATA-3 binding at the Il10 promoter 1.1 kbp upstream of the transcriptional start site. Published GATA-3 ChIP-Seq data were obtained from the Gene Expression Omnibus database (accession no. GSM523226) (SI Appendix) (65). (H) Frequencies of IL-4–, IL-5–, and IL13–producing cells in adoptively transferred WT Th2 and Tbx21−/− Th2 cells ex vivo on day 8 after LCMV infection. All experiments were performed at least twice, and each experimental group included n ≥ 3. Data are representative or pooled and expressed as mean ± SEM. Asterisks indicate statistically significant differences as analyzed by t test (**P < 0.01). ns, not significant in all figures.
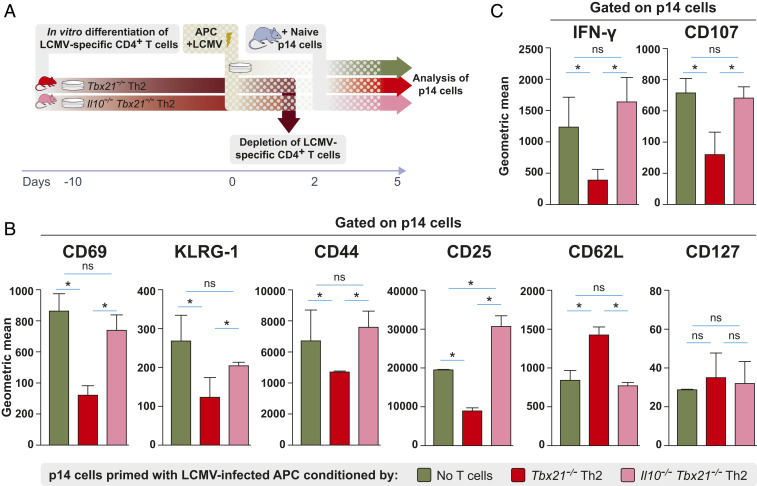
Tbx21−/− Th2 Cells Suppress Not Only Naive but Also Memory LCMV-Specific CD8+ T Cells In Vivo.
Since Tbx21−/− Th2 cells harbor a strong suppressive potential on primary LCMV-specific CD8+ T cell responses in vivo, we investigated whether Tbx21−/− Th2 cells could also suppress CD8+ T cell memory responses. To answer this question, we isolated LCMV-specific memory CD8+ T cells (memory p14) from previously infected mice and (co)transferred them with or without LCMV-specific Tbx21−/− Th2 cells into naive WT mice (Fig. 3A). To quantitatively compare the suppression capacity of LCMV-specific Tbx21−/− Th2 cells during primary and secondary responses in parallel, we additionally transferred naive LCMV-specific CD8+ T cells (naive p14) alone or together with Tbx21−/− Th2 cells into C57BL/6 mice that were subsequently infected with LCMV (Fig. 3A). Interestingly, Tbx21−/− Th2 cells strongly suppressed the cotransferred memory p14 cells, shown as frequencies and absolute numbers as compared to nontransferred control mice that only received memory p14 cells (Fig. 3B). As expected, the expansion of naive p14 cells was also significantly reduced in both frequencies and absolute numbers when cotransferred with Tbx21−/− Th2 cells (Fig. 3B). Normalized frequencies and absolute numbers of the progeny of both naive and memory p14 cells found in the spleen of recipient mice revealed that the reduction effect of cotransferred Tbx21−/− Th2 cells on memory p14 cells was as strong as the effect on naive p14 cells (Fig. 3C). Thus, memory CD8+ T cells are suppressed as potently as naive CD8+ T cells by cotransferred Tbx21−/− Th2 cells in vivo.
Fig. 3.
Tbx21−/− Th2 cells suppress not only naive but also memory LCMV-specific CD8+ T cells in vivo. (A) Schematic experimental layout to assess the suppression of memory p14 cells by LCMV-specific Tbx21−/− Th2 cells. Memory p14 cells were isolated from previously infected mice at day 20 postinfection and subsequently cotransferred with or without LCMV-specific Tbx21−/− Th2 cells into secondary WT recipient mice followed by LCMV infection of the secondary hosts. (B) Frequencies and absolute cell numbers of transferred naive and memory p14 cells with or without cotransfer of Tbx21−/− Th2 cells at day 8 postinfection. (C) Normalized expansion of p14 cells and its suppression by the cotransfer of Tbx21−/− Th2 cells calculated on the frequencies and absolute cell numbers shown in B. (D) Frequencies and absolute cell numbers of IFN-γ–producing p14 cells with or without cotransfer of Tbx21−/− Th2 cells in the spleen after restimulation ex vivo with GP33 peptide. All experiments were performed at least twice, and each experimental group included n ≥ 3. Data are representative and expressed as mean ± SEM. Asterisks indicate statistically significant differences as analyzed by one-way ANOVA with Bonferroni’s post test (*P < 0.05, **P < 0.01, ***P < 0.001).
Moreover, we investigated whether transferred Tbx21−/− Th2 cells could impact the functionality of memory p14 cells in vivo. To this end, we assessed IFN-γ production in p14 recipient mice in the presence or absence of Tbx21−/− Th2 cells. A significant reduction in the frequencies and absolute numbers of IFN-γ–producing p14 cells was observed when Tbx21−/− Th2 cells were cotransferred (Fig. 3D). In summary, Tbx21−/− Th2 cells harbor strong suppressive potential enabling them to hamper the expansion and functionality not only of naive but also of memory CD8+ T cells in vivo.
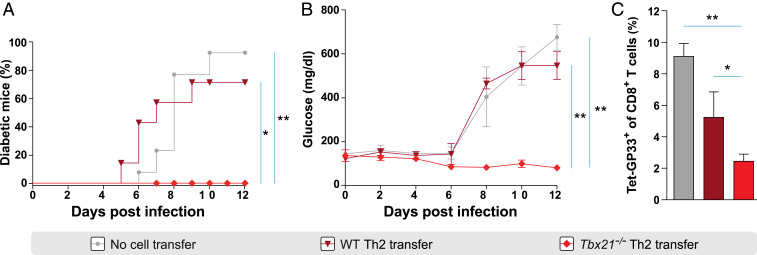
Tbx21−/− Th2 but Not WT Th2 Cells Protect Mice against Type 1 Diabetes Development.
The strong suppressive potential of Tbx21−/− Th2 cells prompted us to investigate their in vivo relevance during the development of proinflammatory autoimmune responses. For this, we used the virus-induced type 1 diabetes (T1D) model of rat insulin promoter–glycoprotein (RIP-GP) mice that express the glycoprotein of LCMV in pancreatic β-cells. T cell responses induced after LCMV infection lead to pancreatic β-cell destruction and rapid development of T1D (36). Since Tbx21−/− Th2 cells strongly suppress LCMV-specific CD8+ T cells, we hypothesized that adoptively transferred Tbx21−/− Th2 but not WT Th2 cells could protect RIP-GP mice from the development of diabetes in vivo. Remarkably, adoptively transferred Tbx21−/− Th2 but not WT Th2 cells protected RIP-GP mice against the development of T1D. All control mice and 70% of the WT Th2 cell recipient mice became diabetic by day 10 p.i. (Fig. 4A). Tbx21−/− Th2 cell recipients maintained blood glucose levels of ∼100 mg/dL, while WT Th2 cell recipients and control mice displayed levels over 400 mg/dL at day 8 p.i. (Fig. 4B). Furthermore, frequencies of CD8+ T cells specific to the dominant LCMV GP epitope in the blood of Tbx21−/− Th2 cell recipients were significantly decreased compared to both WT Th2 cell recipients and nontransferred control RIP-GP mice after infection (Fig. 4C). In summary, Tbx21−/− Th2 but not WT Th2 cells protected RIP-GP mice from diabetes development associated with a suppression of the expansion of LCMV-specific CD8+ T cells.
Fig. 4.
Tbx21−/− Th2 but not WT Th2 cells protect mice against type 1 diabetes development. (A) Percentage of diabetes development in RIP-GP recipients of Tbx21−/− Th2 and WT Th2 cells and control RIP-GP mice without cell transfer until day 12 post LCMV infection. P values were calculated by log-rank test. (B) Blood glucose levels in RIP-GP recipients of Tbx21−/− Th2 and WT Th2 cells and control RIP-GP mice without cell transfer until day 12 postinfection. P values are from day 12 postinfection. (C) Frequencies of endogenous GP33-tetramer+ CD8+ T cells in RIP-GP recipients of Tbx21−/− Th2 and WT Th2 cells and nontransferred control RIP-GP mice in the blood at day 8 postinfection. All experiments were performed at least twice, and each experimental group included n ≥ 3. Data are pooled and expressed as mean ± SEM. Asterisks indicate statistically significant differences as analyzed by one-way ANOVA with Bonferroni’s post test (*P < 0.05, **P < 0.01).
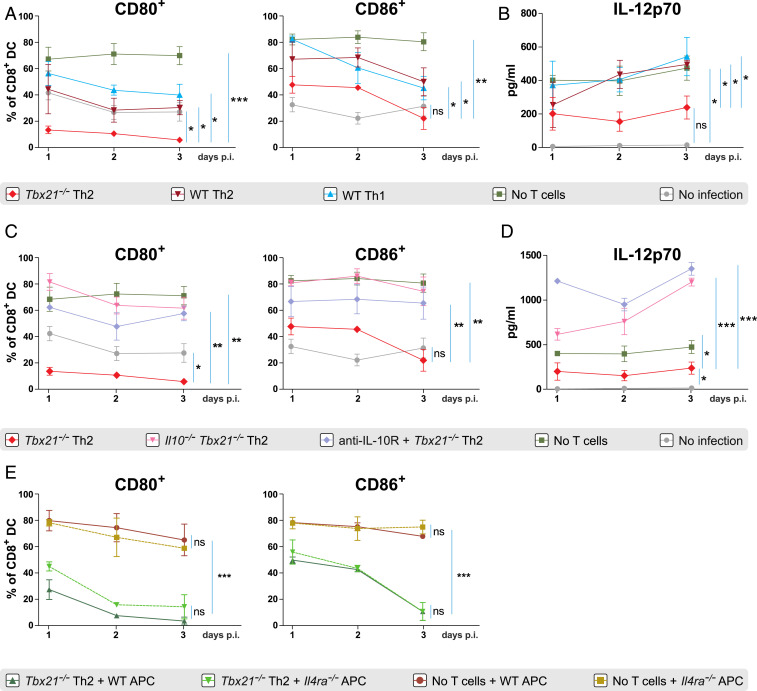
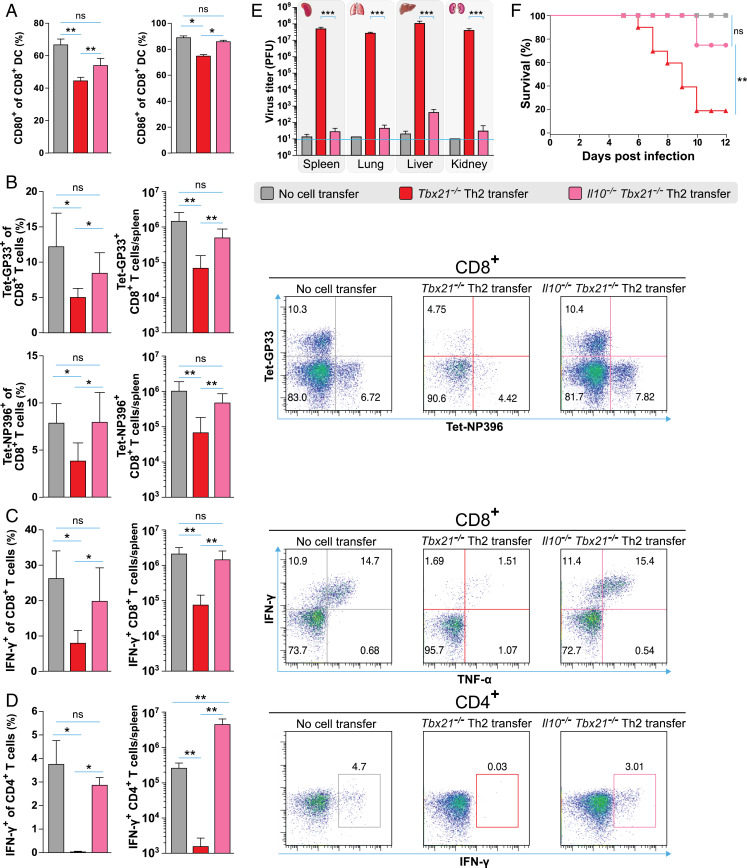
Tbx21−/− Th2 Cells Down-Regulate the Costimulatory Capacity of DCs after LCMV Infection In Vitro.
To investigate the molecular mechanisms underlying the suppressive potential of Tbx21−/− Th2 cells, we designed an LCMV infection model in vitro in which an enriched CD11c+ cell fraction of splenocytes was cocultured with various subsets of differentiated T helper cells. We hypothesized that Tbx21−/− Th2 cells impair the activation and maturation of DCs and this in turn hinders a proper CD8+ T cell activation after LCMV infection. CD8-expressing DCs are particularly important for the activation of CD8+ T cells during viral infections (37). Therefore, we assessed the expression of the costimulatory molecules CD80 and CD86 on CD8+CD11c+ DCs that were previously cocultured with LCMV-specific Tbx21−/− Th2, LCMV-specific WT Th2, and LCMV-specific WT Th1 cells after LCMV infection. Strong up-regulation of CD80 and CD86 expression on CD8+ DC was observed after 1, 2, and 3 d post LCMV infection. However, in the presence of Tbx21−/− Th2 cells, DCs failed to up-regulate the expression of costimulatory molecules shown by the reduced CD80+ and CD86+ frequencies compared to that of DCs in the presence of WT Th2 and WT Th1 cells and in infected DCs without T cells (Fig. 5A) and the displayed lower mean fluorescence intensity of both molecules (SI Appendix, Fig. S6). Expression of CD40, CD70, and OX40L was not modified by the presence of Tbx21−/− Th2 cells in vitro after LCMV infection (data not shown).
Fig. 5.
Tbx21−/− Th2 cells down-regulate the costimulatory capacity of DCs after LCMV infection in vitro. (A) Frequencies of CD80 and CD86 expression on CD8+CD11c+ DCs cocultured with LCMV-specific Tbx21−/− Th2, WT Th2, or WT Th1 cells after 1, 2, and 3 d of LCMV infection in vitro. (B) IL-12p70 concentrations in supernatants of the cocultures in A. (C) Frequencies of CD80 and CD86 expression on CD8+CD11c+ DC cocultured with LCMV-specific Tbx21−/− Th2 or Il10−/−Tbx21−/− Th2 or Tbx21−/− Th2 cells plus anti-IL-10R antibody after 1, 2, and 3 d of LCMV infection. (D) IL-12p70 concentrations in supernatants of the cocultures in C. (E) Frequencies of CD80 and CD86 expression on CD8+CD11c+ WT or Il4ra−/− DCs cocultured with LCMV-specific Tbx21−/− Th2 cells after 1, 2, and 3 d of LCMV infection. Infected as well as uninfected CD8+CD11c+ DCs without T cells were used as positive and negative controls, respectively. All experiments were performed at least twice, and each experimental group included n ≥ 3. Data are pooled and expressed as mean ± SEM. Asterisks indicate statistically significant differences as analyzed by one-way ANOVA with Bonferroni’s post test at day 3 postinfection (*P < 0.05, **P < 0.01, ***P < 0.001).
Apart from the up-regulation of costimulatory molecules, the cytokine production of DCs is critical for the generation of effector CD8+ T cells after LCMV infection (38). Therefore, we examined IL-12 production by CD8+ DCs at days 1, 2, and 3 postinfection. Interestingly, IL-12 production was significantly decreased in the presence of Tbx21−/− Th2 cells compared to that of DCs in the presence of WT Th2 and WT Th1 cells and in infected DCs without T cells (Fig. 5B). These findings collectively suggest that Tbx21−/− Th2 but not WT Th2 cells impair two crucial events during DC maturation, the up-regulation of their costimulatory molecules, and their IL-12 production.
IL-10 from Tbx21−/− Th2 Cells Mediates the Down-Regulation of Costimulatory Molecules on DCs In Vitro.
IL-10 is known to suppress T cell responses as well as to limit proper activation of DCs (16, 39). To investigate whether IL-10 production by Tbx21−/− Th2 cells could impair DC activation, we generated Il10−/−Tbx21−/− LCMV-specific CD4+ T cell transgenic mice to assess the expression of CD80 and CD86 on DCs as well as their IL-12 production after LCMV infection. CD80 and CD86 down-regulation on CD8+ DCs observed in the presence of Tbx21−/− Th2 cells after infection was completely reversed when DCs were infected in the presence of Il10−/−Tbx21−/− Th2 cells (Fig. 5C). In addition, we blocked IL-10R on DCs and infected them with LCMV in the presence of Tbx21−/− Th2 cells. Similarly, the expression of CD80 and CD86 on CD8+ DCs was restored (Fig. 5C) and their IL-12 production was significantly increased in the presence of the IL-10R blocking antibody (Fig. 5D). Furthermore, CD80 and CD86 mean fluorescence intensities were also restored in the presence of Il10−/−Tbx21−/− Th2 cells as well as during IL-10R blockade compared to the levels in the presence of Tbx21−/− Th2 cells (SI Appendix, Fig. S7). These data indicate that IL-10 produced by Tbx21−/− Th2 cells directly impaired DC activation after LCMV infection.
Next, we investigated the potential role of additional cytokines produced by Tbx21−/− Th2 cells such as IL-4 and IL-13 in the suppression of DC activation using our coculture system. For this, we compared the expression of the costimulatory molecules CD80 and CD86 on WT DCs vs. IL-4Rα–deficient DCs lacking both IL-4 and IL-13 signaling, in the presence or absence of Tbx21−/− Th2 cells after LCMV infection. The suppression of CD80 and CD86 expression in the presence of Tbx21−/− Th2 cells was equally pronounced on WT DCs as on IL-4Rα–deficient DCs at days 1, 2, and 3 post LCMV infection (Fig. 5E). Taken together, these data demonstrate that Tbx21−/− Th2 cells impair DC activation via IL-10 but not through IL-4 or IL-13 production in vitro.
Tbx21−/− Th2 Cells Impair CD8+ T Cell Activation via IL-10 In Vitro.
Impaired DC activation by Tbx21−/− Th2 cells via IL-10 might in turn limit CD8+ T cell activation after LCMV infection. To test this hypothesis, we infected DCs with LCMV either in the presence of LCMV-specific Tbx21−/− Th2 or Il10−/−Tbx21−/− Th2 cells for 2 d. After 2 d, the CD4+ Th2 cells were depleted from the cocultures. These conditioned DCs were used to subsequently activate p14 cells (Fig. 6A). Priming of p14 cells by LCMV-infected DCs conditioned with Tbx21−/− Th2 cells resulted in significantly reduced expression of CD69, KLRG-1, CD44, and CD25 compared to p14 cells primed by DCs conditioned with Il10−/−Tbx21−/− Th2 cells or LCMV-infected DCs without Th2 cell conditioning (Fig. 6B). In addition, p14 cells primed by DCs conditioned with Tbx21−/− Th2 cells displayed significantly reduced expression of IFN-γ and the degranulation marker CD107a compared to p14 cells activated by DCs conditioned with Il10−/−Tbx21−/− Th2 cells (Fig. 6C). In summary, our data demonstrate that Tbx21−/− Th2 cell-derived IL-10 impairs DC maturation, and subsequently these conditioned DCs lead to poor CD8+ T cell activation after LCMV infection.
Fig. 6.
Tbx21−/− Th2 cells impair CD8+ T cell activation via IL-10 in vitro. (A) Schematic experimental layout to investigate the effect of LCMV-infected DCs previously conditioned by Tbx21−/− Th2 or Il10−/−Tbx21−/− Th2 cells on p14 cell activation in vitro. After 2 d of LCMV infection, CD4+ LCMV-specific T cells were depleted from T cell–DC cocultures. These conditioned DCs were subsequently cocultured with p14 cells in the presence of GP33 for 3 additional days. (B) Expression of surface markers CD69, KLRG-1, CD44, CD25, CD62L, and CD127 as well as (C) IFN-γ and CD107a expression (geometric mean of fluorescence intensity) by p14 cells after activation in vitro with LCMV-infected DCs that were previously conditioned with Tbx21−/− Th2 cells or Il10−/−Tbx21−/− Th2 cells. All experiments were performed at least twice, and each experimental group included n ≥ 3. Data are pooled and expressed as mean ± SEM. Asterisks indicate statistically significant differences as analyzed by one-way ANOVA with Bonferroni’s post test (*P < 0.05).
Tbx21−/− Th2 Cells Impair the Activation of DCs and T Cells via IL-10 In Vivo.
Lastly, we investigated whether Tbx21−/− Th2 cells could also impair DC activation after LCMV infection in vivo. To address this question, Il10−/−Tbx21−/− Th2 or Tbx21−/− Th2 cells were transferred into WT mice that were subsequently infected with LCMV. Consistent with our in vitro data, significantly reduced frequencies of CD80+ and CD86+ CD8+ DC subsets were observed in the spleen of Tbx21−/− Th2 cell recipient mice compared to that of control mice at day 3 postinfection (Fig. 7A). In contrast, Il10−/−Tbx21−/− Th2 cell recipient mice displayed frequencies of CD80+ and CD86+ CD8+ DC subsets comparable to that of nontransferred control mice after LCMV infection (Fig. 7A). Next, we asked whether Tbx21−/− Th2 cells could also impair T cell activation after LCMV infection via IL-10 in vivo. In contrast to Tbx21−/− Th2 cell recipients, Il10−/−Tbx21−/− Th2 cell recipients displayed similar GP33- and NP396-tetramer+ CD8+ T cell frequencies and absolute numbers in the spleen compared to that of nontransferred control mice at the peak of infection (Fig. 7B). Furthermore, IFN-γ production by virus-specific CD8+ and CD4+ T cells of Il10−/−Tbx21−/− Th2 cell recipients was not impaired in contrast to that of recipients of IL-10–competent Tbx21−/− Th2 cells at the peak of infection (Fig. 7 C and D). Accordingly, Il10−/−Tbx21−/− Th2 cell recipients display significantly lower viral loads in all organs tested as compared to Tbx21−/− Th2 cell recipient mice at day 8 postinfection (Fig. 7E). Moreover, Tbx21−/− Th2 cell recipients exhibited a wasting syndrome, with constant weight loss and reduced survival compared to Il10−/−Tbx21−/− Th2 cell recipient mice (Fig. 7F). These data collectively show that Tbx21−/− Th2 cells mediate LCMV-specific T cell immunosuppression via IL-10 in vivo.
Fig. 7.
Tbx21−/− Th2 cells impair the activation of DCs and T cells via IL-10 in vivo. (A) Frequencies of CD80 and CD86 expression on CD8+CD11c+ DCs in the spleen of Tbx21−/− Th2 and Il10−/−Tbx21−/− Th2 cell recipients and nontransferred control mice at day 3 postinfection. (B) Frequencies and absolute cell numbers (Left) as well as representative fluorescence-activated cell sorting (FACS) plots (Right) of GP33- and NP396-tetramer+ CD8+ T cells in the spleen of Tbx21−/− Th2 and Il10−/−Tbx21−/− Th2 cell recipients and nontransferred control mice at day 8 postinfection. (C) Frequencies and absolute cell numbers of IFN-γ–producing CD8+ T and (D) CD4+ T cells in the spleen of Tbx21−/− Th2 and Il10−/−Tbx21−/− Th2 cell recipients and nontransferred control mice at day 8 postinfection after restimulation ex vivo with GP33 or GP64 peptides, respectively (Left). Representative FACS plots of C and D (Right). (E) LCMV titers in various organs of Tbx21−/− Th2 and Il10−/−Tbx21−/− Th2 cell recipients and nontransferred control mice at day 8 postinfection as determined by plaque assay. (F) Survival plots of Tbx21−/− Th2 and Il10−/−Tbx21−/− Th2 cell recipients and nontransferred control mice until day 12 postinfection. The log-rank test was used for statistical evaluation of survival (Kaplan–Meier survival curves). All experiments were performed at least twice, and each experimental group included n ≥ 3. Data are representative and expressed as mean ± SEM. Asterisks indicate statistically significant differences as analyzed by one-way ANOVA with Bonferroni’s post test (A–E) (*P < 0.05, **P < 0.01, ***P < 0.001).
Discussion
Autoimmunity results from the interplay of several factors, including a genetic predisposition and the loss of central or peripheral tolerance. A better understanding of the molecular mechanisms to suppress autoreactive responses and the development of novel therapeutic strategies that target memory T responses are still needed to treat autoimmune diseases. Experimental approaches to suppress pathogenic T cells during primary effector responses have been reported (40). Recently, Treg cells were shown to suppress naive T cells by removing the complex of cognate peptide and major histocompatibility complex class II from the DC surface (41). However, suppression of memory T responses has been difficult to achieve and represents a major hurdle to long-term transplant survival, tolerance, and for the treatment of autoimmune diseases (42). Memory T cells are thought to be resistant to conventional immunosuppression therapies and costimulation blockade (43). Efforts to suppress memory CD8+ T cell responses have been pursued by genetically targeted antigen expression on steady-state DCs (30). However, memory CD8+ T cell inactivation was slow, and memory CD8+ T cells still expanded under these conditions.
Here we report that T-bet–deficient Th2 but not WT Th2 cells strongly suppress proinflammatory responses, and we have dissected the mechanisms underlying this immunosuppression. First, adoptive transfer of Tbx21−/− Th2 but not WT Th2 cells strongly reduced the frequency and functionality of LCMV-specific CD8+ T cells postinfection. Second, Tbx21−/− Th2 cells suppressed not only naive but also memory CD8+ T cells after LCMV infection. Third, IL-10 but not IL-4 or IL-13 produced by Tbx21−/− Th2 cells mediated T cell immunosuppression, and no FoxP3 expression was observed. We show both in vivo and in vitro that IL-10–producing Tbx21−/− Th2 cells down-regulated CD80 and CD86 expression on conventional DC subsets. Impaired DC activation in turn led to a defective CD8+ T cell activation. Lastly, we show that Tbx21−/− Th2 but not WT Th2 cells protected mice from type 1 diabetes development.
It has been suggested that Th1 cytokines promote or exacerbate the course of some autoimmune disorders, including T1D (44, 45), whereas Th2 cytokines harbor an antiinflammatory potential (46). Therefore, it was proposed that shifting Th1-driven autoimmune responses toward a Th2-like response might ameliorate the course of some autoimmune diseases (31). Using the RIP-GP mouse model of T1D, we show that adoptive transfer of LCMV-specific Tbx21−/− Th2 but not WT Th2 cells strongly suppressed CD8+ T cell responses and protected RIP-GP mice from developing diabetes in vivo. In the RIP-GP model, the up-regulation of major histocompatibility complex (MHC) class II molecules associated with the attraction and activation of antigen-presenting cells (APCs) to the islets occurs as soon as 2 d after LCMV infection of transgenic mice (47). This murine model of diabetes is nonreversible due to rapid and strong CD8+ T cell infiltration in the pancreas and subsequent pancreatic β-cell destruction after LCMV infection (48). Therefore, a therapeutic rather than a protective approach through Tbx21−/− Th2 cell transfer when diabetes is already established is unlikely to be successful.
Although several pathways that mediate immunosuppression have been proposed, IL-10 and its downstream effectors are the best-characterized mechanisms in the regulation of inflammatory immune responses (49). IL-10 production has been shown to be regulated by IL-4, STAT6, GATA-3, and c-Maf in murine Th2 cells (50–52). However, the regulation of IL‐10 production in human T cells is complex and still not completely understood. Similar to murine Th2 cells, GATA-3 is also an important transcription factor in regulating human Th2 cell differentiation (53) and GATA-3 mRNA was preferentially expressed in developing human Th2 cells, whereas T-bet mRNA was selectively expressed in developing Th1 human cells (54). A potential direct or indirect role of T-bet in human IL-10 regulation has not been addressed so far. Transfection of T-bet into Th2 cells induced high levels of IFN-γ and suppressed IL-5; however, IL-10 production was not examined (55). In our study, Tbx21−/− Th2 but not WT Th2 cells stably maintained high GATA-3 expression and IL-10 production upon LCMV infection. We hypothesize that the enhanced IL-10 production in Tbx21−/− Th2 cells might result from enhanced GATA-3 expression levels observed in Tbx21−/− Th2 cells compared to WT Th2 cells ex vivo upon LCMV infection as shown in Fig. 2D. GATA-3 plays a key role in instructing Il10 gene expression and stabilizing the Il10 locus in primary CD4+ T cells, and GATA-3 expression correlated with the levels of IL-10 and changes of the chromatin structure at the Il10 locus (51). In addition, it has been demonstrated that GATA-3 induces Th2 cell differentiation (56). Consistently, ChIP-Seq tracks of in vitro differentiated murine WT Th2 cells show a prominent GATA-3 binding peak in the Il10 promoter (Fig. 2G).
IL-10 is largely known for its immunomodulatory properties on different types of cells (16). Although the regulatory properties of IL-10 have been well characterized, therapies of autoimmune diseases with IL-10 have not yet been successful in clinical trials (57, 58). Long-term IL-10 supplementation via gene therapy strategies have not been approved in part due to some adverse effects such as the development of a preanemic condition after short-term IL-10 supplementation (59). The ability of IL-10 to modulate inflammatory responses might be influenced by several factors, including access to the affected tissue, duration, and local concentrations. Effective delivery has been a major obstacle hampering IL-10 immunotherapies. In our in vitro model of LCMV infection, we found that the presence of Tbx21−/− Th2 but not WT Th2 cells led to the suppression of CD80 and CD86 on CD8+ DCs after infection (Fig. 5A and SI Appendix, Fig. S6). Likewise, adoptively transferred Tbx21−/− Th2 but not Il10−/−Tbx21−/− Th2 cells inhibited the up-regulation of CD80 and CD86 on CD8+ DCs in vivo (Fig. 7A). Moreover, activation of naive p14 cells with conditioned APCs previously cultured in the presence of LCMV-specific Tbx21−/− Th2 but not Il10−/−Tbx21−/− Th2 cells, was also strongly impaired (Fig. 6). Thus, we conclude that the immunosuppression of CD8+ T cell responses induced by IL-10–producing Tbx21−/− Th2 cells was primarily mediated via APCs. However, IL-10 can also directly suppress CD8+ T cell activation by modifying CD8+ T cell glycosylation in chronic viral infections (60). Although the relevance of this mechanism in an acute LCMV infection is unclear, we cannot rule out additional direct inhibitory effects of IL-10 on CD8+ T cells.
During LCMV infection, naive CD8+ T cells divide and differentiate into effector cells that acquire the ability to eliminate infected cells. It has been hypothesized that the strength and duration of T cell receptor (TCR) engagement, costimulation, and cytokine signaling drive the differentiation of CD8+ T cells toward terminal effector cells (TECs) or memory precursor cells (MPCs) (61–63). After LCMV infection, Tbx21−/− Th2 cells not only suppressed the frequency of antigen-specific CD8+ T cells but also modified their phenotype. Instead of displaying an effector phenotype at the peak of the infection, both GP33- and NP396-tetramer+ CD8+ T cells showed a memory precursor-like phenotype characterized by low expression of CD44 but high expression of CD62L and CD127. In addition, activated CD8+ T cells displayed high levels of Eomes but low levels of T-bet at day 8 postinfection. The apparent inverse expression of T-bet and Eomes in both endogenous CD8+ and CD4+ T cells observed in Tbx21−/− Th2 cell recipients (SI Appendix, Fig. S4) may at least in part be due to the impaired expansion of activated CD44+CD62L− and antigen-specific CD8+ T cells (Fig. 1B). In contrast to recipients of WT Th1 and WT Th2 cells and control mice without cell transfer, antigen-specific CD8+ T cells in Tbx21−/− Th2 cell recipients failed to expand, which resulted in very few IFN-γ–producing T cells as depicted in Fig. 1C. Hence, T-bet and Eomes geometric mean differences observed in CD8+ and CD4+ T cells in Tbx21−/− Th2 and WT Th1 cell recipients might not result from a deregulated T-bet/Eomes expression at the single cell level but rather from the impaired expansion of activated T cells observed in Tbx21−/− Th2 cell recipient mice. We also hypothesize that the impaired maturation of DCs mediated by IL-10–producing Tbx21−/− Th2 cells modified the cytokine milieu during CD8+ T cell priming. As shown in Fig. 5, IL-12 production by DCs was strongly reduced in the presence of Tbx21−/− Th2 but not in the presence of WT Th2 cells or WT Th1 cells after LCMV infection in vitro. It has been demonstrated that IL-12 repressed Eomes expression in antigen-specific CD8+ T cells during infection. In contrast, maximal induction of T-bet in CD8+ T cells required IL-12 signaling (64). Therefore, we believe that the poor activation of DCs and a modified cytokine milieu caused by the presence of Tbx21−/− Th2 cells might modify the effector CD8+ T cell phenotype to a rather memory-like phenotype at the peak of LCMV infection (SI Appendix, Figs. S1 and S4).
We are aware that translating the immunosuppressive potential of Tbx21−/− Th2 cells from bench to bedside may be very challenging. However, recent advances in gene deletion using the CRISPR-Cas9 system might open new avenues to apply these results in clinical settings. Moreover, mechanisms to stabilize Th2 cells in vivo and to maintain their immunosuppressive potential on proinflammatory responses need to be further investigated. Collectively, our data indicate that engineered Tbx21−/− Th2 cells harbor a remarkable immunomodulatory capacity and might be considered as an attractive alternative for future interventions for the treatment of autoimmune diseases and for the suppression of memory responses.
Materials and Methods
Mice.
C57BL/6 (WT), Tbx21−/−, Il4ra−/−, and RIP-LCMV-GP mice were bred on a C57BL/6 background. SMARTA1 TCR-transgenic (tg) mice, which express a TCR specific for the LCMV glycoprotein epitope GP61-80 were crossed to Il10−/−Tbx21−/− mice to generate TCR-tg mice on the respective mutant backgrounds. For details see SI Appendix, Supplementary Material. All animal experiments were performed in accordance with the German law for animal protection with permission from the local veterinary offices.
Adoptive T Cell Transfers.
For CD4+ T cell transfers, SMARTA1 LCMV-specific T cells of Tbx21−/−, Il10−/−Tbx21−/−, and WT Thy1.1+ tg mice of the respective knockout backgrounds (6 to 8 wk old) were purified by staining with biotinylated antibodies against CD8 (53-6.7), CD11c (N418), CD11b (M1/70), CD19 (1D3), NK1.1 (PK136), Gr-1 (RB6-8C5), and CD25 (7D4), followed by anti-biotin microbeads and magnetic depletion (Miltenyi Biotec). For each recipient, 1 × 106 cells of the biotin-negative fraction were transferred i.v. LCMV-specific CD8+ T cells from p14 mice (6 to 8 wk old) were purified by staining with biotinylated antibodies against CD4 (L3T4), CD11c (N418), CD11b (M1/70), CD19 (1D3), NK1.1 (PK136), Gr-1 (RB6-8C5), and CD25 (7D4), followed by anti-biotin microbeads and magnetic depletion (Miltenyi Biotec).
Statistical Analysis.
GraphPad Prism (v5.02 and v7) software was used for data analysis. Statistical significance was determined by unpaired two-tailed Student’s t test. All experiments were performed at least twice, and each experimental group included n ≥ 3. Data are expressed as mean ± SEM. More than two groups were compared via one-way ANOVA with Bonferroni’s post test for multiple comparisons. P = 0.01 to 0.05 was considered statistically significant (*), P = 0.001 to 0.01 as very significant (**), and P < 0.001 as extremely significant (***); ns, not significant.
Supplementary Material
Acknowledgments
We thank S. Ebel and I. Panse for expert technical assistance. This work was supported by the German Research Foundation (SFB650, grant TP28, and grants LO 1542/3-1 and LO 1542/4-1 to M.L. and CRC-TRR241 grant to A.N.H.), the European Union (FP7, Marie Curie ITN QuanTI, grant TP6 to M.L.), Volkswagen Foundation (Lichtenberg fellowships to M.L. and A.N.H.), and the Willy Robert Pitzer Foundation (Osteoarthritis Research Program to M.L.). M.M. and A.N.H. are Berlin Institute of Health (BIH)–Charité clinical scientists funded by the Charité–Universitätsmedizin Berlin and the BIH.
Footnotes
The authors declare no competing interest.
This article is a PNAS Direct Submission. J.J.O. is a guest editor invited by the Editorial Board.
This article contains supporting information online at https://www.pnas.org/lookup/suppl/doi:10.1073/pnas.2002787118/-/DCSupplemental.
Data Availability.
All study data are included in the article and/or supporting information.
References
- 1.Cooper G. S., Bynum M. L., Somers E. C., Recent insights in the epidemiology of autoimmune diseases: Improved prevalence estimates and understanding of clustering of diseases. J. Autoimmun. 33, 197–207 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Walsh S. J., Rau L. M., Autoimmune diseases: A leading cause of death among young and middle-aged women in the United States. Am. J. Public Health 90, 1463–1466 (2000). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Lleo A., Invernizzi P., Gao B., Podda M., Gershwin M. E., Definition of human autoimmunity–Autoantibodies versus autoimmune disease. Autoimmun. Rev. 9, A259–A266 (2010). [DOI] [PubMed] [Google Scholar]
- 4.Edinger M., Hoffmann P., Regulatory T cells in stem cell transplantation: Strategies and first clinical experiences. Curr. Opin. Immunol. 23, 679–684 (2011). [DOI] [PubMed] [Google Scholar]
- 5.Trenado A., et al., Ex vivo-expanded CD4+CD25+ immunoregulatory T cells prevent graft-versus-host-disease by inhibiting activation/differentiation of pathogenic T cells. J. Immunol. 176, 1266–1273 (2006). [DOI] [PubMed] [Google Scholar]
- 6.Tanaka H., et al., Successful immunotherapy of autoimmune cholangitis by adoptive transfer of forkhead box protein 3(+) regulatory T cells. Clin. Exp. Immunol. 178, 253–261 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bluestone J. A., et al., Type 1 diabetes immunotherapy using polyclonal regulatory T cells. Sci. Transl. Med. 7, 315ra189 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Abbas A. K., Lohr J., Knoechel B., Nagabhushanam V., T cell tolerance and autoimmunity. Autoimmun. Rev. 3, 471–475 (2004). [DOI] [PubMed] [Google Scholar]
- 9.Sagoo P., et al., Human regulatory T cells with alloantigen specificity are more potent inhibitors of alloimmune skin graft damage than polyclonal regulatory T cells. Sci. Transl. Med. 3, 83ra42 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Campbell D. J., Koch M. A., Phenotypical and functional specialization of FOXP3+ regulatory T cells. Nat. Rev. Immunol. 11, 119–130 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.García-González P., Ubilla-Olguín G., Catalán D., Schinnerling K., Aguillón J. C., Tolerogenic dendritic cells for reprogramming of lymphocyte responses in autoimmune diseases. Autoimmun. Rev. 15, 1071–1080 (2016). [DOI] [PubMed] [Google Scholar]
- 12.Boros P., Ochando J. C., Chen S. H., Bromberg J. S., Myeloid-derived suppressor cells: Natural regulators for transplant tolerance. Hum. Immunol. 71, 1061–1066 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Yang M., Rui K., Wang S., Lu L., Regulatory B cells in autoimmune diseases. Cell. Mol. Immunol. 10, 122–132 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Fleming B. D., Mosser D. M., Regulatory macrophages: Setting the threshold for therapy. Eur. J. Immunol. 41, 2498–2502 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Liu Y., Chen N., Chen G., You P., The protective effect of CD8+CD28- T suppressor cells on the acute rejection responses in rat liver transplantation. Transplant. Proc. 39, 3396–3403 (2007). [DOI] [PubMed] [Google Scholar]
- 16.Moore K. W., de Waal Malefyt R., Coffman R. L., O’Garra A., Interleukin-10 and the interleukin-10 receptor. Annu. Rev. Immunol. 19, 683–765 (2001). [DOI] [PubMed] [Google Scholar]
- 17.Weiner H. L., Induction and mechanism of action of transforming growth factor-beta-secreting Th3 regulatory cells. Immunol. Rev. 182, 207–214 (2001). [DOI] [PubMed] [Google Scholar]
- 18.Golovina T. N., et al., CD28 costimulation is essential for human T regulatory expansion and function. J. Immunol. 181, 2855–2868 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Haanstra K. G., van der Maas M. J., ’t Hart B. A., Jonker M., Characterization of naturally occurring CD4+CD25+ regulatory T cells in rhesus monkeys. Transplantation 85, 1185–1192 (2008). [DOI] [PubMed] [Google Scholar]
- 20.Hoffmann P., Ermann J., Edinger M., Fathman C. G., Strober S., Donor-type CD4(+)CD25(+) regulatory T cells suppress lethal acute graft-versus-host disease after allogeneic bone marrow transplantation. J. Exp. Med. 196, 389–399 (2002). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hilkens C. M., Isaacs J. D., Tolerogenic dendritic cell therapy for rheumatoid arthritis: Where are we now? Clin. Exp. Immunol. 172, 148–157 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.O’Shea J. J., Ma A., Lipsky P., Cytokines and autoimmunity. Nat. Rev. Immunol. 2, 37–45 (2002). [DOI] [PubMed] [Google Scholar]
- 23.Di Ianni M., et al., Tregs prevent GVHD and promote immune reconstitution in HLA-haploidentical transplantation. Blood 117, 3921–3928 (2011). [DOI] [PubMed] [Google Scholar]
- 24.Trzonkowski P., et al., First-in-man clinical results of the treatment of patients with graft versus host disease with human ex vivo expanded CD4+CD25+CD127- T regulatory cells. Clin. Immunol. 133, 22–26 (2009). [DOI] [PubMed] [Google Scholar]
- 25.Canavan J. B., et al., Developing in vitro expanded CD45RA+ regulatory T cells as an adoptive cell therapy for Crohn’s disease. Gut 65, 584–594 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Dall’Era M.et al.; Autoimmunity Centers of Excellence , Adoptive Treg cell therapy in a patient with systemic lupus erythematosus. Arthritis Rheumatol. 71, 431–440 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Afzali B., et al., Relative resistance of human CD4(+) memory T cells to suppression by CD4(+) CD25(+) regulatory T cells. Am. J. Transplant. 11, 1734–1742 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Schneider A., et al., The effector T cells of diabetic subjects are resistant to regulation via CD4+ FOXP3+ regulatory T cells. J. Immunol. 181, 7350–7355 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Zeng Y. Q., Lu C., Dai Z., Editorial: Memory T cells: Effectors, regulators, and implications for transplant tolerance. Front. Immunol. 7, 7 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kenna T. J., Thomas R., Steptoe R. J., Steady-state dendritic cells expressing cognate antigen terminate memory CD8+ T-cell responses. Blood 111, 2091–2100 (2008). [DOI] [PubMed] [Google Scholar]
- 31.Christen U., von Herrath M. G., Manipulating the type 1 vs type 2 balance in type 1 diabetes. Immunol. Res. 30, 309–325 (2004). [DOI] [PubMed] [Google Scholar]
- 32.Löhning M., Richter A., Radbruch A., Cytokine memory of T helper lymphocytes. Adv. Immunol. 80, 115–181 (2002). [DOI] [PubMed] [Google Scholar]
- 33.Zhou L., Chong M. M., Littman D. R., Plasticity of CD4+ T cell lineage differentiation. Immunity 30, 646–655 (2009). [DOI] [PubMed] [Google Scholar]
- 34.Hegazy A. N., et al., Interferons direct Th2 cell reprogramming to generate a stable GATA-3(+)T-bet(+) cell subset with combined Th2 and Th1 cell functions. Immunity 32, 116–128 (2010). [DOI] [PubMed] [Google Scholar]
- 35.Peine M., et al., Stable T-bet(+)GATA-3(+) Th1/Th2 hybrid cells arise in vivo, can develop directly from naive precursors, and limit immunopathologic inflammation. PLoS Biol. 11, e1001633 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Ohashi P. S., et al., Ablation of “tolerance” and induction of diabetes by virus infection in viral antigen transgenic mice. Cell 65, 305–317 (1991). [DOI] [PubMed] [Google Scholar]
- 37.den Haan J. M., Lehar S. M., Bevan M. J., CD8(+) but not CD8(-) dendritic cells cross-prime cytotoxic T cells in vivo. J. Exp. Med. 192, 1685–1696 (2000). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Raué H. P., Beadling C., Haun J., Slifka M. K., Cytokine-mediated programmed proliferation of virus-specific CD8(+) memory T cells. Immunity 38, 131–139 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Brooks D. G., et al., Interleukin-10 determines viral clearance or persistence in vivo. Nat. Med. 12, 1301–1309 (2006). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Sarween N., et al., CD4+CD25+ cells controlling a pathogenic CD4 response inhibit cytokine differentiation, CXCR-3 expression, and tissue invasion. J. Immunol. 173, 2942–2951 (2004). [DOI] [PubMed] [Google Scholar]
- 41.Akkaya B., et al., Regulatory T cells mediate specific suppression by depleting peptide-MHC class II from dendritic cells. Nat. Immunol. 20, 218–231 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Wu Z., et al., Homeostatic proliferation is a barrier to transplantation tolerance. Nat. Med. 10, 87–92 (2004). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Zhai Y., Meng L., Gao F., Busuttil R. W., Kupiec-Weglinski J. W., Allograft rejection by primed/memory CD8+ T cells is CD154 blockade resistant: Therapeutic implications for sensitized transplant recipients. J. Immunol. 169, 4667–4673 (2002). [DOI] [PubMed] [Google Scholar]
- 44.Liblau R. S., Singer S. M., McDevitt H. O., Th1 and Th2 CD4+ T cells in the pathogenesis of organ-specific autoimmune diseases. Immunol. Today 16, 34–38 (1995). [DOI] [PubMed] [Google Scholar]
- 45.von Herrath M. G., Oldstone M. B., Interferon-gamma is essential for destruction of beta cells and development of insulin-dependent diabetes mellitus. J. Exp. Med. 185, 531–539 (1997). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Charlton B., Lafferty K. J., The Th1/Th2 balance in autoimmunity. Curr. Opin. Immunol. 7, 793–798 (1995). [DOI] [PubMed] [Google Scholar]
- 47.von Herrath M., Holz A., Pathological changes in the islet milieu precede infiltration of islets and destruction of beta-cells by autoreactive lymphocytes in a transgenic model of virus-induced IDDM. J. Autoimmun. 10, 231–238 (1997). [DOI] [PubMed] [Google Scholar]
- 48.Winer S., et al., Autoimmune islet destruction in spontaneous type 1 diabetes is not beta-cell exclusive. Nat. Med. 9, 198–205 (2003). [DOI] [PubMed] [Google Scholar]
- 49.Saraiva M., Vieira P., O’Garra A., Biology and therapeutic potential of interleukin-10. J. Exp. Med. 217, e20190418 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Chang H. D., et al., Expression of IL-10 in Th memory lymphocytes is conditional on IL-12 or IL-4, unless the IL-10 gene is imprinted by GATA-3. Eur. J. Immunol. 37, 807–817 (2007). [DOI] [PubMed] [Google Scholar]
- 51.Shoemaker J., Saraiva M., O’Garra A., GATA-3 directly remodels the IL-10 locus independently of IL-4 in CD4+ T cells. J. Immunol. 176, 3470–3479 (2006). [DOI] [PubMed] [Google Scholar]
- 52.Gabryšová L., et al., c-Maf controls immune responses by regulating disease-specific gene networks and repressing IL-2 in CD4+ T cells. Nat. Immunol. 19, 497–507 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Skapenko A., et al., GATA-3 in human T cell helper type 2 development. J. Exp. Med. 199, 423–428 (2004). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Kaneko T., et al., Chromatin remodeling at the Th2 cytokine gene loci in human type 2 helper T cells. Mol. Immunol. 44, 2249–2256 (2007). [DOI] [PubMed] [Google Scholar]
- 55.Lametschwandtner G., et al., Sustained T-bet expression confers polarized human TH2 cells with TH1-like cytokine production and migratory capacities. J. Allergy Clin. Immunol. 113, 987–994 (2004). [DOI] [PubMed] [Google Scholar]
- 56.Zheng W., Flavell R. A., The transcription factor GATA-3 is necessary and sufficient for Th2 cytokine gene expression in CD4 T cells. Cell 89, 587–596 (1997). [DOI] [PubMed] [Google Scholar]
- 57.Chernoff A. E., et al., A randomized, controlled trial of IL-10 in humans. Inhibition of inflammatory cytokine production and immune responses. J. Immunol. 154, 5492–5499 (1995). [PubMed] [Google Scholar]
- 58.Roberti M. L., et al., Immunomodulating treatment with low dose interleukin-4, interleukin-10 and interleukin-11 in psoriasis vulgaris. J. Biol. Regul. Homeost. Agents 28, 133–139 (2014). [PubMed] [Google Scholar]
- 59.Tilg H., Ulmer H., Kaser A., Weiss G., Role of IL-10 for induction of anemia during inflammation. J. Immunol. 169, 2204–2209 (2002). [DOI] [PubMed] [Google Scholar]
- 60.Smith L. K., et al., Interleukin-10 directly inhibits CD8+ T cell function by enhancing N-glycan branching to decrease antigen sensitivity. Immunity 48, 299–312.e5 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Intlekofer A. M., et al., Effector and memory CD8+ T cell fate coupled by T-bet and eomesodermin. Nat. Immunol. 6, 1236–1244 (2005). [DOI] [PubMed] [Google Scholar]
- 62.Joshi N. S., et al., Inflammation directs memory precursor and short-lived effector CD8(+) T cell fates via the graded expression of T-bet transcription factor. Immunity 27, 281–295 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Teixeiro E., et al., Different T cell receptor signals determine CD8+ memory versus effector development. Science 323, 502–505 (2009). [DOI] [PubMed] [Google Scholar]
- 64.Takemoto N., Intlekofer A. M., Northrup J. T., Wherry E. J., Reiner S. L., Cutting edge: IL-12 inversely regulates T-bet and eomesodermin expression during pathogen-induced CD8+ T cell differentiation. J. Immunol. 177, 7515–7519 (2006). [DOI] [PubMed] [Google Scholar]
- 65.Wei G., et al., Genome-wide analyses of transcription factor GATA3-mediated gene regulation in distinct T cell types. Immunity 35, 299–311 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
All study data are included in the article and/or supporting information.