Significance
Understanding species’ eco-evolutionary responses to novel competitive interactions and shifting thermal regimes has become essential under global change. However, we lack insight into these dynamics because experimental evolution studies often focus on populations of individual species. Using a large-scale field experiment with two competing species of drosophilid flies, we show that rapid evolution in response to competition can alter a species’ evolutionary trajectory when exposed to a seasonally changing climate. Our results demonstrate that interactions with competitors, including invasive species, can shape evolution when climatic conditions change. More broadly, we provide empirical, field-based evidence that a legacy of recent adaptation can shape subsequent evolutionary trajectories.
Keywords: interspecific competition, adaptation, seasonal evolution, invasive species, Drosophila melanogaster
Abstract
Eco-evolutionary dynamics will play a critical role in determining species’ fates as climatic conditions change. Unfortunately, we have little understanding of how rapid evolutionary responses to climate play out when species are embedded in the competitive communities that they inhabit in nature. We tested the effects of rapid evolution in response to interspecific competition on subsequent ecological and evolutionary trajectories in a seasonally changing climate using a field-based evolution experiment with Drosophila melanogaster. Populations of D. melanogaster were either exposed, or not exposed, to interspecific competition with an invasive competitor, Zaprionus indianus, over the summer. We then quantified these populations’ ecological trajectories (abundances) and evolutionary trajectories (heritable phenotypic change) when exposed to a cooling fall climate. We found that competition with Z. indianus in the summer affected the subsequent evolutionary trajectory of D. melanogaster populations in the fall, after all interspecific competition had ceased. Specifically, flies with a history of interspecific competition evolved under fall conditions to be larger and have lower cold fecundity and faster development than flies without a history of interspecific competition. Surprisingly, this divergent fall evolutionary trajectory occurred in the absence of any detectible effect of the summer competitive environment on phenotypic evolution over the summer or population dynamics in the fall. This study demonstrates that competitive interactions can leave a legacy that shapes evolutionary responses to climate even after competition has ceased, and more broadly, that evolution in response to one selective pressure can fundamentally alter evolution in response to subsequent agents of selection.
Although ecological and evolutionary dynamics have traditionally been studied as independent processes assumed to proceed on fundamentally different timescales, it is now widely recognized that evolution often occurs rapidly enough to shape ecological outcomes (1–3). There is a growing interest in understanding the eco-evolutionary dynamics that result (4, 5), motivated in part by their potential importance in determining species’ fates under global environmental change (6, 7).
Climate is a principal abiotic pressure that species face in the wild that can exert strong selection capable of driving rapid ecological and evolutionary change (8, 9). Understanding species’ evolutionary responses to climatic conditions has become essential, as temperature, its variability, and the frequency of extreme weather events increase under global change (10). Unfortunately, this understanding remains limited by a lack of experimental tests that place species in the complex and competitive environments in which ecology and evolution actually occur (11, 12). This represents a critical knowledge gap, as species confronted with changing climatic regimes not only face native competitors, but may also face novel competitors in the form of invasive species and species migrating in response to climate change (13, 14).
We have several reasons to expect that selection imposed by competitors could shape species’ ecological and evolutionary responses to climate. First, most species live embedded in communities of competitors, rendering these interactions a likely source of selection in nature. Second, interspecific competition is widely recognized as a key driver of ecological (15, 16) and macroevolutionary dynamics (17, 18). Finally, a handful of experiments have demonstrated that species can rapidly adapt to interspecific competition (2, 19–21). Nonetheless, given that experimental evaluations of rapid evolution tend to focus on single-species populations (22, 23) or selection imposed by consumers or disease (24, 25), we have little understanding of how evolution in response to interspecific competition affects species’ abilities to persist in or adapt to new thermal regimes.
Through changes in the genetic composition and phenotypic traits of populations, rapid evolution in response to competition could alter a species’ ecological trajectory, evolutionary trajectory, or both. We would expect rapid adaptation to competition to influence ecological trajectories under a shifting climate if competition drives the evolution of a phenotype, such as body size, that also influences individual performance and therefore population dynamics as temperatures change (26, 27). Selection from competition could be exerted directly via aggressive interactions with a competitor or indirectly through changes in the availability of shared resources. Studies that have experimentally demonstrated the effects of rapid evolution in response to interspecific competition have identified shifts in phenotypic traits (19, 28) that can affect population dynamics by altering birth and death rates (2, 29). Moreover, adaptive responses to competition have been shown to alter species’ population trajectories when they are also faced with changing environmental conditions, including CO2 enrichment (23, 30).
In addition to these ecological consequences of adapting to competitors, such adaptation could also alter species’ evolutionary trajectories when faced with shifting climatic conditions (31). This could arise through several mechanisms. First, theory indicates that a reduction in population size and strong selection caused by competition can reduce standing genetic variation, which could hinder adaptation to a changing climate (31–33). Second, by altering the genetic composition of populations (2, 34), adaptation to interspecific competition could influence both the magnitude and the direction of evolutionary change when organisms are exposed to novel climatic conditions (31). Traits that link genetic change and competitive performance are likely to be complex and polygenic (35–38), and, as such, the evolution of these traits may be particularly affected by epistasis and pleiotropy (39, 40). As a result, adaptation to interspecific competition could have cryptic but far-reaching consequences for subsequent evolutionary trajectories in response to changing climate if competition drives changes in allele frequencies at loci underlying variation in climate-relevant traits, or if genetic correlations link phenotypes selected under competition with those that affect fitness in a changing climate (41, 42). However, theory examining how evolutionary responses to competition can affect subsequent evolutionary responses to a changing climate remains scarce (27, 43), and the more general links between rapid adaptation in response to the changing selective agents described above have yet to be tested in a natural context.
We tested how rapid evolution in response to interspecific competition influences ecological and evolutionary dynamics in a seasonal climate using a large-scale field-based experimental evolution study with the vinegar fly Drosophila melanogaster and its invasive competitor Zaprionus indianus. The interactions between D. melanogaster and Z. indianus in the seasonal climate of the northeastern United States provide an excellent natural context in which to evaluate the eco-evolutionary interactions between competition and climate. D. melanogaster maintains resident populations throughout the year in temperate North American orchards (35, 44). After emerging from diapause each spring, populations expand and rapidly evolve under warm summer conditions while feeding and laying eggs on fallen fruit (36, 37, 45). Then in fall and early winter, populations gradually decline and evolve under cooling conditions (35, 36, 46).
In contrast, Z. indianus has invaded tropical regions across the globe and now seasonally invades the northeastern United States from more southern latitudes (47). Compared to D. melanogaster, Z. indianus is larger-bodied, less cold-tolerant, and slower to develop (48). In both its native and invasive range, it competes with D. melanogaster adults for food and oviposition space on rotting fruit and with D. melanogaster larvae for food during development (48). Because of its cold intolerance, Z. indianus suffers high mortality and reproductive arrest as temperatures drop in the fall (49, 50), leaving fall D. melanogaster populations to continue to reproduce and adapt to fall conditions in the absence of their interspecific competitor. It is not known how selection imposed by competition with Z. indianus over the summer affects D. melanogaster and shapes its ecology and evolution in the cooler fall.
We conducted an experimental evolution study with replicate fly populations in an experimental orchard that mimics our focal species’ primary northeastern US habitat. The field mesocosms that we used experience natural temperature fluctuations and contain many of the predators and microbes that co-occur with local natural populations of D. melanogaster (37, 45). To examine the consequences of rapid evolution in response to interspecific competition on ecological and evolutionary dynamics in the fall, we first allowed replicate populations of D. melanogaster to grow and evolve in the presence or absence of Z. indianus for approximately six generations over the summer (Fig. 1). At the end of summer, we removed Z. indianus, equalized abundances of D. melanogaster across populations, and allowed the populations to continue their ecological and evolutionary dynamics through the fall (approximately three generations). We quantified the ecological (population dynamic) consequences of our treatments with weekly censuses of relative fly abundances throughout the summer and fall. We quantified the evolutionary consequences of our treatments by measuring 10 key phenotypes of D. melanogaster collected at the end of summer and end of the fall and then reared for two generations in a common garden to remove plastic responses to treatments or field conditions.
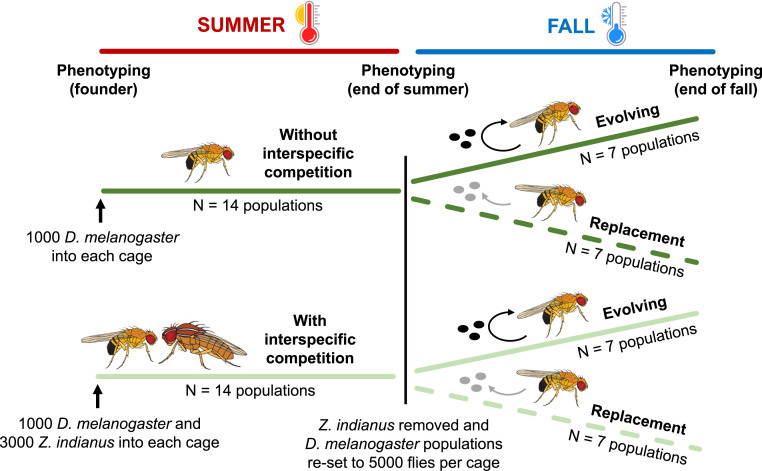
Fig. 1.
Experimental design to determine the effect of rapid evolution in response to interspecific competition on the ecological and evolutionary trajectory of D. melanogaster in a cool fall climate. Each replicate population consisted of a large outdoor cage containing thousands (up to 100,000) of genetically diverse flies. At each “phenotyping” time point,10 fly phenotypes were measured on each replicate population after two generations in a common garden environment. In evolving populations, eggs laid in the field experiment were allowed to develop into adult flies, whereas in replacement populations, eggs laid in the field experiment were replaced by eggs laid by laboratory populations in order to prevent intergenerational adaptation to fall conditions. Colors and dashing of lines to distinguish treatments are also used in Figs. 2–4.
The mechanisms that drive ecological and evolutionary patterns can be difficult to untangle in cases where ecological and evolutionary dynamics occur simultaneously (1, 51), and this is further complicated by the polygenic and multiphenotypic nature of D. melanogaster’s adaptive responses to climatic and biotic conditions (35, 36, 45, 52, 53). We therefore implemented an additional treatment in the fall phase of the experiment to provide insight into the mechanisms underlying the effects of competition on ecological and evolutionary responses to fall climate. In the fall, we effectively stopped intergenerational adaptation to fall conditions in half of our populations by replacing all eggs laid in field mesocosms with eggs laid by populations of flies collected from the experiment at the end of the summer and maintained in a nonseasonal laboratory environment (hereafter called “replacement” populations) (2, 45) (Fig. 1 and Methods). From an ecological perspective, this replacement treatment allowed us to determine the effect of adaptation to competition on fall population dynamics in both the presence and absence of further intergenerational adaptation to fall conditions. From an evolutionary perspective, it allowed us to evaluate the extent to which responses depended on intergenerational genetic change (e.g., recombination reducing negative epistatic or pleiotropic effects of adaptation or cumulative effects of selection across generations) versus recurrent selection of standing genetic variation within individual cohorts.
We predicted that if competition with Z. indianus and cold fall temperatures exert opposing selection on D. melanogaster (e.g., opposing effects on body size or development time), evolution to interspecific competition would accelerate fall population decline. This could arise if, for example, the presence of slower-developing Z. indianus exerts selection for faster larval development that allows D. melanogaster to avoid larval competition but is detrimental under cold conditions (54, 55). If, instead, competition and climate were to select in the same direction, evolution to interspecific competition could slow fall population decline. This could occur if, for example, competition with the large-bodied Z. indianus for oviposition space selects for large adult body size in D. melanogaster that is beneficial under cold conditions. These expectations, of course, depend on simple relationships between genetic change, trait change, and success under competition and climate. Because the complex genetic architecture underlying fitness-associated traits is likely to generate complex links between adaptation to different selective pressures, we also predicted more generally that any divergent phenotypic and genetic changes resulting from adaptation to the summer competitive environment would shape the outcome of adaptation to subsequent fall conditions.
Results
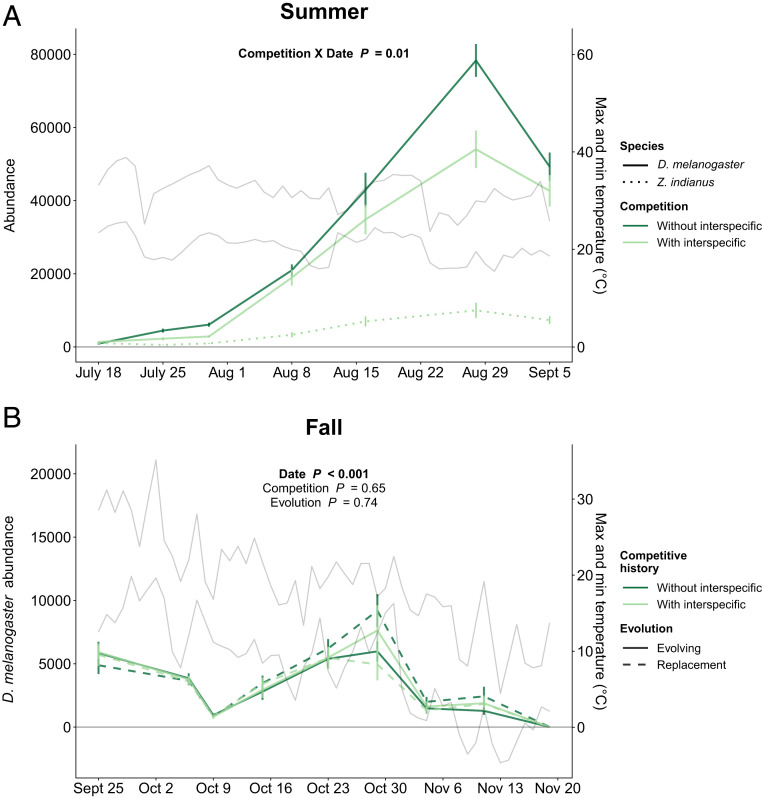
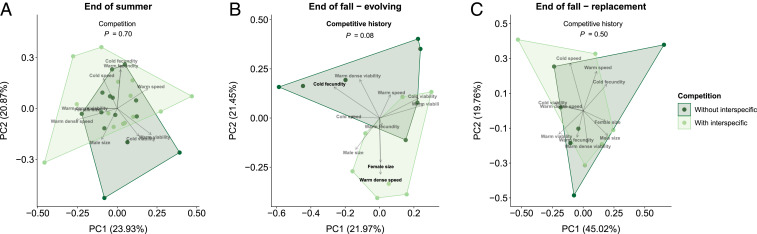
Interspecific competition with Z. indianus in the summer reduced D. melanogaster abundances by 10 to 53%, depending on date (competition × date interaction, χ2 = 6.49, P = 0.011) (Fig. 2A and SI Appendix, Fig. S1A). In the interspecific competition treatment, D. melanogaster was always more abundant than Z. indianus, but Z. indianus was maintained at >15% of the total fly community throughout the summer (Fig. 2 and SI Appendix, Fig. S2). Counter to our expectations, there was no effect of competition with Z. indianus on D. melanogaster’s multivariate trait evolution over the summer (F1,26 = 0.72, P = 0.70; Fig. 3A).
Fig. 2.
Fly population dynamics in (A) summer and (B) fall. Plotted points show estimated adult fly population sizes at seven summer census time points and nine fall census time points (treatment means ±1 SE). Reported statistics evaluate the effect of the treatments on D. melanogaster populations. Gray lines show the average daily minimum and maximum temperatures in the experiment, measured using data loggers placed inside cages. n = 14 replicates per treatment in summer, and n = 7 replicates per treatment in fall (data for each individual population are shown in SI Appendix, Fig. S1).
Fig. 3.
The effect of competition on the evolution of D. melanogaster phenotypes. Multivariate axes were drawn from 10 phenotypes measured on flies at (A) the end of summer (all populations evolving), (B) the end of fall from evolving populations (in which field-laid eggs were allowed to develop into adult flies), and (C) the end of fall from replacement populations (in which field-laid eggs were replaced by eggs laid by laboratory populations to prevent intergenerational adaptation to fall conditions). n = 14 replicates per treatment in summer, and n = 7 replicates per treatment in fall. All traits were measured after two generations in a common garden laboratory environment to remove to remove the effects of plastic responses to treatments and to the field environment. Boldface in (B) indicates the three traits that were significantly different between populations with or without a history of interspecific competition (Fig. 4). Reported statistical results evaluate the effect of competition or competitive history on the populations’ locations in multivariate trait space, analyzed using a permutational MANOVA approach.
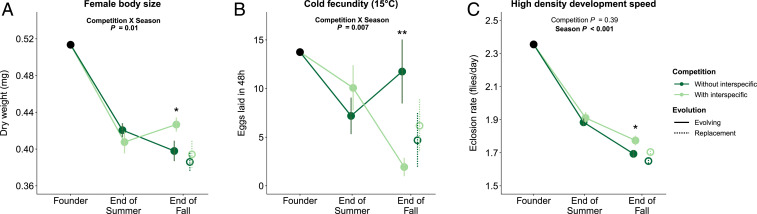
By contrast, a history of competition over the summer did affect the trajectory of D. melanogaster trait evolution in the fall. “Evolving” fall populations with different competitive histories showed marginally different multivariate trait evolution by the end of fall (F1,12 = 1.62, P = 0.082; Fig. 3B). When we specifically tested whether evolutionary trajectories from the end of summer to the end of fall depended on whether or not populations had been subjected to Z. indianus over the summer, we found significant competition by season interactions for female body size (Fig. 4A, χ2 = 6.24, P = 0.012) and cold fecundity (Fig. 4B, χ2 = 7.17, P = 0.0074). As a consequence, by the end of fall, flies with a history of interspecific competition had lower cold fecundity (t = −2.87, df = 12, P = 0.014; Fig. 4B) and marginally larger female body size (t = 2.13, df = 12, P = 0.055; Fig. 4A) than flies with no history of interspecific competition, both of which contributed to the marginally significant multivariate trait differentiation between flies with different competitive histories (Fig. 3B). The effect of competitive history on the evolution of fecundity under cold conditions was of comparable magnitude to the evolutionary response to the season itself (Fig. 4B). An additional trait—development speed at high density—showed no significant interaction between competition treatment and season, but, by the end of fall, populations with a history of interspecific competition had evolved marginally faster development (t = 2.16, df = 12, P = 0.051), as the small difference between competition treatments at the end of summer was strengthened over the course of the fall (Fig. 4C).
Fig. 4.
Three D. melanogaster traits with fall evolutionary trajectories that were affected by competitive history: (A) female body size, (B) cold fecundity, and (C) high density development speed. Points show trait values for the founding population (a single black point), the mean of 14 populations per treatment at the end of the summer, and the mean of 7 populations per treatment at the end of the fall (Fig. 1). Solid points show evolving populations (all populations in the summer, half of the populations in the fall), and solid lines connecting these points show the evolutionary trajectories of evolving populations. Dotted lines and open circles show replacement populations in which all field-laid eggs were replaced by eggs laid by laboratory populations to prevent intergenerational adaptation to fall conditions. End of summer and end of fall traits were measured on D. melanogaster after two generations in a common garden laboratory environment to remove the effects of plastic responses to treatments and to the field environment. Reported statistical results evaluate the effect of season and competitive treatment on traits of evolving populations. Asterisks indicate the effect of competitive history on end of fall phenotypes (**P < 0.05, *P < 0.1). Error bars show 1 SE from the mean. Data for each individual population are shown in SI Appendix, Fig. S3.
Interestingly, the effect of summer competition on the trajectory of fall evolution depended on the evolution treatment that populations were subjected to in the fall. In contrast to the evolutionary legacy of competition that we observed in evolving populations, the trajectory of fall multivariate trait evolution in replacement populations was not affected by competitive history (F1,9 = 0.93, P = 0.54; Fig. 3C). This, combined with the observation that trait change did occur in the fall in these replacement populations (Fig. 4; details in SI Appendix), indicates that intergenerational mechanisms of adaptation under the fall selection regime were a critical determinant of the evolutionary legacy of competition.
In contrast to the effects of summer competition on genetically based phenotypic change in the fall, there was no effect of competition on the end-of-fall body size of flies that were collected directly from field cages and were therefore subject to the effects of both genetic and plastic responses to treatments and the field environment (both sexes: P > 0.1) (SI Appendix, Fig. S4).
Finally, counter to our expectations, there was no effect of a history of competition with Z. indianus on the population dynamics of D. melanogaster in the fall. D. melanogaster populations declined significantly over the course of the fall (date: χ2 = 33.83, P < 0.001), but population dynamics were not affected by competitive history (competition treatment: χ2 = 0.21, P = 0.65), nor were they affected by whether flies experienced intergenerational adaptation to fall conditions (evolution treatment: χ2 = 0.11, P = 0.74; Fig. 2B and SI Appendix, Fig. S1B).
Discussion
Rapid evolution in response to interspecific competition in the summer altered the evolutionary, but not ecological, trajectory of D. melanogaster populations when they were subsequently exposed to cold fall conditions. Populations of D. melanogaster with a history of interspecific competition evolved in the fall to be larger, develop faster, and have lower cold fecundity than flies with no history of interspecific competition (Figs. 3B and 4). Evolutionary divergence between treatments occurred after Z. indianus had been removed from the experiment, indicating that competitive interactions can leave a legacy that shapes evolutionary dynamics even after the competitor is no longer present.
Surprisingly, there was no effect of competitive history on ecological dynamics (population trajectories) in the fall (Fig. 2B) and no evidence of evolution in response to interspecific competition in any of the fly phenotypes that we measured at the end of the summer (Figs. 3A and 4). While previous research investigating rapid evolutionary responses to competition has focused on the immediate ecological and evolutionary consequences (phenotypic or genetic change) (19, 23, 30) and, less commonly, on the subsequent ecological consequences (2, 3, 56), we show here that effects on subsequent evolution are also possible. Specifically, our results demonstrate that there can be knock-on effects of evolution in response to competitors on subsequent evolution in response to another selective pressure. This finding contributes to a fuller understanding of eco-evolutionary dynamics and reveals an evolutionary interaction between competition and climate that could be exacerbated by global change.
Our finding that competitive history shaped D. melanogaster’s evolutionary trajectory in the fall, despite a lack of observed phenotypic change in response to interspecific competition during the summer, raises two interesting and related questions. First, what is the evolutionary mechanism that caused a history of interspecific competition to affect subsequent evolution? And second, how can exposure to an ecological pressure that results in no obvious evolved phenotypic change subsequently alter the trajectory of evolution?
The answer to the first question is more straightforward. The divergent fall evolutionary trajectories across our two competitive history treatments, combined with the parallel evolutionary trajectories across replicate populations within each treatment in the fall (Fig. 4), point to selection as the most likely mechanism driving our fall evolutionary responses. Selection is the most likely mechanism to cause both parallel trait change across independent populations within treatments and divergent trait change among experimental treatments (22, 57). Likewise, divergent selection across treatments would depend on our treatments having different selective environments, and, indeed, the finding that Z. indianus (Fig. 2A) lowered D. melanogaster abundances indicates that our two competition treatments did impose different ecological conditions over the summer. Divergent selection between the two competition treatments in the summer that then affected subsequent evolutionary trajectories when flies were exposed to fall conditions is the most likely explanation for patterns of evolutionary change that we observed in the fall. Drift, an alternative mechanism of evolutionary change, is much less likely to have contributed to the observed divergence between treatments. Genetic drift would be expected to cause stochastic divergence across replicate populations, not treatment-level phenotypic differences (58, 59). It is also unlikely that summer competition hindered subsequent fall evolution by reducing population sizes and restricting genetic diversity, as large D. melanogaster population sizes were maintained throughout the experiment, even in our interspecific competition treatment (minimum of 1,000 individuals to maximum of 54,000 individuals; Fig. 2A).
This, however, raises the question of how selection by competitors with no observed concurrent phenotypic response in the summer could drive subsequent evolutionary change in the fall. One explanation is that we may have simply missed a critical phenotype that evolved in response to the competitive environment in the summer and then drove evolutionary divergence in the fall (41, 60). It is a well-recognized challenge in eco-evolutionary research that trait measurements often fail to capture evolutionary responses because the a priori identification of the traits on which selection will act, and the choice of appropriate common garden conditions under which to measure these traits, is exceedingly difficult (41, 60–62). While we measured many traits and targeted those likely to respond to competition and temperature, many potentially relevant phenotypes, and many conditions under which to measure them, were by necessity not captured. These include phenotypes that can evolve rapidly, such as longevity, starvation tolerance, larval competitive ability, egg size, lipid content, chill recovery, and larval feeding rate (35, 63, 64). Furthermore, evolved differences in physiological responses or phenotypes that are challenging to assay on a large scale, such as behavior, toxicology, immunity, and ion transport would also have gone undetected (52, 65). While we focused our efforts on tracking the ecological and phenotypic consequences of competition and climate, future research could use genomic data to determine the extent and architecture of genetic divergence in response to these two agents of selection and to uncover connections between the genetic and phenotypic changes that they produce (53).
The specific genetic basis of evolution in response to summer conditions could also have contributed to the observed evolutionary divergence in the fall. As a result of pleiotropy or epistasis, differences in the complex selective landscapes in each treatment could lead to systematic differences in the subset of loci that underlie adaptation. These genetic differences, even in the absence of phenotypic divergence, would produce parallel genomic divergence in the summer that could influence the trajectory of evolution in the fall (39, 41, 42). Indeed, genetic differences that are phenotypically cryptic under one set of conditions have been shown to contribute to rapid evolution when environmental conditions change (66, 67). Such cryptic evolutionary links are likely common in both natural systems and in the experimental evolution studies that investigate them (41, 66).
Comparisons between our two fall evolution treatments provide additional insight into the mechanisms underlying the evolutionary responses that we observed. We found that competitive history affected fall evolutionary trajectories in our evolving treatment, but not in our treatment for which intergenerational adaptation to fall conditions was prevented by egg replacements (Fig. 3B vs. Fig. 3C). This finding indicates that evolutionary mechanisms that occur across generations (e.g., recombination, epistasis, cumulative effects of selection across generations) were necessary for fall evolutionary trajectories to diverge based on competitive history. Our replacement populations started the fall with the same genetic diversity and experienced the same abiotic selective environment as our evolving populations, but their evolutionary response to fall conditions was limited to recurrent selection on standing genetic variation. In contrast, evolving populations experienced recombination over multiple generations, which can reduce negative epistasis and pleiotropy (68, 69) and lead to a greater response to selection. This result highlights how evolutionary processes that require multiple generations of selection and allow for recombination and other mechanisms that can alter the genetic architecture of complex traits, can meaningfully affect adaptation. Our results further suggest that studies that examine adaptation in response to selection within a single generation may underestimate adaptive potential (70).
Another unexpected result from this experiment was that, even though flies exhibited divergent phenotypic evolution based on competitive history by the end of the fall, this phenotypic divergence did not influence fall population dynamics (Fig. 2B). There are several possible explanations for why we did not observe an ecological response to divergent fall evolution based on competitive history. First, it is not obvious which combination of evolved phenotypes observed at the end of fall would confer an adaptive advantage expected to slow population declines under cooler, low-density fall conditions (Fig. 2B). A history of interspecific competition caused flies to evolve larger female body size and lower cold fecundity over the fall. While large body size has been associated with higher fitness under cold conditions (71, 72), low cold fecundity is likely to reduce short-term fitness as temperatures drop in the fall, but could increase overwinter survival if associated with the induction of reproductive diapause (46).
Second, the seasonal dynamics of this system may simply not have allowed enough time for phenotypic effects to translate into demographic responses; had climatic conditions allowed our populations to persist longer, or had populations been tracked into the following spring or with renewed exposure to competitors, we may have observed a demographic effect of the observed fall phenotypic changes (56, 73, 74). Finally, female flies that were collected directly from field cages, and thus had phenotypes that were a product of both genetic and plastic responses to our treatments and the field environment, were smaller overall and did not show any effect of competitive history on body size (SI Appendix, Fig. S4). Field conditions are more resource-limited than laboratory conditions, and the evolved trait differences apparent after two generations in a common garden may have been muted in the field by a general plastic response to field conditions. Future work could examine whether this plastic response is adaptive (i.e., flies are smaller in the field because it is disadvantageous to be large under field conditions) or due simply to resource limitation (i.e., poor conditions constraining growth in the field) (41, 75). Regardless of the mechanism, our results suggest that phenotypic change that occurs very rapidly may not always produce a detectable demographic signal, and highlight the need for more research linking rapid phenotypic evolution to population responses. This finding further counters the traditional view that ecological dynamics are fast relative to evolutionary change by suggesting that, in certain situations, rapid evolution may foster further evolutionary change before ecological responses are observed (5, 76).
In addition to implications for understanding eco-evolutionary dynamics, our findings also expand our understanding of community responses to nonnative species introductions and climate change. Our results add to the growing body of evidence that invasive species can induce rapid evolutionary responses in their native or naturalized counterparts (77–79). Past work on invasive competitors has focused primarily on character displacement (56, 79), but our work suggests the possibility of more cryptic impacts; here, the negative ecological impacts of the invasive species were apparent immediately (Fig. 2A), but the evolutionary impacts did not emerge until our focal species subsequently evolved in response to a change in climatic conditions (Figs. 3B and 4). More broadly, our finding that an invasive species only impacted evolutionary dynamics once our focal species was exposed to a seasonally changing climate indicates that we may be underestimating the impacts of the invasive species on the evolutionary fate of native populations.
Species are constantly subject to complex and shifting adaptive landscapes as seasons progress and competitors emerge, migrate, and die off, and the need to understand the implications of these dynamics has never been more pressing than under current global change (7). Our findings provide evidence for evolutionary interactions between climate and competition that are rarely considered and emphasize the need for eco-evolutionary research conducted under natural field conditions that incorporates both the biotic and abiotic drivers of global change (11, 12). As we have shown, the integration of ecological and evolutionary processes will be required to fully understand how biological systems respond to global change.
Methods
Study Species.
We used D. melanogaster as our focal species and Z. indianus as its competitor. These two species can be easily distinguished at both the adult and the egg stages. We created a genetically diverse D. melanogaster founder population for our experiment by mixing 150 wild-derived isofemale lines collected from two orchards in southeastern Pennsylvania in the summer and fall of 2017 and 2018. Lines were kept in vials at room temperature and maintained by serial transfer until May 2019, when we created the founder population for our field experiment by randomly selecting 10 adult females from each line and allowing their offspring to mate and recombine for three generations of unrestricted population expansion in laboratory conditions. This created a single founder population from which flies for our experimental populations were selected. While the use of a single founding population results in lower genetic diversity across the experiment than would multiple independent founding populations, this approach allowed evolution to proceed from a common starting point. Moreover, our methods ensured a genetically diverse founder population, and experimental populations remained completely independent once the experiment started. Given the high level of genetic diversity in our experimental populations and the timescale of adaptation in our experiment, we assume that evolutionary change occurred primarily via selection on standing variation rather than though de novo mutations. We created our experimental Z. indianus population by combining 10 males and 10 females from each of 100 isofemale lines collected from orchards in Pennsylvania and Florida, allowing flies to breed in a common cage and then maintaining this genetically diverse population in the laboratory at large population sizes by serial transfer until the start of the experiment.
Orchard Experiment.
We conducted the study in an experimental orchard at the University of Pennsylvania in Philadelphia. The orchard consisted of cleared land with 28 metal framed walk-in cages (1.8 m3) covered with insect screen (Bioquip) (SI Appendix, Fig. S8). Each cage contained a dwarf peach tree and a shelving unit to hold fly food and developing eggs and had clover as ground cover (SI Appendix, Fig. S8). Peach trees provided habitat and heat refuge for the flies, but all peaches were removed before ripening during the experiment and were not a source of food or an egg-laying substrate. We conducted the experiment in two phases: summer and fall (Fig. 1).
Summer Phase.
In the summer we imposed two treatments: interspecific competition (Z. indianus and D. melanogaster added) and no interspecific competition (only D. melanogaster added) (Fig. 1). In the summer each treatment was replicated 14 times for a total of 28 cages. We assigned treatments to cages in an alternating pattern throughout the orchard. We initiated the summer phase on July 9 by adding 500 adult male and 500 adult female D. melanogaster flies to all cages, and 3,000 mixed-sex adult Z. indianus to each of the 14 interspecific competition cages (details in SI Appendix). Fly populations in each cage were fed 400 mL of Drosophila media (modified Bloomington Stock Center cornmeal recipe) in a 1.5-lb aluminum loaf pan three times per week (SI Appendix, Fig. S8). Flies laid eggs on this food over the course of 2 or 3 d, and then pans were covered until flies eclosed, at which point adult flies were released into the general population (details in SI Appendix). In order to maintain high levels of interspecific competition, we supplemented each interspecific competition cage with ∼1,000 laboratory-reared adult Z. indianus once per week in August (5 wk). Five loaf pans of developing D. melanogaster eggs (i.e., 1.5 wk of development) were discarded from all field cages in mid-July as a result of food spoilage.
The summer phase lasted for 9 wk (July 9 to September 12th), which was just over six full generations (egg to laying adult) for D. melanogaster in warm summer conditions.
Fall Phase.
In the fall phase there was only one species of fly (D. melanogaster), and we imposed four treatments: two levels of competitive history (history of interspecific competition or no history of interspecific competition) crossed with two levels of evolution (evolving to outdoor conditions or eggs replaced to prevent intergenerational adaptation in response to outdoor conditions—described below). Each experimental replicate (cage containing a fly population) was maintained independently throughout the entire experiment (summer and fall), with half of the 14 summer interspecific competition and half of the 14 summer noninterspecific competition cages randomly assigned to be evolving in the fall, and the other half randomly assigned to be nonevolving (Fig. 1). Therefore, each of the four fall treatments was replicated seven times for a total of 28 cages. In the fall, we used the same cages that we had used in the summer, so that the offspring of our 28 replicate populations went back into the cage from which their parents had been collected at the end of the summer. Although this method of placing each fly population back into the same cage meant that changes in predators or microbes over the summer could potentially carry over to affect fall evolution, we have no indication that this occurred. Moreover, the resource-based carry-over effects between time points observed in other experimental systems (80, 81) are not possible in our study because we supplied all fly food at a constant rate throughout the summer and fall.
To establish these summer-adapted D. melanogaster populations for the fall phase, we collected D. melanogaster eggs (>20,000 eggs on 10 food pans per cage) and adults from each of our 28 cages at the end of the summer (Fig. 1) and then killed all remaining flies in the orchard (Fig. 1; details in SI Appendix). We then placed 5,000 adult D. melanogaster flies that originated from each summer cage back into that same cage at the start of the fall phase. As such, each population was kept independent through the entire experiment.
Starting the fall phase with the same number of D. melanogaster in each cage (rather than letting cage-level differences in abundance carry through from summer to fall) allowed us to isolate the effects of rapid evolution in response to summer competition on ecological and evolutionary dynamics when flies were exposed to a cooler fall climate by removing the effect of summer competition on abundances. The culling of D. melanogaster and the removal of Z. indianus in early fall simulates an early fall frost event that would be lethal for cold-intolerant Z. indianus.
Of the 5,000 adult flies placed back into each cage, 4,000 were flies that eclosed from eggs collected from field cages at the end of the summer (some of these eggs were also used to create the indoor populations for our nonevolving treatment—described below), and 1,000 were collected directly from their field cage prior to the end of the summer phase. We included these field-collected flies in order to incorporate any plastic responses to field conditions in our fall populations, to reduce the low probability of a genetic bottleneck caused by egg collection, and to avoid hindering an evolutionary response to fall conditions by maximizing the amount of genetic variation in each population that was carried over from summer to fall.
To implement our fall evolution treatment, we either allowed adaptive intergenerational changes in genotype frequency in response to fall conditions (evolving treatment) or prevented them (replacement treatment). For our evolving treatment, we used the normal summer feeding and egg-rearing protocol described above, with eggs laid in the food and left to develop into adult flies that were then released back into the cage. For our replacement treatment, we counted all of the eggs laid in the food three times per week and replaced them with the same number of eggs laid by summer-adapted populations kept in the laboratory (Fig. 1) (45). Each of our 14 outdoor nonevolving fall D. melanogaster populations had a corresponding indoor laboratory population consisting of ∼5,000 D. melanogaster flies that had eclosed from the eggs collected at the end of the summer phase from that cage (described above), thereby keeping replicates independent. Indoor cages were kept at room temperature with a 12 light:12 dark photoperiod. Therefore, in the replacement treatment, intergenerational genetic change in response to fall field conditions was prevented because all eggs that were permitted to develop into adult flies in the outdoor cages were the F1 offspring of flies collected at the end of the summer. Almost all flies in these indoor cages survived throughout the entire fall phase, so no new generations were required to maintain indoor populations, reducing the potential for evolution in response to laboratory conditions. While the aim of this treatment was to prevent intergenerational adaptation to cold fall conditions, these populations were still subject to truncation selection on each cohort of eggs that were put into the field, which could act through selective mortality of eggs, larvae, or adults. The fall phase lasted 7 wk (September 23 to November 11), which was approximately three full generations for D. melanogaster in these cooler fall conditions.
Population Size Estimates.
To estimate fly abundances in orchard cages in the summer and fall, we took photos of delineated sections of cage roofs roughly once per week (seven summer censuses and nine fall censuses), counted the number of flies in the photos, and multiplied these counts by a calibrated constant to get an estimate of total population size (details in SI Appendix). While the abundances that we report are thus estimates of total population size and not exact counts, we were primarily interested in differences in abundance across treatments.
Phenotyping.
To assess the effect of our treatments on the evolution of ecologically relevant traits, we measured 10 fitness-associated phenotypes on D. melanogaster at the beginning of the summer phase (founder population), the end of the summer phase, and the end of the fall phase. Flies phenotyped at the beginning of the summer came from the single founder population, and so this time point has a single value for each phenotype (Fig. 4). By contrast, at the end of summer and fall, flies from each population (cage) were phenotyped.
On September 10 to 11 (end of summer phase) and November 6 to 8 (end of fall phase) we collected eggs from each orchard cage and brought them into the laboratory to be reared for two generations under common garden conditions (room temperature and a 12 light:12 dark photoperiod). We did this in order to remove plastic responses to our treatments and the field environment, although epigenetic effects could have potentially persisted for longer than two generations (82). We then measured 10 phenotypes: body size (female and male), early life fecundity (warm [25 °C] and cold [15 °C] conditions), and larval development speed and egg viability, each under three different conditions (35, 37) (details in SI Appendix). The three conditions for larval development speed and egg viability were: 1) cold (15 °C) low density (50 eggs), 2) warm (25 °C) low density, and 3) warm high density (500 eggs). These three larval development environments mimic the range of conditions experienced by developing larvae in the experiment: warm high density simulates summer conditions, warm low density simulates early summer and early fall, and cold low density simulates late fall. Finally, we also measured female and male body size on flies collected directly from each cage (no common garden) in order to determine the effect of our treatments on flies subject to plasticity in response to the field environment. We measured all phenotypes on three replicates per cage (to account for vial effects), and replicates were then averaged and the cage-level mean was used as the experimental unit in all analyses.
Data Analysis.
To determine the effect of competition on adult D. melanogaster abundances over time in the summer, we ran a linear mixed-effect model with competition treatment and survey date as fixed effects, estimated D. melanogaster abundance as the response, and cage as a random factor (n = 14 for each of the two treatments). We repeated this analysis with the fall data with the evolution treatment as an additional fixed effect (n = 7 for each of the four treatments).
To determine the effect of competition on multivariate trait evolution, we used a permutational multivariate analysis of variance (MANOVA) to analyze the Euclidean distance between competition treatments in multivariate trait space derived from our 10 measured phenotypes. We ran separate analyses for the end of summer populations (n = 14 for each treatment), end of fall replacement populations (n = 7 for each treatment), and end of fall evolving populations (n = 7 for each treatment). We implemented this analysis using the Adonis function in the R package vegan (999 permutations). To determine which specific traits contributed to the marginally significant effect of competitive history on D. melanogaster multivariate trait evolution that we detected in our fall evolving populations, we used two sample t tests to test for a difference in each phenotype between fall evolving populations with and without a history of Z. indianus.
To determine the effect of competition on D. melanogaster phenotypic evolution for each trait, and to see whether this effect depended on season, we ran separate linear mixed-effects models for each of our 10 fly phenotypes. Each model had competition treatment and season as fixed effects, the phenotype as the response, and cage as a random factor. In addition, we included the competition by season interaction to evaluate whether a history of competition affected the trajectory of trait evolution from summer to fall. Because we were interested in the effect of competition on evolved phenotypic changes, we excluded the 14 replacement fall cages from these analyses. Specifying cage as a random factor in an intercept-only mixed effects model accounted for the nonindependence of the 14 evolving populations that were carried through from the summer to the fall, and accounted for cage-level heterogeneity in evolutionary trajectories.
To determine the effect of competition on the body size of flies subject to plasticity in response to our treatments and to the field environment, and to see whether this effect depended on season, we ran linear mixed-effects models with the same fixed and random effects described above for female and male body size of flies collected directly from our field cages.
All analyses were conducted in R (version 3.5.3), and all figures were made using the package ggplot2.
Supplementary Material
Acknowledgments
We thank D. MacArthur-Waltz, Y. Babore, H. Oken, A. Goldfischer, and E. Brud for assistance in the field and laboratory. We thank the J.M.L. and P.S. research groups for valuable feedback. Funding was provided by a Natural Sciences and Engineering Research Council of Canada Postdoctoral Fellowship (to T.N.G.), Princeton Start-Up funds (to J.M.L.), and NIH Grant R01GM100366 (to P.S.).
Footnotes
The authors declare no competing interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at https://www.pnas.org/lookup/suppl/doi:10.1073/pnas.2015772118/-/DCSupplemental.
Data Availability.
Excel file data have been deposited in “Competitive history shapes rapid evolution in a seasonally changing climate” (https://doi.org/10.5061/dryad.w6m905qn7). All data supporting this research are available on the Dryad Digital Repository (83).
References
- 1.Hairston N. G., Ellner S. P., Geber M. A., Yoshida T., Fox J. A., Rapid evolution and the convergence of ecological and evolutionary time. Ecol. Lett. 8, 1114–1127 (2005). [Google Scholar]
- 2.Hart S. P., Turcotte M. M., Levine J. M., Effects of rapid evolution on species coexistence. Proc. Natl. Acad. Sci. U.S.A. 116, 2112–2117 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Turcotte M. M., Reznick D. N., Hare J. D., The impact of rapid evolution on population dynamics in the wild: Experimental test of eco-evolutionary dynamics. Ecol. Lett. 14, 1084–1092 (2011). [DOI] [PubMed] [Google Scholar]
- 4.Hendry A. P., Eco-evolutionary Dynamics (Princeton University Press, 2020). [Google Scholar]
- 5.Schoener T. W., The newest synthesis: Understanding the interplay of evolutionary and ecological dynamics. Science 331, 426–429 (2011). [DOI] [PubMed] [Google Scholar]
- 6.Norberg J., Urban M. C., Vellend M., Klausmeier C. A., Loeuille N., Eco-evolutionary responses of biodiversity to climate change. Nat. Clim. Chang. 2, 747–751 (2012). [Google Scholar]
- 7.Urban M. C., De Meester L., Vellend M., Stoks R., Vanoverbeke J., A crucial step toward realism: Responses to climate change from an evolving metacommunity perspective. Evol. Appl. 5, 154–167 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Barrett R. D., et al., Rapid evolution of cold tolerance in stickleback. Proc. Biol. Sci. 278, 233–238 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Fryxell D. C., et al., Recent warming reduces the reproductive advantage of large size and contributes to evolutionary downsizing in nature. Proc. Biol. Sci. 287, 20200608 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.IPCC: Climate Change 2014: Synthesis Report . Contribution of Working Groups I, II and III to the Fifth Assessment Report of the Intergovernmental Panel on Climate Change, Core Writing Team, R.K. Pachauri and L.A. Meyer, Eds. (IPCC, Geneva, Switzerland, 2014).
- 11.De Meester L., et al., Analysing eco‐evolutionary dynamics: The challenging complexity of the real world. Funct. Ecol. 33, 43–59 (2019). [Google Scholar]
- 12.Hendry A. P., A critique for eco‐evolutionary dynamics. Funct. Ecol. 33, 84–94 (2019). [Google Scholar]
- 13.Alexander J. M., Diez J. M., Hart S. P., Levine J. M., When climate reshuffles competitors: A call for experimental macroecology. Trends Ecol. Evol. 31, 831–841 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Williams J. W., Jackson S. T., Novel climates, no‐analog communities, and ecological surprises. Front. Ecol. Environ. 5, 475–482 (2007). [Google Scholar]
- 15.Chesson P., Mechanisms of maintenance of species diversity. Annu. Rev. Ecol. Syst. 31, 343–366 (2000). [Google Scholar]
- 16.Tilman D., Resource Competition and Community Structure (Princeton University Press, 1982). [PubMed] [Google Scholar]
- 17.Dieckmann U., Doebeli M., On the origin of species by sympatric speciation. Nature 400, 354–357 (1999). [DOI] [PubMed] [Google Scholar]
- 18.Schluter D., The Ecology of Adaptive Radiation (Oxford University Press, 2000). [Google Scholar]
- 19.Grant P. R., Grant B. R., Evolution of character displacement in Darwin’s finches. Science 313, 224–226 (2006). [DOI] [PubMed] [Google Scholar]
- 20.Schluter D., Experimental evidence that competition promotes divergence in adaptive radiation. Science 266, 798–801 (1994). [DOI] [PubMed] [Google Scholar]
- 21.terHorst C. P., Miller T. E., Levitan D. R., Evolution of prey in ecological time reduces the effect size of predators in experimental microcosms. Ecology 91, 629–636 (2010). [DOI] [PubMed] [Google Scholar]
- 22.Bolnick D. I., Barrett R. D., Oke K. B., Rennison D. J., Stuart Y. E., (Non) parallel evolution. Annu. Rev. Ecol. Evol. Syst. 49, 303–330 (2018). [Google Scholar]
- 23.Collins S., Competition limits adaptation and productivity in a photosynthetic alga at elevated CO2. Proc. Biol. Sci. 278, 247–255 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Agrawal A. A., Hastings A. P., Johnson M. T., Maron J. L., Salminen J.-P., Insect herbivores drive real-time ecological and evolutionary change in plant populations. Science 338, 113–116 (2012). [DOI] [PubMed] [Google Scholar]
- 25.Duffy M. A., et al., Ecological context influences epidemic size and parasite-driven evolution. Science 335, 1636–1638 (2012). [DOI] [PubMed] [Google Scholar]
- 26.Jones A. G., A theoretical quantitative genetic study of negative ecological interactions and extinction times in changing environments. BMC Evol. Biol. 8, 119 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Barraclough T. G., How do species interactions affect evolutionary dynamics across whole communities? Annu. Rev. Ecol. Evol. Syst. 46, 25–48 (2015). [Google Scholar]
- 28.terHorst C. P., Experimental evolution of protozoan traits in response to interspecific competition. J. Evol. Biol. 24, 36–46 (2011). [DOI] [PubMed] [Google Scholar]
- 29.Joshi A., Thompson J. N., Alternative routes to the evolution of competitive ability in two competing species of Drosophila. Evolution 49, 616–625 (1995). [DOI] [PubMed] [Google Scholar]
- 30.Lawrence D., et al., Species interactions alter evolutionary responses to a novel environment. PLoS Biol. 10, e1001330 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Osmond M. M., de Mazancourt C., How competition affects evolutionary rescue. Philos. Trans. R. Soc. Lond. B Biol. Sci. 368, 20120085 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Johansson J., Evolutionary responses to environmental changes: How does competition affect adaptation? Evolution 62, 421–435 (2008). [DOI] [PubMed] [Google Scholar]
- 33.Barrett R. D., Schluter D., Adaptation from standing genetic variation. Trends Ecol. Evol. 23, 38–44 (2008). [DOI] [PubMed] [Google Scholar]
- 34.Listmann L., Hattich G. S., Matthiessen B., Reusch T. B., Eco-evolutionary interaction in competing phytoplankton: Nutrient driven genotype sorting likely explains dominance shift and species responses to CO2. Front. Mar. Sci. 7, 634 (2020). [Google Scholar]
- 35.Behrman E. L., Watson S. S., O’Brien K. R., Heschel M. S., Schmidt P. S., Seasonal variation in life history traits in two Drosophila species. J. Evol. Biol. 28, 1691–1704 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Bergland A. O., Behrman E. L., O’Brien K. R., Schmidt P. S., Petrov D. A., Genomic evidence of rapid and stable adaptive oscillations over seasonal time scales in Drosophila. PLoS Genet. 10, e1004775 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Rudman S. M., et al., Microbiome composition shapes rapid genomic adaptation of Drosophila melanogaster. Proc. Natl. Acad. Sci. U.S.A. 116, 20025–20032 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Mackay T. F., et al., The Drosophila melanogaster genetic reference panel. Nature 482, 173–178 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Huang W., et al., Epistasis dominates the genetic architecture of Drosophila quantitative traits. Proc. Natl. Acad. Sci. U.S.A. 109, 15553–15559 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Shao H., et al., Genetic architecture of complex traits: Large phenotypic effects and pervasive epistasis. Proc. Natl. Acad. Sci. U.S.A. 105, 19910–19914 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Kinnison M. T., N. G. Hairston, Jr, Hendry A. P., Cryptic eco-evolutionary dynamics. Ann. N. Y. Acad. Sci. 1360, 120–144 (2015). [DOI] [PubMed] [Google Scholar]
- 42.Paaby A. B., Rockman M. V., The many faces of pleiotropy. Trends Genet. 29, 66–73 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Kopp M., Matuszewski S., Rapid evolution of quantitative traits: Theoretical perspectives. Evol. Appl. 7, 169–191 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Schmidt P. S., Conde D. R., Environmental heterogeneity and the maintenance of genetic variation for reproductive diapause in Drosophila melanogaster. Evolution 60, 1602–1611 (2006). [PubMed] [Google Scholar]
- 45.Rajpurohit S., et al., Adaptive dynamics of cuticular hydrocarbons in Drosophila. J. Evol. Biol. 30, 66–80 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Schmidt P. S., Paaby A. B., Reproductive diapause and life-history clines in North American populations of Drosophila melanogaster. Evolution 62, 1204–1215 (2008). [DOI] [PubMed] [Google Scholar]
- 47.Comeault A. A., et al., Genetic diversity and thermal performance in invasive and native populations of African fig flies. Mol. Biol. Evol. 37, 1893–1906 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.van der Linde K., et al., First records of Zaprionus indianus (Diptera: Drosophilidae), a pest species on commercial fruits from Panama and the United States of America. Fla. Entomol. 89, 402–404 (2006). [Google Scholar]
- 49.Araripe L., Klaczko L., Moreteau B., David J., Male sterility thresholds in a tropical cosmopolitan drosophilid, Zaprionus indianus. J. Therm. Biol. 29, 73–80 (2004). [Google Scholar]
- 50.Gibert P., et al., Drosophila as models to understand the adaptive process during invasion. Biol. Invasions 18, 1089–1103 (2016). [Google Scholar]
- 51.Ellner S. P., Geber M. A., N. G. Hairston, Jr, Does rapid evolution matter? Measuring the rate of contemporary evolution and its impacts on ecological dynamics. Ecol. Lett. 14, 603–614 (2011). [DOI] [PubMed] [Google Scholar]
- 52.Behrman E. L., et al., Rapid seasonal evolution in innate immunity of wild Drosophila melanogaster. Proc. Biol. Sci. 285, 20172599 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Rudman S. M., et al., What genomic data can reveal about eco-evolutionary dynamics. Nat. Ecol. Evol. 2, 9–15 (2018). [DOI] [PubMed] [Google Scholar]
- 54.Harshman L. G., Hoffmann A. A., Laboratory selection experiments using Drosophila: What do they really tell us? Trends Ecol. Evol. 15, 32–36 (2000). [DOI] [PubMed] [Google Scholar]
- 55.Yadav P., Sharma V. K., Correlated changes in life history traits in response to selection for faster pre-adult development in the fruit fly Drosophila melanogaster. J. Exp. Biol. 217, 580–589 (2014). [DOI] [PubMed] [Google Scholar]
- 56.Germain R. M., Srivastava D., Angert A. L., Evolution of an inferior competitor increases resistance to biological invasion. Nat. Ecol. Evol. 4, 419–425 (2020). [DOI] [PubMed] [Google Scholar]
- 57.Colosimo P. F., et al., Widespread parallel evolution in sticklebacks by repeated fixation of Ectodysplasin alleles. Science 307, 1928–1933 (2005). [DOI] [PubMed] [Google Scholar]
- 58.Lande R., Natural selection and random genetic drift in phenotypic evolution. Evolution 30, 314–334 (1976). [DOI] [PubMed] [Google Scholar]
- 59.Wright S., Evolution in Mendelian populations. Genetics 16, 97–159 (1931). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Germain R. M., Williams J. L., Schluter D., Angert A. L., Moving character displacement beyond characters using contemporary coexistence theory. Trends Ecol. Evol. 33, 74–84 (2018). [DOI] [PubMed] [Google Scholar]
- 61.Arnold S. J., Wade M. J., On the measurement of natural and sexual selection: Theory. Evolution 38, 709–719 (1984). [DOI] [PubMed] [Google Scholar]
- 62.Irschick D. J., Measuring performance in nature: Implications for studies of fitness within populations. Integr. Comp. Biol. 43, 396–407 (2003). [DOI] [PubMed] [Google Scholar]
- 63.Azevedo R. B., French V., Partridge L., Life-history consequences of egg size in Drosophila melanogaster. Am. Nat. 150, 250–282 (1997). [DOI] [PubMed] [Google Scholar]
- 64.Joshi A., Mueller L. D., Evolution of higher feeding rate in Drosophila due to density-dependent natural selection. Evolution 42, 1090–1093 (1988). [DOI] [PubMed] [Google Scholar]
- 65.Edwards A. C., Rollmann S. M., Morgan T. J., Mackay T. F., Quantitative genomics of aggressive behavior in Drosophila melanogaster. PLoS Genet. 2, e154 (2006). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Le Rouzic A., Carlborg O., Evolutionary potential of hidden genetic variation. Trends Ecol. Evol. 23, 33–37 (2008). [DOI] [PubMed] [Google Scholar]
- 67.Schlichting C. D., Hidden reaction norms, cryptic genetic variation, and evolvability. Ann. N. Y. Acad. Sci. 1133, 187–203 (2008). [DOI] [PubMed] [Google Scholar]
- 68.Felsenstein J., The evolutionary advantage of recombination. Genetics 78, 737–756 (1974). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Kondrashov A. S., Deleterious mutations and the evolution of sexual reproduction. Nature 336, 435–440 (1988). [DOI] [PubMed] [Google Scholar]
- 70.Otto S. P., Lenormand T., Resolving the paradox of sex and recombination. Nat. Rev. Genet. 3, 252–261 (2002). [DOI] [PubMed] [Google Scholar]
- 71.McCabe J., Partridge L., An interaction between environmental temperature and genetic variation for body size for the fitness of adult female Drosophila melanogaster. Evolution 51, 1164–1174 (1997). [DOI] [PubMed] [Google Scholar]
- 72.Partridge L., Barrie B., Fowler K., French V., Evolution and development of body size and cell size in Drosophila melanogaster in response to temperature. Evolution 48, 1269–1276 (1994). [DOI] [PubMed] [Google Scholar]
- 73.Hausch S. J., Fox J. W., Vamosi S. M., Coevolution of competing Callosobruchus species does not stabilize coexistence. Ecol. Evol. 7, 6540–6548 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Pimentel D., Feinberg E. H., Wood P. W., Hayes J. T., Selection, spatial distribution, and the coexistence of competing fly species. Am. Nat. 99, 97–109 (1965). [Google Scholar]
- 75.Monaghan P., Early growth conditions, phenotypic development and environmental change. Philos. Trans. R. Soc. Lond. B Biol. Sci. 363, 1635–1645 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Reznick D. A., Bryga H., Endler J. A., Experimentally induced life-history evolution in a natural population. Nature 346, 357–359 (1990). [Google Scholar]
- 77.Carroll S. P., Boyd C., Host race radiation in the soapberry bug: Natural history with the history. Evolution 46, 1052–1069 (1992). [DOI] [PubMed] [Google Scholar]
- 78.Strauss S. Y., Ecological and evolutionary responses in complex communities: Implications for invasions and eco‐evolutionary feedbacks. Oikos 123, 257–266 (2014). [Google Scholar]
- 79.Stuart Y. E., et al., Rapid evolution of a native species following invasion by a congener. Science 346, 463–466 (2014). [DOI] [PubMed] [Google Scholar]
- 80.Brunner F. S., Anaya-Rojas J. M., Matthews B., Eizaguirre C., Experimental evidence that parasites drive eco-evolutionary feedbacks. Proc. Natl. Acad. Sci. U.S.A. 114, 3678–3683 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Matthews B., Aebischer T., Sullam K. E., Lundsgaard-Hansen B., Seehausen O., Experimental evidence of an eco-evolutionary feedback during adaptive divergence. Curr. Biol. 26, 483–489 (2016). [DOI] [PubMed] [Google Scholar]
- 82.Bošković A., Rando O. J., Transgenerational epigenetic inheritance. Annu. Rev. Genet. 52, 21–41 (2018). [DOI] [PubMed] [Google Scholar]
- 83.Grainger T. N., Rudman S. M., Schmidt P., Levine J. M., Competitive history shapes rapid evolution in a seasonal climate, Dryad Digital Repository, 10.5061/dryad.w6m905qn7. Deposited 26 November 2020. [DOI] [PMC free article] [PubMed]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Excel file data have been deposited in “Competitive history shapes rapid evolution in a seasonally changing climate” (https://doi.org/10.5061/dryad.w6m905qn7). All data supporting this research are available on the Dryad Digital Repository (83).