Significance
The APL2012 trial is currently the largest randomized, multicenter clinical study of APL based on ATRA-ATO treatment with risk stratification. We enrolled 855 newly diagnosed APL patients during December 2012 to December 2017 for careful follow-up. All patients received ATRA-ATO–based protocols for remission induction. At the consolidation phase, the key part of the trial, ATO was used to replace or reduce chemotherapy in a risk-stratified way. Patients were then treated with ATRA-ATO as maintenance. The results indicated not only the noninferiority of ATO compared to intensive chemotherapy in survival but also an advantage in adverse effects. The trial provides support for the ATO regimen as a standard for making further refinements in the treatment of this highly curable disease.
Keywords: acute promyelocytic leukemia, all-trans retinoic acid, arsenic trioxide, risk stratification, consolidation therapy
Abstract
As all-trans retinoic acid (ATRA) and arsenic trioxide (ATO) are widely accepted in treating acute promyelocytic leukemia (APL), deescalating toxicity becomes a research hotspot. Here, we evaluated whether chemotherapy could be replaced or reduced by ATO in APL patients at different risks. After achieving complete remission with ATRA-ATO–based induction therapy, patients were randomized (1:1) into ATO and non-ATO groups for consolidation: ATRA-ATO versus ATRA–anthracycline for low-/intermediate-risk patients, or ATRA-ATO–anthracycline versus ATRA–anthracycline–cytarabine for high-risk patients. The primary end point was to assess disease-free survival (DFS) at 3 y by a noninferiority margin of –5%; 855 patients were enrolled with a median follow-up of 54.9 mo, and 658 of 755 patients could be evaluated at 3 y. In the ATO group, 96.1% (319/332) achieved 3-y DFS, compared to 92.6% (302/326) in the non-ATO group. The difference was 3.45% (95% CI –0.07 to 6.97), confirming noninferiority (P < 0.001). Using the Kaplan–Meier method, the estimated 7-y DFS was 95.7% (95% CI 93.6 to 97.9) in ATO and 92.6% (95% CI 89.8 to 95.4) in non-ATO groups (P = 0.066). Concerning secondary end points, the 7-y cumulative incidence of relapse (CIR) was significantly lower in ATO (2.2% [95% CI 1.1 to 4.2]) than in non-ATO group (6.1% [95% CI 3.9 to 9.5], P = 0.011). In addition, grade 3 to 4 hematological toxicities were significantly reduced in the ATO group during consolidation. Hence, ATRA-ATO in both chemotherapy-replacing and -reducing settings in consolidation is not inferior to ATRA–chemotherapy (https://www.clinicaltrials.gov/, NCT01987297).
The treatment of all-trans retinoic acid (ATRA) combined with anthracycline-based chemotherapy has remarkably improved the prognosis of patients with acute promyelocytic leukemia (APL), achieving over 90% complete remission (CR) and 60 to 80% long-term survival (1–6). For patients relapsed from ATRA-chemotherapy, arsenic trioxide (ATO) was initially used as salvage therapy and showed a satisfactory outcome (7–9). Then, the treatment of newly diagnosed APL with an ATRA-ATO combination therapy was reported in 2004, which demonstrated curative effects in 90% of patients (10–13). The advantage of ATO as the front-line treatment of APL has been further validated by a number of international working groups (14–18). Meanwhile, an exploratory study on ATRA-ATO with or without gemtuzumab ozogamicin (GO, the cytotoxic agent calicheamicin linked an anti-CD33 monoclonal antibody) by the MD Anderson Cancer Center suggested that a deescalating cytotoxic regimen might be feasible for APL patients (14, 19).
A large body of evidence has been obtained to show that both ATRA and ATO target the APL-specific PML-RARA oncoprotein and the two agents may exert a synergistic effect in achieving a curative clinical effect in most APL (2, 9). However, in our previous studies, though ATRA-ATO were used as main therapeutic agents for induction, the consolidation was based on chemotherapy rather than ATO, which could cause life-threatening myelosuppression and cardiotoxicity (10, 11). Besides, risk-stratified treatment had not been introduced, leading to probable overtreatment for low- risk (a white blood cell [WBC] count ≤ 10 × 109/L and a platelet count > 40 × 109/L) to intermediate-risk (a WBC count ≤ 10 × 109/L and a platelet count ≤ 40 × 109/L) patients (20). These issues warranted further clinical investigations to address the role of ATRA-ATO in consolidation and to adapt the treatment protocols to distinct clinical risks. In order to optimize the treatment protocols by reducing their relevant toxicities and costs, as well as further improving therapeutic efficacy and tolerance, we proposed a multicenter randomized trial, APL2012, deriving from our previous ATRA-ATO–based therapy taking into consideration of Sanz risk stratification (20). The objective of this study was to examine whether chemotherapy could be replaced or reduced in consolidation therapy by ATO in patients with APL at different risks.
Results
Enrollment and Patient Characteristics.
A total of 901 patients with suspected APL were screened from 6 December 2012 to 31 December 2017; 46 patients were excluded due to disqualification for inclusion criteria (13 were negative for RT-PCR or real-time qPCR [RQ-PCR] detection of PML-RARA gene transcripts, while 17 did not meet other inclusion criteria) or refusal to participate in the study (n = 16). A total of 855 patients were enrolled in this trial. The major clinical characteristics of all patients are provided in Table 1.
Table 1.
Baseline characteristics
| Characteristic | Total (n = 855)* | ATO group (n = 382) | Non-ATO group (n = 373) |
| Median age, y (range) | 40 (18-65) | 39 (18-64) | 39 (18-65) |
| Sex, no. (%) | |||
| Male | 448 (52.4) | 196 (51.3) | 194 (52.0) |
| Female | 407 (47.6) | 186 (48.7) | 179 (48.0) |
| WBC, × 109/L (range) | 3.12 (0.20–165.97) | 2.81 (0.20–134.23) | 3.40 (0.36–161.70) |
| WBC, × 109/L, no. (%) | |||
| 0–9.9 | 588 (68.8) | 262 (68.6) | 258 (69.2) |
| 10–49.9 | 201 (23.5) | 90 (23.6) | 88 (23.6) |
| 50–99.9 | 52 (6.1) | 25 (6.5) | 23 (6.2) |
| ≥ 100 | 14 (1.6) | 5 (1.3) | 4 (1.1) |
| Hemoglobin, g/L (range) | 85 (27-167) | 84 (27-159) | 88 (36-149) |
| Platelet count, × 109/L (range) | 27 (2-194) | 27 (2-187) | 30 (2-194) |
| Sanz risk, no. (%) | |||
| Low | 191 (22.3) | 91 (23.8) | 85 (22.8) |
| Intermediate | 397 (46.4) | 171 (44.8) | 173 (46.4) |
| High | 267 (31.2) | 120 (31.4) | 115 (30.8) |
| ECOG score, no. (%) | |||
| 0–2 | 721 (84.3) | 335 (87.7) | 317 (85.0) |
| 3–4 | 134 (15.7) | 47 (12.3) | 56 (15.0) |
| Blasts in bone marrow, no. (%) | 85 (21-98) | 85 (24-98) | 85 (21-97) |
A total of 855 APL patients were enrolled in the trial. After achieving complete remission, 382 and 373 patients were randomly assigned to ATO and non-ATO group, respectively.
Induction Therapy.
Among the 855 patients, 8 were not evaluable because they were lost to follow-up without any information on response to remission induction. Among the 847 evaluable patients, 34 (4.0%) died during induction therapy: 20 from severe hemorrhage (17 from central nervous system [CNS] hemorrhage and three from pneumorrhagia), 8 from severe infection, 3 from cerebral infarction, 2 from differentiation syndrome (DS) accompanied with pulmonary infection, and 1 from unknown cause. The rates of early death were 0.5% (1/189), 4.5% (18/396), and 5.7% (15/262) in low-, intermediate-, and high-risk groups, respectively (P = 0.016). Hematological CR was achieved in all of the remaining 813 (96.0%) evaluable cases and the median time to CR was 42 d (range, 21 to 79 d). As such, CR rates were 99.5% (188/189) in the low-risk, 95.5% (378/396) in the intermediate-risk, and 94.3% (247/262) in the high-risk group, respectively, with median time of 41 d (range, 22 to 73 d), 43 d (range, 21 to 71 d) and 42 d (range, 21 to 79 d) to CR in the three risk groups, respectively. Notably, during induction therapy, 15 low-risk patients received chemotherapy due to significant leukocytosis. In contrast, 22 intermediate-risk patients with leukocytosis and 1 high-risk patient did not receive chemotherapy owing to the agile management by researchers. Another high-risk patient was prevented from chemotherapy because of severe cardiac dysfunction. Among all these 39 patients, 2 died during induction phase, 1 withdrew before randomization, 1 was lost for follow-up after randomization, and the remaining 35 entered into randomization and consolidation therapy.
Randomization and Consolidation Therapy.
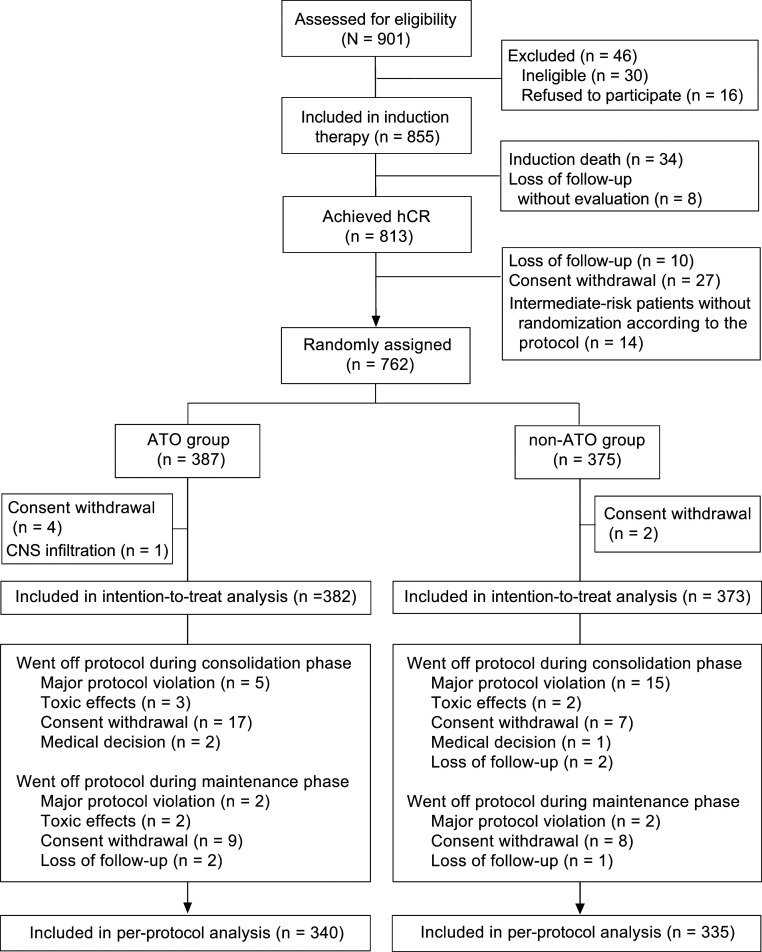
Of the 813 patients who achieved CR, 10 were lost for follow-up, 27 withdrew consent before randomization; 14 intermediate-risk patients whose WBC count was consistently below 10 × 109/L during the induction therapy were not randomized into the ATO or non-ATO group according to the protocol (excluded from intention-to-treat [ITT] analysis) but received the same consolidation therapy as the ATO group. Therefore, 762 patients were randomly assigned to the ATO group (n = 387) or non-ATO group (n = 375). Four patients in the ATO group and two in the non-ATO group withdrew after randomization, and one in the ATO group was diagnosed with CNS infiltration before consolidation therapy and received salvage therapy instead of the assigned treatment. As a result, the ITT analysis included a total of 755 randomized patients (382 in the ATO group vs. 373 in the non-ATO group) who complied with at least one course of consolidation therapy. The baseline characteristics of patients in both arms are also provided in Table 1, and the randomization and disposition of patients are illustrated in Fig. 1.
Fig. 1.
Enrollment, randomization, and disposition of patients. hCR, hematological complete remission.
During the consolidation therapy, 54 cases went off protocol because of major protocol violation (n = 20), toxic intolerance (n = 5), consent withdrawal (n = 24), medical decision (n = 3), and loss of follow-up (n = 2). However, out of these patients, 31 underwent molecular analysis of bone marrow samples and then entered into maintenance therapy and the ITT analysis.
Maintenance Therapy.
At the end of consolidation therapy, one patient died from hepatic failure of unknown reason without molecular analysis, so there were 731 patients tested for PML-RARA. Two patients in the non-ATO group relapsed at the end of consolidation therapy (one high-risk patient with hematological relapse and one low-risk case with molecular relapse) and three patients had persistently positive PML-RARA transcripts. Thus, 726 (99.3%) of 731 patients achieved molecular complete remission (mCR) and were continuously treated with maintenance therapy; 26 patients went off the maintenance protocol (Fig. 1). At the last follow-up, 675 patients adhered to the protocol (per-protocol [PP] population).
Events of Resistance, Relapse, and Death in CR.
Among the three cases with persistently positive PML-RARA transcripts, one intermediate-risk patient was initially allocated to the ATO group and was crossed over to the non-ATO group to receive three more courses of consolidation therapy of high-risk, followed by autologous hematopoietic stem cell transplantation (HSCT) and subsequent mCR. The other two were both high-risk patients in the non-ATO group. One patient achieved mCR after salvage therapy, while the other died of CNS relapse soon.
A total of 30 patients relapsed after CR, 8 in the ATO group vs. 22 in the non-ATO group. In terms of risk categories, 5 belonged to low-, 8 to intermediate-, and 17 to high-risk groups, respectively. As for the types of relapse, 19 patients had a single type of relapse, with molecular relapse in 5, hematological relapse in 11, and CNS relapse in 3 (including the case who failed to achieve mCR and progressed to CNS relapse as mentioned above) cases, respectively. The remaining 11 patients had multiple types of relapse, including six molecular plus CNS relapses, and five hematological plus CNS relapses. Of note, among 14 patients with CNS relapse, 2 cases were at low risk, 3 at intermediate risk, and 9 at high risk. The relapses occurred 4.4 to 49.0 (median 13.7) mo after achieving CR. 29 (96.7%) out of the 30 patients relapsed within 36 mo after CR. Twenty-seven patients received salvage therapy including ATRA, ATO, and chemotherapy in all cases and HSCT (four autologous and three allogeneic) in seven cases; the other three died before any treatment could be given. Among the 27 receiving salvage therapy, 8 died due to disease progression, 2 were lost for follow-up, and the remaining 17 were alive in mCR by the last follow-up.
Furthermore, the disease in three patients (one high-risk in the non-ATO group and two intermediate-risk in the ATO group) was transformed to secondary acute myeloid leukemia, and all of them died of disease progression. Of note, one intermediate-risk patient did not receive anthracycline during induction therapy although leukocytes increased more than 10 × 109/L. Another three patients suffered from secondary tumor, two cases of gastric cancer and one lung cancer, but only one with gastric cancer survived till the last follow-up and the other two eventually died. Except for the above five cases of death and one case of death from liver failure as previously mentioned there were two additional deaths in CR, one ascribed to cerebral hemorrhage and the other suicide due to depression. The data of all the eight patients who died in CR from causes unrelated to APL are listed in SI Appendix, Table S1.
Primary End Point.
The last follow-up was performed on 29 February 2020 and the median follow-up time from CR was 54.9 mo (range, 0.7 to 84.9 mo). The noninferiority analysis was carried out in 755 patients (382 in the ATO group vs. 373 in the non-ATO group). Because 658 out of these 755 cases (87.2%) could be evaluated at 3 y, which had reached the expected sample size of 598, the data of the study could be analyzed with sufficient statistical stringency. Among the 658 cases, 96.1% (319 of 332) of the patients in the ATO group remained disease-free at 3 y, compared to 92.6% (302 of 326) in the non-ATO group. For the remaining 97 patients, 83 were censored for disease-free survival (DFS) before 3 y, and 14 were lost for follow-up within 3 y. The percentage difference in the DFS rate at 3 y between the two groups was thus 3.45% (95% CI –0.07 to 6.97). The lower limit of the 95% CI was greater than the –5% noninferiority margin, confirming thus noninferiority of the ATO group (P < 0.001). In addition, sensitivity analysis also supported this conclusion (SI Appendix, Table S2).
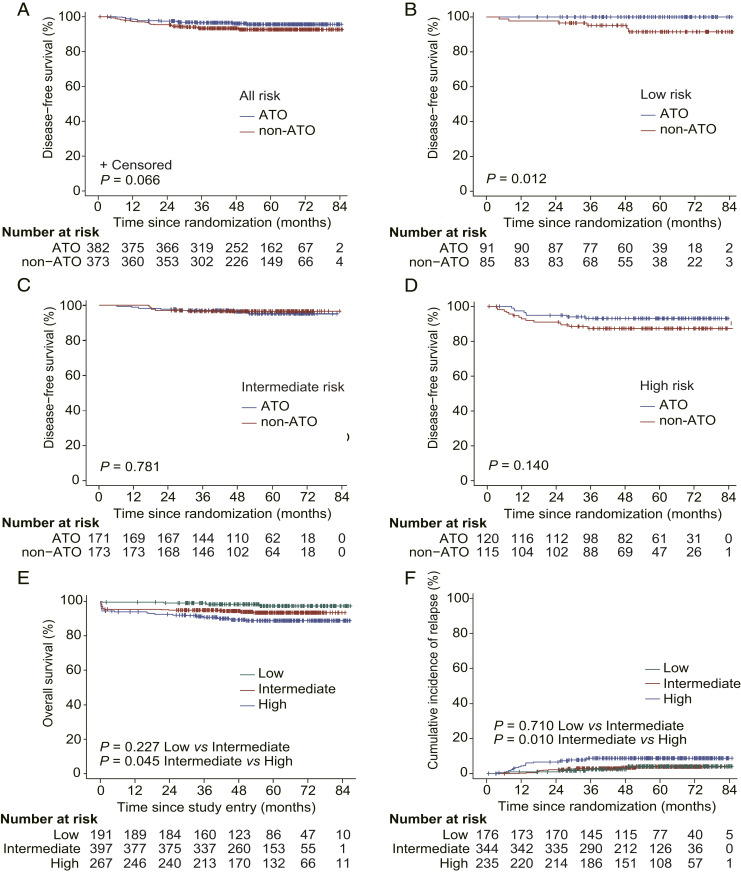
Using the Kaplan–Meier method, all outcome estimates calculated by ITT analysis are listed in Table 2, and outcome curves are shown in Fig. 2. The estimated 7-y DFS rates (when treated as a time-to-event variable) in ATO and non-ATO groups were 95.7% (95% CI 93.6 to 97.9) and 92.6% (95% CI 89.8 to 95.4, P = 0.066), respectively. In low-risk patients, DFS was significantly different between ATO and non-ATO groups (100% vs. 91.6% [95% CI 85.2 to 98.4], P = 0.012). Considering the four times of comparisons for DFS, namely the whole cohort, low-, intermediate- and high-risk subgroups, the adjusted P value should be equal to 0.048 (0.012 multiplied by 4), still less than the 5% significance level. However, there was no significant difference between the two groups in intermediate-risk (95.2% [95% CI 91.7 to 98.8] vs. 96.5% [95% CI 93.8 to 99.3], P = 0.781) and high-risk patients (93.2% [95% CI 88.7 to 97.9] vs. 87.4% [95% CI 81.5 to 93.8], P = 0.140). As for low- and intermediate-risk combined cohort, DFS also showed no significant difference between ATO and non-ATO groups (96.9% [95% CI 94.5 to 99.2] vs. 94.8% [95% CI 91.9 to 97.8], P = 0.200; SI Appendix, Table S3).
Table 2.
Clinical outcomes
| Total (n = 755) | ATO group (n = 382) | Non-ATO group (n = 373) | HR (95% CI) | P value | |
| probability, % (95% CI) | probability, % (95% CI) | probability, % (95% CI) | |||
| 3-y DFS | |||||
| Low risk | 97.6 (95.3–100) | 100 | 95.1 (90.5–99.9) | NA | 0.012 |
| Intermediate risk | 96.8 (94.9–98.7) | 97.1 (94.6–99.6) | 96.5 (93.8–99.3) | 1.17 (0.39–3.47) | 0.781 |
| High risk | 90.4 (86.6–94.3) | 93.2 (88.7–97.9) | 87.4 (81.5–93.8) | 0.52 (0.22–1.24) | 0.14 |
| Total | 95.0 (93.4–96.6) | 96.5 (94.7–98.4) | 93.4 (90.9–96.0) | 0.55 (0.29–1.04) | 0.066 |
| 7-y DFS | |||||
| Low risk | 95.9 (92.7–99.2) | 100 | 91.6 (85.2–98.4) | NA | 0.012 |
| Intermediate risk | 95.8 (93.6–98.1) | 95.2 (91.7–98.8) | 96.5 (93.8–99.3) | 1.17 (0.39–3.47) | 0.781 |
| High risk | 90.4 (86.6–94.3) | 93.2 (88.7–97.9) | 87.4 (81.5–93.8) | 0.52 (0.22–1.24) | 0.14 |
| Total | 94.2 (92.4–95.9) | 95.7 (93.6–97.9) | 92.6 (89.8–95.4) | 0.55 (0.29–1.04) | 0.066 |
| 7-y OS | |||||
| Low risk | 98.4 (96.1–100) | 100 | 96.7 (92.2–100) | NA | 0.150 |
| Intermediate risk | 97.8 (96.0–99.6) | 96.9 (93.8–100) | 98.7 (97.0–100) | 1.96 (0.36–10.69) | 0.438 |
| High risk | 94.9 (91.9–97.9) | 94.5 (90.3–98.9) | 95.3 (91.3–99.4) | 1.11 (0.34–3.64) | 0.864 |
| Total | 97.0 (95.7–98.4) | 96.9 (94.9–98.8) | 97.2 (95.4–99.1) | 1.07 (0.44–2.63) | 0.883 |
| 7-y CIR | |||||
| Low risk | 2.9 (1.3–6.7) | 0 | 6.6 (2.6–16.9) | NA | <0.001 |
| Intermediate risk | 2.4 (1.2–4.9) | 1.2 (0.3–4.5) | 3.5 (1.7–7.1) | 0.34 (0.07–1.68) | 0.186 |
| High risk | 7.6 (4.9–11.7) | 5.1 (2.5–10.4) | 9.9 (6.1–16.1) | 0.50 (0.19–1.35) | 0.173 |
| Total | 4.1 (2.8–6.1) | 2.2 (1.1–4.2) | 6.1 (3.9–9.5) | 0.35 (0.16–0.79) | 0.011 |
The Kaplan–Meier method was used to estimate the survival above. Treatment protocol of ATO group (Low risk: ATRA+ATO, Intermediate risk: ATRA+ATO, High risk: ATRA+ATO+IDA/DNR). Treatment protocol of non-ATO group (Low risk: ATRA+IDA/DNR, Intermediate risk: ATRA+IDA/DNR, High risk: ATRA+IDA/DNR+Ara-C). NA, not applicable.
Fig. 2.
Kaplan–Meier plot of clinical outcomes. Disease-free survival curves of patients in ATO or non-ATO groups (A) with low-, intermediate-, and high-risk disease, respectively (B–D). Overall survival of 855 patients enrolled (E) and cumulative incidence of relapse (F) with low-, intermediate-, and high-risk disease. Treatment protocol of ATO group (Low risk: ATRA+ATO, Intermediate risk: ATRA+ATO, High risk: ATRA+ATO+IDA/DNR). Treatment protocol of non-ATO group (Low risk: ATRA+IDA/DNR, Intermediate risk: ATRA+IDA/DNR, High risk: ATRA+IDA/DNR+Ara-C). IDA, idarubicin; DNR, daunorubicin; Ara-C, cytarabine.
Secondary End Points.
The estimated 7-y overall survival (OS) rates for 855 patients enrolled with low-, intermediate-, and high-risk APL were 97.4% (95% CI 94.8 to 100), 93.5% (95% CI 91.0 to 96.1), and 88.9% (95% CI 85.0 to 92.9), respectively (P = 0.227 for low- vs. intermediate-risk and P = 0.045 for intermediate- vs. high-risk; Fig. 2E). However, between ATO and non-ATO groups, there was no difference at each risk. The estimated 7-y cumulative incidence of relapse (CIR) rates were 2.9% (95% CI 1.3 to 6.7), 2.4% (95% CI 1.2 to 4.9), and 7.6% (95% CI 4.9 to 11.7) in low-, intermediate-, and high-risk groups, respectively (P = 0.710 for low- vs. intermediate-risk and P = 0.010 for intermediate- vs. high-risk; Table 2 and Fig. 2F). Remarkably, the 7-y CIR rate was 2.2% (95% CI 1.1 to 4.2) in the ATO group, which was significantly lower than that of the non-ATO group (6.1% [95% CI 3.9 to 9.5], P = 0.011; Table 2 and SI Appendix, Fig. S1). When the 7-y CIRs of the chemotherapy-replacing (low-/intermediate-risk) and chemotherapy-reducing (high-risk) patients were separately analyzed it could be seen that a statistically significantly reduced CIR was seen in the ATO group compared to the non-ATO group only in the former setting but not in the latter one (SI Appendix, Table S3).
Additional Analyses.
Likewise, among 675 patients in the PP population adhered to the protocol, 593 (87.9%) could be evaluated at 36 mo for the primary analysis. In the ATO group, 96.6% (286 of 296) of the patients remained disease-free at 3 y, compared to 92.9% (276 of 297) in the non-ATO group. The between-group percentage difference of 3-y DFS was 3.69% (95% CI 0.12 to 7.26). The lower limit of the 95% CI was also greater than the –5% noninferiority margin, in support of the noninferiority of the ATO group (P < 0.001). Sensitivity analysis of PP population also provided evidence for the noninferiority result (SI Appendix, Table S4). The time-to-event survival data of PP population are listed in SI Appendix, Table S5.
Adverse Events during Consolidation Therapy.
The incidences of grade 3 to 4 neutropenia were generally lower in the ATO group, especially among low- to intermediate-risk patients, which were 20.0 to 36.6% in the ATO group vs. 86.8 to 92.9% in the non-ATO group (P < 0.0001 in each course of consolidation therapy). Grade 3 to 4 thrombocytopenia among low- and intermediate-risk patients was noticed in 0 to 3.8% and 49.4 to 65.8% of ATO and non-ATO groups, respectively, whereas in high-risk patients it was 6.3 to 60.4% vs. 94.3 to 98.9% (P < 0.0001 in each course). Grade 3 to 4 infection and febrile neutropenia were also significantly more frequent in each non-ATO group (Table 3).
Table 3.
Incidence of grade 3 to 4 hematological toxic effects during consolidation treatment
| First consolidation | Second consolidation | Third consolidation | ||||||||
| ATO group | Non-ATO group | P value | ATO group | Non-ATO group | P value | ATO group | Non-ATO group | P value | ||
| Risk | no. (%) | no. (%) | no. (%) | no. (%) | no. (%) | no. (%) | ||||
| Neutropenia | ||||||||||
| Low risk | 28/78 (35.9) | 35/38 (92.1) | <0.0001 | 15/75 (20.0) | 33/38 (86.8) | <0.0001 | NA | NA | NA | |
| Intermediate risk | 53/145 (36.6) | 79/85 (92.9) | <0.0001 | 34/132 (25.8) | 70/78 (89.7) | <0.0001 | 27/112 (24.1) | NA | NA | |
| High risk | 106/111 (95.5) | 90/90 (100) | 0.113 | 99/106 (93.4) | 91/91 (100) | 0.035 | 28/95 (29.5) | 84/88 (95.5) | <0.0001 | |
| Thrombocytopenia | ||||||||||
| Low risk | 0/78 | 22/38 (57.9) | <0.0001 | 1/75 (1.3) | 25/38 (65.8) | <0.0001 | NA | NA | NA | |
| Intermediate risk | 1/145 (0.7) | 42/85 (49.4) | <0.0001 | 5/132 (3.8) | 51/78 (65.4) | <0.0001 | 3/112 (2.7) | NA | NA | |
| High risk | 48/111 (43.2) | 89/90 (98.9) | <0.0001 | 64/106 (60.4) | 88/91 (96.7) | <0.0001 | 6/95 (6.3) | 83/88 (94.3) | <0.0001 | |
| Infection | ||||||||||
| Low risk | 11/78 (14.1) | 12/39 (30.8) | 0.032 | 4/76 (5.3) | 12/39 (30.8) | <0.0001 | NA | NA | NA | |
| Intermediate risk | 15/145 (10.3) | 23/87 (26.4) | 0.001 | 14/133 (10.5) | 18/78 (23.1) | 0.014 | 5/112 (4.5) | NA | NA | |
| High risk | 25/110 (22.7) | 43/90 (47.8) | <0.0001 | 29/106 (27.4) | 42/93 (45.2) | 0.009 | 5/95 (5.3) | 35/88 (39.8) | <0.0001 | |
| Febrile neutropenia | ||||||||||
| Low risk | 3/78 (3.8) | 9/37 (24.3) | 0.002 | 1/75 (1.3) | 15/36 (41.7) | <0.0001 | NA | NA | NA | |
| Intermediate risk | 6/145 (4.1) | 23/86 (26.7) | <0.0001 | 7/133 (5.3) | 23/78 (29.5) | <0.0001 | 6/112 (5.4) | NA | NA | |
| High risk | 21/109 (19.3) | 52/89 (58.4) | <0.0001 | 24/106 (22.6) | 39/91 (42.9) | 0.002 | 3/95 (3.2) | 50/89 (56.2) | <0.0001 | |
Treatment protocol of ATO group (Low risk: ATRA+ATO, Intermediate risk: ATRA+ATO, High risk: ATRA+ATO+IDA/DNR). Treatment protocol of non-ATO group (Low risk: ATRA+IDA/DNR, Intermediate risk: ATRA+IDA/DNR, High risk: ATRA+IDA/DNR+Ara-C). The denominators differ for the various toxic effects because of variations in the total numbers of patients returning data for each effect. NA, not applicable.
Most cases of hepatic toxicity (as measured by elevated alanine aminotransferase [ALT] or aspartate aminotransferase [AST]) were in grade 1 to 2, with more frequent incidence in the ATO group (26.1%) than in the non-ATO group (17.9%, P < 0.0001); only seven cases of grade 3 to 4 hepatic toxicity occurred (0.3% vs. 0.5%, P = 0.861) during the entire consolidation phase. In all cases, hepatic adverse effects were resolved with temporary discontinuation of ATO or chemotherapy. Meanwhile, the incidence of prolonged QT corrected (QTc) intervals was significantly higher in the ATO group (4.0%) than in the non-ATO group (0.4%) across the whole consolidation therapy (P < 0.0001). Most cases were in grade 1 to 2 and only two cases in ATO group were in grade 3, with no life-threatening cardiac arrhythmia observed. The incidences of kidney dysfunction, hyperlipidemia, and rash were not significantly different between the two groups in each risk category (Table 4).
Table 4.
Incidence of nonhematological toxic effects during consolidation treatment
| First consolidation | Second consolidation | Third consolidation | |||||||
| ATO group | Non-ATO group | P value | ATO group | Non-ATO group | P value | ATO group | Non-ATO group | P value | |
| Risk | no. (%) | no. (%) | no. (%) | no. (%) | no. (%) | no. (%) | |||
| Raised liver ALT or AST (grade 1–2) | |||||||||
| Low risk | 30/84 (35.7) | 13/77 (16.9) | 0.007 | 12/78 (15.4) | 15/77 (19.5) | 0.501 | NA | NA | NA |
| Intermediate risk | 47/157 (29.9) | 30/157 (19.1) | 0.026 | 27/136 (19.9) | 17/151 (11.3) | 0.044 | 14/114 (12.3) | NA | NA |
| High risk | 43/114 (37.7) | 26/112 (23.2) | 0.018 | 37/113 (32.7) | 22/108 (20.4) | 0.038 | 24/101 (23.8) | 18/104 (17.3) | 0.252 |
| Raised liver ALT or AST (grade 3–4) | |||||||||
| Low risk | 0/84 | 0/77 | NA | 1/78 (1.3) | 0/77 | 1.000 | NA | NA | NA |
| Intermediate risk | 0/157 | 1/157 (0.6) | 0.500 | 0/136 | 0/151 | NA | 1/114 (0.9) | NA | NA |
| High risk | 1/114 (0.9) | 1/112 (0.9) | 1.000 | 0/113 | 1/108 (0.9) | 0.489 | 0/101 | 1/104 (1.0) | 1.000 |
| Hypercholesterolemia (grade 1–2) | |||||||||
| Low risk | 15/60 (25.0) | 8/54 (14.8) | 0.176 | 10/56 (17.9) | 8/54 (14.8) | 0.666 | NA | NA | NA |
| Intermediate risk | 23/123 (18.7) | 16/109 (14.7) | 0.414 | 20/99 (20.2) | 16/94 (17.0) | 0.571 | 16/87 (18.4) | NA | NA |
| High risk | 13/77 (16.9) | 5/79 (6.3) | 0.039 | 14/79 (17.7) | 10/79 (12.7) | 0.375 | 14/70 (20.0) | 18/79 (22.8) | 0.68 |
| Hypercholesterolemia (grade 3–4) | |||||||||
| Low risk | 0/60 | 3/54 (5.6) | 0.206 | 0/56 | 0/54 | NA | NA | NA | NA |
| Intermediate risk | 2/123 (1.6) | 0/109 | 0.500 | 2/99 (2.0) | 0/94 | 0.498 | 1/87 (1.1) | NA | NA |
| High risk | 1/77 (1.3) | 0/79 | 0.494 | 0/79 | 1/79 (1.3) | 1.000 | 1/70 (1.4) | 1/79 (1.3) | 1.000 |
| Hypertriglyceridemia (grade 1–2) | |||||||||
| Low risk | 32/58 (55.2) | 24/54 (44.4) | 0.257 | 29/54 (53.7) | 26/55 (47.3) | 0.502 | NA | NA | NA |
| Intermediate risk | 58/114 (50.9) | 49/102 (48.0) | 0.677 | 45/89 (50.6) | 50/89 (56.2) | 0.453 | 40/81 (49.4) | NA | NA |
| High risk | 37/71 (52.1) | 38/75 (50.7) | 0.861 | 38/76 (50.0) | 30/76 (39.5) | 0.192 | 37/65 (56.9) | 31/76 (40.8) | 0.056 |
| Hypertriglyceridemia (grade 3–4) | |||||||||
| Low risk | 0/58 | 5/54 (9.3) | 0.056 | 3/54 (5.6) | 3/55 (5.5) | 1.000 | NA | NA | NA |
| Intermediate risk | 10/114 (8.8) | 8/102 (7.8) | 0.805 | 4/89 (4.5) | 4/89 (4.5) | 1.000 | 4/81 (4.9) | NA | NA |
| High risk | 3/71 (4.2) | 2/75 (2.7) | 0.95 | 3/76 (3.9) | 5/76 (6.6) | 0.716 | 4/65 (6.2) | 3/76 (3.9) | 0.832 |
| Prolonged QTc interval (all grades) | |||||||||
| Low risk | 2/72 (2.8) | 0/66 | 0.497 | 2/66 (3.0) | 1/65 (1.5) | 1.000 | NA | NA | NA |
| Intermediate risk | 9/150 (6.0) | 1/135 (0.7) | 0.037 | 6/130 (4.6) | 0/122 | 0.047 | 2/108 (1.9) | NA | NA |
| High risk | 3/99 (3.0) | 1/101 (1.0) | 0.599 | 7/103 (6.8) | 0/100 | 0.023 | 2/95 (2.1) | 0/95 | 0.477 |
| Rash (all grades) | |||||||||
| Low risk | 2/83 (2.4) | 0/79 | 0.497 | 4/78 (5.1) | 0/79 | 0.125 | NA | NA | NA |
| Intermediate risk | 4/159 (2.5) | 2/158 (1.3) | 0.686 | 2/137 (1.5) | 2/153 (1.3) | 1.000 | 2/118 (1.7) | NA | NA |
| High risk | 4/116 (3.4) | 2/114 (1.8) | 0.695 | 5/113 (4.4) | 6/110 (5.5) | 0.723 | 1/99 (1.0) | 8/104 (7.7) | 0.049 |
| Raised creatinine (all grades) | |||||||||
| Low risk | 0/83 | 0/78 | NA | 0/78 | 0/77 | NA | NA | NA | NA |
| Intermediate risk | 1/157 (0.6) | 1/158 (0.6) | 1.000 | 0/136 | 0/152 | NA | 1/113 (0.9) | NA | NA |
| High risk | 2/115 (1.7) | 0/113 | 0.498 | 0/113 | 1/109 (0.9) | 0.491 | 0/100 | 1/104 (1.0) | 1.000 |
Treatment protocol of ATO group (Low risk: ATRA + ATO, Intermediate risk: ATRA + ATO, High risk: ATRA + ATO + IDA/DNR). Treatment protocol of non-ATO group (Low risk: ATRA + IDA/DNR, Intermediate risk: ATRA + IDA/DNR, High risk: ATRA+IDA/DNR+Ara-C). The denominators differ for the various toxic effects because of variations in the total numbers of patients returning data for each effect. NA, not applicable.
Discussion
In this large-scale clinical trial including 855 APL patients, we found that ATRA-ATO–based consolidation therapy was not inferior to ATRA–chemotherapy in terms of long-term survival, and thus the chemotherapy-free and cytarabine-free strategies could be used for patients in low-/intermediate-risk and high-risk groups, respectively.
The extraordinary effect of ATRA-ATO targeting APL oncoprotein PML-RARA and its clinical result has aroused great interest among hematologists (2, 21, 22). The APL0406 trial pioneered by an Italian–German group demonstrated the advantages of ATRA-ATO therapy over ATRA–chemotherapy at both induction and consolidation phases in low- to intermediate-risk APL patients (23, 24). We actually launched a similar initiative after achieving a 90% long-term DFS rate with an ATRA-ATO–chemotherapy triad protocol in pilot investigations (11, 13, 25). In the design of APL2012, we incorporated the available therapeutic response and stratification data (20) and decided to lay the emphasis on the effects of ATO at the consolidation phase. Like the APL0406 trial, patients of both low- and intermediate-risk categories in our study have benefited from the therapeutic effects of ATO in replacement of anthracyclines in the consolidation phase. However, different from the ATRA–chemotherapy arm of APL0406, ATO was used in the induction and maintenance therapy for our patients in the non-ATO group. It was thus not surprising to see that the outcome of our non-ATO group was also quite satisfactory, with a 7-y DFS rate of 94.8% (SI Appendix, Fig. S2 and Table S3), which was significantly higher than 72-mo DFS of the ATRA–chemotherapy group (77.4%) in APL0406 (26). Our study confirmed that chemotherapy-free or “replacing” strategy was feasible among low- and intermediate-risk patients after induction therapy.
However, high-risk patients still bear unsatisfactory prognosis with both early death and relapse representing the treatment bottleneck. Even though researchers from the 22 participating hospitals in our trial all had a wealth of experience in diagnosing and treating APL patients, the rate of early death was 4.0% for the whole cohort, similar to the 30-d mortality rate of 4% and 6% in ATRA-ATO and ATRA–chemotherapy arms in the UK AML17 trial, respectively (27). The main causes of early death included severe hemorrhage, severe infection, cerebral infarction, and DS. Hence, early identification of these factors and quick therapeutic intervention are required. With regard to the curative therapy for high-risk patients, there were controversies on the benefit of mid- or high-dose cytarabine in reducing relapse when ATRA was introduced into APL treatment (1, 3, 4, 6). On the contrary, the advantages of ATO in improving long-term survival with tolerable side effects have been well proved by several clinical trials (18, 28, 29), inspiring us to replace cytarabine with ATO in high-risk patients. Our results showed that long-term DFS and CIR in the chemotherapy “reducing” ATO group were comparable to those in the non-ATO group which contained cytarabine, while the incidence of grade 3 to 4 hematological adverse effects was significantly reduced, indicating that the replacement of cytarabine with ATO was a better choice for high-risk patients. We noticed that in the UK AML17 study high-risk patients in the ATO group received neither cytarabine nor anthracyclines after induction therapy, and they could still achieve quite satisfactory results, with no relapse in 30 high-risk patients in the ATO group (27). This result suggested that postinduction chemotherapy-free treatment might also be applicable in high-risk patients, though larger sample size and longer follow-up are desired. Of interest, a prospective clinical trial (APOLLO trial) has been launched in European countries to verify whether the chemotherapy-free strategy can be extended to high-risk patients after chemotherapy-containing remission induction. Indeed, the APL2012 trial further indicated the importance of ATO in reducing relapse in APL and confirmed the feasibility of a chemotherapy “reducing” approach at consolidation in high-risk patients, thus supporting a further step toward the chemotherapy-free strategy, which may eventually become the new standard of care for most high-risk patients in the future.
In addition to the therapeutic benefits, our study also confirmed advantages of the ATO group over the non-ATO group in significantly reducing hematological side-effect profiles. As for the common nonhematological toxicities of ATO, hepatic toxicity and QTc prolongation were more frequently observed in the ATO group compared with the non-ATO group, but these adverse effects in most cases were in grade 1 to 2 and controllable. Hence, our results may further enrich the treatment strategy consensus of the international APL clinical research community (30). Regarding the long-term toxicity of ATO, our previous studies including an 83-mo follow-up in patients with ATRA-ATO treatment (10–12) revealed no major organ damage except for low-degree hepatic steatosis. In APL2012, the total dosage of ATO used in the chemotherapy “reducing” high-risk cases was slightly higher than that in our initial trial (10) (see Patients and Methods for details), and long-term chronic adverse effects will be reported later once the 5-y follow-up of the last enrolled patients is reached.
There are some limitations in the present work. The study was started in 2012 and lasted for a long time to execute enrollment and investigate the primary end point. The result for low-/intermediate-risk patients is mostly confirmatory today. As for high-risk patients, we hereby recommend a chemotherapy-deescalating strategy by replacing cytarabine with ATO, while anthracycline was reserved. Whether it would be superior or inferior to a completely chemotherapy-free postinduction strategy for most high-risk patients, as was reported in the UK AML17 trial, requires further exploration.
In conclusion, the APL2012 trial provides a strong support for using the ATO group regimen in this study as a main component through all phases of APL treatment.
Patients and Methods
Study Design and Enrollment.
This is a prospective, randomized, open-label, multicenter, noninferiority pragmatic trial for patients with newly diagnosed APL. Eligible patients between 18 and 65 y of age were enrolled from 22 hospitals in China (SI Appendix). Initial screening was based on the morphological diagnosis, and the confirmation of PML-RARA fusion gene by RT-PCR or RQ-PCR (10), or t(15;17) chromosomal translocation by karyotype analysis or fluorescence in situ hybridization, was crucial for subsequent enrollment.
Other inclusion criteria were an Eastern Cooperative Oncology Group (ECOG) performance status score of 0 to 4, an ALT and AST level below two times the normal upper limit, a bilirubin level of 35 μmol/L or lower, a creatinine level of 150μmol/L or lower, and a normal cardiac function. Exclusion criteria were CNS infiltration, severe heart disease (acute myocardial infarction or heart failure), concurrent active malignancy, tuberculosis or HIV infection, QTc interval over 450 ms, contraindication or allergy to anthracyclines or other agents in the protocol, drug addiction or mental disorders, and pregnant or breastfeeding women. Written informed consent was signed by each participant before study entry. This study was approved by the Ethics Committee of Ruijin Hospital in Shanghai, China. The trial was conducted in accordance with the Declaration of Helsinki.
Treatment Protocol and Randomization.
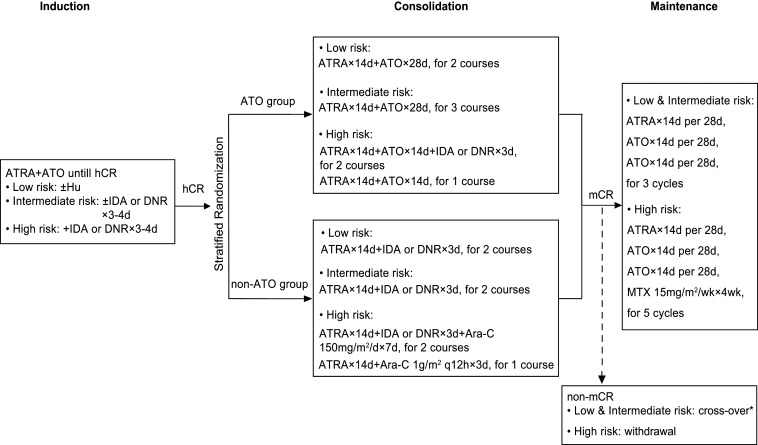
Patients all received ATRA-ATO–based induction therapy. Anthracycline was added to all the patients of high risk (a WBC count at diagnosis >10 × 109/L) and those with intermediate-risk disease but experiencing leukocytosis (a WBC count ≥10 × 109/L) during induction. Chemotherapy was not involved in low-risk patients, although hydroxyurea (Hu) was applied to manage leukocytosis (Fig. 3).
Fig. 3.
Treatment protocol and patients’ allocation. ATRA 25 mg⋅m−2⋅d−1; ATO 0.16 mg⋅kg−1⋅d−1; IDA 8 mg⋅m−2⋅d−1; DNR 45 mg⋅m−2⋅d−1. *For low- and intermediate-risk patients who did not achieve mCR after consolidation therapy at two successive times of detection within 1 mo apart, they would be enrolled cross-over in the other group to receive three more courses of high-risk consolidation therapy. IDA, idarubicin; DNR, daunorubicin; Ara-C, cytarabine; hCR, hematological complete remission.
Upon achieving CR, all patients were randomly assigned (1:1) to the ATO group or non-ATO group for consolidation therapy. In the ATO group, low- and intermediate-risk patients received two and three courses of ATRA plus ATO, respectively, while patients with high-risk disease received two courses of ATRA plus ATO and anthracycline and one course of ATRA plus ATO. In the non-ATO group, low- and intermediate-risk patients both received two courses of ATRA plus anthracycline, while patients of high-risk APL were treated with two courses of ATRA plus anthracycline and cytarabine and one course of ATRA plus middose cytarabine.
Patients with mCR after consolidation therapy entered into maintenance therapy. Low- and intermediate-risk patients received three cycles of ATRA and ATO sequential treatment, while those of high-risk received five cycles of ATRA, ATO, and methotrexate (MTX) treatment. Thus, the total days of ATO infusion for low-, intermediate-, and high-risk patients in the two arms were ∼168 vs. 112, 196 vs. 112, and 210 vs. 168 d. Compared to our previous trials with a total of 180 d (10), in the current study the total days and thus the total doses of ATO groups in intermediate-risk and high-risk cases were 8% and 17% higher. For those who did not achieve mCR after consolidation therapy at two successive times of detection within 1 mo apart, low- and intermediate-risk patients would be enrolled cross-over in the other group to receive three more courses of high-risk consolidation therapy, while high-risk ones withdrew and received salvage therapy. After that, patients who were still positive for PML-RARA would be withdrawn from the study and subject to salvage treatment.
ATRA 25 mg/m2 per d (10) was given in an oral divided dose till CR, and at the same dose for 14 d in each consolidation and maintenance course. ATO was given intravenously at a dose of 0.16 mg/kg (10 mg maximum) per day till CR, and then at the same dose for 28 d in each subsequent course, except that patients at high-risk ATO were given for 14 d in each consolidation course. Prophylaxis of CNS leukemia (cytarabine 50 mg + dexamethasone 5 mg ± MTX 10 mg intrathecal injection) was given in a total of three times to patients at low risk after achieving CR and six times to those at intermediate and high risks. Guidelines for the prevention and management of coagulopathy, leukocytosis, hematological and nonhematological toxicities, and follow-up assessments were predefined in the protocol. Intravenous dexamethasone was administered at a dose of 5 to 10 mg/d to patients with slight to moderate DS. In the presence of severe DS, ATRA and ATO were temporarily discontinued and intravenous dexamethasone was administered up to 20 mg/d until WBC count went below 10 × 109/L and the disappearance of signs and symptoms for a minimum of 3 d.
Randomization and Masking.
Randomization was supported by Shanghai Clinical Research Center. A stratified block randomization, according to Sanz risk, was performed by a trial statistician for each participating hospital. The information of randomization was put into opaque sealed envelopes and distributed to each center. Once a patient had achieved hematological CR after induction therapy, the investigator would open the corresponding random envelope of the patient and allocate the patient into the ATO group or non-ATO group accordingly. Intermediate-risk patients whose WBC count was consistently below 10 × 109/L during the whole induction therapy were assigned to the ATO group without randomization. Patients and study staff were not masked to treatments, but the data analysis and assessment of outcomes were performed in a masked manner. No pharmaceutical company was involved in the design, data collection or analysis of the trial, or the drafting of the manuscript.
Study End Points and Definitions.
The primary end point was DFS at 3 y. Secondary end points included CR after induction therapy, mCR after consolidation therapy, early death, OS, CIR, hematological and nonhematological toxicities. CR, OS, and relapse (including hematological, extramedullary, and molecular relapse) were defined according to the criteria recommend by the international working group (31). DFS was defined as the time from randomization to any relapse, or persistent positivity of PML-RARA after consolidation therapy, or any secondary malignancies, or death of any reason. CIR was defined as the cumulative incidence from CR to any relapse, considering death in CR as a competing risk. mCR was defined as the absence of detectable PML-RARA transcripts by nested RT-PCR or RQ-PCR in two successive bone marrow samples. Early death referred to death in the induction phase from the entry into the treatment. Hematological and nonhematological toxicities were assessed according to the Common Terminology Criteria for Adverse Events Version 4.0 (National Cancer Institute).
Statistical Analysis.
The primary objective of the trial was to demonstrate the noninferiority of the ATO group to the non-ATO group in terms of 3-y DFS rate, which was analyzed as binomial outcomes rather than as time-to-event outcomes (23, 32). Based on a 95% of expected DFS rate in the non-ATO group, and on prerequisite conditions including a noninferiority margin of –5%, a follow-up period of 3 y, a one-sided 2.5% of type I error probability, and an 80% of power the sample size calculated by PASS 11.0 software (NCSS) established that 598 evaluable cases (1:1 per group) were required to draw a noninferiority conclusion. The target sample size was increased to 628 to allow for an expected drop-out rate of 5%. Since randomization was administered after achieving CR, a portion of patients would not be randomized due to early death (in this study set as 10%) and drop-out during induction therapy (5%), a total of 738 cases were finally required for enrollment.
All efficacy analyses were based on the ITT principle. Noninferiority could be concluded if the lower bound of the 95% CI for the between-group difference of 3-y DFS rates was not lower than –5%. A PP analysis including patients who completed their assigned treatments as scheduled was also performed for the primary end point. The sensitivity tests of both ITT and PP population were performed taking into account patients lost for follow-up.
DFS was also estimated as time-to-event outcomes using the Kaplan–Meier method, as well as OS, and compared between groups by the log-rank test. With the competing risk of death in CR, CIR was compared by Fine and Gray’s competing risk analysis. The χ2 test or the Fisher’s exact test was used to compare dichotomous variables. The t test was performed for comparison of continuous variables. All analyses in this study were performed as being two-sided at the 5% significance level except for the noninferiority hypothesis, and no adjustments have been made for multiple comparisons. SAS version 9.4 (SAS Institute Inc.) was adopted for all analyses. See details of the study protocol in SI Appendix. The trial was registered on ClinicalTrials.gov, number NCT01987297.
Supplementary Material
Acknowledgments
This work was supported by grants from the National High-tech Research and Development [863] Program of China (2012AA02A505) (J.-M.L.), the National Natural Science Foundation of China (81770144) (J.-M.L.), (81800141) (L.C.), (81870110) (H.-M.Z.), (81890994) (K.-K.W.), Shanghai Collaborative Innovation Program on Regenerative Medicine and Stem Cell Research (2019CXJQ01) (S.-J.C.), the Overseas Expertise Introduction Project for Discipline Innovation (111 Project, B17029) (S.-J.C.), Double First-Class Project of Shanghai Jiao Tong University (WF510162602) (S.-J.C.), and Shanghai Guangci Translational Medical Research Development Foundation (S.-J.C.). We thank Shanghai Clinical Research Center. Our thanks go to all international colleagues working on APL.
Footnotes
The authors declare no competing interest.
This article contains supporting information online at https://www.pnas.org/lookup/suppl/doi:10.1073/pnas.2020382118/-/DCSupplemental.
Data Availability.
All data relevant to this manuscript are included in the main text or SI Appendix. Further detailed data on patients that support the findings of this study have been deposited in the Open Science Framework (https://osf.io/6h9de).
References
- 1.Adès L., et al., Treatment of newly diagnosed acute promyelocytic leukemia (APL): A comparison of French-Belgian-Swiss and PETHEMA results. Blood 111, 1078–1084 (2008). [DOI] [PubMed] [Google Scholar]
- 2.Wang Z. Y., Chen Z., Acute promyelocytic leukemia: From highly fatal to highly curable. Blood 111, 2505–2515 (2008). [DOI] [PubMed] [Google Scholar]
- 3.Lengfelder E.et al.; German AML Cooperative Group , High dose ara-C in the treatment of newly diagnosed acute promyelocytic leukemia: Long-term results of the German AMLCG. Leukemia 23, 2248–2258 (2009). [DOI] [PubMed] [Google Scholar]
- 4.Sanz M. A.et al.; PETHEMA and HOVON Groups , Risk-adapted treatment of acute promyelocytic leukemia based on all-trans retinoic acid and anthracycline with addition of cytarabine in consolidation therapy for high-risk patients: Further improvements in treatment outcome. Blood 115, 5137–5146 (2010). [DOI] [PubMed] [Google Scholar]
- 5.Lo-Coco F.et al.; Italian GIMEMA Cooperative Group , Front-line treatment of acute promyelocytic leukemia with AIDA induction followed by risk-adapted consolidation for adults younger than 61 years: Results of the AIDA-2000 trial of the GIMEMA group. Blood 116, 3171–3179 (2010). [DOI] [PubMed] [Google Scholar]
- 6.Burnett A. K.et al.; United Kingdom National Cancer Research Institute Acute Myeloid Leukaemia Subgroup , Inclusion of chemotherapy in addition to anthracycline in the treatment of acute promyelocytic leukaemia does not improve outcomes: Results of the MRC AML15 trial. Leukemia 27, 843–851 (2013). [DOI] [PubMed] [Google Scholar]
- 7.Shen Z. X., et al., Use of arsenic trioxide (As2O3) in the treatment of acute promyelocytic leukemia (APL): II. Clinical efficacy and pharmacokinetics in relapsed patients. Blood 89, 3354–3360 (1997). [PubMed] [Google Scholar]
- 8.Soignet S. L., et al., Complete remission after treatment of acute promyelocytic leukemia with arsenic trioxide. N. Engl. J. Med. 339, 1341–1348 (1998). [DOI] [PubMed] [Google Scholar]
- 9.Mi J. Q., Li J. M., Shen Z. X., Chen S. J., Chen Z., How to manage acute promyelocytic leukemia. Leukemia 26, 1743–1751 (2012). [DOI] [PubMed] [Google Scholar]
- 10.Shen Z. X., et al., All-trans retinoic acid/As2O3 combination yields a high quality remission and survival in newly diagnosed acute promyelocytic leukemia. Proc. Natl. Acad. Sci. U.S.A. 101, 5328–5335 (2004). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hu J., et al., Long-term efficacy and safety of all-trans retinoic acid/arsenic trioxide-based therapy in newly diagnosed acute promyelocytic leukemia. Proc. Natl. Acad. Sci. U.S.A. 106, 3342–3347 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Zhu H., et al., All-trans retinoic acid and arsenic combination therapy benefits low-to-intermediate-risk patients with newly diagnosed acute promyelocytic leukaemia: A long-term follow-up based on multivariate analysis. Br. J. Haematol. 171, 277–280 (2015). [DOI] [PubMed] [Google Scholar]
- 13.Zhu H., et al., The 12-year follow-up of survival, chronic adverse effects, and retention of arsenic in patients with acute promyelocytic leukemia. Blood 128, 1525–1528 (2016). [DOI] [PubMed] [Google Scholar]
- 14.Ravandi F., et al., Effective treatment of acute promyelocytic leukemia with all-trans-retinoic acid, arsenic trioxide, and gemtuzumab ozogamicin. J. Clin. Oncol. 27, 504–510 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Gore S. D., et al., Single cycle of arsenic trioxide-based consolidation chemotherapy spares anthracycline exposure in the primary management of acute promyelocytic leukemia. J. Clin. Oncol. 28, 1047–1053 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Mathews V., et al., Single-agent arsenic trioxide in the treatment of newly diagnosed acute promyelocytic leukemia: Long-term follow-up data. J. Clin. Oncol. 28, 3866–3871 (2010). [DOI] [PubMed] [Google Scholar]
- 17.Ghavamzadeh A., et al., Phase II study of single-agent arsenic trioxide for the front-line therapy of acute promyelocytic leukemia. J. Clin. Oncol. 29, 2753–2757 (2011). [DOI] [PubMed] [Google Scholar]
- 18.Iland H. J.et al.; Australasian Leukaemia and Lymphoma Group , All-trans-retinoic acid, idarubicin, and IV arsenic trioxide as initial therapy in acute promyelocytic leukemia (APML4). Blood 120, 1570–1580, quiz 1752 (2012). [DOI] [PubMed] [Google Scholar]
- 19.Estey E., et al., Use of all-trans retinoic acid plus arsenic trioxide as an alternative to chemotherapy in untreated acute promyelocytic leukemia. Blood 107, 3469–3473 (2006). [DOI] [PubMed] [Google Scholar]
- 20.Sanz M. A., et al., Definition of relapse risk and role of nonanthracycline drugs for consolidation in patients with acute promyelocytic leukemia: A joint study of the PETHEMA and GIMEMA cooperative groups. Blood 96, 1247–1253 (2000). [PubMed] [Google Scholar]
- 21.de Thé H., Chen Z., Acute promyelocytic leukaemia: Novel insights into the mechanisms of cure. Nat. Rev. Cancer 10, 775–783 (2010). [DOI] [PubMed] [Google Scholar]
- 22.Mi J. Q., Chen S. J., Zhou G. B., Yan X. J., Chen Z., Synergistic targeted therapy for acute promyelocytic leukaemia: A model of translational research in human cancer. J. Intern. Med. 278, 627–642 (2015). [DOI] [PubMed] [Google Scholar]
- 23.Lo-Coco F.et al.; Gruppo Italiano Malattie Ematologiche dell’Adulto; German-Austrian Acute Myeloid Leukemia Study Group; Study Alliance Leukemia , Retinoic acid and arsenic trioxide for acute promyelocytic leukemia. N. Engl. J. Med. 369, 111–121 (2013). [DOI] [PubMed] [Google Scholar]
- 24.Platzbecker U., et al., Improved outcomes with retinoic acid and arsenic trioxide compared with retinoic acid and chemotherapy in non-high-risk acute promyelocytic leukemia: Final results of the randomized Italian-German APL0406 trial. J. Clin. Oncol. 35, 605–612 (2017). [DOI] [PubMed] [Google Scholar]
- 25.Shen Y., et al., Mutations of epigenetic modifier genes as a poor prognostic factor in acute promyelocytic leukemia under treatment with all-trans retinoic acid and arsenic trioxide. EBioMedicine 2, 563–571 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Cicconi L., et al., Long-term results of all-trans retinoic acid and arsenic trioxide in non-high-risk acute promyelocytic leukemia: Update of the APL0406 Italian-German randomized trial. Leukemia 34, 914–918 (2020). [DOI] [PubMed] [Google Scholar]
- 27.Burnett A. K.et al.; UK National Cancer Research Institute Acute Myeloid Leukaemia Working Group , Arsenic trioxide and all-trans retinoic acid treatment for acute promyelocytic leukaemia in all risk groups (AML17): Results of a randomised, controlled, phase 3 trial. Lancet Oncol. 16, 1295–1305 (2015). [DOI] [PubMed] [Google Scholar]
- 28.Powell B. L., et al., Arsenic trioxide improves event-free and overall survival for adults with acute promyelocytic leukemia: North American Leukemia Intergroup Study C9710. Blood 116, 3751–3757 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Abaza Y., et al., Long-term outcome of acute promyelocytic leukemia treated with all-trans-retinoic acid, arsenic trioxide, and gemtuzumab. Blood 129, 1275–1283 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Sanz M. A., et al., Management of acute promyelocytic leukemia: Updated recommendations from an expert panel of the European LeukemiaNet. Blood 133, 1630–1643 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Cheson B. D.et al.; International Working Group for Diagnosis, Standardization of Response Criteria, Treatment Outcomes, and Reporting Standards for Therapeutic Trials in Acute Myeloid Leukemia , Revised recommendations of the international working group for diagnosis, standardization of response criteria, treatment outcomes, and reporting standards for therapeutic trials in acute myeloid leukemia. J. Clin. Oncol. 21, 4642–4649 (2003). [DOI] [PubMed] [Google Scholar]
- 32.Zhu H. H., et al., Oral arsenic plus retinoic acid versus intravenous arsenic plus retinoic acid for non-high-risk acute promyelocytic leukaemia: A non-inferiority, randomised phase 3 trial. Lancet Oncol. 19, 871–879 (2018). [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
All data relevant to this manuscript are included in the main text or SI Appendix. Further detailed data on patients that support the findings of this study have been deposited in the Open Science Framework (https://osf.io/6h9de).