Abstract
Retinoid therapy transformed response and survival outcomes in acute promyelocytic leukemia (APL) but has demonstrated only modest activity in non-APL forms of acute myeloid leukemia (AML). The presence of natural retinoids in vivo could influence the efficacy of pharmacologic agonists and antagonists. We found that natural RXRA ligands, but not RARA ligands, were present in murine MLL-AF9-derived myelomonocytic leukemias in vivo and that the concurrent presence of receptors and ligands acted as tumor suppressors. Pharmacologic retinoid responses could be optimized by concurrent targeting of RXR ligands (e.g., bexarotene) and RARA ligands (e.g., all-trans retinoic acid), which induced either leukemic maturation or apoptosis depending on cell culture conditions. Co-repressor release from the RARA:RXRA heterodimer occurred with RARA activation, but not RXRA activation, providing an explanation for the combination synergy. Combination synergy could be replicated in additional, but not all, AML cell lines and primary samples, and was associated with improved survival in vivo, although tolerability of bexarotene administration in mice remained an issue. These data provide insight into the basal presence of natural retinoids in leukemias in vivo and a potential strategy for clinical retinoid combination regimens in leukemias beyond APL.
Introduction
The retinoic acid receptors (RAR) and retinoid X receptors (RXR) are ligand-activated transcription factors that influence hematopoietic stem cell self-renewal and differentiation.1-3 Transcriptional activation of the retinoid receptors is ligand dependent.4 Therefore, the potential activity of retinoid receptors in leukemogenesis may be altered depending on the availability of natural ligands within a leukemic population, and retinoid receptors might act as either tumor suppressors or oncogenes depending on the ligand context. However, in leukemia, it is not known whether these receptors are exposed to natural activating ligands in vivo, or whether different forms of leukemia might contain different quantities of functional ligands.
There are three different RAR and RXR isoforms (α, β, and γ) that are differently expressed in hematopoietic cells.5,6 RARA and RXRA expression are dynamically regulated during myeloid maturation, with highest mRNA expression in mature neutrophils.7 RAR function as obligate heterodimers with RXR, whereas RXR can form either homodimers or heterodimers with other orphan nuclear receptors (e.g., peroxisome proliferator-activated receptors [PPAR], liver X receptors [LXR], etc.).4 RAR-RXR dimers bind DNA with high affinity at specific retinoic acid response elements (RARE) in target gene promoters/enhancers.8 RAR-RXR acts as a transcriptional repressor by binding co-repressor complexes composed by nuclear receptor co-repressor (N-CoR) and the silencing mediator for retinoid and thyroid hormone receptors (SMRT) and recruiting histone deacetylases (HDAC). Local histone deacetylation then facilitates chromatin condensation and gene silencing. Ligand binding alters the heterodimer conformation, displacing the co-repressors and facilitating binding of co-activator complexes composed by p160 family (TIF-2/SRC-1/RAC3) with histone acetylase activity (HAT). Local histone acetylation then facilitates chromatin decondensation and gene transcription activation.9,10
All-trans retinoic acid (ATRA) has been proposed as the natural ligand for RAR. Multiple natural ligands have been proposed for RXR, including modified retinoic acids (9-cis retinoic acid and 9-cis-13,14-dihydroretinoic acid) and long-chain fatty acids (C22:6, C22:5; C20:4, and C24:5), which are available in diverse tissues as well as in serum.11-15 Natural RXRA, but not RARA, ligands are present in normal hematopoietic cells in vivo under steady-state conditions, with preferential distribution in myeloid cells (Gr1+) and following myeloid stress resulting from granulocytecolony stimulating factor (GCSF) treatment.14
ATRA (tretinoin) has transformed the outcomes of M3-acute promyelocytic leukemia (APL).16,17 However, outcomes with ATRA in non-APL AML have yielded mixed results.18 Bexarotene is a pan-RXR-activating ligand, which is approved for the treatment of cutaneous T-cell lymphoma (CTCL).19 In small exploratory studies of non- APL AML, bexarotene demonstrated evidence of activity, but only in a small proportion of patients.20,21
We used an in vivo reporter assay to explore whether retinoid receptors in leukemia cells are transcriptionally active (and therefore might be best targeted with antagonists) or transcriptionally inactive (and therefore might be best targeted with agonists). Our results show that natural ligands for RXRA, but not RARA, are present at low levels in vivo in primary mouse myelomonocytic leukemia cells where they exhibit tumor suppressor phenotypes.
Methods
Hematopoietic cell culture
Mouse BM Kit+ cells were isolated using an Automacs Pro (Miltenyl Biotec, San Diego, CA, USA) and plated in RPMI 1640 medium, 15% fetal bovine serum (FBS), Scf (50 ng/mL), IL3 (10 ng/mL), Flt3 (25 ng/mL), Tpo (10 ng/mL), L-glutamine (2 mM), sodium pyruvate (1 mM), HEPES buffer (10 mM), penicillin/streptomycin (100 units/mL), β-mercaptoethanol (50 mM). MLL-AF9 leukemia was cultured in a similar media, but without Flt3, or Tpo. MOLM-13 were grown in RPMI1640 and 20% FBS; THP-1 in RPMI1640, 10% FBS, 0.05 mM MetOH; MONOMAC-6 in RPMI1640, 10% FBS, 2 mM L-glutamine, 2 mM NEAA, 1 mM sodium pyruvate, 10 ug/mL human insulin; OCI-AML-3 in α-MEM and 20% FBS. Fluorescence was detected on a FACS Scan, Gallios instrument (Beckman Coulter, Brea, CA, USA) or ZE5 Cell Analyzer (Biorad, Hercules, CA, USA).
Primary acute myeloid leukemia samples
All cryopreserved AML samples were collected as part of a study approved by the University of Helsinki after patients provided informed consent in accordance with the Declaration of Helsinki. Thawed BM mononuclear cells were suspended in 87.5% RPMI 1640 medium plus 12.5% HS5 stromal cells conditioned media, and supplemented with 10% FBS, L-glutamine (2 mM) and penicillin/streptomycin (100 units/mL). 10,000 cells/well in 25 mL were plated on 384-well plates containing bexarotene and ATRA. The cells were incubated with the drugs for 96 h after which cell viability was measured with CellTiter-Glo (Promega, Madison, WI, USA).
Mice
UAS-GFP and Mx1-Cre x Rxraflox/flox x Rxrbflox/flox mice were bred as previously described.22,23 pIpC treatment was intraperitoneal injection (IP) with 300 μg/mouse; four doses were given every other day. Rxra and Rxrb deletion were confirmed by polymerase chain reaction (PCR). Bexarotene was administrated by oral gavage, suspended in sterile α-tocopherol suspension or in corn oil, 1 mg per mouse per day for 5 days/week. Lox-stop-Lox YFP mice were a gift from Todd Fehniger, Washington University. Dnmt3a- R878H/FLT3-ITD mouse leukemia cells were generated by Angela Verdoni and were a gift from Timothy Ley, Washington University (T Ley, unpublished material, 2019). To generate this line, hematopoietic cells derived from Dnmt3a R878H mosaic mice were transduced with an MSCV-FLT3-ITD-IRES-GFP retrovirus, and transplanted into multiple recipient mice. AML developing in these mice were confirmed to express the R878H allele by RNAseq, with approximately 50% of all reads coming from the mutant allele (T Ley, personal communication 2019 ). The AML sample (AML- 1) examined in these studies is associated with rapid lethality in secondary transplants (median 48 days in syngeneic B6 animals) and an immature myeloid immunophenotype (Cd117+ and partial expression of Gr-1 and Cd11b) (O di Martino personal observation, 2020). Tet2-KO/FLT3-ITD leukemia cells were a gift from Ross Levine, Memorial Sloan Kettering Cancer Center. The Washington University Animal Studies Committee approved all animal experiments.
Study approval
All animal procedures were approved by the Institutional Animal Care and Use Committee of Washington University. All cryopreserved human AML samples were collected as part of a study approved by the University of Helsinki after patients provided informed consent in accordance with the Declaration of Helsinki.
Results
RXRA natural ligands are present in MLL-AF9 myeloid leukemia cells in vivo
RARA and RXRA are preferentially expressed in mature myeloid cells and this pattern is reflected in a biased expression in M4/M5 AML where RARA and RXRA expression correlates with markers of maturation7 (Online Supplementary Figure S1). Therefore, we hypothesized that the availability of natural retinoids in hematopoietic malignancies might also be myeloid biased.
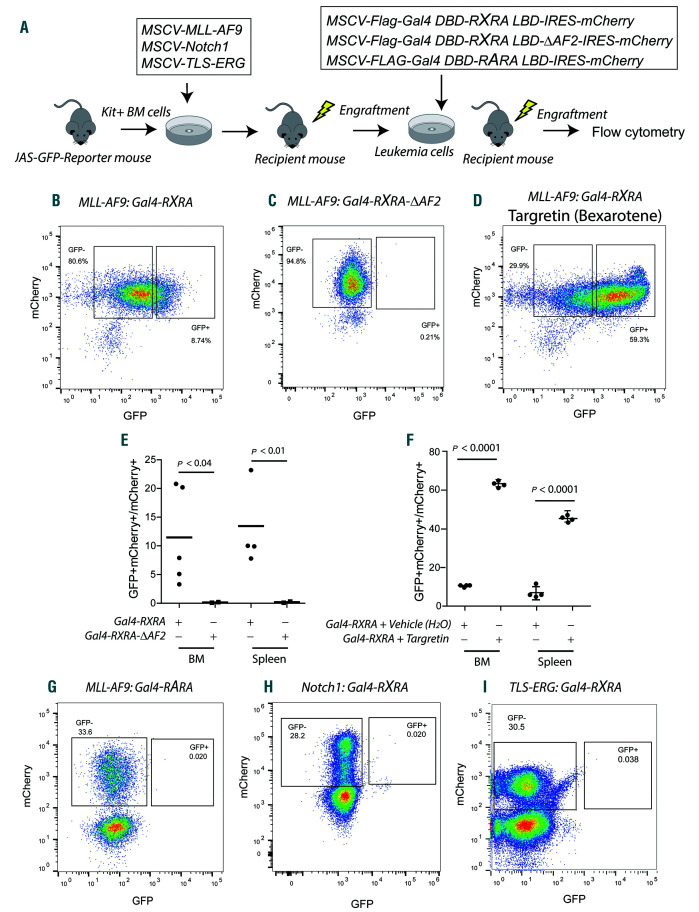
We evaluated in vivo retinoid ligand availability using a retroviral model of myelomonocytic leukemia and a reporter assay we had previously characterized.14,22 UAS-GFP bone marrow (BM) cells first were transduced with a retrovirus expressing MLL-AF9 and transplanted into sublethally irradiated recipient mice (see Figure 1A). Once leukemia emerged (i.e., UAS-GFP x MLL-AF9), these cells were transduced with a second retrovirus (MSCV-Flag-Gal4 DBD-RXRA LBD-IRES-mCherry) and subsequently transplanted into sublethally irradiated recipients. Using this strategy, leukemia cells with active, natural RXRA ligands express GFP (Online Supplementary Figure S2A and B). Ex vivo, the reporter was sensitive to low nM concentrations of retinoids and exhibited specificity between RARA and RXRA ligands (Online Supplementary Figure S2D-F). Although the RXRA LBD is used in this assay, the ligand-binding pocket is highly conserved between RXRA, RXRB, and RXRG, and no RXR subtype-specific compounds have yet been identified, suggesting that natural ligands that activate RXRA are likely to cross-react with RXRB and RXRG.25We noted the presence of mCherry+GFP+ cells in both BM and spleen from multiple mice transplanted using three different primary MLL-AF9 leukemias and engrafted into a total of five different recipients (Figure 1B and E). As a negative control, we transduced UAS-GFP x MLL-AF9 leukemia cells with a retrovirus expressing Gal4-RXRA-DAF2, which contains a deletion of the RXRA C-terminal helix 12 (AF2 domain); this mutation is able to bind to RXR ligands, but does not transactivate the reporter (Figure 1C and E, and Online Supplementary Figure S2C).14
Figure 1.
Retinoid X receptors (RXR) natural ligands are present in MLL-AF9 myeloid leukemia in vivo. (A) Schema for bone marrow (BM) transplant procedure. Kit+ cells isolated from the BM of UAS-GFP mice using magnetic-activated cell sorting (MACS) were transduced with MSCV-MLL-AF9, MSCV-Notch1 or MSCV-TLS-ERG retroviruses and then injected into sublethally irradiated recipient mice. When leukemia emerged, recipient mice were sacrificed and their leukemic cells harvested. Leukemia cells were transduced with MCSV-Flag-Gal4-RXRA-IRES-mCherry (Gal4-RXRA), MSCV-Flag-Gal4-RXRA-ΔAF2-IRES-mCherry (Gal4-RXRA-DAF2), or MSCVFlag- Gal4-RARA-IRES-mCherry (Gal4-RARA) and then injected into sublethally irradiated recipient mice. After leukemia engraftment, leukemia cells were harvested and the ratio of mCherry+GFP+ versus total mCherry+ cells was evaluated by flow cytometry. (B and C) Representative data showing GFP and mCherry intensity in MLL-AF9 leukemia cells. (D) Representative GFP and mCherry expression in mice transplanted with MLL-AF9-derived leukemic cells transduced with Gal4-RXRA and treated with Targretin (bexarotene, 50 mg/kg) by oral gavage administration for 2 days. (E) Combined results from mice transplanted with MLL-AF9-derived leukemia transduced with Gal4-RXRA (circles; n=5 recipient mice) or RXRA-DAF2 (squares; n=3 recipient mice). (F) Combined results from mice transplanted with MLL-AF9- derived leukemia cells transduced with Gal4-RXRA and gavaged with bexarotene (Targretin) (squares; n=4 recipient mice) or water (vehicle) (circles; n=4 recipient mice). (G) Representative GFP and mCherry intensity in MLL-AF9-derived leukemia cells transduced with Gal4-RARA and transplanted into recipient mice (n=3 recipient mice). (H and I) Representative GFP and mCherry intensity in leukemia cells derived with MSCV-Notch1 (n=5 recipient mice) or MSCV-TLS-ERG (n=4 recipient mice) retroviruses, transduced with Gal4-RXRA, and transplanted into recipient mice. t-test with Welch’s correction.
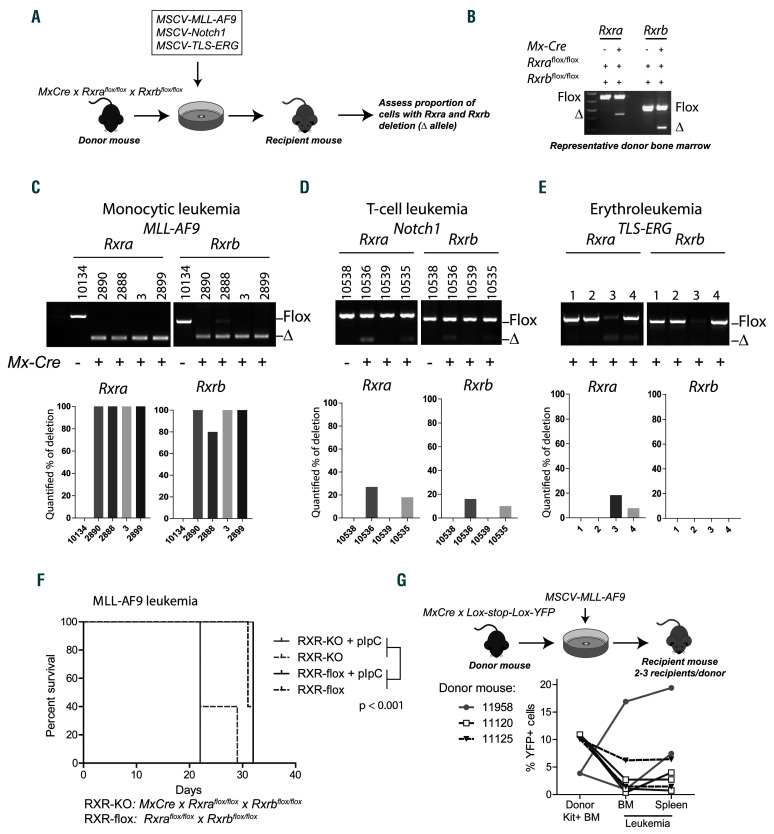
Figure 2.
Retinoid X receptors (RXR) act as tumor suppressors in mouse MLL-AF9 leukemias. (A) Schema for leukemia transplant procedure. Rxraflox/flox x Rxrbflox/flox x Mx-Cre bone marrow (BM) cells were collected from the donor mice and transduced as indicated and injected into sublethally irradiated recipient mice. Upon leukemia engraftment, the proportion of Rxra and Rxrb deletion was assessed by polymerase chain reaction (PCR). (B) PCR results from representative donor BM cells with “Flox” indicating the retained and “Δ” the deleted allele. (C-E) PCR analysis of Rxra and Rxrb alleles from leukemias that emerged from individual mice. Bar graphs display the quantified percentage of deleted alleles in each unique leukemia. ImageJ software was used for quantification analysis. (F) Kaplan-Meier survival curve of mice injected with Rxraflox/flox x Rxrbflox/flox x Mx-Cre MLL-AF9 leukemic cells (RXR-KO) or Rxraflox/flox x Rxrbflox/flox MLL-AF9 leukemic cells (RXR-flox). Each cohort consisted of five mice. Indicated cohorts were treated with three doses of pIpC on days 5-10 (solid lines). (G) Schema for BM transplant procedure of Loxstop- Lox-YFP x Mx-Cre mice and YFP evaluation of the hematopoietic cell. Kit+ BM cells from Lox-stop-Lox-YFP x Mx-Cre donor mice were harvested (n=3 donor mice) and transduced with MSCV-MLL-AF9 retrovirus, then injected into sublethally irradiated recipient mice (n=7 recipient mice). The percentage of yellow fluorescent protein (YFP) was quantified by flow cytometry in BM cells before transduction and after leukemia emerged. Each line represents results from an individual recipient mouse.
In vivo, a significative proportion of cells remained mCherry+GFP– (Figure 1B). To determine whether RXRA transactivation could be further stimulated, leukemic RXRA reporter mice were orally gavaged for 2 days with 50 mg/kg bexarotene using a clinical formulation with improved solubility (Targretin). Bexarotene is a pan-RXR agonist and results were compared with vehicle control (water). We observed that bexarotene treatment further augmented RXRA-dependent GFP expression (Figure 1D and F). In contrast, we did not observe evidence of natural RARA ligands following transplantation of UAS-GFP x MLL-AF9 leukemia cells transduced with Gal4-RARA (Figure 1G), suggesting the absence of RARA ligands, in MLL-AF9 leukemia cells in vivo.
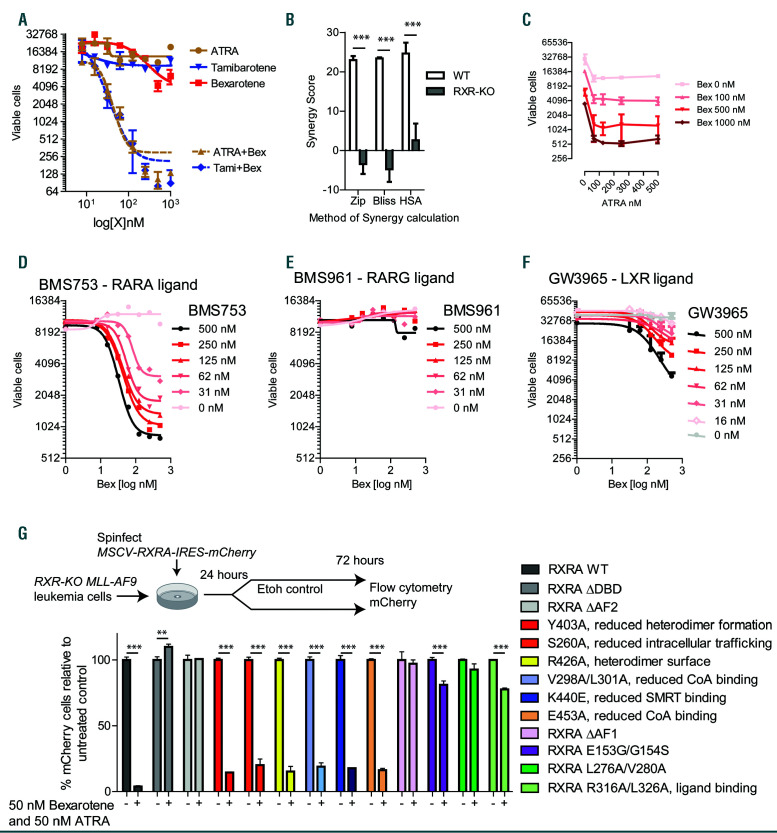
Figure 3.
Pharmacologic targeting of natural retinoic acid receptor (RAR)A and retinoid X receptor (RXR)A ligands blocks MLL-AF9 proliferation in vitro. (A) MLLAF9 leukemia cells derived from UAS-GFP bone marrow (BM) and transduced with MSCV-Flag-Gal4-RXRA-IRES-mCherry retrovirus (MLL-AF9 Gal4-RXRA cells) were treated as indicated, replated after 48 hours (h), and total viable cells in 50 mL assessed in duplicate after 96 total h of treatment at indicated doses. (B) MLL-AF9 RXR-flox (wild-type, WT) or RXR-KO (Rxra/Rxrb deficient) leukemia cells (see Figure 2) were treated with all-trans retinoic acid (ATRA) and bexarotene for 96 total h and the synergy was calculated by SynergyFinder software24 using three different mathematical calculators for synergy versus additive effects (Zip, Bliss, and HAS). In these calculations, results >1 suggest mathematical synergy, although larger values are typically required for biologically relevant synergy. (C) MLL-AF9 Gal4-RXRA cells were treated as indicated, replated after 48 h, and total viable cells in 50 mL were assessed in duplicate after 96 total h of treatment. (D-F) MLL-AF9 leukemia cells were treated as indicated, replated after 48 h, and total viable cells in 50 mL assessed in duplicate after 96 h of total treatment. (G) Rxra/Rxrb deficient MLLAF9 leukemia cells (RXR-KO) were transduced with retrovirus encoding MSCV-RXRA (full length)-IRES-mCherry or retrovirus with indicated RXRA mutations. Mutations and published mutation effects are indicated. 24 h after retroviral transduction, cells were treated in triplicate with 50 nM ATRA/bexarotene, and the proportion of mCherry+ cells was assessed relative to untreated control population after 72 h. **P<0.01, ***P<0.001, t-test with Welch’s correction relative to control.
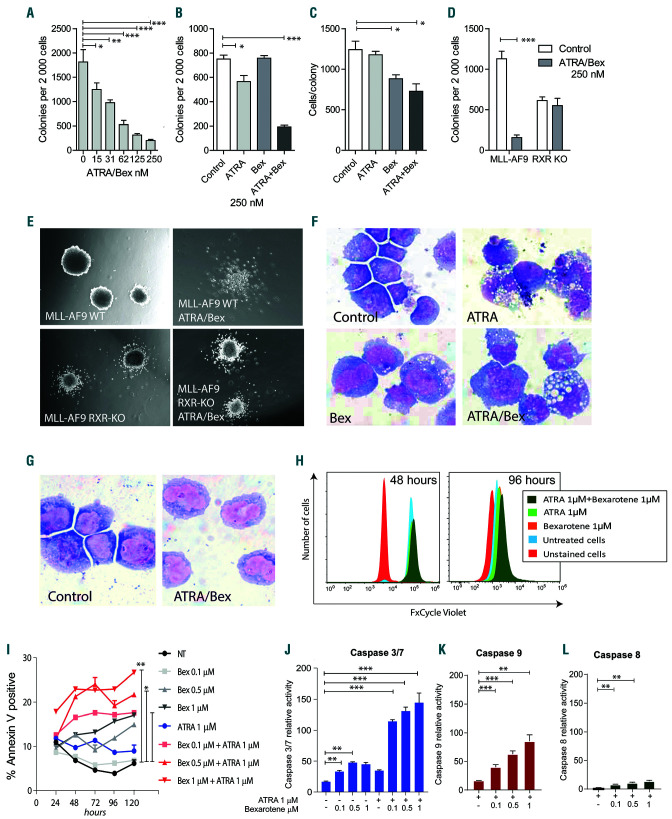
Figure 4.
Differentiation and apoptosis induced by all-trans retinoic acid (ATRA)/bexarotene. (A-D) Colony forming units (CFU) in methylcellulose per 2,000 MLL-AF9 leukemia cells treated as indicated and assessed in triplicate. (E) Photographs of MLL-AF9 colonies treated as indicated for 7 days. (F) Cytospin preparation of MLL-AF9 leukemia cells treated with or without ATRA and bexarotene for 96 hours (h) stained with Wright-Giemsa. (G) Cytospin preparation of MLL-AF9 leukemia cells that remains after treatment with ATRA/bexarotene and stained with Wright-Giemsa. (H) Cell division analysis of MLL-AF9 leukemia. On day 0, cells were stained with FxCycle Violet, and retained dye was assessed at indicated time points by flow cytometry. (I) Annexin V staining of MLL-AF9 leukemia cells after 24, 48, 72, 96, and 120 h of ATRA and bexarotene treatment in triplicate. (J-L) Relative activity of caspases 3/7, 9, and 8 in MLL-AF9 leukemia cells after 48 h of ATRA and bexarotene treatment in triplicate. *P<0.05, **P<0.01, ***P<0.001, t-test with Welch’s correction relative to control.
Figure 5.
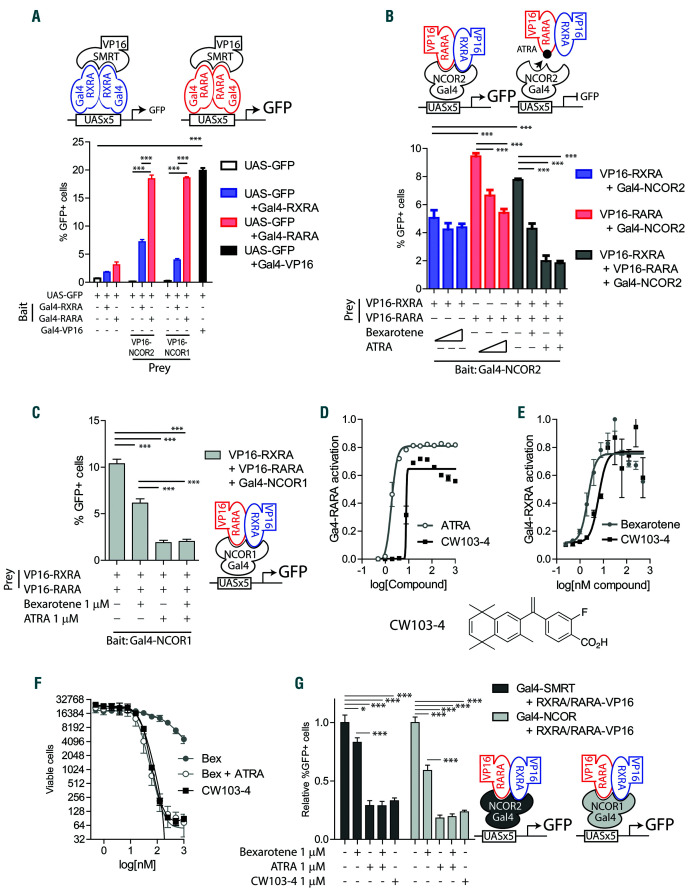
Interaction of co-repressors NCOR and SMRT with natural retinoic acid receptor (RAR)A : retinoid X receptor (RXR)A heterodimers. (A) Mammalian twohybrid assay schema. 293T cells co-transfected with plasmids encoding the reporter: UAS-GFP; “bait”: Gal4-RXRA or Gal4-RARA; and “prey”: VP16-SMRT or VP16-NCOR. The percentage of GFP+ cells was assessed 48 hours (h) after transfection by flow cytometry in triplicate. A vector encoding Gal4-VP16 fusion was used as a positive control. (B) Reversely, 293T cells were co-transfected with plasmids encoding the reporter: UAS-GFP; “bait”: Gal4-SMRT; and “prey”: VP16-RARA and/or VP16-RXRA. Cells were treated with increasing concentrations of all-trans retinoic acid (ATRA) and bexarotene (0, 100 nM and 1 mM) in triplicate. The percentage of GFP+ cells was assessed 48 h after transfection by flow cytometry. (C) 293T cells were co-transfected with plasmids encoding the reporter: UAS-GFP; “bait”: Gal4-NCOR; and “prey”: VP16-RARA and/or VP16-RXRA. Cells were treated with ATRA and bexarotene (1 mM) in triplicate. The percentage of GFP+ cells was assessed 48 h after transfection by flow cytometry. (D and E) MLL-AF9 leukemia cells derived from UAS-GFP bone marrow and transduced with MSCV-Flag-Gal4-RXRA-IRESmCherry retrovirus (MLL-AF9 Gal4-RXRA cells) or MSCV-Flag-Gal4-RARA-IRES-mCherry retrovirus (MLL-AF9 Gal4-RARA cells) were treated as indicated, replated and total viable cells in 50 mL assessed after 96 total h of treatment in duplicate. (F) MLL-AF9 cells were treated as indicated, replated and total viable cells in 50 mL assessed after 96 total h of treatment in duplicate. (G) 293T cells were co-transfected with plasmids encoding the reporter: UAS-GFP; “bait”: Gal4-NCOR or Gal4- SMRT; and “prey”: VP16-RARA and/or VP16-RXRA. Cells were treated with ATRA, bexarotene, and CW103-4 as indicated in triplicate. The percentage of GFP+ cells was assessed 48 h after transfection by flow cytometry. *P<0.05, ***P<0.001, t-test with Welch’s correction.
Figure 6.
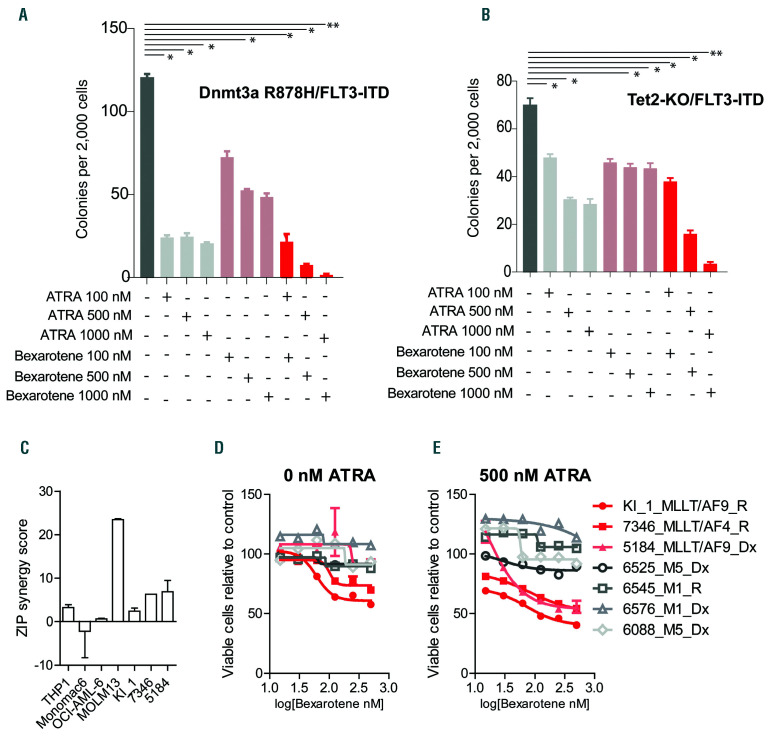
All-trans retinoic acid (ATRA)/bexarotene in different leukemia cell lines and in human acute myeloid leukemia (AML) ex vivo. (A and B) Colony forming units (CFU) in methylcellulose per 2,000 Dnmt3a R878H/FLT3-ITD and Tet2-KO/FLT3-ITD leukemia cells treated as indicated for 7 days in duplicate. (C) Zip synergy score of ATRA and bexarotene interactions calculated by SynergyFinder.24 (D and E) Cell viability of primary human AML leukemia samples treated for 96 hours ex vivo with ATRA and bexarotene at indicated concentrations assessed in duplicate by CellTiter-Glo (CTG). Dx: sample acquired at initial diagnosis; R: sample acquired at relapse. *P<0.05, **P<0.01, t-test relative to control.
To determine whether natural RXRA ligands were present in other forms of murine leukemia, we repeated these studies in leukemias derived using activated Notch1 (T-cell leukemia)26 and TLS-ERG (erythroleukemia).27 In neither was the presence of mCherry+GFP+ cells observed (Figure 1H and I).
RXR acts as tumor suppressors in mouse MLL-AF9 leukemias
Given the presence of natural RXRA ligands in MLLAF9 leukemia, we sought to determine whether activated endogenous mouse Rxrs contribute to leukemic growth in vivo. We transduced Mx-Cre x Rxraflox/flox x Rxrbflox/flox BM cells with MLL-AF9 retrovirus, transplanted these into sublethally irradiated recipient mice, and derived subsequent leukemias (see Figure 2A). Of note, Rxrg expression is low to absent in hematopoietic cells (Online Supplementary Figure S1G).28 We performed our initial retroviral transduction and transplantation in the absence of polyinosinic:polycytidylic acid (pIpC) treatment. However, low levels of native interferons induced Mx-Cre activity in a subset of cells, and a small proportion of BM cells exhibited Rxra and Rxrb deletion prior to transduction and transplantation (Figure 2B).23 Unexpectedly, all MLLAF9- derived primary leukemias emerged with almost 100% deletion of both Rxra and Rxrb alleles even in the absence of any pIpC injections (Figure 2C), suggesting a strong positive selection for the D/D (deleted) alleles. In contrast, repeat experiments deriving Notch1 T-cell leukemia and TLS-ERG erythroleukemias showed near complete retention of Rxraflox/flox and Rxrbflox/flox alleles (Figure 2D and E). We performed secondary transplantation of MLL-AF9 leukemia cells derived from Mx-Cre x Rxraflox/flox x Rxrbflox/flox and from Rxraflox/flox x Rxrbflox/flox mice using 1x106 leukemia cells per recipient. Because these tumors were transplanted immediately, we were unaware of their deletion status and separately treated cohorts of mice with pIpC or control intending to determine whether the deleted allele augmented growth. We subsequently noted that primary tumors derived from Mx-Cre+ mice already carried deleted alleles and that these Rxra/Rxrb null (RXR-KO) leukemias resulted in shorter latency than tumors derived from Mx-Cre– mice, and that further stimulation by pIpC did not impact survival (Figure 2F). This suggests that RXR-KO leukemia cells grow more quickly in vivo than their wild-type counterparts, and could, therefore, have outgrown the wild-type populations during the primary transplantations. To exclude the possibility that MLL-AF9 leukemogenesis or hematopoietic transplantation could non-specifically activate Mx-Cre and induce deletion of the Rxr alleles, we crossed Mx-Cremice with Lox-stop-Lox- YFP reporter mice and derived seven independent MLL-AF9 leukemias using three individual donors. The leukemia cells that emerged were not associated with increased YFP expression, suggesting that Mx-Cre activation does not routinely occur following viral MLL-AF9 leukemogenesis and transplantation (Figure 2G).
Pharmacologic targeting RXRA and RARA in vitro
In vitro, bexarotene exerted dose-dependent growth inhibition in UAS-GFP x MLL-AF9 x Gal4-RXRA leukemia cells, although the effect was modest (2-4 fold) (Figure 3A, red line). Because Rxrs act as heterodimers with other orphan receptors, we screened for interactions with additional ligands. The combination of bexarotene with either ATRA or tamibarotene (an RARA-specific agonist) lead to profound, dose-dependent growth inhibition while singleagent RARA ligands resulted in only modest (2-4 fold) growth inhibition (Figure 3A, blue and brown lines). Response and synergy were absent in RXR-KO MLL-AF9 leukemia cells (Figure 3B). The effect of ATRA on leukemic growth inhibition reached a plateau at 60-100 nM, whereas increasing concentrations of bexarotene were associated with increasing growth reduction up to 1 mM (Figure 3C). After 5 days of retinoids, we noted the proportion of GFP+ cells and the GFP median fluorescence intensity (MFI) was significantly reduced (Online Supplementary Figure S3A and B), suggesting that the most RXR responsive cells experienced the greatest negative selection by retinoid treatment.
We tested a series of additional nuclear receptor ligands for anti-leukemic activity. A broad range of RXR ligands cooperated with ATRA to induce anti-leukemic activity and again displayed only modest single-agent activity (Online Supplementary Figure S4A and B). The RARA-specific agonist BMS753, but not the RARG-specific agonist BMS961, cooperated with bexarotene to inhibit MLL-AF9 cell growth (Figure 3D and E). RXR weak agonists and antagonists (LG100754 and HX531) were insufficient to cooperate with ATRA or tamibarotene (RARA specific agonist) and the pan-RAR inverse agonist BMS493 inhibited the effect of combination ATRA and bexarotene (Online Supplementary Figure S4C and D). Finally, the PPARA agonist GW7647, PPARG agonist pioglitazone, and the LXR agonist GW3965 had modest effects with bexarotene (Figure 3F and Online Supplementary Figure S4E and F).
RXRA domains required for retinoid sensitivity
To map the structural domains of RXRA required for anti-leukemic activity, we retrovirally re-expressed RXRA in RXR-KO MLL-AF9 leukemia cells (Figure 3G). mCherry expression identified transduced cells. A series of RXRA deletions and mutations were generated and the position of the LBD mutations are highlighted within an available RXRA crystal structure (Protein Database: 4K4J) (Online Supplementary Figure S5A and B). The expression of the RXRA mutants was confirmed by western blot (Online Supplementary Figure S5C). In cells transduced with fulllength RXRA, bexarotene and ATRA induced a strong decrease in the proportion of mCherry+ cells after 48 h (Figure 3G), consistent with a strong retinoid sensitivity among cells expressing wild-type RXRA. We found that mutants lacking the AF1, DBD, or AF2 domain and mutations reported to: disrupt the DNA binding zinc finger (E153G/G154S),29 reduce co-activator binding (L276A/V280A),30 or abrogate ligand binding (R316A/L326A),31 were unresponsive to retinoids (Figure 3G). Additional mutations reported to: reduce co-activator binding (E453A, V298A/L301A),32 reduce intracellular trafficking of RXRA (S260A),33 obstruct the heterodimer interface (Y402A, R426A)34 or reduce co-repressor binding to RXRA (K440E),32 largely retained sensitivity to retinoids (Figure 3G). These data demonstrate the necessity of specific, functional RXRA domains (AF1, DBD, and AF2), as well as RXRA co-activator-interacting moieties for the activity of the RARA:RXRA heterodimer.
Maturation and apoptosis induced by ATRA/bexarotene
Retinoids have been associated with myeloid maturation programs.35-38 We observed that different culture conditions influenced the effect of retinoid-induced maturation and apoptosis in MLL-AF9 leukemia. When grown in methylcellulose, combination ATRA/bexarotene reduced colony-forming capacity in a dose-dependent manner (Figure 4A); these effects were associated with changes in the number of colonies, the size of the colonies (smaller), colony morphology (diffusion of cell clustering), and cellular cytomorphology (acquisition of vacuoles), consistent with loss of self-renewal and maturation (Figure A-F). Again, these phenotypes were absent in RXR-KO leukemia cells and single-agent retinoids resulted in modest effects. However, when MLL-AF9 leukemia cells were grown in liquid culture, retinoid combinations did not induce cytomorphologic changes or cell cycle exit, but rather, they induced time- and dose-dependent apoptosis and activation of intrinsic caspase pathways (Caspases 3/7 and 9) (Figure 4G-L).
Co-repressor binding to RARA:RXRA heterodimers
The synergy observed between ATRA and bexarotene suggests different effects within the RARA:RXRA heterodimer. In the absence of retinoids, the RARA:RXRA heterodimer binds to co-repressors such as the nuclear receptor co-repressor (NCOR1) and silencing mediator of retinoic acid and thyroid hormone receptor (SMRT, aka NCOR2).39-42 Using a mammalian two-hybrid assay in 293T cells,43 we noted that RARA resulted in greater transcriptional activity with either co-repressor (SMRT) versus RXRA, suggesting a stronger affinity of RARA to corepressors than RXRA (Figure 5A). Similarly, when the bait and prey strategy was reversed (Figure 5B), ATRA led to greater reporter inhibition than bexarotene, with as great an effect as the combination of ATRA and bexarotene (Figure 5B). Similar results were noted using NCOR as bait (Figure 5C), suggesting that co-repressor interactions with RARA:RXRA heterodimers are dominated by interactions with RARA.
Figure 7.
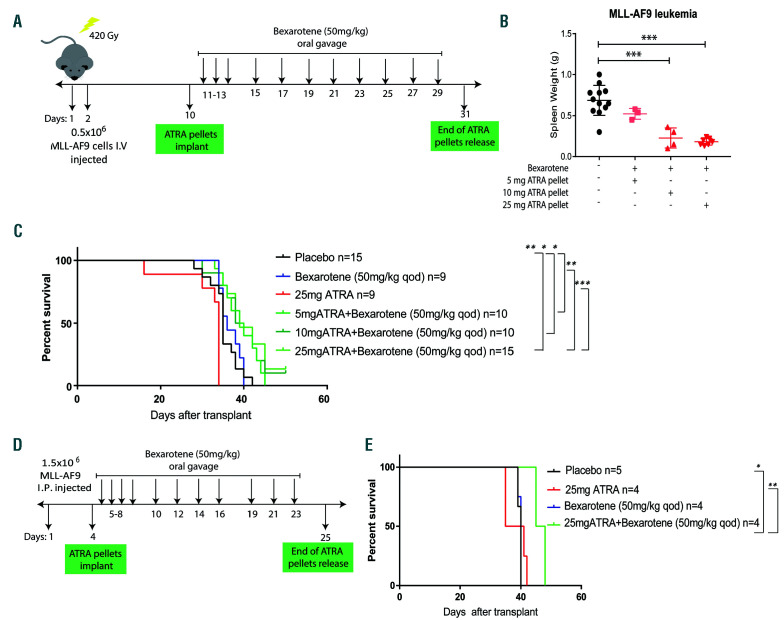
Concurrent all-trans retinoic acid (ATRA)/bexarotene treatment reduces MLL-AF9 leukemia burden in vivo. (A) Schema for leukemia transplant procedure and in vivo treatment. Sublethally irradiated FVB mice were transplanted with 0.5x106 MLL-AF9 leukemia cells. Ten days later the mice were divided in four different cohorts and treated with 21-day release ATRA pellets. 5 mg: n=5 (~0.23 mg/day), 10 mg: n=5 (~0.5 mg/day), and 25 mg: n=8 (~1.2 mg/day). One day after pellet implantation, bexarotene (50 mg/kg) was delivered by oral gavage as indicated. A control cohort was implanted with placebo pellets and treated with the vehicle gavage (n=12). IV: intravenous. (B) The mice were sacrificed 21 days from pellet implantation and the spleen weight analyzed. (C) Kaplan-Meier survival curve analysis of mice transplanted with MLL-AF9 cells and treated as indicated in (A). (D) Schema for leukemia transplant procedure and mice treatment. FVB mice were transplanted with 1.5x106 MLL-AF9 leukemia cells by intraperitoneal (IP) injection. Five days later the mice were divided in four different cohorts and implanted with 21- day release ATRA pellets 25 mg (~1.2 mg/day) or placebo pellets. One day after pellet implantation, bexarotene (Targretin, 50 mg/kg) was delivered by oral gavage as indicated. (E) Kaplan-Meier survival curve analysis of mice transplanted with MLL-AF9 cells and treated as indicated in (E). *P<0.05, **P<0.01, ***P<0.001, t-test.
A bexarotene derivative (CW103-4) had been identified with potential dual RARA/RXRA activity.44-46 CW103-4 has improved murine pharmacokinetics compared with bexarotene (peak plasma concentration of 152,955.83 vs. 18,633.33 ng/mL and area under the curve of 51,531 vs. 8,523 ng/mL).43 However, this is associated with a 5-fold increase in triglycerides 24 h after treatment,43 making it an interesting tool compound, but not an obvious clinical therapy. We assessed this compound to determine whether its dual-affinity might provide single-agent activity in vitro. Indeed, CW103-4 exhibited dual RARA and RXRA activation in UAS-GFP x MLL-AF9 leukemia cells, and was capable of ATRA-independent anti-leukemic activity (Figure 5D-F and Online Supplementary Figure S6AD). Specifically, CW103-4 induced proliferative capacity reduction and apoptosis mediated by caspases 3/7 activation (Online Supplementary Figure S6A-D). Interestingly, CW103-4 also induced consistent single-agent co-repressor release (Figure 5G). To determine whether these effects might occur through LXRs or PPARD, we assessed reporter activation and found no evidence of cross-reactivity with these receptors (Online Supplementary Figure S6EG). These results suggest that single-agent bexarotene is ineffective to induce co-repressor release from the RARA:RXRA heterodimer, and that co-repressor release results primarily from RARA activation, providing an explanation for the anti-leukemic combination synergy (Figure 3A and B).
ATRA/bexarotene responses in diverse acute myeloid leukemias
To determine whether retinoid combination efficacy was limited to MLL-AF9 models or might extend to other leukemias, we examined the effect of combination ATRA/bexarotene on the proliferative capacity of two additional primary murine AML. The first was derived in the Timothy Ley lab using a Dnmt3a R878H knock-in allele and BM cells transduced with MSCV-FLT3-ITD virus containing GFP (Dnmt3a R878H/FLT3-IT (T Ley, unpublished observations, 2019). The second was derived in the Ross Levine lab using germline Tet2 deficiency crossed with a germline FLT3-ITD allele (Tet2-KO x FLT3-ITD).47 Both primary mouse leukemias could be grown transiently in methylcellulose, and we observed anti-leukemic activity of combination retinoids, with limited effects by single agents (Figure 6A and B). We examined cell growth of several human AML cell lines and primary human AML following ATRA/bexarotene treatment, noting low nM IC50 combination ATRA/bexarotene in THP1, Monomac6, OCI-AML-3, and MOLM13 (1.3±1.2, 19.2±1.7, 5.0±1.1, and 15.8±1.2, respectively), with evidence of synergy in THP1 and MOLM13, both of which contain MLL translocations (Figure 6C). Slightly higher IC50 values were observed for CW103-4 (8±1.1, 43±1.2, 20±1.1, and 24±1.4, respectively). We also assessed retinoid responses in a series of primary AML samples collected at diagnosis or relapse and noted evidence of response and modest synergy in samples with MLL translocations (KI_1, 7346, and 5184) (Figure 6D and E).
ATRA/bexarotene activity in vivo
To determine whether retinoid combinations could be transitioned into in vivo activity, we first assessed the potential tolerability of the combination on normal hematopoiesis. Two cohorts of 5 wild-type mice were treated with ATRA/bexarotene by oral gavage or vehicle control. No differences were noted in the peripheral blood counts after 3 weeks of treatment (Online Supplementary Figure S7A-E), suggesting tolerance of the combination by normal hematopoiesis.
Subsequently, we tested the effect of combination retinoids on MLL-AF9 leukemia in vivo. In our first studies, we engrafted MLL-AF9 leukemia cells using sublethal irradiation and we treated mice with slow-release ATRA/bexarotene pellets implanted subcutaneously. However, this lead to wound dehiscence and loss of the pellets (data not shown). Second, four cohorts of mice were treated with 21 days ATRA release pellets (placebo, 5 mg, 10 mg, and 25 mg, which provide an average of 0.23 mg, 0.5 mg, and 1.2 mg/day, respectively). The day after pellet implantation, all ATRA cohorts received bexarotene by oral gavage (in α-tocopherol, 50 mg/kg, which is reported to be better tolerated as a vehicle than corn oil during serial administration)48 (Figure 7A). However, tolerance of the combination by the mice was quite poor, as they exhibited reduced activity the day after gavage, ruffled fur, weight loss, and poor wound healing, and bexarotene could be administered only every other day (qod) (Online Supplementary Figure S7F). After 21 days from pellet implantation, the mice were sacrificed and the spleen weight was analyzed. This end-point was designed to isolate leukemic growth from confounding factors of toxicity. We observed a significant dose-dependent effect of combination retinoid treatment in reducing the tumor burden (spleen weight) of treated mice compared to the control cohort (Figure 7B). Of note, the in vivo steady-state serum concentration of ATRA resulting from 10 mg 21-day release pellets is reported as 100-220 nM,49 and the 5 mg pellets did not provide a sufficient dose to inhibit leukemic growth.
Third, we suspected that some of the toxicity might be due to retinoid-induced radiation recall, a known complication of retinoids,50,51 and tolerability might be improved using a micronized formula of bexarotene found in the clinical Targretin formulation.52 We found that MLL-AF9 leukemia cells engrafted consistently following IP injection without irradiation conditioning, with improved fur ruffling, improved wound healing, and no weight loss (Online Supplementary Figure S7G). However, the mice again exhibited reduced activity the day after gavage and administration was again reduced to qod due to tolerability. Despite this, the combination of ATRA/bexarotene was associated with improved survival (Figure 7D and E). This study was repeated a fourth time with similar modest, but significant, improvement in survival and tolerability issues that persisted (data not shown). Thus, retinoid delivery to mice in the context of leukemic models remains a challenge, but is still associated with a statistical and reproducible survival advantage, although modest.
Discussion
Retinoid therapy has transformed the treatment of APL.16,17 Retinoids have been explored in different clinical trials in non-APL, and their action has repeatedly been shown, although never to the extent seen in APL.18
We previously found that natural RXRA ligands are present in mouse hematopoietic cells in vivo, that they are biased toward myeloid cells and dynamically regulated under myeloid stress (e.g., GCSF treatment).14 Consistent with those findings, in this study we found also that a malignant myeloid stressor (MLL-AF9 transformation) was associated with natural in vivo RXRA ligands. In vivo natural RXRA ligands resulted in incomplete reporter activation, and pharmacologic doses of ligands could activate the reporter further under both basal conditions14 and leukemic stress (Figure 1). In contrast to these RXRA results, we have been unable to detect natural RARA ligands (Figure 1G).14,22
We found that MLL-AF9 cells that carried deleted Rxra and Rxrb alleles had a competitive advantage during MLLAF9 leukemogenesis (Figure 2C and F). Whereas Notchderived T-cell leukemias and TLS-ERG-derived erythroleukemia were not associated with in vivo natural RXRA ligands, and also were not associated with a competitive advantage among cells with Rxra or Rxrb deletion (Figures 1 and 2). Thus, the presence versus absence of active natural ligands may determine whether RXR exert tumor suppressor activity.
RXR form heterodimers with a wide range of orphan nuclear receptors. Other groups have reported co-operativity of RXR ligands with either RARA ligands37 or LXR ligands.53MLL-AF9 leukemia cells appeared most sensitive to the combination of RARA and RXR ligands (Figure 3). Culture conditions influenced retinoid responses, and we observed that combinations of retinoids induced either maturation or apoptosis depending on whether the leukemia cells were grown in methylcellulose or liquid culture (Figure 4). The relevant cell signals that influence retinoid responses in these two settings still have to be defined.
The synergy of ATRA and bexarotene can be understood within the context of co-repressor interactions with the RARA:RXRA heterodimer. Other studies of the RARA:RXRA heterodimer identified a subordinate role for RXR.32,39,41,42 Consistent with those results, mammalian two-hybrid assays demonstrated that RARA bound more effectively to co-repressors, that ATRA was more efficient than bexarotene at releasing co-repressors from RARA:RXRA, and that these limitations could be overcome using a bexarotene-derivative compound with dual RARA/RXRA activity (CW103-4) (Figure 5). Thus, the ATRA-dependent release of co-repressor from the RARA:RXRA heterodimer may account for the synergy observed between ATRA and bexarotene.
Within the RARA:RXRA heterodimer, specific RXRA domains appear necessary for anti-leukemic effects, including the AF1, DBD, and AF2, as well as amino acids responsible for ligand-binding and co-activator recruitment (Figure 3G). Thus, although ligand activation of RXR may be subordinate to RAR, RXR play an active, not a passive role in anti-leukemic response to ligands.
Other studies have suggested that biomarkers may identify subsets of retinoid-sensitive patients.54,55 We note that expression of RARA and RXRA are co-ordinately regulated in AML with highest expression in M4/M5-FAB AML (Online Supplementary Figure S1). Cell lines and primary AML samples suggest that combination retinoids may be active in leukemias beyond the mouse MLL-AF9 model and a potential bias in sensitivity within this group of patients (Figure 6). Larger studies will be required to accurately determine the specificity and sensitivity of these biomarkers as predictors of retinoid responses in non-APL AML. We have established an on-going ex vivo biomarker study of primary AML samples (clinicaltrials.gov identifier: NCT04263181) to further address this issue.
Although both ATRA and bexarotene are well-tolerated orally-available compounds, approved by the US Food and Drug Administration, we found it difficult to provide mice with a sufficient dose to enable us to observe any big effects (Figure 7). Both oral ATRA and bexarotene are poorly soluble, have short serum halflives (1 and 7 h, respectively), and their BM concentrations may be locally reduced by stroma expression of P450 enzymes.56 Given the short serum half-life of bexarotene, our administration of bexarotene (given every other day) is likely associated with a suboptimal area under the curve and incomplete receptor activation in vivo. Although ATRA is highly active against APL, administration and in vivo activity of ATRA in mouse models of APL have been surprisingly modest. For example, across multiple mouse models, ATRA improved median survival in secondary transplants: 53 days versus 31 days,57 35 versus 25 days,58 approximately 90 days versus approximately 50 days,59 approximately 75 days versus approximately 35 days,60 approximately 45 days versus approximately 35 days,61 and approximately 130 days versus approximately 85 days.49 In contrast, Westervelt et al. found that a liposomal formulation of ATRA (that is now no longer commercially available) resulted in much higher levels of ATRA in mice than ATRA slow-release pellets, and in 88% long-term survival. Therefore, additional chemical or formulaic modifications may be required to optimize in vivo retinoids as anti-leukemic therapeutics, and the combination of free acid ATRA and bexarotene is not sufficient to overcome these limitations.
In summary, we find evidence of natural RXRA, but not RARA ligands in myelomonocytic MLL-AF9 mouse leukemia in vivo where the concurrent presence of receptors and active ligands acts as tumor suppressors. RARA and RXRA are co-ordinately expressed in myelomonocytic leukemias, and optimal retinoid activation appears to require concurrent activation of both elements in the RARA:RXRA heterodimer, providing a further step toward improved retinoid therapeutics in AML.
Supplementary Material
Acknowledgments
We thank the Alvin J. Siteman Cancer Center at Washington University School of Medicine and Barnes-Jewish Hospital in St. Louis, MO, USA for the use of the Flow Cytometry Core. The Siteman Cancer Center is supported in part by an NCI Cancer Center Support Grant P30 CA91842. We thank High- Throughput Screening Center at Washington University School of Medicine in St. Louis, MO, USA. We thank Deborah Laflamme, Conner York, and Julie Richie for technical assistance.
Funding Statement
Funding: This work was supported by NIH R01 HL128447 (to JSW), NIH P50 CA171963 (Project 1, to JSW and DRP) by the Siteman Investment Program (to JSW), and grants from the Spanish Ministerio de Ciencia e Innovación (MCI) (SAF2015-71878-REDT-NurCaMeIn, RTI2018-095928-B100) (to MR). The CNIC is supported by the MCI and the Pro CNIC Foundation and is a Severo Ochoa Center of Excellence (SEV- 2015-0505).
References
- 1.Bushue N, Wan YJ. Retinoid pathway and cancer therapeutics. Adv Drug Deliv Rev. 2010;62(13):1285-1298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Oren T, Sher JA, Evans T. Hematopoiesis and retinoids: development and disease. Leuk Lymphoma. 2003;44(11):1881-1891. [DOI] [PubMed] [Google Scholar]
- 3.Kastner P, Lawrence HJ, Waltzinger C, Ghyselinck NB, Chambon P, Chan S. Positive and negative regulation of granulopoiesis by endogenous RARalpha. Blood. 2001;97(5):1314-1320. [DOI] [PubMed] [Google Scholar]
- 4.Evans RM, Mangelsdorf DJ. Nuclear receptors, RXR, and the Big Bang. Cell. 2014; 157(1):255-266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Purton LE, Dworkin S, Olsen GH, et al. RARgamma is critical for maintaining a balance between hematopoietic stem cell selfrenewal and differentiation. J Exp Med. 2006;203(5):1283-1293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lefebvre P, Benomar Y, Staels B. Retinoid X receptors: common heterodimerization partners with distinct functions. Trends Endocrinol Metab. 2010;21(11):676-683. [DOI] [PubMed] [Google Scholar]
- 7.Network TCGAR. Genomic and epigenomic landscapes of adult de novo acute myeloid leukemia. N Engl J Med. 2013; 368(22):2059-2074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Loinder K, Soderstrom M. The nuclear receptor corepressor (N-CoR) modulates basal and activated transcription of genes controlled by retinoic acid. J Steroid Biochem Mol Biol. 2003;84(1):15-21. [DOI] [PubMed] [Google Scholar]
- 9.Martino OD, Welch JS. Retinoic acid receptors in acute myeloid leukemia therapy. Cancers (Basel). 2019;11(12):1915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Altucci L, Gronemeyer H. The promise of retinoids to fight against cancer. Nat Rev Cancer. 2001;1(3):181-193. [DOI] [PubMed] [Google Scholar]
- 11.Arnold SL, Amory JK, Walsh TJ, Isoherranen N. A sensitive and specific method for measurement of multiple retinoids in human serum with UHPLCMS/ MS. J Lipid Res. 2012;53(3):587-598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Jones JW, Pierzchalski K, Yu J, Kane MA. Use of fast HPLC multiple reaction monitoring cubed for endogenous retinoic acid quantification in complex matrices. Anal Chem. 2015;87(6):3222-3230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lengqvist J, Mata De Urquiza A, Bergman AC, et al. Polyunsaturated fatty acids including docosahexaenoic and arachidonic acid bind to the retinoid X receptor alpha ligand-binding domain. Mol Cell Proteomics. 2004;3(7):692-703. [DOI] [PubMed] [Google Scholar]
- 14.Niu H, Fujiwara H, di Martino O, et al. Endogenous retinoid X receptor ligands in mouse hematopoietic cells. Sci Signal. 2017;10(503):eaan1011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ruhl R, Krzyzosiak A, Niewiadomska-Cimicka A, et al. 9-cis-13,14-dihydroretinoic acid is an endogenous retinoid acting as RXR ligand in mice. PLoS Genet. 2015;11(6):e1005213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lo-Coco F, Avvisati G, Vignetti M, et al. Retinoic acid and arsenic trioxide for acute promyelocytic leukemia. N Engl J Med. 2013;369(2):111-121. [DOI] [PubMed] [Google Scholar]
- 17.Wang ZY, Chen Z. Acute promyelocytic leukemia: from highly fatal to highly curable. Blood. 2008;111(5):2505-2515. [DOI] [PubMed] [Google Scholar]
- 18.Brown G, Hughes P. Retinoid differentiation therapy for common types of acute myeloid leukemia. Leuk Res Treatment. 2012;2012:939021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Querfeld C, Rosen ST, Guitart J, et al. Comparison of selective retinoic acid receptor- and retinoic X receptor-mediated efficacy, tolerance, and survival in cutaneous T-cell lymphoma. J Am Acad Dermatol. 2004;51(1):25-32. [DOI] [PubMed] [Google Scholar]
- 20.Tsai DE, Luger SM, Andreadis C, et al. A phase I study of bexarotene, a retinoic X receptor agonist, in non-M3 acute myeloid leukemia. Clin Cancer Res. 2008;14(17):5619-5625. [DOI] [PubMed] [Google Scholar]
- 21.Welch JS, Niu H, Uy GL, et al. A phase I dose escalation study of oral bexarotene in combination with intravenous decitabine in patients with AML. Am J Hematol. 2014;89(8):E103-108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Niu H, Chacko J, Hadwiger G, Welch JS. Absence of natural intracellular retinoids in mouse bone marrow cells and implications for PML-RARA transformation. Blood Cancer J. 2015;5:e284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Menendez-Gutierrez MP, Roszer T, Fuentes L, et al. Retinoid X receptors orchestrate osteoclast differentiation and postnatal bone remodeling. J Clin Invest. 2015;125(2):809-823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ianevski A, He L, Aittokallio T, Tang J. SynergyFinder: a web application for analyzing drug combination dose-response matrix data. Bioinformatics. 2017; 33(15):2413-2415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Alvarez S, Alvarez R, Khanwalkar H, et al. Retinoid receptor subtype-selective modulators through synthetic modifications of RARgamma agonists. Bioorg Med Chem. 2009;17(13):4345-4359. [DOI] [PubMed] [Google Scholar]
- 26.Pear WS, Aster JC, Scott ML, et al. Exclusive development of T cell neoplasms in mice transplanted with bone marrow expressing activated Notch alleles. J Exp Med. 1996;183(5):2283-2291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Carmichael CL, Metcalf D, Henley KJ, et al. Hematopoietic overexpression of the transcription factor Erg induces lymphoid and erythro-megakaryocytic leukemia. Proc Natl Acad Sci U S A. 2012;109(38):15437-15442 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ricote M, Snyder CS, Leung HY, Chen J, Chien KR, Glass CK. Normal hematopoiesis after conditional targeting of RXRalpha in murine hematopoietic stem/progenitor cells. J Leukoc Biol. 2006;80(4):850-861. [DOI] [PubMed] [Google Scholar]
- 29.Temple KA, Cohen RN, Wondisford SR, Yu C, Deplewski D, Wondisford FE. An intact DNA-binding domain is not required for peroxisome proliferator-activated receptor gamma (PPARgamma) binding and activation on some PPAR response elements. J Biol Chem. 2005;280(5):3529-3540. [DOI] [PubMed] [Google Scholar]
- 30.Leo C, Yang X, Liu J, Li H, Chen JD. Role of retinoid receptor coactivator pockets in cofactor recruitment and transcriptional regulation. J Biol Chem. 2001;276(25):23127-23134. [DOI] [PubMed] [Google Scholar]
- 31.Hiromori Y, Aoki A, Nishikawa J, Nagase H, Nakanishi T. Transactivation of the human retinoid X receptor by organotins: use of site-directed mutagenesis to identify critical amino acid residues for organotininduced transactivation. Metallomics. 2015;7(7):1180-1188. [DOI] [PubMed] [Google Scholar]
- 32.Ghosh JC, Yang X, Zhang A, et al. Interactions that determine the assembly of a retinoid X receptor/corepressor complex. Proc Natl Acad Sci U S A. 2002;99(9):5842-5847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Jusu S, Presley JF, Kremer R. Phosphorylation of human retinoid X receptor alpha at Serine 260 impairs its subcellular localization, receptor interaction, nuclear mobility, and 1alpha,25-dihydroxyvitamin D3-dependent DNA binding in Ras-transformed keratinocytes. J Biol Chem. 2017;292(4):1490-1509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Vivat-Hannah V, Bourguet W, Gottardis M, Gronemeyer H. Separation of retinoid X receptor homo- and heterodimerization functions. Mol Cell Biol. 2003;23(21):7678-7688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Welch JS, Klco JM, Varghese N, Nagarajan R, Ley TJ. Rara haploinsufficiency modestly influences the phenotype of acute promyelocytic leukemia in mice. Blood. 2011;117(8):2460-2468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kastner P, Chan S. Function of RARalpha during the maturation of neutrophils. Oncogene. 2001;20(49):7178-7185. [DOI] [PubMed] [Google Scholar]
- 37.Kizaki M, Dawson MI, Heyman R, et al. Effects of novel retinoid X receptor-selective ligands on myeloid leukemia differentiation and proliferation in vitro. Blood. 1996;87(5):1977-1984. [PubMed] [Google Scholar]
- 38.Collins SJ. Retinoic acid receptors, hematopoiesis and leukemogenesis. Curr Opin Hematol. 2008;15(4):346-351. [DOI] [PubMed] [Google Scholar]
- 39.Altucci L, Rossin A, Hirsch O, et al. Rexinoid-triggered differentiation and tumor-selective apoptosis of acute myeloid leukemia by protein kinase A-mediated desubordination of retinoid X receptor. Cancer Res. 2005;65(19):8754-8765. [DOI] [PubMed] [Google Scholar]
- 40.Lammi J, Perlmann T, Aarnisalo P. Corepressor interaction differentiates the permissive and non-permissive retinoid X receptor heterodimers. Arch Biochem Biophys. 2008;472(2):105-114. [DOI] [PubMed] [Google Scholar]
- 41.Pogenberg V, Guichou JF, Vivat-Hannah V, et al. Characterization of the interaction between retinoic acid receptor/retinoid X receptor (RAR/RXR) heterodimers and transcriptional coactivators through structural and fluorescence anisotropy studies. J Biol Chem. 2005;280(2):1625-1633. [DOI] [PubMed] [Google Scholar]
- 42.Hu X, Li Y, Lazar MA. Determinants of CoRNR-dependent repression complex assembly on nuclear hormone receptors. Mol Cell Biol. 2001;21(5):1747-1758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Zhang J, Hu X, Lazar MA. A novel role for helix 12 of retinoid X receptor in regulating repression. Mol Cell Biol. 1999;19(9):6448-6457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Hanish BJ, Hackney Price JF, Kaneko I, et al. A novel gene expression analytics-based approach to structure aided design of rexinoids for development as next-generation cancer therapeutics. Steroids. 2018;135:36-49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Jurutka PW, Kaneko I, Yang J, et al. Modeling, synthesis, and biological evaluation of potential retinoid X receptor (RXR) selective agonists: novel analogues of 4-[1- (3,5,5,8,8-pentamethyl-5,6,7,8-tetrahydro- 2-naphthyl)ethynyl]benzoic acid (bexarotene) and (E)-3-(3-(1,2,3,4-tetrahydro- 1,1,4,4,6-pentamethylnaphthalen-7- yl)-4-hydroxypheny l)acrylic acid (CD3254). J Med Chem. 2013;56(21):8432-8454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Marshall PA, Jurutka PW, Wagner CE, et al. Analysis of differential secondary effects of novel rexinoids: select rexinoid X receptor ligands demonstrate differentiated side effect profiles. Pharmacol Res Perspect. 2015;3(2):e00122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Shih AH, Jiang Y, Meydan C, et al. Mutational cooperativity linked to combinatorial epigenetic gain of function in acute myeloid leukemia. Cancer Cell. 2015;27(4):502-515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Neophytou CM, Constantinou AI. Drug delivery innovations for enhancing the anticancer potential of vitamin E isoforms and their derivatives. Biomed Res Int. 2015;2015:584862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Westervelt P, Pollock JL, Oldfather KM, et al. Adaptive immunity cooperates with liposomal all-trans-retinoic acid (ATRA) to facilitate long-term molecular remissions in mice with acute promyelocytic leukemia. Proc Natl Acad Sci U S A. 2002;99(14):9468-9473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Malik SM, Collins B, Pishvaian M, Ramzi P, Marshall J, Hwang J. A phase I trial of bexarotene in combination with docetaxel in patients with advanced solid tumors. Clin Lung Cancer. 2011;12(4):231-236. [DOI] [PubMed] [Google Scholar]
- 51.Noble S, Wagstaff AJ. Tretinoin. A review of its pharmacological properties and clinical efficacy in the topical treatment of photodamaged skin. Drugs Aging. 1995;6(6):479-496. [DOI] [PubMed] [Google Scholar]
- 52.Tesseur I, Lo AC, Roberfroid A, et al. Comment on “ApoE-Directed Therapeutics Rapidly Clear β-Amyloid and Reverse Deficits in AD Mouse Models”. Science. 2013;340(6135):924-e. [DOI] [PubMed] [Google Scholar]
- 53.Sanchez PV, Glantz ST, Scotland S, Kasner MT, Carroll M. Induced differentiation of acute myeloid leukemia cells by activation of retinoid X and liver X receptors. Leukemia. 2014;28(4):749-760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.McKeown MR, Corces MR, Eaton ML, et al. Super-enhancer analysis defines novel epigenomic subtypes of non-APL AML including an RARalpha dependency targetable by SY-1425, a potent and selective RARalpha agonist. Cancer Discov. 2017;7(10):1136-1153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Mugoni V, Panella R, Cheloni G, et al. Vulnerabilities in mIDH2 AML confer sensitivity to APL-like targeted combination therapy. Cell Res. 2019;29(6):446-459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Hernandez D, Palau L, Norsworthy K, et al. Overcoming microenvironment-mediated protection from ATRA using CYP26-resistant retinoids. Leukemia. 2020;34(11):3077-3081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Keeshan K, Vieugue P, Chaudhury S, et al. Co-operative leukemogenesis in acute myeloid leukemia and acute promyelocytic leukemia reveals C/EBPalpha as a common target of TRIB1 and PML/RARA. Haematologica. 2016;101(10):1228-1236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Lu Y, Yan JS, Xia L, et al. 2-bromopalmitate targets retinoic acid receptor alpha and overcomes all-trans retinoic acid resistance of acute promyelocytic leukemia. Haematologica. 2019;104(1):102-112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Lallemand-Breitenbach V, Guillemin MC, Janin A, et al. Retinoic acid and arsenic synergize to eradicate leukemic cells in a mouse model of acute promyelocytic leukemia. J Exp Med. 1999;189(7):1043-1052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Omidvar N, Maunakea ML, Jones L, et al. PML-RARalpha co-operates with Sox4 in acute myeloid leukemia development in mice. Haematologica. 2013;98(3):424-427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Coltella N, Valsecchi R, Ponente M, Ponzoni M, Bernardi R. Synergistic leukemia eradication by combined treatment with retinoic acid and HIF inhibition by EZN-2208 (PEG-SN38) in preclinical models of PML-RARalpha and PLZFRARalpha- driven leukemia. Clin Cancer Res. 2015;21(16):3685-3694. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.