To the Editor,
COVID-19 is an ongoing pandemic condition caused by SARS-CoV-2 infection, characterized by a broad spectrum of clinical signs, ranging from asymptomatic or mild to severe illness that requires hospitalization and mechanical ventilation [1]. Currently, diagnosis of COVID-19 depends only by the detection of SARS-CoV-2 RNA by real-time polymerase chain reaction (PCR), which is considered the “gold standard” to achieve the diagnosis [2].
Compelling evidence revealed the ability of SARS-CoV-2 to cause widely different pathological conditions, including hematological, cardiovascular, renal, gastrointestinal, and neurological signs [1]. These systemic clinical manifestations of COVID-19 suggest the possibility that the spread of SARS-CoV-2 within extra-pulmonary sites may occur via blood flow. In this scenario, over the last few months several research groups have investigated the presence of viral particles in the blood of SARS-CoV-2 infected subjects [1,3,4]. For instance, detection of viral RNA in the sera from COVID-19 patients has been observed; indeed, Chen et al. revealed that serological levels of SARS-CoV-2 RNA from COVID-19 patients associated with high interleukin (IL)-6 concentration and poor prognosis [5]. On the contrary, Zheng and co-workers reported no significantly correlation between serum viral RNA levels and the grade of disease severity in COVID-19 patients [3]. It is likely that these contrasting results are due to the high instability of viral RNA molecules [4], underlying the requirement of other more sensitive assays.
Very recent published evidence suggested that detection of viral proteins in the blood might represent a novel approach to improve diagnosis of COVID-19 [6,7], the association between serum levels of SARS-CoV-2 antigens and inflammatory molecules is not completely explored, to date.
Here, we evaluated the circulating levels of SARS-CoV-2 N-antigen in serum from hospitalized COVID-19 patients and its correlation with inflammatory status, measured as C-Protein Reactive (CRP) levels.
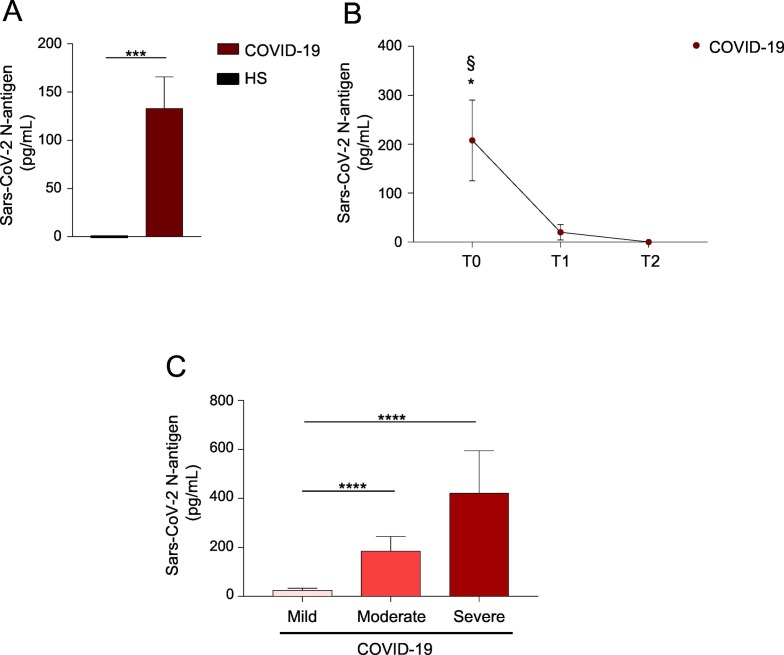
To this aim, serum samples from a large cohort (n = 233) of hospitalized COVID-19 patients and from healthy individuals (n = 61) (see Supplementary Table 1 for baseline characteristics) were analyzed to detect SARS-CoV-2 N-antigen, by using Lumipulse® G SARS-CoV-2 Ag CLEIA. COVID-19 subjects expressed SARS-CoV-2 N-antigen in the blood, while no detectable amounts were observed in healthy individuals (Fig. 1A). Specificity of this assay was 100% (95% confidence interval (CI) 98.4–100) and sensitivity was 62% (95% CI 56.4–68.0). Further, in a subgroup of COVID-19 subjects (n = 36), evaluation of SARS-CoV-2 N-antigen from time of diagnosis (T0) to recovery (T2) revealed that circulating levels of the N-antigen progressively decline overtime (Fig. 1B).
Fig. 1.
Detection of SARS-CoV-2 N-antigen in the serum of COVID-19 patients associates with inflammation.
(A) Bar histogram showing SARS-CoV-2 N-antigen concentrations (pg/mL) in serum of COVID-19 and healthy subjects (HS). ***P < 0.001 by Mann-Whitney U test. (B) SARS-CoV-2 N-antigen concentrations in COVID-19 patients from diagnosis (T0), to clinical improvement (T1) and until recovery (T2). *P < 0.05 T0 versus T1; §P < 0.01 T0 versus T2 by one-way ANOVA test, multiple comparison corrected for Bonferroni's test. (C) Bar histogram showing SARS-CoV-2 N-antigen concentration in COVID-19 patients stratified on the basis of serum CRP levels, as mild (≤ 5 mg/L), moderate (6–20 mg/L) and severe (21–80 mg/L) disease. ****P < 0.0001 by Mann-Whitney U test. Data are shown as mean ± S.E.M..
The N-protein is a highly immunogenic and abundantly expressed protein during SARS-CoV-2 infection, which participates in RNA package and viral replication [8]. It is likely that both N-protein and viral RNA circulate in peripheral blood, as viral particles. However, compared to the high instability of viral RNA [4], proteins are more stable with a lower rate of degradation, thus persisting in the circulation longer that viral RNA. In this study the presence in the serum of both SARS-CoV-2 N-antigen and RNA was found only in a subgroup of the above-mentioned COVID-19 patients (data not shown), supporting the concept that detection of protein is more sensitive compared to RNA measurement.
Other research groups have recently measured SARS-CoV-2 antigens in the blood of COVID-19 affected individuals, by using different commercial assays [6,7]. Among these, Ogata and co-workers detected SARS-CoV-2 N and spike (S)1 antigens in the plasma of patients with COVID-19, and found that concentrations of S1 antigen positively associated with intensive care unit (ICU) admission [7].
Similarly, we observed that the serum levels of SARS-CoV-2 N-antigen was higher in COVID-19 patients admitted to ICU (Supplementary Table 2).
Next, patients were stratified on the basis of serum CRP levels, as mild (≤ 5 mg/L), moderate (6–20 mg/L) and severe (21–80 mg/L) COVID-19 subjects (Supplementary Table 3) to ascertain a possible association between serum SARS-CoV-2 N-antigen and inflammatory status. This analysis indicated that serum SARS-CoV-2 N-antigen concentration progressively increased together with inflammatory status and disease severity, as patients with higher levels of CRP had high concentration of SARS-CoV-2 N-antigen in the blood (Fig. 1C, and Supplementary Table 3). Our study is the first associating SARS-CoV-2 N-antigen concentration in the blood of COVID-19 patients with inflammatory status and disease severity, measured as CRP levels. It is well known that CRP correlated with the inflammation status, thus it can activate the complement, enhance phagocytosis and favor the release of pro-inflammatory cytokines [9]. Further, CRP concentrations are not affected by demographical factors such as age, sex and physical condition [10]. Recent findings revealed that CRP levels are positively associated with lung lesion and disease severity in COVID-19 patients, suggesting that higher levels of CRP reflect a more severe disease and worse prognosis [10]. Therefore, it may be a suitable marker for the diagnosis and assessment of severe lung injury in COVID-19 individuals.
Although it is not clear whether circulation of viral proteins impact on physiopathology of COVID-19, detection of SARS-CoV-2 N-antigen may have important clinical implications. For example, it may represent an innovative molecular biomarker to monitor COVID-19 patients with different systemic inflammatory response. However, additional studies are required to define whether the presence of viral particles in the blood could account for systemic diffusion and multi-organ involvement in SARS-CoV-2 infection.
In conclusion, our findings revealed that serum levels of SARS-CoV-2 N-protein associated with inflammation, disease severity and overtime progression in hospitalized COVID-19 patients. Despite detection of SARS-CoV-2 N-protein in the blood cannot be use as diagnostic tool, it may represent an additional valuable approach to help in monitoring COVID-19 viral load and disease progression.
Author contribution
S.B., E.P. and S.S. performed most of the experiments and data analyses; V.M. and N.P. performed molecular experiments and data analyses; F.P. and S.B. analyzed the data and interpreted the results; Li.A, A.S. and C.N. provided human samples; F.P., S.B., A.S., M.B., G.M., M.G. and Lu.A. were involved in the data discussion; F.P., G.M., M.G. and Lu.A. designed the study and wrote the manuscript.
Declaration of Competing Interest
The authors declare no conflict of interest.
Acknowledgments
We thank Montagna M. and De Simone S. for technical assistant. This paper was supported by grants from Fondazione Italiana Sclerosi Multipla (FISM, n° 2016/R/18 and 2018/S/5), Progetti di Rilevante Interesse Nazionale (PRIN, n° 2017 K55HLC 001) and Ministero della Salute (n° RF-2019-12371111) for G.M.; European Foundation for the Study of Diabetes (EFSD)/Novo Nordisk Programme for Diabetes Research in Europe 2020 for M.G..
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.clim.2021.108720.
Appendix A. Supplementary data
Supplementary material
References
- 1.Huang C., Wang Y., Li X., Ren L., Zhao J., Hu Y., Zhang L., Fan G., Xu J., Gu X., Cheng Z., Yu T., Xia J., Wei Y., Wu W., Xie X., Yin W., Li H., Liu M., Xiao Y., Gao H., Guo L., Xie J., Wang G., Jiang R., Gao Z., Jin Q., Wang J., Cao B. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet. 2020;395:497–506. doi: 10.1016/S0140-6736(20)30183-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kevadiya B.D., Machhi J., Herskovitz J., Oleynikov M.D., Blomberg W.R., Bajwa N., Soni D., Das S., Hasan M., Patel M., Senan A.M., Gorantla S., McMillan J., Edagwa B., Eisenberg R., Gurumurthy C.B., Reid S.P.M., Punyadeera C., Chang L., Gendelman H.E. Diagnostics for SARS-CoV-2 infections. Nat. Mater. 2021 doi: 10.1038/s41563-020-00906-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Zheng S., Fan J., Yu F., Feng B., Lou B., Zou Q., Xie G., Lin S., Wang R., Yang X., Chen W., Wang Q., Zhang D., Liu Y., Gong R., Ma Z., Lu S., Xiao Y., Gu Y., Zhang J., Yao H., Xu K., Lu X., Wei G., Zhou J., Fang Q., Cai H., Qiu Y., Sheng J., Chen Y., Liang T. Viral load dynamics and disease severity in patients infected with SARS-CoV-2 in Zhejiang province, China, January-march 2020: retrospective cohort study. BMJ. 2020;369:m1443. doi: 10.1136/bmj.m1443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Chang L., Yan Y., Wang L. Coronavirus disease 2019: coronaviruses and blood safety. Transfus. Med. Rev. 2020;34:75–80. doi: 10.1016/j.tmrv.2020.02.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Chen X., Zhao B., Qu Y., Chen Y., Xiong J., Feng Y., Men D., Huang Q., Liu Y., Yang B., Ding J., Li F. Detectable serum severe acute respiratory syndrome coronavirus 2 viral load (RNAemia) is closely correlated with drastically elevated interleukin 6 level in critically ill patients with coronavirus disease 2019. Clin. Infect. Dis. 2020;71:1937–1942. doi: 10.1093/cid/ciaa449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hingrat Q.L., Visseaux B., Laouenan C., Tubiana S., Bouadma L., Yazdanpanah Y., Duval X., Burdet C., Ichou H., Damond F., Bertine M., Benmalek N., Choquet C., Timsit J.F., Ghosn J., Charpentier C., Descamps D., Houhou-Fidouh N., C.-C.S.G. French Covid Cohort Management Committee, C.C.S.G. Members of the French, V.C.S.G.P.I. Member of the Co, C. Steering, V.C.C.C. Co, Coordination, A. statistical, L. Virological, C. Biological, Partners, Sponsor, Genetic Detection of SARS-CoV-2 N-antigen in blood during acute COVID-19 provides a sensitive new marker and new testing alternatives. Clin. Microbiol. Infect. 2020 doi: 10.1016/j.cmi.2020.11.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ogata A.F., Maley A.M., Wu C., Gilboa T., Norman M., Lazarovits R., Mao C.P., Newton G., Chang M., Nguyen K., Kamkaew M., Zhu Q., Gibson T.E., Ryan E.T., Charles R.C., Marasco W.A., Walt D.R. Ultra-sensitive serial profiling of SARS-CoV-2 antigens and antibodies in plasma to understand disease progression in COVID-19 patients with severe disease. Clin. Chem. 2020 doi: 10.1093/clinchem/hvaa213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Cong Y., Ulasli M., Schepers H., Mauthe M., V'Kovski P., Kriegenburg F., Thiel V., de Haan C.A.M., Reggiori F. Nucleocapsid protein recruitment to replication-transcription complexes plays a crucial role in coronaviral life cycle. J. Virol. 2020;94 doi: 10.1128/JVI.01925-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Sproston N.R., Ashworth J.J. Role of C-reactive protein at sites of inflammation and infection. Front. Immunol. 2018;9:754. doi: 10.3389/fimmu.2018.00754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Wang L. C-reactive protein levels in the early stage of COVID-19. Med. Mal. Infect. 2020;50:332–334. doi: 10.1016/j.medmal.2020.03.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary material