Abstract
Background
COVID-19 is a multisystemic disorder. Hematologic and cardiovascular involvement of COVID-19 causes thromboembolic events across multiple organs which mainly manifest as venous thromboembolism, and rarely, peripheral arterial thromboembolic events. In-situ thrombosis of a healthy, non-atherosclerotic native artery is rare, and COVID-19 has been reported to be a cause of this phenomenon. We aimed to report our institutional experience with COVID-19 patients who developed acute limb ischemia (ALI) during hospitalization or after discharge.
Methods
This was a single-center cross-sectional study. Records of all patients ≥18 years of age admitted to a tertiary center with a confirmed diagnosis of COVID-19 infection between September 1 and December 31, 2020 were retrospectively examined. Data regarding patient demographics, co-morbidities and outcomes were collected. Patients were followed-up during index hospitalization and for 30 days postdischarge. Acute limb ischemia was diagnosed by means of duplex ultrasound and computed tomography angiography in the presence of a clinical suspicion.
Results
A total of 681 consecutive patients (38.5% women) were hospitalized with a confirmed diagnosis of COVID-19 during the study period. Median age was 63 years (IQR, 52–74). In-hospital mortality occurred in 94 (13.8%) patients. Ninety (13.2%) patients required intensive care unit admission at some point of their hospital stay. Six (0.9%) patients (one woman) with a median age of 62 years experienced ALI (IQR, 59–64.3). All patients were receiving low molecular weight heparin when they developed ALI. The median of duration between COVID-19 diagnosis and ALI symptom onset was 13 days (IQR, 11.3–14). Three patients underwent emergent surgical thrombectomy combined with systemic anticoagulation, and 3 received systemic anticoagulation alone. Two patients with ALI did not survive to hospital discharge. Among survivors, 1 patient underwent bilateral major amputations, and another underwent a minor amputation within 1 month of hospital discharge. Symptoms of ALI completely resolved in 2 patients without sequelae.
Conclusions
COVID-19 is a multisystemic disorder with involvement of hematologic and cardiovascular systems. Despite widespread use of thromboprophylaxis, hospitalized patients with COVID-19 are at increased risk of ALI, and subsequent limb loss or even death.
Introduction
At the end of 2019, a novel coronavirus, later named as severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), caused an outbreak emerging from Wuhan, China.1., 2., 3. The outbreak was soon declared as a global pandemic by the World Health Organization.4 The disease caused by SARS-CoV-2, coronavirus disease 2019 (COVID-19), is a multi-systemic disorder primarily involving respiratory, hematologic and cardiovascular systems.5 Hematologic and cardiovascular involvement of COVID-19 causes thromboembolic events across multiple organs which mainly manifest as venous thromboembolism (VTE).6., 7., 8. Peripheral arterial involvement is relatively rare.6., 7., 8.
Acute limb ischemia (ALI) is defined as a sudden decrease in arterial perfusion of an extremity associated with a threat to the viability of the extremity.9 In patients with limb ischemia, a symptom duration of less than 2 weeks is considered as acute.9 If not promptly recognized, ALI may cause major tissue or limb loss, or death.9 Etiology of ALI includes in-situ thrombosis of atherosclerotic plaques, embolization from proximal sources, acute failure of stents or grafts, iatrogenic thromboembolism, acute aortic syndromes, and traumatic arterial injuries.9 The most common sources of embolization are left atrial thrombus in patients with atrial fibrillation (AF), left ventricular mural thrombus after myocardial infarction (MI), prosthetic heart valves, cardiac vegetations, and aortic or peripheral arterial aneurysms.9 In-situ thrombosis of a healthy, nonatherosclerotic native artery is rare, and COVID-19 has been reported to be a cause of this phenomenon.10., 11., 12., 13.
The purpose of this study was to report our institutional experience with COVID-19 patients who developed ALI during hospitalization or after discharge.
Methods
This was a single-center cross-sectional study. Institutional Research Ethics Committee approved the study protocol (reference number, 514/192/55), and waived the need for informed consent due to retrospective design. All procedures related to the study were conducted in accordance with the ethical standards of the Helsinki Declaration. Records of all patients ≥18 years of age admitted to Department of Infectious Diseases at Kartal Dr. Lutfi Kirdar City Hospital with a confirmed diagnosis of COVID-19 infection between September 1 and December 31, 2020 were retrospectively examined. Patients younger than 18 years of age, patients with an unconfirmed diagnosis of COVID-19, and pregnant women were excluded. Data regarding patient demographics, co-morbidities and outcomes were collected. Patients were followed-up during index hospitalization and for 30 days postdischarge. All patients received low molecular weight heparin (LMWH) during hospital stay, and for 1 month after discharge if they did not have active bleeding or a high risk profile for major bleeding. Prophylactic dose LMWH was given if D-dimer level was <1000 ng/mL, and therapeutic doses were given if a patient had a D-dimer level of ≥1000 ng/mL or additional risk factors for venous or arterial thromboembolic events. Acute limb ischemia was diagnosed by means of duplex ultrasound (DUS) and computed tomography angiography (CTA) in the presence of a clinical suspicion. Absence of arterial flow on DUS imaging of an acutely threatened extremity prompted further investigation with CTA, which provided information regarding the extent of the occluded arterial segment, collaterals, and inflow and outflow target vessels. Continuous variables are presented as median (interquartile range [IQR]). Categorical variables are presented as absolute numbers (n) and proportions (%).
Results
A total of 681 consecutive patients were hospitalized with a confirmed diagnosis of COVID-19 during the study period (Table 1 ). Of those, 262 (38.5%) were women. The median age was 63 years (IQR, 52–74). In-hospital mortality occurred in 94 (13.8%) patients. Ninety (13.2%) patients required intensive care unit (ICU) admission at some point of their hospital stay. Sixteen (2.3%) patients were lost to follow-up after discharge. There were 339 (49.8%) patients with hypertension, 227 (33.3%) with diabetes, 92 (13.5%) with chronic obstructive pulmonary disease, 90 (13.2%) with ischemic heart disease, 87 (12.8%) with active malignancy, and 36 (5.3%) with chronic kidney disease.
Table 1.
Patients' demographics, co-morbidities and outcomes
| Variable | Value |
|---|---|
| Demographics | |
| Female sex, n (%) | 262 (38.5%) |
| Age (years), median (IQR) | 63 (52–74) |
| Co-morbidities, n (%) | |
| Hypertension | 339 (49.8%) |
| Diabetes | 227 (33.3%) |
| COPD | 92 (13.5%) |
| Ischemic heart disease | 90 (13.2%) |
| Active malignancy | 87 (12.8%) |
| Chronic kidney disease | 36 (5.3%) |
| ICU admission, n (%) | 90 (13.2%) |
| In-hospital all-cause mortality, n (%) | 94 (13.8%) |
COPD, chronic obstructive pulmonary disease; ICU, intensive care unit; IQR, interquartile range.
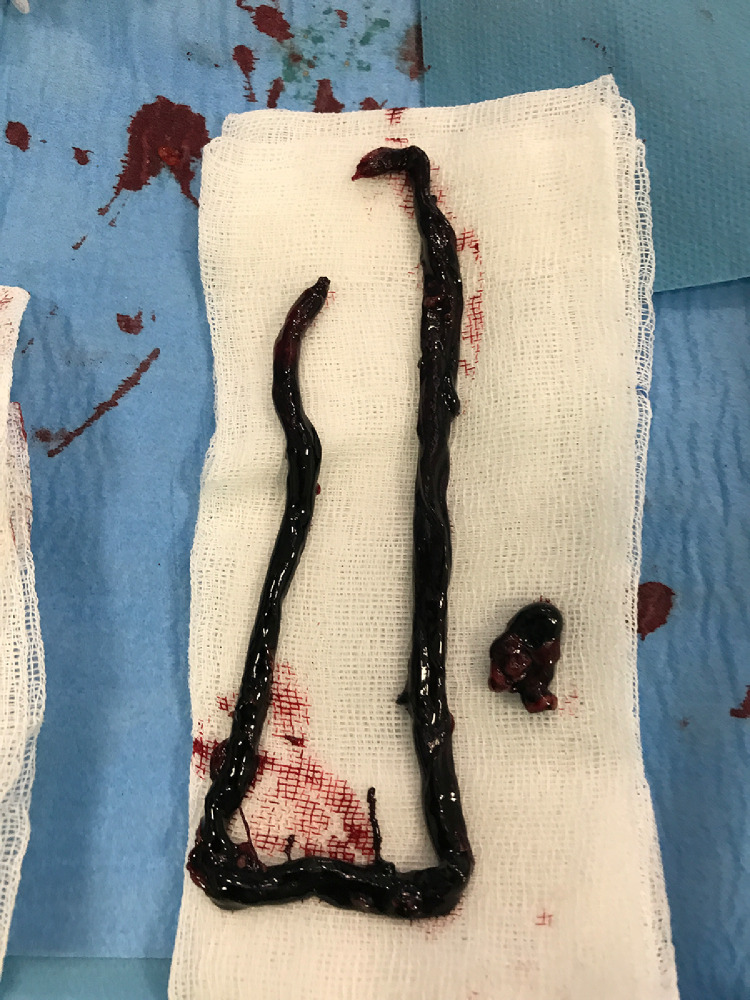
Six (0.9%) patients (one woman) with a median age of 62 years experienced ALI (IQR, 59–64.3) (Table 2 ). One of them had chronic peripheral artery disease (PAD), and none had atrial fibrillation, recent MI, prosthetic heart valves, endocarditis, or arterial aneurysms. Two patients were ex-smokers. The median of duration between COVID-19 diagnosis and ALI symptom onset was 13 days (IQR, 11.3–14). All patients were receiving LMWH injections when they developed ALI. Ischemia was clinically classified as Rutherford grade I in 1 patient, grade IIA in 2 patients, grade IIB in 2 patients, and grade III in 1 patient (Fig. 1 ).14 The involved segment was right ilio-femoral in 2 patients, bilateral popliteal in 2, left infrapopliteal in one, and left upper limb in one (Fig. 2 ). Three patients underwent emergent surgical thrombectomy followed by systemic anticoagulation with unfractionated heparin (UFH) infusion, and three received systemic anticoagulation with UFH infusion alone. Thrombus material obtained during thrombectomy procedures appeared more adhesive and viscous than our previous experience with ALI patients (Fig. 3 ). Two patients with ALI did not survive to hospital discharge. Among survivors, one patient underwent bilateral major amputations, and another underwent a minor amputation within one month of hospital discharge. Symptoms of ALI completely resolved in 2 patients without sequelae (Table 3 ).
Table 2.
Characteristics of patients with acute limb ischemia
| Variable | Value |
|---|---|
| Demographics | |
| Female sex, n (%) | 1 (16.7%) |
| Age (years), median (IQR) | 62 (59–64.3) |
| Risk factors, n (%) | |
| Peripheral artery disease | 1 (16.7%) |
| Atrial fibrillation | 0 (0%) |
| Recent myocardial infarction | 0 (0%) |
| Heart valve prosthesis | 0 (0%) |
| Endocarditis | 0 (0%) |
| Aortic aneurysm | 0 (0%) |
| Peripheral arterial aneurysm | 0 (0%) |
| Smoking, n (%) | |
| Ex- | 2 (33.3%) |
| Current | 0 (0%) |
| Time from COVID-19 diagnosis to ALI symptom onset (days), median (IQR) | 13 (11.3–14) |
| D-dimer level (ng/mL), median (IQR) | |
| On hospital admission | 715 (640–1127.5) |
| Highest | 7,085 (1712.5–13,942.5) |
| LMWH dose, n (%) | |
| Prophylactic dose | 2 (33.3%) |
| Therapeutic dose | 4 (66.7%) |
| Clinical grade of ALI,a n (%) | |
| I | 1 (16.7%) |
| IIA | 2 (33.3%) |
| IIB | 2 (33.3%) |
| III | 1 (16.7%) |
| Management of ALI, n (%) | |
| Systemic anticoagulation with UFH alone | 3 (50%) |
| Thrombectomy and systemic anticoagulation with UFH | 3 (50%) |
| Outcome, n (%) | |
| In-hospital mortality | 2 (33.3%) |
| Major amputation | 1 (16.7%) |
| Minor amputation | 1 (16.7%) |
| Cure | 2 (33.3%) |
According to Rutherford and colleagues.14
ALI, acute limb ischemia; LMWH, low molecular weight heparin; UFH, unfractionated heparin.
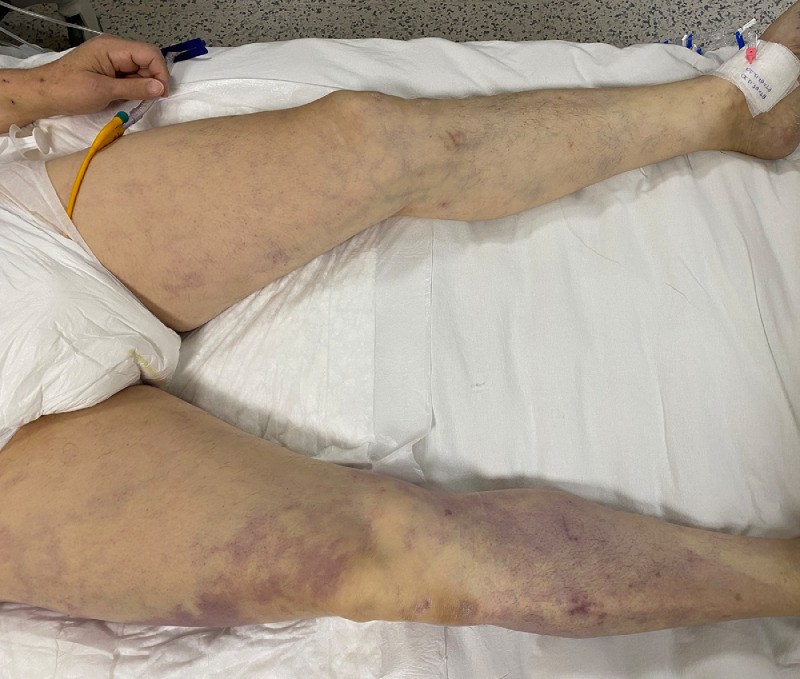
Fig. 1.
Clinical appearance of acute limb acute limb ischemia in a hospitalized patient with COVID-19 (Patient #2).
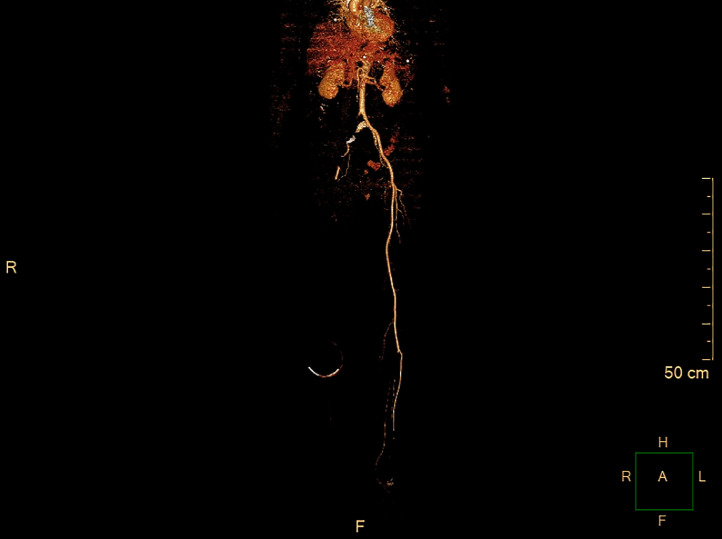
Fig. 2.
Computed tomography angiography demonstrating acute iliofemoral arterial occlusion in Patient #2.
Fig. 3.
Thrombus material obtained during surgical thrombectomy procedure performed on Patient #2.
Table 3.
Detailed characteristics of patients with acute limb ischemia
| Patient #1 | Patient #2 | Patient #3 | Patient #4 | Patient #5 | Patient #6 | |
|---|---|---|---|---|---|---|
| Sex | Male | Male | Male | Male | Female | Male |
| Age, years | 62 | 70 | 62 | 65 | 20 | 58 |
| Risk factors for ALI | None | None | None | PAD | None | None |
| Smoking | Ex-smoker | None | None | Ex-smoker | None | None |
| ALI symptom onset,a days | 12 | 8 | 14 | 11 | 21 | 14 |
| Initial D-dimer, ng/mL | 670 | 1,630 | 630 | 1,250 | 760 | 340 |
| Highest D-dimer, ng/mL | 950 | 4,000 | 10,170 | 35,000 | 760 | 15,200 |
| LMWH dose | Prophylactic | Therapeutic | Therapeutic | Therapeutic | Prophylactic | Therapeutic |
| Clinical grade of ALIb | IIA | IIB | III | IIA | I | IIB |
| Involved segment | Infrapopliteal | Iliofemoral | Iliofemoral | Popliteal | Upper limb | Popliteal |
| Involved side | Left | Right | Right | Bilateral | Left | Bilateral |
| Management of ALI | Thrombectomy and systemic anti-coagulation | Thrombectomy and systemic anti-coagulation | Systemic anti-coagulation alone | Systemic anti-coagulation alone | Systemic anti-coagulation alone | Thrombectomy and systemic anti-coagulation |
| Outcome | Minor amputation | Death | Death | Cure | Cure | Major amputation |
Time from COVID-19 diagnosis to ALI symptom onset.
According to Rutherford and colleagues.14
ALI, acute limb ischemia; LMWH, low molecular weight heparin; PAD, peripheral artery disease.
Discussion
The present study adds to the growing evidence that COVID-19 infection is associated with an increased incidence of ALI. We observed a 0.9% incidence of ALI in hospitalized COVID-19 patients in a tertiary center, a rate similar to previous COVID-19 series,8 , 15., 16., 17. much higher than general population,18 and even higher than hospitalized cancer patients.19 An early report by Bellosta and colleagues20 estimated an almost 6-fold increase in ALI cases in 2020 compared to 2019. Beyond that, occurrence of arterial thromboembolic events is associated with an increased overall mortality rate among hospitalized COVID-19 patients.8 , 15 , 17 In the present series, 2/6 of COVID-19 patients who developed ALI died.
Most patients with ALI in our study were young, 5/6 of them did not have any of the typical causes of ALI, and all were under LMWH when they developed ALI symptoms. Furthermore, all 3 patients who underwent surgery appeared to have relatively healthy and non-atherosclerotic vessels. This finding is in correlation with previous reports.7 , 11 , 12 , 16 , 20., 21., 22. The only patient with a considerable risk factor for ALI had chronic PAD with mild claudication and bilateral iliofemoral stenotic lesions on hospital admission. He developed ischemic rest pain on both legs 11 days after an initial diagnosis of COVID-19, and a CTA revealed de novo thrombosis of bilateral popliteal arteries. He was hemodynamically stable, and responded well to systemic UFH with complete resolution of ALI symptoms without surgical intervention. Whereas the prevalent PAD may have contributed to occurrence of acute ischemic symptoms in this patient, acute onset of bilateral popliteal artery occlusion in the absence of low perfusion state made us believe that ALI was associated with hypercoagulable state caused by COVID-19 infection. Rey and colleagues16 compared COVID-19-positive and COVID-19-negative patients who presented with acute arterial thrombotic events during the same time period, and reported a lower cardiovascular risk profile in the COVID-19 group. Our finding, along with results from previous studies, supports the evidence that COVID-19 infection may cause arterial thromboembolic events in low-risk patients despite the use of aggressive thromboprophylaxis.7 , 11 , 12 , 16 , 20., 21., 22. We believe clinicians should keep a low threshold to initiate therapeutic doses of anticoagulants in hospitalized COVID-19 patients since prophylactic doses appear inadequate to prevent venous or arterial thromboembolism in this population.
We failed to restore limb perfusion in 2/3 patients despite open surgical thrombectomy, resulting in minor amputation in one patient, and bilateral major amputations in the other. Others also reported lower success rate than anticipated in COVID-19 patients presenting with ALI.11 , 15 , 20 , 23
Several mechanisms are involved in the pathogenesis of thromboembolic events associated with COVID-19. SARS-CoV-2 triggers a hyper-inflammatory response which in turn results in a hyper-coagulable state characterized by increased D-dimer, fibrinogen, and fibrin degradation products, increased coagulation factor activity (notably factors V, VIII and von Willebrand factor), decreased antithrombin levels, increased activation of platelets and neutrophils.11 , 21 , 24 There is also direct viral infection of endothelial cells via the angiotensin converting enzyme 2 receptor.25 A combination of these factors contributes to the development of arterial thromboembolic events in COVID-19 patients.
Due to absence of atherosclerotic disease and lack of collateral circulation, younger adults may be more prone to development of ALI symptoms in the presence of an acute occlusion, whereas older adults better tolerate acute thrombosis of an already diseased limb artery owing to their prevalent collaterals. In addition, age is associated with increased mortality among COVID-19 patients,3 therefore it is possible that some of the older patients in the study population did not survive long enough to experience ALI. It can also be speculated that the hyper-inflammatory response caused by SARS-CoV-2 is more prominent in younger adults due to their more robust immune system.
The present study has some limitations. The study design is observational and descriptive without a control group, therefore conclusions about causation cannot be made. Additionally, the limited number of patients with ALI makes it impossible to assess risk factors for ALI in hospitalized COVID-19 patients. Asymptomatic ALI cases may have been missed since radiological investigation was only performed in the presence of clinical suspicion. Other arterial thromboembolic events with similar pathophysiology such as stroke and visceral ischemia were beyond the scope of this study and were not investigated. Finally, a more comprehensive assessment of arterial thromboembolic events during COVID-19 would require inclusion of both hospitalized and non-hospitalized COVID-19 patients.
Conclusion
COVID-19 is a multi-systemic disorder with involvement of hematologic and cardiovascular systems. Despite widespread use of thromboprophylaxis, hospitalized patients with COVID-19 are at increased risk of ALI, and subsequent limb loss or even death.
References
- 1.Munster VJ, Koopmans M, van Doremalen N, et al. A novel coronavirus emerging in China - key questions for impact assessment. N Engl J Med. 2020;382:692–694. doi: 10.1056/NEJMp2000929. [DOI] [PubMed] [Google Scholar]
- 2.Coronaviridae Study Group of the International Committee on Taxonomy of Viruses. The species Severe acute respiratory syndrome-related coronavirus: classifying 2019-nCoV and naming it SARS-CoV-2. Nat Microbiol. 2020;5:536–544. doi: 10.1038/s41564-020-0695-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Doganay F, Elkonca F, Seyhan AU, et al. Shock index as a predictor of mortality among the Covid-19 patients. Am J Emerg Med. 2020;40:106–109. doi: 10.1016/j.ajem.2020.12.053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Cucinotta D, Vanelli M. WHO declares COVID-19 a pandemic. Acta Biomed. 2020;91:157–160. doi: 10.23750/abm.v91i1.9397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Roberts CM, Levi M, McKee M, et al. COVID-19: a complex multisystem disorder. Br J Anaesth. 2020;125:238–242. doi: 10.1016/j.bja.2020.06.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Cheruiyot I, Kipkorir V, Ngure B, et al. Arterial thrombosis in Coronavirus Disease 2019 patients: a rapid systematic review. Ann Vasc Surg. 2021;70:273–281. doi: 10.1016/j.avsg.2020.08.087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.de Roquetaillade C, Chousterman BG, Tomasoni D, et al. Unusual arterial thrombotic events in Covid-19 patients. Int J Cardiol. 2021;323:281–284. doi: 10.1016/j.ijcard.2020.08.103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bilaloglu S, Aphinyanaphongs Y, Jones S, et al. Thrombosis in hospitalized patients with COVID-19 in a New York City health system. JAMA. 2020;324:799–801. doi: 10.1001/jama.2020.13372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Björck M., Earnshaw J.J., Acosta S., et al. Editor's choice-European society for vascular surgery (ESVS) 2020 clinical practice guidelines on the management of acute limb ischaemia. Eur J Vasc Endovasc Surg. 2020;59:173–218. doi: 10.1016/j.ejvs.2019.09.006. [DOI] [PubMed] [Google Scholar]
- 10.Topcu AC, Ariturk C, Yilmaz A. Acute limb ischemia in a COVID-19 patient. Thrombosis Update. 2021;2 doi: 10.1016/j.tru.2020.100031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Indes JE, Koleilat I, Hatch AN, et al. Early experience with arterial thromboembolic complications in patents with COVID-19. J Vasc Surg. 2021;73(2):381–389.e1. doi: 10.1016/j.jvs.2020.07.089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Perini P, Nabulsi B, Massoni CB, et al. Acute limb ischaemia in two young, non-atherosclerotic patients with COVID-19. Lancet. 2020;395:1546. doi: 10.1016/S0140-6736(20)31051-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gomez-Arbelaez D, Ibarra-Sanchez G, Garcia-Gutierrez A, et al. COVID-19-related aortic thrombosis: a report of four cases. Ann Vasc Surg. 2020;67:10–13. doi: 10.1016/j.avsg.2020.05.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Rutherford RB, Baker JD, Ernst C, Johnston KW, et al. Recommended standards for reports dealing with lower extremity ischemia: revised version. J Vasc Surg. 1997;26:517–538. doi: 10.1016/s0741-5214(97)70045-4. [DOI] [PubMed] [Google Scholar]
- 15.Etkin Y, Conway AM, Silpe J, et al. Acute arterial thromboembolism in patients with COVID-19 in the New York City Area. Ann Vasc Surg. 2021;70:290–294. doi: 10.1016/j.avsg.2020.08.085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Rey JR, Caro-Codón J, Poveda Pineda D, et al. Arterial thrombotic complications in hospitalized patients with COVID-19. Rev Esp Cardiol. 2020;73:769–771. doi: 10.1016/j.rec.2020.05.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Malas MB, Naazie IN, Elsayed N, et al. Thromboembolism risk of COVID-19 is high and associated with a higher risk of mortality: a systematic review and meta-analysis. EClinicalMedicine. 2020;29 doi: 10.1016/j.eclinm.2020.100639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Howard DP, Banerjee A, Fairhead JF, et al. Population-based study of incidence, risk factors, outcome, and prognosis of ischemic peripheral arterial events: implications for prevention. Circulation. 2015;132:1805–1815. doi: 10.1161/CIRCULATIONAHA.115.016424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Khorana AA, Francis CW, Culakova E, et al. Thromboembolism in hospitalized neutropenic cancer patients. J Clin Oncol. 2006;24:484–490. doi: 10.1200/JCO.2005.03.8877. [DOI] [PubMed] [Google Scholar]
- 20.Bellosta R, Luzzani L, Natalini G, et al. Acute limb ischemia in patients with COVID-19 pneumonia. J Vasc Surg. 2020;72:1864–1872. doi: 10.1016/j.jvs.2020.04.483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Helms J, Tacquard C, Severac F, et al. High risk of thrombosis in patients with severe SARS-CoV-2 infection: a multicenter prospective cohort study. Intensive Care Med. 2020;46:1089–1098. doi: 10.1007/s00134-020-06062-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lari E, Lari A, AlQinai S, et al. Severe ischemic complications in Covid-19-A case series. Int J Surg Case Rep. 2020;75:131–135. doi: 10.1016/j.ijscr.2020.09.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Bozzani A, Arici V, Tavazzi G, et al. Acute arterial and deep venous thromboembolism in COVID-19 patients: Risk factors and personalized therapy. Surgery. 2020;168:987–992. doi: 10.1016/j.surg.2020.09.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Han H, Yang L, Liu R, et al. Prominent changes in blood coagulation of patients with SARS-CoV-2 infection. Clin Chem Lab Med. 2020;58:1116–1120. doi: 10.1515/cclm-2020-0188. [DOI] [PubMed] [Google Scholar]
- 25.Varga Z, Flammer AJ, Steiger P, et al. Endothelial cell infection and endotheliitis in COVID-19. Lancet. 2020;395:1417–1418. doi: 10.1016/S0140-6736(20)30937-5. [DOI] [PMC free article] [PubMed] [Google Scholar]