Abstract
Advances in the isolation and sequencing of ancient DNA have begun to reveal the population histories of both people and dogs. Over the last 10,000 y, the genetic signatures of ancient dog remains have been linked with known human dispersals in regions such as the Arctic and the remote Pacific. It is suspected, however, that this relationship has a much deeper antiquity, and that the tandem movement of people and dogs may have begun soon after the domestication of the dog from a gray wolf ancestor in the late Pleistocene. Here, by comparing population genetic results of humans and dogs from Siberia, Beringia, and North America, we show that there is a close correlation in the movement and divergences of their respective lineages. This evidence places constraints on when and where dog domestication took place. Most significantly, it suggests that dogs were domesticated in Siberia by ∼23,000 y ago, possibly while both people and wolves were isolated during the harsh climate of the Last Glacial Maximum. Dogs then accompanied the first people into the Americas and traveled with them as humans rapidly dispersed into the continent beginning ∼15,000 y ago.
Keywords: archaeology, genetics, domestication, dogs, peopling of the Americas
Dogs were the first domesticated species and the only animal known to enter into a domestic relationship with people during the Pleistocene (1–4). Recent genetic analyses of ancient dog remains and of the archaeological and genetic records of ancient people have demonstrated that the spatiotemporal patterning of specific dog mitochondrial lineages are often correlated with the known dispersal of human groups at different times and places.
For instance, a study of mitochondrial signatures derived from ancient Near Eastern and European dogs has demonstrated that a specific haplogroup arrived in Europe as dogs dispersed out of the Near East along with farmers (5). The first dogs to arrive in New Zealand did so with newly arriving Polynesians (6). People and dogs also dispersed together in the North American Arctic, where dogs carrying a specific mitochondrial DNA (mtDNA) signature (haplogroup A2a; refs. 7 and 8) accompanied Paleo-Inuit groups as they moved into the region ∼5,000 y ago (5 ka). Subsequently, the arrival of Inuit groups into the same region ∼1 ka was accompanied by the introduction of a dog population that carried novel mtDNA signatures (A1a and A1b; ref. 7).
These correlations between the dispersals of people and specific dog lineages may have begun much earlier, perhaps soon after dogs became domesticated from a gray wolf ancestor in Eurasia, though precisely where and how many times that process took place remains unknown. The archaeologically documented presence of dogs in the Americas by at least 10 ka (9) suggests that dogs accompanied the early human groups who moved from northeast Asia across the Bering Land Bridge (Beringia) into the Americas. On the basis of current archaeological and genetic evidence, this movement likely took place before 15 ka (10, 11). Here, we take advantage of this record and newly available evidence from humans and dogs in late Pleistocene Siberia, Beringia, and North America to assess the likelihood that the first people to reach and disperse across the Americas did so in tandem with their dogs. This analysis allows us to better understand that dispersal process and present a hypothesis for the temporal and geographic origins of domestic dogs.
The First Dogs
Numerous archaeometric approaches have been applied to document the interaction between wolves, dogs, and people in order to establish the time frame and geography of dog domestication. These studies have shown, first, that dogs were the earliest animal domesticated, and the only species that entered into a domestic relationship with people during the Pleistocene (1, 2, 12, 13). Second, the specific wolf population from which dogs derived appears to be extinct (1, 14, 15). Finally, genetic and archaeological evidence from modern and ancient dogs and wolves demonstrates that dog domestication took place in Eurasia (16–18). Many other aspects of dog domestication, including the circumstances under which the relationship began, the time frame, and the number and location(s) of potential independent domestication regions, remain unresolved (19–21).
The shift in human–wolf interactions that led to domestication has been addressed through a wide variety of approaches, and there are ongoing debates over when the first recognizably domestic dog appeared. The earliest generally accepted dog dates to ∼15 ka (from the site of Bonn-Oberkassel, discussed below). However, claims for the existence of domestic dogs as early as 40 ka (22–28) have been made on the basis of morphological (22, 24–27), isotopic (22, 29), genetic (22, 28, 30), and contextual assessments (24, 31) of ancient canid remains. Yet, none of these potential domestication markers is fail-safe, owing to the fact that wolves and early domesticated dogs can be difficult to distinguish from each other.
For example, common morphological markers used to identify domestication such as tooth crowding, skull size, and reduced snout length often fail to clearly distinguish dogs from wolves (32–36). Isotopic signatures, and their dietary inferences, have been used to identify early dogs, though these have also been questioned given that the isotopic variation is often consistent with wolf diets (3). In addition, genetic analyses of these proposed early dogs have demonstrated that they do not belong to the same lineages as ancient or modern dogs (30, 37). While genomic estimates from multiple studies place the split time within wolf lineages, including the one that ultimately gave rise to dogs, to between ∼40 and 27 ka (14, 38), this timing is unlikely to reflect the initiation of the domestication process (1, 39). Finally, sites with purported domestic dogs have been questioned based on an absence of carnivore gnawing (40) or pups (41), features taken to indicate the presence of a living, breeding dog population.
Accordingly, claims for domestic dogs at the Belgian site of Goyet (22, 42, 43) have been disputed since their cranial and dental morphologies do not exclude the possibility that they are wolves (3, 32–36, 44, 45). mtDNA analyses of these canids also showed that they belong to an ancient European wolf lineage that is genetically highly divergent from any dog haplogroup (30). Similarly, genetic analyses of proposed Paleolithic dogs from the sites of Ulakhan Sular (23), Tumat (46), Razboinichya (27), Berelekh (23), Kostenki 8 (23), and Eliseevichi (25) have shown these canids to be more closely related to ancient and modern wolves than they are to dogs (30, 37, 47).
At least one of the purported dogs from the Czech site of Předmostí (24, 48) has also shown a genetic affinity to wolves over dogs (37). The domestic designation of these canids has likewise been questioned based on analyses of their dental and cranial morphologies (12, 32–36, 44, 45). Isotopic (29) and dental microwear (31) analyses of the diet of the proposed dogs at the site may also fall within the range of variation of the local wolf populations (12). Additionally, Předmostí lacks evidence for carnivore gnawing or pups (40, 41), raising further questions about the presence of a dog population at the site.
The challenge for all claims of late Pleistocene dogs has been to show conclusively, across several lines of evidence, that the specimen(s) in question can be clearly distinguished from contemporaneous wolves (3). Here, we take a conservative approach and only include those canids whose taxonomic status is unambiguously domestic.
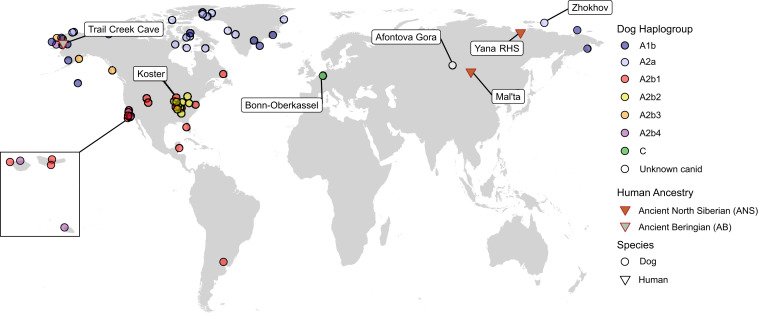
The earliest generally accepted remains of a domestic dog, based on a convergence of morphological, genetic, isotopic, and contextual evidence, comes from the site of Bonn-Oberkassel in Germany, dated to ∼15 ka (4, 30) (Fig. 1). The morphology and genetics of this young dog clearly distinguish it from local wolves. Its coburial with humans and the evidence for its care after suffering an illness also suggest it was a dog. Claims for contemporaneous domestic dogs have also been made at sites in France (49), Germany (50), Israel (51), Italy (52), and Switzerland (53). Based on their morphology and context, additional potential dogs may be present at Pleistocene Siberian sites such as Afontova Gora, Diuktai Cave, and Verkholenskaia Gora (9, 23), although their status has yet to be established. In the Americas, the earliest confirmed archaeological dog remains, based on combined morphological, genetic, isotopic, and contextual evidence, are from the Koster and Stilwell II sites, which have been dated to ∼10 ka (9, 16).
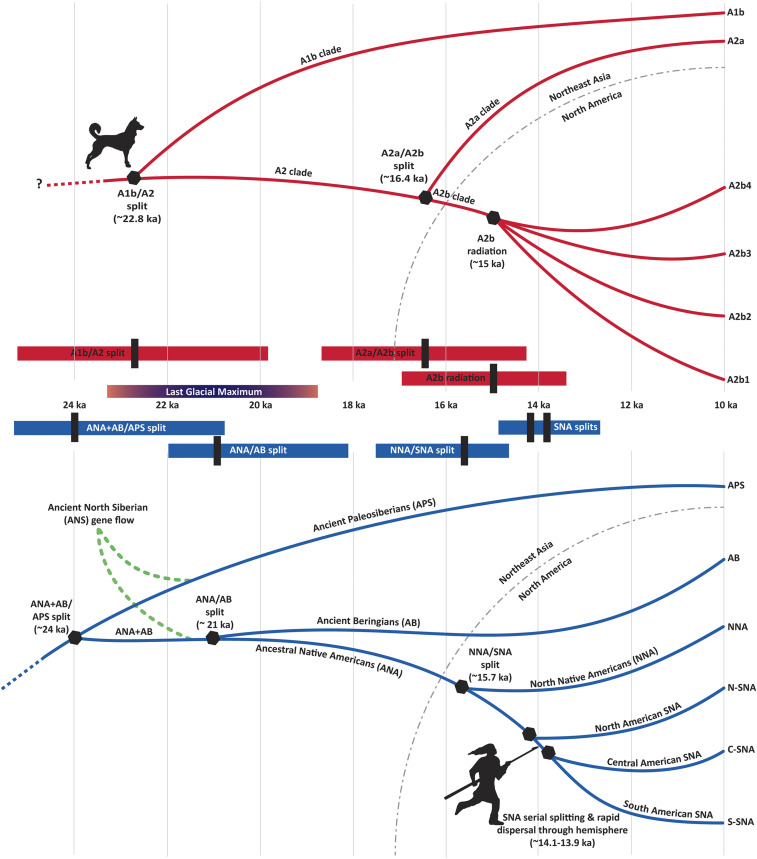
Fig. 1.
Lineages and estimated population divergence times (hexagons) for dogs (Top, in red), and humans (Bottom, in blue), for the period from ∼24,000 to 10,000 y ago. Divergence times are shown as point estimates. Confidence intervals for the respective point estimates are shown in the horizontal red bars (dogs) and blue bars (humans) in the center of the figure, which shows as well the span of the LGM, ∼23,000 to 19,000 y ago.
From a genetic perspective, hundreds of ancient and modern canid mitochondrial and nuclear genomes have been sequenced. Analyses of the nuclear data indicate that all dogs represent a genetically homogeneous group that possesses varying degrees of ancestry from three major ancestral lineages: a western Eurasian lineage (mostly found in European, Indian, and African dogs); an east Asian lineage (e.g., dingoes); and an Arctic lineage (e.g., huskies and ancient American dogs) (16). A recent study of dozens of ancient dog genomes suggests that these lineages were all established by at least 11 ka (54).
mtDNA data indicate that the vast majority of modern dogs fall into one of four monophyletic haplogroups (A, B, C, or D), with the majority belonging to haplogroup A (SI Appendix, Fig. S1). Recent ancient DNA studies demonstrated that all precontact dogs in the Americas south of the Arctic possess a unique mitochondrial haplogroup (A2b) that is nested within mitochondrial haplogroup A and which has since virtually disappeared (∼0.5%; ref. 16) in modern dogs inside and outside of the Americas (16–18, 55). Within A2b are four additional and well-supported monophyletic subhaplogroups, A2b1 through A2b4 (16), which are only found in the Americas. Of those four haplogroups, A2b1 is pan-American (possessed by dogs from California’s Channel Islands to Argentina), while the other three haplogroups are more geographically restricted, given current data (Fig. 2).
Fig. 2.
A map depicting the sites and lineages mentioned in the text. Human and dog lineages are denoted (see references in the text).
Molecular clock analyses have revealed the timing associated with splits within haplogroup A (SI Appendix, Fig. S1). First, the split between lineages A1b and A2 at the base of the haplogroup is estimated to date to ∼22.8 ka (95% CI 26 to 19.7 ka; ref. 7). This timing, which represents the oldest known coalescence between two dog mitochondrial lineages, suggests that dogs were domesticated several thousand years prior to their first appearance in the archaeological record. This estimate may have been affected by later gene flow between wolves and dogs, though this possibility is less likely since these haplogroups have not been found in any ancient or modern wolves, and because wolf–dog admixture appears to have been uncommon (54). Regardless, this suggested early time frame indicates that dogs were likely domesticated by the time humans crossed into the Americas.
The First People in the Americas
Our understanding of the origins and antiquity of the first people to reach the Americas has advanced significantly over the past decade through the identification of new archaeological sites and the generation of ancient human genomes from individuals in the Americas and northeast Asia.
Genomic evidence indicates that Native American ancestry can be traced to a population that is currently estimated to have diverged from an East Asian ancestor ∼30 ka (95% CI 36.4 to 26.8 ka; ref. 56) (all age estimates in this section are based on nuclear DNA, except where noted). Around 24 ka (95% CI 27.9 to 20.9 ka based on nuclear data and 24.9 to 18.4 ka based on mitochondrial data; ref. 57), that population then split into at least two groups. One group identified as Ancient Paleosiberians (APS) appears to have remained in far northeast Asia, while the other group became the basal branch of Native Americans (56). Both groups subsequently, and separately, received gene flow (∼24 ka; Fig. 1) from Ancient North Siberians (ANS), a population whose ancestors are detected archaeologically at the Yana RHS site in far northern Siberia (∼31.6 ka; Fig. 2) and the Mal’ta site near Lake Baikal (∼24 ka; Fig. 2; ref. 56).
From the time of the Last Glacial Maximum (LGM, ∼23 to 19 ka), the basal branch of Native Americans appears to have been isolated in northeast Asia, where they remained before departing for the Americas. There is currently no evidence of subsequent gene flow with other populations in the region, though this does not preclude the possibility of interaction with other groups that is not visible genetically. This period of isolation, by virtue of where it is suspected to have occurred, is known as the Beringian Standstill (58). Estimates of its duration based on nuclear DNA, Y chromosome, and mtDNA vary, but overall they indicate this episode may have lasted as little as 2,400 y to as long as 9,000 y (10, 57–59).
During this period of isolation, current evidence indicates that perhaps ∼21 ka (95% CI 21.9 to 18.1 ka) this basal branch split into at least two distinctive populations: Ancient Beringians (AB) and Ancestral Native Americans (ANA) (60). Both the AB and ANA populations crossed into eastern Beringia (present-day Alaska) after their split, though the timing of their dispersal(s), whether they moved simultaneously, and how long they may have maintained a degree of gene flow once they diverged from one another remain unclear (60, 61). Although both populations reached Alaska, to date no genomic evidence of the AB has been found south of Alaska, or in any Alaskan populations after ∼9 ka (the radiocarbon age of the AB individual from the Trail Creek Cave site, Alaska; Fig. 2; ref. 10).
The ANA lineage, on the other hand, reached North America south of the continental ice sheets, after diversifying ∼15.7 ka (95% CI 17.5 to 14.6 ka; ref. 60) into northern (NNA) and southern (SNA) branches (62). The NNA/SNA split must have taken place well after ANA had physically separated from the population represented by the AB lineage (57, 60), since NNA and SNA groups are genetically equidistant to AB (otherwise, one or the other of the groups would have been closer to AB). It is inferred that this split took place as the ANA were moving south from Alaska.
The estimated time of the split, and the archaeological evidence for people in the Americas ∼15 ka (e.g., refs. 11 and 61), implies that the route to the Americas south of the continental ice sheets must have been along the Pacific coast. The alternative—an interior route between the ice sheets (the “ice-free corridor”)—had yet to open and did not yet support the plant and animal resources necessary for human foragers (11, 63, 64). How much earlier people may have arrived in the Americas is unclear: Archaeological evidence provides only a minimum age, since the oldest sites that have been found are not the oldest on the continent (61). Genetic estimates provide a maximum value, since the peopling process must postdate the basal split of Native Americans, which could have been as early as 27.9 ka. It is noteworthy, however, that there are no archaeological sites in the Americas that can be shown to securely predate or were occupied during the LGM.
Once people arrived south of the ice sheets, the NNA branch appears to have had a relatively limited geographic spread. The SNA branch, however, radiated throughout the hemisphere, and as they dispersed they diverged genetically, starting ∼14.1 ka (95% CI 14.9 to 13.2 ka; refs. 10, 62, and 65).
Reconciling Lineage Branching in Late-Pleistocene Humans and Dogs
The Americas were one of the last regions of the world to be settled by people, and based on the antiquity of dogs in North America it is possible that the first people had canine companions with them when entering this new landscape (9, 66). Dogs were part of a larger cultural repertoire that may have assisted humans in rapidly dispersing into and throughout the Northern Hemisphere (67). Though people could have come to the Americas without them, dogs must have entered along with people. It is also reasonable to assume that, when human populations split from one another, they took their dogs with them. Thus, by aligning their respective population splits (Fig. 1), we can identify the timing of their tandem late-Pleistocene movements.
Perhaps not surprisingly, there are challenges in comparing divergence estimates obtained for dogs and humans. The dates relevant to the introduction of dogs in the Americas were derived from mitochondrial data and represent ancestral coalescence events that predate population splits, especially if the ancestral population was large. All ancient American dogs outside of the Arctic, however, belong to the same lineage (A2b) which coalesces with an ancient Siberian dog lineage ∼16.4 ka (95% CI 18.6 to 14.3 ka; refs. 7 and 16) (SI Appendix, Fig. S1). Although this time provides an upper bound for the introduction of dogs in the Americas, evidence for a population bottleneck associated with the founding of the ancient American dog lineage (18) suggests that this age may, in fact, be close to the population split between ancient Siberian and American dogs. The deepest coalescence event among A2b lineages dates back to ∼15 ka (95% CI 16.9 to 13.4 ka; ref. 7). Given that the A2b haplogroup is virtually absent from outside of the Americas, it is likely that its deepest coalescence took place in the ancestral population of American dogs. This time can, thus, be interpreted as a lower bound for divergence between American and Siberian dogs.
This time frame is remarkably consistent with that of the first peopling of the Americas (Fig. 1) and there are several key divergence nodes in common. First, the deepest coalescence event among dogs at ∼26 to 19.7 ka (16) is contemporaneous with the split between APS and ANA/AB at ∼27.9 to 20.9 ka (56). This correspondence suggests that dogs were already domesticated around the time ANA ancestry was established. Second, the coalescence of the A2b (American dog) and A2a (Siberian and American Arctic dog) lineages, at ∼18.6 to 14.3 ka (7, 16), and the deepest coalescence event within A2b, at ∼16.9 to 13.4 ka (7), overlap with the split time between the two major Native American lineages (NNA/SNA), at ∼17.5 to 14.6 ka (60). This indicates that the radiation of the major human and dog lineages in the Americas was contemporaneous, suggesting that they diverged in tandem. This evidence, combined with the antiquity of dog remains in North America (∼10 ka; ref. 9), and the lack of later human migration into the Americas until the early to middle Holocene (between 9 and 5 ka; ref. 68), indicates that dogs crossed Beringia during the Pleistocene and were present south of the continental ice sheets by the time the A2b lineage radiated at ∼15 ka, coincident with the widespread and rapid dispersal of the SNA lineage.
Either ANA or AB could have brought dogs into the Americas since there is archaeological evidence supporting the notion that both groups crossed the land bridge. However, they may not have crossed at the same time. AB are associated with a distinctive microblade/microcore stone tool technology (69, 70) seen at the eastern Siberian site of Diuktai Cave dated to ∼16.8 ka, from which it spread northeastward into western Beringia, ultimately reaching Alaska by ∼14.2 ka (site of Swan Point; ref. 71). Yet, by that time people using a very different technology had already been in the Americas south of the continental ice sheets for more than a millennium and had begun to disperse throughout the hemisphere (10). Although caution is always appropriate when drawing links between stone tool traditions across space and time (similar tools readily occur as a result of convergence), or between stone tools and human populations, this evidence suggests that, by the time AB arrived in Alaska ANA and dogs had already passed through that region. This, in turn, implies that ANA were the people who first brought dogs into the Americas.
Dog Domestication in Siberia
These parallels in the population divergences of humans and dogs place constraints that allow us to reevaluate previously proposed narratives for the origins of dogs and suggest a hypothesis for the timing and geographic location of dog domestication. On one hand, the split time estimated between wolf lineages, including the one that gave rise to dogs, provides an upper bound for domestication at ∼40 ka (14, 38). On the other hand, since we established that dogs likely crossed Beringia with the initial human arrivals, the archaeological evidence of people in the Americas by ∼15 ka (11, 72, 73) provides a lower bound for dog domestication. Combined with evidence that indicates that dogs were not domesticated in the Americas (16), this points to dogs having been present in Siberia prior to 15 ka.
Ancient human genome studies have identified multiple genetically divergent groups that were in Siberia/western Beringia within that time frame. This includes the ANS, APS, and the basal branch of Native Americans, which, after ∼21 ka, split into ANA and AB (Fig. 1). Ancient genomic data indicates that there was no significant gene flow among these Siberian groups after ∼23 ka, or with groups from outside Siberia from ∼39 ka (56). During this same period there is also a paucity of archaeological sites in arctic and subarctic Siberia and Beringia (71, 74, 75). Together, this evidence suggests that human populations in the region must have been small and living in relative isolation. They appear to have remained so up to the time when ANA and AB (separately) crossed into the Americas.
This evidence for little to no interaction with communities outside of Siberia raises the question of how ANA acquired the dogs that accompanied them into the Americas. One possible explanation is that dogs were domesticated from a wolf population somewhere in Siberia or western Beringia during the late Pleistocene, and before ANA crossed into the Americas. Previous studies have suggested on the basis of genetic evidence that dogs became domesticated in either East Asia (76), Europe (30), Central Asia (77), or in more than one of these locations independently (39). If dogs were domesticated in western Eurasia, then their spread eastward into Siberia would have required a far-ranging movement of people. Although possible, this seems unlikely given that western and eastern Eurasian human populations had already diverged ∼39 ka (95% CI 45.8 to 32.2 ka) (56, 75, 78).
Any of the groups known to have been in Siberia during the LGM (ANA, AB, APS, ANS, and their ancestral lineages; Fig. 1) may have domesticated dogs. Dogs associated with ANA, however, do not represent a basal lineage but instead cluster with Arctic dogs (7, 16, 67), suggesting they were not the initial domesticated population. Likewise, although APS could have domesticated dogs, there is no genomic evidence of their interaction with ANA (though meetings could have taken place and not been recorded either archaeologically or genetically). Similarly, dogs may have been domesticated by AB, but there is currently no genetic evidence of their interacting with ANA. Nevertheless, dogs could have been domesticated by their shared ancestral lineage prior to its split at ∼21 ka.
By process of elimination and for several other reasons, ANS therefore represents the more likely population to have initiated the domestication process. For example, genomic analyses of ANS individuals at the Siberian sites of Mal’ta (∼24 ka) and Afontova Gora (∼17 ka) show evidence for late Pleistocene gene flow from these populations into both ancient Native American (Fig. 1) and western Eurasian lineages (79). This provides a mechanism for the transfer of dogs into different groups and thus their movement both east and west following their domestication. Potential late Paleolithic dogs have also been identified at Afontova Gora (80, 81), possibly representing the basal lineage, though the genomes of these canids have yet to be analyzed. This scenario also fits with a recent study supporting a single origin for domestic dogs (54) and reconciles the presence of dogs in western Eurasia, the Near East, and the Americas by ∼15 ka.
Dog domestication in Siberia during the LGM provides a plausible context for the process. Climatic conditions may have brought human and wolf populations (37) into close proximity within refugial areas, given their attraction to the same prey species. Increasing interactions between the two, perhaps resulting from the mutual scavenging of kills, or from wolves drawn to the detritus of human campsites (82, 83), may have initiated a shift in the relationship between the species, eventually leading to dog domestication. A number of recently identified potential late-Pleistocene dogs from the region, including those from Afontova Gora (80) and Diuktai Cave (9, 23), offer an opportunity to test this hypothesis.
Conclusions
The archaeological evidence for both early humans and dogs in Siberia and the Americas is sparse. The ability to isolate and sequence ancient DNA from the few individuals that have been recovered is gradually providing new insights into the populations that initially moved east over the Bering Land Bridge and into the Americas. Dog mitochondrial sequences reflect the history of just a single locus, and genomic sequences are necessary to reconstruct their population history. Nonetheless, the coalescence time estimates of their mtDNA lineages suggest that dogs and humans share a correlated history of population divergences and migration from Siberia into the Americas. More specifically, we suggest that the first people to enter the Americas likely did so with their dogs. The subsequent geographic dispersal and genetic divergences within each population suggest that where people went, dogs went.
The convergence of the early genetic histories of people and dogs in Siberia and Beringia suggests that this may be the region where humans and wolves first entered into a domestic relationship. The oldest time to most recent common ancestor of the A haplogroup suggests that this process had already begun by 26–19.7 ka, which precedes the first unequivocal dogs in the Eurasian archaeological record by ∼11,000 to 4,000 y. The vast expanse of the region, combined with limited excavation, may explain the absence of earlier dog remains in Siberia. Future analyses of the handful of existing putative dogs, such as those from the site of Afontova Gora (80), are necessary to test this hypothesis.
Since their emergence from wolves, dogs have played a wide variety of roles within human societies, many of which are specifically tied to the lifeways of cultures worldwide. Future archaeological research, combined with numerous scientific techniques, will no doubt reveal how the emerging mutual relationship between people and dogs led to their successful dispersal across the globe.
Supplementary Material
Acknowledgments
A.R.P. was funded by the European Union’s Seventh Framework Programme for research, technological development and demonstration under grant agreement 609412. T.R.F. was funded by the European Union’s EU Framework Programme for research and innovation Horizon 2020 under grant agreement 676154, and funding for the Qimmeq project came from the Velux Foundations and the Aage og Johanne Louis-Hansens Fond. L.A.F.F. and G.L. were supported by a European Research Council grants (ERC-2013-StG-337574-UNDEAD and ERC-2019-StG-853272-PALAEOFARM) and Natural Environmental Research Council grants (NE/K005243/1 and NE/K003259/1). D.J.M. is supported by the Quest Archaeological Research Fund. K.E.W. was supported by a Wenner Gren Foundation grant and with R.S.M. an NSF (BCS-1540336) grant and is currently supported by NIH grant awarded to Dr. Emilia Huerta-Sanchez (1R35GM128946-01). The authors thank Victor Moreno-Mayar, Yun S. Song, and the editor and the reviewers for helpful advice.
Footnotes
The authors declare no competing interest.
This article is a PNAS Direct Submission. T.G.S. is a guest editor invited by the Editorial Board.
This article contains supporting information online at https://www.pnas.org/lookup/suppl/doi:10.1073/pnas.2010083118/-/DCSupplemental.
Data Availability.
All study data are included in the article and/or SI Appendix.
References
- 1.Freedman A. H., et al., Genome sequencing highlights the dynamic early history of dogs. PLoS Genet. 10, e1004016 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Larson G., et al., Rethinking dog domestication by integrating genetics, archeology, and biogeography. Proc. Natl. Acad. Sci. U.S.A. 109, 8878–8883 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Perri A., A wolf in dog’s clothing: Initial dog domestication and Pleistocene wolf variation. J. Archaeol. Sci. 68, 1–4 (2016). [Google Scholar]
- 4.Janssens L., et al., A new look at an old dog: Bonn-Oberkassel reconsidered. J. Archaeol. Sci. 92, 126–138 (2018). [Google Scholar]
- 5.Ollivier M., et al., Dogs accompanied humans during the Neolithic expansion into Europe. Biol. Lett. 14, 20180286 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Greig K., et al., Complete mitochondrial genomes of New Zealand’s first dogs. PLoS One 10, e0138536 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ameen C., et al., Specialized sledge dogs accompanied Inuit dispersal across the North American Arctic. Proc. Biol. Sci. 286, 20191929 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Brown S. K., Darwent C. M., Sacks B. N., Ancient DNA evidence for genetic continuity in arctic dogs. J. Archaeol. Sci. 40, 1279–1288 (2013). [Google Scholar]
- 9.Perri A., et al., New evidence of the earliest domestic dogs in the Americas. Am. Antiq. 84, 68–87 (2019). [Google Scholar]
- 10.Moreno-Mayar J. V., et al., Early human dispersals within the Americas. Science 362, eaav2621 (2018). [DOI] [PubMed] [Google Scholar]
- 11.Davis L. G., et al., Late upper paleolithic occupation at Cooper’s Ferry, Idaho, USA, ∼16,000 years ago. Science 365, 891–897 (2019). [DOI] [PubMed] [Google Scholar]
- 12.Perri A., A wolf in dog ’ s clothing: Initial dog domestication and Pleistocene wolf variation. J. Archaeol. Sci. 68, 1–4 (2016). [Google Scholar]
- 13.Janssens L., et al., A new look at an old dog: Bonn-Oberkassel reconsidered. J. Archaeol. Sci. 92, 126–138 (2018). [Google Scholar]
- 14.Skoglund P., Ersmark E., Palkopoulou E., Dalén L., Ancient wolf genome reveals an early divergence of domestic dog ancestors and admixture into high-latitude breeds. Curr. Biol. 25, 1515–1519 (2015). [DOI] [PubMed] [Google Scholar]
- 15.Frantz L. A. F., et al., Genomic and archaeological evidence suggest a dual origin of domestic dogs. Science 352, 1228–1231 (2016). [DOI] [PubMed] [Google Scholar]
- 16.Ní Leathlobhair M., et al., The evolutionary history of dogs in the Americas. Science 361, 81–85 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Leonard J. A., et al., Ancient DNA evidence for old world origin of new world dogs. Science 298, 1613–1616 (2002). [DOI] [PubMed] [Google Scholar]
- 18.Witt K. E., et al., DNA analysis of ancient dogs of the Americas: Identifying possible founding haplotypes and reconstructing population histories. J. Hum. Evol. 79, 105–118 (2015). [DOI] [PubMed] [Google Scholar]
- 19.Thalmann O., Perri A. R., “Paleogenomic inferences of dog domestication” in Paleogenomics: Genome-Scale Analysis of Ancient DNA, Lindqvist C., Rajora O. P., Eds. (Springer International Publishing, 2019), pp. 273–306. [Google Scholar]
- 20.Larson G., Bradley D. G., How much is that in dog years? The advent of canine population genomics. PLoS Genet. 10, e1004093 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Frantz L. A. F., Bradley D. G., Larson G., Orlando L., Animal domestication in the era of ancient genomics. Nat. Rev. Genet. 21, 449–460 (2020). [DOI] [PubMed] [Google Scholar]
- 22.Germonpré M., et al., Fossil dogs and wolves from palaeolithic sites in Belgium, the Ukraine and Russia: Osteometry, ancient DNA and stable isotopes. J. Archaeol. Sci. 36, 473–490 (2009). [Google Scholar]
- 23.Germonpré M., et al., Palaeolithic and prehistoric dogs and Pleistocene wolves from Yakutia: Identification of isolated skulls. J. Archaeol. Sci. 78, 1–19 (2017). [Google Scholar]
- 24.Germonpré M., Lázničková-Galetová M., Sablin M. V., Palaeolithic dog skulls at the Gravettian Předmostí site, the Czech Republic. J. Archaeol. Sci. 39, 184–202 (2012). [Google Scholar]
- 25.Sablin M. V., Khlopachev G. A., The earliest ice age dogs: Evidence from Eliseevichi 1. Curr. Anthropol. 43, 795–799 (2002). [Google Scholar]
- 26.Camarós E., Münzel S. C., Cueto M., Rivals F., Conard N. J., The evolution of Paleolithic hominin–carnivore interaction written in teeth: Stories from the Swabian Jura (Germany). J. Archaeol. Sci. Rep. 6, 798–809 (2016). [Google Scholar]
- 27.Ovodov N. D., et al., A 33,000-year-old incipient dog from the Altai Mountains of Siberia: Evidence of the earliest domestication disrupted by the last glacial maximum. PLoS One 6, e22821 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Druzhkova A. S., et al., Ancient DNA analysis affirms the canid from Altai as a primitive dog. PLoS One 8, e57754 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Bocherens H., et al., Reconstruction of the Gravettian food-web at Předmostí I using multi-isotopic tracking (13C, 15N, 34S) of bone collagen. Quat. Int. 359-360, 211–228 (2015). [Google Scholar]
- 30.Thalmann O., et al., Complete mitochondrial genomes of ancient canids suggest a European origin of domestic dogs. Science 342, 871–874 (2013). [DOI] [PubMed] [Google Scholar]
- 31.Prassack K. A., DuBois J., Lázničková-Galetová M., Germonpré M., Ungar P. S., Dental microwear as a behavioral proxy for distinguishing between canids at the Upper Paleolithic (Gravettian) site of Předmostí, Czech Republic. J. Archaeol. Sci. 115, 105092 (2020). [Google Scholar]
- 32.Janssens L., Perri A., Crombé P., Van Dongen S., Lawler D., An evaluation of classical morphologic and morphometric parameters reported to distinguish wolves and dogs. J. Archaeol. Sci. Rep. 23, 501–533 (2019). [Google Scholar]
- 33.Drake A. G., et al., Three-dimensional geometric morphometric analysis of fossil canid Mandibles and skulls. Sci. Rep. 7, 9508 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Drake A. G., Coquerelle M., Colombeau G., 3D morphometric analysis of fossil canid skulls contradicts the suggested domestication of dogs during the late Paleolithic. Sci. Rep. 5, 8299 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Boudadi-Maligne M., Escarguel G., A biometric re-evaluation of recent claims for Early Upper Palaeolithic wolf domestication in Eurasia. J. Archaeol. Sci. 45, 80–89 (2014). [Google Scholar]
- 36.Ameen C., et al., A landmark-based approach for assessing the reliability of mandibular tooth crowding as a marker of dog domestication. J. Archaeol. Sci. 85, 41–50 (2017). [Google Scholar]
- 37.Loog L., et al., Ancient DNA suggests modern wolves trace their origin to a Late Pleistocene expansion from Beringia. Mol. Ecol. 29, 1596–1610 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Botigué L. R., et al., Ancient European dog genomes reveal continuity since the Early Neolithic. Nat. Commun. 8, 16082 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Frantz L. A. F., et al., Genomic and archaeological evidence suggest a dual origin of domestic dogs. Science 352, 1228–1231 (2016). [DOI] [PubMed] [Google Scholar]
- 40.Wilczyński J., et al., Friend or foe? Large canid remains from pavlovian sites and their archaeozoological context. J. Anthropol. Archaeol. 59, 101197 (2020). [Google Scholar]
- 41.Perri A., Sazelova S., “The role of large canids: Preliminary variabilities forming the population structure in Moravia (Dolni Vestonice II)” in Dolní Vestonice II: Chronostratigraphy, Paleoethnology, Paleoanthropology, Svoboda J., Ed. (Academy of Sciences of the Czech Republic, Institute of Archaeology, 2016), pp. 138–146. [Google Scholar]
- 42.Germonpré M., et al., Palaeolithic dogs and the early domestication of the wolf: A reply to the comments of. J. Archaeol. Sci. 40, 786–792 (2013). [Google Scholar]
- 43.Galeta P., Lázničková-Galetová M., Sablin M., Germonpré M., Morphological evidence for early dog domestication in the European Pleistocene: New evidence from a randomization approach to group differences. Anat. Rec. (Hoboken) 304, 42–62 (2020). [DOI] [PubMed] [Google Scholar]
- 44.Crockford S. J., Kuzmin Y. V., Comments on Germonpré et al., Journal of Archaeological Science 36, 2009 “Fossil dogs and wolves from Palaeolithic sites in Belgium, the Ukraine and Russia: osteometry, ancient DNA and stable isotopes”, and Germonpré, Lázkičková-Galetová, and Sablin, Journal of Archaeological Science 39, 2012 “Palaeolithic dog skulls at the Gravettian Předmostí site, the Czech Republic.”. J. Archaeol. Sci. 39, 2797–2801 (2012). [Google Scholar]
- 45.Morey D. F., In search of paleolithic dogs: A quest with mixed results. J. Archaeol. Sci. 52, 300–307 (2014). [Google Scholar]
- 46.Кандыба А. В., Федоров С. Е., Дмитриев А. И., Местонахождение Сыалах-новый археологический объект позднегонеоплейстоцена Сибирской Арктики. Проблемы археологии 21, 90–93 (2015). [Google Scholar]
- 47.Ramos-Madrigal J., et al., Genomes of Pleistocene Siberian wolves uncover multiple extinct wolf lineages. Curr. Biol. In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Germonpré M., Lázničková-Galetová M., Losey R. J., Räikkönen J., Sablin M. V., Large canids at the Gravettian Předmostí site, the Czech Republic: The mandible. Quat. Int. 359-360, 261–279 (2015). [Google Scholar]
- 49.Pionnier-Capitan M., et al., New evidence for upper palaeolithic small domestic dogs in south-western Europe. J. Archaeol. Sci. 38, 2123–2140 (2011). [Google Scholar]
- 50.Musil R., “Domestication of wolves in central European Magdalenian sites” in Dogs Through Time: An Archaeological Perspective, Crockford S. J., Ed. (BAR International Series, British Archaeological Reports, Oxford, 2000), vol. 889, pp. 21–28. [Google Scholar]
- 51.Tchernov E., Valla F. F., Two new dogs, and other natufian dogs, from the southern levant. J. Archaeol. Sci. 24, 65–95 (1997). [Google Scholar]
- 52.Boschin F., et al., The first evidence for Late Pleistocene dogs in Italy. Sci. Rep. 10, 13313 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Morel P., et al., Un Campement Magdalénien au Bord du Lac de Neuchâtel: Étude Archéozoologique (Secteur 1) (Musée cantonal d’archéologie, 1997). [Google Scholar]
- 54.Bergström A., et al., Origins and genetic legacy of prehistoric dogs. Science 370, 557–564 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.van Asch B., et al., Pre-Columbian origins of native American dog breeds, with only limited replacement by European dogs, confirmed by mtDNA analysis. Proc. Biol. Sci. 280, 20131142 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Sikora M., et al., The population history of northeastern Siberia since the Pleistocene. Nature 570, 182–188 (2019). [DOI] [PubMed] [Google Scholar]
- 57.Llamas B., et al., Ancient mitochondrial DNA provides high-resolution time scale of the peopling of the Americas. Sci. Adv. 2, e1501385 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Tamm E., et al., Beringian standstill and spread of Native American founders. PLoS One 2, e829 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Pinotti T., et al., Y chromosome sequences reveal a short Beringian standstill, rapid expansion, and early population structure of native American founders. Curr. Biol. 29, 149–157.e3 (2019). [DOI] [PubMed] [Google Scholar]
- 60.Moreno-Mayar J. V., et al., Terminal Pleistocene Alaskan genome reveals first founding population of Native Americans. Nature 553, 203–207 (2018). [DOI] [PubMed] [Google Scholar]
- 61.Meltzer D. J., First Peoples in a New World: Populating Ice Age America (Cambridge University Press, 2021). [Google Scholar]
- 62.Rasmussen M., et al., The genome of a Late Pleistocene human from a Clovis burial site in western Montana. Nature 506, 225–229 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Dillehay T. D., et al., Monte verde: Seaweed, food, medicine, and the peopling of south America. Science 320, 784–786 (2008). [DOI] [PubMed] [Google Scholar]
- 64.Froese D., Young J. M., Norris S. L., Margold M., Availability and viability of the ice-free corridor and pacific coast routes for the peopling of the Americas. SAA Archaeol. Rec. 19, 27–33 (2019). [Google Scholar]
- 65.Posth C., et al., Reconstructing the deep population history of Central and South America. Cell 175, 1185–1197.e22 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Fiedel S. J., Man’s best friend–mammoth’s worst enemy? A speculative essay on the role of dogs in paleoindian colonization and megafaunal extinction. World Archaeol. 37, 11–25 (2005). [Google Scholar]
- 67.Sinding M. S., et al., Arctic-adapted dogs emerged at the Pleistocene-Holocene transition. Science 368, 1495–1499 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Flegontov P., et al., Palaeo-Eskimo genetic ancestry and the peopling of Chukotka and North America. Nature 570, 236–240 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Potter B. A., Irish J. D., Reuther J. D., McKinney H. J., New insights into eastern beringian mortuary behavior: A terminal Pleistocene double infant burial at upward sun river. Proc. Natl. Acad. Sci. U.S.A. 111, 17060–17065 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Potter B. A., Irish J. D., Reuther J. D., Gelvin-Reymiller C., Holliday V. T., A terminal Pleistocene child cremation and residential structure from eastern Beringia. Science 331, 1058–1062 (2011). [DOI] [PubMed] [Google Scholar]
- 71.Goebel T., Potter B., “First traces” in The Oxford Handbook of the Prehistoric Arctic, Friesen T. M., Mason O. K., Eds. (Oxford University Press, 2016), p. 223. [Google Scholar]
- 72.Waters M. R., et al., Pre-Clovis projectile points at the debra L. Friedkin site, Texas-implications for the late Pleistocene peopling of the Americas. Sci. Adv. 4, eaat4505 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Jenkins D. L., et al., Geochronology, archaeological context, and DNA at the paisley caves. Paleoamerican Odyssey 32, 485–510 (2013). [Google Scholar]
- 74.Kuzmin Y. V., Keates S. G., Siberia and neighboring regions in the last glacial maximum: Did people occupy northern Eurasia at that time? Archaeol. Anthropol. Sci. 10, 111–124 (2018). [Google Scholar]
- 75.Graf K. E., “Siberian odyssey” in Paleoamerican Odyssey, Graf K. E., Ketron C. V., Waters M. R., Eds. (Texas A&M University Press, 2014), pp. 65–80. [Google Scholar]
- 76.Wang G.-D., et al., Out of southern east Asia: The natural history of domestic dogs across the world. Cell Res. 26, 21–33 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Shannon L. M., et al., Genetic structure in village dogs reveals a Central Asian domestication origin. Proc. Natl. Acad. Sci. U.S.A. 112, 13639–13644 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Lipson M., Reich D., A working model of the deep relationships of diverse modern human genetic lineages outside of Africa. Mol. Biol. Evol. 34, 889–902 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Raghavan M., et al., Upper Palaeolithic Siberian genome reveals dual ancestry of Native Americans. Nature 505, 87–91 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Kuzmin Y. V., Mammalian fauna from palaeolithic sites in the upper Yenisei river basin (southern Siberia): Review of the current zooarchaeological evidence. Int. J. Osteoarchaeol. 21, 218–228 (2011). [Google Scholar]
- 81.Germonpré M., Sablin M. V., “Humans and mammals in the Upper Palaeolithic of Russia” in The Oxford Handbook of Zooarchaeology, Russ H., Albarella U., Vickers K., Rizzetto M., Viner-Daniels S., Eds. (Oxford University Press, 2017), pp. 25–38. [Google Scholar]
- 82.Olsen S. J., Origins of the Domestic Dog: The Fossil Record (University of Arizona Press, 1985). [Google Scholar]
- 83.Zeder M. A., “Pathways to animal domestication” in Biodiversity in Agriculture: Domestication, Evolution, and Sustainability, Harlan J. R., et al., Eds. (Cambridge University Press, 2012), pp. 227–259. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
All study data are included in the article and/or SI Appendix.