Abstract
Mucormycosis is an invasive fungal infection (IFI) due to several species of saprophytic fungi, occurring in patients with underlying co-morbidities (including organ transplantation). During the ongoing Coronavirus disease 2019 (COVID-19) pandemic, there have been increasing reports of bacterial and fungal co-infections occurring in COVID-19 patients, including COVID-19 associated pulmonary aspergillosis (CAPA). We describe a case of mucormycosis occurring after COVID-19, in an individual who received a recent heart transplant for severe heart failure. Two months after heart transplant, our patient developed upper respiratory and systemic symptoms and was diagnosed with COVID-19. He was managed with convalescent plasma therapy and supportive care. Approximately three months after COVID-19 diagnosis, he developed cutaneous mucormycosis at an old intravascular device site. He underwent extensive surgical interventions, combined with broad-spectrum antifungal therapy. Despite the aggressive therapeutic measures, he died after a prolonged hospital stay. In this case report, we also review the prior well-reported cases of mucormycosis occurring in COVID-19 patients and discuss potential mechanisms by which COVID-19 may predispose to IFIs. Similar to CAPA, mucormycosis with COVID-19 may need to be evaluated as an emerging disease association. Clinicians should be vigilant to evaluate for invasive fungal infections such as mucormycosis in patients with COVID-19 infection.
Keywords: Mucormycosis, Zygomycosis, Rhizopus microsporus, Cardiac transplant, Coronavirus disease 2019 (COVID-19), SARS-CoV-2
Introduction
Mucormycosis (previously called Zygomycosis) is the term used for invasive fungal infections (IFIs) occurring due to saprophytic environmental fungi–Rhizpous (most common), Mucor, Cunninghamella, Aposphysomyces, Licitheimia (Absidia), Saksenaea, Rhizomucor] [1]. Mucormycosis can have at least six different clinical presentations–rhinocerebral, pulmonary, cutaneous, gastrointestinal, disseminated and miscellaneous [2]. Cutaneous mucormycosis is the third most common manifestation (after rhinocerebral and pulmonary) [3] and is seen in 19% of patients with mucormycosis [1].
The common risk factors for mucormycosis include hematologic malignancies, solid organ transplant recipients (SOTRs), stem cell transplantation, prolonged and severe neutropenia, poorly controlled diabetes mellitus (DM), iron overload, deferoxamine therapy, major trauma, prolonged corticosteroid use, illicit intravenous drug use, neonatal prematurity, malnutrition and potential nosocomial sources (including bandages and intravascular devices) [1], [3], [4]. Mucormycosis comprises 2–6% of IFIs [4], [5]. The multi-center prospective TRANSNET study reported that the cumulative incidence of mucormycosis was 0.07% in SOTRs at the end of one year [5].
The ongoing Coronavirus disease 2019 (COVID-19) pandemic, due to the novel severe-acute-respiratory-syndrome-coronavirus-2 (SARS-CoV-2), has caused more than 110 million cases and more than 2.4 million deaths globally [6]. There are increasing reports of the occurrence of bacterial and fungal co-infections in COVID-19 patients [7].
We describe a heart transplant recipient who developed cutaneous mucormycosis infection after COVID-19. We review the literature surrounding the occurrence of mucormycosis after COVID-19, as well as explore potential mechanisms.
Case
A sixty-eight-year-old gentleman underwent orthotopic heart transplantation in February 2020. The donor was cytomegalovirus-seronegative with recipient seropositive; both donor and recipient were Toxoplasma-seronegative. His post-operative course was complicated by prolonged intubation, paroxysmal atrial fibrillation, hyperglycemia requiring intravenous insulin, and acute renal failure requiring continuous renal replacement therapy (that resolved prior to discharge). He was discharged March 2020 on prednisone oral (PO) 10 mg/d, mycophenolate mofetil (MMF) PO 250 mg twice daily (BID), tacrolimus PO 4 mg in morning/5 mg in evening and prophylactic antimicrobials (atovaquone, nystatin, valganciclovir).
He was readmitted two months later with complaints of non-productive cough with non-bloody diarrhea for one week; fevers (highest temperature 38.6 °Celsius) for two days; and a one-day history of sternal wound discharge. His family members reported fevers with respiratory symptoms.
His past medical history was significant for coronary artery disease (treated with coronary artery bypass graft and stenting); ischemic cardiomyopathy leading to American College of Cardiology/American Heart Association (ACC/AHA) stage D chronic systolic heart failure necessitating intra-aortic balloon pump (IABP) and left ventricular assist device placement, followed by heart transplant; type-2-DM, hypertension, chronic kidney disease, obstructive sleep apnea and prior smoking.
On evaluation, he was afebrile and hemodynamically stable. Systemic examination revealed dehiscence of lower aspect of sternal incision, with minimal foul-smelling serosanguinous drainage. Respiratory and cardiovascular system examination was otherwise unremarkable. Admission laboratory testing revealed white blood cell count 7.820 × 109/L (normal range {N}: 3.8–10.5 × 109/L), lymphocyte count 0.2 × 109/L (N: 1.0–3.0 × 109/L) and elevated inflammatory markers–C-reactive protein 1612.38 nmol/L (N: 0–3.81 nmol/L), procalcitonin 0.72 μg/L (N: 0.02–0.10 μg/L), lactate dehydrogenase 6083.33 nmol/(s•L) [N: 833.33–4033.33 nmol/(s•L), ferritin 1.15 nmol/L (N: 0.07–0.90 nmol/L) and D-dimer 1194 μg/L DDU (N: ≤ 229 μg/L DDU). Computed tomography imaging (CT) of chest/abdomen/pelvis revealed central and peripheral ground-glass opacities in right middle, lingular and bilateral lower lobes, with bilateral interlobular septal thickening in lower lobes. There were no fluid collections noted at the sternotomy site in the CT scan. Nasopharyngeal testing by reverse-transcriptase-polymerase-chain-reaction detected SARS-Cov-2 virus, confirming the diagnosis of COVID-19.
Infectious diseases service was consulted for assistance in management. Given the ongoing use of immunosuppressive agents, he was not eligible for use of interleukin-inhibitors. He met criteria for enrollment in the antiviral remdesivir trial; however, study recruitment had closed at that time. Initiation of oral hydroxychloroquine (per the institutional COVID-19 protocol at the time) was held due to concurrent amiodarone use and prolonged QTc-interval. He received one convalescent plasma infusion after inclusion in the ongoing trial. He was started on empiric intravenous (IV) vancomycin and meropenem for suspected sternal wound infection. Plastics surgery performed bedside sternal wound debridement, followed by wound vacuum therapy (“wound vac”) placement. Sternal wound cultures grew Morganella morganni and Enterobacter cloacae, with blood cultures also revealing growth of Morganella.
His hospital course was further complicated by encephalopathy, respiratory decompensation necessitating intubation and mechanical ventilation; acute kidney injury necessitating hemodialysis; and recurrent infections (vancomycin-resistant Enterococcus faecium bacteremia, Klebsiella variicola sternal wound infection, coagulase-negative Staphylococcus species bacteremia). He required debridement of sternal wound with reconstruction using pectoralis major muscle flaps. Given the multiple infections, MMF was discontinued and cyclosporine was initiated. He was evaluated by the Rheumatology service for polyarticular gout symptoms. He received a three-day course of IV methylprednisolone, followed by a fifteen-day oral prednisone taper. His prednisone dose was subsequently decreased to 7.5 mg/d PO, so fungal prophylaxis (nystatin) was discontinued per institutional protocol. He underwent bedside tracheostomy one month after admission, with decannulation performed two months after admission. After completing the antibiotic courses, he underwent chest wall reconstruction using omental flap and split-thickness skin grafting.
Approximately three months after admission, purplish skin discoloration with fluctuant swelling was noted in the right axilla, at the prior IABP catheter insertion site. CT chest revealed a 6.4 × 5.3 cm fluid collection along the anterior right upper chest wall, with extensive inflammatory changes in the chest wall and surrounding tissues (Fig. 1 ). There was a 1.3 cm right subclavian artery pseudoaneurysm at the vascular graft site (concerning for mycotic aneurysm) (Fig. 2 ). 75 ml of brown fluid was aspirated from the collection by interventional radiology. A rare mold with broad coenocytic/aseptate hyphae was noted on preliminary cultures and was sent to the state reference laboratory for further evaluation. He was started on IV liposomal amphotericin B 550 mg/d and posaconazole delayed-release PO 300 mg/d. Serum markers for fungal infections were normal–(1–3)-β-D-Glucan level 43 pg/mL (N: < 60 pg/mL) and Aspergillus (Galactomannan) antigen < 0.5 index (N: < 0.5). The state laboratory identified the fungus as Rhizopus microsporus.
Fig. 1.
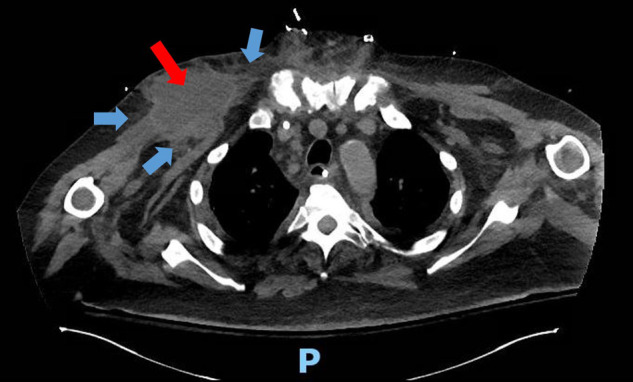
Computed tomography (CT) scan–transverse view of thoracic cavity. Fluid collection noted in right anterior chest wall (red arrow), with inflammatory infiltrate in surrounding tissues (blue arrows). The letter P denotes the posterior orientation of the patient.
Fig. 2.
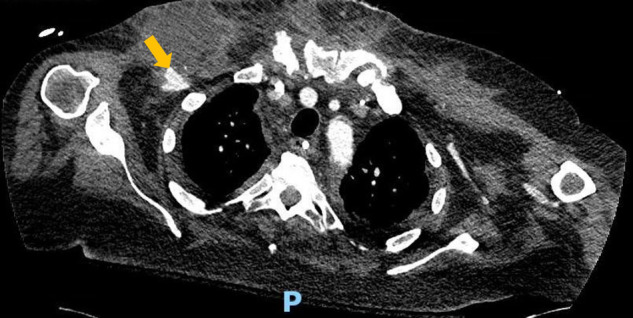
Computed tomography (CT) scan–transverse view of thoracic cavity. A right subclavian artery pseudoaneurysm is noted adjacent to the right chest wall collection (yellow arrow). The letter P denotes the posterior orientation of the patient.
He underwent exploration and debridement of the thoracic cavity in the operating room. The mold was tunneling under the omentum, extending deep in the right chest cavity and into the musculature. It involved an area of approximately 15 × 5 × 4 cm in size, with burrowing towards the sternal wound. Resection of the subclavian artery and potentially infected prosthetic graft was also performed, with primary repair of the proximal and distal ends. After debridement, wound vac was applied to the sternal wound. Operative sternal wound cultures redemonstrated growth of R. microsporus, while subclavian graft cultures were negative.
His hospital course was further complicated by vancomycin-resistant E. faecium and later daptomycin-resistant E. faecium sternal wound infections; chest wall abscesses with Enterobacter cloacae; and development of sacral decubitus ulcers (managed with local wound care). He eventually developed polymicrobial septic shock (with vancomycin-resistant E. faecium, daptomycin-resistant E. faecium and multi-drug-resistant K. variicola). He was intubated for hypercapneic respiratory failure and worsening encephalopathy. He had worsening vasopressor requirements and unfortunately died on day one-hundred-and-seventy-five of hospital stay.
Discussion
Patients with skin barrier disruptions (burns, trauma, catheter insertion, injections) or persistent skin maceration are at increased risk for cutaneous mucormycosis [2]. The fungus can invade into adjacent fat, muscle, fascia, and even bone, while secondary vascular invasion and hematogenous spread are less common [1], [3]. However, cutaneous mucormycosis with hematogenous dissemination has high fatality rates [1]. Four general principles are critical for managing mucormycosis: rapid diagnosis, reversal of underlying predisposing factors (if possible), surgical debridement of infected tissue and appropriate antifungal therapy [2].
IFIs are being increasingly reported in COVID-19 patients [8], [9], [10], with COVID-19-associated pulmonary aspergillosis (CAPA) being recognized as a new disease entity [9], [10]. There is concern that an IFI may alter the natural history of COVID-19 [8], [9]–higher mortality rates are reported in patients with both IFIs and COVID-19 [8], [9], [10].
We performed a literature review in Medline® (PubMed) bibliographic database for English language articles (search terms “Mucormycosis COVID-19”, “Mucormycosis SARS-Cov-2”, “fungal COVID-19”, “fungal SARS-Cov-2”) and found seven similar cases of invasive mucormycosis [11], [12], [13], [14], [15], [16], [17] (Table 1 ). A diabetic patient received high-dose corticosteroids for COVID-19 management and developed rhino-orbital mucormycosis [11]. Mucor was diagnosed from nasal biopsy and subsequent culture. Another patient presented with left-sided ptosis and altered mental status [12]. Laboratory testing revealed diabetic ketoacidosis (with previously undiagnosed DM) and COVID-19, with maxillary and ethmoidal sinus disease and multiple areas of cerebral infarction and ischemia noted on imaging. Mucor was demonstrated in intra-operative sinus cultures. Disseminated mucormycosis (involving lungs, hilar lymph nodes, brain and kidney) was noted on post-mortem examination of a twenty-two-year-old COVID-19 patient who died within 27 days after symptom onset [13]. An elderly hypertensive patient [14] presented with symptoms suggestive of COVID-19 and developed melena with severe anemia soon after admission. Esophagogastroduodenoscopy revealed two large gastric ulcers with dirty debris covering a hemorrhagic base. Ulcer biopsy confirmed mucormycosis, but the patient died before initiation of antifungal therapy. A forty-nine-year-old male [15] who had received immunosuppressive therapies (dexamethasone and tocilizumab) for COVID-19 developed acute-onset dyspnea. Imaging revealed a tension pneumothorax with bronchopleural fistula. Intra-operatively, an empyema with fungal elements was noted, with Rhizopus growing on subsequent cultures. A poorly controlled diabetic developed severe COVID-19 and rhino-orbital mucormycosis, necessitating retrobulbar anti-fungal injections [16]. Lastly, an intubated COVID-19 patient developed a new pulmonary lesion, with bronchial aspirate revealing Rhizopus. The authors hypothesized that in their patient with no classic risk factors for IFIs, severe COVID-19 may have led to lymphopenia and immune system alterations, predisposing to mucormycosis [17]. All patients expired despite anti-fungal therapy and surgery.
Table 1.
Reported cases of mucormycosis occurring in patients with COVID-19 infection.
| Patient | # 1 | # 2 | # 3 | # 4 | # 5 | # 6 | # 7 | # 8 |
|---|---|---|---|---|---|---|---|---|
| Age/Sex | 68/M | 60/M | 33/F | 22/M | 86/M | 49/M | 60/M | 66/M |
| Past Medical History | 1. ICM 2. HF s/p OHT 3. DM 4. HTN CKD |
DM | 1. HTN 2. Asthma |
1. Obesity Hypothyroidism |
HTN | None | 1. DM 2. Asthma 3. HTN HLD |
HTN |
| Initial Presenting symptoms | 1. Fever 2. Cough 3. Diarrhea |
1. Fever 2. Malaise 3. Tachypnea 4. Dyspnea |
1. Altered mental status 2. Cough 3. Tachypnea 4. Vomiting 5. Left-sided ptosis |
1. COVID-19 pneumonia (not specified) 2. Subacute right middle cerebral artery infarct |
1. Diarrhea 2. Cough 3. Dyspnea 4. Fever |
1. Fever 2. Cough 3. Dyspnea |
1. Dyspnea Hypoxia |
COVID-19 symptoms (not specified) |
| COVID-19 treatment | 1. Convalescent plasma 2. Supportive care |
1. Meropenem 2. Oseltamivir 3. Methylprednisolone 4. Dexamethasone 5. Enoxaparin 6. Supportive care |
1. Remdesivir 2. Convalescent plasma 3. Supportive care |
1. Linezolid 2. Meropenem 3. Tigecycline 4. Caspofungin 5. Argatroban |
1. Ceftriaxone 2. Azithromycin 3. Oseltamivir 4. Hydrocortisone 5. Supportive care |
1. Ceftriaxone 2. Azithromycin 3. Dexamethasone 4. Remdesivir 5. Tocilizumab 6. Enoxaparin Supportive care |
1. Remdesivir 2. Convalescent plasma 3. Dexamethasone 4. Supportive care |
1. Hydroxychloroquine 2. Lopinavir-Ritonavir Supportive care |
| Clinical presentation of Mucormycosis | Cutaneous | Rhino-orbital | Rhino-orbital-cerebral | Disseminated | Gastrointestinal | Pulmonary (necrotic empyema) | Rhino-orbital | Pulmonary |
| Diagnosis | Culture of aspirate fluid culture and operative specimen | Biopsy and culture of nasal specimen | Culture of sinus operative specimen | Post-mortem | Biopsy of gastric ulcer | Pathology and culture of operative empyema specimen | Biopsy and culture of nasal specimen | Microscopy of bronchial aspirate |
| Fungal species isolated | Rhizopus microsporus | NA | NA | NA | NA | Rhizopus species | Rhizopus species | Rhizopus species |
| Likely precipitating factors | 1. Post-OHT 2. Use of immunosuppressive therapy 3. DM 4. Corticosteroid use |
1. DM 2. Corticosteroid use |
1. Undiagnosed DM Diabetic keto-acidosis |
Obesity | Corticosteroid use | Corticosteroid use | Uncontrolled DM | Unclear |
| Antifungal treatment | 1. AMB Posaconazole |
AMB | AMB | None (Post-mortem diagnosis) |
None (Death prior to institution of antifungals) |
AMB | 1. AMB 2. Caspofungin 3. Retrobulbar AMB injections |
1. AMB Isavuconazole |
| Patient outcome | Death | Death | Death | Death | Death | Death | Death | Death |
| Reference | Current Case | 11 | 12 | 13 | 14 | 15 | 16 | 17 |
AMB: liposomal Amphotericin B; CAD: coronary artery disease; CKD: chronic kidney disease; COVID-19: Coronavirus disease 2019; Cr: serum creatinine level; CRP: C-reactive protein; D-dimer: D-dimer assay level; DM: diabetes mellitus; F: female; HF; heart failure; HTN: Hypertension; ICM: ischemic cardiomyopathy; M: male; NA: not available; OHT: orthotopic heart transplant; s/p: status post.
The pathophysiology of secondary IFI in COVID-19 patients is not known at this time–there are several potential mechanisms, all of which may have contributed to the development of mucormycosis in our patient.
-
•
Firstly, COVID-19 is associated with extensive pulmonary parenchymal disease [13]. Post-mortem examination of COVID-19 patients has demonstrated significant diffuse alveolar damage, hyaline membrane formation, interstitial lymphocyte infiltration and vascular microthrombi formation [13]. These pulmonary changes may take weeks to resolve and thus may serve as a nidus for fungal infection [18].
-
•
Secondly, COVID-19 is associated with severe immune system abnormalities–reduced CD4+ and CD8+ T-lymphocyte counts; elevated inflammatory cytokines [interleukin (IL)-2R, IL-6, IL-10, tumor necrosis factor-alpha] [19], [20]. As seen in influenza, this dysregulation may promote pathogenesis of IFIs [19].
-
•
Thirdly, severe COVID-19 results in mechanical ventilation and prolonged intensive care unit stay–this may predispose to bacterial co-infections and IFIs [7].
-
•
Finally, the fungal co-infection may have occurred secondary to immunosuppressive medication use for COVID-19 management. In one retrospective study of nine COVID-19 patients with Aspergillus isolated in clinical specimens, seven patients had received corticosteroids and five patients had received IL-6-antagonist tocilizumab [9]. The corticosteroid dexamethasone has shown mortality benefits in COVID-19 patients requiring supplemental oxygen [21]. However, the potential risk of fungal infection cannot be ruled out; recent studies reported that the use of corticosteroids predisposed to an increased risk of aspergillosis [8], [10].
To the best of our knowledge, this is the first documented case of mucormycosis occurring in a heart transplant recipient with prior COVID-19. Our patient was diabetic, with a recent transplant and immunosuppressive medication use. He also received a brief corticosteroid course for gout flare. Thus, similar to previously described cases, our patient had severe COVID-19, use of immunosuppressive therapies and multiple risk factors for the development of mucormycosis (Table 1). Similar to the reports of CAPA occurring in COVID-19 patients [9], [10], the potential link between mucormycosis and COVID-19 needs to be investigated further. Additionally, with the pandemic continuing for more than a year since the initial recognition of SARS-CoV-2, clinicians must evaluate for secondary infections and co-infections in COVID-19 patients. A strategy (as suggested by Song et al.) for recognizing the risk factors for IFIs and ensuring their timely diagnosis and management must be considered for implementation in COVID-19 patient-care protocols [19]. Early identification of fungal co-infections may significantly reduce morbidity and mortality [12].
Conclusion
Clinicians should be vigilant to evaluate for mucormycosis in patients with COVID-19 infection. Further research is needed to evaluate the potential link between these two infections.
Ethics statement
The work described has been carried out in accordance with The Code of Ethics of the World Medical Association (Declaration of Helsinki) for experiments involving humans. The research met our institutional definition of a case report (a medical chart review of 3 or fewer patients) and thus insitutional research board review was not needed. As we have used anonymized images and we have removed all patient identifiers, consent was not obtained.
Disclosure of interest
The authors declare that they have no competing interest.
References
- 1.Roden M.M., Zaoutis T.E., Buchanan W.L., et al. Epidemiology and outcome of zygomycosis: a review of 929 reported cases. Clin Infect Dis. 2005;41:634–653. doi: 10.1086/432579. [DOI] [PubMed] [Google Scholar]
- 2.Spellberg B., Edwards J., Jr., Ibrahim A. Novel perspectives on mucormycosis: pathophysiology, presentation, and management. Clin Microbiol Rev. 2005;18(3):556–569. doi: 10.1128/CMR.18.3.556-569.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Petrikkos G., Skiada A., Lortholary O., Roilides E., Walsh T.J., Kontoyiannis D.P. Epidemiology and clinical manifestations of mucormycosis. Clin Infect Dis. 2012;54(1):S23–S34. doi: 10.1093/cid/cir866. [DOI] [PubMed] [Google Scholar]
- 4.Rammaert B., Lanternier F., Zahar J.R., et al. Healthcare-associated mucormycosis. Clin Infect Dis. 2012;54(Suppl. 1) doi: 10.1093/cid/cir867. S44-54. [DOI] [PubMed] [Google Scholar]
- 5.Pappas P.G., Alexander B.D., Andes D.R., et al. Invasive fungal infections among organ transplant recipients: results of the Transplant-Associated Infection Surveillance Network (TRANSNET) Clin Infect Dis. 2010;50(8):1101–1111. doi: 10.1086/651262. [DOI] [PubMed] [Google Scholar]
- 6.World Health Organization Coronavirus disease 2019 (COVID-19) dashboard. Available online: https://covid19.who.int (Accessed February 20, 2021).
- 7.Lansbury L., Lim B., Baskaran V., Lim W.S. Co-infections in people with COVID-19: a systematic review and meta-analysis. J Infect. 2020;81(2):266–275. doi: 10.1016/j.jinf.2020.05.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.White P.L., Dhillon R., Cordey A., et al. A national strategy to diagnose COVID-19 associated invasive fungal disease in the ICU. Clin Infect Dis. 2020 doi: 10.1093/cid/ciaa1298. ciaa1298, [published online ahead of print, 2020 Aug 29] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Nasir N., Farooqi J., Mahmood S.F., Jabeen K. COVID-19 associated pulmonary aspergillosis (CAPA) in patients admitted with severe COVID-19 pneumonia: an observational study from Pakistan. Mycoses. 2020;63:766–770. doi: 10.1111/myc.13135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bartoletti M., Pascale R., Cricca M., et al. PREDICO study group. Epidemiology of invasive pulmonary aspergillosis among COVID-19 intubated patients: a prospective sudy. Clin Infect Dis. 2020 doi: 10.1093/cid/ciaa1065. ciaa1065, [published online ahead of print, 2020 Jul 28] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Mehta S., Pandey A. Rhino-orbital mucormycosis associated with COVID-19. Cureus. 2020;12(9):e10726. doi: 10.7759/cureus.10726. Published 2020 Sep 30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Werthman-Ehrenreich A. Mucormycosis with orbital compartment syndrome in a patient with COVID-19. Am J Emerg Med. 2020 doi: 10.1016/j.ajem.2020.09.032. S0735-6757(20)30826-3 [published online ahead of print, 2020 Sep 16] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hanley B., Naresh K.N., Roufosse C., et al. Histopathological findings and viral tropism in UK patients with severe fatal COVID-19: a post-mortem study. Lancet Microbe. 2020;1(6):e245–e253. doi: 10.1016/S2666-5247(20)30115-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Monte Junior E.S.D., Santos M.E.L.D., Ribeiro I.B., et al. Rare and fatal gastrointestinal mucormycosis (Zygomycosis) in a COVID-19 patient: a case report. Clin Endosc. 2020 doi: 10.5946/ce.2020.180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Placik D.A., Taylor W.L., Wnuk N.M. Bronchopleural fistula development in the setting of novel therapies for acute respiratory distress syndrome in SARS-CoV-2 pneumonia. Radiol Case Rep. 2020;15(11):2378–2381. doi: 10.1016/j.radcr.2020.09.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Mekonnen Z.K., Ashraf D.C., Jankowski T., et al. Acute invasive rhino-orbital mucormycosis in a patient with COVID-19-Associated Acute Respiratory Distress Syndrome. Ophthalmic Plast Reconstr Surg. 2020:10. doi: 10.1097/IOP.0000000000001889. 1097/IOP.0000000000001889, [published online ahead of print, 2020 Nov 19] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Pasero D., Sanna S., Liperi C., et al. A challenging complication following SARS-CoV-2 infection: a case of pulmonary mucormycosis [published online ahead of print, 2020 Dec 17] Infection. 2020:1–6. doi: 10.1007/s15010-020-01561-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kanne J.P., Little B.P., Chung J.H., Elicker B.M., Ketai L.H. Essentials for Radiologists on COVID-19: An Update-Radiology Scientific Expert Panel. Radiology. 2020;296(2):E113–E114. doi: 10.1148/radiol.2020200527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Song G., Liang G., Liu W. Fungal co-infections associated with global COVID-19 pandemic: a clinical and diagnostic perspective from China. Mycopathologia. 2020;185(4):599–606. doi: 10.1007/s11046-020-00462-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Gangneux J.P., Bougnoux M.E., Dannaoui E., Cornet M., Zahar J.R. Invasive fungal diseases during COVID-19: we should be prepared. J Mycol Med. 2020;30(2):100971. doi: 10.1016/j.mycmed.2020.100971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.RECOVERY Collaborative Group. Horby P., Lim W.S., Emberson J.R., et al. Dexamethasone in hospitalized patients with Covid-19–Preliminary Report. N Engl J Med. 2020 doi: 10.1056/NEJMoa2021436. NEJMoa2021436. [DOI] [PMC free article] [PubMed] [Google Scholar]


