Significance
Animal skins are complex, highly specialized surfaces that are decorated with a variety of small structural features, whose functional benefits are often unknown. We investigate the microscopic features present on snake skins—which serve as the only interface between these animals and their environments—and we discover that distantly related sidewinding vipers have a unique structure that is distinct from other snakes. We develop a mathematical model that links structure to function and provides insight into evolutionary and behavioral adaptation in limbless locomotion.
Keywords: snake, locomotion, evolution, structure, function
Abstract
The small structures that decorate biological surfaces can significantly affect behavior, yet the diversity of animal–environment interactions essential for survival makes ascribing functions to structures challenging. Microscopic skin textures may be particularly important for snakes and other limbless locomotors, where substrate interactions are mediated solely through body contact. While previous studies have characterized ventral surface features of some snake species, the functional consequences of these textures are not fully understood. Here, we perform a comparative study, combining atomic force microscopy measurements with mathematical modeling to generate predictions that link microscopic textures to locomotor performance. We discover an evolutionary convergence in the ventral skin structures of a few sidewinding specialist vipers that inhabit sandy deserts—an isotropic texture that is distinct from the head-to-tail-oriented, micrometer-sized spikes observed on a phylogenetically broad sampling of nonsidewinding vipers and other snakes from diverse habitats and wide geographic range. A mathematical model that relates structural directionality to frictional anisotropy reveals that isotropy enhances movement during sidewinding, whereas anisotropy improves movement during slithering via lateral undulation of the body. Our results highlight how an integrated approach can provide quantitative predictions for structure–function relationships and insights into behavioral and evolutionary adaptations in biological systems.
Small surface features, ubiquitous in biological systems, can have profound and diverse functional consequences (1). While developing a link between microscopic structure and macroscopic function is challenging in general, some progress has been made in specific systems, where surface-characterization techniques are combined with mathematical or physical modeling. For example, nanostructured and microstructured patterns on biological surfaces manipulate interactions with light and can greatly affect appearance (2, 3); microscopic hair-like structures enhance adhesion (4) and manipulate fluid flows (5); microscopic denticles on shark skin affect fluid flows (6, 7); and microscopic cilia on bird feathers help to stabilize wing shape and improve flight mechanics (8). Here, we build upon the approach taken in these studies, combining surface-characterization techniques and mathematical modeling as a part of a comparative study across snake species from diverse habitats (Fig. 1) to develop an understanding of how putative evolutionary adaptations may affect snake locomotion.
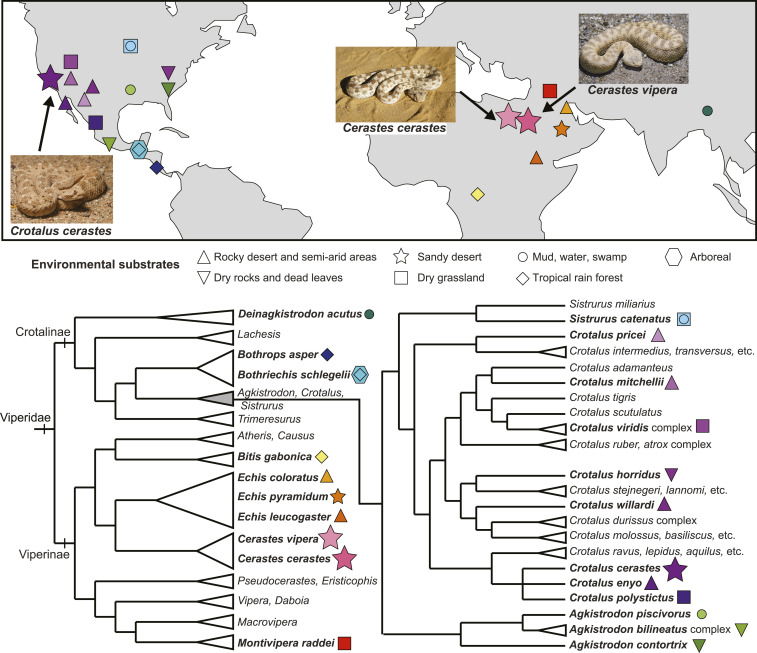
Fig. 1.
Geography and phylogeny of vipers. (Upper) World map showing approximate geographic locations of 22 viper species. Symbols indicate natural substrates, and colors distinguish species within a habitat. (Lower) Phylogeny of viperid snakes (data from ref. 9), with the pitviper (Crotalinae) clade expanded to the right (data from refs. 9 and 10). we show a conservative polytomy for the topology of the subclade (Cr. cerastes, Cr. enyo, and Cr. polystictus). Bold text and colored symbols (consistent with map labels) identify species investigated here. Cladistic and geographic patterns underscore the independent, convergent nature of sidewinding locomotion (bolded stars) in the American sidewinder (Cr. cerastes), a crotaline pitviper, and the African sidewinding viperine vipers (Cerastes spp.). Each snake shown is approximately 35 cm in length. Cr. Cerastes and Ce. Vipera images credit: Wolfgang Wuster (photographer). Ce. cerastes image credit: iStock/acceptfoto. Map image credit: VectorStock/SpicyTruffel.
Limbless locomotion is achieved through the use of properly coordinated sequences of body curvatures that push on the surrounding environment to generate propulsive forces—an interaction that in terrestrial situations is primarily mediated by the highly frictional interface between the body’s ventral surface and the substrate. A common strategy for generating movement [and one that all snakes are capable of (11)] is lateral undulation—in which an animal creates and subsequently propagates lateral body bends from head to tail, producing overall displacements primarily in the cranial direction. An example of this movement pattern in Chionactis occipitalis (Colubridae) (Fig. 2A), a desert specialist, is shown for two cycles of lateral undulation in Fig. 2B.
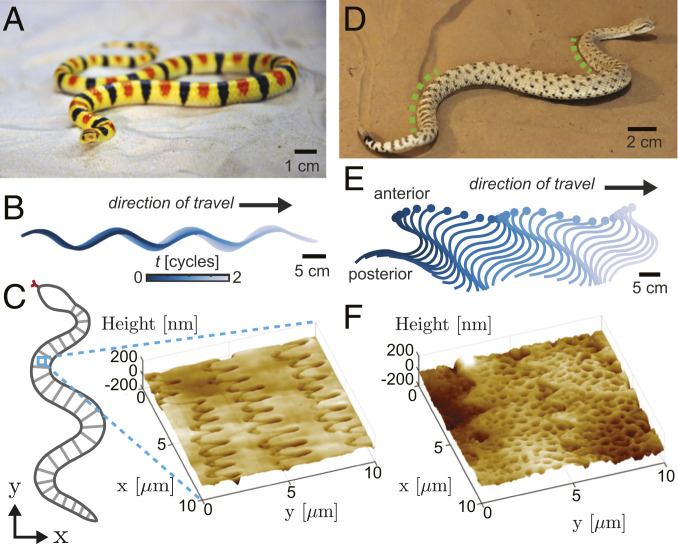
Fig. 2.
Undulatory locomotion modes of two sand-specialist snakes. (A) The shovel-nosed snake (Colubridae: Ch. occipitalis). Reprinted from ref. 46. (B) Time trace of splined points along the body as the snake moves on 300-m glass beads via lateral undulation. (C, Left) Schematic of the underside of a snake; transverse lines represent ventral scales with the blue box highlighting that our scan regions are subscale in size (not to scale). (C, Right) AFM scan of microscopic structure within one ventral scale of Ch. occipitalis. (D) The sidewinder (Viperidae: Cr. cerastes). Green dashed lines indicate regions of body–substrate contact. (E) Time trace of splined points along the body as the snake sidewinds on natural sand. (F) AFM scan of microscopic structure within one ventral scale of Cr. cerastes. We note that the posterior–anterior axis in C and F is aligned with the y axis, and larger y values are closer to the head of the animal.
Some snakes have adopted modifications to this mode of movement, where a coupled, but independently modulated, vertical wave of body bending is also generated and propagated from head to tail. Depending on the environment and lifting pattern, this vertical wave can act to modulate environmental contact—leading to enhanced speed through drag reduction during typical slithering locomotion (12), improved maneuverability on sandy slopes through weight distribution modulations that help reduce avalanching events during sidewinding (13)—and has even been shown to stabilize the aerial glides of arboreal snakes (14). Here, we focus on the differences between sidewinding specialists and other vipers that primarily use other modes of locomotion.
During sidewinding, a phase-shifted vertical traveling wave creates nearly static contact patches with the substrate that, when coupled to the lateral wave, enables the animal to move forward at an angle (relative to the cranial–caudal axis) in a continuing series of steps (13, 15) (Fig. 2 D and E). Specialization for sidewinding has evolved a small number of times and has been best characterized in the sidewinder rattlesnake (Crotalus cerastes), a pitviper from North American deserts, and in several true vipers (e.g., Cerastes species [spp.], Pseudocerastes spp., and Bitis spp.) from African and Middle Eastern deserts (16). Despite the unique mode of locomotion, the only morphologically distinctive feature identified thus far is a shorter musculus semispinalis-spinalis, a muscle likely involved in lifting portions of a snake’s body (17, 18).
Atomic force microscopy (AFM) surface characterization of the ventral scales from shed skins of Ch. occipitalis (Fig. 2C) revealed a structurally anisotropic texture in which the epidermal skin cells have caudally directed, elevated, regular, micrometer-sized spikes we refer to as microspicules; previous literature has inconsistently used other terms for these structures (e.g., microfibrils, denticles, or digits) that we feel offer poor or misleading anatomical characterizations (19–21). The anatomy seen in Ch. occipitalis is qualitatively similar to those found in previous studies of many nonviperid alethinophidian snakes (19, 20, 22–30). This configuration of the microspicules produces longitudinal structural anisotropy on the ventral surface that is closely associated with lateral undulation and with other types of cranially oriented movement. In contrast, we found that Cr. cerastes possessed a different, more isotropic texture with greatly reduced microspicules and large impressions; similar, but smaller, structures in other species that have been referred to as pits or holes (21) (Fig. 2F). To investigate whether these differences in textures relate to movement patterns or details of the environmental interactions, we characterized the microscopic textures on the ventral scales of a broad range of species within the viper family (7 Viperinae and 15 Crotalinae). Fig. 3 A and B, Left show m m regions on three nonsidewinding species and three sidewinding species. We observed qualitatively similar textures in the nonsidewinding crotalines (pitvipers), comprising regularly arranged, elongated, sharply pointed, caudally protruding microspicules oriented along the longitudinal axis of the body; we consider this to be the ancestral (plesiomorphic) morphology for the clade (Fig. 3A). The main differences in textures across species are quantitative, primarily intramicrospicule and intermicrospicule length variations (Fig. 3A and SI Appendix, Table S1). In contrast, Cr. cerastes (Fig. 3 B, Upper Left) has a remarkably different (derived) morphology—greatly reduced microspicules and the presence of isotropic pits—that is distinct from close relatives Crotalus polystictus (Fig. 3A, Upper Left) and Crotalus enyo (SI Appendix, Fig. S1 and Table S1).
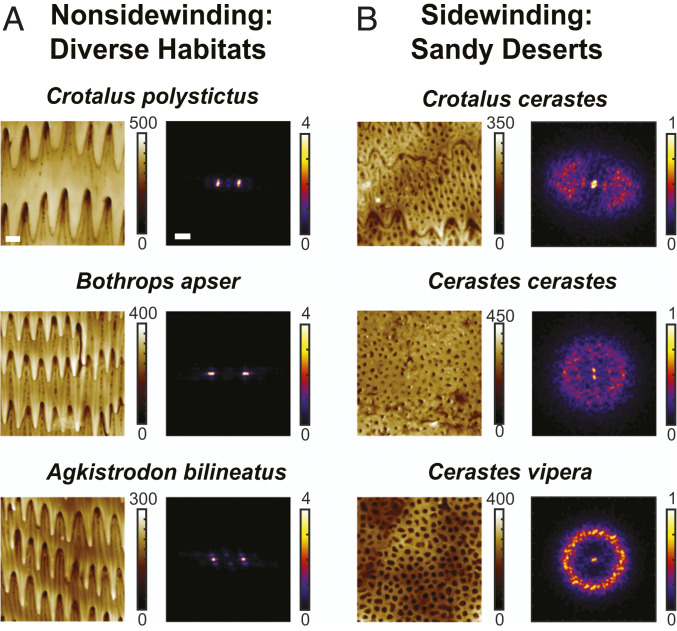
Fig. 3.
Microscopic ventral scale structures. AFM scans were performed to examine the structure of a subsample of a single scale (as illustrated by the light blue box in the schematic of Fig. 2C). Three 10-m × 10-m scans (color indicates height in nanometers) and their corresponding 2D power spectra are shown for three representative nonsidewinding species (A) and three sidewinding species (B). All scans are oriented such that the head (tail) is toward the top (bottom) of the page. The bright spots in the power spectra for nonsidewinding species confirm the presence of a strong structural anisotropy oriented along the body across species and environments. For all sidewinding species scanned, the power spectra are more isotropic, indicating a lack of (or reduced) structural anisotropy.
Our smaller sampling of Viperinae (true vipers) also revealed a general pattern of caudally projecting microspicules, but with more variability in size and shape across species than is evident in pitvipers (SI Appendix, Figs. S1 and S2 and Table S1). While the ancestral morphology of the viperine clade is not clear from our sampling, we find that the North African sidewinding vipers, Cerastes cerastes and Cerastes vipera, have distinct textures—marked by the absence of microspicules and the presence of isotropic pits—that are morphologically similar to that of the American sidewinder, Cr. cerastes (Fig. 3B).
The convergent evolution of similar isotropic textures in the North American sidewinder rattlesnake (Cr. cerastes) and in distantly related North African sidewinding specialists (Cerastes spp.) under similar environmental conditions suggests adaptation. Moreover, the fact that a laterally undulating sand specialist (Ch. occipitalis) retains an anisotropic condition (Fig. 2C) suggests that isotropy is related specifically to sidewinding locomotion, rather than to sandy habitats. The species that use sidewinding as their primary mode of locomotion differ in the degree to which they show derived morphology: North American Cr. cerastes have greatly reduced microspicules and enlarged, cratered, epidermal pits, whereas in North African Cerastes spp., the microspicules are completely absent, and the enlarged epidermal pits are more smoothly bounded than in the American sidewinder. That the North American species appears somewhat less morphologically specialized is consistent with the context of Earth’s geologic history and viperid evolutionary history. Fossil dunes indicate that sandy desert conditions appeared in the Sahara region at least 7 million years ago, and the geologic record shows that arid conditions have cyclically prevailed since (31, 32). In contrast, the Mojave Desert of North America first accumulated sand only about 15,000 to 20,000 years ago (33, 34). Additionally, an ancestral state reconstruction shows that the common ancestor of North African Cerastes spp. had most likely evolved specialization for sidewinding by about 16 million years ago, while the most recent common ancestor of North American sidewinders and their sister species (about 11 million years ago) probably did not sidewind (16).
In addition to the three sidewinding specialists, Echis pyramidum sidewinds regularly and proficiently on sand, but uses other types of locomotion on nonsand substrates, while Echis coloratus can sidewind when frightened or when placed on shifting or smooth substrates, though it lives in rocky habitats (35–37). The ventral surface of E. pyramidum lacks microspicules and has a low structural anisotropy (SI Appendix, Fig. S1). The ventral surface of E. coloratus does not resemble that of the specialized sidewinders.
Although we were unable to obtain samples for sidewinding species in other genera, we can make predictions as to their degree of morphological specialization. Pseudocerastes spp. and Eristicophis macmahoni should possess highly specialized scale morphology, similar to Cerastes spp., considering that their shared ancestor about 21 million years ago was likely a specialized sidewinder (16). Sidewinding specialization in an ancestor of Bitis peringueyi and Bitis caudalis may have evolved more recently, perhaps only 6 million or 7 million years ago (16). Their ventral scales may therefore show only partial loss of the anisotropic condition, similar to Cr. cerastes. Echis carinatus may similarly show incomplete specialization in scale microtexture. If future studies confirm our predictions, the results would indicate that the evolution of sidewinding behavior likely preceded the evolution of highly specialized scale morphology.
We quantitatively compared microtextures by computing the two-dimensional (2D) power spectrum of a 20-m 20-m AFM scan region for each species (Fig. 3 A And B, Right; SI Appendix, Fig. S1). Sharp peaks in the power spectra result from periodic structures, with peak locations identifying both dominant feature lengths and corresponding directions of periodicity. For the nonsidewinding species investigated, the strongest peaks are oriented along the horizontal axis, indicating that the lateral structure is the dominant feature in the array of microspicules. In contrast, the structures of the three sidewinding species sampled lack a strong direction dependence, as indicated by the nearly radially symmetric appearance of the power spectra in Fig. 3B. To quantify the observed differences in the textures, we created a structural anisotropy index, , that compares the angular variation in the power spectra along the dominant structural direction and the orthogonal direction.
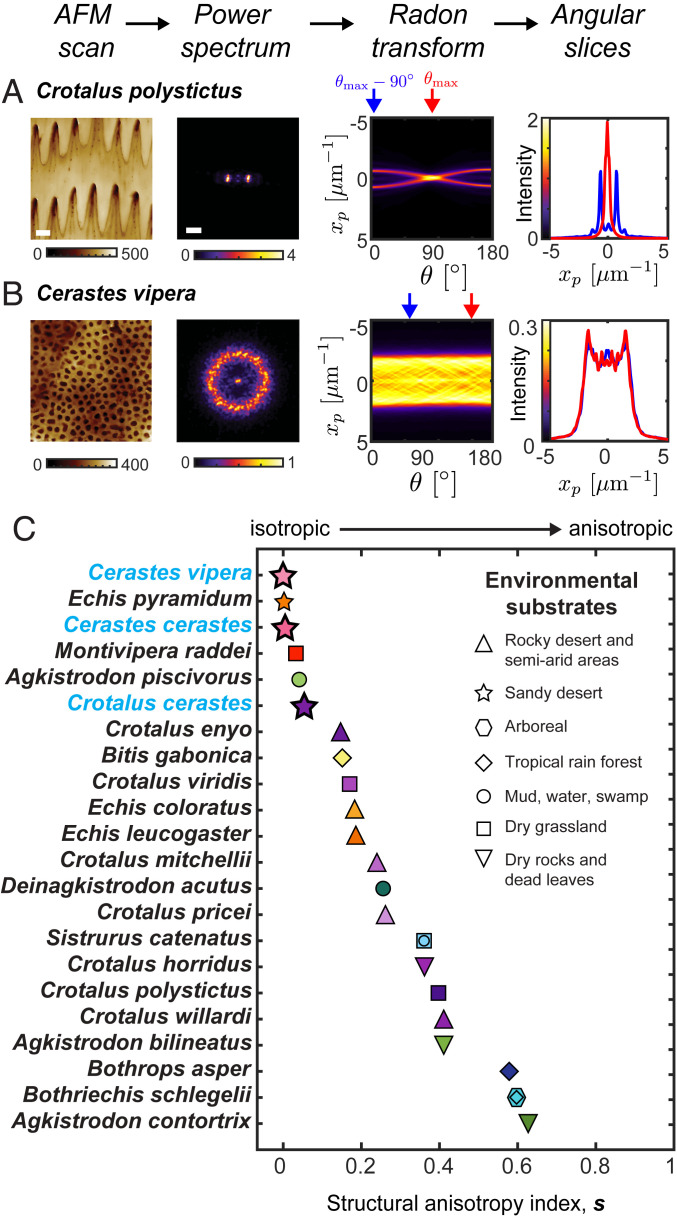
To determine , we computed the radon transform of each power spectrum and defined the dominant structural direction by the brightest pixel value; was then defined as the Jensen–Shannon divergence (38) of two slices through the radon transform: the one along the dominant direction and the one orthogonal to the dominant direction (Fig. 4 A and B). The three sidewinding specialists investigated here all have low values of () and are among the lowest values of all vipers sampled, indicating that the microtextures are more isotropic than most species we investigated. We note that is bounded between zero and one, with zero indicating the two directions have identical features and one indicating maximal dissimilarity (see Materials and Methods for details). The relative isotropy of the evidently nonsidewinding viperine Montivipera raddei (Fig. 4C) is an interesting result and suggests that microstructural evolution in the viperine clade is worthy of subsequent investigation. Low anisotropy in the pitviper Agkistrodon piscivorus may be associated with relaxed selection for anisotropy due to its semiaquatic habits and/or use of muddy substrates, and this also invites further investigation.
Fig. 4.
Quantification of structural anisotropy. We define a structural anisotropy index, , by taking the radon transform of the power spectrum and comparing the radial profiles of the radon transform along the dominant angular direction, , and the orthogonal direction, . (A and B) These steps are shown for Cr. polystictus (A) and Ce. vipera (B). (C) The Jensen–Shannon divergence of the two radon profiles provides a quantification of the structural anisotropy (see Materials and Methods for details). The three sidewinding specialists investigated here (labeled with blue text) have nearly isotropic microtextures.
Previous AFM investigations of the structurally anisotropic microspicule textures have measured a drag-direction-dependent microscopic friction coefficient, with smaller values for forward movement (from head to tail) along the body (19, 23, 26–30, 39). Larger friction coefficients have consistently been reported for backward (tail to head) movement, and, while there has been some sensitivity to experimental details, some studies have reported similarly large friction coefficients for lateral movement across the body in both natural and artificial microspicule structures (23, 26–29). Previously reported macroscopic measurements of friction are qualitatively consistent with microscopic measurements, reporting a larger value for backward movement relative to forward movement (12, 40) and a larger value for lateral movement relative to forward movement (12). However, thus far, hypotheses about the mechanical importance of these textures for locomotion have focused on the forward/backward anisotropy.
Like previous researchers, we expect that the direction-dependent friction coefficient results from the structural anisotropy. However, given the dominance of the lateral features, as indicated by the power spectra in Fig. 3A (and SI Appendix, Fig. S1), we hypothesized that the lateral/forward anisotropy is important particularly for lateral undulation, which is common for snakes with these anisotropic structures. We developed a mathematical model of a snake and used a modified granular resistive force theory (RFT) (41–43) to model environmental interactions and systematically probe how frictional anisotropy affects locomotion (Materials and Methods). To decouple the anisotropy associated with flowable substrates (e.g., see ref. 44) from the hypothesized contribution of microstructural features present on the body, we modeled the substrate interactions with kinetic Coulomb friction (45).
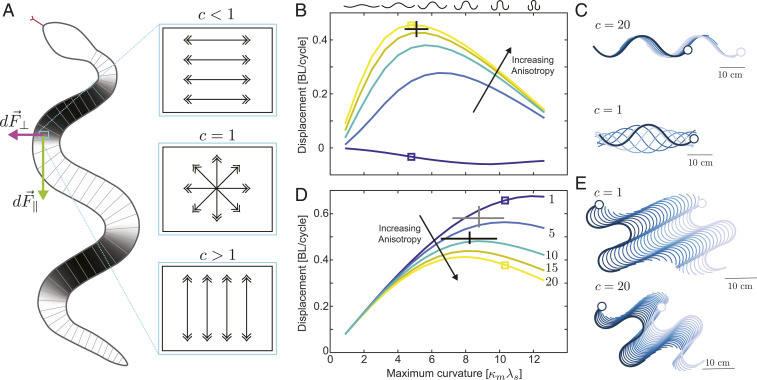
Our hypothesized relationship between structure and friction is shown in Fig. 5A; arrows in the enlarged regions to the right identify directions associated with lower friction. We define a frictional anisotropy coefficient, , as the ratio of the lateral (locally transverse to the body midline) to longitudinal friction coefficients (locally aligned with the body midline). If = 1 (i.e., standard kinetic Coulomb friction), then movement along all directions results in the same frictional force. If , the transverse friction is larger than the longitudinal friction. If , then transverse friction would be lower than longitudinal friction, though, to our knowledge, this condition has not been experimentally observed in snakes. We note that, as with other implementations of RFT, there is no forward (head to tail)/backward (tail to head) frictional anisotropy in our model.
Fig. 5.
Performance varies with anisotropy. (A) Schematic of the model. The body is divided into small segments, each of which experiences an environmental force, with components both parallel and perpendicular to the long axis of the body. Arrow sketches in A, Right show a hypothesized connection between microspicule structure and relative ease of movement, where arrows indicate the direction associated with the smallest friction coefficient. For lateral undulation, all parts of the body are in contact with the ground. In sidewinding, the contact pattern changes throughout a gait cycle (13, 15) with sections of body alternately in contact with (black areas on snake schematic), or lifted above (white areas) the substrate. (B) Model-predicted displacements (in body lengths [BL] per undulation cycle) for lateral undulation as a function of maximum body curvature. Curves of different colors show predictions for different anisotropy values. (C) Model-generated kinematics for one cycle of movement for lateral undulation for c = 20 (Upper) and c = 1 (Lower). Curvatures and predicted displacements are highlighted by the square points in B. (D) Model-predicted displacements (BL/cycle) for sidewinding as a function of maximum body curvature, with color indicating anisotropy value. (E) Model-generated kinematics for one cycle of movement via sidewinding for c = 1 (Upper) and c = 20 (Lower). Curvatures and predicted displacements are highlighted by the square points in D.
For lateral undulation, all body segments were modeled to maintain substrate contact throughout movement; therefore, all segments were treated equally and experienced nonzero environmental reaction forces. Fig. 5B shows predicted displacements as a function of maximal nondimensional body curvature, , for a snake with waves along the body (see Materials and Methods for more detail). For each curvature investigated, we found that lateral undulation performance was enhanced by higher values. While we do not know the relationship between the microscopic structure and overall frictional anisotropy (or how substrate anisotropy may contribute to the overall frictional anisotropy), the experimentally measured performance of Ch. occipitalis moving on sand (the black cross in Fig. 5B; originally reported in refs. 44 and 45) is bounded by model predictions for high anisotropy values (), and the range of observed curvature values nearly coincide with model-predicted peak performance per cycle. Examination of model-generated trajectories with curvature values within the range typically used by Ch. occipitalis (Fig. 2 A and B; refs. 44 and 46) reveals that when anisotropy is high (), the snake is predicted to move forward about 0.5 body lengths over the course of one undulation cycle (Fig. 5 C, Upper). However, when , nonzero locally transverse velocity components cause lateral (sideways) slip that hinders locomotion and prevents forward displacement (Fig. 5 C, Lower).
To adapt our model for sidewinding, we introduced a smoothly varying posture-dependent contact function (ranging from zero to one) that is consistent with previous characterizations of substrate contact in Cr. cerastes (13, 15, 45). This contact function multiplies the forces of each infinitesimal segment and specifies how much each segment experiences substrate forces (with zero corresponding to fully lifted and one corresponding to full-contacted segments, respectively; see ref. 45 and Materials and Methods for more detail). The grayscale coloration of the snake schematic in Fig. 5A shows the prescribed contact pattern associated with this posture, with black regions on the ground and white regions fully lifted.
Predicted displacements for sidewinding are shown in Fig. 5D as a function of maximal body curvature for locomotors with waves along the body. We note that all aspects of the model, aside from the variable contact pattern, are the same as the lateral undulation model. We predict enhanced performance for each curvature as anisotropy is decreased (i.e., for smaller values of ; Fig. 5D). Previously reported animal performance on both sand and smooth wood (Fig. 5D, black and gray crosses, respectively) revealed that animals displace further when moving on rigid substrates (bounded by model predictions for ), despite using similar curvatures on both hard and soft substrates (45); we attributed this difference in performance to an additional contribution to the overall frictional anisotropy arising from the flowable sand substrate. Examination of model-generated trajectories associated with curvature values used by the American sidewinder [Cr. cerastes (15, 45)] revealed that high anisotropy () prevents slipping locally perpendicular to the body midline and, therefore, induces a velocity component along the midline and directed toward the tail (i.e., a backward slip) that opposes the overall direction of motion to achieve force balance. This backward slip hinders forward progress by decreasing the net displacement possible over the course of a cycle (Fig. 5 E, Lower). However, when there is no anisotropy (), the force balance produces a velocity component orthogonal to the overall direction of motion (i.e., a sideways slip) that does not oppose forward progress and, therefore, results in larger displacements over the course of an undulation cycle (Fig. 5 E, Upper).
Our modeling results reveal that a high lateral/longitudinal frictional anisotropy improves predicted forward displacements during lateral undulation, which we propose is provided (at least in part) by the periodic array of caudally projected microspicules observed on the ventral surfaces of laterally undulating snakes. This hypothesis is consistent with previous microscopic and macroscopic ventral friction measurements (12, 19, 23, 26–29, 39, 40). Our model-predicted displacements are in agreement with biological observations (13, 15, 44, 45), despite having excluded the backward/forward anisotropy that has been the focus of previous AFM studies. Our results, along with the quantitative agreement achieved by other RFT models (40–42, 45) (none of which include a backward/forward anisotropy), suggest that the lateral/longitudinal anisotropy is more important for undulatory locomotion than backward/forward anisotropy. Our model predicts that higher values of lateral/longitudinal anisotropy lead to enhanced performance for lateral undulation, which we posit is a consequence of the anisotropic microstructure. RFT modeling of sidewinding reveals that, unlike lateral undulation, performance enhanced by the lack of a lateral/longitudinal anisotropy. Previously reported biological sidewinding performance is in quantitative agreement with predictions for isotropic friction, which we hypothesize results from the observed isotropic ventral microstructure.
In this paper, we examined the microtextures present on the ventral surface of shed viper skins. In most snakes investigated, we found variations of a longitudinally oriented, caudally projecting, periodic array of microspicules. Surprisingly, we found two independent origins of a derived, nearly isotropic, pitted ventral microtexture in three viper species (Fig. 3B) that coincide with specialization for sidewinding locomotion, suggesting adaptation. These results provide a prediction for expected structural features on other sidewinding specialists that we did not have access to for this study. Though we do not yet have a precise quantitative mapping from structural anisotropy to friction, we created a model in which frictional anisotropy can be systematically varied. Our model revealed that anisotropic frictional interactions are beneficial for lateral undulation, but detrimental for sidewinding, suggesting that the nearly isotropic microtextures observed on sidewinders may enhance their locomotor performance. Previous work investigating the direction-dependent nature of nonsidewinding microtextures found a larger anisotropy when skin samples were mounted with soft backing (27), suggesting that frictional properties may be enhanced by the compliant soft tissues under the skin. It would be interesting to explore the potential dependence of the skin friction on the mechanical properties of underlying substrate, as well as the degree to which muscle-activation patterns may be able to control frictional interactions through modulations of microspicule protrusion. Previous studies have also noted the presence of external lipid layers on the ventral scales of least some snake species (47)—it would be interesting to explore the potential benefits of surface coatings (e.g., lubrication, traction, and/or wear mitigation) and how they may affect environmental interactions and frictional anisotropy. Finally, while other modes of limbless locomotion were outside the scope of this study, our approach could be used to explore other potential relationships and functional benefits of microstructures for other locomotor modes and, more broadly, other animal behaviors that are essential for survival.
Materials and Methods
Surface Characterization.
Surface-topography images of skins were acquired by an AFM (Bruker Multimode 8) with a silicon nitride tip (Bruker Scanasyst-Air) under peak-force tapping mode in air. Samples with adherent dust (Ce. cerastes and Ce. vipera) were cleaned for 30 min by ultrasonicating in detergent (5% Cole-Parmer micro-90 in water). Water-rinsed and air-dried samples were then mounted on a glass substrate. No change was observed for the surface topography of a noncontaminated sample from Crotalus viridis before and after the cleaning treatment, demonstrating that the cleaning protocol did not alter the intrinsic surface.
We note that our study was not designed to examine variation in microstructure with regard to ontogeny or anatomical position, nor among individuals (reviewed by refs. 20, 26, and 48). Samples from a central point of midbody unwrinkled ventral scales from naturally shed skins of adult snakes were mounted for AFM analyses (per ref. 20, figure 13a). Qualitative and quantitative measures and terms used are consistent with ref. 21, however, choosing the term “microspicules” rather than the terms “digits,” “microfibrils,” or “denticles” used in some previous works. The impressions variously referred to as “pits” or “holes” (21) are common features in many snakes, but their function and homology are unclear (21, 22).
Data Analysis.
To identify dominant structural features present in each AFM scan, we computed the power spectrum from a 20-m 20-m region. Scan images were rescaled and converted to 8-bit unsigned integer images in Matlab, and then background variation was removed by using a contrast-limited adaptive histogram equalizer function (adapthisteq, with the image divided into 25 equally sized regions). The 2D power spectra were computed after each image was converted to a double-precision array, and the overall average was subtracted from the image. Each power spectrum was smoothed by using a 2D Gaussian filter (with ). All power spectra are shown in SI Appendix, Fig. S1.
We computed the radon transforms (SI Appendix, Fig. S3) to quantify the angular dependencies of the of each 2D power spectrum. We identified , the angle associated with the brightest pixel in each radon transform, and took vertical slices through the radon transform at and . We compared these two slices through the radon transform using the Jensen–Shannon divergence (38), a measure of the distinguishability of two distributions that is bounded between zero (indistinguishable) and one (perfectly distinguishable) (SI Appendix, Fig. S3).
Modeling.
RFT assumes that forces along a deforming body are decoupled, and, therefore, a locomotor can be divided into many infinitesimal segments that can be treated independently. Further, in dissipation-dominated environments, where inertial forces are negligible, the net force on a body is zero at every moment in time, yielding
| [1] |
Here, and are the tangential and normal components, respectively, of the environmental force acting on an infinitesimal segment of the body (sketch in Fig. 5A).
| [2] |
| [3] |
where is the coefficient of kinetic friction, is the mass of the segment, m/, and and are unit vectors locally tangent and normal to the body, respectively. We introduced and varied , an anisotropy factor, to explore how the predicted performance depends on the magnitude of the normal forces relative to the tangential forces.
Snake Geometry.
To parameterize the time-varying shape of the snake, we linked 100 small segments together end-to-end. Connections between segments were treated as position-controlled lateral joints, with angle at position prescribed to vary sinusoidally along the body and through time, , creating a serpenoid curve (49):
| [4] |
where is the number of waves along the body, is the total number of segments along the body, and and are the time-varying angle amplitudes that produce a traveling wave propagated from head to tail, with temporal frequency and amplitude .
We note that animal-curvature values from previous studies were reported in terms , a nondimensional quantity originally defined in ref. 40. is the local curvature along the body, is the maximal value of , and is the arc-length of one wave. To relate to joint angles, , we note that is equal to , the total body length divided by the number of waves along the body. is given by , where is the length of a single body segment (given by ), is the local tangent angle along the body, and is equivalent to . Given these relations, reduces to , and is equal to the maximum value of .
Changing Contact.
Previous work (15) revealed that the three-dimensional pose of Cr. cerastes could be represented by a horizontal wave coupled to a phase-shifted vertical wave that sets the environmental contact condition. To properly couple the vertical wave to the in-plane shape, we introduced
| [5] |
where is the position along the body, is the number of waves on the body, is the total length of the body, and and describe the in-plane wave shape. To set the contact using the vertical wave description, , we defined the smoothly varying function,
| [6] |
Here, sets the local fraction of the environmental force experienced as a function of position, , along the body, sets contact width, and sets the sharpness of the on/off ground transition along the body.
To be consistent with previous observations of Cr. cerastes, and were chosen so that, when averaged over a completed gait cycle, approximately of the animal’s body is on the ground (13).
Supplementary Material
Acknowledgments
We thank Elisa Riedo for the use of AFM facilities and for helpful discussions; Kelimar Diaz Cruz for assistance with collection of initial AFM data; Sarah Huskisson for organizing the collection of shed skins; Gordon Berman and Stanislav Gorb for helpful discussions; and the reviewers for thoughtful comments that improved this manuscript. Samples were generously donated by Albuquerque BioPark Zoo, Riverbanks Zoo, Kentucky Reptile Zoo, and the Herpetology Department of Zoo Atlanta. This research was supported by the Georgia Tech Elizabeth Smithgall Watts Fund; NSF Physics of Living Systems Grants PHY-1205878 and PHY-1150760; Army Research Office Grant W911NF-11-1-0514; and the Dunn Family Professorship.
Footnotes
The authors declare no competing interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at https://www.pnas.org/lookup/suppl/doi:10.1073/pnas.2018264118/-/DCSupplemental.
Data Availability.
Matlab files data have been deposited in the Open Science Framework (https://doi.org/10.17605/OSF.IO/KJ9TV; ref. 50).
References
- 1.Gorb S. N., Functional Surfaces in Biology: Little Structures with Big Effects (Springer Science & Business Media, Dordrecht, Netherlands, 2009), vol. 1. [Google Scholar]
- 2.Vukusic P., Sambles J. R., Photonic structures in biology. Nature 424, 852–855 (2003). [DOI] [PubMed] [Google Scholar]
- 3.Sharma V., Crne M., Park J. O., Srinivasarao M., Structural origin of circularly polarized iridescence in jeweled beetles. Science 325, 449–451 (2009). [DOI] [PubMed] [Google Scholar]
- 4.Autumn K., Peattie A. M., Mechanisms of adhesion in geckos. Integr. Comp. Biol. 42, 1081–1090 (2002). [DOI] [PubMed] [Google Scholar]
- 5.Nasto A., Brun P. T., Hosoi A., Viscous entrainment on hairy surfaces. Phys. Rev. Fluids 3, 024002 (2018). [Google Scholar]
- 6.Oeffner J., Lauder G. V., The hydrodynamic function of shark skin and two biomimetic applications. J. Exp. Biol. 215, 785–795 (2012). [DOI] [PubMed] [Google Scholar]
- 7.Lauder G. V., et al. , Structure, biomimetics, and fluid dynamics of fish skin surfaces. Phys. Rev. Fluids 1, 060502 (2016). [Google Scholar]
- 8.Matloff L. Y., et al. , How flight feathers stick together to form a continuous morphing wing. Science 367, 293–297 (2020). [DOI] [PubMed] [Google Scholar]
- 9.Alencar L. R., et al. , Diversification in vipers: Phylogenetic relationships, time of divergence and shifts in speciation rates. Mol. Phylogenet. Evol. 105, 50–62 (2016). [DOI] [PubMed] [Google Scholar]
- 10.Reyes-Velasco J., Meik J. M., Smith E. N., Castoe T. A., Phylogenetic relationships of the enigmatic longtailed rattlesnakes (Crotalus ericsmithi, C. lannomi, and C. stejnegeri). Mol. Phylogenet. Evol. 69, 524–534 (2013). [DOI] [PubMed] [Google Scholar]
- 11.Lillywhite H. B., How Snakes Work: Structure, Function and Behavior of the World’s Snakes (Oxford University Press, Oxford, UK, 2014). [Google Scholar]
- 12.Hu D. L., Nirody J., Scott T., Shelley M. J., The mechanics of slithering locomotion. Proc. Natl. Acad. Sci. U.S.A. 106, 10081–10085 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Marvi H., et al. , Sidewinding with minimal slip: Snake and robot ascent of sandy slopes. Science 346, 224–229 (2014). [DOI] [PubMed] [Google Scholar]
- 14.Yeaton I. J., Ross S. D., Baumgardner G. A., Socha J. J., Undulation enables gliding in flying snakes. Nat. Phys. 16, 974–982 (2020). [Google Scholar]
- 15.Astley H. C., et al. , Modulation of orthogonal body waves enables high maneuverability in sidewinding locomotion. Proc. Natl. Acad. Sci. U.S.A. 112, 6200–6205 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Tingle J. L., Facultatively sidewinding snakes and the origins of locomotor specialization. Integr. Comp. Biol. 60, 202–214 (2020). [DOI] [PubMed] [Google Scholar]
- 17.Jayne B. C., Comparative morphology of the semispinalis-spinalis muscle of snakes and correlations with locomotion and constriction. J. Morphol. 172, 83–96 (1982). [DOI] [PubMed] [Google Scholar]
- 18.Tingle J., Gartner G., Jayne B., T. Garland, Jr, Ecological and phylogenetic variability in the spinalis muscle of snakes. J. Evol. Biol. 30, 2031–2043 (2017). [DOI] [PubMed] [Google Scholar]
- 19.Hazel J., Stone M., Grace M., Tsukruk V., Nanoscale design of snake skin for reptation locomotions via friction anisotropy. J. Biomech. 32, 477–484 (1999). [DOI] [PubMed] [Google Scholar]
- 20.Schmidt C. V., Gorb S. N., Snake scale microstructure: Phylogenetic significance and functional adaptations. Zoologica 157, 1–106 (2012). [Google Scholar]
- 21.Arrigo M. I., et al. , Phylogenetic mapping of scale nanostructure diversity in snakes. BMC Evol. Biol. 19, 91 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Gower D. J., Scale microornamentation of uropeltid snakes. J. Morphol. 258, 249–268 (2003). [DOI] [PubMed] [Google Scholar]
- 23.Benz M. J., Kovalev A. E., Gorb S. N., “Anisotropic frictional properties in snakes” in Bioinspiration, Biomimetics, and Bioreplication 2012, A. Lakhtakia, Ed. (International Society for Optics and Photonics, SPIE, 2012), pp. 256–261. [Google Scholar]
- 24.Abdel-Aal H., Vargiolu R., Zahouani H., El Mansori M., Preliminary investigation of the frictional response of reptilian shed skin. Wear 290, 51–60 (2012). [Google Scholar]
- 25.Abdel-Aal H. A., El Mansori M., Tribological analysis of the ventral scale structure in a python regius in relation to laser textured surfaces. Surf. Topogr. Metrol. Prop. 1, 015001 (2013). [Google Scholar]
- 26.Berthé R., Westhoff G., Bleckmann H., Gorb S., Surface structure and frictional properties of the skin of the Amazon tree boa Corallus hortulanus (Squamata, Boidae). J. Comp. Physiol. 195, 311–318 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Baum M. J., Kovalev A. E., Michels J., Gorb S. N., Anisotropic friction of the ventral scales in the snake Lampropeltis getula californiae. Tribol. Lett. 54, 139–150 (2014). [Google Scholar]
- 28.Baum M. J., Heepe L., Gorb S. N., Friction behavior of a microstructured polymer surface inspired by snake skin. Beilstein J. Nanotechnol. 5, 83–97 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Baum M. J., Heepe L., Fadeeva E., Gorb S. N., Dry friction of microstructured polymer surfaces inspired by snake skin. Beilstein J. Nanotechnol. 5, 1091–1103 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Wu W., et al. , Variation of the frictional anisotropy on ventral scales of snakes caused by nanoscale steps. Bioinspir. Biomim. 15, 056014 (2020). [DOI] [PubMed] [Google Scholar]
- 31.Vignaud P., et al. , Geology and palaeontology of the Upper Miocene Toros-Menalla hominid locality, Chad. Nature 418, 152–155 (2002). [DOI] [PubMed] [Google Scholar]
- 32.Schuster M., et al. , The age of the Sahara Desert. Science 311, 821 (2006). [DOI] [PubMed] [Google Scholar]
- 33.Wells S. G., McFadden L. D., Dohrenwend J. C., Influence of Late Quaternary climatic changes on geomorphic and pedogenic processes on a desert piedmont, Eastern Mojave Desert, California. Quat. Res. 27, 130–146 (1987). [Google Scholar]
- 34.Clarke M., Infra-red stimulated luminescence ages from aeolian sand and alluvial fan deposits from the eastern Mojave Desert, California. Quat. Sci. Rev. 13, 533–538 (1994). [Google Scholar]
- 35.Mendelssohn H., On the biology of the venomous snakes of Israel II. Isr. J. Zool. 14, 185–212 (1965). [PubMed] [Google Scholar]
- 36.Gans C., Mendelssohn H., “Sidewinding and jumping progression of vipers” in Toxins of Animal and Plant Origin, De Vries A., Kochva E., Eds. (Gordan and Breach, Science Publishers, Inc., New York, NY, 1971), pp. 17–38. [Google Scholar]
- 37.Spawls S., Branch B., The Dangerous Snakes of Africa (Ralph Curtis Books, Sanibel Island, FL, 1995). [Google Scholar]
- 38.Cover T. M., Elements of Information Theory (John Wiley & Sons, Hoboken, NJ, 1999). [Google Scholar]
- 39.Filippov A. E., Gorb S. N., “Anisotropic friction in biological systems” in Combined Discrete and Continual Approaches in Biological Modelling (Biologically Inspired Systems, Springer, Cham, Switzerland, 2020), vol. 16, pp. 143–175. [Google Scholar]
- 40.Sharpe S. S., et al. , Locomotor benefits of being a slender and slick sand swimmer. J. Exp. Biol. 218, 440–450 (2015). [DOI] [PubMed] [Google Scholar]
- 41.Maladen R. D., Ding Y., Li C., Goldman D. I., Undulatory swimming in sand: Subsurface locomotion of the sandfish lizard. Science 325, 314–318 (2009). [DOI] [PubMed] [Google Scholar]
- 42.Zhang T., Goldman D. I., The effectiveness of resistive force theory in granular locomotion. Phys. Fluids 26, 101308 (2014). [Google Scholar]
- 43.Askari H., Kamrin K., Intrusion rheology in grains and other flowable materials. Nat. Mater. 15, 1274–1279 (2016). [DOI] [PubMed] [Google Scholar]
- 44.Schiebel P. E., et al. , Mitigating memory effects during undulatory locomotion on hysteretic materials. eLife 9, e51412 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Rieser J. M., et al. , Geometric phase and dimensionality reduction in locomoting living systems. arXiv [Preprint] (2019) https://arxiv.org/abs/1906.11374 (accessed 1 July 2020).
- 46.Schiebel P. E., et al. , Mechanical diffraction reveals the role of passive dynamics in a slithering snake. Proc. Natl. Acad. Sci. U.S.A. 116, 4798–4803 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Baio J. E., et al. , Evidence of a molecular boundary lubricant at snakeskin surfaces. J. R. Soc. Interface 12, 20150817 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Klein M. C. G., Gorb S. N., Ultrastructure and wear patterns of the ventral epidermis of four snake species (Squamata, Serpentes). Zoology 117, 295–314 (2014). [DOI] [PubMed] [Google Scholar]
- 49.Hirose S., Biologically Inspired Robots: Serpentile Locomotors and Manipulators (Oxford University Press, Oxford, UK, 1993). [Google Scholar]
- 50.Rieser J. M., et al. , Snake microstructure. Open Science Framework. https://osf.io/kj9tv/. Deposited 17 November 2020. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Matlab files data have been deposited in the Open Science Framework (https://doi.org/10.17605/OSF.IO/KJ9TV; ref. 50).