Significance
Alphaviruses are mosquito-borne RNA viruses that cause rash, arthritis, and neurologic disease. Despite a continued risk of outbreaks, there are no licensed interventions for any alphavirus. For progress in control, an understanding of the molecular targets that affect virus replication and virulence is essential. This paper characterizes a conserved macrodomain in the virulence factor nonstructural protein 3 (nsP3). We discovered that the macrodomain ADP-ribosylhydrolase activity is critical for controlling the composition of cellular condensates, specifically through regulating the localization of translation factors, during viral infection. Given that this macrodomain is conserved across alphaviruses and coronaviruses and that the associated enzymatic activity is critical for virulence, our work may open avenues for developing a class of antiviral therapeutics.
Keywords: ADP-ribosylation, stress granules, biomolecular condensates, alphavirus, macrodomain
Abstract
While biomolecular condensates have emerged as an important biological phenomenon, mechanisms regulating their composition and the ways that viruses hijack these mechanisms remain unclear. The mosquito-borne alphaviruses cause a range of diseases from rashes and arthritis to encephalitis, and no licensed drugs are available for treatment or vaccines for prevention. The alphavirus virulence factor nonstructural protein 3 (nsP3) suppresses the formation of stress granules (SGs)—a class of cytoplasmic condensates enriched with translation initiation factors and formed during the early stage of infection. nsP3 has a conserved N-terminal macrodomain that hydrolyzes ADP-ribose from ADP-ribosylated proteins and a C-terminal hypervariable domain that binds the essential SG component G3BP1. Here, we show that macrodomain hydrolase activity reduces the ADP-ribosylation of G3BP1, disassembles virus-induced SGs, and suppresses SG formation. Expression of nsP3 results in the formation of a distinct class of condensates that lack translation initiation factors but contain G3BP1 and other SG-associated RNA-binding proteins. Expression of ADP-ribosylhydrolase–deficient nsP3 results in condensates that retain translation initiation factors as well as RNA-binding proteins, similar to SGs. Therefore, our data reveal that ADP-ribosylation controls the composition of biomolecular condensates, specifically the localization of translation initiation factors, during alphavirus infection.
Biomolecular condensates are prevalent in cells and critical for a range of cellular functions, including RNA metabolism, embryonic cell fate specification, and neuronal activity (1–3). While condensates often dynamically exchange components with the surrounding milieu, the overall composition of these cellular structures remains distinct (4). How cells control the specific composition of these condensates remains unclear. Stress granules (SGs), one of the best characterized biomolecular condensates, are RNA–protein assemblies formed in response to a variety of environmental cues (1). While SG composition can vary with the type of stress cue (5), certain common components, such as Ras GTP-activating protein-binding proteins G3BP1/2, are essential for formation of SGs (6, 7). Dysregulation of SG formation and disassembly is implicated in the pathogenesis of diseases, including viral infection, cancer, and neurodegeneration (2, 8–10).
SG formation and disassembly are tightly regulated during viral infection, often reflecting cellular translation status (11–14). In the early phase of many viral infections, the presence of double-stranded viral RNAs (vRNAs) activate protein kinase R (PKR), resulting in eIF2α phosphorylation, messenger RNA (mRNA) translation inhibition, and formation of SGs enriched with translation initiation factors such as eIF3b. However, in later infection stages, many viruses instead suppress SG formation or disassemble SGs altogether. The mechanisms underlying this switch, and its physiological function, remain unclear.
SG formation and disassembly are regulated by posttranslational modifications of proteins, including those that conjugate simple chemical groups, attach polypeptides, and add nucleotides as in the case of ADP-ribosylation (15–21). ADP-ribosylation refers to the addition of one or more ADP-ribose units onto proteins (22–24). In humans, ADP-ribosylation is accomplished primarily by a family of 17 ADP-ribosyltransferases, commonly known as poly(ADP-ribose) polymerases (PARPs). SG components are specifically ADP-ribosylated, and ADP-ribose polymers [i.e., poly(ADP-ribose) or PAR], five PARPs and two isoforms of the degradative enzyme PAR glycohydrolase (PARG) have been localized to these condensates (17, 25–27). Overexpression of these PARPs and PARG isoforms induces and suppresses SG formation, respectively, while PARG knockdown delays SG disassembly (17, 26). The noncovalent interaction between PAR and proteins facilitates SG targeting (25–27). For example, PAR-mediated targeting regulates TDP-43 localization to SGs and prevents the formation of pathological aggregates in amyotrophic lateral sclerosis (26, 27).
The mosquito-borne alphaviruses, which cause a range of diseases from rashes and arthritis to encephalitis, induce SG formation early in infection and later initiate SG disassembly (11, 14, 28, 29). Previous studies have identified the alphaviral nonstructural protein 3 (nsP3), a key factor for virus replication and virulence (30–32), as able to suppress SG formation (28, 33–35). The alphaviral nsP3 is a tripartite protein composed of a highly conserved macrodomain (MD) in the N terminus, a central zinc-binding domain (ZBD), and a C-terminal hypervariable domain (HVD; ref. 30). Recent studies indicate that the HVD, which is of low complexity, directs alphaviral nsP3 binding to host SG proteins (30, 36). For example, the HVD of chikungunya virus (CHIKV) binds the essential SG components G3BP1 and G3BP2 (33, 37). Given that nsP3 expression increases over the course of viral infection, it has been proposed that nsP3 sequesters G3BP1/2, resulting in the suppression of SG formation during the late phase of infection (28, 29, 34).
Here, we report that the expression of the G3BP-binding HVD alone does not suppress SG formation; rather, expression of the N-terminal MD alone can trigger the suppression of this biomolecular condensate. The structural integrity of SGs is dependent on ADP-ribosylation (17), and we and others recently found that the viral MD can remove single ADP-ribose groups, and possibly PAR, from ADP-ribosylated proteins (31, 38–40). We therefore hypothesized that MD ADP-ribosylhydrolase activity is required to suppress SG formation across stress conditions, with G3BP1 being a key target substrate. Indeed, we find that MD ADP-ribosylhydrolase activity is critical for disassembling SGs formed by G3BP1 expression and during viral infection. Consistent with this premise, live cell imaging revealed that SGs persist in cells infected with a hydrolase-deficient recombinant CHIKV. ADP-ribosylhydrolase activity is required for altering the composition of biomolecular condensates in nsP3-expressing or virus-infected cells and specifically regulates translation factor localization. Together, these data argue that nsP3 ADP-ribosylhydrolase activity modulates SG formation, disassembly, and composition.
Results
Two Distinct Classes of Biomolecular Condensates Formed during Alphavirus Infection.
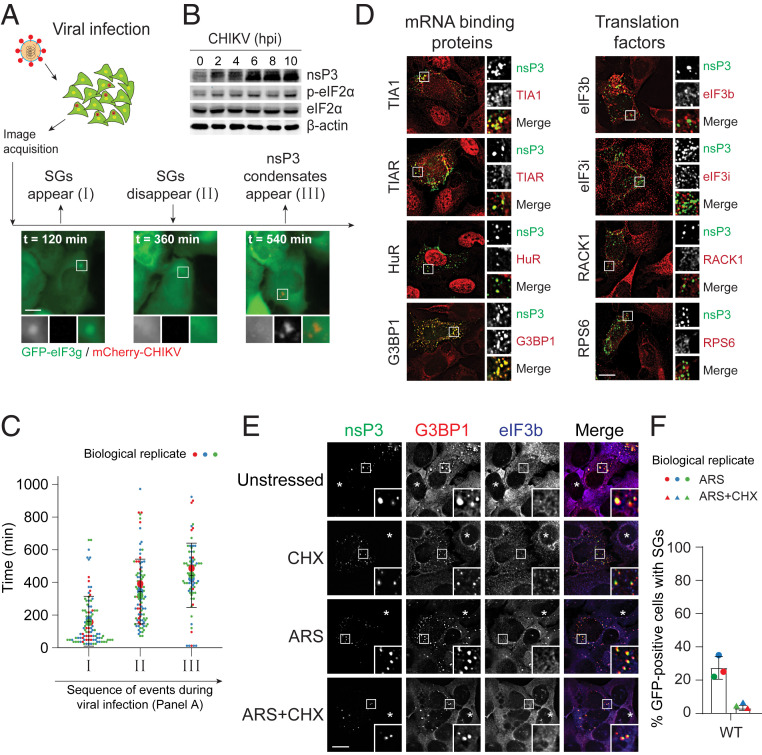
In the early phase of alphaviral infection, the presence of double-stranded vRNAs activates PKR, resulting in eIF2α phosphorylation, mRNA translation inhibition, and SG formation (11–14). SGs are aggregates of stalled translation initiation complexes, indicated by the presence of translation initiation factors as condensates (5). To monitor SG kinetics during viral infection, U2OS cells stably expressing GFP-tagged translation initiation factor eIF3g (U2OSGFP-eIF3g) were infected with CHIKV with mCherry-tagged nsP3. SGs were induced at the early stage of infection, but these translation factor-enriched condensates disassembled as the infection progressed (Fig. 1A and Movie S1) despite persistent eIF2α phosphorylation (Fig. 1B). These data suggest an active mechanism to suppress the formation of SGs.
Fig. 1.
Two distinct classes of biomolecular condensates form during alphavirus infection. (A) U2OS cells stably expressing GFP-eIF3g were infected with WT CHIKVmCherry at MOI of 10, subjected to live cell imaging with the time-lapse interval of 12 min over 14 h. Images shown are the snapshots of different infection stages. (B) U2OS cells were either mock-infected or infected with CHIKV. Twelve hours postinfection, cells were lysed and blotted against indicated antibodies. (C) Violin plot shows the distribution of time points (minutes) representing SG appearance, disappearance, and nsP3 condensate appearance. Ninety-seven cells from three independent experiments were included, and the time points at which SGs appeared/disappeared and nsP3 condensates appeared were measured within each cell. Error bars correspond to SD. (D) U2OS cells were infected with CHIKV WT at MOI of 1. At 6 h postinfection (hpi), cells were fixed and immunostained for nsP3 and indicated SG proteins. (E) U2OS cells transfected with GFP-tagged nsP3WT were either unstressed, treated with 100 μg/mL cycloheximide (CHX), 0.2 mM arsenite alone (ARS), or cotreated with 0.2 mM arsenite and 100 μg/mL cycloheximide (ARS+CHX) for 30 min. Cells were then fixed and immunostained for G3BP1 (red) and eIF3b (blue). Asterisks indicate untransfected cells. (F) Bar graph shows the percentage of GFP-positive cells with SGs from three independent experiments. Error bars correspond to mean ± SD. (Scale bars, 10 μm.)
Live cell imaging further revealed that another class of condensates containing the viral protein nsP3 form after SG disappearance, as signified by the absence of any eIF3g-containing condensates (Fig. 1 A and C and Movie S1). Immunofluorescence data demonstrated that these nsP3-containing condensates (hereby termed nsP3 condensates) possess SG-associated RNA-binding proteins such as G3BP1, TIA1, TIAR, and HuR, but not SG-associated translation factors, including eIF3b, eIF3i, RACK1, and RPS6 (Fig. 1D). Notably, these nsP3 condensates can be formed by the expression of this alphaviral nsP3 protein alone in unstressed and stressed cells, and their formation is not sensitive to cycloheximide—a translation elongation inhibitor (5) (Fig. 1E). Cycloheximide traps mRNAs along with translation factors in polysomes, thus decreasing the availability of mRNAs for SG formation and, thereby, promoting SG disassembly (5). Therefore, bona fide SGs disappear upon cycloheximide treatment. However, nsP3 condensates persisted in the presence of cycloheximide (Fig. 1E, CHX). In addition, these nsP3 condensates formed in the absence of the essential SG components G3BP1/2 (SI Appendix, Fig. S1A). These nsP3 condensates are, therefore, distinct in composition and properties from SGs.
Notably, nsP3 expression also suppresses the formation of SGs, even in the presence of the classic SG inducer arsenite (28, 33–35). In untransfected cells, eIF3b and G3BP1 colocalized as SGs after arsenite treatment (Fig. 1E, asterisk, ARS). In nsP3-transfected cells, only 27% of cells have SGs (i.e., condensates stained both positive with eIF3b and G3BP1; Fig. 1F). The suppression of SGs is thought to result from sequestration of the essential SG component G3BP1 and its paralog G3BP2 by nsP3 binding through its C-terminal HVD (33, 34, 37, 41–43). However, it is unclear whether G3BP sequestration by the HVD is sufficient to suppress SGs because other host cell factors also bind the nsP3 HVD (30, 41, 44). In addition, cellular G3BP1/2 are highly abundant, challenging a model based on complete sequestration (SI Appendix, Table S1).
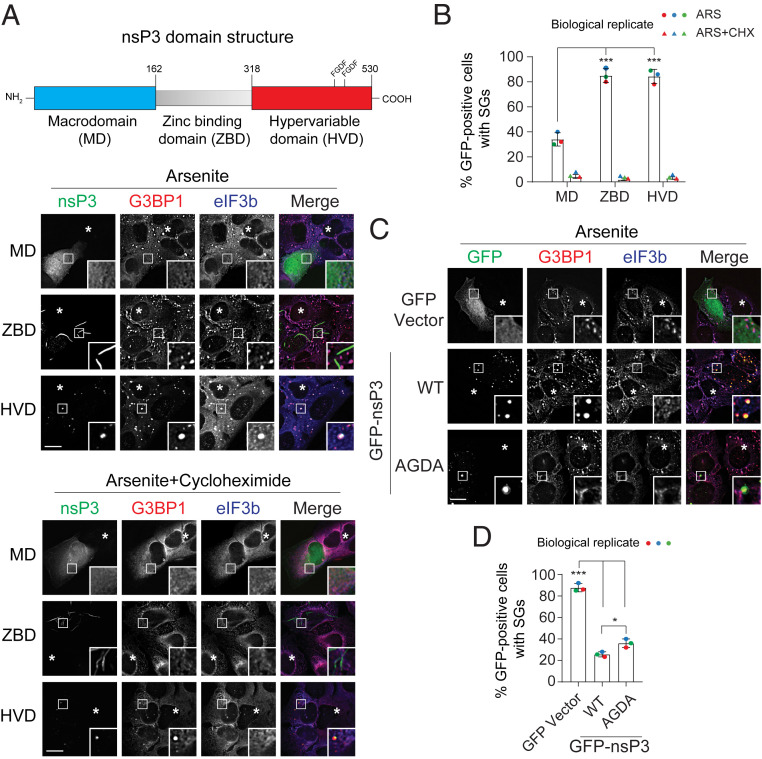
nsP3 Macrodomain Suppresses SG Formation and Hypervariable Domain Facilitates Condensate Formation.
To determine whether the HVD alone is sufficient to suppress SG formation, we overexpressed a GFP-tagged HVD fragment (318–530) in U2OS cells (Fig. 2A and SI Appendix, Fig. S1B) and HeLa cells (SI Appendix, Fig. S1C), exposed the cells to arsenite, and examined the colocalization of nsP3HVD with SG components G3BP1 and eIF3b. In contrast to cells expressing full-length nsP3 (Fig. 1E), eIF3b and G3BP1 colocalized as SGs in 85% of cells transfected with nsP3HVD and these nsP3HVD-containing condensates were sensitive to cycloheximide (Fig. 2 A and B). After cycloheximide treatment, the nsP3HVD no longer colocalized with the translation factor eIF3b, suggesting that the nsP3HVD-containing condensates formed upon arsenite treatment were indeed SGs (Fig. 2A). Notably, nsP3HVD can still form G3BP1-positive but eIF3b-negative condensates in the absence of arsenite. Unlike SGs, these condensates were cycloheximide-insensitive (SI Appendix, Fig. S1D). These data indicate that the low-complexity HVD alone is insufficient to suppress SG formation, but this domain possesses the ability to form condensates.
Fig. 2.
nsP3 macrodomain suppresses SG formation. (A) Schematic representation of the nsP3 domain architecture. U2OS cells transfected with GFP-tagged nsP3MD, nsP3ZBD, or nsP3HVD were either treated with 0.2 mM arsenite alone or cotreated with 100 μg/mL cycloheximide for 30 min. Cells were then processed and immunostained for SG markers G3BP1 (red) and eIF3b (blue). Asterisks indicate untransfected cells. (B) Bar graph shows the percentage of GFP-positive cells with SGs. ***P < 0.005, two-tailed, unpaired Student’s t test. Error bars correspond to mean ± SD with three independent experiments. (C) U2OS cells transfected with GFP vector, GFP-tagged nsP3WT, or nsP3AGDA were treated with 0.2 mM arsenite for 30 min and immunostained for G3BP1 (red) and eIF3b (blue). Asterisks indicate untransfected cells. (D) Bar graph shows the percentage of GFP-positive cells with SGs. *P < 0.05, ***P < 0.001, two-tailed, unpaired Student’s t test. Error bars correspond to mean ± SD with n = 3. (Scale bars, 10 μm.)
Unlike its stress-specific colocalization with eIF3b, nsP3HVD colocalized with G3BP1 to form condensates in both unstressed and arsenite-stressed cells likely through direct interaction (Fig. 2A and SI Appendix, Fig. S1D). The nsP3 C-terminal HVD has two FGDF motifs that bind strongly to the NTF2-like (NTF2L) domain of G3BP1. To further test whether G3BP1 binding to the nsP3 HVD is critical for suppressing SG formation, we mutated the two FGDF motifs to AGDA and analyzed the ability of full-length nsP3 to suppress SG formation. Consistent with previous reports, nsP3AGDA no longer colocalized with G3BP1 and G3BP2 in either unstressed or stressed cells (Fig. 2C and SI Appendix, Fig. S1E). Yet, nsP3AGDA still formed condensates in both conditions, further indicating that this class of condensates is distinct from SGs. Upon arsenite treatment, SG formation was still suppressed in nsP3AGDA-expressing cells (36%) (Fig. 2 C and D). Taken together, these data indicated that regions(s) other than the nsP3 HVD are responsible for suppressing SG formation.
To examine which domains were linked to SG suppression, we next examined the central ZBD (163-317) of nsP3, which positively regulates alphaviral replication and transcription (45). Overexpressed nsP3ZBD formed filament-like structures in cells and did not suppress SG formation (Fig. 2 A and B) (28). Given that the N-terminal MD can catalyze removal of ADP-ribosylation (31, 38), a modification essential for structural integrity of SGs (17), we tested whether the MD of nsP3 (nsP3MD) is responsible for loss of SGs. Similar to full-length nsP3, overexpression of the MD fragment (1–162) inhibited SG formation upon arsenite treatment (Fig. 2 A and B), as well as after exposure to the mitochondrial stressor clotrimazole or endoplasmic reticulum stressor thapsigargin (SI Appendix, Fig. S1 F and G; ref. 5). However, unlike full-length nsP3, nsP3MD did not colocalize with G3BP1 (Fig. 2A), presumably due to lack of the G3BP-binding HVD region. These data suggest that while the nsP3HVD is sufficient for forming condensates with SG components G3BP1 and eIF3b, nsP3MD plays a critical role in regulating association with the SG components such as G3BP1 and eIF3b.
ADP-Ribosylhydrolase Activity Controls Cellular Condensate Composition.
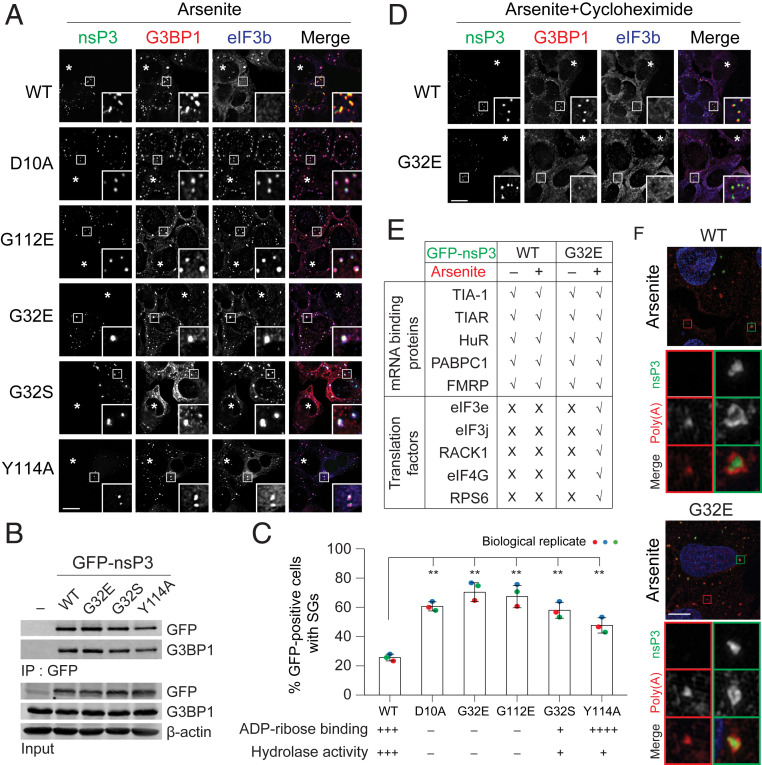
To dissect which functions of nsP3MD are responsible for suppressing SG formation (Fig. 3), we examined three classes of MD mutants (31): 1) no ADP-ribose–binding and negligible hydrolase activity (D10A, G32E, and G112E); 2) weak ADP-ribose binding and weak hydrolase activity (G32S); and 3) weak hydrolase activity, but stronger ADP-ribose–binding than WT (Y114A). Similar to WT nsP3, all of these MD mutant nsP3s colocalized with G3BP1 in condensates with or without arsenite (Fig. 3A and SI Appendix, Fig. S2A). Consistently, no differences were observed in G3BP1 association with WT and mutant nsP3s representative of the three different classes, arguing that ADP-ribosylhydrolase activity does not regulate nsP3 association with G3BP1 (Fig. 3B). However, unlike WT, mutants colocalized with eIF3b upon arsenite treatment (Fig. 3 A and C), indicating that these nsP3 mutants do not suppress SG formation.
Fig. 3.
ADP-ribosylhydrolase activity of nsP3 suppresses SG formation. (A) U2OS cells transfected with GFP-tagged nsP3WT or different nsP3 point mutants (D10A, G32E, G32S, G112E, and Y114A) were treated with 0.2 mM arsenite for 30 min and immunostained for G3BP1 (red) and eIF3b (blue). Asterisks indicate untransfected cells. (B) 293F cells were transfected with either GFP vector or GFP-tagged nsP3WT, nsP3G32E, nsP3G32S, or nsP3Y114A. After 24-h transfection, cells were pelleted, lysed, immunoprecipitated using anti-GFP antibodies, and the immunoprecipitates were blotted against G3BP1 and GFP antibodies. (C) Bar graph shows the percentage of GFP-positive cells with SGs in A. (Lower) ADP-ribose binding and hydrolase activity of the mutants tested (31). **P < 0.01, two-tailed, unpaired Student’s t test. Error bars correspond to mean ± SD with n = 3. (D) Cells transfected with GFP-tagged nsP3WT or nsP3G32E were cotreated with 0.2 mM arsenite and 100 μg/mL cycloheximide for 30 min. Cells were then immunostained for G3BP1 (red) and eIF3b (blue). Asterisks indicate untransfected cells. (E) Table summarizes the colocalization of nsP3 (WT or G32E) with different mRNA binding proteins and translation factors as shown in SI Appendix, Fig. S3A. (F) Superresolution microscopy indicates how the distribution of poly(A)+ mRNA signal overlaps with nsP3 WT or G32E mutant. U2OS cells transfected with GFP-tagged nsP3WT or nsP3G32E were with 0.2 mM arsenite for 30 min and hybridized with oligo(dT) probes, followed by staining for GFP. Green and red boxes indicate the condensates from nsP3-transfected and untransfected cells, respectively. (Scale bars, 10 μm.)
Using G32E as a model, we further showed that mutant nsP3/eIF3b/G3BP1 condensates were SGs because they: 1) were formed in response to multiple stresses, such as arsenite, clotrimazole, and thapsigargin (Fig. 3A and SI Appendix, Fig. S2 B and C); 2) were sensitive to cycloheximide (Fig. 3D); and 3) contained all tested SG-associated RNA-binding proteins and translation factors (Fig. 3E and SI Appendix, Fig. S3A). Similar to SGs, mutant condensates were enriched with poly(A)+ at its core based on superresolution microscopy analyses (Fig. 3F). Because all tested mutants impact ADP-ribosylhydrolase activity, nsP3-mediated suppression of SG formation likely requires this catalytic activity.
In stressed cells, WT nsP3 colocalized with all RNA-binding proteins that were tested but none of the translation factors. In contrast, nsP3G32E colocalized with all tested SG-associated RNA-binding proteins and translation factors in stressed cells (Fig. 3A and SI Appendix, Fig. S3A). The observed difference could be due to mutant nsP3, but not WT, associating with SG components prior to stress conditions. To test this possibility, we examined the colocalization of WT and mutant nsP3 with all of these SG components in unstressed cells. Both WT and G32E mutant nsP3 colocalized with all tested RNA-binding proteins, but not translation factors, in unstressed cells (Fig. 3E and SI Appendix, Fig. S3A). Taken together, we observed two classes of condensates with differential sensitivity to nsP3 ADP-ribosylhydrolase activity in the presence and absence of stress (Table 1). In unstressed cells, nsP3 condensates form independent of its ADP-ribosylhydrolase activity. Upon stress, WT nsP3 exists in condensates that lack translation factors and its ADP-ribosylhydrolase suppresses SG formation. In contrast, hydrolase-deficient nsP3 forms SGs with canonical components such as G3BP1 and eIF3b. Therefore, the enzymatic activity regulates the composition of cellular condensates. Specifically, nsP3 ADP-ribosylhydrolyase activity segregates SG-associated RNA-binding proteins from translation factors during stress.
Table 1.
Composition and property of biomolecular condensates observed in this study
| Condensates | Formation | Example figure | Tested condensate components | Properties |
| Stress granules | Induced by stress (e.g., arsenite, clotrimazole, thapsigargin), viral infection, or G3BP1 expression | 1A | RBPs (e.g., G3BP) and translation factors (e.g., eIF3b) | Integrity can be disrupted by cycloheximide or nsP3 ADP-ribosyhydrolase |
| Expression of nsP3 HVD domain in the presence of stress | 2A (HVD) | nsP3 HVD domain, RBPs (e.g., G3BP) and translation factors (e.g., eIF3b) | Integrity can be disrupted by cycloheximide | |
| Expression of hydrolase-deficient nsP3 in the presence of stress | 3A | Hydrolase-deficient nsP3, RBPs (e.g., G3BP) and translation factors (e.g., eIF3b) | Integrity can be disrupted by cycloheximide | |
| nsP3 condensates | Expression of WT nsP3 in the presence or absence of stress | 1E | WT nsP3 and RBPs (e.g., G3BP) | Insensitive to cycloheximide |
| Expression of hydrolase-deficient mutant nsP3 in the absence of stress | S2A | Hydrolase-deficient nsP3 and RBPs (e.g., G3BP) | Insensitive to cycloheximide | |
| Expression of nsP3 HVD domain in the absence of stress | S1D | nsP3 HVD domain, G3BP1 | Insensitive to cycloheximide |
The function of nsP3 resembles host cell PARG, which also possesses ADP-ribosylhydrolase activity but cleaves ribose-ribose bonds within the PAR polymer. Similar to nsP3, PARG isoforms (PARG99 and particularly PARG102) suppress SG formation (17). nsP3-mediated suppression of SG formation is stronger, however, than PARG102, even when the latter was expressed at a higher level than nsP3 (SI Appendix, Fig. S3 B–D). Also similar to the nsP3 MD alone, PARG does not colocalize with G3BP1 to form condensates, likely because it lacks a G3BP1-binding motif (Fig. 2A and SI Appendix, Fig. S3 B, E, and F). Consistent with these findings, expressing only the MD carrying the G32 mutation did not suppress SG formation nor form G3BP1-containing condensates (SI Appendix, Fig. S3 E and F). These data altogether suggest that although nsP3MD does not possess the ability to form condensates, its ADP-ribosylhydrolase activity is sufficient to suppress SG formation.
nsP3 Reduces ADP-Ribosylation of the Essential SG Component G3BP1.
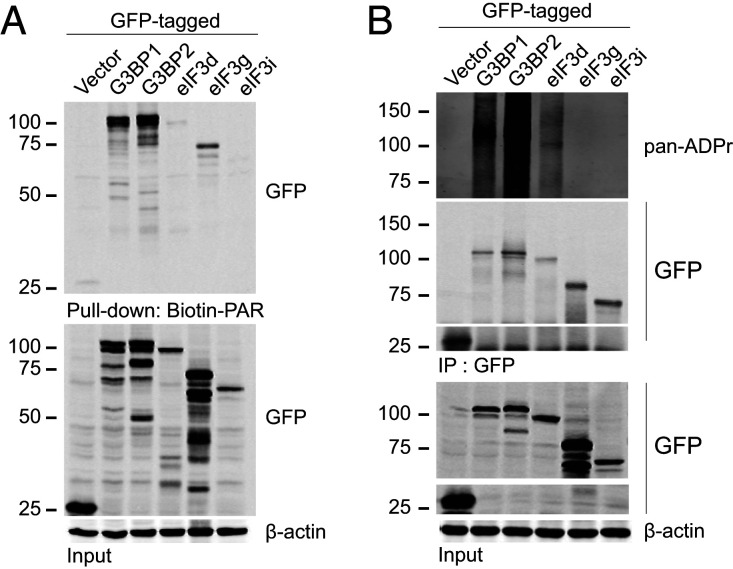
Next, we examined the possible targets of nsP3 ADP-ribosylhydrolase activity that alter SG composition and structural integrity. Given that nsP3 ADP-ribosylhydrolase activity regulates the association between SG-associated RNA-binding proteins and translation factors, we wondered whether nsP3 disrupts the interaction network in SGs formed by protein–ADP-ribose–protein interactions. We therefore hypothesized that some of these SG components are ADP-ribosylated while others bind to ADP-ribose noncovalently. To test whether these SG components are ADP-ribose binders, we used biotinylated PAR to pull down complexes from cell lysates expressing either GFP only, GFP-tagged G3BP1, G3BP2, eIF3d, eIF3g, or eIF3i. Consistent with previous results showing that G3BP1 binds to PAR (46), biotinylated PAR pulled down GFP-tagged G3BP1, but not GFP alone (Fig. 4A). In addition, G3BP2 and both translation factors eIF3d and eIF3g can also bind to PAR noncovalently (Fig. 4A). To test whether SG-associated RNA-binding proteins and translation factors are differentially ADP-ribosylated, we transfected GFP-tagged G3BP1, G3BP2, eIF3d, eIF3g, or eIF3i into cells, immunoprecipitated the cell lysates using anti-GFP antibodies, and probed the immunoprecipitates with a reagent that detects ADP-ribosylation (Fig. 4B). G3BP1, G3BP2, and eIF3d, but not eIF3g and eIF3i, were ADP-ribosylated in cells, where prominent ADP-ribosylation signals appeared at and above the expected molecular weight of the respective proteins. The signals appeared as smears because of the heterogeneous number of ADP-ribose units added onto these proteins. Therefore, these data suggest that specific SG components are ADP-ribosylated while others can bind noncovalently to ADP-ribose polymers. By removing ADP-ribosylation from specific SG substrates, nsP3 may disrupt protein–ADP-ribose–protein interactions critical for SG formation.
Fig. 4.
Differential PAR-binding ability and ADP-ribosylation of SG components. (A) 293F cells were transfected with either GFP vector or GFP-tagged G3BP1, G3BP2, eIF3d, eIF3g, or eIF3i for 36 h. Cells were then pelleted, lysed, and incubated with 100 pmol Biotin-PAR and streptavidin beads. The streptavidin pulldown was then blotted with anti-GFP antibodies. (B) 293F cells transfected with either GFP vector or GFP-tagged G3BP1, G3BP2, eIF3d, eIF3g, or eIF3i for 36 h. Cells were then pelleted, lysed, and immunoprecipitated using anti-GFP antibodies. The immunoprecipitates were then blotted with pan-ADPr reagent.
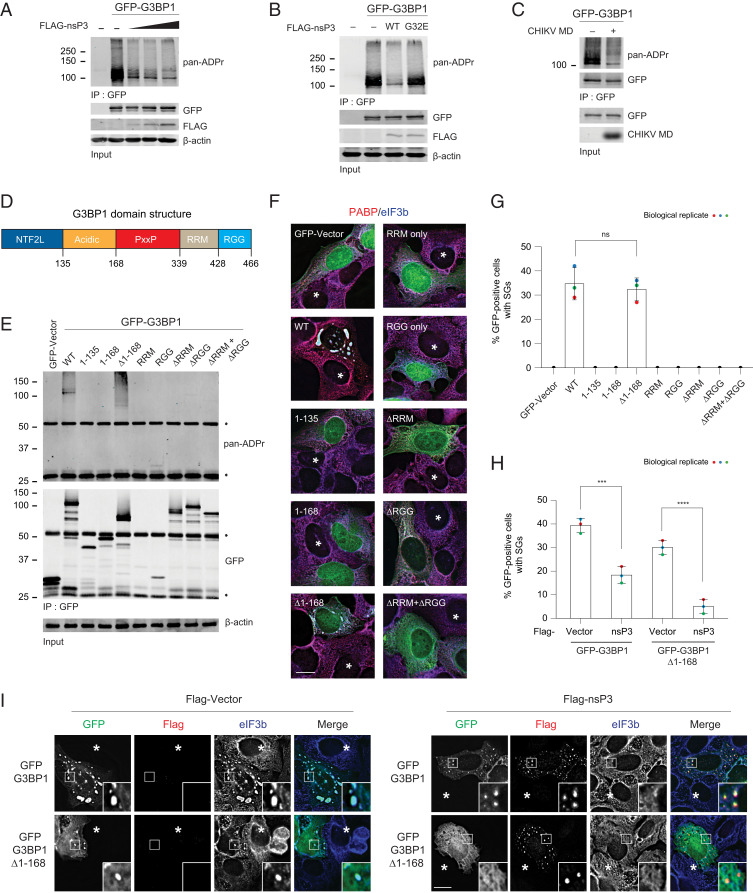
We next tested whether G3BP1 is one of the nsP3 ADP-ribosylhydrolase targets, given that it interacts directly with nsP3 (33, 34, 37, 41–43), is an essential component of SG formation across stress triggers (6), and plays a critical role in alphavirus infection (33, 47). To test this possibility, we expressed GFP-G3BP1 alone or with increasing amounts of FLAG-tagged nsP3 WT. GFP-G3BP1 was then immunoprecipitated and probed for ADP-ribosylation. Upon coexpression of WT nsP3, the ADP-ribosylation signal associated with G3BP1 was reduced in a dose-dependent manner as well as in both unstressed and multiple stress conditions (Fig. 5A and SI Appendix, Fig. S4A). Reduction of the ADP-ribose signal was not observed when the G32E mutant nsP3 was coexpressed (Fig. 5B). In vitro incubation of the recombinant WT MD alone was sufficient to reduce G3BP1 ADP-ribosylation (Fig. 5C). These data together suggest that ADP-ribosylhydrolase activity residing in the nsP3 MD is responsible for reducing ADP-ribosylation associated with the essential SG component G3BP1.
Fig. 5.
nsP3 reduces ADP-ribosylation of the essential SG component G3BP1. (A) 293F cells were transfected with either GFP, GFP-tagged G3BP1 alone, or cotransfected with GFP-tagged G3BP1 with increasing concentration of FLAG-tagged nsP3WT. After 24-h transfection, cells were pelleted, lysed, immunoprecipitated using anti-GFP antibodies, and immunoblotted with pan-ADPr reagent. (B) 293F cells were transfected with GFP, GFP-tagged G3BP1 alone, or cotransfected GFP-tagged G3BP1 with either FLAG-tagged nsP3WT or nsP3G32E. Twenty-four hours posttransfection, cells were pelleted, lysed, immunoprecipitated using anti-GFP antibodies, and immunoblotted with pan-ADPr reagent. (C) 293F cells transfected with GFP-tagged G3BP1 were lysed and immunoprecipitated using anti-GFP antibodies. The immunoprecipitates were split into two halves and incubated either with buffer or 5 μg CHIKV MD for 1 h at 37 °C. After incubation, the beads were washed and blotted with pan-ADPr reagent. (D) Schematic representation of G3BP1 domain structure. (E–G) U2OS G3BP1/2 double knockout (dKO) cells were transfected with either GFP vector or GFP-tagged G3BP1 constructs for 36 h. (E) Cells were then either lysed, immunoprecipitated using anti-GFP antibodies, and blotted with pan-ADPr reagent (asterisks indicate heavy and light chain) or (F) permeabilized, fixed, and immunostained for PABP (red) and eIF3b (blue). Asterisks indicate untransfected cells. (G) Bar graph shows the percentage of GFP-positive cells with SGs. ns, nonsignificant, two-tailed, unpaired Student’s t test. Error bars correspond to mean ± SD with n = 3. (H and I) U2OS G3BP1/2 dKO cells were cotransfected in two combinations: 1) FLAG-vector with GFP-tagged G3BP1 or G3BP1 ∆1–168, and 2) FLAG-tagged nsP3 with GFP-tagged G3BP1 or G3BP1 ∆1–168. At 36 h posttransfection, cells were fixed and stained for FLAG (red) and eIF3b (blue). Asterisks indicate untransfected cells. Bar graph shows the percentage of GFP-positive cells with SGs. ***P < 0.001, ****P < 0.0001, two-tailed, unpaired Student’s t test. Error bars correspond to mean ± SD with n = 3. (Scale bars, 10 μm.)
G3BP1 has an N-terminal NTF2L domain, followed by an acidic region, PxxP, RRM, and C-terminal RGG domain (Fig. 5D). To identify the region undergoing G3BP1 ADP-ribosylation, we transiently transfected U2OS G3BP1/2 knockout cells with different G3BP1 domain constructs tagged with GFP and then probed ADP-ribosylation after GFP immunoprecipitation. We found that ADP-ribosylation was prominently associated with full-length WT and Δ1–168 G3BP1 (NTF2L and acidic domain deleted) (Fig. 5E). Intriguingly, SGs only formed in cells expressing G3BP1 constructs associated with ADP-ribosylation (i.e., with full-length or Δ1–168 G3BP1, but not other domains) (Fig. 5 F and G). These data suggest that NTF2L and acidic domain were not required for ADP-ribosylation of G3BP1 nor SG formation.
In line with previous observations that ADP-ribosylation is critical for SG formation (17, 26), SGs formed upon expression of either GFP-G3BP1 or Δ1–168 mutant in G3BP1/2 knockout cells were also suppressed by nsP3 (Fig. 5 H and I). Besides suppressing SGs, the nsP3 hydrolase activity can also disassemble preformed SGs induced by expressing G3BP1 (48, 49). Compared with the vector control or G32E mutant nsP3 transfected into G3BP1-expressing cells, the numbers of SGs (stained positive with G3BP1 and eIF3b) in WT nsP3-transfected cells were reduced by approximately twofold (SI Appendix, Fig. S4B). These data altogether suggest that the nsP3 ADP-ribosylhydrolase activity reduces G3BP1 ADP-ribosylation, thereby regulating SG formation and disassembly.
nsP3 Macrodomain ADP-Ribosylhydrolase Activity Suppresses the Formation of SGs during Viral Infection.
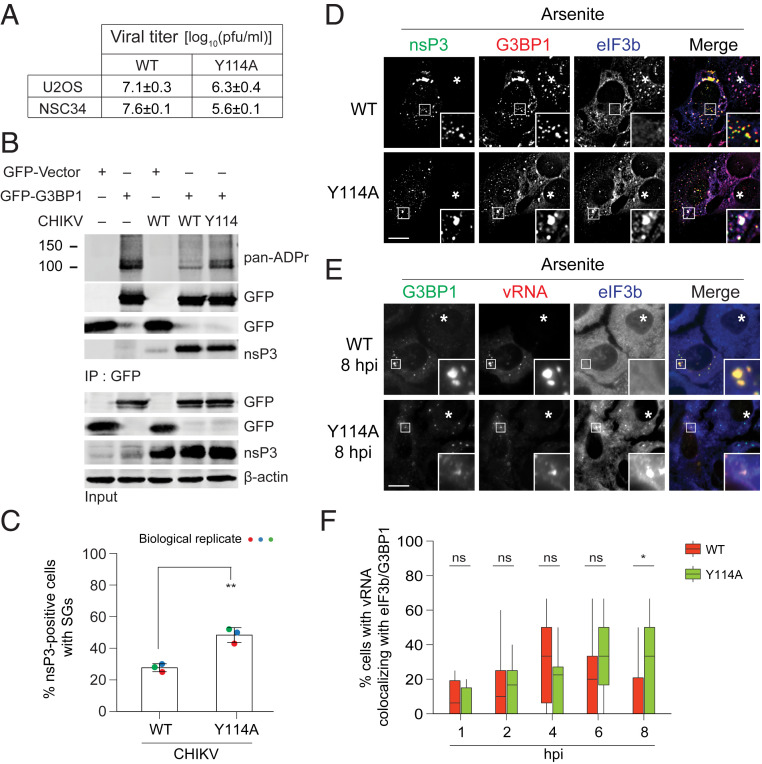
To test the physiological relevance of nsP3 ADP-ribosylhydrolase activity, we compared how the WT and mutant MD hydrolase affect G3BP1 ADP-ribosylation and SG formation in CHIKV-infected cells. Because CHIKV carrying nsP3MD mutations (D10A, G32E, G112E) that severely compromise ADP-ribose binding and hydrolase activities are not viable (31), we focused on viable CHIKV nsP3MD mutants such as G32S and Y114A. These latter mutants have diminished, but not absent, hydrolase activity and have reduced titers compared with WT virus in cell cultures (ref. 31; Fig. 6A). Particularly, the Y114A mutant was used as a comparison because it has replication kinetics similar to WT, producing comparable amounts of vRNA (50). Upon infection with WT CHIKV, G3BP1 ADP-ribosylation was reduced (Fig. 6B). However, similar reduction of G3BP1 ADP-ribosylation was not observed in cells infected with the Y114A mutant virus, indicating that G3BP1 is an endogenous target of nsP3 ADP-ribosylhydrolase during infection.
Fig. 6.
ADP-ribosylhydrolase activity of nsP3 suppresses SG formation during alphavirus infection. (A) Viral titers of WT and Y114A mutant in NSC34 (31) and U2OS cells analyzed at 24 hpi. Data are plotted as plaque-forming units (pfu)/mL ± SEM. (B) 293T cells transfected with GFP vector or GFP-G3BP1 for 12 h were either mock infected or infected with CHIKVWT or CHIKVY114A at MOI of 1 for 24 h. At 24 hpi, cells were lysed, immunoprecipitated using anti-GFP antibodies, and blotted with pan-ADPr reagent. (C and D) U2OS cells were infected with either CHIKVWT or CHIKVY114A at MOI of 1. At 5.5 hpi, cells were treated with 0.2 mM arsenite for 30 min and immunostained for nsP3 (green), G3BP1 (red), and eIF3b (blue). Asterisks indicate untransfected cells. Bar graph shows the percentage of virus-infected cells (nsP3-positive) with SGs. **P < 0.01, two-tailed, unpaired Student’s t test. Error bars correspond to mean ± SD with n = 3. (E) U2OS cells were infected with either CHIKVWT or CHIKVY114A at MOI of 5. At 7.5 hpi, cells were treated with 0.2 mM arsenite for 30 min. Treated cells were then fixed and hybridized with vRNA probes (red), followed by immunostaining for G3BP1 (green) and eIF3b (blue). Asterisks indicate untransfected cells. (F) Box plot shows the percentage of cells with vRNA colocalizing with eIF3b/G3BP1 in Fig. 6E and SI Appendix, Fig. S5E. About 20 cells from each time point were quantified for colocalization. *P < 0.05, ns, nonsignificant, two-tailed, unpaired Student’s t test. (Scale bars, 10 μm.)
To compare the ability of WT and Y114A mutant viruses to suppress SG formation, infected cells were examined at 5.5 h postinfection (hpi) when nsP3 appears as condensates (SI Appendix, Fig. S5A). These cells were then treated with arsenite for an additional 0.5 h (a time point when ∼95% of uninfected cells have developed SGs). Consistent with experiments using nsP3 protein expression constructs (Figs. 1E and 3A), WT CHIKV infection suppressed SG formation. Indeed, only 27% of nsP3-positive cells had canonical (G3BP1/eIF3b double-positive) SGs (Fig. 6 C and D). In contrast, 49% of Y114A nsP3-positive cells contained SGs (Fig. 6 C and D). The result generalized across stress triggers: colocalization of mutant nsP3, eIF3b, and G3BP1 was observed with clotrimazole and thapsigargin (SI Appendix, Fig. S5 B and C). The mutant nsP3/eIF3b/G3BP1 condensates formed during viral infection were also cycloheximide-sensitive, underlining their similarity to SGs (SI Appendix, Fig. S5D). These data suggest that nsP3 ADP-ribosylhydrolase activity suppresses the formation of SGs during viral infection.
As nsP3 ADP-ribosylhydrolase activity alters the condensate localization of poly(A)+ mRNA upon stress (Fig. 3F), we characterized localization of the viral genome (vRNA), which is polyadenylated, and tested whether the enzymatic activity affects its localization. Time-course single-molecule fluorescence in situ hybridization (smFISH) experiments revealed that vRNA increasingly associated with G3BP1-containing condensates (Fig. 6E and SI Appendix, Fig. S5E). Some of them also contain eIF3b, indicating that these condensates are SGs (Fig. 6 E and F and SI Appendix, Fig. S5 E and F). For WT virus, the percentage of cells with vRNA-containing SGs peaked at 4 hpi and declined thereafter (Fig. 6F). In contrast, vRNA-containing SGs persisted at 6 and 8 hpi for the hydrolase-deficient mutant Y114A (Fig. 6 E and F and SI Appendix, Fig. S5 E and F). Therefore, these data suggest that nsP3 ADP-ribosylhydrolase activity controls the composition of these vRNA-containing SGs.
nsP3 Macrodomain ADP-Ribosylhydrolase Activity Regulates the Disassembly of Virus-Induced SGs.
SGs induced during early stages of alphaviral infection are disassembled as infection progresses (11–14). Because expression of nsP3 (either by transfection or infection) suppresses SG formation (Figs. 1E and 6D) and disassembles preformed G3BP1-induced SGs through ADP-ribosylhydrolase activity (SI Appendix, Fig. S4B), we next tested whether this same mechanism regulates later SG disassembly during virus infection.
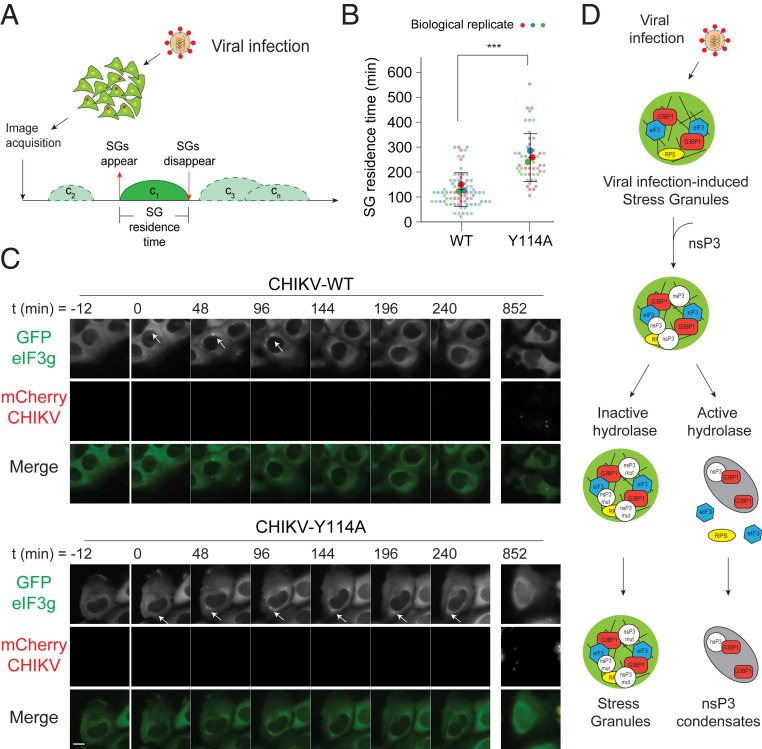
To monitor SG kinetics in live cells, we used the U2OSGFP-eIF3g stable cell line (Fig. 1A) or, as a negative control, U2OS cells stably expressing GFP (U2OSGFP). These cells were imaged every 12 min for 14 h (Fig. 7A). Infection with WT and nsP3 mutant CHIKVmCherry resulted in SG formation, visualized as GFP-positive condensates, in U2OSGFP-eIF3g cells (Fig. 7 B and C and Movie S2). GFP-positive condensates were not observed in U2OSGFP or mock-infected U2OSGFP-eIF3g cells over the same time-course (SI Appendix, Fig. S6 A and B). Consistent with fixed cell analyses of other alphaviruses (51, 52), SGs disassembled at later stages of CHIKV infection. However, these SGs were disassembled with different kinetics in cells infected with WT versus mutant viruses (Fig. 7 B and C and Movie S2). To quantify the dynamics of SG formation and disassembly in a given cell (i.e., SG residence time in cell c1, c2, c3...cn), we defined t = 0 as the first appearance of SGs (Fig. 7A). SG residence time was shorter (152 ± 67 min) in WT virus-infected cells when compared with Y114A (281 ± 95 min) mutant virus-infected cells (Fig. 7 B and C). Because reducing ADP-ribosylhydrolase activity increases SG residence time, we conclude that ADP-ribosylhydrolase activity is critical for regulating SG disassembly during CHIKV infection.
Fig. 7.
ADP-ribosylhydrolase activity of nsP3 regulates SG disassembly. (A) Schematic representation of the live cell imaging experiment setup. (B and C) U2OS cells stably expressing GFP-eIF3g were infected with either WT or Y114A CHIKVmCherry at MOI of 10 and subjected to live cell imaging with the time-lapse interval of 12 min over 14 h. Images shown are the time-points at which no SGs were observed (t = −12 min), followed by the time-point at which SGs were observed (t = 0 h). White arrows indicate SGs. (Scale bar, 10 μm.) Violin plot shows the distribution of average SG residence time (minutes) in cells infected with WT and Y114A CHIKVmCherry. ***P < 0.001, two-tailed, unpaired Student’s t test. WT (n = 51) and Y114A (n = 77) infected cells from three independent experiments were included to measure the SG residence time. Error bars correspond to SD. (D) Model of virus infection-induced SG assembly and disassembly regulated by nsP3 ADP-ribosylhydrolase activity.
Discussion
Here, we report that both SG formation and disassembly can be regulated by the MD of alphaviral protein nsP3 through its ADP-ribosylhydrolase activity. Expression of the MD alone, but not a catalytically dead mutant MD, suppressed SG formation. Live cell imaging revealed that the MD ADP-ribosylhydrolase activity determines the residence time of virus-induced SGs, with their prolonged presence in cells infected with catalytically deficient mutant viruses. It was previously proposed that SG disassembly is mediated through the nsP3-mediated sequestration of G3BP1/2 (28, 29, 34). Although we confirmed the association of the nsP3 HVD and G3BP1, which is central to the sequestration model, expression of the HVD domain alone did not suppress SG formation. In contrast, full-length nsP3 carrying HVD FGDF mutations (which reduce G3BP1/2 interaction) and the intact MD alone can suppress SG formation. Moreover, G3BP1 mutant lacking the NTF2L domain, which binds HVD, can still form SGs, and the formation of these SGs can still be suppressed by nsP3. It is therefore unlikely that nsP3 suppresses SG formation in CHIKV-infected cells through G3BP1/2 sequestration alone.
Instead, we propose that nsP3 regulates SG formation through both C-terminal HVD interactions with SG proteins and an N-terminal MD-catalyzed decrease in ADP-ribosylation. As nsP3 concentration increases over the course of infection, the accompanying increase in ADP-ribosylhydrolase activity facilitates disassembly of preexisting SGs and suppresses the formation of new ones in later stages of the replication cycle (Fig. 7D).
What might the function of SG disassembly by nsP3 ADP-ribosylhydrolase during late stages of alphavirus infection be? Expression of WT nsP3 results in all tested SG-associated translation factors (eIF3b, eIF3i, eIF3e, eIF3j, RACK1, eIF4G1, and RPS6) separating from all tested SG-associated RNA-binding proteins (G3BP1, G3BP2, TIA-1, TIAR, HuR, PABP, and FMRP). Meanwhile nsP3 remains associated with the RNA-binding proteins as a class of condensates that is distinct from SGs. Yet, expression of ADP-ribosylhydrolase–deficient nsP3 forms SG condensates with translation factors as well as RNA-binding proteins. Therefore, the enzymatic activity determines whether RNA-binding proteins associate with translation factors, thereby regulating the formation of two distinct classes of cellular condensates that have apparently similar composition but distinct properties (see Table 1 for comparisons).
Our data indicate that some protein–protein interactions within SGs are mediated by PAR, which are covalently conjugated to components like G3BP1 and G3BP2, whereas other components (such as eIF3g and G3BP1) bind noncovalently to this polymer. nsP3 appears to alter the composition of condensates through removing ADP-ribosylation associated with the essential SG component G3BP1 and potentially other ADP-ribosylated components, a mechanism that is predicted to significantly affect the function of the resulting condensate. The observed pattern of protein retention revealed that nsP3 ADP-ribosylhydrolase activity promotes the release of translation components from SGs, likely needed for viral structural protein translation during late infection stages (Fig. 7D). This possibility is consistent with recent data showing that nsP3 ADP-ribosylhydrolase activity is critical for the switch from translation of host proteins and viral nonstructural proteins from genomic RNA to translation of the viral structural proteins from a subgenomic RNA during the later stages of viral replication (50, 53). Our smFISH data further revealed that the vRNA genome is temporarily colocalized with SGs at an early stage of infection, and that ADP-ribosylhydrolase activity is required for reducing association between vRNA genome and translation factors (cf. Fig. 6 E and F). This redistribution of host translation factors, vRNA genome, and ribosomal proteins may be required for efficient virus structural protein translation at later stages of infection (11).
Compared with other protein modifications that regulate SG dynamics, ADP-ribosylation is unique in that the polymeric form (i.e., PAR) is able to seed low-complexity region-containing proteins to form protein condensates in vitro (26, 27, 54–56). The complete removal of PAR from ADP-ribosylated proteins requires two steps: the degradation by PARG of the polymeric chain down to single ADP-ribose units conjugated to proteins, followed by the hydrolysis of the final, proximal ADP-ribose groups from proteins (57–59). The alphaviral nsP3 MD efficiently hydrolyzes single ADP-ribose groups from ADP-ribosylated proteins in vitro but inefficiently removes PAR chains (31, 38). Previous studies localized PARG to SGs, where its expression level controls the formation and disassembly of SGs (17). Therefore, endogenous PARG likely mediates the breakdown of PAR in SGs, followed by the action of nsP3 ADP-ribosylhydrolase, which removes the final ADP-ribose. Given that nsP3 has a stronger effect than PARG in suppressing SG formation and nsP3 removes ADP-ribose from acidic residues (31), the removal of specific mono-ADP-ribosylation is likely a rate-limiting step in SG disassembly. The observed SG suppression by nsP3 could be due to its blocking of the initiation of PAR formation, thus preventing not only adding single but also multiple ADP-ribose units to form PAR critical for SG formation.
Given that ADP-ribosylhydrolase activity is conserved across all MD-containing RNA viruses (60, 61), including coronaviruses, it is possible that these viruses regulate SG formation and disassembly through a common mechanism by removing ADP-ribosylation.
Materials and Methods
Cell Culture, Chemicals, and Transfection.
U2OS and HeLa cells were obtained from American Type Culture Collection and 293F cells from Invitrogen. Cells were maintained in Dulbecco’s modified Eagle medium (DMEM) (Gibco) containing 10% heat-inactivated fetal bovine serum (FBS) (Gibco, Life Technologies). In all experiments, plasmids were transfected using JetPrime from Polyplus (U2OS) or 293fectin from Gibco (293F) as per the manufacturer’s protocol. The following drugs were used for stress induction: Arsenite (Sigma, S7400); Clotrimazole (Sigma, C6091); Thapsigargin (Sigma, T9033); Cycloheximide (Sigma, C7698).
Plasmid Generations.
EGFP-C1-nsP3 WT, EGFP-C1-nsP3 MD, EGFP-C1-nsP3 ZBD, EGFP-C1-nsP3 HVD, EGFP-C1-nsP3 D10A, EGFP-C1-nsP3 G32E, EGFP-C1-nsP3 G32S, EGFP-C1-nsP3 G112E, EGFP-C1-nsP3 Y114A, EGFP-C1-nsP3 AGDA, EGFP-C1-eIF3g, pCI-neo-Flag-nsP3 WT, pCI-neo-Flag-nsP3 G32E, pCI-neo-Flag-nsP3 MD were constructed by restriction enzyme-based method or Gibson assembly using primers listed in SI Appendix, Table S2.
Generation of U2OSGFP and U2OSGFP-eIF3g Stable Cell Lines.
U2OS cells transfected with pEGFP vector alone or pEGFP-eIF3g were maintained in G418 (neomycin) at 0.5 mg/mL for several days to eliminate untransfected parental U2OS cells. Medium was changed every 2 d to replenish drugs and remove dead cells. Cells were then trypsinized and diluted for single-clone selection based on manual analysis of GFP expression under the microscope.
Viruses.
CHIKV 181/25 WT and mutant strains were prepared as described previously (50). Viral stocks were grown in BHK21 cells, and titers were determined by plaque formation in Vero cells as described previously (31).
Immunoblot Analysis.
Cells were lysed in RIPA buffer (50 mM Tris-Cl pH 8.0, 150 mM NaCl, 0.1% sodium dodecyl sulfate [SDS], 1% Nonidet P-40, 1 mM EDTA as well as protease inhibitors 5 mM NaF and 1 mM phenylmethylsulfonyl fluoride [PMSF]) for 15 min on ice, followed by centrifugation at 14,000 rpm for 15 min, 4 °C. Protein samples were acetone-precipitated for at least 1 h at −20 °C. Precipitates were centrifuged at 13,000 rpm, 4 °C for 15 min, and the air-dried pellets were then diluted in 1× SDS sample buffer. The samples were resolved in polyacrylamide gel electrophoresis and blotted with appropriate primary antibodies (SI Appendix, Table S3).
Immunofluorescence.
U2OS (∼4 × 104) or HeLa (∼1 × 105) cells grown on coverslips were treated with the indicated stressors. Following the stress treatment, cells were washed twice with 1× PBS, fixed with 4% paraformaldehyde for 15 min, permeabilized with ice-cold methanol for 10 min, and washed twice with 1× PBS. The cells were then blocked with 5% normal horse serum in 1× PBS containing 0.02% sodium azide for 1 h at room temperature (RT). All primary antibodies (SI Appendix, Table S3) were diluted in blocking buffer and incubated with cells overnight at 4 °C, followed by three washes with 1× PBS, 10 min each. Next, appropriate secondary antibodies were diluted in blocking buffer (1:500), incubated with cells for 1 h at RT, washed three times with 1× PBS, and the coverslips were mounted on glass slides using Prolong Gold. All experiments were performed at least thrice.
Image Quantitation.
For all quantitation, random 40× fields were chosen and a total of between 80 and 120 cells were counted per condition. To quantify the number of SGs in cells transfected with various GFP-tagged nsP3 constructs, cells with eIF3b foci in GFP-positive cells were scored as SG-positive. To quantify the number of SGs in virus infection, eIF3b foci in nsP3-stained cells were scored as SG-positive. All experiments were repeated three independent times.
Live Cell Imaging.
U2OSGFP or U2OSGFP-eIF3g cells were seeded 1 d prior to the experiment at a concentration of ∼1 × 104 cells/mL on a 2-well chambered cover glass (Nunc Lab-Tek) and were cultured in DMEM, 10% (vol/vol) FBS at 37 °C, and 5% CO2 in a humidified incubator. On the day of the experiment, cells were infected with either CHIKV WT or Y114A mutants at a multiplicity of infection (MOI) of 10 and incubated at 4 °C for 1 h, followed by incubation at 37 °C for 1 h. Cells were then washed twice with 1× PBS, and fresh CO2-independent DMEM containing 20% FBS was added. Infected cells were imaged using a DeltaVision Elite system (GE Healthcare) microscope equipped with ×40 (1.516 N.A. oil) immersion objectives, a high-speed CCD Camera (Cool SNAP HQ2), appropriate filter sets for FITC/mCherry, and an incubation chamber (37 °C and 80% humidity). Images were acquired at 12-min intervals for 14 h, controlled by the SoftWorx suite (GE Healthcare). A total of 15–20 random fields were chosen and imaged, and the presence of SG was monitored as microscopically visible condensates in the FITC channel. To calculate the SG residence time, the duration between the appearance and disappearance of SG was manually analyzed for a given cell. The SG residence time was calculated as the summation of t1 = 0 min, t2 = 12 min, t3 = 24 min, tn = n min (where, t1 is the earliest frame at which SG appeared; and tn is the first frame at which SG disappeared).
Simultaneous smFISH and Immunofluorescence to Detect vRNA.
Immunofluorescence staining for SG proteins was coupled with single-molecule imaging of vRNA using the previously published protocol (62). A set of 40 oligonucleotide probes (each 20 nucleotides [nt] long) was designed specifically against nsP3 RNA (SI Appendix, Table S4). Each probe was designed to have a 3′ amino modification (LGC Biosearch Technologie). The probes were then pooled and coupled en mass with Texas Red, and coupled probes were purified using HPLC (63). Briefly, U2OS cells grown on glass coverslips were infected with CHIKV WT or Y114A mutant for 1 h, 2 h, 4 h, 6 h, 8 h, and then treated with 0.2 mM arsenite for 30 min. Following arsenite treatment, cells were washed twice with 1× PBS, fixed with 4% paraformaldehyde for 15 min, and permeabilized with 70% ethanol. The cells were washed with 1× PBS twice and incubated with bovine serum albumin (BSA) for 1 h at RT. Coverslips were incubated with primary antibodies for G3BP1 or eIF3b overnight at 4 °C and then washed three times with 1× PBS, 10 min each followed by incubation with appropriate secondary antibodies for 1 h at RT. The coverslips were then washed with 1× PBS three times, and smFISH protocol was followed as described previously (63). Briefly, coverslips were washed with 2× SSC buffer with 20% formamide and then hybridized with smFISH probes in a 20% hybridization buffer overnight at 37 °C in a humidified chamber. After hybridization, coverslips were washed four times with 2× SSC buffer containing 20% formamide, counterstained with DAPI, and mounted using deoxygenated mounting media. The coverslips were imaged with 100× oil objective using a Nikon TiE Inverted epi Fluorescence microscope equipped with a pixis 1024b camera (Princeton Instruments). The images were obtained using Metamorph imaging software and analyzed using custom-written programs in MATLAB (Mathworks Inc.) as described previously (64).
smFISH on poly(A)+ mRNA.
Poly(A) FISH was performed using 35-nt-long dT LNA probes containing amine reactive group (synthesized by Exiqon) and labeled with far red dye Cy5 (GEPA25001) or red dye Cy3.5 (GEPA23501) from Sigma. For labeling, one dye pack was resuspended in 30 µL of freshly prepared 0.1 M sodium bicarbonate solution (pH 8.4) and was used to label 20 µg of dT LNA probes. The labeled probes were purified from free dyes using the QIAGEN nucleotide removal kit (catalog no. 28304). smFISH was performed as described in ref. 65. Briefly, cells transfected with GFP-nsP3 WT or G32E were fixed using 4% paraformaldehyde in 1× PBS and kept in 70% ethanol at −20 °C. Before hybridization, coverslips were rehydrated in 1× PBS and submerged in 10% formamide/2× SSC for 10 min. Hybridization mix was prepared using 10 ng of probes and 40 mg of salmon sperm DNA/transfer RNA were resuspended in 10% formamide, 2× SSC and 10% dextran sulfate solution containing 2 mM ribonucleoside vanadyl complexes and 0.1 mg/mL BSA. Hybridization was carried out for 3 h at 37 °C in dark. After hybridization, coverslips were washed twice with 10% formamide, 2× SSC for 30 min at 37 C. Thereafter, the coverslips were rinsed in 1× PBS containing 0.5 µg/mL DAPI for 5 min followed by two rinses with 1× PBS and mounted using ProLong Gold.
The superresolution images were acquired using a Zeiss Elyra PS.1 system equipped with a 63× oil objective, an Andor EMCCD iXon3 DU-885 CSO VP461 camera (1004 × 1002 pixels), the following lasers: 50 mW 405 nm HR diode, 100 mW 488 nm HR diode, 100 mW 561 nm HR DPSS, 150 mW 642 nm HR diode and the following filter sets: DAPI: BP420-480 + LP750 (Zeiss SR cube 07), Cy2: BP495-590 + LP750 (Zeiss SR cube 13), Cy3: LP570 (Zeiss SR cube 14), Cy5: LP655 (Zeiss SR cube 10). Each image was acquired using three grid rotations using a grid size of 42 mm for all channels.
Immunoprecipitation.
293F cells were spun down at 400 × g for 3 min at 4 °C, washed once with cold 1× PBS, pelleted and lysed in cold lysis buffer (CLB) (50 mM Hepes pH 7.4, 150 mM NaCl, 1 mM MgCl2, 1 mM ethylene glycol-bis(β-aminoethyl ether)-N,N,N′,N′-tetraacetic acid, 1% Triton X-100 supplemented with 1 mM NaF, 1 mM PMSF, 1 mM adenosine 5′-diphosphate (hydroxymethyl)pyrrolidinediol). Cell lysates were mixed at 4 °C for 15 min, spun down for 15 min at 13,000 rpm, and the supernatant fluid was collected in a new tube. The cleared lysates were added to preincubated anti-GFP (3E6, Invitrogen)–DYNA magnetic beads (10004D, Invitrogen) complex and incubated for 2 h at RT. The beads were washed once with CLB, twice with high-salt CLB (300 mM NaCl), followed by a final wash with CLB. The precipitates were then boiled with 1× SDS sample buffer for 10 min at ∼85 °C. All experiments were performed at least thrice. For Biotin-PAR pull-down, 293F cells transfected with appropriate plasmids for 24 h were pelleted, lysed in CLB for 15 min. Cell lysates were cleared by centrifugation at 13,000 rpm for 15 min, and the supernatant was collected in a new tube. 100 pmol biotin-labeled PAR (66) and 50 µL of streptavidin magnetic beads were added to each sample and incubated for 2 h at 4 °C. After incubation, the beads were washed once with CLB, twice with high-salt CLB (300 mM NaCl), followed by a final wash with CLB. The precipitates were then boiled with 1× SDS sample buffer for 10 min at ∼85 °C.
Statistical Analysis.
Data were presented as mean ± SD and groups compared using two-tailed unpaired Student’s t test and Kolmogorov–Smirnov test. P < 0.05 was considered statistically significant. All statistical analyses were performed using Graphpad Prism8.
Supplementary Material
Acknowledgments
We thank Drs. Phillip Sharp, Nancy Kedersha, Lucas Reineke, Lyle McPherson, and members of the A.K.L.L. laboratory for their critiques of the manuscript. We thank Dr. Nancy Kedersha and Dr. Paul Anderson for G3BP1/2 dKO cells and G3BP1 constructs, and Dr. Andres Merits for nsP3 antibodies. We also thank Morgan Dasovich for synthesizing the Biotin–PAR and Debra Hauer for construction of the nsP3 mCherry-tagged viruses. This work was supported by a Johns Hopkins Catalyst Award (to A.K.L.L.), pilot grants from the Johns Hopkins University School of Medicine Sherrilyn and Ken Fisher Center for Environmental Infectious Disease (to A.K.J., D.E.G., and A.K.L.L.), NIH Grants R56AI137264 (to D.E.G. and A.K.L.L.) and R01GM104135 (to A.K.L.L.), and Canadian Institutes of Health Research Grants PJT-148932 (to D.Z.) and UDRF_SI_19A00244 (to M.B.). D.Z. is a Fonds de Recherche du Québec-Santé Chercheur-boursier Senior Research Scholar, and S.A. holds a Fonds de Recherche du Québec-Santé Doctoral Fellowship.
Footnotes
The authors declare no competing interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at https://www.pnas.org/lookup/suppl/doi:10.1073/pnas.2021719118/-/DCSupplemental.
Data Availability.
All study data are included in the article and/or supporting information.
References
- 1.Ivanov P., Kedersha N., Anderson P., Stress granules and processing bodies in translational control. Cold Spring Harb. Perspect. Biol. 11, 032813 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ramaswami M., Taylor J. P., Parker R., Altered ribostasis: RNA-protein granules in degenerative disorders. Cell 154, 727–736 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Seydoux G., The P granules of C. elegans: A genetic model for the study of RNA-protein condensates. J. Mol. Biol. 430, 4702–4710 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Buchan J. R., Parker R., Eukaryotic stress granules: The ins and outs of translation. Mol. Cell 36, 932–941 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kedersha N., Anderson P., Mammalian stress granules and processing bodies. Methods Enzymol. 431, 61–81 (2007). [DOI] [PubMed] [Google Scholar]
- 6.Kedersha N., et al., G3BP-Caprin1-USP10 complexes mediate stress granule condensation and associate with 40S subunits. J. Cell Biol. 212, 845–860 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Matsuki H., et al., Both G3BP1 and G3BP2 contribute to stress granule formation. Genes Cells 18, 135–146 (2013). [DOI] [PubMed] [Google Scholar]
- 8.Grabocka E., Bar-Sagi D., Mutant KRAS enhances tumor cell fitness by upregulating stress granules. Cell 167, 1803–1813.e12 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kim H. J., et al., Mutations in prion-like domains in hnRNPA2B1 and hnRNPA1 cause multisystem proteinopathy and ALS. Nature 495, 467–473 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Reineke L. C., Lloyd R. E., Diversion of stress granules and P-bodies during viral infection. Virology 436, 255–267 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.McCormick C., Khaperskyy D. A., Translation inhibition and stress granules in the antiviral immune response. Nat. Rev. Immunol. 17, 647–660 (2017). [DOI] [PubMed] [Google Scholar]
- 12.Onomoto K., Yoneyama M., Fung G., Kato H., Fujita T., Antiviral innate immunity and stress granule responses. Trends Immunol. 35, 420–428 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ruggieri A., et al., Dynamic oscillation of translation and stress granule formation mark the cellular response to virus infection. Cell Host Microbe 12, 71–85 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Tsai W.-C., Lloyd R. E., Cytoplasmic RNA granules and viral infection. Annu. Rev. Virol. 1, 147–170 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hofweber M., Dormann D., Friend or foe - post-translational modifications as regulators of phase separation and RNP granule dynamics. J. Biol. Chem. 294, 7137–7150 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Jayabalan A. K., et al., NEDDylation promotes stress granule assembly. Nat. Commun. 7, 12125 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Leung A. K. L., et al., Poly(ADP-ribose) regulates stress responses and microRNA activity in the cytoplasm. Mol. Cell 42, 489–499 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ohn T., Kedersha N., Hickman T., Tisdale S., Anderson P., A functional RNAi screen links O-GlcNAc modification of ribosomal proteins to stress granule and processing body assembly. Nat. Cell Biol. 10, 1224–1231 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Saito M., et al., Acetylation of intrinsically disordered regions regulates phase separation. Nat. Chem. Biol. 15, 51–61 (2019). [DOI] [PubMed] [Google Scholar]
- 20.Tsai W.-C., Reineke L. C., Jain A., Jung S. Y., Lloyd R. E., Histone arginine demethylase JMJD6 is linked to stress granule assembly through demethylation of the stress granule-nucleating protein G3BP1. J. Biol. Chem. 292, 18886–18896 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Wippich F., et al., Dual specificity kinase DYRK3 couples stress granule condensation/dissolution to mTORC1 signaling. Cell 152, 791–805 (2013). [DOI] [PubMed] [Google Scholar]
- 22.Gupte R., Liu Z., Kraus W. L., PARPs and ADP-ribosylation: Recent advances linking molecular functions to biological outcomes. Genes Dev. 31, 101–126 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hottiger M. O., SnapShot: ADP-ribosylation signaling. Mol. Cell 58, 1134–1134.e1 (2015). [DOI] [PubMed] [Google Scholar]
- 24.Palazzo L., Mikoč A., Ahel I., ADP-ribosylation: New facets of an ancient modification. FEBS J. 284, 2932–2946 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Catara G., et al., PARP1-produced poly-ADP-ribose causes the PARP12 translocation to stress granules and impairment of Golgi complex functions. Sci. Rep. 7, 14035 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Duan Y., et al., PARylation regulates stress granule dynamics, phase separation, and neurotoxicity of disease-related RNA-binding proteins. Cell Res. 29, 233–247 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.McGurk L., et al., Poly(ADP-Ribose) prevents pathological phase separation of TDP-43 by promoting liquid demixing and stress granule localization. Mol. Cell 71, 703–717.e9 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Fros J. J., et al., Chikungunya virus nsP3 blocks stress granule assembly by recruitment of G3BP into cytoplasmic foci. J. Virol. 86, 10873–10879 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Panas M. D., et al., Viral and cellular proteins containing FGDF motifs bind G3BP to block stress granule formation. PLoS Pathog. 11, e1004659 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Götte B., Liu L., McInerney G. M., The enigmatic alphavirus non-structural protein 3 (nsP3) revealing its secrets at last. Viruses 10, 105 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.McPherson R. L., et al., ADP-ribosylhydrolase activity of Chikungunya virus macrodomain is critical for virus replication and virulence. Proc. Natl. Acad. Sci. U.S.A. 114, 1666–1671 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Park E., Griffin D. E., Interaction of Sindbis virus non-structural protein 3 with poly(ADP-ribose) polymerase 1 in neuronal cells. J. Gen. Virol. 90, 2073–2080 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kim D. Y., et al., New world and old world alphaviruses have evolved to exploit different components of stress granules, FXR and G3BP proteins, for assembly of viral replication complexes. PLoS Pathog. 12, e1005810 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Panas M. D., et al., Sequestration of G3BP coupled with efficient translation inhibits stress granules in Semliki Forest virus infection. Mol. Biol. Cell 23, 4701–4712 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Remenyi R., Roberts G. C., Zothner C., Merits A., Harris M., SNAP-tagged chikungunya virus replicons improve visualisation of non-structural protein 3 by fluorescence microscopy. Sci. Rep. 7, 5682 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Frolov I., Kim D. Y., Akhrymuk M., Mobley J. A., Frolova E. I., Hypervariable domain of eastern equine encephalitis virus nsP3 redundantly utilizes multiple cellular proteins for replication complex assembly. J. Virol. 91, e00371-17 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Panas M. D., Ahola T., McInerney G. M., The C-terminal repeat domains of nsP3 from the Old World alphaviruses bind directly to G3BP. J. Virol. 88, 5888–5893 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Eckei L., et al., The conserved macrodomains of the non-structural proteins of Chikungunya virus and other pathogenic positive strand RNA viruses function as mono-ADP-ribosylhydrolases. Sci. Rep. 7, 41746 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Fehr A. R., et al., The conserved coronavirus macrodomain promotes virulence and suppresses the innate immune response during severe acute respiratory syndrome coronavirus infection. MBio 7, e01721-16 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Li C., et al., Viral macro domains reverse protein ADP-ribosylation. J. Virol. 90, 8478–8486 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Cristea I. M., et al., Tracking and elucidating alphavirus-host protein interactions. J. Biol. Chem. 281, 30269–30278 (2006). [DOI] [PubMed] [Google Scholar]
- 42.Frolova E., et al., Formation of nsP3-specific protein complexes during Sindbis virus replication. J. Virol. 80, 4122–4134 (2006). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Gorchakov R., Garmashova N., Frolova E., Frolov I., Different types of nsP3-containing protein complexes in Sindbis virus-infected cells. J. Virol. 82, 10088–10101 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Meshram C. D., et al., Multiple host factors interact with the hypervariable domain of chikungunya virus nsP3 and determine viral replication in cell-specific mode. J. Virol. 92, 491 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Gao Y., Goonawardane N., Ward J., Tuplin A., Harris M., Multiple roles of the non-structural protein 3 (nsP3) alphavirus unique domain (AUD) during Chikungunya virus genome replication and transcription. PLoS Pathog. 15, e1007239 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Isabelle M., Gagné J.-P., Gallouzi I.-E., Poirier G. G., Quantitative proteomics and dynamic imaging reveal that G3BP-mediated stress granule assembly is poly(ADP-ribose)-dependent following exposure to MNNG-induced DNA alkylation. J. Cell Sci. 125, 4555–4566 (2012). [DOI] [PubMed] [Google Scholar]
- 47.Scholte F. E. M., et al., Stress granule components G3BP1 and G3BP2 play a proviral role early in Chikungunya virus replication. J. Virol. 89, 4457–4469 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Tourrière H., et al., The RasGAP-associated endoribonuclease G3BP assembles stress granules. J. Cell Biol. 160, 823–831 (2003). [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 49.Bosco D. A., et al., Mutant FUS proteins that cause amyotrophic lateral sclerosis incorporate into stress granules. Hum. Mol. Genet. 19, 4160–4175 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Abraham R., et al., ADP-ribosyl-binding and hydrolase activities of the alphavirus nsP3 macrodomain are critical for initiation of virus replication. Proc. Natl. Acad. Sci. U.S.A. 115, E10457–E10466 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Ng C. S., et al., Encephalomyocarditis virus disrupts stress granules, the critical platform for triggering antiviral innate immune responses. J. Virol. 87, 9511–9522 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Fung G., et al., Production of a dominant-negative fragment due to G3BP1 cleavage contributes to the disruption of mitochondria-associated protective stress granules during CVB3 infection. PLoS One 8, e79546 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Akhrymuk I., Frolov I., Frolova E. I., Sindbis virus infection causes cell death by nsP2-induced transcriptional shutoff or by nsP3-dependent translational shutoff. J. Virol. 92, 491 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Altmeyer M., et al., Liquid demixing of intrinsically disordered proteins is seeded by poly(ADP-ribose). Nat. Commun. 6, 8088 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Leung A. K. L., Poly(ADP-ribose): An organizer of cellular architecture. J. Cell Biol. 205, 613–619 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Leung A. K. L., Poly(ADP-ribose): A dynamic trigger for biomolecular condensate formation. Trends Cell Biol. 30, 370–383 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Barkauskaite E., Jankevicius G., Ahel I., Structures and mechanisms of enzymes employed in the synthesis and degradation of PARP-dependent protein ADP-ribosylation. Mol. Cell 58, 935–946 (2015). [DOI] [PubMed] [Google Scholar]
- 58.Feijs K. L. H., Forst A. H., Verheugd P., Lüscher B., Macrodomain-containing proteins: Regulating new intracellular functions of mono(ADP-ribosyl)ation. Nat. Rev. Mol. Cell Biol. 14, 443–451 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Pascal J. M., Ellenberger T., The rise and fall of poly(ADP-ribose): An enzymatic perspective. DNA Repair (Amst.) 32, 10–16 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Fehr A. R., Jankevicius G., Ahel I., Perlman S., Viral macrodomains: Unique mediators of viral replication and pathogenesis. Trends Microbiol. 26, 598–610 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Leung A. K. L., McPherson R. L., Griffin D. E., Macrodomain ADP-ribosylhydrolase and the pathogenesis of infectious diseases. PLoS Pathog. 14, e1006864 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Bayer L. V., Batish M., Formel S. K., Bratu D. P., Single-molecule RNA in situ hybridization (smFISH) and immunofluorescence (IF) in the Drosophila egg chamber. Methods Mol. Biol. 1328, 125–136 (2015). [DOI] [PubMed] [Google Scholar]
- 63.Batish M., Tyagi S., Fluorescence in situ imaging of dendritic RNAs at single-molecule resolution. Curr. Protoc. Neurosci. 89, e79 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Markey F. B., Ruezinsky W., Tyagi S., Batish M., Fusion FISH imaging: Single-molecule detection of gene fusion transcripts in situ. PLoS One 9, e93488 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Adivarahan S., et al., Spatial organization of single mRNPs at different stages of the gene expression pathway. Mol. Cell 72, 727–738.e5 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Ando Y., et al., ELTA: Enzymatic labeling of terminal ADP-ribose. Mol. Cell 73, 845–856.e5 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
All study data are included in the article and/or supporting information.