Abstract
Objective: Despite recent advances in molecular biology and genetics, the development of intracranial aneurysms (IA) is still poorly understood. Elucidation of the processes occurring in the IA wall is essential for a better understanding of IA pathophysiology. We sought to analyze the current evidence from histological, molecular and genetic studies of IA. Methods: We systematically searched PubMed, Scopus, Web of Science and Cochrane Library for articles published before Mar 1, 2019 reporting on different diagnostic markers in human IA specimens. Expression of the markers in IA wall (vs. healthy arterial wall) and association with the rupture status were analyzed. The quality of the included studies and the level of the evidence for the markers were incorporated into the final data assessment. Results: We included 123 studies reporting on analyses of 3476 IA (median 19 IA/study) published between 1966 and 2018. Based on microscopic, biochemical, genetic and biomechanical analyses, data on 358 diagnostic targets in the IA wall were collected. We developed a scale to distribute the diagnostic markers according to their specificity for IA or healthy arterial wall, as well as for ruptured or unruptured IA. We identified different functional pathways, which might reflect the intrinsic and extrinsic processes underlying IA pathophysiology. Conclusions: Multiple histological and molecular markers and the related functional pathways contributing to the development of IA might present promising targets for future therapeutic interventions. Because of small numbers of IA samples in each study, 89% of the analyzed diagnostic markers presented with the lowest level of evidence. This underlines the need for the initiation of a multi‐centric prospective histological IA register for pooled data analysis.
Keywords: histology, immunohistochemistry, intracranial aneurysm, genetic, molecular, marker, rupture, formation, inflammation
INTRODUCTION
Except for syndromal and infectious cases, saccular intracranial aneurysms (IA) are usually slowly growing vascular lesions. While IA may remain mostly asymptomatic over years, their sudden rupture leads to heavy burden of subarachnoid hemorrhage with poor outcome despite maximal treatment 16.
The knowledge of risk factors for IA development is essential. Large population‐based observational studies identified that increasing age, female sex, arterial hypertension, smoking, drug abuse, familial predisposition and certain syndromal and non‐syndromal disorders are associated with a higher probability of IA formation and rupture 5, 11, 15.
At the same time, the exact pathophysiologic processes underlying this IA dynamic are still poorly understood. Previous clinical and experimental studies addressing the aberrations in the IA wall strongly contributed to a better understanding of the background of IA formation. Current hypotheses of IA genesis are based on the chronic vascular injury as a result of long‐lasting hemodynamic stress with subsequent inflammatory cascades resulting in degeneration and reorganization of the affected arterial wall 8, 13, 21. In addition, role of parent artery morphology in development of IA was also previously discussed 14, 26.
Currently, there is still no clear differentiation between the cellular and molecular pathways leading to rupture or to stabilization of the IA wall. This question is of particular interest, since some IA are considered to have a neglectable risk of rupture justifying their lifetime surveillance without a treatment 24. In addition, knowledge of clinically relevant diagnostic markers might be helpful for the development of better diagnostic methods to identify rupture‐prone IA, or even open the door for a medical treatment of the pathologically altered intracranial vasculature.
In this systematic review, we aimed to analyze the current evidence of histological, molecular and genetic studies addressing the biological changes occurring in the wall of human IA. Special attention is paid to the discrimination between the pathophysiological processes contributing to IA growth and rupture.
MATERIALS AND METHODS
Search strategy and selection criteria
This systematic review was performed according to the PRISMA guidelines. PubMed, Web of Science, Scopus and Cochrane Library databases were systematically searched to identify all clinical studies published before Mar 1, 2019 that reported on molecular and histological analyses of human IA tissue specimens. The used search terms contained different combinations of the following keywords: “histo*,” “tissue,” “immunohistochemistry,” “immunofluorescence,” “immunostaining,” “*microscopy,” “cell,” “wall,” “brain” “cerebral,” “intracranial” and “aneurysm” (see Table S1 in the Supporting Information). After the inclusion of the search results within an electronic database and exclusion of the duplicate records, the titles and abstracts (and, if necessary, the full texts) of the studies were independently screened by RJ and TFD to assess their eligibility. Reference lists of relevant publications were screened for additional articles in the same manner. Any disagreement was resolved by a consensus discussion. The review was restricted to the studies published in English.
The inclusion criteria for the eligible studies were (i) any kind of diagnostic evaluation of human IA wall tissue as described by the authors; and (ii) the study results based on at least three different IA specimens. Studies (i) sing the series of infectious, post‐traumatic or non‐saccular IA (ii), containing the cases with IA related to syndromal/systemic disorders like polycystic kidney disease, sickle‐cell disease, Ehlers‐Danlos syndrome, Marfan's syndrome, Loeys–Dietz syndrome, Moyamoya disease, phakomatoses etc., or (iii) describing solely the changes in the IA wall after the endovascular or microsurgical treatment, were excluded from the review (see the detailed flow chart in Figure 1).
Figure 1.
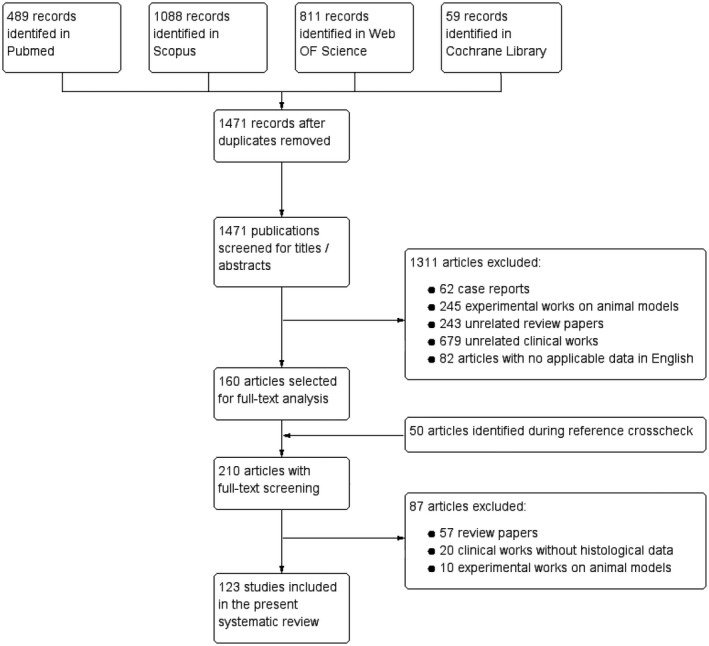
Flow diagram showing selection of eligible studies.
Data analysis
Data collection
Data extraction were performed by RJ and the quality was controlled by LR. The following data (when available) were collected from the included studies for further analysis:
Publication and population characteristics: publication year and journal title; geographic origin of the cohort (country and the location of the research center), demographic characteristics (age and sex), the first and the last year of enrolment; study design; number, type (ruptured IA [RIA]/unruptured IA [UIA]), morphology and radiographic characteristics of the analyzed IA; number, location and origin of non‐IA (nIA) tissue samples used as control group (the same patients with IA, other patients with IA or patients without IA); method of tissue collection (autopsy, intraoperative or by endovascular biopsy); data assessment method (descriptive, univariate or multivariate statistics).
Characteristics of assessed diagnostic markers: the full name and abbreviation of the marker(s), diagnostic method(s) used for experiments, addressed endpoints (expression in IA vs. nIA, in RIA vs. UIA, other endpoints as specified by the authors) and the results of these correlations.
All analyzed markers were recorded into an electronic register. Where identifiable, overlapping results from the same cohorts were excluded. The following information was collected within the database: biomarker name, abbreviation, source studies, total number of IA, results from different studies regarding the evaluated endpoints, summary conclusion based on the cumulative evidence, as well as the level of evidence (as described below), data on various functional pathways (not only IA‐related) where the marker is or might be involved (using the information from the GeneCards® and UniProt® databases). The definitions of the involved pathways were focused on describing both the related processes (inflammation, proliferation and cell migration etc.) and the effector of the pathway (atherosclerosis, microbiomes and extracellular matrix remodeling etc.).
Quality assessment of the studies
To assess the potential for selection and information bias, a quality score was calculated for each included study, based on an assessment form adapted from the previous publications 15 (0–20 points, see Table S2 in the Supporting Information). Those articles with a score ≥ 14 points were regarded as good‐quality studies. The quality score values were incorporated into assessment of the evidence level for diagnostic markers. The quality of the studies was independently evaluated by RJ and TFD.
The level of evidence for diagnostic markers
The level of evidence for each IA marker was assigned to one of four classes: (i) high, (ii) moderate, (iii) low and (iv) very low evidence. Criteria for classification were defined according to the GRADE (Grading of Recommendations Assessment, Development and Evaluation) guidelines with further adaptation to the scope of the review (see Table S3 in the Supporting Information). The evaluation of the evidence level was performed independently by RJ and LR.
Cumulative data assessment were not limited by the evidence level. However, the associations based on the data with high or moderate evidence level were reported separately.
Study endpoints and classification of the diagnostic markers and functional pathways
Our primary review endpoint was the association between the evaluated diagnostic markers and the following two endpoints: (i) IA wall vs. non‐IA vessel specimen (control tissues, nIA) and (ii) RIA vs. UIA. If available, the summary effect was assessed also for other endpoints (secondary review endpoints): associations between different diagnostic markers, radiographic characteristics of IA (size and irregularity), findings in magnetic resonance imaging (MRI) and computational fluid dynamics (CFD), aspirin intake, smoking history, carbon birth dating and time left since IA clipping etc.
The marker was considered characteristic for the review endpoint(s) if the study(‐ies) reported on significant associations, without conflicting results from good‐quality studies. For the data based on low‐quality studies, the presence of significant and congruent results from over two‐thirds of the total population was required to assign the marker as characteristic. The remaining cases were regarded as non‐characteristic for the review endpoint(s). Thereafter, the markers with significant summary results for the primary review endpoints were divided into four groups: nIA > IA (Group 1); IA > nIA (Group 2); UIA > RIA (Group 3); RIA > UIA (Group 4). Finally, the functional pathways of the markers were analyzed with regard to their distribution in each arm of the primary review endpoints. The results were expressed in percentages.
Statistical analysis
Study and population characteristics were analyzed using PRISM software (version 5.0, GraphPad Software Inc, San Diego, CA, USA). Differences between continuous variables were analyzed using the Student's t‐test for normally distributed and the Mann–Whitney U test for non‐normally distributed data. Variables were expressed as medians with ranges, absolute numbers and percentages, when appropriate. Differences with a P‐value of 0.05 or less were regarded as statistically significant.
RESULTS
Study characteristics
Of 1521 non‐duplicating records identified through the databases search (n = 1471) and reference list crosscheck (n = 50), 123 studies were included into this systematic review. The flow chart in Figure 1 shows the selection process of the eligible studies (see also Table S4 in the Supporting Information for the full list of the included studies).
The included studies were performed in 75 neurovascular centers from 18 countries. The majority of the studies presented cohorts from China (n = 28), followed by Finland (n = 22), USA (n = 20), Japan (n = 18) and Italy (n = 8), whereas the centers from the remaining countries published 1–4 articles.
The median number of IA per each study was 19 (range 3–404). The total number of IA samples examined in the experiments was 3476. However, when accounting potential overlapping of study results from the similar cohorts of the same institutions, the summary samples count might have been considerably lower (n = 2456 IA). Of note, the exclusion of the overlapping results was complicated because the majority of the studies did not report on their enrollment years.
Comparisons between RIA and UIA were reported in 45 studies. Alongside with IA samples, 71 studies performed the identical analyses on nIA vessel wall tissues consisting of intracranial or extracranial arterial segments, mostly of the superficial temporal artery. These studies presented the data on the differences between IA and not IA‐affected vessels. Finally, 32 studies analyzed the associations between the markers and secondary review endpoints (radiographic IA characteristics, other diagnostic markers etc.).
The samples of IA were collected mostly during microsurgical clipping (n = 95) or by autopsy (n = 17). In nine studies, the series consisted of mixed samples (intraoperative and autopsy). One study was based on endovascular biopsy of IA, another study did not report on the sampling method.
The publications showed a heterogeneous study quality ranging between 4 and 19 points. The median value of the quality score was 13 points. Only 48 studies (39%) scored ≥ 14 points and were regarded as good‐quality studies.
The studies were published between 1966 and 2018. There was an increase in the annual number of publications over the recent 20 years: 81.3% of the articles (n = 100) were published between 1999 and 2018. In this period, the median number of IA per each study increased from 15 to 19.5 (P = 0.5745), and the median quality of the studies from 11 to 13 points (P = 0.0012).
Summary on diagnostic markers
Using electron and light microscopy, as well as biochemical, genetic and other analyses, a total of 358 histological and molecular markers of IA were analyzed. Regarding the review endpoints, the most frequently used diagnostic method was the polymerase chain reaction (n = 218), followed by immunostaining (n = 92), western blotting (n = 61) and microscopy (n = 45).
The basic diagnostic evaluation addressed the morphological changes occurring in the IA wall like loss of the anatomic layer structure of the arterial wall, reorganization and remodeling, presence of intraluminal thrombosis and atherosclerotic lesions etc. The genetic markers (n = 208) constituted the largest group of the diagnostic targets. Biochemical analyses were used to identify 141 more diagnostic markers. Finally, six studies performed biomechanical tests on the IA tissues (like tension, straining and strength measurements or testing of mechanical integrity).
In median, each diagnostic marker was evaluated only in one study (ranging 1–16) and upon 12 IA samples (ranging 3–811). In particular, genetic markers were evaluated on significantly lower number of IA in comparison to nongenetic markers (12 vs. 25 IA/marker, P < 0.0001). This heterogeneity of the data quality resulted in different levels of evidence supporting the potential association of markers with one of the review endpoints. The vast majority of the tested markers (n = 326, 88.6%) showed the lowest evidence level (Class IV). The evidence level was even lower for genetic markers (Class IV = 99.1%). Only for the following assessments, there was a high (Class I) or at least moderate (Class II) level of evidence: increased apoptosis, loss of smooth muscle cell layer and endothelial layer damage, as well as infiltration of M2‐macrophages and activity of matrix metalloproteinase‐9 in IA and RIA (vs. nIA and UIA, respectively), increase of intraluminal thrombosis, lower number of collagen fibers and atherosclerotic lesions in RIA.
Functional pathways
Analysis of functional characteristics of the collected diagnostic markers revealed over 20 different functional pathways linked with the significant markers. Basically, there were pathophysiologic processes widely acknowledged to occur during IA formation, such as apoptosis, inflammation, oxidative stress, cell adhesion, thrombosis, iron binding & transport and proliferation etc. However, there were also various pathways, unspecific and/or uncommon for the process of IA formation: antimicrobial activity, calcium ion channels, lipid & glucose metabolism, atherosclerosis, hormonal pathways, cellular migration, cytoskeleton regulation, “wound healing” and “tumor markers” etc. Several markers were reported to be involved in multiple pathways. Figure 2 shows the most relevant functional pathways in relation (in percentage) to the primary endpoint arms (see Table S5 in the Supporting Information for the enhanced list of the pathways).
Figure 2.
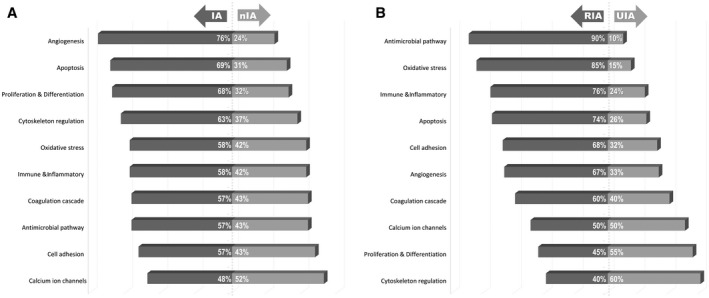
Distribution of the most common 10 functional pathways between the primary review endpoints (in percentages). A. For intracranial aneurysms (IA) vs. non‐IA (nIA) control specimens. B. For ruptured (RIA) vs. unruptured IA (UIA). For the enhanced list of the functional pathways see the Table S5 in Supporting Information.
Primary review endpoints
Except for four targets (ApoB100, CNN, OxLDL and 4‐PH), all markers were tested for the associations with one or both primary review endpoints. Accordingly, 150 markers were overexpressed (ie, characteristic for IA) and 98 were under‐expressed in the IA wall (ie, characteristic for nIA). There were fewer markers showing differences regarding the rupture status of IA: 96 parameters were characteristic for RIA and 55 for UIA.
Depending on the relation to both primary endpoints, the significant results were distributed into four categories and presented in Table 1.
Table 1.
The full list of histological and molecular IA markers in each group. Abbreviation: Gene‐based markers are highlighted with an asterisk. The full names for the abbreviations of the histological and molecular markers are available in the Table S7 in Supporting Information.
| Group 1: nIA > IA |
|
AT1, AT2, ACE, CASP8, CAV1, DPD, DPD + PYD/Collagen, DES, FLN‐C, ITGA1, ITGA2, IL‐10, MLCK, MYL9, SMTN, SORBS2, VCL, WWOX *ADH1C, *AHF (*F8), *AOC3, *ANXA2P1, *ANXA2P3, *APOL3, *BCL2, *BST2, *BCYRN1, *CMYA5, *CD97, *CCL15, *CXCL12, *CXCL14, *CFD, *CPNE9, *DPT, *FTH1P3, *FYN, *GJB6, *GREB1, *GBP2, *HSP90AA6P, *HSP90AB3P, *HIST1H3J, *JCHAIN, *IFITM4P, *IFIH1, *MHC1 (*HLA‐B), *MHC1 (*HLA‐C), *MHC1 (*HLA‐E), *MHC1 (*HLA‐F), *HLA‐G, *HLA‐DRB3, *KCNT1, *MASP2, *MATN2, *MARCH8, *MFAP4, *MGLL, *MMRN2, *MBP, *MYL9, *NFATC3, *NF‐κB2, *NR3C1, *OTUB1, *PLN, *PLA2G4C, *PRELP, *PTGDS, *PTGER4, *PSMB8, *PSMB9, *PRKRA, *RETREG1, *RPL13AP20, *SERPIND1, *STAT3, *SLC13A3, *SORBS1, *SWAP70T1, *TAPBIN1, *TNC, *TCF7, *TMEM45B, *TPI1P2, *TNS1, *vWF |
| Group 2: IA > nIA |
|
AZU1, CD62E, CD4+, CD8+, FADD, Gelatin lysis, HA, MMP‐1, MMP‐2, MMP‐3, MT1‐MMP, MCP1 (CCL2), NGF, PLG, PSF, RAI, S100A4, SMOC1, TM9SF1, tPA (uPA), VCAM1, XO (XAQ) *A2M, *ACTB, *AJ, *ATF5, *AG, *BPI, *BCR, *BLM, *CAM, *CCC, *CCRI, *CC/RGCC, *CHST15, *CLDN5, *CLIP2, *CPZ, *CTSB, *DEFB1, *DVAF‐, *E2F4, *FAK, *FBN2, *FLRT3, *GJ, *H19, *HAAO, *HAMP, *HNF4, *HNF6, *HP, *HRH2, *IKBKG, *INS, *JUN, *LILRB2, *LTEM, *LIFR, *LTD, *LTP, *LYZ, *LOXL1, *LOXL2, *LOXL3, *LOXL4, *MHC2 (*HLA‐A), *MAPK, *MMP13, *MMP16, *MG, *MEX3B, *MCP1 (*CCL2), *MTND6P4, *mTOR, *MUC3B, *MYH11, *NK, *NLRI, *NUFIP2, *NIFK, *PAX5, *PTHLH, *POSTN, *PIK3R5, *PLVAP, *PLEK, *RAC, *SAMSN1, *SFRP2, *SCG2, *SERPINA1, *SNARE, *SOX4, *SMOC1, *TJ, *TLR4, *HTF4, *TGF‐β, *TOG1, *TMEM132B, *TWIST1, *VEGF, *WAS |
| Group 3: UIA > RIA |
|
Presence of atherosclerosis, Presence of SMC layer, Presence of Collagen, Resistance to biomechanical tests, AHF (F8), AHSG, ASPN (PLAP1), Bad (Phospho‐Bad), BGN, CAT, CHST14, CRAT, CRABP1, CXCL12 (SDF1), DKZp686H1812, DKZp686K06110, DPT (TRAMP), HLA‐A, HLA‐B, HSPG, IL6, mTOR (Phospho‐mTOR), OLFML1, OLFML3, OPN, PINCH, PLC, PTGDS, vWF *BCL2L1, *BCL2, *CAMK2A, *CD69, *CDKN2A, *cDNA FLJ51023, *cDNA FLJ58762 *COL, *DDN, *DOK6, *ENG, *FAM110C, *HES1, *HLA‐DPB1, *IGHG4, *KRT17, *KLF2, *KLF12, *KLF15, *MYOZ3, *PACSIN1, *RXFP1, *TIMP2, *TIMP1, *TIMP3, *TIMP4,*TPH1, *TIE1, *VCAM1 |
| Group 4: RIA > UIA |
|
Apoptosis, Endothelial layer damage, Intraluminal Thrombosis, AA1 (CC1), ALOX12, ASC, CASP1, C1q, C3b, C3c, C3d, C4b, C5b, CASP9, CC1, CD45+, CD68+, CD163+, CD3, CD45RO (CD45RB), CTSD, CTSG, COX‐2, DEFA3, ECP, ECRP, ELA2, EPO, FN, GP1BB, GPA, HLA‐DR, HMGB1, HO1, ICAM1, IgM, IgG, IgA, IL‐1β, ITGAM (CD11b), ITGA2B, ITGB3, JNK (MAPK), KLF5, Ki67, Laminin, LF, LTF, MAC, MDA, MMP9, MPO, NCF1C, NCF4, NF‐κB, NLRP3, PMP2, PNUT, PGE2, PYCARD, RAC2, RETN, S100P, SERPINF2, TC1, TGF‐α, TLR, VEGF *ADAMTS1, *ALDOB, *AGT, *ATP1B2, *BAX, *BID, *CD163, *CD300C, *CD300E, *cDNA FLJ57667, *CLEC5A, *CTSD, *DAXX, *ETS, *FAS, *HPSE, *HIF1A, *KCNA5, *MARCO, *MMP2, *MMP14, *MT1X, *MT1E, *MT1G, *MT1M, *MT1A, *MT2A, *MT1B, *MPO, *NQO1, *NOS1, *NOS1AP, *OSCAR, *OLR1, *PDCD5, *RYR2, *S100A8, *S100A9, *S100A12, *SEMA3A, *TFAP2A, *TGFBI, *TNF‐α (*TNFRSF1A, *TNFRSF1B) |
Secondary review endpoints
Forty‐four markers were tested for possible correlations with other study endpoints as defined by the authors. The largest subgroup (n = 15) of these analyses consisted of associations between the different markers. The morphologic characteristics of IA (size and irregularity) were addressed for 13 molecular/histological parameters. Nine diagnostic markers were evaluated with regard to brain imaging (MRI and CFD). Among other analyzed endpoints, the data on associations with aspirin intake, smoking history, carbon birth dating and time passed since IA clipping were also collected. The detailed information on the associations with the above‐mentioned secondary endpoints is reported in Table S6 in Supporting Information.
DISCUSSION
Development and rupture of IA presents a cascade of pathophysiological processes quasi documented in the IA wall. In this systematic review, we identified over 350 diagnostic markers analyzed in the IA wall, and described over 20 associated functional pathways. Depending on their over‐ or under‐expression in IA (vs. nIA) and RIA (vs. UIA), we classified the markers and the linked pathways into according categories. The presented results contribute to a better understanding of IA pathophysiology and outline future perspectives for clinical and experimental researches on IA.
Clinical value of the diagnostic markers in the IA wall
We identified a huge number of histological and molecular targets addressed by the researchers in the IA wall. For the clarification of their functional background and clinical relevance, this plethora of IA tissue markers necessitates an effect‐oriented systematization. In accordance with the relation to the primary review endpoints, the markers were categorized into four groups. The diagnostic markers in the Groups 1 and 2 reflect the process of transformation from healthy arterial wall to IA. The diagnostic targets most likely contributing to further progress and rupture of IA are summarized in the Group 4. In contrast, the Group 3 includes the markers, which might be linked with reparative processes counteracting IA rupture.
The functional role of the reported diagnostic markers in the process of IA genesis is of paramount importance. Further improvements of brain imaging tools might enable a non‐histological (non‐invasive) identification of the relevant tissue markers in the vessel wall to detect the rupture‐prone IA. The categorization of markers into the specific groups, as proposed in our manuscript, might serve as a kind of “traffic light” for simple distinction between the histological and molecular markers reflecting the destructive or reparative processes during IA formation.
Functional pathways contributing to IA formation
Alongside with the assessment of the associations between the markers and review endpoints, we also looked for different functional pathways related to the investigated diagnostic targets. The majority of the diagnostic markers (nearly 80%) were referred to more than one functional group.
The inflammatory markers (n = 111) presented the largest group in our review. This is in line with the current knowledge and theories on basically inflammation‐mediated IA genesis 8, 13, 21. Inflammatory response is a common reaction on any local tissue injury 6. Hemodynamic stress is considered the major trigger of inflammatory processes in the arterial wall, starting with the infiltration of inflammatory cells: monocytes, neutrophils, lymphocytes and, probably, mast cells 19. Here, they undergo further differentiation and synthesize the inflammatory cytokines and immunoglobulins, as well as activate the complement system. Moreover, vascular inflammation contributes to the alteration of the endothelial function, disruption of the internal elastic lamina and collagen matrix, vasa vasorum activation and proliferation 6, 8. Therefore, inflammation promotes other pathophysiologic mechanisms involved in IA formation like oxidative stress, cell adhesion, intraluminal thrombosis and apoptosis, which in turn, facilitate vascular inflammation leading to a kind of vicious circle 6, 8, 9. In summary, these processes prompt irreversible anatomical remodeling and functional alteration of the damaged vessel, eventually resulting in IA rupture 1.
Along with IA formation, vascular inflammation in large vessels contributes to other vasculopathies. In particular, atherosclerosis is considered not only a disorder of lipid accumulation in the arterial wall, but also as a consequence of the chronic vascular inflammation 25. Our review found that many histological and molecular markers in the IA wall are also involved in atherosclerosis formation. Apparently, inflammatory processes responsible for progression of atherosclerosis largely overlap with those underlying IA formation 2. At the same time, because of different triggers and target layers in the arterial wall, vascular inflammation leads to distinct consequences during IA and atherosclerosis formation (weakening of the vessel wall with further dilatation and rupture or progressive luminal stenosis and occlusion, respectively) 20.
Alongside with the above‐mentioned pathways widely acknowledged as IA contributors, several less IA‐common pathways summarized in our review are worth mentioning. In particular, molecular markers related to antimicrobial activity were more characteristic for RIA, than for UIA. The potential role of microbial exposure on the development of IA, probably via microbiome‐triggered inflammation, has already been discussed in the literature 12. Another association of IA‐related markers was found in the pathway(s) linked to tyrosine kinase activity. These enzymes are responsible for the phosphorylation of tyrosine residues in proteins and have been reported to be involved in cellular transformation processes and fibroblast activity 17. Moreover, our manuscript highlights interactions of the IA markers with hormonal pathways, especially in those related to estrogen metabolism. This finding supports the previous clinical and experimental data on the impact of female sex hormones on formation, growth and rupture of IA 4.
In addition, the relation between the functional pathways and rupture status of IA is of great interest. In particular, UIA samples were enriched with the markers related to the extracellular matrix remodeling/cytoskeleton regulation, calcium ion channels, proliferation and cell growth, as well as wound healing. The knowledge of these pathways is of paramount importance, since they might explain the pathophysiologic background of reparative processes in the IA wall increasing its stability. And vice versa, the highly RIA‐typical pathways (such as antimicrobial activity, oxidative stress, iron binding and transport, inflammatory and apoptosis) outline the mechanisms for critical vessel wall damage leading to IA rupture. It can be assumed that the fate of rupture or non‐rupture of IA depends on the balance between the above‐mentioned opposing processes occurring in the IA wall. In Figure 3, we summarize the current evidence on the causative factors, functional pathways and resulting pathophysiologic processes related to IA formation 2, 3, 7, 8, 10, 13, 21, 22.
Figure 3.
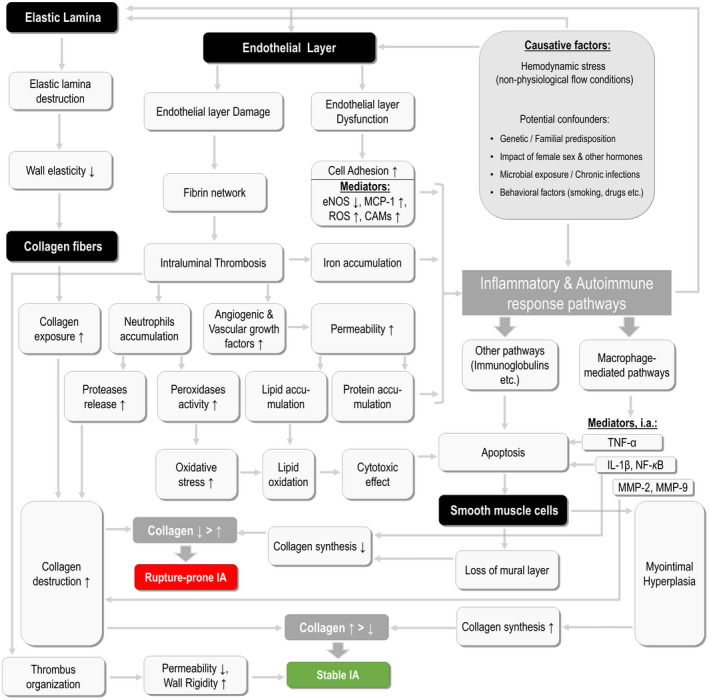
Schematic presentation of the major pathophysiological processes and causative factors involved in intracranial aneurysm (IA) development. This model summarizes the current evidence on the etiologies and consecutive mechanisms leading to IA formation, as well as shows the processes determining the rupture of IA. The anatomic targets within the arterial wall are highlighted with black background, whereat the processes are shown with gray background. Abbreviations: eNOS = endothelial nitric oxide synthase, MCP‐1 = monocyte chemotactic protein 1, ROS = reactive oxygen species, CAMs = cell adhesion molecules, TNF‐α = tumor necrosis factor alpha, IL‐1β = interleukin 1 beta, NF‐kB = nuclear factor kappa b, MMP‐2/‐9 = matrix metalloproteinase‐2/‐9.
The inhibition or excitation of these pathways might provide a basis for non‐invasive (pharmaceutical) treatment of IA. Some therapeutic substances have already been tested experimentally in animal models of IA 18, but their clinical implication is rather the matter of future researches. To date, there is a prospective multi‐centric randomized open‐label clinical trial (PROTECT‐U) trying to assess the protective effects of aspirin intake and a strict blood pressure measurement on the risk of growth and rupture of IA 23.
Study limitations
This manuscript is the first systematic review of the current evidence on all histological and molecular markers assessed on the human IA samples. Unfortunately, it reveals the limited quality of the data for the majority of the tested markers. The results presented must be interpreted with a certain a sense of proportion. Many of the markers with potential clinical relevance should be re‐evaluated prospectively on larger patient cohorts.
CONCLUSIONS
The presented results contribute to a better understanding of IA pathophysiology. Multiple functional pathways involved in the development of IA may present promising targets for future diagnostic evaluations and therapeutic interventions. Because of the high prevalence of monocentric studies, addressing different markers on a small numbers of IA samples, the summary data lacks proper integrality and is limited as a consequence of a low level of evidence. This stresses the urge need for a multi‐centric prospective histological IA register for pooled data analysis.
CONFLICT OF INTERESTS
None.
Supporting information
Table S1. The summary on the search terms used in each academic library.
Table S2. Quality assessment score.
Table S3. Estimation of the Level of Evidence for histological biomarkers.
Table S4. The full list of the studies included to the systematic review.
Table S5. The full list of the functional pathways with the appropriate prevalence for each study endpoint arm.
Table S6. The full list of associations between the histological markers and secondary review endpoints.
Table S7. The full list of histological markers and their abbreviations.
DATA AVAILABILITY STATEMENT
The data that support the findings of this study are available under the following link: https://figshare.com/articles/SUPPLEMENTARY_TABLES_E1-E7/11854356.
References
- 1. Ammirati E, Moroni F, Pedrotti P, Scotti I, Magnoni M, Bozzolo EP et al (2014) Non‐invasive imaging of vascular inflammation. Front Immunol 5:399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Chalouhi N, Ali MS, Jabbour PM, Tjoumakaris SI, Gonzalez LF, Rosenwasser RH et al (2012) Biology of intracranial aneurysms: role of inflammation. J Cerebr Blood Flow Metab 32:1659–1676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Chalouhi N, Hoh BL, Hasan D (2013) Review of cerebral aneurysm formation, growth, and rupture. Stroke 44:3613–3622. [DOI] [PubMed] [Google Scholar]
- 4. Desai M, Wali AR, Birk HS, Santiago‐Dieppa DR, Khalessi AA (2019) Role of pregnancy and female sex steroids on aneurysm formation, growth, and rupture: a systematic review of the literature. Neurosurg Focus 47:E8. [DOI] [PubMed] [Google Scholar]
- 5. Etminan N, Brown RD Jr, Beseoglu K, Juvela S, Raymond J, Morita A et al (2015) The unruptured intracranial aneurysm treatment score: a multidisciplinary consensus. Neurology 85:881–889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Fisher CL, Demel SL (2019) Nonsteroidal anti‐inflammatory drugs: a potential pharmacological treatment for intracranial aneurysm. Cerebrovas Dis Extra 9:31–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Frosen J (2014) Smooth muscle cells and the formation, degeneration, and rupture of saccular intracranial aneurysm wall–a review of current pathophysiological knowledge. Transl Stroke Res 5:347–356. [DOI] [PubMed] [Google Scholar]
- 8. Frosen J, Cebral J, Robertson AM, Aoki T (2019) Flow‐induced, inflammation‐mediated arterial wall remodeling in the formation and progression of intracranial aneurysms. Neurosurg Focus 47:E21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Frösen J, Tulamo R, Heikura T, Sammalkorpi S, Niemelä M, Hernesniemi J et al (2013) Lipid accumulation, lipid oxidation, and low plasma levels of acquired antibodies against oxidized lipids associate with degeneration and rupture of the intracranial aneurysm wall. Acta Neuropathol Commun 1:71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Frosen J, Tulamo R, Paetau A, Laaksamo E, Korja M, Laakso A et al (2012) Saccular intracranial aneurysm: pathology and mechanisms. Acta neuropathologica 123:773–786. [DOI] [PubMed] [Google Scholar]
- 11. Greving JP, Wermer MJ, Brown RD Jr, Morita A, Juvela S, Yonekura M et al (2014) Development of the PHASES score for prediction of risk of rupture of intracranial aneurysms: a pooled analysis of six prospective cohort studies. Lancet Neurol 13:59–66. [DOI] [PubMed] [Google Scholar]
- 12. Hallikainen J, Lindgren A, Savolainen J, Selander T, Jula A, Narhi M et al (2019) Periodontitis and gingival bleeding associate with intracranial aneurysms and risk of aneurysmal subarachnoid hemorrhage. Neurosurg Rev [Epub ahead of print; doi: 10.1007/s10143-019-01097-1]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Hosaka K, Hoh BL (2014) Inflammation and cerebral aneurysms. Transl Stroke Res 5:190–198. [DOI] [PubMed] [Google Scholar]
- 14. Ikedo T, Kataoka H, Minami M, Hayashi K, Miyata T, Nagata M et al (2019) Sequential inward bending of arterial bifurcations is associated with intracranial aneurysm formation. World Neurosurg 129:e361–e366. [DOI] [PubMed] [Google Scholar]
- 15. Jabbarli R, Dinger TF, Darkwah Oppong M, Pierscianek D, Dammann P, Wrede KH et al (2018) Risk factors for and clinical consequences of multiple intracranial aneurysms: a systematic review and meta‐analysis. Stroke 49:848–855. [DOI] [PubMed] [Google Scholar]
- 16. Jabbarli R, Pierscianek D, Rolz R, Darkwah Oppong M, Kaier K, Shah M et al (2019) Endovascular treatment of cerebral vasospasm after subarachnoid hemorrhage. Neurology 93:e458–e466. [DOI] [PubMed] [Google Scholar]
- 17. Radha V, Nambirajan S, Swarup G (1996) Association of Lyn tyrosine kinase with the nuclear matrix and cell‐cycle‐dependent changes in matrix‐associated tyrosine kinase activity. Eur J Biochem 236:352–359. [DOI] [PubMed] [Google Scholar]
- 18. Sawyer DM, Amenta PS, Medel R, Dumont AS (2015) Inflammatory mediators in vascular disease: identifying promising targets for intracranial aneurysm research. Mediators Inflammation 2015:896283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Signorelli F, Sela S, Gesualdo L, Chevrel S, Tollet F, Pailler‐Mattei C et al (2018) Hemodynamic stress, inflammation, and intracranial aneurysm development and rupture: a systematic review. World Neurosurg 115:234–244. [DOI] [PubMed] [Google Scholar]
- 20. Syed MBJ, Fletcher AJ, Dweck MR, Forsythe R, Newby DE (2019) Imaging aortic wall inflammation. Trends Cardiovasc Med 29:440–448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Tulamo R, Frosen J, Hernesniemi J, Niemela M (2018) Inflammatory changes in the aneurysm wall: a review. J NeuroInterv Surg 10(Suppl. 1):i58–i67. [DOI] [PubMed] [Google Scholar]
- 22. Turjman AS, Turjman F, Edelman ER (2014) Role of fluid dynamics and inflammation in intracranial aneurysm formation. Circulation 129:373–382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Vergouwen MD, Rinkel GJ, Algra A, Fiehler J, Steinmetz H, Vajkoczy P et al (2018) Prospective randomized open‐label trial to evaluate risk factor management in patients with unruptured intracranial aneurysms: study protocol. Int J Stroke 13:992–998. [DOI] [PubMed] [Google Scholar]
- 24. Wiebers DO, Whisnant JP, Huston J 3rd et al (2003) Unruptured intracranial aneurysms: natural history, clinical outcome, and risks of surgical and endovascular treatment. Lancet 362:103–110. [DOI] [PubMed] [Google Scholar]
- 25. Yamashita T, Sasaki N, Kasahara K, Hirata K (2015) Anti‐inflammatory and immune‐modulatory therapies for preventing atherosclerotic cardiovascular disease. J Cardiol 66:1–8. [DOI] [PubMed] [Google Scholar]
- 26. Zhang X, Yao ZQ, Karuna T, He XY, Wang XM, Li XF et al (2019) The role of wall shear stress in the parent artery as an independent variable in the formation status of anterior communicating artery aneurysms. Eur Radiol 29:689–698. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1. The summary on the search terms used in each academic library.
Table S2. Quality assessment score.
Table S3. Estimation of the Level of Evidence for histological biomarkers.
Table S4. The full list of the studies included to the systematic review.
Table S5. The full list of the functional pathways with the appropriate prevalence for each study endpoint arm.
Table S6. The full list of associations between the histological markers and secondary review endpoints.
Table S7. The full list of histological markers and their abbreviations.
Data Availability Statement
The data that support the findings of this study are available under the following link: https://figshare.com/articles/SUPPLEMENTARY_TABLES_E1-E7/11854356.


