Abstract
Recent studies have demonstrated that the brain is equipped with a lymphatic drainage system that is actively involved in parenchymal waste clearance, brain homeostasis and immune regulation. However, the exact anatomic drainage routes of brain lymph fluid (BLF) remain elusive, hampering the physiological study and clinical application of this system. In this study, we systematically dissected the anatomy of the BLF pathways in a rat model. Moreover, we developed a protocol to collect BLF from the afferent lymphatic vessels of deep cervical lymph nodes (dcLNs) and cerebrospinal fluid (CSF) from the fourth ventricle. Nuclear magnetic resonance spectroscopy showed that BLF contains more metabolites than CSF, suggesting that BLF might be a more sensitive indicator of brain dynamics under physiological and pathological conditions. Finally, we identified several metabolites as potential diagnostic biomarkers for glioma, Parkinson's disease and CNS infectious diseases. Together, these data may provide insight into the physiology of the lymphatic system in the brain and into the clinical diagnosis of CNS disorders.
Keywords: brain lymph fluid (BLF), glioma, metabolome, nuclear magnetic resonance (NMR), Parkinson's disease (PD)
Introduction
The lymphatic system maintains immune surveillance 8, 11, 35 and tissue homeostasis via the recycling of interstitial fluid (ISF) 1, 28. For a long time, the brain was considered to lack a dedicated and conventional lymphatic system. Recently, emerging evidences have demonstrated that the brain has a lymphatic drainage system that is actively involved in parenchymal waste clearance, brain homeostasis and immune regulation 3, 37, 38. The lymphatic drainage system in the brain consists of a brain‐wide glymphatic (glial‐lymphatic) pathway, which includes the periarterial and perivenous spaces and glymphatic CSF‐ISF flow in the brain parenchyma, and cerebrospinal fluid (CSF) drainage routes, which include dural‐sinus‐lined meningeal lymphatic vessels and olfactory/cervical lymphatic routes. In the glymphatic pathway, CSF flows into the brain along periarterial spaces and subsequently into the brain interstitium, carrying metabolic products from ISF; then, CSF flows toward the perivascular spaces 22, 23, 46, 47, 58. In the CSF drainage routes, CSF is transported mainly to meningeal lymphatic vessels or to perineural spaces across the cribriform plate leading to the nasal mucosa 4, 13, 27, 29, 39, 41 and subsequently into cervical lymph nodes 3, 38, 42. These findings confirmed the presence of lymphatic vasculature in the brain, which serves as a draining route from brain ISF to peripheral lymph nodes. However, the exact anatomic drainage routes of brain lymph fluid (BLF) from cranial bone to peripheral lymph nodes need to be more precisely characterized.
The brain can produce different metabolic products under physiologic and pathological conditions, such as neurodegeneration, tumors and neuroinflammatory diseases initiated by neurotropic viruses or bacteria such as tubercle bacillus. These metabolic waste products need to be efficiently eliminated to maintain proper functioning in the brain. Some differentially produced metabolites have been considered diagnostic biomarkers 19, 54, 55. In most organs, venous return is a major drainage route for metabolic products. In the case of the brain, the blood–brain barrier (BBB), which separates circulating blood cells and solutes from the brain parenchyma and ISF 5, 16, makes venous blood in the brain only a component of micromolecular metabolites. Thus, CSF, instead of venous blood in the brain, has been widely considered a major diagnostic target for brain diseases 7, 30, 33, 59. Owing to the great permeability of lymphatic vessels, it is reasonable to hypothesize that there are more brain metabolites in BLF than in CSF, and brain metabolites in BLF vary dynamically and may reflect more accurate physiological and pathological conditions of the brain. Therefore, monitoring the BLF of brain metabolites provides new insight into the clinical diagnosis of central nervous system (CNS) diseases.
The aim of this study is, therefore, to systematically dissect the brain lymph drainage routes to cervical lymph nodes and probe the potential novel diagnostic biomarkers from BLF for CNS diseases.
Materials and Methods
Animals
Male Sprague Dawley rats (weighing over 220 g) were purchased from Hubei Disease Control and Prevention Center and were housed in temperature‐ and humidity‐controlled rooms with a 12/12 h light/dark cycle (lights on at 7:00 am). All procedures were performed by following the regulations of the Institutional Animal Care and Use Committee of Huazhong Agricultural University (ethical review number: HZAURA‐2015‐007, approved by The Scientific Ethic Committee of Huazhong Agricultural University). Animals in the same experimental group were selected from different cages to assure randomization.
Animal anesthesia
Compound anesthetic agents were used for anesthetizing animals. Briefly, 10% urethane and 2% chloral hydrate were mixed with 3 mg/mL xylazine (Sigma‐Aldrich, USA) at a ratio of 3:1, diluted with ddH2O and stored at 4°C in the short term or −20°C in the long term. For a short operation period (less than 5 min), isoflurane was used instead of compound anesthesia.
Evans blue injection
Rats were anesthetized with the compound anesthetic agent (0.9 mL/100 g, i.p.), and then 10 or 20 μL of 10% Evans blue (Sigma‐Aldrich, USA) was injected into the left lateral ventricle (AP: −0.95, ML: −1.9, DV: 3.8) using a stereotaxic apparatus (RWD, China). Thirty minutes after injection, facial and cervical skin were removed.
BLF sample collection
For BLF sample collection, cervical skin was incised, and muscles were separated bluntly to expose deep cervical lymph nodes. In conjunction with the circulation of Evans blue, brain‐related lymphatic vessels were dyed to distinguish them from other vessels. The afferent vessels of the dcLNs were selected for the BLF sample collection. With the help of a needle hook, a single lymphatic vessel was isolated and cut to collect 30–100 μL of BLF. A 100 μL microsyringe (Gaoge, China) was used to collect the BLF.
CSF sample collection
The CSF was collected by making a skin incision between the ears, and blunt dissection was performed at the base of the cranial muscles to expose the foramen magnum. Stroke‐physiological saline solution (0.9% NaCl) was used to wash blood away from the foramen magnum. A 1 mL microsyringe was used to penetrate the fourth cerebral ventricle through the foramen magnum at an angle of 20–30 degrees in the horizontal plane for collection of 100 μL of CSF.
Glioma animal model
Rats were anesthetized with compound anesthetic agent (0.9 mL/100 g, i.p.) and mounted on a stereotaxic frame. A total of 104 C6 glioma strain cells (Hualianke Company, China) were injected into the right striatum at three sites (AP: 0.95, ML: 2.6, DV: 3.5), (AP: 0.95, ML: 2.6, DV: 4.0) and (AP: 0.95, ML: 2.6, DV: 4.5) through a 10 μL microsyringe (Gaoge, China) at a rate of 0.5 μL/min. Then, the same amount of PBS was injected into the counterpart regions of control rats. After the infusion, the microsyringe was left at the target site for an additional 10 min to allow diffusion. At 21 days postoperation, careful attention was paid to monitoring the pathological status of the rats, and weights were documented each day. When the rats appeared weak and their weight dropped significantly (over 10 g per day), BLF was collected immediately. After collection, the rats were perfused with PBS and 4% PFA. Then, the brains were extracted and fixed in 4% PFA overnight. The success of glioma model construction was further confirmed by the appearance of glioma tissue in the striatum by brain paraffin sectioning and H&E staining.
Virus infection model
Rats were anesthetized by isoflurane inhalation (RWD, China). A GFP‐expressing pseudorabies virus 152 (PRV152) was injected intramuscularly into the forelimbs, with 10 μL (virus titer 10E8) injections on each side. Then, the same amount of PBS was injected into the counterpart region of control rats. At 120 h postinjection, the rats were anesthetized with a compound anesthetic agent (0.9 mL/100 g, i.p.) for BLF collection. Then, rats were perfused with PBS and 4% PFA. Brains were extracted and fixed in 4% PFA.
Parkinson's disease animal model
Following anesthetization of rats with the compound anesthetic agent (0.9 mL/100 g, i.p.), 6‐OHDA was used to construct the PD model via intracerebral injection at three sites (AP: 0.95, ML: 2.6, DV: 3.5), (AP: 0.95, ML: 2.6, DV: 4.0) and (AP: 0.95, ML: 2.6, DV: 4.5) into the right striatum. A total of 10 μL 6‐OHDA (1 μg/μL; Sigma, USA) in 0.9% NaCl solution with 0.02% ascorbic acid was used for infusion into the brain via a 10 μL microsyringe at a rate of 0.5 μL/min. The same amount of PBS was injected into the counterpart regions of control rats. After infusion, we left the microsyringe at the target site for an additional 10 min to allow diffusion. Three weeks after the injection, the effectiveness was assessed using the test of contralateral rotational behavior induced by apomorphine (0.5 mg/kg; i.m.) dissolved in 0.9% NaCl solution at a concentration of 1 mg/mL. The lesion was considered successful if the rat made over 210 rotations per 30 min in the contralateral direction. The rats with successful lesions were used for lymphatic fluid collection. After collection, the rats were perfused with PBS and 4% PFA, and the brains were extracted for fixation in 4% PFA.
Sample preparation for NMR and 1H NMR spectroscopy
BLF and CSF samples (approximately 30 µl) were separately mixed with 30 µL of phosphate buffer solution for NMR analysis. The phosphate buffer (45 µM) prepared by dissolving K2HPO4/NaH2PO4≈4:1 into 0.9% NaCl deuterated water (D2O) was used to lock the frequency of the spectrometer during sample acquisition. All the NMR experiments were conducted on a Bruker Avance III 600 MHz NMR spectrometer equipped with an inverse detection cryoprobe (Bruker Biospin, einstetten, Germany) at 298 K. The Carr–Purcell–Meiboom–Gill pulse program ((RD−90°− (τ−180°−τ)n‐acquisition); τ = 350 μs, n = 100) with water suppression pulse sequences was used to suppress NMR signals from macromolecules with a shorter T2 relaxation time and to retain signals from low‐molecular weight metabolites with longer T2 relaxation times. Each BLF and CSF spectrum with a spectral width of 20 ppm contained 128 scans, and each scan consisted of 32 k data points. For the purpose of signal assignments, a set of two‐dimensional NMR spectra, 1H–1H correlation spectroscopy (COSY), 1H–1H total correlation spectroscopy (TCOSY), 1H J‐resolved, 1H–13C heteronuclear single quantum correlation (HSQC) and 1H–13C multiple bond correlation spectra (HMBC), were recorded as described in a previous study 14, 15, 57.
NMR spectrum processing and statistical analysis
The processing of all NMR spectra was conducted with Topspin software. Zero‐filled to 128 k points and exponential widow functions with 1 Hz line‐broadening were applied to all spectra prior to Fourier transformation, followed by manual phase and baseline adjustments. The spectral regions of 0.8–8.8 ppm were integrated into bins of 0.002 ppm with Amix software. The processed data were further exposed to Multiple Univariate Data Analysis (MUDA) using MATLAB script. A previous study described the detailed MUDA analysis 18. In short, the MUDA analysis was based on either Student's t‐test or nonparametric tests. The difference in metabolite concentrations in the groups is presented in a differential metabolome plot, in which statistically significant metabolites (P < 0.05) are colored red.
Sample preparation and H&E staining
All rat brains were fixed in 4% PFA, following the standard principles for dehydration, waxing, and embedding of brain samples. The tissue sections were prepared with an Ultra‐Thin Semiautomatic Microtome (Leica, Germany) or a freezing microtome (Leica, Germany). Brain slices were 4 μm thick for paraffin sections and 20 μm thick for frozen sections. To verify the construction of each model, H&E staining was performed.
Immunohistochemistry and immunofluorescence
Sections were baked in a 37°C dryer for 12 h and in a 60°C dryer for 1 h. Sections were then deparaffinized in xylene and rehydrated in descending dilutions of ethanol. For paraffin‐embedded sections, we performed antigen retrieval procedures in the microwave, with circulation for 2 min under moderate heat and 8 min at room temperature for a total of 3 times. Slices were washed with PBS and blocked with 2% BSA at room temperature for 1 h. Anti‐tyrosine hydroxylase (TH) (ab6211) antibody was used to confirm the decrease in TH‐positive neurons in the right striatum. Briefly, 1:400 diluted primary antibody was added to the slices and incubated overnight at 4°C. Biotinylated anti‐rabbit IgG was used as a secondary antibody, and DAB substrate was used for staining these sections. Anti‐LYVE1 primary antibody (Abcam, USA) was used to confirm the lymphatic vessel characteristics of cervical LN‐related ducts. Then, 1:400 diluted anti‐LYVE1 was added to the ducts and incubated overnight at 4°C. A 1:300 diluted secondary antibody (Alexa Fluor 488, Thermo Fisher Scientific, USA) was then incubated for 2 h at room temperature. After secondary antibody washing, tissue sections were mounted with 50% glycerol. Immunofluorescence images were taken by fluorescence microscopy (Olympus, Japan).
Results
Dissection of the BLF drainage anatomic routes from the cranium to dcLNs and scLNs
To systemically delineate the BLF drainage anatomic routes from the cranium to cervical lymph nodes, different amounts of Evans blue were injected into the lateral ventricle to optimize the visualization of BLF drainage (Figure 1A) (n = 3). We found that waiting 30 min after injection of 10 μL Evans blue was enough time to visualize the bilateral deep cervical lymph nodes (dcLNs) and related vessels (Figure 1B) (n = 5). For superficial cervical lymph nodes (scLNs), the optimal injection condition was 20 μL Evans blue, as the scLNs remained gray or slightly blue with an injection of 10 μL Evans blue (Figure 1C) (n = 5). Figure 1B,D shows that dcLN‐ and scLN‐related BLF vessels obviously extended into separate pathways. dcLN‐related interlacing afferent ducts arose near the internal jugular vein, stretched along the trachea in the neck and medial pterygoid bone in the base of the skull, and eventually exited via foramina at the base of the skull (Figure 1B). scLN‐related afferent ducts extended along the masseter muscle under the facial and cervical skin. A portion of scLN ducts crossed through the infra‐orbital foramen, while the others exited via the nasal cavities. Finally, both ducts traveled in a retrograde fashion to the cribriform plate at the front of the base of the skull (Figure 1E). The integrated BLF drainage routes from the cranium to dcLNs and scLNs are illustrated in Figure 1F.
Figure 1.
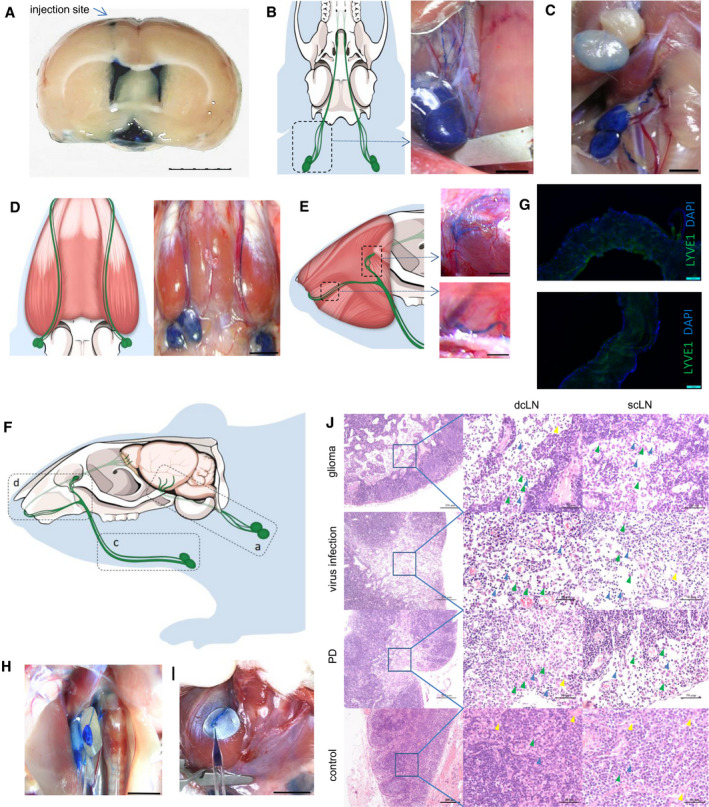
Anatomical structures of rat BLF drainage routes to cervical lymph nodes. A, Evans blue was injected into the lateral ventricle to optimize the visualization of BLF drainage.(n = 3) Bar: 5 mm. B, 30 min after, 10 μL of 10% Evans blue was injected into the lateral ventricle, dcLNs and their afferent vessels were dyed blue, whereas scLNs and their vessels remained gray or slightly blue C. (n = 5) Bars: 2 mm. D, 30 min after injection of 20 μL Evans blue, scLNs and their afferent vessels became dark blue after dcLNs. (n = 5) Bar: 5 mm. E, A portion of scLN afferent vessels crossed through the infraorbital foramen, while the others exited via the nasal cavities. Bars: 2 mm. F, Schematic diagram of rat BLF drainage routes from the cranium to cervical lymph nodes. G, Immunofluorescence of dcLN‐ and scLN‐related afferent vessels for LYVE‐1. Bars: 100 μm. H, Collection of BLF from dcLN afferent vessels. Bar: 5 mm. I, Collection of BLF from scLN afferent vessels. Bar: 5 mm. J, Both dcLNs and scLNs connected to brain lymphatic vessels appeared to have pathological changes in brain glioma, virus infection and PD models, including the expansion of the subcapsular sinus, increasing of macrophages and lymphocytes, decreasing of desmocytes in the subcapsular sinus. Green arrowheads: macrophages, blue arrowheads: lymphocytes, yellow arrowheads: desmocytes Bars: 200 μm and 50 μm.
The lymphatic characteristics of dcLN‐ and scLN‐related afferent vessels were further examined by assessing the presence of the classic lymphatic vessel marker, lymphatic vessel endothelial hyaluronan receptor 1 (LYVE‐1). Immunofluorescence staining of the vessels showed that LYVE‐1‐positive lymphatic vessels drained BLF from the cranium to cervical lymph nodes (Figure 1G). Moreover, we developed a protocol to collect original BLF before dcLNs to avoid the degradation of brain metabolites in lymph nodes, as shown in Figure 1H. By taking advantage of this strategy, we collected, to our knowledge, the first BLF sample from dcLNs. The BLF with Evans blue dye could be collected from the afferent vessels of the dcLNs (Figure 1H) and scLNs (Figure 1I). These data further support that the identified vessels are indeed BLF drainage routes.
It is conceivable that pathological brain conditions (such as glioma) could influence the pathophysiological condition of dcLNs and scLNs, as the first lymph nodes of BLF drainage. To test this hypothesis, we established a rat glioma animal model by injecting C6 glioma cells in the rat striatum and investigated the morphology of dcLNs and scLNs. Both dcLNs and scLNs connected to brain lymphatic vessels appeared to have pathological changes (Figure 1J), such as an expansion of the subcapsular sinus, an increasing number of macrophages and lymphocytes, as well as a decreasing number of desmocytes, in the subcapsular sinus. Similar LNs pathological changes were observed under virus infection and PD conditions. For LNs in PD model, the expansion of the subcapsular sinus were smaller than that of the glioma and virus models, probably duo to PD progressed slowly and caused less inflammation in LNs. These data further indicate that BLF can drain towards dcLNs and scLNs. Notably, we observed that the dcLN pathway might be the first relay of BLF, as dcLNs became blue prior to scLNs, although both are associated with the lymphatic drainage system in the brain and are active in response to brain disorders.
Comparison of Metabolites in BLF and CSF
As dcLNs were demonstrated as the first relay of BLF, we collected 30 μL or more BLF from the afferent vessels of the dcLNs (n = 8), as well as the same volume of CSF from the fourth ventricle (n = 9), and these samples were subsequently subjected to NMR analysis. A total of 31 metabolites were identified from the NMR spectra in BLF (Figure 2A, Table S1), and these metabolites were further validated by various 2D NMR spectra. The spectra of the BLF contained signals from glucose, organic acids (eg, lactate, acetoacetate and N,N‐dimethylglycine), amino acids (eg, alanine, leucine, valine, isoleucine, lysine, tyrosine, glycine, histidine, methionine and phenylalanine), glycoprotein (eg, O‐acetyl glycoprotein and N‐acetyl glycoprotein) and tricarboxylic acid (TCA) intermediates (citrate and succinate). Out of 31 metabolites, only 21 metabolites were detected in CSF (Figure 2B). Student's t‐test was used to evaluate the significance of difference in metabolites between CSF (n = 9) and BLF (n = 8). Twenty out of 21 metabolites in BLF had a significantly higher concentration than in CSF (P < 0.01) (Figure 2C). These NMR results showed that more metabolites could be detected in BLF than in CSF, suggesting that BLF was enriched with more metabolites and may more accurately reflect the pathophysiological status of the brain.
Figure 2.
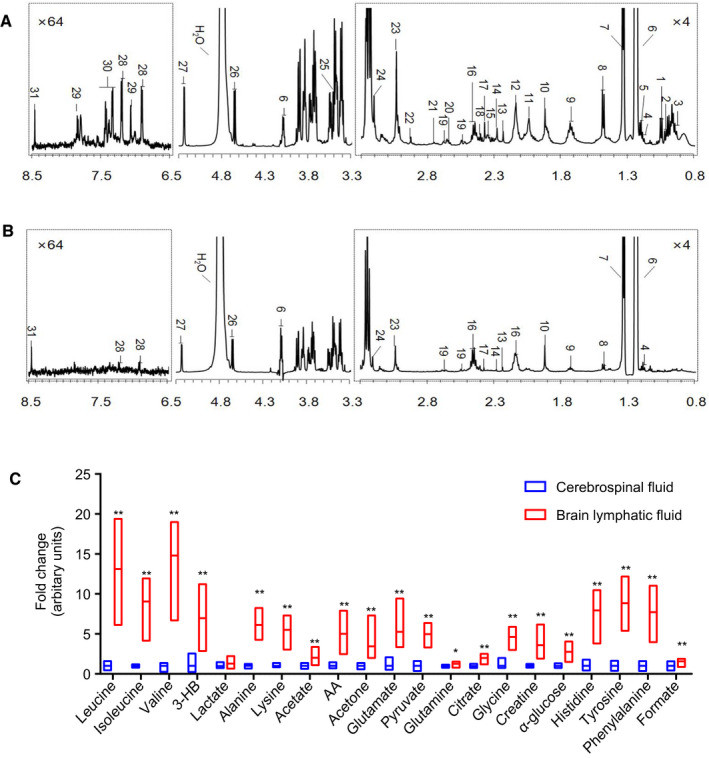
1H NMR spectra of BLF and CSF from healthy rats, and concentrations of metabolites in BLF and CSF. A, The spectrum of BLF. The spectral regions of δ 0.8‐3.3 and δ 6.5‐8.5 were amplified 4 and 64 times, respectively, compared with the region of δ 3.3‐5.3. A total of 31 metabolites were identified from the NMR spectra in BLF. (n = 8). B, The spectrum of CSF. A total of 21 metabolites were detected in CSF. Metabolite keys are listed in Table S1. (n = 9). C, The relative concentration difference in metabolites between BLF and CSF from healthy rats, suggesting that BLF was enriched with more metabolites. Student's t‐test was used to evaluate the significance of difference in metabolites between CSF (n = 9) and BLF (n = 8). *P < 0.05, **P < 0.01.
BLF metabolome fluctuation under glioma conditions
Glioma is the most common malignant tumor of the central nervous system. To investigate the BLF metabolome of the brain in glioma, the rat brain glioma tumor model was established by injecting C6 cells into the right striatum (n = 8). As shown in Figure 3A,B, there were a number of glioma cells and lymphatic cells in the section of striatum of the brain, as shown by H&E staining. Next, we collected 30 μL BLF from the ipsilateral afferent lymphatic vessels of dcLNs and subjected the samples to NMR. Multiple univariate data analysis (MUDA) and Student's t‐test were used to evaluate the significance of difference in metabolite concentrations between control rats (n = 8) and glioma rats (n = 8). Figure 3C,D demonstrated that the concentrations of ten metabolites (lactate, pyruvate, 3‐hydroxybutyrate (3‐HB), N‐acetyl‐glycoprotein (N‐AG), O‐acetyl‐glycoprotein (O‐AG), acetone, acetoacetate (AA), creatine, α‐glucose and phenylalanine) were significantly different between glioma BLF samples and control samples. As shown in Figure 2C, among the 10 metabolites, 2 metabolites (N‐AG and O‐AG) could be detected in BLF but not in CSF. Among the remaining eight metabolites, seven metabolites (pyruvate, 3‐HB, acetone, AA, creatine, α‐glucose, phenylalanine) had significantly lower relative concentrations in CSF than in BLF. These ten metabolites might potentially be used as biomarkers for glioma diagnosis in the future.
Figure 3.
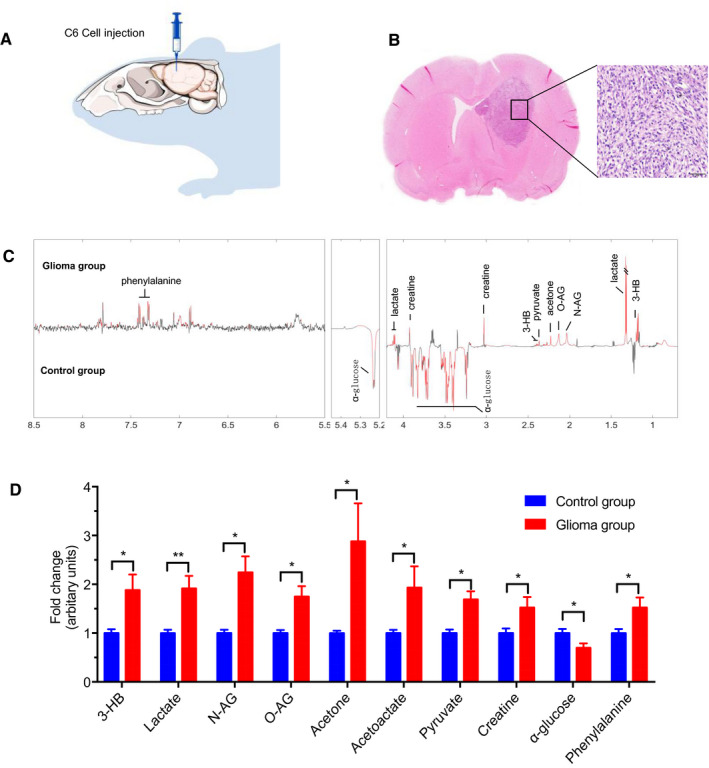
Brain morphologic changes and BLF metabolome fluctuations under glioma conditions. A, Injection of C6 glioma cells in the right striatum. (n = 8). B, Twenty‐two days after the injection, glioma tissue was found in the striatum with a number of glioma cells and lymphatic cells. Bar: 50 μm. C, Differential‐metabograms of BLF from control rats (n = 8) and glioma rats (n = 8). Metabolites with red color are significantly changed between groups. Multiple univariate data analysis (MUDA) was used to evaluate the significance of difference in metabolite concentrations between groups. D, Relative concentrations of ten metabolites (3‐hydroxybutyrate (3‐HB), lactate, N‐acetyl‐glycoprotein (N‐AG), O‐acetylglycoprotein (O‐AG), acetone, acetoacetate (AA), pyruvate, creatine, α‐glucose, and phenylalanine) showing significant differences between glioma BLF samples and control samples. Student's t‐test was used to evaluate the significance of difference in metabolites between two groups of samples. *P < 0.05, **P < 0.01.
BLF metabolic disturbances with viral infection
Next, we aimed to investigate the BLF metabolome during neurotropic viral infection. To this end, a neurotropic virus, the pseudorabies virus expressing GFP, PRV152, was injected into rat forelimbs as a neurotropic virus infection model 53 (n = 9). As shown in Figure 4A,B, the GFP fluorescence signal in hippocampus and cerebral cortex M1 zone neurons validated the successful infection of PRV152. Then, 30 μL of BLF was collected from the ipsilateral afferent lymphatic vessels of dcLNs and subjected to NMR analysis. Then MUDA and Student's t‐test were used to evaluate the significance of difference in metabolite concentrations between control rats (n = 10) and viral infection rats (n = 9). The NMR data demonstrated that two metabolic disturbances (dimethylamine (DMA), N,N‐dimethylglycine (DMG)) were significantly (P < 0.05) lower and Glutamine was significantly higher (P < 0.05) in the BLF of the viral infection group than in the BLF of the control group (Figure 4C,D). According to Figure 2C, two metabolites (DMA and DMG) were detected in BLF but not in CSF. While glutamine was also found in CSF, it was significantly lower (P < 0.01) than in BLF.
Figure 4.

BLF metabolic disturbances with viral infection. A, Virus infection models. A neurotropic virus, the pseudorabies virus expressing GFP, PRV152, was injected into rat forelimbs as a brain viral infection model. (n = 9). B, Four days after infection, infected neurons were observed in the hippocampus and cerebral cortex M1 zone. Bars: 100 μm. C, Differential‐metabograms of BLF from control rats (n = 10) and viral infection rats (n = 9). Metabolites with red color are significantly changed between groups. MUDA were used to evaluate the significance of difference in metabolite concentrations between groups. D, In the BLF of the viral infection group, dimethylamine (DMA) and N,N‐dimethylglycine (DMG) were significantly lower, while glutamine was significantly higher than that of the control group. Student's t‐test was used to evaluate the significance of difference in metabolites between control rats and viral infection rats. *P < 0.05.
BLF metabolome in Parkinson's disease (PD) models
To establish a PD model, 6‐hydroxydopamine (6‐OHDA) was injected into the right striatum (Figure 5A) (n = 7). The PD model was validated by the assay of contralateral rotational behavior induced by apomorphine, and it resulted in sharply decreased tyrosine hydroxylase (TH)‐positive neurons in the striatum 3 weeks after injection (Figure 5B,C). BLF was collected only from rats with a successful behavior assay. NMR, MUDA and Student's t‐test analysis showed that four brain metabolites (N‐AG, O‐AG, AA and α‐glucose) were significantly different between the PD model (n = 7) and control groups (n = 8) (Figure 5D). According to Figure 2C, N‐AG and O‐AG were detected in BLF but not in CSF. While α‐glucose and AA could also be detected in CSF, their concentrations were significantly lower (P < 0.01) than those in BLF. These four metabolites might potentially be used as biomarkers for PD diagnosis in the future.
Figure 5.
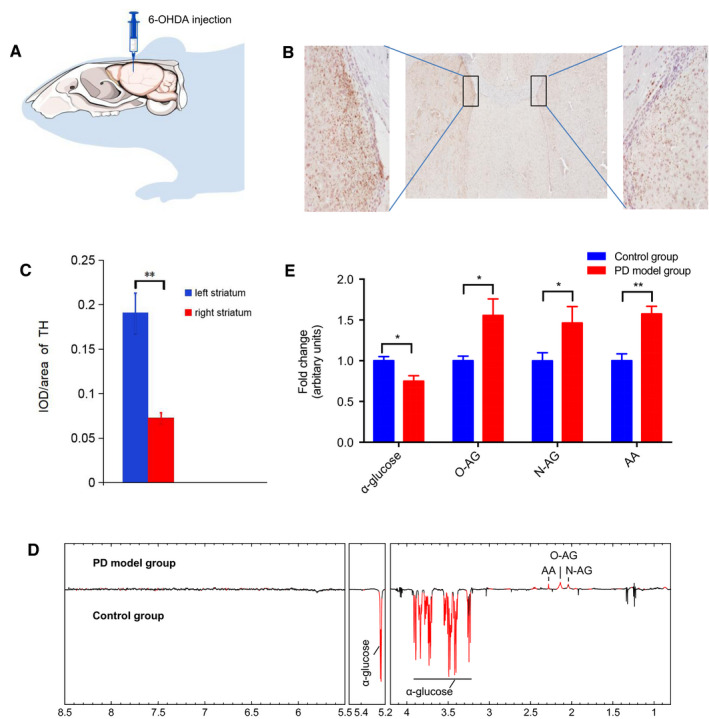
The BLF metabolome under the condition of Parkinson's disease. A, Brain infection with 6‐hydroxydopamine (6‐OHDA) to selectively destroy dopaminergic and noradrenergic neurons in the right striatum. (n = 7). B,C, 21 days after the infection, TH‐positive neurons decreased sharply in the right striatum compared to the left striatum. Bars: 200 μm. D, Differential‐metabograms of BLF from control rats (n = 8) and 6‐OHDA injection rats (n = 7). Metabolites with red color are significantly changed between groups. MUDA were used to evaluate the significance of difference in metabolite concentrations between groups. E, Relative change fold of significant metabolites (acetoacetate (AA), N‐acetylglycoprotein (N‐AG), O‐acetyl‐glycoprotein (O‐AG) and α‐glucose). Student's t‐test was used to evaluate the significance of difference in metabolites between between the PD group and control group. *P < 0.05, **P < 0.01.
Discussion
The extracellular fluid of the brain consists of CSF, which exists in the ventricles, subarachnoid space and ISF in the extracellular spaces of the brain parenchyma. While recent studies have established the concept that the glymphatic system carries brain metabolic products from ISF to CSF, it still remains elusive how exactly the CSF eliminates brain waste products. An obvious way that this elimination occurs is via dural sinuses, through which brain waste products are transported to the bloodstream. Another important elimination route is via brain lymphatic vessels that are located in the dura mater, which lines the dural sinuses 31, 38, or along the cranial nerves 2. Recently, experimental evidence has suggested that meningeal lymphatic vessels can even invade the brain parenchyma to guide vascular regeneration under cerebrovascular injury conditions 12. Therefore, brain lymphatic vessels play an important role in collecting brain metabolites, draining waste products into nearby lymph nodes 38 and helping the brain maintain homeostasis. Moreover, it has been demonstrated that brain lymphatic clearance dysfunction could participate in the pathogenesis of neurodegenerative diseases 10, 60. In line with this, we demonstrated that the BLF metabolomes from a PD model group and a control group were indeed different.
While recent evidence has demonstrated that the brain has a lymphatic drainage system for parenchymal waste clearance, brain homeostasis and immune regulation 3, 37, 38, the detailed anatomy of this pathway has not been systematically investigated. Previous studies showed that CSF was drained from the subarachnoid space to dcLNs but not to scLNs 3 by injecting 5 μL Evans blue i.c.v. into mice and PEG‐IRDye into the brain parenchyma of Prox1‐GFP mice. In our study, we demonstrated that both the bilateral dcLNs and scLNs were labeled with Evans blue dye 30 min after injection with 20 μL of Evans blue into the rat brain. This discrepancy could be due to the injection amount of the dyes. Anatomical findings and histological results have revealed that the inferior nasal turbinate contains numerous lymphatic tissues and is connected to the nasolacrimal duct ensheathed by a dense network of lymphatics 36. Furthermore, there is ample evidence suggesting that CSF drains via the cribriform plate into the nasal mucosa 24, 32, 56. Together, these results suggest the existence of lymphatic outflow routes of CSF (namely, BLF) in the nasal cavity. Notably, we showed that intranasal BLF subsequently drained into scLNs.
Our study systematically delineated the pathways of BLF drainage and demonstrated that this sophisticated lymphatic vasculature mainly comprised two main routes: (i) dural lymphatic vessels that flow from the dura mater via foramina at the base of the skull into dcLNs; and (ii) channels lining the olfactory nerves that deliver fluid into the nasal mucosa and then to scLNs. Our findings characterized the final step in BLF drainage of ISF from the brain parenchyma into peripheral LNs. We speculated that BLF was preferentially drained via the dcLN route, as the dcLN pathway became blue prior to scLN pathway after i.c.v. injection. scLN route might carry out a small amount of BLF as a supplementary pathway of BLF drainage under elevated intracranial pressure. The drainage of CSF is not only significant for volume regulation but also for the clearance of brain metabolic waste products. Accumulating studies have demonstrated that CSF dynamics can reflect many pathological conditions of the brain and are clinically applied for the diagnosis of many diseases, including hydrocephalus 17, 34, intracerebral hemorrhage 21, 51, idiopathic intracranial hypertension 45, 50, large hemispheric stroke 6, 40, subarachnoid hemorrhage 9, 43, traumatic brain injury 25, 26 and brain aging 49, 52. In fact, soluble amyloid beta‐protein (Aβ) and apolipoprotein E levels in CSF are significantly lower in patients with definite Alzheimer's disease (AD) 20, 44. Moreover, it has been shown that there is a substantial decrease in the levels of total secreted β‐amyloid precursor protein (sAPP), sAPP‐alpha (sAPPα) and Aβ1, 2, 3, 4, 5, 6, 7, 8, 9, 10, 11, 12, 13, 14, 15, 16, 17, 18, 19, 21, 22, 23, 24, 25, 26, 27, 28, 29, 30, 31, 32, 33, 34, 35, 36, 37, 38, 39, 40, 41, 42, 43 in the CSF of normal pressure hydrocephalus (NPH) patients who may develop AD‐like pathology 48. However, CSF contains only a small portion of the metabolites in the brain and thus is a great limitation to accurately reflecting the pathophysiological status of the brain.
Due to the great permeability of lymphatic vessels, BLF may accumulate more kinds of brain metabolites than CSF. Thus, BLF might have a better capacity than CSF to accurately reflect the pathophysiological status of the brain. In the current study, we developed a protocol to collect BLF from the afferent vessels of dcLNs and compared brain metabolites between BLF and CSF by NMR. Our data showed that 31 brain metabolites could be detected in BLF, whereas only 21 metabolites could be recognized in CSF, suggesting that nearly 48% more metabolites could be found in BLF than in CSF. For those brain metabolites detected in both BLF and CSF, approximately 95% of them had higher concentrations in BLF than in CSF. Together, these data suggest that BLF might contain more potential biomarkers than CSF for the diagnosis of brain diseases. Accordingly, we identified BLF creatine as a potential diagnostic biomarker for glioma. In line with this, brain parenchymal creatine has been used in the clinical diagnosis of glioma, further supporting the potential promise of this diagnostic approach 19, 54, 55. Moreover, we identified DMA, DMG and glutamine as potential diagnostic biomarkers for viral infection and N‐AG, O‐AG, α‐glucose and AA as biomarkers for Parkinson's disease. While BLF has great potential for brain disease diagnosis, further minimally invasive surgery studies need to be performed to develop a strategy for the collection of BLF samples in human patients. The optimization of this strategy may require a long period of time, as many years have been needed to establish the surgery for CSF collection.
Together, the results our study systematically delineated the pathways of BLF drainage, and these findings may pave the way for the physiological study of the lymphatic system in the brain. We also developed a method to collect BLF and demonstrated that BLF has a better capacity than CSF to accurately reflect the pathophysiological status of the brain. Using this approach, we identified several potential diagnostic biomarkers for glioma, Parkinson's disease and CNS infection. These findings may provide new insights into the diagnosis of CNS disorders.
Conflict of Interest
The authors have declared that they have no conflict of interest.
Supporting information
Table S1. The metabolites detected in the spectra of BLF and CSF with assessments achieved using a series of 2D NMR spectra.
Acknowledgments
This work was financially supported by the Fundamental Research Funds for the Central Universities [grant numbers: 2662017PY105, 510318177, 2662018PY025].
Contributor Information
Yunte Deng, Email: tmdytok@163.com, Email: yaozhaohui2004@126.com, Email: yuejunqiu@hotmail.com, Email: gcao@mail.hzau.edu.cn.
Zhaohui Yao, Email: yaozhaohui2004@126.com.
Junqiu Yue, Email: yuejunqiu@hotmail.com.
Gang Cao, Email: gcao@mail.hzau.edu.cn.
Data Availability Statement
All data generated or analyzed during this study are included in this article.
References
- 1. Alitalo K (2011) The lymphatic vasculature in disease. Nat Med 17:1371–1380. [DOI] [PubMed] [Google Scholar]
- 2. Antila S, Karaman S, Nurmi H, Airavaara M, Voutilainen MH, Mathivet T et al (2017) Development and plasticity of meningeal lymphatic vessels. J Exp Med 214:3645–3667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Aspelund A, Antila S, Proulx ST, Karlsen TV, Karaman S, Detmar M et al (2015) A dural lymphatic vascular system that drains brain interstitial fluid and macromolecules. J Exp Med 212:991–999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Bakker EN, Bacskai BJ, Arbel‐Ornath M, Aldea R, Bedussi B, Morris AW et al (2016) Lymphatic clearance of the brain: perivascular, paravascular and significance for neurodegenerative diseases. Cell Mol Neurobiol 36:181–194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Bar‐Klein G, Lublinsky S, Kamintsky L, Noyman I, Veksler R, Dalipaj H et al (2017) Imaging blood‐brain barrier dysfunction as a biomarker for epileptogenesis. Brain 140:1692–705. [DOI] [PubMed] [Google Scholar]
- 6. Beck C, Kruetzelmann A, Forkert ND, Juettler E, Singer OC, Köhrmann M et al (2014) A simple brain atrophy measure improves the prediction of malignant middle cerebral artery infarction by acute DWI lesion volume. J Neurol 261:1097–1103. [DOI] [PubMed] [Google Scholar]
- 7. Begcevic I, Brinc D, Brown M, Martinez‐Morillo E, Goldhardt O, Grimmer T et al (2018) Brain‐related proteins as potential CSF biomarkers of Alzheimer's disease: a targeted mass spectrometry approach. J Proteomics 182:12–20. [DOI] [PubMed] [Google Scholar]
- 8. Betterman KL, Harvey NL (2016) The lymphatic vasculature: development and role in shaping immunity. Immunol Rev 271:276–292. [DOI] [PubMed] [Google Scholar]
- 9. Brinker T, Seifert V, Stolke D (1990) Acute changes in the dynamics of the cerebrospinal fluid system during experimental subarachnoid hemorrhage. Neurosurgery 27:369–372. [DOI] [PubMed] [Google Scholar]
- 10. Carare RO, Hawkes CA, Jeffrey M, Kalaria RN, Weller RO (2013) Review: cerebral amyloid angiopathy, prion angiopathy, CADASIL and the spectrum of protein elimination failure angiopathies (PEFA) in neurodegenerative disease with a focus on therapy. Neuropathol Appl Neurobiol 39:593–611. [DOI] [PubMed] [Google Scholar]
- 11. Card CM, Yu SS, Swartz MA (2014) Emerging roles of lymphatic endothelium in regulating adaptive immunity. J Clin Invest 124:943–952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Chen J, He J, Ni R, Yang Q, Zhang Y, Luo L (2019) Cerebrovascular injuries induce lymphatic invasion into brain parenchyma to guide vascular regeneration in zebrafish. Dev Cell 49:697–710. [DOI] [PubMed] [Google Scholar]
- 13. Da Mesquita S, Fu Z, Kipnis J (2018) The meningeal lymphatic system: a new player in neurophysiology. Neuron 100:375–388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Dai H, Xiao C, Liu H, Hao F, Tang H (2010) Combined NMR and LC‐DAD‐MS analysis reveals comprehensive metabonomic variations for three phenotypic cultivars of Salvia miltiorrhiza Bunge. J Proteome Res 9:1565–1578. [DOI] [PubMed] [Google Scholar]
- 15. Dai H, Xiao C, Liu H, Tang H (2010) Combined NMR and LC‐MS analysis reveals the metabonomic changes in Salvia miltiorrhiza Bunge induced by water depletion. J Proteome Res 9:1460–1475. [DOI] [PubMed] [Google Scholar]
- 16. Daneman R, Prat A (2015) The blood‐brain barrier. Cold Spring Harb Perspect Biol 7:a020412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Desai B, Hsu Y, Schneller B, Hobbs JG, Mehta AI, Linninger A (2016) Hydrocephalus: the role of cerebral aquaporin‐4 channels and computational modeling considerations of cerebrospinal fluid. Neurosurg Focus 41:E8. [DOI] [PubMed] [Google Scholar]
- 18. Duan Y, An Y, Li N, Liu B, Wang Y, Tang H (2013) Multiple univariate data analysis reveals the inulin effects on the high‐fat‐diet induced metabolic alterations in rat myocardium and testicles in the preobesity state. J Proteome Res 12:3480–3495. [DOI] [PubMed] [Google Scholar]
- 19. Durmo F, Rydelius A, Cuellar Baena S, Askaner K, Lätt J, Bengzon J et al (2018) Multivoxel 1H‐MR spectroscopy biometrics for preoprerative differentiation between brain tumors. Tomography 4:172–181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. van Etten ES, Verbeek MM, van der Grond J, Zielman R, van Rooden S, van Zwet EW et al (2017) beta‐Amyloid in CSF: biomarker for preclinical cerebral amyloid angiopathy. Neurology 88:169–176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Huang M, Dong XQ, Hu YY, Yu WH, Zhang ZY (2010) High S100B levels in cerebrospinal fluid and peripheral blood of patients with acute basal ganglial hemorrhage are associated with poor outcome. World J Emerg Med 1:22–31. [PMC free article] [PubMed] [Google Scholar]
- 22. Iliff JJ, Wang M, Liao Y, Plogg BA, Peng W, Gundersen GA et al (2012) A paravascular pathway facilitates CSF flow through the brain parenchyma and the clearance of interstitial solutes, including amyloid beta. Sci Transl Med 4:147ra111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Jessen NA, Munk ASF, Lundgaard I, Nedergaard M (2015) The glymphatic system: a beginner's guide. Neurochem Res 40:2583–2599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Johanson CE, Duncan JA, Klinge PM, Brinker T, Stopa EG, Silverberg GD (2008) Multiplicity of cerebrospinal fluid functions: new challenges in health and disease. Cerebrospinal Fluid Res 5:10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Johanson C, Johanson N (2016) Merging transport data for choroid plexus with blood‐brain barrier to model CNS homeostasis and disease more effectively. CNS Neurol Disord Drug Targets 15:1151–1180. [DOI] [PubMed] [Google Scholar]
- 26. Johanson C, Stopa E, Baird A, Sharma H (2011) Traumatic brain injury and recovery mechanisms: peptide modulation of periventricular neurogenic regions by the choroid plexus‐CSF nexus. J Neural Transm (Vienna) 118:115–133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Johnston M, Zakharov A, Papaiconomou C, Salmasi G, Armstrong D (2004) Evidence of connections between cerebrospinal fluid and nasal lymphatic vessels in humans, non‐human primates and other mammalian species. Cerebrospinal Fluid Res 1:2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Kerjaschki D (2014) The lymphatic vasculature revisited. J Clin Invest 124:874–877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Kida S, Pantazis A, Weller RO (1993) CSF drains directly from the subarachnoid space into nasal lymphatics in the rat. Anatomy, histology and immunological significance. Neuropathol Appl Neurobiol 19:480–488. [DOI] [PubMed] [Google Scholar]
- 30. Koscova S, Zakova Slivarichova D, Tomeckova I, Melicherova K, Stelzer M, Janakova A et al (2017) Cerebrospinal fluid biomarkers in the diagnosis of Creutzfeldt‐Jakob disease in Slovak patients: over 10‐Year period review. Mol Neurobiol 54:5919–5927. [DOI] [PubMed] [Google Scholar]
- 31. Kuo PH, Stuehm C, Squire S, Johnson K (2018) Meningeal lymphatic vessel flow runs countercurrent to venous flow in the superior sagittal sinus of the human brain. Tomography 4:99–104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Laman JD, Weller RO (2013) Drainage of cells and soluble antigen from the CNS to regional lymph nodes. J Neuroimmune Pharmacol 8:840–856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Lattanzio F, Abu‐Rumeileh S, Franceschini A, Kai H, Amore G, Poggiolini I et al (2017) Prion‐specific and surrogate CSF biomarkers in Creutzfeldt‐Jakob disease: diagnostic accuracy in relation to molecular subtypes and analysis of neuropathological correlates of p‐tau and Aβ42 levels. Acta Neuropathol 133:559–578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Laubner S, Ondreka N, Failing K, Kramer M, Schmidt MJ (2015) Magnetic resonance imaging signs of high intraventricular pressure–comparison of findings in dogs with clinically relevant internal hydrocephalus and asymptomatic dogs with ventriculomegaly. BMC Vet Res 11:181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Liao S, von der Weid PY (2015) Lymphatic system: an active pathway for immune protection. Semin Cell Dev Biol 38:83–89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Lohrberg M, Wilting J (2016) The lymphatic vascular system of the mouse head. Cell Tissue Res 366:667–677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Louveau A, Da MS, Kipnis J (2016) Lymphatics in neurological disorders: a neuro‐lympho‐vascular component of multiple sclerosis and Alzheimer's disease? Neuron 91:957–973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Louveau A, Smirnov I, Keyes TJ, Eccles JD, Rouhani SJ, Peske JD et al (2015) Structural and functional features of central nervous system lymphatic vessels. Nature 523:337–341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Lowhagen P, Johansson BB, Nordborg C (1994) The nasal route of cerebrospinal fluid drainage in man. A light‐microscope study. Neuropathol Appl Neurobiol 20:543–550. [DOI] [PubMed] [Google Scholar]
- 40. Minnerup J, Wersching H, Ringelstein EB, Heindel W, Niederstadt T, Schilling M et al (2011) Prediction of malignant middle cerebral artery infarction using computed tomography‐based intracranial volume reserve measurements. Stroke 42:3403–3409. [DOI] [PubMed] [Google Scholar]
- 41. Murtha LA, Yang Q, Parsons MW, Levi CR, Beard DJ, Spratt NJ, McLeod DD (2014) Cerebrospinal fluid is drained primarily via the spinal canal and olfactory route in young and aged spontaneously hypertensive rats. Fluids Barriers CNS 11:12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Noé FM, Marchi N (2019) Central nervous system lymphatic unit, immunity, and epilepsy: is there a link? Epilepsia Open 4:30–39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Paradot G, Baledent O, Gondry‐Jouet C, Meyer ME, Le Gars D (2006) Cerebrospinal fluid flow imaging in the meningeal hemorrhage. Neurochirurgie 52:323–329. [DOI] [PubMed] [Google Scholar]
- 44. Pirttila T, McHta PD, Soininen H, Kim KS, Heinonen O, Paljarvi L et al (1996) Cerebrospinal fluid concentrations of soluble amyloid beta‐protein and apolipoprotein E in patients with Alzheimer's disease: correlations with amyloid load in the brain. Arch Neurol 53:189–193. [DOI] [PubMed] [Google Scholar]
- 45. Preuss M, Hoffmann KT, Reiss‐Zimmermann M, Hirsch W, Merkenschlager A, Meixensberger J, Dengl M (2013) Updated physiology and pathophysiology of CSF circulation–the pulsatile vector theory. Childs Nerv Syst 29:1811–1825. [DOI] [PubMed] [Google Scholar]
- 46. Raper D, Louveau A, Kipnis J (2016) How do meningeal lymphatic vessels drain the CNS? Trends Neurosci 39:581–586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Rasmussen MK, Mestre H, Nedergaard M (2018) The glymphatic pathway in neurological disorders. Lancet Neurol 17:1016–1024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Ray B, Reyes PF, Lahiri DK (2011) Biochemical studies in Normal Pressure Hydrocephalus (NPH) patients: change in CSF levels of amyloid precursor protein (APP), amyloid‐beta (Abeta) peptide and phospho‐tau. J Psychiatr Res 45:539–947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Schmid Daners M, Knobloch V, Soellinger M, Boesiger P, Seifert B, Guzzella L, Kurtcuoglu V (2012) Age‐specific characteristics and coupling of cerebral arterial inflow and cerebrospinal fluid dynamics. PLoS ONE 7:e37502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Schob S, Dieckow J, Fehrenbach M, Peukert N, Weiss A, Kluth D et al (2016) The cerebral surfactant system and its alteration in hydrocephalic conditions. PLoS ONE 11:e0160680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Shapira Y, Artru AA, Lam AM (1992) Changes in the rate of formation and resistance to reabsorption of cerebrospinal fluid during deliberate hypotension induced with adenosine or hemorrhage. Anesthesiology 76:432–439. [DOI] [PubMed] [Google Scholar]
- 52. Stoquart‐ElSankari S, Balédent O, Gondry‐Jouet C, Makki M, Godefroy O, Meyer ME (2007) Aging effects on cerebral blood and cerebrospinal fluid flows. J Cereb Blood Flow Metab 27:1563–1572. [DOI] [PubMed] [Google Scholar]
- 53. Sun L, Tang Y, Yan K, Yu J, Zou Y, Xu W et al (2019) Differences in neurotropism and neurotoxicity among retrograde viral tracers. Mol Neurodegener 14:8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Usinskiene J, Ulyte A, Bjørnerud A, Venius J, Katsaros VK, Rynkeviciene R et al (2016) Optimal differentiation of high‐ and low‐grade glioma and metastasis: a meta‐analysis of perfusion, diffusion, and spectroscopy metrics. Neuroradiology 58:339–350. [DOI] [PubMed] [Google Scholar]
- 55. Wang Q, Zhang J, Xu W, Chen X, Zhang J, Xu B (2017) Role of magnetic resonance spectroscopy to differentiate high‐grade gliomas from metastases. Tumour Biol 39:1010428317710030. [DOI] [PubMed] [Google Scholar]
- 56. Weller RO, Djuanda E, Yow HY, Carare RO (2009) Lymphatic drainage of the brain and the pathophysiology of neurological disease. Acta Neuropathol 117:1–14. [DOI] [PubMed] [Google Scholar]
- 57. Xiao C, Dai H, Liu H, Wang Y, Tang H (2008) Revealing the metabonomic variation of rosemary extracts using 1H NMR spectroscopy and multivariate data analysis. J Agric Food Chem 56:10142–10153. [DOI] [PubMed] [Google Scholar]
- 58. Xie L, Kang H, Xu Q, Chen MJ, Liao Y, Thiyagarajan M et al (2013) Sleep drives metabolite clearance from the adult brain. Science 342:373–377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Zhong X, Wang J, Carlsson C, Okonkwo O, Zetterberg H, Li L (2019) A strategy for discovery and verification of candidate biomarkers in cerebrospinal fluid of preclinical Alzheimer's disease. Front Mol Neurosci 11:483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Zou W, Pu T, Feng W, Lu M, Zheng Y, Du R et al (2019) Blocking meningeal lymphatic drainage aggravates Parkinson's disease‐like pathology in mice overexpressing mutated α‐synuclein. Transl Neurodegener 8:7. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1. The metabolites detected in the spectra of BLF and CSF with assessments achieved using a series of 2D NMR spectra.
Data Availability Statement
All data generated or analyzed during this study are included in this article.


