Abstract
Sudden unexpected death in epilepsy (SUDEP) is mechanistically complex and one probable cause is seizure‐related respiratory dysfunction. Medullary respiratory regulatory nuclei include the pre‐Bötzinger complex (pre‐BötC) in the ventrolateral medulla (VLM), the medullary raphé nuclei (MR) and nucleus of solitary tract in the dorsomedial medulla (DMM). The region of the VLM also contains intermingled tyrosine hydroxylase (TH) catecholaminergic neurones which directly project to the pre‐BötC and regulate breathing under hypoxic conditions and our aim was to evaluate these neurones in SUDEP cases. In post‐mortem cases from three groups [SUDEP (18), epilepsy controls (8) and non‐epilepsy controls (16)] serial sections of medulla (obex + 2 to + 13 mm) were immunolabeled for TH. Three regions of interest (ROI) were outlined (VLM, DMM and MR) and TH‐immunoreactive (TH‐IR) neurones were evaluated using automated detection for overall labeling index (neurones and processes) and neuronal densities and compared between groups and relative to obex level. C‐fos immunoreactivity was also semi‐quantitatively evaluated in these regions. We found no significant difference in the density of TH‐IR neurones or labeling index between the groups in all regions. Significantly more TH‐IR neurones were present in the DMM region than VLM in non‐epilepsy cases only (P < 0.01). Regional variations in TH‐IR neurones with obex level were seen in all groups except SUDEP. We also identified occasional TH neurones in the MR region in all groups. There was significantly less c‐fos labeling in the VLM and MR in SUDEP than non‐epilepsy controls but no difference with epilepsy controls. In conclusion, in this series we found no evidence for alteration of total medullary TH‐IR neuronal numbers in SUDEP but noted some differences in their relative distribution in the medulla and c‐fos neurones compared to control groups which may be relevant to the mechanism of death.
Keywords: catecholamingergic, c‐fos, SUDEP, TH, TPH, ventrolateral medulla
Impaired brainstem autonomic and cardio‐respiratory networks are implicated in SUDEP. In a post‐mortem series of SUDEP and controls we studied the chatecholaminergic (TH+) medullary neurones. We found no evidence for alteration of total medullary neuronal numbers in SUDEP but noted some differences in their relative distribution in the medulla compared to control groups.
Abbreviations
- BP
blood pressure
- DMM
dorsomedial medulla
- DMNV
dorsal motor nucleus of the vagus
- HR
heart rate
- IR
immunoreactivity
- MR
medullary median raphé
- NTS
nucleus tractus solitarius
- Pre‐BötC
pre‐Bötzinger complex
- TH
tyrosine hydroxylase
- TPH
tryptophan hydroxylase
- ST
solitary tract
- SUDEP
sudden unexpected death in epilepsy
- VLM
ventrolateral medulla
Introduction
People with epilepsy are more likely to die prematurely. One major cause is sudden unexpected death in epilepsy (SUDEP) with an annual rate exceeding one case per thousand with epilepsy (40). SUDEP is mechanistically complex and involves various risk factors with highly probabilistic effects, making it impossible to precisely predict individual risk (7). Prevention may come through better understanding of the pathophysiological mechanisms and there are recent clinical, neuroimaging and experimental data to support seizure‐related autonomic dysfunction, in particular impaired cardio‐respiratory regulation (7, 33).
Autonomic phenomena are not uncommon manifestations of seizures; sympathetic outflow dominates after a seizure with self‐limiting effects on blood pressure (BP) (27) and heart rate (HR) (12); altered baroreflex sensitivity in the ictal period is also recognized (8, 11). Irregular breathing (38) and peri‐ictal central apnea associated with hypoxemia (6, 21, 43) have been recorded during seizure monitoring and regarded as risk factors for SUDEP. In rare monitored seizure deaths, depressed respiration, and non‐tachyarrhythmic cardiac dysfunction with terminal apnea preceding terminal asystole were reported (33). Evidence from structural and volumetric MRI studies (26, 29) as well as functional MRI (2) support the hypothesis that autonomic brain regions, including the brainstem, are altered in SUDEP.
There is a relative paucity of neuropathological post‐mortem data addressing any alterations in autonomic nuclei. We have recently reported in the ventral lateral medulla (VLM), a region that encompasses the putative inspiratory excitatory neurones of the pre‐Bötzinger complex (Pre‐BötC), alterations of somatostatin and galanergic networks as well as neuromodulatory serotonergic neurones in the medullary raphé (MR) in SUDEP (31). Catecholaminergic neuronal groups in the medulla are identified in histological sections with immunoreactivity (IR) for Tyrosine hydroxylase (TH), the rate‐limiting enzyme in the synthesis of DOPA; these include neurones in the VLM (C1 adrenergic neurones) that form the putative vasomotor centre regulating BP but also influencing respiratory drive (10). Experimentally, local projections have been identified from C1 neurones to pre‐BötC that stimulate breathing in hypoxic conditions (16). C1 neuronal activation also causes sleep‐state dependent cardio‐respiratory arousal (1), of potential relevance to SUDEP as many deaths occur during sleep. A second, regionally distinct, group of TH‐IR neurones recognized in the medulla of mammals are the C2/C3 catecholaminergic neurones in the dorsal medial medulla (DMM) in the vicinity of the nucleus tractus solitarius (NTS), although less is known regarding their vasomotor regulatory functions (36). There is experimental evidence that seizures increase VLM C1 neuronal activity (37), equivalent to that observed in hypoxia (17). It has been proposed that C1 activation is the mechanism underlying increased BP and sympathetic activity during seizures (38). Experimental depletion of catecholaminergic neurones in the VLM impairs respiratory responses (24) and a reduction in medullary TH‐IR has been reported in sudden infant death syndrome (SIDS) series (28, 30), a condition with circumstantial similarities to SUDEP.
There are no published human studies of medullary catecholaminergic neurones in epilepsy. In a post‐mortem series our aim was to quantify and explore changes in TH‐IR neurones in a SUDEP series compared to control groups.
Methods and Materials
Case selection
Medullae from 46 post‐mortem cases were selected from the Epilepsy Society Brain and Tissue Bank (ESBTB) at UCL, through Brain UK (pathology department at Derriford Hospital, Plymouth) and the MRC sudden death brain bank in Edinburgh (Table 1). Tissue from all cases was retained with era‐appropriate consent. The project has ethics approval. Post‐mortem intervals and fixation times were recorded. The cases were acquired from mainly adult post‐mortem examinations conducted between 1999 and 2017 with median age 40 years and included three main groups: SUDEP (18), an epilepsy control group without sudden death (10) and a non‐epilepsy control group (16), as used in a previous studies (31). Regarding genetic characterization, Dravet syndrome cases (7) with confirmed SCN1A mutations were included in both SUDEP (2) and epilepsy control groups (3) (Table 1); the remaining epilepsy cases were not genotyped. The SUDEP group included 11 definite SUDEP cases (complete and negative autopsy including toxicology), the remaining 7 being probable or possible SUDEP. The non‐epilepsy control group included 12 cases with sudden death (non‐neurological, non‐epilepsy sudden death). In the SUDEP groups there were cases with recent onset of seizures in the last two years prior to death the epilepsy control group was selected accordingly and there was no significant difference in mean age of onset of seizures and epilepsy duration between the groups (Table 1). Presumed symptomatic causes of epilepsy in the control group identified were hippocampal sclerosis (1), tumor (1) and heterotopia (1) and causes of death included aspiration, congestive cardiac failure, sepsis, convulsive/non‐convulsive status epilepticus (4); in three cases a head only post‐mortem examination was carried out but the deaths were witnessed and not sudden and there was no neuropathological cause of death.
Table 1.
Clinical and pathology data from the 46 cases studied.
| Groups subgroups (N = total in group) | Age at death mean (range) years | Age at seizure onset mean (range) years duration | Gender M:F | Mean mid obex level of medulla (range) mm | Fixation times/PM intervals mean (range) days | Number of TH interval sections analysed per case (mean) |
|---|---|---|---|---|---|---|
| SUDEP (N = 18) | 33.6 (1–53) | 10.5 (0.5–39); 19 (1–46) | 12:6 | 6 (2–13) | 35 (5–98); 3 (1–7) | 8.5 |
| D‐SUDEP (N = 11) | 34 (11–51) | 11.3 (0.6–26);18.2 (1–39) | 6:5 | 5.5 (2–9) | 41 (18–98); 2.7 (1–5) | 8.1 |
| Epilepsy controls (N = 10) | 58 (5–86) | 25 (0.5–84); 27 (0.5–78) | 7:3 | 6.6 (3–11) | 41 (1–90); 2.4 (1–5) | 9 |
| Dravet syndrome (N = 7) * | 18 (1–47) | 18 (1–47); 17 (1–46) | 4:3 | 7 (3.5–13) | 48 (40–57); 1.2 (1–2) | 8 |
| Non‐epilpesy controls N = 16 | 42 (23–80) | – | 10:6 | 6 (2.5–10) | 23 (7–94); 3.4 (1–6) | 7 |
| NESD (N = 12) | 36.8 (23–45) | 8:4 | 6.3 (3–10) | 20.3 (7–94); 3.1 (1–6) | 6.7 | |
| Statistical differences between groups | P = 0.009 | N/S | N/S | N/S | N/S | N/S |
DS = Dravet syndrome; NESD = non‐epilepsy sudden death; N/S = not significant; D‐SUDEP = definite SUDEP.
In the DS group two cases had definite SUDEP, three had other cause of deaths and in two cases the cause of death was not further classified due to limited post‐mortem data; these data have been included to the SUDEP and epilepsy controls rows.
Tissue preparation
In all cases, a single 5 mm thick formalin‐fixed paraffin‐embedded tissue block was selected from medulla (axial level between obex + 2 to + 13 mm). Serial sections were cut through the block at 20μm thickness using the Tissue‐Tek Auto Section automated microtome (Sakura Finetek, U.S.A. Inc) and every tenth section was stained with cresyl violet to determine the corresponding obex level using, according to the atlas (Paxinos and Huang 1995). A second set of serial sections at 200 µm intervals through the medulla were immunolabeled with TH using standard immunohistochemistry (1: 750 Abcam ab112) and a further single section from the mid‐obex region was immunolabeled with c‐fos (1: 50 Santa cruz sc‐166940). In selected cases from SUDEP, epilepsy control, and non‐epilepsy control groups sections were double labeled with TH and tryptophan hydroxylase TPH (TPH2 1:100, Abcam ab121013), TH and c‐fos, and TPH and c‐fos to compare the distribution of catecholaminergic, serotonergic, and activated neurones in the regions studied. Details of all immunohistochemistry protocols are included in the Supporting File.
The slides were scanned at 40x magnification using a Leica SCN400 slide scanner (Leica Microsystems, Wetzlar, Germany). Three regions of interest (ROI) were defined using Definiens Tissue Studio (Definiens AG, Munich, Germany) image analysis (Supporting Figure S1A): (i) MR, (ii) the VLM to include C1 neurones, and (iii) DMM to include the Nucleus Tractus Solitarius (NTS) region and C2/3 neurones. Anatomical landmarks were used in order to reproduce consistent ROI in all cases. An algorithm based on staining intensity threshold and the size, shape, and separation of cellular structures was used with Definiens developer to calculate both the immunostained area (neurones and processes) and also the number of labeled neurones in each ROI (further detailed in supplemental method and Supporting Figure S1B,C). These values were subsequently used to calculate both the labeling index (total labeling of cells and processes/total ROI area) and neuronal density (cells per area) which were averaged for one side. Double labeling co‐localization was evaluated qualitatively with confocal imaging using ZEISS LSM 880 laser scanning microscope with Airyscan and Zeiss Axio Imager Z2 fluorescent microscope (Zeiss, Göttingen, Germany) in each ROI. C‐fos labeling was assessed on the single section and evaluated semi‐quantitatively in the MR, VLM and DMM regions; 0 = virtually no neuronal labeling, 1 = occasional positive neurones, 2 = many positive neurones.
Statistical analysis was carried out with SPSS (version 22, IBM) for non‐parametric tests to compare data between the three main cause of groups (Kruskall–Wallis and Mann–Whitney tests), regions within groups (Wilcoxon tests), clinical correlations (Spearman’s rho test and Kendal’s tau), and regression analysis to factor for multiple independent variables as age and fixation times; P values of <0.05 (with correction for multiple comparisons) were regarded as significant. In addition, the Dravet subgroup was similarly compared to other main cause of death groups and definite‐SUDEP compared to non‐epilepsy sudden death subgroup (Table 1). For graphical representation of data, Graphpad Prism 7 (University of California, San Diego) was used.
Results
TH immunohistochemistry
TH‐IR neurones formed symmetrical bands extending dorsomedially to ventrolaterally across the medulla (Figure 1A–C). In the VLM region dorsal to the inferior olive, intense cytoplasmic labeling of medium to large multipolar, fusiform and triangular neurones with mainly two to three coarse dendrites was seen (Figure 1D), with axons extending along the band to the lateral medullary surface. In the DMM, TH positive neurones and processes were concentrated in the NTS surrounding the solitary tract and the dorsal motor nucleus of the vagus (DMNV) (Figure 1E). In addition, medial to the solitary tract and ventrolateral to the XIIth cranial nerve nucleus, prominent TH positive axonal tracts running longitudinally to the axial plane in the dorsomedial tegmentum were identified (Figure 1G), as well as intense labeling in the area postrema in lower obex levels. We also noted occasional single positive neurones, in addition to axons and processes, in the MR region (Figure 1F); these were noted in all cause of death groups but not present in all cases, appearing more frequent in rostral obex levels.
Figure 1.
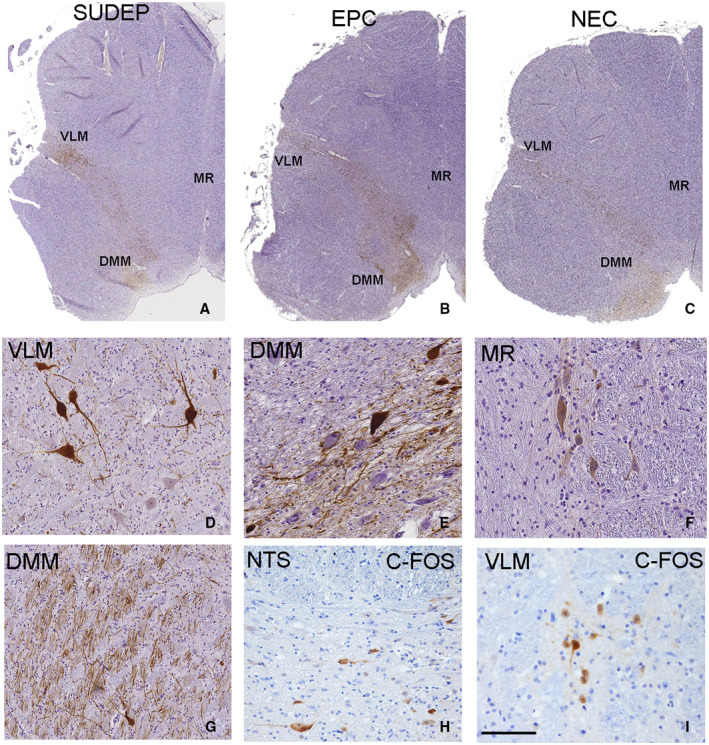
Tyrosine hydroxylase (TH) and c‐fos immunostaining in medulla and delineation of regions of interest. A–C. A low power images of section of medulla with band TH neuronal labeling extending from the dorsal medial medulla region (DMM) (arrowheads) to the ventrolateral medulla (VLM) region (arrows) with axons extending to the lateral medullary surface is shown in SUDEP (A), epilepsy control (EPC) (B) and non‐epilepsy controls (NEC) (C) all at mid obex levels. D. Medium to large multipolar, fusiform and triangular neurones with mainly two to three coarse dendrites was seen with TH labeling in the VLM region intermingled with negative neurones. E. In the DMM region positive neurones enmeshed in axons and processes intermingled with immunonegative neurones was noted. F. Distinct TH labeling of neurones in the medullary median Raphé (MR) regions was noted in some cases including in this case with Dravet syndrome.G. Longitudinal and transverse TH‐positive axonal tracts near the XII nucleus. H. C‐fos labeling of neurones in the DMM region and I. in the VLM region was noted in a proportion of all cause of death groups. VLM = ventrolateral medulla, MR = median Raphé, DMM = dorsal medial medulla. Bar is approximately equivalent to 1 mm (A‐C), 75 microns in D, E, F, H and 200 microns in G, I.
In view of the finding of TH‐IR neurones in the MR region we carried out double labeling with TPH to highlight their relationship to serotonergic neurones. Representative cases from each group and different obex levels were included. A relative predominance of TH cells in the VLM and TPH cells in the MR was apparent in all cases but neurones of both types were visible in either regions (Figure 2A). There was mainly little overlap of expression in the VLM (Figure 2A,E) but occasional neurones with both TH and TPH positivity were noted in some cases (Figure 2B). Double labeling also confirmed small numbers of TH positive neurones and processes in the MR in proximity to TPH positive neurones (Figure 2C) with occasional apparent co‐localization (Figure 2D).
Figure 2.
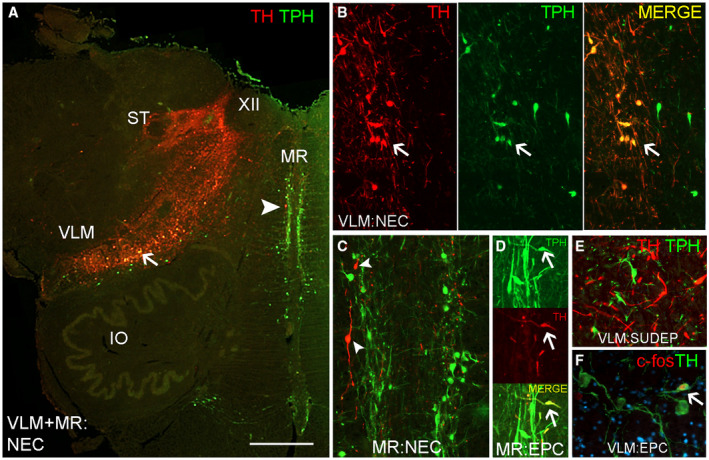
Double labeling of Tyrosine hydroxylase (TH), Tryptophan hydroxylase (TPH) and c‐fos neuronal populations in the ventrolateral medulla and medullary Raphé. A. Low power view through the medulla at obex 7 mm showing dominance of TH labeling in the ventral lateral medulla (VLM) to dorsomedial medulla (DMM) as a band; TPH labeling is dominant in the medullary Raphé (MR). The positions of the solitary tract (ST) and XIIth cranial nerve nucleus are highlighted. Occasional double labeled neurones are shown in the VLM (arrow and in figure B) and scattered single labeled TH neuron in the Raphé (arrowhead and shown in C). B. VLM showing co‐localization of labeling with TH and TPH (arrow) as well as cells positive for TH or TPH alone. C. Medullary Raphé with TH positive neurones (arrowheads). D. Medullary Raphé with rare co‐localization of cellular labeling for TH and TPH (arrow). E. VLM region with distinct TH and TPH positive neurones intermingled. F. TH neurones with c‐fos nuclear labeling in the VLM region (arrow). EPC = epilepsy‐control, NEC = non‐epilepsy control. Bar is approximately equivalent to A = 2.5 mm and other figures 250 microns.
Quantitative analysis TH neurones
There were no significant differences in the TH labeling index or neuronal density in the VLM, DMM or MR ROI between the SUDEP and epilepsy and non‐epilepsy control groups (Table 2). This was also the finding when definite‐SUDEP cases and non‐epilepsy sudden death controls were compared. The only observed difference was for higher TH neuronal density in the MR ROI in Dravet syndrome cases compared to the SUDEP group without Dravet (P < 0.05).
Table 2.
TH quantification shown as labelling index (LI) and neuronal densities (ND) in the medullary regions of interest for all obex levels and c‐fos semi‐quantitative analysis in main cause of death groups (SUDEP, Epilepsy controls, non‐epilepsy controls) and subgroups (Definite SUDEP, Dravet and non‐epilepsy sudden death).
| Group/region | SUDEP* | Definite SUDEP | Epilepsy controls* | Dravet syndrome | Non‐epilepsy controls (NEC) | Sudden death NEC |
|---|---|---|---|---|---|---|
| VLM LI Mean (SD) | 6.7 (3.5) | 7.2 (4.1) | 7.8 (6.5) | 6.5 (2.1) | 6.2 (2.1) | 6.3 (2.1) |
| MR LI Mean (SD) | 1.7 (1.7) | 1.5 (1.4) | 1.4 (1.7) | 2.1 (2.0) | 1.9 (1.7) | 1.9 (1.7) |
| DMM LI Mean (SD) | 8.0 (3.7) | 7.8 (3.9) | 9.9 (6.2) | 6.7 (3.1) | 8.8 (4.4) | 9.0 (4.5) |
| VLM ND (cells/mm2) Mean (SD) | 3.4 (1.5) | 3.8 (1.8) | 4.3 (2.1) | 2.9 (1.0) | 5.1 (5.3) | 3.6 (1.2) |
| MR ND (cells/mm2) Mean (SD) | 0.98 (0.7) | 0.99 (0.8) | 1.1 (0.9) | 1.3 (0.5) | 1.6 (1.3) | 1.7 (1.4) |
| DMM ND (cells/mm2) Mean (SD) | 3.9 (1.6) | 3.9 (1.1) | 5.1 (5.3) | 3.4 (2.1) | 4.0 (1.8) | 3.8 (1.6) |
| C‐fos (VLM) Semi‐quant Mean (SD) | 0.39 (0.6) | 0.36 (0.67) | 0.67 (0.7) | 0.17 (0.4) | 1.21 (0.8) | 1.3 (0.82) |
| C‐fos (MR) Semi‐quant Mean (SD) | 0.06 (0.23) | 0.09 (0.3) | 0.11 (0.33) | 0 (0) | 0.71 (0.9) | 0.9 (0.9) |
| C‐fos (DMM) Semi‐quant Mean (SD) | 0.5 (0.7) | 0.45 (0.68) | 0.22 (0.44) | 0.17 (0.4) | 1.07 (0.9) | 1.2 (1.03) |
These columns also include cases in the Dravet group (see Table 1). VLM, ventrolateral medulla, MR median raphe, DMM dorsomedial medulla (which includes the region of the nucleus of the solitary tract and solitary tract). LI shown as × 103 and ND (shown as cells/mm2). See methods for c‐fos semi‐quantitative scoring.
In all groups, the TH labeling index and neuronal density was significantly higher in the VLM compared to the MR (P = 0.01 to <0.0001) and also the DMM compared to the MR (P = 0.02 to <0.0001) (Table 2). The TH labeling index was also significantly higher in the DMM than VLM ROI in the non‐epilepsy controls (P < 0.01) but this difference was not present in SUDEP and epilepsy control groups (Figure 3).
Figure 3.
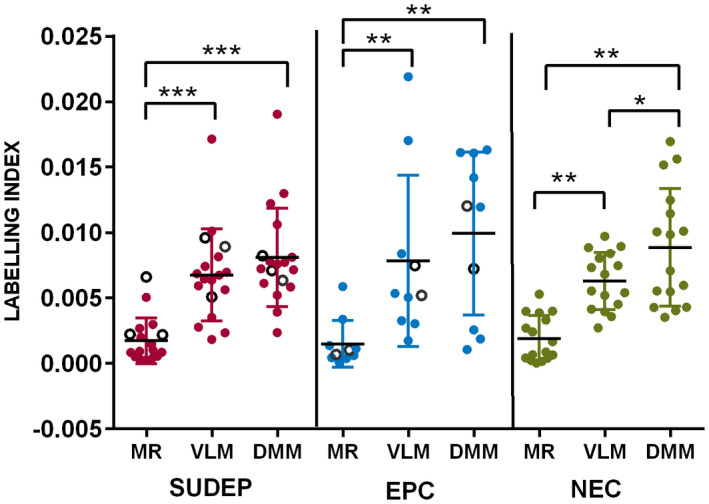
Medullary Tyrosine hydroxylase in cause of death groups. There were no significant differences in TH labeling index between the groups SUDEP, epilepsy controls (EPC) and non‐epilepsy controls (NEC) for all regions (VLM, ventrolateral medulla, MR median Raphé, and DMM, dorsomedial medulla which includes the NTS region of the nucleus of the solitary tract). The Dravet syndrome cases are indicated with open circles in the SUDEP and EPC groups. Within each cause of death groups, the TH labeling index was significantly higher in the VLM compared to the MR and also the DMM compared to the MR. (This was also noted for neuronal density, see Table 2). The TH labeling index was also significantly higher in DMM than VLM ROI in the non‐epilepsy controls but this difference was not present in SUDEP and epilepsy control groups. (*P = 0.01, **P = 0.005 to 0.001, ***P < 0.0001).
Relationship of TH neurones with obex level
The mean mid‐obex level varied between cause of death groups as these included archival cases where often only one block from the medulla was available; mid‐obex levels were not significantly different between cause of death groups (Table 1). However, we also evaluated any relationship of TH cells relative to the obex level at 2 mm intervals from obex + 1 mm to 13 mm. In the non‐epilepsy controls higher TH densities and labeling indices were noted for the DMM in the caudal medulla (Figure 4A,B), for the MR in the rostral medulla (Figure 4C,D) and in the VLM measurements peaked at obex 7–8 mm (Figure 4E,F). Statistical analysis did not show significant differences for TH measurements at any obex level between the cause of death groups. A significant negative correlation of TH labeling index (P = 0.02) and neuronal density (P = 0.03) was shown in the DMM with higher obex levels in non‐epilepsy controls (P = 0.01) (Figure 4A), a positive correlation between TH labeling index in the MR and higher obex level in epilepsy controls (P = 0.02) and in the Dravet group a positive correlation of TH neuronal density in the VLM with higher obex level (P = 0.01). There was no correlation between labeling index or neuronal density in any region in relation to obex level in the SUDEP group.
Figure 4.
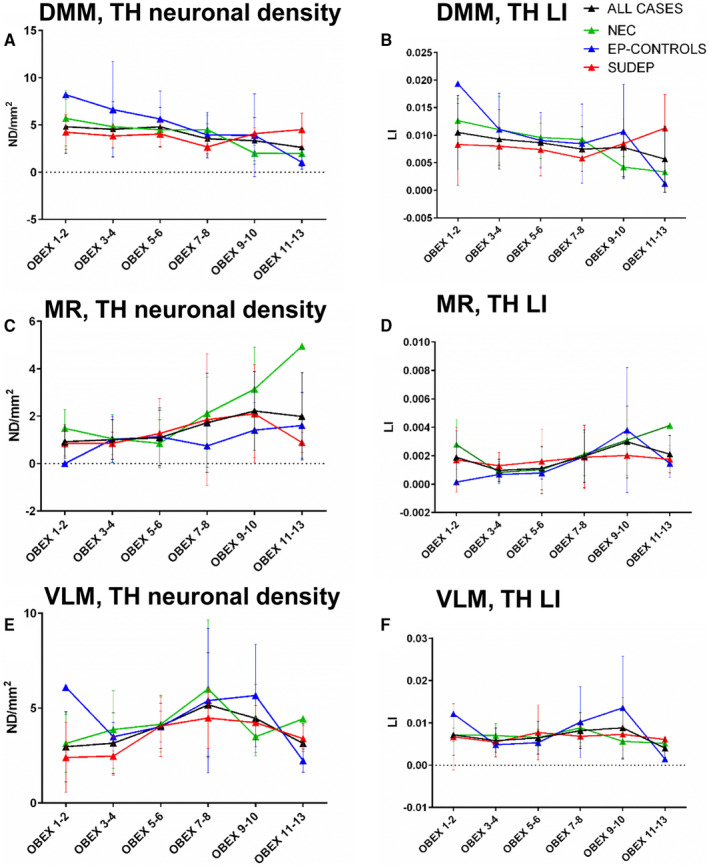
Tyrosine hydroxylase (TH) labeling with respect to obex level. Graphical representation of the mean neuronal density (A,C,E) and labeling index (LI) (B,D,F) values shown at 2 mm increments of medullary obex from + 1 mm (caudal) to + 13 mm (rostral) for DMM (A,B), MR (C,D) and VLM (E,F) region in non‐epilepsy controls (NEC), epilepsy controls (Ep‐controls) and SUDEP cases. The black line indicates the pooled data for all cases. In the non‐epilepsy controls, higher TH densities and labeling indices were noted for the DMM in the caudal medulla for the MR in the rostral medulla and in the VLM measurements peaked at obex 7–8 mm. Statistical analysis did not show significant differences for TH measurements at any obex level between the cause of death groups. A significant negative correlation of TH labeling index (P = 0.02) and neuronal density (P = 0.03) was shown in the DMM with higher obex levels in non‐epilepsy controls (P = 0.01). A positive correlation between TH labeling index in the MR and higher obex level in epilepsy controls (P = 0.02). There was no correlation between labeling index or neuronal density in any region in relation to obex level in the SUDEP group. VLM = ventrolateral medulla, MR = median Raphé, DMM = dorsal medial medulla. Error bars represent the standard deviation of measurements.
TH: clinical‐pathological correlations
There was a positive correlation between the age at death and the TH neuronal density in the DMM and VLM in all cases (P = 0.014 and P = 0.048). Within cause of death groups this correlation was present in SUDEP cases in the DMM region only (P = 0.012) (Supporting Figure S2). There were significant differences in the age at death in the groups, with younger ages in SUDEP cases (Table 1) but multiple regression analysis did not show significant differences in TH LI or densities between the groups when age was factored. There was no correlation with TH measurements in ROIs and the age of onset of seizures and epilepsy duration in any groups. Significantly lower TH labeling index was noted in males than females (P = 0.024). Males were over‐represented in all cause of death groups and there was no significant difference in the gender split in each group (Table 1); further analysis of cases split for gender did not identify significant differences in TH labeling index or neuronal density in any region between the cause of death groups. There was a negative correlation with VLM labeling index and post‐mortem interval (P = 0.012) but not with fixation times in any region. The post‐mortem intervals and fixation times varied but were not significantly different between the cause of death groups (Table 1); using multiple regression analysis to factor fixation times and post‐mortem interval, there were still no significant differences in TH labeling index or neuronal density in any region between the cause of death groups.
C‐fos labeling in the medulla
C‐fos mainly labeled neurones, with nuclear and some cytoplasmic positivity, highlighting rare to many neurones in the DMM and the NTS (Figure 1H) and the VLM (Figure 1I) with occasional neurones in the MR region; c‐fos‐IR neurones were also noted in the arcuate nucleus in some cases but other nuclei, including inferior olivary nuclei and XIIth cranial nucleus which were virtually negative. Double labeling of TPH or TH with c‐fos confirmed occasional co‐localized neurones in the VLM (Figure 2F).
C‐fos neuronal labeling was not present in all cases, being observed in 47% of SUDEP, 17% of DS, 78% of non‐epilepsy controls, and 57% of epilepsy controls but with a similar distribution. Semi‐quantitative analysis showed significantly higher labeling in non‐epilepsy sudden deaths than definite SUDEP cases in the MR (P = 0.005) and VLM (P = 0.006) (Table 2) but there were no difference between epilepsy controls and other groups. Although we noted robust c‐fos neuronal labeling in surgical control tissues that were formalin fixed for 5 months (data not shown) there was a negative correlation with c‐fos labeling and longer fixation times in the post‐mortem series (P < 0.05) but a positive correlation with post‐mortem interval (P < 0.05) (Supporting Figure S3). Using multiple regression analysis to factor fixation times and post‐mortem interval, significant differences remained between non‐epilepsy sudden deaths and definite SUDEP cases in the MR (P = 0.01) and VLM (P = 0.03).
Discussion
We did not identify differences in the number of TH‐IR catecholaminergic neurones in the medulla in SUDEP compared to control groups. This is in contrast with a recent study where we showed a reduction of somatostatin/neurokinin1‐IR neurones in the pre‐BötC/VLM region and TPH labeling in the VLM and MR in SUDEP in a similar cohort. Our findings suggest that catecholaminergic neuronal medullary neurones are preserved in SUDEP. We did note a difference in the relative distribution of TH‐IR neurones between the VLM and DMM region in epilepsy cases compared to controls and in relation to obex level in SUDEP cases. We also identified TH‐IR neurones in the MR and co‐expression of TPH and TH in a minority of neurones. This highlights the complex integration of medullary catecholaminergic systems with respiratory regulatory groups and subtle regional distribution differences that may still impact on autonomic dysregulation during seizures.
The VLM catecholaminergic neurones, including C1 adrenergic neurones, are critical for the regulation of arterial BP but also orchestrate a range of physiological stress responses, including breathing regulation in hypoxia (10) and sleep‐state dependent cardiorespiratory arousal (1), both of potential relevance to SUDEP. Our finding however of preserved medullary catecholaminergic neurones in SUDEP, contrasts with Multiple System Atrophy (MSA) sudden death cases where loss of VLM TH‐IR neurones was observed in some (5, 39) but not all studies (32). SIDS also shares circumstantial similarities with SUDEP, with deaths often occurring at night, in the prone position and negative post‐mortem examinations. A reduction of TH‐IR neurones in the VLM, DMNV, and NTS has been reported in some (28, 30) but not in all SIDS series (35). Methodological differences need to be considered when drawing comparisons between post‐mortem studies; many use single sections at one level of the medulla, do not account for obex level, use different antibodies or detection methods for TH and have applied semi‐quantitative estimates of neuronal number (23). In this series we have used serial sections through the medulla and whole slide scanning image analysis for unbiased evaluation of the entire regions of interest. Through alignment with obex levels we have demonstrated detailed rostral‐caudal variations in TH‐IR densities in the VLM, DMM, and MR in the human medulla as also alluded to in earlier descriptive studies (18, 19).
Preservation of TH neurones in SUDEP, however, does not exclude catecholaminergic neuronal dysfunction. C‐fos, an early/immediate gene is an established marker of recent neuronal and synaptic activation, with mRNA expression appearing within 30 minutes following activation and protein at 2 hours (15), including following a seizure (34). C‐fos is extensively used in experimental epilepsy to determine the distribution and time‐course of acute seizure‐related brain injury (4, 14, 25). Following experimental seizures, c‐fos immunoreactivity in TH‐IR neurones in the VLM and dorsal vagal complex mirrored that observed following hypoxia (17) and was similar to the distribution following experimentally induced hypotension; this could suggest that seizures themselves directly induce TH neuronal activity. C‐fos expression was also noted in NTS and VLM TH and serotonergic neurones following induction of the protective laryngeal chemoreflex which induces respiratory inhibition, bradycardia, and hypertension (44). A previous post‐mortem study of sudden unexplained death, showed c‐fos‐expressing neurones in the dorsal medulla (hypoglossal nucleus and/or the dorsal vagal nucleus) but not in control groups (13). In the current study, we noted that c‐fos in the medulla mainly corresponded to autonomic regions enriched with TH and TPH neurones, and showed occasional co‐expression of c‐fos with TH and TPH. This pattern could suggest regionally selective neuronal c‐fos activation in the period prior to death rather than global factors such as pre‐mortem hypoxia, seizures, or spreading depolarization. However, c‐fos interpretation requires caution, particularly in post‐mortem tissues; lack of expression does not preclude neuronal activation, it is not expressed after prolonged or chronic activity (15, 34) and multiple potential pre‐morbid factors complicate any analysis (14). Therefore, although significantly fewer c‐fos neurones were noted in SUDEP compared to non‐epilepsy sudden death controls this may not implicate deficient autonomic neuronal activation.
We noted differences in the distribution of medullary TH neurones between study groups, including along the dorsal‐ventral aspect in epilepsy cases and rostro‐caudal aspect in SUDEP. TH‐expressing medullary neurones are among the first neurones generated, identified at 4.5 weeks gestation (42), forming distinct ventral and dorsal groups but also an ‘intermediate group’ of immature neurones at 14.5 weeks (22). The dorsal group mature into C2/3 neurones and the ventral groups to the C1neurones in the VLM, but the fate of the intermediate neurones is unknown. Maturational delay in medullary autonomic neurones has been proposed in SIDS (23) and we noted increased TH‐IR with age at death, more particularly in the SUDEP group which could indicate ongoing/delayed maturation or possibly modulation, in response to chronic intermittent seizures and hypoxia, as in experimental studies (9).We also identified TH neurones in the MR region, with higher densities in rostral levels and significantly higher TH neuronal density in the Dravet syndrome cases compared to SUDEP. Furthermore, we noted occasional neurones that co‐expressed both TH and TPH2; catecholaminergic and serotonergic neurones of the medulla are generally recognized as distinct populations with limited co‐expression (3, 41, 45). Difference in the distribution and phenotype of TH medullary neurones in epilepsy and SUDEP groups compared to controls may have functional and connectivity implications but require further investigation.
The limitations of our studies include the variation of tissue fixation times and protocols as tissue was obtained from different eras and centers and post‐mortem delays with potential effects on immunostaining, particularly c‐fos. We included a wide age range and in three cases (age 79,80 and 84; in EPC (2) and NEC groups) age‐related phosphorylated‐tau (Braak stage II) and small vessel disease was observed in sampled brain regions and we cannot exclude a neurodegenerative contribution of neuronal loss in the medulla in these cases. TH identifies all medullary catecholaminergic neurones, and further studies to explore differences in functional subsets of TH‐IR neurones, including adrenergic [Phenylethanolamine N‐methyltransferase (PNMT)], noradrenergic [Dopa‐β‐hydroxylase (DPH)] and dopaminergic populations, as in SIDS studies (20), is required in SUDEP in addition to study of other catecholaminergic nuclei, including the locus coeruleus.
In summary, although we have no conclusive evidence for alteration of medullary TH neuronal numbers in SUDEP from this series, differences in their relative distribution and c‐fos activation were observed in the findings. Functional alterations of medullary catecholaminergic neurones in SUDEP cannot be excluded at this stage which requires further interrogation.
Conflict of Interest
None of the authors has any conflict of interest to disclose. We confirm that we have read the Journal’s position on issues involved in ethical publication and affirm that this report is consistent with those guidelines.
Supporting information
Fig S1
Figure S1. Image analysis regions of interest and cell detection. A. Low power image through medulla section with the regions of interest (ROI) as defined with image analysis software (TH labeling is shown as orange overlay). A line was drawn from the central recess of the fourth ventricle to the dorsomedial edge of the inferior olive nucleus. A second line is drawn from the central recess of the fourth ventricle to the lateral edge of the solitary tract. A third line is drawn from the lateral edge of the solitary tract parallel to the first line to the lateral medullar surface. The fourth line joined the first and third line, tracing the medullary surface. This made a quadrilateral shape which was then divided at the midpoint to form two smaller ROI, the ventrolateral medulla (VLM) ROI and the dorsomedial medulla (DMM) which included part of the nucleus of tractus solitarius (NTS). The third ROI (medullary raphé, MR) was a rectangle formed by the midline, extending from ventricle to the level of the olive with a lateral width of 1.4 mm to include the Raphé obscurus. All ROI were drawn on both sides of the medulla or on one side in hemi‐brainstem samples; measured values were averaged for one side. B. In the VLM distinct neuronal labeling of medium to large size fusiform and triangular neurones with thick dendritic processes and small numbers of intervening axons. C. The same images thresholded with the image analyses to define overall labeling (neurones and processes) and to distinguish single neurones (outlined in red). Method detailed in supplementary method file.
Fig S2
Figure S2. Scatter graph of TH neuronal densities in the DMM and age at death. There was a positive correlation for all cases but when split into the three cause of death groups this correlation remained significant only in the SUDEP group. (Dotted line shows linear regression, P = 0.0037).
Fig S3
Figure S3. C‐Fos labeling in relation to post‐mortem interval and fixation time. Semi‐quantitative scores for the three main cause of death groups (SUDEP, epilepsy controls (EPC) and non‐epilepsy controls (NEC) shown for the three regions of interest (DMM = dorsomedial medulla, VLM = ventrolateral medulla, MR = median raphé) at increasing intervals of fixation time (FXT) and post‐mortem intervals (PMI). There was a negative correlation with c‐fos labeling and longer fixation times in the post‐mortem series (P < 0.05) but a positive correlation with post‐mortem interval (P < 0.05).
Supplementary Material
Acknowledgments
UCL is part of the Center for SUDEP Research (CSR) and supported through the National Institute of Neurological Disorders And Stroke of the National Institutes of Health (Award Numbers neuropathology of SUDEP: 5U01NS090415 and SUDEP admin core grant: U01‐NS090405). Epilepsy Society supports SMS, and through the Katy Baggott Foundation, supports the UCL Epilepsy Society Brain and Tissue Bank. This work was undertaken at UCLH/UCL who received a proportion of funding from the Department of Health’s NIHR Biomedical Research Centres funding scheme. We are very grateful for provision of additional SUDEP and control material for this study from the following resources: The MRC Sudden Death Brain Bank in Edinburgh (cases detailed in additional methods file). Tissue samples were also obtained from David Hilton at Derriford Hospital as part of the UK Brain Archive Information Network (BRAIN UK) which is funded by the Medical Research Council and Brain Tumour Research.
Data Availability Statement
The data that support the findings of this study are available from the corresponding author upon reasonable request.
References
- 1. Abbott SB, Coates MB, Stornetta RL, Guyenet PG (2013) Optogenetic stimulation of c1 and retrotrapezoid nucleus neurons causes sleep state‐dependent cardiorespiratory stimulation and arousal in rats. Hypertension 61:835–841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Allen LA, Harper RM, Lhatoo S, Lemieux L, Diehl B (2019) Neuroimaging of sudden unexpected death in epilepsy (SUDEP): insights from structural and resting‐state functional MRI Studies. Front Neurol 10:185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Arai R, Karasawa N, Nagatsu T, Nagatsu I (1995) Exogenous L‐5‐hydroxytryptophan is decarboxylated in neurons of the substantia nigra pars compacta and locus coeruleus of the rat. Brain Res 669:145–149. [DOI] [PubMed] [Google Scholar]
- 4. Barros VN, Mundim M, Galindo LT, Bittencourt S, Porcionatto M, Mello LE (2015) The pattern of c‐Fos expression and its refractory period in the brain of rats and monkeys. Front Cell Neurosci 9:72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Benarroch EE, Schmeichel AM, Low PA, Boeve BF, Sandroni P, Parisi JE (2005) Involvement of medullary regions controlling sympathetic output in Lewy body disease. Brain 128(Pt 2):338–344. [DOI] [PubMed] [Google Scholar]
- 6. Bruno E, Maira G, Biondi A, Richardson MP (2018) Ictal hypoxemia: a systematic review and meta‐analysis. Seizure 63:7–13. [DOI] [PubMed] [Google Scholar]
- 7. Devinsky O, Hesdorffer DC, Thurman DJ, Lhatoo S, Richerson G (2016) Sudden unexpected death in epilepsy: epidemiology, mechanisms, and prevention. Lancet Neurol 15:1075–1088. [DOI] [PubMed] [Google Scholar]
- 8. Esmaeili B, Kaffashi F, Theeranaew W, Dabir A, Lhatoo SD, Loparo KA (2018) Post‐ictal modulation of baroreflex sensitivity in patients with intractable epilepsy. Front Neurol 9:793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Givan SA, Cummings KJ (2016) Intermittent severe hypoxia induces plasticity within serotonergic and catecholaminergic neurons in the neonatal rat ventrolateral medulla. J Appl Physiol 120:1277–1287. [DOI] [PubMed] [Google Scholar]
- 10. Guyenet PG, Stornetta RL, Bochorishvili G, Depuy SD, Burke PG, Abbott SB (2013) C1 neurons: the body's EMTs. Am J Physiol Regul Integr Comp Physiol 305:R187–R204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Hampel KG, Elger CE, Surges R (2017) Impaired baroreflex sensitivity after bilateral convulsive seizures in patients with focal epilepsy. Front Neurol 8:210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Hampel KG, Jahanbekam A, Elger CE, Surges R (2016) Seizure‐related modulation of systemic arterial blood pressure in focal epilepsy. Epilepsia 57:1709–1718. [DOI] [PubMed] [Google Scholar]
- 13. Hayashi M, Sakuma H (2016) Immunohistochemical analysis of brainstem lesions in the autopsy cases with severe motor and intellectual disabilities showing sudden unexplained death. Front Neurol 7:93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Herrera DG, Robertson HA (1996) Activation of c‐fos in the brain. Prog Neurogibol 50:83–107. [DOI] [PubMed] [Google Scholar]
- 15. Hudson AE (2018) Genetic reporters of neuronal activity: c‐Fos and G‐CaMP6. Methods Enzymol 603:197–220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Kang JJ, Liang WH, Lam CS, Huang XF, Yang SJ, Wong‐Riley MT et al (2017) Catecholaminergic neurons in synaptic connections with pre‐Botzinger complex neurons in the rostral ventrolateral medulla in normoxic and daily acute intermittent hypoxic rats. Exp Neurol 287(Pt 2):165–175. [DOI] [PubMed] [Google Scholar]
- 17. Kanter RK, Strauss JA, Sauro MD (1996) Comparison of neurons in rat medulla oblongata with fos immunoreactivity evoked by seizures, chemoreceptor, or baroreceptor stimulation. Neuroscience 73:807–816. [DOI] [PubMed] [Google Scholar]
- 18. Kitahama K, Denoroy L, Goldstein M, Jouvet M, Pearson J (1988) Immunohistochemistry of tyrosine hydroxylase and phenylethanolamine N‐methyltransferase in the human brain stem: description of adrenergic perikarya and characterization of longitudinal catecholaminergic pathways. Neuroscience 25:97–111. [DOI] [PubMed] [Google Scholar]
- 19. Kitahama K, Sakamoto N, Jouvet A, Nagatsu I, Pearson J (1996) Dopamine‐beta‐hydroxylase and tyrosine hydroxylase immunoreactive neurons in the human brainstem. J Chem Neuroanat 10:137–146. [DOI] [PubMed] [Google Scholar]
- 20. Kopp N, Chigr F, Denoroy L, Gilly R, Jordan D (1993) Absence of adrenergic neurons in nucleus tractus solitarius in sudden infant death syndrome. Neuropediatrics 24:25–29. [DOI] [PubMed] [Google Scholar]
- 21. Lacuey N, Zonjy B, Hampson JP, Rani MRS, Zaremba A, Sainju RK et al (2018) The incidence and significance of periictal apnea in epileptic seizures. Epilepsia 59:573–582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Lorke DE, Kwong WH, Chan WY, Yew DT (2003) Development of catecholaminergic neurons in the human medulla oblongata. Life Sci 73:1315–1331. [DOI] [PubMed] [Google Scholar]
- 23. Machaalani R, Waters KA (2014) Neurochemical abnormalities in the brainstem of the Sudden Infant Death Syndrome (SIDS). Paediatr Respir Rev 15:293–300. [DOI] [PubMed] [Google Scholar]
- 24. Malheiros‐Lima MR, Takakura AC, Moreira TS (2017) Depletion of rostral ventrolateral medullary catecholaminergic neurons impairs the hypoxic ventilatory response in conscious rats. Neuroscience 351:1–14. [DOI] [PubMed] [Google Scholar]
- 25. Mraovitch S, Calando Y (1999) Interactions between limbic, thalamo‐striatal‐cortical, and central autonomic pathways during epileptic seizure progression. J Comp Neurol 411:145–161. [PubMed] [Google Scholar]
- 26. Mueller SG, Nei M, Bateman LM, Knowlton R, Laxer KD, Friedman D et al (2018) Brainstem network disruption: a pathway to sudden unexplained death in epilepsy? Hum Brain Mapp 39:4820–4830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Nass RD, Hampel KG, Elger CE, Surges R (2019) Blood pressure in seizures and epilepsy. Front Neurol 10:501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Obonai T, Yasuhara M, Nakamura T, Takashima S (1998) Catecholamine neurons alteration in the brainstem of sudden infant death syndrome victims. Pediatrics 101:285–288. [DOI] [PubMed] [Google Scholar]
- 29. Ogren JA, Tripathi R, Macey PM, Kumar R, Stern JM, Eliashiv DS et al (2018) Regional cortical thickness changes accompanying generalized tonic‐clonic seizures. Neuroimage Clin 20:205–215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Ozawa Y, Takashima S (2002) Developmental neurotransmitter pathology in the brainstem of sudden infant death syndrome: a review and sleep position. Forensic Sci Int 130(Suppl):S53–S59. [DOI] [PubMed] [Google Scholar]
- 31. Patodia S, Somani A, O'Hare M, Venkateswaran R, Liu J, Michalak Z et al (2018) The ventrolateral medulla and medullary raphe in sudden unexpected death in epilepsy. Brain 141:1719–1733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Riku Y, Watanabe H, Mimuro M, Iwasaki Y, Ito M, Katsuno M et al (2017) Non‐motor multiple system atrophy associated with sudden death: pathological observations of autonomic nuclei. J Neurol 264:2249–2257. [DOI] [PubMed] [Google Scholar]
- 33. Ryvlin P, Nashef L, Lhatoo SD, Bateman LM, Bird J, Bleasel A et al (2013) Incidence and mechanisms of cardiorespiratory arrests in epilepsy monitoring units (MORTEMUS): a retrospective study. Lancet Neurology 12:966–977. [DOI] [PubMed] [Google Scholar]
- 34. Sauvage M, Kitsukawa T, Atucha E (2019) Single‐cell memory trace imaging with immediate‐early genes. J Neurosci Methods 326:108368. [DOI] [PubMed] [Google Scholar]
- 35. Sawaguchi T, Ozawa Y, Patricia F, Kadhim H, Groswasser J, Sottiaux M et al (2003) Catecholaminergic neurons in the brain‐stem and sleep apnea in SIDS victims. Early Hum Dev 75(Suppl):S41–S50. [DOI] [PubMed] [Google Scholar]
- 36. Sevigny CP, Bassi J, Williams DA, Anderson CR, Thomas WG, Allen AM (2012) Efferent projections of C3 adrenergic neurons in the rat central nervous system. J Comp Neurol 520:2352–2368. [DOI] [PubMed] [Google Scholar]
- 37. Silveira DC, Schachter SC, Schomer DL, Holmes GL (2000) Flurothyl‐induced seizures in rats activate Fos in brainstem catecholaminergic neurons. Epilepsy Res 39:1–12. [DOI] [PubMed] [Google Scholar]
- 38. Simon RP (2010) Heart and lung in the postictal state. Epilepsy Behav 19:167–171. [DOI] [PubMed] [Google Scholar]
- 39. Tada M, Kakita A, Toyoshima Y, Onodera O, Ozawa T, Morita T et al (2009) Depletion of medullary serotonergic neurons in patients with multiple system atrophy who succumbed to sudden death. Brain 132(Pt 7):1810–1819. [DOI] [PubMed] [Google Scholar]
- 40. Thurman DJ, Logroscino G, Beghi E, Hauser WA, Hesdorffer DC, Newton CR et al (2017) The burden of premature mortality of epilepsy in high‐income countries: a systematic review from the Mortality Task Force of the International League Against Epilepsy. Epilepsia 58:17–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Vandenbergh DJ, Mori N, Anderson DJ (1991) Co‐expression of multiple neurotransmitter enzyme genes in normal and immortalized sympathoadrenal progenitor cells. Dev Biol 148:10–22. [DOI] [PubMed] [Google Scholar]
- 42. Verney C (1999) Distribution of the catecholaminergic neurons in the central nervous system of human embryos and fetuses. Microsc Res Tech 46:24–47. [DOI] [PubMed] [Google Scholar]
- 43. Vilella L, Lacuey N, Hampson JP, Rani MRS, Sainju RK, Friedman D et al (2018) Postconvulsive central apnea as a biomarker for sudden unexpected death in epilepsy (SUDEP). Neurology 92:e171–e182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Wang X, Guo R, Zhao W (2015) Distribution of fos‐like immunoreactivity, catecholaminergic and serotoninergic neurons activated by the laryngeal chemoreflex in the medulla oblongata of rats. PLoS One 10:e0130822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Weihe E, Depboylu C, Schutz B, Schafer MK, Eiden LE (2006) Three types of tyrosine hydroxylase‐positive CNS neurons distinguished by dopa decarboxylase and VMAT2 co‐expression. Cell Mol Neurobiol 26:659–678. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Fig S1
Figure S1. Image analysis regions of interest and cell detection. A. Low power image through medulla section with the regions of interest (ROI) as defined with image analysis software (TH labeling is shown as orange overlay). A line was drawn from the central recess of the fourth ventricle to the dorsomedial edge of the inferior olive nucleus. A second line is drawn from the central recess of the fourth ventricle to the lateral edge of the solitary tract. A third line is drawn from the lateral edge of the solitary tract parallel to the first line to the lateral medullar surface. The fourth line joined the first and third line, tracing the medullary surface. This made a quadrilateral shape which was then divided at the midpoint to form two smaller ROI, the ventrolateral medulla (VLM) ROI and the dorsomedial medulla (DMM) which included part of the nucleus of tractus solitarius (NTS). The third ROI (medullary raphé, MR) was a rectangle formed by the midline, extending from ventricle to the level of the olive with a lateral width of 1.4 mm to include the Raphé obscurus. All ROI were drawn on both sides of the medulla or on one side in hemi‐brainstem samples; measured values were averaged for one side. B. In the VLM distinct neuronal labeling of medium to large size fusiform and triangular neurones with thick dendritic processes and small numbers of intervening axons. C. The same images thresholded with the image analyses to define overall labeling (neurones and processes) and to distinguish single neurones (outlined in red). Method detailed in supplementary method file.
Fig S2
Figure S2. Scatter graph of TH neuronal densities in the DMM and age at death. There was a positive correlation for all cases but when split into the three cause of death groups this correlation remained significant only in the SUDEP group. (Dotted line shows linear regression, P = 0.0037).
Fig S3
Figure S3. C‐Fos labeling in relation to post‐mortem interval and fixation time. Semi‐quantitative scores for the three main cause of death groups (SUDEP, epilepsy controls (EPC) and non‐epilepsy controls (NEC) shown for the three regions of interest (DMM = dorsomedial medulla, VLM = ventrolateral medulla, MR = median raphé) at increasing intervals of fixation time (FXT) and post‐mortem intervals (PMI). There was a negative correlation with c‐fos labeling and longer fixation times in the post‐mortem series (P < 0.05) but a positive correlation with post‐mortem interval (P < 0.05).
Supplementary Material
Data Availability Statement
The data that support the findings of this study are available from the corresponding author upon reasonable request.


