Abstract
Neuroinflammation is thought to play a pivotal role in the pathogenesis of periventricular white matter (PWM) damage (PWMD) induced by neonatal sepsis. Because the complement cascade is implicated in inflammatory response, this study was carried out to determine whether C3a is involved in PWMD, and, if so, whether it would induce axonal hypomyelination. Furthermore, we explored if C3a would act through its C3a receptor (C3aR) and thence inhibit maturation of oligodendrocyte precursor cells (OPCs) via the WNT/β‐catenin signal pathway. Sprague Dawley (SD) rats aged 1 day were intraperitoneally injected with lipopolysaccharide (LPS) (1 mg/kg). C3a was upregulated in activated microglia and astrocytes in the PWM up to 7 days after LPS injection. Concomitantly, enhanced C3aR expression was observed in NG2+ oligodendrocytes (OLs). Myelin proteins including CNPase, PLP, MBP and MAG were significantly reduced in the PWM of 28‐day septic rats. The number of PLP+ and MBP+ cells was markedly decreased. By electron microscopy, myelin sheath thickness was thinner and the average g‐ratios were higher. This was coupled with an increase in number of NG2+ cells and decreased number of CC1+ cells. Olig1, Olig2 and SOX10 protein expression was significantly reduced in the PWM after LPS injection. Very strikingly, C3aRa administration for the first 7 days could reverse the above‐mentioned pathological alterations in the PWM of septic rats. When incubated with C3a, expression of MBP, CNPase, PLP, MAG, Olig1, Olig2, SOX10 and CC1 in primary cultured OPCs was significantly downregulated as opposed to increased NG2. Moreover, WNT/β‐catenin signaling pathway was found to be implicated in inhibition of OPCs maturation and differentiation induced by C3a in vitro. As a corollary, it is speculated that C3a in the PWM of septic rats is closely associated with the disorder of OPCs differentiation and maturation through WNT/β‐catenin signaling pathway, which would contribute ultimately to axonal hypomyelination.
Keywords: complement 3a, hypomyelination, microglia, sepsis, WNT/β‐catenin signaling
Abbreviations
- C3a
complement 3 a
- C3aR
complement 3a receptor
- C3aRa
complement 3a receptor antagonist
- CC1
CEACAM1a
- CNPase
2′, 3′‐cyclic nucleotide 3′‐phosphodiesterase
- CNS
central nervous system
- GFAP
glial fibrillary acidic protein
- LPS
lipopolysaccharide
- MAG
myelin associated glycoprotein
- MBP
myelin basic protein
- NG2
NG2 chondroitin sulfate proteoglycan
- Olig1/2
oligodendrocyte transcription factor 1/2
- OLs
oligodendrocytes
- OPCs
oligodendrocyte progenitor cells
- PLP
proteolipid protein
- PWM
periventricular white matter
- PWMD
periventricular white matter damage
- TMEM119
transmembrane protein 119
Introduction
Neonatal sepsis is one of the most common causes of newborn death especially preterm as well as very low birth weight infants, accounting for more than 30% of neonatal mortality 22, 51. Therefore, prevention of neonatal sepsis in infants born very preterm (<32 weeks' gestation) is clearly desirable as this would contribute to reducing mortality and morbidity 22, 59. There is ample evidence indicating that neonatal sepsis can lead to long‐term disability, including cerebral palsy, cognitive deficit, delayed mental and psychomotor, vision and hearing impairment 1, 8, 26, 30, 31, 37. Periventricular white matter damage (PWMD) is a distinctive pathological feature of brain injury in septic neonates 38, 63. It is closely associated with long‐term neurological deficits. The pathogenesis of PWMD is complex and remains obscure. In our previous studies, we reported that PWMD induced by neonatal sepsis was associated with microglial activation, diffuse reactive astrogliosis, axonal injury, oligodendrocyte progenitor apoptosis and myelination disturbances. 34, 65 Neuroinflammation elicited by sepsis plays a key role in the pathogenesis of PWMD 9, 38, 65. It is well recognized that PWMD is accompanied by prominent neuroinflammation manifested by microgliosis, reactive astrogliosis and elevated levels of proinflammatory cytokines 11, 50, 65. Recent studies have provided strong evidence that innate immune response caused by microglia activation may lead to the developmental impairment of the central nervous system 3, 48.
The complement system plays a pivotal role in the innate immunity of CNS in aging and disease progression 16, 23, 48. The complement pathway is an important regulatory factor of innate immunity. C3, one of the central complement factors, was cleaved by C3 invertase in two fragments of C3a and C3b respectively, which lead to downstream events by binding to their receptors C3aR and CR3 71. Previous studies have shown that some resident cells such as macrophages and glial cells may generate complement components 2, 52, 54. It is relevant to note that C3a and C5a receptors are localized in different kinds of glial cells, neurons and neural progenitor cells 6. Of the various glial cell types, activated microglia and astrocytes are innate immune cells which play a key role in neuroinflammation through complement receptors 2, 6, 34, 52, 54, 65. It has been reported in a recent study that complement C3 and C3aR were activated in Alzheimer's disease and this had led to the tauopathy conditions and the synapse loss. Interestingly, this is associated with the activated microglia and astroglia 16. Of note, C3/C3a‐null mice or treatment with C3aR inhibitor would contribute to preventing microglial activation and protecting the neurodevelopment from neuroimpairment events 48. These studies suggest that C3–C3aR signaling is involved in the CNS inflammatory response.
We reported previously axonal hypomyelination in the PWM of neonatal rats at 14 and 28 days after LPS injection 34, 65. However, the role of the complement system in PWMD in septic neonatal brain has remained obscure and indeed to be explored. This study aims to investigate if C3a would contribute to the pathogenesis of PWMD in septic neonatal brain. First, intense C3a expression was localized in the activated microglia and astrocytes in the PWM after LPS injection. Next, we ascertained whether NG2+ OPCs in septic neonatal brain would be its potential target by expressing complement 3a receptor, and if so, whether the maturation of OPCs would be affected. In this connection, we took into consideration of reduction of myelin sheath thickness as well as inhibition of OPCs maturation associated transcription factors such as Olig1, Olig2 and SOX10. In addition, we sought to elucidate whether C3a would suppress the maturation and differentiation of OPCs in vitro through activating the canonical WNT/β‐catenin signaling pathway relating to myelination failure 7, 69. We report here with supporting experimental evidence that C3a can indeed contribute to inducing axonal hypomyelination in septic neonatal brain through the WNT/β‐catenin signaling thus underscoring the crucial role of C3a in PWMD.
Materials and Methods
Animals
Sprague Dawley (SD) rats (1‐dayold) were obtained from the Laboratory Animal Center of Sun Yat‐sen University (Guangzhou, China). All the rats were housed in standard conditions, 12 h/12 h light/dark cycle, controlled temperature of 23 ± 2°C, and 50%–65% humidity, with free access to standard chow and water, according to the guidelines provided by Sun Yat‐sen University. They were randomly divided into three groups: the control group, the LPS injection group and the LPS + C3aRa injection group. The rats in the septic model group were intraperitoneally injected with lipopolysaccharide (LPS) (1 mg/kg) derived from Escherichia coli 055: B5 (Sigma‐Aldrich, St. Louis, MO, USA Cat. No. L2880), while the control group was intraperitoneally administered with an equal volume of 0.01M phosphate buffered saline (PBS). The rats in the LPS + C3aRa injection group were injected intraperitoneally with C3a receptor antagonist (C3aRa) (SB 290157 (trifluoroacetate salt), MedChemExpress, Cat. No. HY‐101502A) (10mg/kg) 4, 40 at 10 minutes after LPS injection (1 mg/kg). On the following day, the rats in the LPS + C3aRa injection group were injected intraperitoneally with C3aRa once daily for 6d, while the rats in the other two groups were intraperitoneally injected with an equal volume of PBS. The rats in each group were sacrificed at 6 and 24 h, 3, 7, 14 and 28 days. The number of rats in each group is given in Table 1. All rats were anesthetized with pentobarbital (30 mg/kg), and perfused with 0.01M PBS followed by using 4% paraformaldehyde solution. Subsequently, the brain was removed and processed accordingly. All experiments were handled in accordance with an approved protocol from the Institutional Animal Care and Use Committee (IACUC), Guangdong Provincial People's Hospital, Guangdong Province, China (Animal Certificate No.: SYXK2012‐0081) and performed in accordance with IACUC guidelines. All experimental animals were comply followed by ARRIVE guidelines. During the whole experiments, animal suffering was reduced as much as possible.
Table 1.
Number of rats killed at various time points after the LPS intraperitoneal injection (inside the round brackets) or LPS + C3aRa intraperitoneal injection (inside the square brackets) and their age‐matched controls for different experiments (outside the brackets).
| Age | Immunofluorescence | Western blotting | In situ hybridization | Electron microscopy |
|---|---|---|---|---|
| 6 h | 0 | 6 (6) | 0 | 0 |
| 24 h | 5 (5) | 6 (6) | 0 | 0 |
| 3 day | 5 (5) [5] | 6 (6) | 0 | 0 |
| 7 day | 5 (5) [5] | 6 (6) [6] | 0 | 0 |
| 14 day | 5 (5) [5] | 6 (6) [6] | 5 (5) [5] | 0 |
| 28 day | 5 (5) [5] | 6 (6) [6] | 5 (5) [5] | 5 (5) [5] |
Primary culture of OPCs
The cerebral cortex was carefully dissected from the brain of 1‐day‐old SD rats (obtained from Laboratory Animal Center, Sun Yat‐sen University, Guangdong Province, China) and mechanically dissociated into single‐cell suspensions by repeated pipetting. The mixed glia cells were then plated on a 75‐cm2 flask at a density of 1.2 × 106 cells/mL in Dulbecco's modified Eagle's medium/F12 medium (DMEM/F12, Gibco) supplemented with 10% fetal bovine serum (FBS) (Hyclone, Logan, UT).
The mixed cells were cultured for 7–8 days in a humidified 5% CO2 incubator at 37 °C, and half of the medium was replaced every 2 or 3 days. After shaking at 180 revolutions per minute (rpm) and 37°C for 1 h to remove microglial cells, the medium was replaced with fresh DMEM/F12/FBS and the cultured cells were again shaken at 250 rpm and 37°C for 18–20 h to harvest OPCs. The harvested cells were first incubated on a 10‐cm Petri dish for 60 minutes at 37°C to remove mixed astrocytes and microglia. After this, the purified OPCs (5 × 105 cells per well) were plated on a 6‐multiwell culture dish with poly‐L‐lysine (PLL) and cultured in proliferating medium (OPCM)‐containing platelet‐derived growth factor‐AA (PDGF‐AA) and neurotrophin‐3 (NT‐3) 12, 19 for 1 day or in differentiating medium (OPCDM) containing thyroid hormone 66 for 4 days. After treating with OPCM for 1 day, the purity of OPCs was assessed by oligodendrocyte transcription factor 2 (Olig2), a widely used marker for OPCs, and 4′‐6‐diamidino‐2‐phenylindole (DAPI), a nuclear marker of all cells. The OPCs cultures with above 96% purity were used in this study.
Treatment of OPCs Culture OPCs cultures were divided into four groups.
Group I
To study the effects of C3a on differentiation and maturation of OPCs, purified OPCs were cultured in oligodendrocyte precursor cell medium (OPCM) (Sciencell Research Laboratories, USA, Cat. No. 1601) for 1 day at 5% CO2 and 95% air at 37°C. The cells were digested and plated at the same number in OPCM, and then cultured in oligodendrocyte precursor cell differentiation culture medium (OPCDM) (Sciencell Research Laboratories, USA, No.1631) for 4 days at 5% CO2 and 95% air at 37°C. The OPCs were subdivided into the control group (0.01M PBS); 80 ng/mL C3a group; 80 ng/mL C3a + 80 ng/mL C3aRa (SB 290157) group; 80 ng/mL C3aRa (SB 290157) group.
Group II
To study the effects of C3a on the proliferation of OPCs, purified OPCs were cultured in oligodendrocyte precursor cell medium (OPCM) (Sciencell Research Laboratories, USA, Cat. No. 1601) for 1 day at 5% CO2 and 95% air at 37°C. The OPCs were subdivided into the control group (0.01M PBS); 80 ng/mL C3a group; 80 ng/mL C3a + 80 ng/mL C3aRa (SB 290157) group; and 80 ng/mL C3aRa (SB 290157) group. The OPCs in this group was stained with NG2 and Ki‐67.
Group III
To examine the effects of C3a on the WNT/β‐catenin pathway in differentiated OPCs, purified OPCs were cultured in OPCM for 1 day and then replaced with OPCDM. The OPCs were divided into the control group (0.01M PBS) and C3a treatment group (80 ng/mL). The OPCs treated with C3a were subdivided into five subgroups at 15 and 30 min, 1, 2 and 4 h after administration with C3a. The OPCs in the control group was treated with equal volume of PBS.
Group IV
To investigate whether C3a administration would activate WNT/β‐catenin pathway in the OPCs, XAV‐939 (a selective inhibitor against Wnt/β‐catenin‐mediated transcription by tankyrase1/2) (MedChemExpress, Cat. No. HY‐15147) was used to reverse the effect of C3a administration on the differentiation and maturation of OPCs. The OPCs were divided into control group (0.01M PBS), 80 ng/mL C3a group; 80 ng/mL C3a + 80 ng/mL C3aRa (SB 290157) group, 80 ng/mL C3a + 80 ng/mL XAV‐939 group and 80 ng/mL XAV‐939 group. The OPCs in four groups were cultured in OPCDM for 4 days at 5% CO2 and 95% air at 37°C.
Western blot analysis
Proteins were extracted from PWM of the rats at different time points from three groups or primary OPCs subjected to different treatments using a protein extraction kit (Pierce Biotechnology Inc, IL, USA) according to the manufacturer's protocol. Protein concentrations were measured by the Bradford method using bovine serum albumin (BSA) (Sigma‐Aldrich, St Louis, MO, USA) as a standard. Samples of supernatants containing 20 µg of protein were heated to 95°C for 5 minutes and were separated by sodium dodecyl sulfate–polyacrylamide gel electrophoresis in 10% gels in a Mini‐Protein 3 apparatus (Bio‐Rad Laboratories, Hercules, CA, USA). Protein bands were electroblotted onto 0.22‐µm polyvinylidene fluoride membranes (Bio‐Rad) at 1.5 mA/cm2 of membrane for 1 h in Towbin buffer, pH 8.3, to which 20% (v/v) methanol was added. After transfer, the membranes were blocked with 5% (mass/vol) nonfat dried milk in Tris‐buffered saline (TBS) containing 0.05% Tween 20 (TBST) [0.05% (v/v) Tween‐20 in 20 mm Tris‐HCl buffer, pH 7.6, containing 137 mm sodium chloride] for 1 h, then incubated with the primary antibodies according to the manufacturer's recommendations. Incubation with all the primary antibodies listed in Table 2 was carried out overnight at 4°C. After three washes with TBS with 0.1% Tween‐20, the membranes were incubated with the horseradish peroxidase (HRP)‐conjugated secondary antibodies including anti‐rabbit IgG (1:3000, Cell Signaling Technology, 7074) or anti‐mouse IgG (1:3000, Cell Signaling Technology, 7076), or anti‐chicken IgY (1:2000, Abbkine, A21080) for 1 h. Immunoreactivity was detected by Immobilon Western Chemiluminescent HRP Substrate (Millipore, No. WBKLS0100) and visualized by Image Quant LAS500 (GE healthcare life science). Image J software was used to quantify the intensity of the protein band (n = 6 each).
Table 2.
Primary antibodies used in experiments.
| Antibody | Host | Company | Cat. No. | RRID | Application (concentration) |
|---|---|---|---|---|---|
| C3a/C3 | Chicken | Abcam | ab48581 | AB_869211 | WB (1:1000)/IF (1:200) |
| C3aR | Rabbit | Abcam | ab140777 | AB_2687440 | IF (1:100) |
| C3aR | Mouse | Santa Cruz Biotechnology | sc‐133172 | AB_2066736 | WB (1:1000)/IF (1:50) |
| TMEM119 | Rabbit | Abcam | ab209064 | AB_2800343 | IF (1:100) |
| GFAP | Mouse | Abcam | ab4648 | AB_449329 | IF (1:200) |
| CNPase | Mouse | Cell Signaling Technology | 5664 | AB_10705455 | WB (1:1000)/IF (1:200) |
| MBP | Rabbit | Abcam | ab62631 | AB_956157 | WB (1:1000)/IF (1:200) |
| PLP | Rabbit | Abcam | ab28486 | AB_776593 | WB (1:1000) |
| MAG | Mouse | Abcam | ab89780 | AB_2042411 | WB (1:1000) |
| Olig1 | Rabbit | Abcam | ab124908 | AB_10972689 | WB (1:1000) |
| Olig2 | Rabbit | Abcam | ab109186 | AB_10861310 | WB (1:1000)/IF (1:200) |
| SOX10 | Rabbit | Abcam | ab155279 | AB_2650603 | WB (1:1000) |
| NG2 | Mouse | Abcam | ab50009 | AB_881569 | WB (1:1000)/IF (1:200) |
| CC1 | Mouse | Abcam | ab16794 | AB_443473 | WB (1:1000)/IF (1:200) |
| Ki‐67 | Rabbit | Cell Signaling Technology | 9129 | AB_2687446 | IF (1:200) |
| β‐catenin | Rabbit | Abcam | ab32572 | AB_725966 | WB (1:1000) |
| p‐β‐catenin | Rabbit | Cell Signaling Technology | 9561 | AB_331729 | WB (1:1000) |
| Tcf4 | Mouse | Novus | H00006925‐M03 | AB_2199267 | WB (1:1000) |
| Axin2 | Rabbit | Abcam | ab32197 | AB_2290204 | WB (1:1000) |
| β‐actin | Mouse | Cell Signaling Technology | 58169 | AB_2750839 | WB (1:3000) |
| GAPDH | Rabbit | Cell Signaling Technology | 3683 | AB_1642205 | WB (1:3000) |
Double immunofluorescence
The brain was removed after perfusion with 4% PFA, and soaked in the same fixative overnight at 4°C. Formalin fixed brain was then dehydrated and kept in 30% sucrose solution. Frozen sections were cut at 20 μm thickness mounted on glass slides and stored at −20°C in cryoprotectant. Coronal brain sections derived from different time in control, LPS injection group and LPS + C3aRa group were divided into five groups. The brain sections in group Ⅰ were from PWM of rats sacrificed at 24 h, 3 and 7 days after LPS injection and corresponding controls, and incubated with antibody directed against anti‐C3a and anti‐TMEM119 (transmembrane protein 119, a microglia marker) or anti‐GFAP (an astrocyte marker). The brain sections in group II were from PWM of rats sacrificed at 24 h, 3 and 7 days after LPS injection and corresponding controls, and incubated with antibodies directed against C3aR (1:100, Abcam, ab140777) and anti‐NG2 or C3aR (1:50, Santa Cruz Biotechnology, sc‐133172) and anti‐TMEM119. The brain sections in group Ⅲ were from PWM of rats sacrificed at 14 and 28 days after LPS injection or LPS + C3aRa injection or corresponding controls, and incubated with antibody directed against anti‐CNPase. The brain sections in group Ⅳ were from PWM of rats sacrificed at 7, 14 and 28 days after LPS injection or LPS + C3aRa injection or corresponding controls, and incubated with antibodies directed against anti‐CC1 and anti‐NG2. The brain sections in group Ⅴ were from PWM of rats sacrificed at 3, 7, 14 and 28 days after LPS injection or LPS + C3aRa injection or corresponding controls, and incubated with antibodies directed against anti‐NG2 and anti‐Ki‐67. The brain sections in group VI were from PWM of rats sacrificed at 7, 14 and 28 days after LPS injection or LPS + C3aRa injection or corresponding controls, and incubated with antibody directed against Olig2. Incubation with the primary antibodies was overnight at 4°C. The primary antibodies used are listed in Table 2. After washing three times by PBS, the sections were incubated with Alexa‐conjugated secondary antibodies for 1 h at room temperature: Dylight 549 goat anti‐rabbit IgG (1:100, Abbkine, A23320), Dylight 488 goat anti‐mouse IgG (1:100, Abbkine, A23210) and Dylight 555 goat anti‐chicken IgY (1:100, Abcam, ab150170). After washing 3 times with PBS, DAPI (Sigma‐Aldrich, St. Louis. D9542) was used as nuclear staining. Finally, the sections were mounted with a fluorescent mounting medium.
For cultured OPCs, the cells were treated with C3a or C3a + C3aRa or C3aRa for 4 days. After treatment, the cells were fixed in 4% PFA for 30 min, blocked in 1% BSA for 30 minutes and incubated with primary antibodies overnight at 4°C. Immunofluorescence labeling was carried out using primary antibodies directed against anti‐NG2 or anti‐MBP. Along with this and to explore the effects of C3a on the proliferation of OPCs, the cells were treated with C3a or C3a + C3aRa or C3aRa together with OPCM for 1 day; the treated cells were incubated with the primary antibodies anti‐NG2 and anti‐Ki‐67. After this, the cells were incubated with Dylight 549 goat anti‐rabbit IgG (1:100, Abbkine, A23320) and Dylight 488 goat anti‐mouse IgG (1:100, Abbkine, A23210) for 1 h. Finally, the cells were counterstained with DAPI.
Brain sections or primary cell staining were imaged using a fluorescence microscope (Olympus System Microscope Model BX53, Olympus Company, Tokyo, Japan). Quantitative analysis of cell numbers in the PWM was carried out through counting the labeled cells in eight randomly selected microscopic fields respectively, by a blinded observer. C3a+/TMEM119+/DAPI+ and C3a+/GFAP+/DAPI+ immunofluorescence cells were identified and counted at 100× magnification. On the other hand, C3aR+/NG2+/DAPI+, C3aR+/TMEM119+/DAPI+ and NG2+/Ki‐67+/DAPI+ positive cells were counted at 60× magnification, while the NG2+/DAPI+, CC1+/DAPI+ and Olig2+/DAPI+ cells were counted at 40× magnification.
In the primary OPCs, each area of microscopic fields contained about 40 cells in the control and in the OPCs treated with C3a, C3a + C3aRa and C3aRa. The cells with only a blue nucleus were counted as NG2 or MBP negative, while those with a blue nucleus overlapped with green fluorescence were counted as NG2 or MBP positive. In the immunofluorescence staining of anti‐NG2 and anti‐Ki‐67, the cells with a blue nucleus overlapped with green fluorescence were counted as NG2 positive, while the cells with a blue nucleus overlapped with green and red fluorescence were counted as proliferative NG2 positive. The percentage of cells with positive expression for the respective antibodies was calculated and averaged. Each experiment was done in five times.
Electron microscopy and G‐ratio analysis
For electron microscopy, 28d rats from control, LPS injection group and LPS + C3aRa injection group were transcardially flushed with 0.01 M PBS, followed by perfusion with a mixed aldehyde fixative composed of 2% paraformaldehyde and 3% glutaraldehyde in 0.1M phosphate buffer, pH 7.2. After perfusion, the brain was removed and coronal slices (approximately 1 mm thick) were cut. Blocks of corpus callosum were trimmed from these slices. Vibratome sections (Model 3000TM, The Vibratome Company, St. Louis, MO) of 80–100 μm thickness were prepared from these blocks and rinsed overnight in 0.1M phosphate buffer. They were then postfixed in 1% osmium tetraoxide for 2 h, dehydrated, and embedded in Araldite mixture. Ultrathin sections were cut, double stained with uranyl acetate and lead citrate and observed using in a Philips CM 120 electron microscope (FEI Company, Hillsboro, OR). Electron microscopic images were captured at two different magnifications (4000× and 12 000×). Six non‐overlapping electron microscopic images in each group were randomly selected to analyze myelinated axon number, axon diameter, fiber diameter and g‐ratio. G‐ratios of myelinated axons were calculated as the ratio of the diameter of the axon over the diameter of the axon and the myelin sheath. Measurements were used by Image J software and analyzed by GraphPad software.
In situ hybridization
Coronal frozen brain sections (10 μm thick) from 14‐ and 28‐day‐old rats were cut using a cryostat at − 20°C, and stored at − 80°C before use. The standard process of in situ hybridization was followed according to the method as described earlier 34, 65. Briefly, brain sections were incubated with proteinase K (S3004, Dako, Carpinteria, CA, USA) for 10 minutes and then rinsed in distilled water in 96% ethanol and in isopropanol for 5 minutes each. Following this, the brain sections were incubated with 125 μL of hybridization mixtures composed of 15 μL of distilled water, 25 μL of 20× saline sodium citrate (SSC) buffer, 62.5 μL of 50% formamide, 12.5 μL of 50% dextran sulfate, 2.5 μL of Denhardt's solution (D2532, Sigma‐Aldrich, Saint Louis, MO,USA), 6.25 μL of herring sperm DNA (D7290, Sigma‐Aldrich) and 1.25 μL of 3′‐digoxigenin‐conjugated probe (presented from prof. Hui Fu's lab). The probe, forward primer 5′‐AGCCATACAACAGTCAGGGC‐3′, reverse primer 3′‐TTGTAAAACGACGGCCCAGT‐5′, with a concentration of 100 ng/mL, detects a transcript of the 5.8S ribosomal RNA of PLP. The probe, forward primer 5′‐GCCACACATATCTCCAGCGT‐3′, reverse primer 3′‐TGATTTCGACGTGGCAGAGG‐5′, with a concentration of 100 ng/mL, detects a transcript of the 5.8S ribosomal RNA of MBP. The sections were incubated at 95°C for 6 min, immediately chilled in ice, and then incubated at 40°C for 14–16 h in a humidified chamber. After this, the sections were washed in 2× SSC, 1× SSC, and 0.1× SSC buffer for 5 minutes each, followed by incubation with the anti‐digoxigenin antibody conjugated to alkaline phosphatase, diluted in TBS (1:200, 11093274910, Roche Diagnostics, Indianapolis, IN, USA) for 1 h, and then washed in TBS. Visualization was achieved using NBT/BCIP (nitro blue tetrazolium/5‐bromo‐4‐chloro‐3‐indolyl‐phosphate) (11681451001, Roche Diagnostics) for 1 h in the dark. The reaction was stopped with TE buffer (pH 8.0) for 10 minutes and then washed in distilled water. The sections were counterstained with Mayer's hematoxylin and mounted in aqueous medium (Faramount, S3025, Dako).
PLP and MBP positive cells were observed under a laboratory bright‐field microscope (Olympus System Microscope Model BX53, Olympus Company Pte, Tokyo, Japan). Four non‐overlapping regions of the PWM from each animal were photographed at the magnifications of ×40. For each section, five randomly selected microscope fields were analyzed to calculate MBP and PLP‐positive cells under ×40 magnifications.
Statistical analysis
Graphical data were shown using GraphPad Prism (v7) software. All data were analyzed by IBM SPSS 19.0 statistical software (USA). Different statistical methods were used to analyze different types of data. The data in Figures 1, 2, 4, 5 and Figures [Link], [Link], [Link], [Link], [Link], [Link], [Link], [Link], [Link], [Link], [Link], [Link], with time (days post‐LPS; repeated measure) and treatment (vehicle, LPS or C3aRa) as the independent variables were compared using two‐way ANOVA with Tukey's post hoc test. The data in Figure 3 with axon diameter and treatment (vehicle, LPS or C3aRa) as the independent variables were compared using two‐way ANOVA with Tukey's post hoc test. The univariate factor data in Figures 6, 7 and S7 were analyzed by one‐way ANOVA because the data were homogeneity of variance. Statistical significance was defined as P < 0.05.
Figure 1.
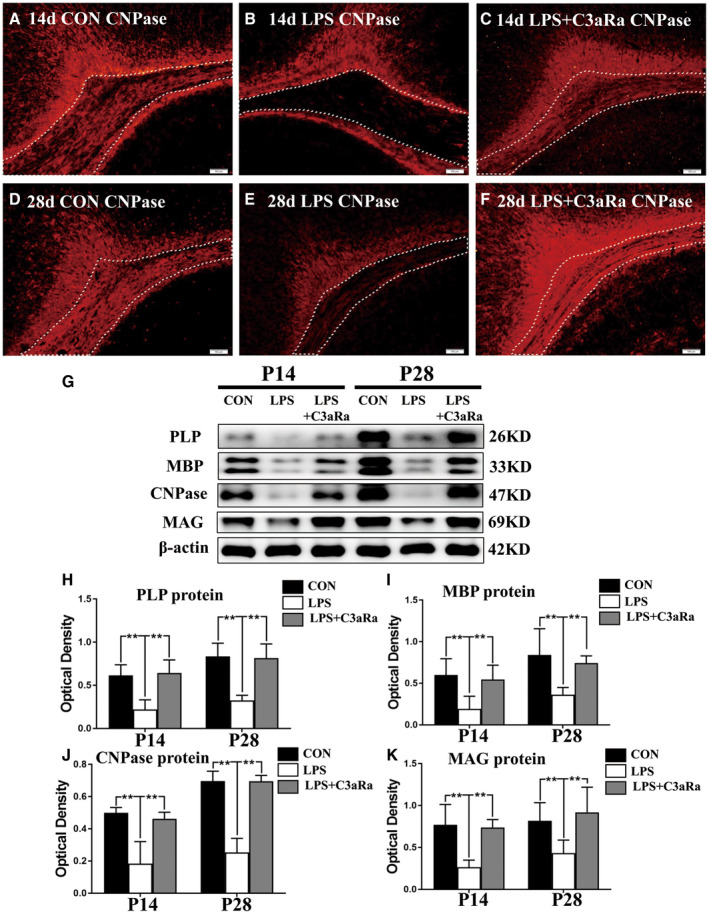
C3aRa administration reverses the expression of myelin‐associated proteins in the PWM of postnatal rats at 14 and 28 days after LPS injection. A–F. Immunohistochemistry (IHC) of CNPase expression in the PWM in postnatal rats at 14 and 28 days after LPS (B, E) or LPS + C3aRa (C, F) injection and their corresponding controls (A, D) at the magnification of ×10 (n = 5) (the area outlined with dotted line). G. Western blot analysis of PLP, MBP, CNPase and MAG protein expression levels in the PWM of postnatal rats at 14 and 28 days after LPS or LPS + C3aRa injection and their corresponding controls. β‐actin served as the loading control (n = 6). H–K. Bar graphs depicting the optical density of CNPase, PLP, MBP and MAG expression shown in G (n = 6). For statistical analysis, two‐way ANOVA with Tukey's post hoc test was used for H, I, J and K and presented as the mean ± standard error of measurement (SEM). Scale bars: 100 μm. *P < 0.05, **P < 0.01.
Figure 2.
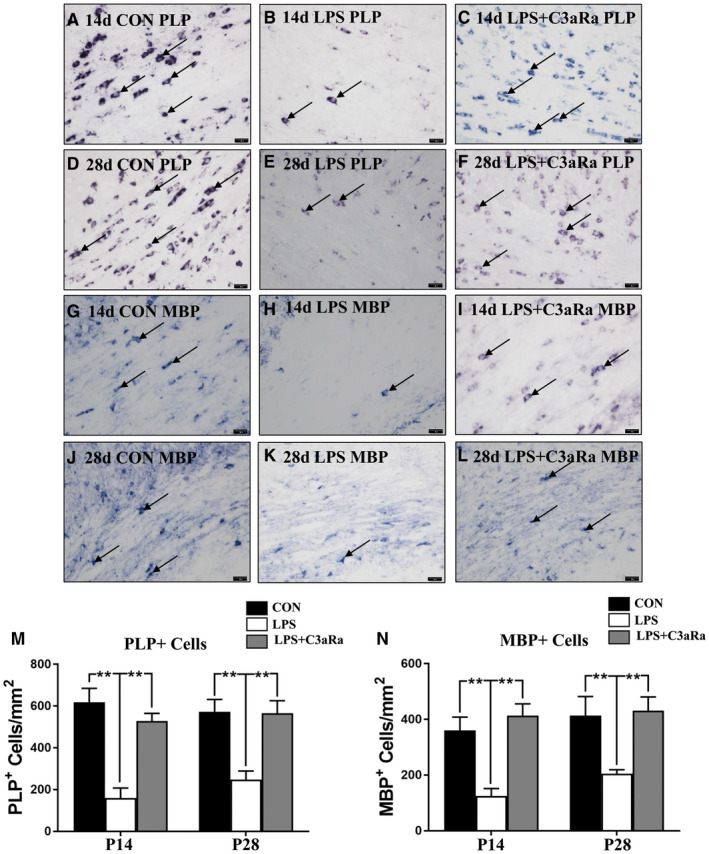
C3aRa administration increase the number of PLP+ and MBP+ oligodendrocytes in the PWM after LPS injection by in situ hybridization. (A–L) In situ hybridization shows the number of PLP+ and MBP+ oligodendrocytes in the PWM at 14 and 28 days after LPS injection, LPS + C3aRa administration and the matching control at the magnification of ×40. Note C3aRa administration reverses the decreased number of PLP+ and MBP+ oligodendrocytes in the PWM induced by LPS exposure at 14 and 28 days (n = 5). Bar graph (M, N) shows the number of PLP+ and MBP+ oligodendrocytes in the PWM at 14 and 28 days using in situ hybridization (n = 5). For statistical analysis, two‐way ANOVA with Tukey's post hoc test was used for M and N and presented as the mean ± standard error of measurement (SEM). Scale bars: 20 μm. *P < 0.05, **P < 0.01.
Figure 4.
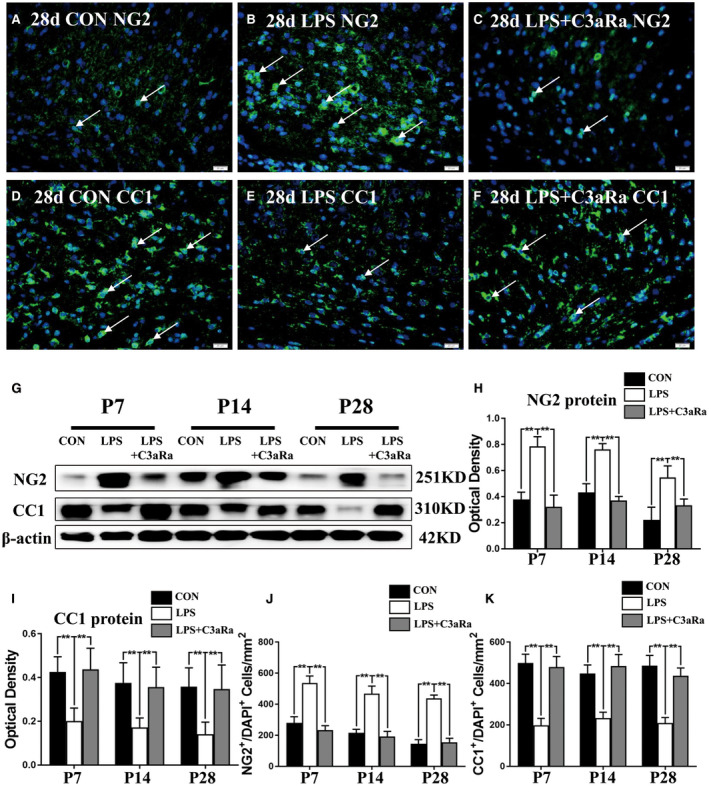
Effect of C3aRa administration on the maturation of OPCs in the PWM of neonatal rats after LPS injection. Immunofluorescence staining showing NG2, CC1 immunoreactive oligodendrocytes (green) and DAPI (blue) in the PWM in postnatal rats at 28 days after LPS injection (B, E), LPS + C3aRa administration (C, F) and their matching controls (A, D) at the magnification of ×40 (n = 5). Panel G shows NG2 (251 kDa), CC1 (310 kDa) and β‐actin (42 kDa) immunoreactive bands (n = 6). (H, I) Bar graphs depict the optical density of NG2 and CC1 expression shown in G (n = 6). (J, K) Bar graphs show the number of NG2+/DAPI+ and CC1+/DAPI+ in the PWM at 7, 14 and 28 days after LPS injection after LPS injection, LPS + C3aRa administration and their matching controls (n = 5). For statistical analysis, two‐way ANOVA with Tukey's post hoc test was used for H, I, J and K and presented as the mean ± standard error of measurement (SEM). Scale bars: 20 μm. *P < 0.05, **P < 0.01.
Figure 5.
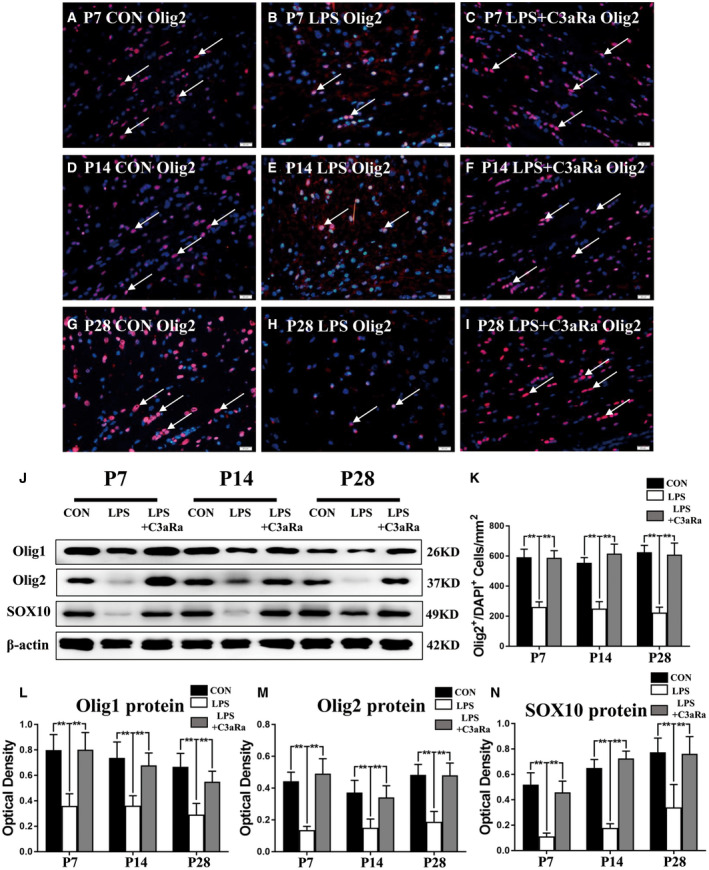
C3aRa administration upregulated the expression of transcription factors involved in oligodendrocyte maturation and differentiation. Immunofluorescence staining showing Olig2 immunoreactive oligodendrocytes (red) and DAPI (blue) in the PWM in postnatal rats at 7, 14 and 28 days after LPS injection (B, E, H) or LPS + C3aRa administration (C, F, I) and their matching controls (A, D, G) at the magnification of ×40 (n = 5). Panel J shows Olig1 (26 kDa), Olig2 (37 kDa), SOX10 (49 kDa) and β‐actin (42 kDa) immunoreactive bands (n = 6). (K) Bar graph shows the number of Olig2+/DAPI+ in the PWM at 7, 14 and 28 days after LPS injection after LPS injection, LPS + C3aRa administration and their matching controls (n = 5). (L–N) Bar graphs depict the optical density of Olig1, Olig2 and SOX10 expression shown in J (n = 6). For statistical analysis, two‐way ANOVA with Tukey's post hoc test was used for K, L, M and N and presented as the mean ± standard error of measurement (SEM). Scale bars: 20 μm. *P < 0.05, **P < 0.01.
Figure 3.
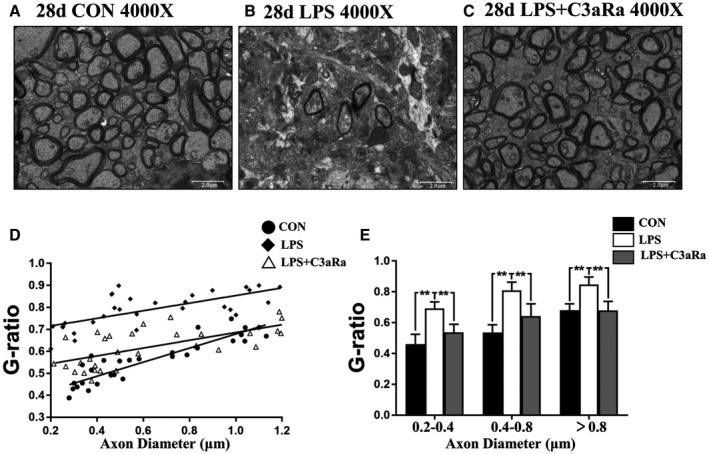
C3aRa administration prevents hypomyelination in the PWM after LPS injection by electron microscope. Electron microscopic images of PWM at the magnification of ×4000. (A–C) show myelinated axonal profiles in transverse section in the PWM at 28 days after the LPS injection (B), LPS + C3aRa administration (C) and their matching controls (A) (n = 5). Scatter plots of g‐ratio against axon diameter in the PWM at 28 days after the LPS injection, LPS + C3aRa administration and corresponding control are shown in bar graph D (n = 30). E is bar graph showing increased g‐ratio of myelinated axons of different diameters in the PWM at 28 days after the LPS injection, LPS + C3aRa administration and corresponding control (n = 10). For statistical analysis, two‐way ANOVA with Tukey's post hoc test was used for E and presented as the mean ± standard error of measurement (SEM). Scale bars: 2 μm. *P < 0.05, **P < 0.01.
Figure 6.
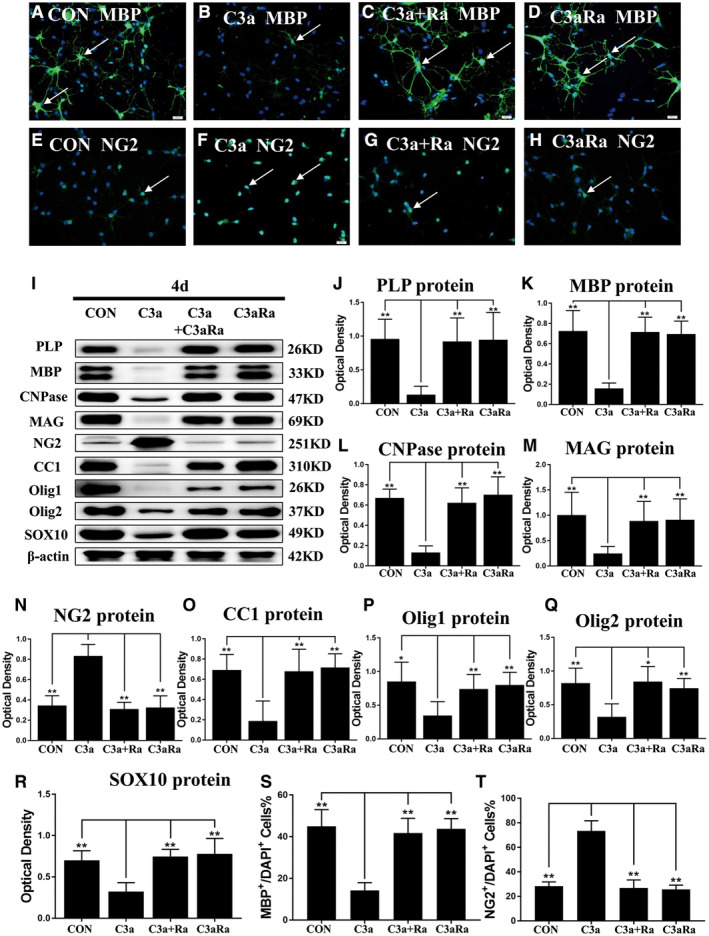
C3a inhibits the differentiation and maturation of OPCs in vitro. Immunofluorescence images of cultured OPCs showing the expression of MBP (A–D, green), NG2 (E–H, green) and DAPI (blue) at 4 days after the C3a, C3a + C3aRa and C3aRa treatment when compared with the corresponding control at the magnification of ×40 (n = 5). Panel I shows the immunoreactive bands of PLP (26 kDa), MBP (33 kDa), CNPase (47 kDa), MAG (69 kDa), NG2 (251 kDa), CC1 (310 kDa), Olig1 (26 kDa), Olig2 (37 kDa), SOX10 (49 kDa) and β‐actin (42 kDa) after C3a administration or C3a + C3aRa treatment or C3aRa treatment and the corresponding control (n = 6). Bar graphs (J–R) show the optical density of protein expression shown in I (n = 6). (S, T) Bar graphs show the percentage of MBP+/DAPI+ and NG2+/DAPI+ cells at 4 d after the C3a, C3a + C3aRa and C3aRa treatment when compared with the corresponding control (n = 5). For statistical analysis, one‐way ANOVA with Tukey's post hoc test was used for J–T and presented as the mean ± standard error of measurement (SEM). Scale bars: 20 μm. *P < 0.05, **P < 0.01.
Figure 7.
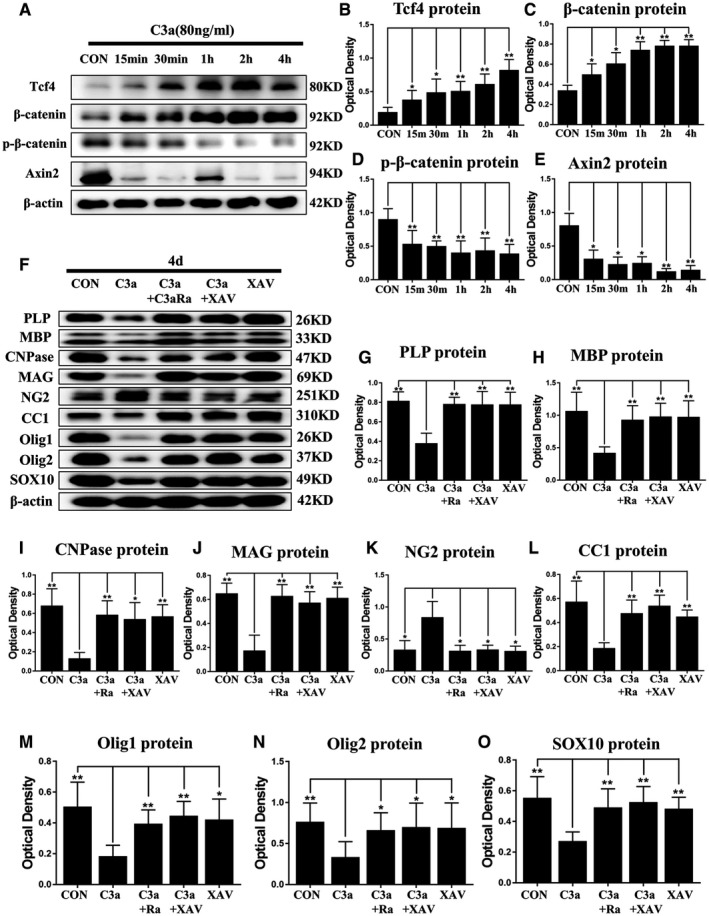
C3a delays the differentiation and maturation of OPCs in vitro by activating the WNT/β‐catenin pathway. C3a administration activates the WNT/β‐catenin pathway in primary culture of OPCs. Panel A shows TCF4, β‐catenin, β‐catenin phosphorylation and AXIN2 immunoreactive bands after C3a treatment for 15 minutes, 30 minutes, 1, 2, and 4 h and the corresponding control (n = 6). Bar graphs (B–E) show the optical density of protein expression in A (n = 6). XAV939, an inhibitor of WNT/β‐catenin pathway, reverses the effect of C3a in primary culture of OPCs. Panel F shows the immunoreactive bands of PLP (26 kDa), MBP (33 kDa), CNPase (47 kDa), MAG (69 kDa), NG2 (251 kDa), CC1 (310 kDa), Olig1 (26 kDa), Olig2 (37 kDa), SOX10 (49 kDa) and β‐actin (42 kDa) after C3a, C3a + C3aRa, C3a + XAV939, XAV939 treatment and the corresponding control (n = 6). Bar graphs (G–O) show the optical density of protein expression in I (n = 6). For statistical analysis, one‐way ANOVA with Tukey's post hoc test was used for B–E and G–O and presented as the mean ± standard error of measurement (SEM). Scale bars: 20 μm. *P < 0.05, **P < 0.01.
Results
C3a and C3aR protein expression in the PWM in experimentally induced septic neonatal rats
To investigate the role of C3a–C3aR signaling in the PWM of neonatal rats injected with LPS, we first examined the inflammatory expression of C3a and C3aR in PWM. Double immunofluorescence showed intense C3a antibody and TMEM119 colocalization in activated microglia in the PWM at 24 h, 3 and 7 days after LPS injection which was evidently enhanced when compared with cells in the corresponding control (Figure S1A–R). Additionally, cell quantification showed that the number of C3a+/TMEM119+/DAPI+ cells was significantly increased in the PWM at 24 h, 3 and 7 days after LPS injection in comparison with the age‐matched control (Figure S1U). Furthermore, double immunofluorescence showed an increase in number of C3a+/GFAP+ fluorescent astrocytes in the PWM at 24 h, 3 and 7 days after LPS injection when compared with the corresponding control (Figure S2A–R). By cell counting, the number of C3a+/GFAP+/DAPI+ cells was significantly increased in the PWM at 24 h, 3 and 7 days after LPS injection in comparison with the age‐matched control (Figure S2S). The immunoreactive bands of C3a protein levels that appeared at approximately 25 kDa increased significantly in optical density at 6, 24 h, 3 and 7 days after LPS injection as compared with the controls (Figure S1S,T). The results showed that increased C3a expression was mainly localized in the activated microglia in the PWM after LPS injection; of note, C3a was also localized in some reactive astrocytes. To ascertain the involvement of the C3a–C3aR signaling in the inflammatory response induced by LPS, we next detected the expression of C3aR in the PWM. By double immunostaining, C3aR was co‐localized in some oligodendrocytes marked by NG2 antibody (Figure S3A–R). The immunoreactivity of C3aR was markedly enhanced at 24 h, 3 and 7 days following LPS injection when compared with their age‐matched groups (Figure S3A–R). Cell counting results showed that the frequency of C3aR+/NG2+/DAPI+ cells was significantly increased in the PWM at 24 h, 3 and 7 days after LPS injection (Figure S3U). Interestingly, C3aR was also co‐localized in some microglia marked with TMEM119; an increase in number of C3aR+/TMEM119+ cells was shown in the PWM at 24 h, 3 and 7 days after LPS injection when compared with the corresponding control (Figure S4A–S).The optical density of immunoreactive bands of C3aR protein in the PWM that appeared at approximately 69 kDa was markedly enhanced at 6 and 24 h, 3 and 7 days after LPS injection (Figure S3S,T). Taken together, the results support that LPS can elicit upregulated expression of C3a in the activated microglia and astrocytes coupled with increased C3aR expression in the OLs and microglia in the PWM in septic neonatal rats. Also, microglia may possess both autocrine and paracrine functions which collectively promote the secretion of C3a after LPS injection.
C3aRa administration attenuated hypomyelination in the PWM in experimentally induced septic neonatal rats
To verify if enhanced C3a expression would induce hypomyelination, we next explored whether C3aRa intraperitoneal injection would prevent hypomyelination in the PWM at 14 and 28 days after LPS injection. The expression of mature myelin sheath associated proteins including CNPase, PLP, MBP and MAG in the PWM was examined. Immunofluorescence staining showed that CNPase expression in the PWM was noticeably reduced in postnatal rats at 14 and 28 days after LPS administration (Figure 1B,E) when compared with the matching controls (Figure 1A,D) (the area outlined with dotted line). Remarkably, C3aRa administration reversed the decrease of CNPase protein expression (Figure 1C,F) (the area outlined with dotted line). The optical density of immunoreactive bands of CNPase, PLP, MBP and MAG protein levels in the PWM that appeared at approximately 47kDa, 26kDa, 33kDa and 69kDa respectively, was significantly reduced at 14 and 28 days after LPS injection in comparison with the corresponding controls (Figure 1G–K). C3aRa intraperitoneal injection reversed the alteration in expression of mature myelin sheath associated proteins as mentioned above (Figure 1G–K).
To further verify the above findings, in situ hybridization with antisense riboprobes targeted at MBP and PLP was performed to examine the number of MBP+ and PLP+ OLs in the PWM. The number of MBP+ and PLP+ OLs in the PWM was significantly declined at 14 and 28 days after LPS injection (Figure 2A,D,G,J) when compared with the corresponding controls (Figure 2B,E,H,K). C3aRa administration increased the number of MBP+ and PLP+ OLs in the PWM significantly at 14 and 28 days after LPS injection (Figure 2C,F,I,L). Cell quantification showed that the number of MBP+ and PLP+ OLs was significantly decreased in the PWM at 14 and 28 days after LPS injection in comparison with the age‐matched control, while C3aRa administration increased the number of MBP+ and PLP+ OLs (Figure 2M,N).
The myelinated axons and myelin sheath thickness in the PWM were analyzed by electron microscopy. Images of axonal profiles at 4000× showed that the number of myelinated axons was significantly decreased along with thinning of the myelin sheath which tended to appear in irregular profiles in the PWM at 28 days in the LPS‐group (Figure 3B) when compared with the matching control (Figure 3A). Increase in number of myelinated axons and thicker myelin sheath became evident in the PWM at 28 days after LPS + C3aRa administration (Figure 3C). The g‐ratio is calculated as the ratio of the axon diameter to the total diameter of axon plus its myelin sheath, which provides a reliable index of axonal myelin sheath thickness. The g‐ratio for myelinated axons values was calculated in the PWM in each group. The scatter plot D demonstrated that the average g‐ratios in the LPS group were significantly increased when compared with their control group (Figure 3D). Bar graph E showed that g‐ratio values became greater in different diameters ranging from 0.2 to 1.2 μm as measured in electron micrographs at 12 000× in LPS group in comparison with the controls (Figure 3E). However, g‐ratio value was reversed after C3aRa + LPS administration when compared with LPS injection (Figure 3D,E). Taken together, the present results indicate that C3aRa injection could recover the thickness and number of myelin sheath to varying extent in the PWM of experimentally induced septic neonatal rats.
C3aRa prevented impairment of oligodendrocyte differentiation and maturation in the PWM in experimentally induced septic neonatal rats
To further explore the effect of C3aRa administration on differentiation and maturation of OPCs, NG2 and CC1 antibodies were used to examine the numerical change of NG2+ and CC1+ OLs in the PWM. Immunofluorescence staining showed that the number of NG2+ OPCs in the PWM was significantly increased at 7, 14 and 28 days following LPS injection (Figure S5B,E; Figure 4B,J) when compared with the age‐matched controls (Figure S5A,D; Figure 4A,J). However, the number of CC1+ OLs in the PWM was significantly decreased at 7, 14 and 28 days after LPS injection (Figure S5G,H,J,K; Figure 4D,E,K). On the contrary, C3aRa administration could reverse the decrease in numbers of NG2+ OPCs and CC1+ OLs induced by LPS injection (Figure S5C,F,I,L; Figure 4C,F,J,K). The immunoreactive bands of NG2 protein levels that appeared at approximately 251kDa increased in optical density at 7, 14 and 28 days after LPS injection when compared with the matching controls (Figure 4G,H). C3aRa administration reversed the increase of NG2 expression induced by LPS injection (Figure 4G,H). The immunoreactive bands of CC1 protein levels that appeared at approximately 310kDa, decreased significantly in optical density at 7, 14 and 28 days after LPS injection as compared with the control groups (Figure 4G,I). C3aRa administration reversed the decline in CC1 expression induced by LPS injection (Figure 4G,I). In addition, there was no significant difference in the number of NG2+/Ki‐67+ cells in the PWM at 3, 7, 14 and 28 days after LPS or LPS + C3aRa injection when compared with the age‐matched groups (Figure S6A–L). The proliferation of OPCs showed no significant change following LPS or LPS + C3aRa injection in vivo (Figure S6M). These results demonstrated that enhanced C3a expression derived from microglia and astrocytes could disturb the differentiation and maturation of OPCs in the PWM in experimentally induced septic neonatal rats after LPS injection.
C3aRa administration increased Olig1, Olig2 and Sox10 expression in the PWM in experimentally induced septic neonatal rats
In the process of differentiation and maturation of OLs, some transcription factors are needed to promote the process, such as Olig1, Olig2, Sox10, Mrf, Nkx2.2 18, 36. The expression of key transcription factors including Olig1, Olig2 and Sox10 was detected using immunofluorescence and western blot. Immunostaining showed that the number of Olig2+ cells was significantly reduced in the PWM at 7, 14, and 28 days after LPS injection (Figure 5B,E,H,K) when compared with the corresponding controls (Figure 5A,D,G,K). However, the number of Olig2+ cells was significantly increased in the PWM at 7, 14 and 28 days after LPS + C3aRa administration (Figure 5C,F,I,K) when compared with rats given LPS injection only (Figure 5B,E,H,K). Western blot showed that the expression level of Olig1, Olig2 and Sox10 was significantly decreased in optical density in the PWM at 7, 14, and 28 days after LPS injection when compared with corresponding controls (Figure 5J,L–N). Conversely, the expression of Olig1, Olig2 and Sox10 was significantly increased in C3aRa + LPS administration group when compared with LPS‐groups (Figure 5J,L–N). Arising from the above, it is suggested that C3aRa administration can prevent the decreased expression of transcription factors Olig1, Olig2 and Sox10 induced by LPS injection, which promote the differentiation and maturation of OLs.
C3a administration inhibited maturation and differentiation of OPCs in vitro
To investigate whether C3a would suppress the maturation and differentiation of primary OPCs in vitro, OPCs were cultured for 4 days using OPCs differentiation medium. They were divided into four groups: C3a (80 ng/mL), C3a + C3aRa (80 ng/mL), C3aRa (80 ng/mL) and equal volume of PBS as control. Immunofluorescence staining showed a significant increase in number of NG2+ OPCs after treatment with C3a for 4 days (Figure 6F,T) when compared with control group (Figure 6E,T), whereas the number of MBP+ OLs was significantly decreased (Figure 6A, B, S). Treatment with C3aRa + C3a significantly increased the number of MBP+ OLs (Figure 6C,S) and decreased the number of NG2+ OPCs (Figure 6G,T) when compared with C3a. There was no significant change in percentage of NG2+/Ki67+/DAPI+ OPCs following treatment with C3a, C3a + C3aRa or C3aRa for 4 days by immunofluorescence staining (Figure S7A–M). These results indicated that C3aRa can revert the inhibition of C3a on differentiation and maturation of primary OPCs but it would not affect or alter the proliferation of OPCs in vitro. The immunoreactive bands of NG2 proteins levels that appeared at approximately 260 kDa increased significantly in optical density at 4 days after treatment with C3a as compared with the controls (Figure 6I,N). The protein expression of CC1, CNPase, MBP, PLP, MAG, Olig1, Olig2 and Sox10 was significantly decreased at 4 days after administration of C3a when compared with the controls (Figure 6I–M,O–R). Administration of C3aRa + C3a reversed the expression of these proteins as mentioned above (Figure 6I–R). It is therefore suggested that C3a treatment can delay the maturation of primary OPCs in vitro.
C3a activated WNT/β‐catenin signaling pathway associated with maturation and differentiation of primary OPCs
Recent studies reported that the activation of WNT/β‐catenin pathways was implicated in the maturation and differentiation of OPCs in vitro and in vivo 7, 70. We therefore examined whether C3a would disturb the maturation and differentiation of OPCs through WNT/β‐catenin pathway in the primary OPCs in differentiation medium. Western blot analysis showed that expression levels of β‐catenin and TCF4 was significantly increased at 15 and 30 minutes, 1, 2 and 4 h after treatment with C3a as compared with the controls (Figure 7A–C), but the protein levels of phosphorylated β‐catenin and Axin2 were significantly decreased (Figure 7A,D,E). These results demonstrated that WNT/β‐catenin could be activated by C3a treatment in primary OPCs in vitro.
Arising from the above, we surmised that C3a might suppress the maturation of OPCs through activating β‐catenin and TCF4 and inhibiting the phosphorylation of β‐catenin and AXIN2. We next investigated whether XAV‐939 (a selective inhibitor against WNT/β‐catenin‐mediated transcription by tankyrase1/2 suppression) would revert the effect of C3a administration on the maturation and differentiation of OPCs. A significant reduction in the protein level of CC1, CNPase, MBP, PLP, MAG, Olig1, Olig2 and Sox10 was found at 4 days after C3a administration when compared with the controls by western blot (Figure 7F–J,L–O). However, the protein expression of NG2 was significantly increased after C3a treatment (Figure 7F, K). Both C3aRa and XAV‐939 could significantly reverse the effects of C3a treatment (Figure 7F–O). These findings therefore suggested that C3a treatment disturbed the maturation and differentiation of OPCs in vitro through activation of WNT/β‐catenin pathway.
Discussion
The PWM in neonates, especially in preterm newborns, is immature and highly vulnerable to hypoxia, ischemia, and inflammation, leading to PWMD 42, 56, 58, 60, 62. There is compelling evidence indicating that besides hypoxia–ischemia, neonatal sepsis is an important risk factor for PWMD 3, 5. We reported in our previous studies in rats that a variety of inflammatory mediators such as IL‐1β was released in the PWM and hippocampus in septic neonatal rats. This indicated that systemic LPS injection had elicited neuroinflammation in CNS, especially in the PWM and hippocampus, where innate immune cells microglia are known to preponderate 34, 65. In addition, emerging evidences demonstrated that the activation of innate immunity and neuroinflammation play a key role in the pathogenesis of AD and related disorders 20. The complement cascade is a highly conserved signaling pathway which is crucial for innate immunity, and that it allows the immune cells to attack membranes or phagocytose pathogen. Previous study has reported that C1q, an initiation component of the complement pathway, tags neuronal synapses for microglial phagocytosis both in development and CNS disease, including pruning of retinal ganglion cell (RGC) synapses for activity‐dependent refinement during postnatal periods 16, 49.
The present results have shown that the protein expression of C3a was upregulated in the PWM after LPS intraperitoneal injection. The increased expression of C3a is likely to be derived from the activated microglia or astrocytes during inflammation. Recent study has reported that neuroinflammation may induce two different phenotypes of reactive astrocytes, namely, A1 and A2 astrocytes 47. A1 phenotype may exert harmful effects because its upregulated expression of many classical complement cascade genes during neuroinflammation or ischemia, which would be destructive to synapses 47. In contrast, A2 astrocytes produce many neurotrophic factors, which is therefore considered to be protective to neurons or oligodendrocytes 47. In human AD, A1 astrocytes make up a large proportion of astrocytes in the prefrontal cortex, 60% of which were C3 positive and likely to aggravate neurodegeneration 46. Likewise, activated microglia have been classified into the M1 and M2 phenotypes in pathological conditions such as LPS stimulation or ischemia/hypoxia 68. The M1 phenotype represents the pro‐inflammatory cellular state, which is coupled with upregulated expression of pro‐inflammatory mediators 68. In contrast, M2 phenotype is endowed with immunosuppressive and neuroprotective properties, which is involved in the generation of anti‐inflammatory and neurotrophic factors 68. In the present study, upregulated expression of C3a was localized primarily in activated microglia. It is noteworthy that C3a was also localized in some astrocytes double labeled with GFAP in the PWM at 3 and 7 days after LPS injection. This indicates that while activated microglia were the main source of C3a at 24 h after LPS intraperitoneal injection, astrocytes also contribute to its production. This would be consistent with our previous report that microglia might contribute to the early phase of cytokine production, whereas astrocytes are implicated in the release of inflammatory mediators at a late phase in brain pathologies 17 over a protracted period.
It is well documented that C3aR expression is upregulated in glial cells, especially the astrocytes and microglia in inflammation 15. Additionally, C3aR expression was transiently localized in the cerebellar granule neurons during the development of the cerebellum 10. This indicates that C3aR expression occurs in both glial cells and neurons. We have shown in this study that C3aR expression is localized on OPCs and activated microglia in the PWM up to 7 days after LPS injection, alluding that complement C3a released by activated microglia and astrocytes may be implicated in PWMD through C3a–C3aR pathway. Thus, C3a not only could amplify inflammatory response through C3aR expressed on microglia through autocrine and paracrine mode, but would also exert a direct effect on the differentiation and maturation of OPCs expressing C3aR. It is conceivable that this would ultimately contribute to PWMD.
A pertinent question arose from this would be whether the C3a–C3aR pathway is involved in axonal hypomyelination in PWMD induced by LPS injection. In this study, C3aRa intraperitoneal injection was adopted to explore whether this would reverse the disruption of axonal myelination caused by LPS administration in the neonatal rats. It is relevant to note that the expression of PLP, MBP, CNPase and MAG, which are marker proteins of mature myelin sheath, was significantly upregulated in the PWM of septic neonatal rats at 14 and 28 days after C3aRa intraperitoneal injection. By electron microscopy, the present results have shown that the frequency of myelinated axons was increased and that the axonal myelin sheath associated with the axons became thicker in the PWM of septic neonatal rats at 28 days after C3aRa administration. These results therefore had demonstrated unequivocally that C3aRa administration could counter the extensive axonal hypomyelination in the PWM induced by LPS intraperitoneal injection. There are two possible mechanisms by which C3aRa intraperitoneal injection might help prevent axonal hypomyelination in the PWM in septic neonatal rats. First, systemic LPS injection causes cerebral endothelial activation, which upregulates the expression of C3a on vascular endothelial cells and contributes to leukocyte–endothelial interactions and leukocyte recruitment 64. Systemic C3aRa administration could prevent the leukocyte–endothelial interactions through C3aR 64. This would lead to the inhibition of leukocyte recruitment to CNS thereby attenuating the inflammatory response and neuroinflammation associated injury in the CNS 64. Second, C3aRa injected intraperitoneally may cross the blood–brain barrier and gain access to the nervous tissue and block C3aR expression on microglia and OPCs. It is relevant to note that C3aRa injected intraperitoneally can attenuate synaptic dysfunction, tau hyperphosphorylation, neuronal morphology and improve neurologic outcome associated with Alzheimer's disease and intracerebral hemorrhage 39, 45, 57. Along with the above, it has been reported that C3a plays an important role in the production of proinflammatory cytokines and chemokines by glial cells 41. It stands to reason therefore that C3aR antagonists may decrease the release of proinflammatory mediators by microglia, such as IL‐1β and TNF‐α. Taken together, it is suggested that C3aR antagonists administered intraperitoneally as demonstrated in the present results may help revert neuroinflammation and disorder of OPCs differentiation and maturation including myelination in neonatal rats with experimentally induced sepsis by LPS.
In the CNS, myelination formation is an intricate process involving interaction between oligodendrocytes and axons. Indeed, differentiation and maturation of OLs, full developmental axons and suitable circumstances of CNS are vital for proper myelin sheath formation 24. Mature oligodendrocytes originate from OPCs, which require the ability to proliferate, migrate and differentiate into myelinating oligodendrocytes 13, 61. The differentiation or maturation of OPCs is regulated by many specific transcription factors, such as Olig1, Olig2, Mash, Myt1, Nkx2.2, Sip1, Sox10 and Mrf 25, 28. It is suggested that downregulated expression of these transcriptional factors would retard OPCs differentiation and maturation leading to axonal hypomyelination in the PWM. In situ hybridization results showed that the number of PLP+ and MBP+ cells was significantly increased in the PWM of septic neonatal rats at 14 and 28 days after C3aRa administration. Immunofluorescence and western blot analyses showed that C3aRa administration could increase the number of CC1+ OLs and decrease the number of NG2+ OPCs in the PWM of septic neonatal rats. Moreover, the transcription factors Olig1, Olig2 and Sox10 were significantly increased in the PWM of septic neonatal rats at 7, 14 and 28 days after C3aRa injection. All in all, these results suggest that C3aRa administration may prevent the disorder of OPCs differentiation and maturation and recover the expression of some OPCs maturation associated transcription factors in the PWM of septic neonatal rats. On the basis of these results, it is justified to suggest that C3a can disrupt the differentiation/maturation of OPCs through C3a–C3aR signaling.
To further investigate whether C3a would directly attenuate the differentiation and maturation of OPCs, primary OPCs were treated with C3a (80 ng/mL) for 4 days to observe expression of MBP and NG2 in vitro. The expression of CNPase, PLP, MBP, MAG, CC1, Olig1, Olig2 and SOX10 was significantly reduced in the OPCs after C3a administration. However, the expression of NG2 was increased. C3aRa administration could revert the alterations of OPCs induced by C3a. It can be safely concluded that C3a administration can suppress the differentiation and maturation of primary OPCs through its receptor C3aR. The underlying mechanism is likely through suppressing the expression of Olig1, Olig2 and SOX10 thereby disrupting the differentiation and maturation of primary OPCs. This is one possible mechanism by which systemic LPS administration can induce axonal hypomyelination in the PWMD in septic rat model.
WNT/β‐catenin/Tcf4 signaling pathway has been implicated in OPCs maturation and differentiation 21, 67, 70. In general, β‐catenin does not accumulate in the cytoplasm under normal physiological condition because an existing complex consisting of AXIN2, APC, PP2A, GSK3, CK1α, could phosphorylate β‐catenin 44. Subsequently, p‐β‐catenin is degraded by proteasome. When the WNT/β‐catenin/Tcf4 signaling pathway is activated, a large amount of β‐catenin accumulates in the cytoplasm, and then binds to TCF4 in the nucleus to activate a series of downstream genes 44. Concurrently, AXIN2 expression is decreased which slows down the degradation of β‐catenin. The activation of WNT/β‐catenin signaling may inhibit OPCs maturation and differentiation 32, 55. Furthermore, pharmacological inhibition of WNT/β‐catenin signaling pathway could promote the differentiation and maturation of OPCs 43. The present results have shown that expression of β‐catenin and Tcf4 was gradually upregulated whereas that of p‐β‐catenin and AXIN2 was decreased when OPCs were treated with C3a for 15 minutes to 4 h. This indicates that C3a administration could activate the Wnt/β‐catenin/Tcf4 signaling pathway in OPCs. XAV‐939, a selective inhibitor to Wnt/β‐catenin signaling pathway by tankyrase1/2 inhibition 14, 35, could upregulate the expression of CNPase, PLP, MBP, MAG, CC1, Olig1, Olig2 and SOX10 and decrease the expression of NG2 in OPCs treated with C3a. As reported by Han, C3a could lead to a significant phosphorylation of AKT and the nuclear accumulation of β‐catenin in tubular cells 33. Phosphorylated AKT can activate β‐catenin/TCF4 transcriptional activity by the indirect stabilization of β‐catenin through GSK‐3β inhibition and by directly inhibiting the phosphorylation of β‐catenin 53. Therefore, it is speculated that C3a binding to C3aR may activate and phosphorylate AKT, which is associated with the activation of WNT/β‐catenin signaling pathway. Similarly, IL‐1β binds to and activates the IL‐1R, which recruits MyD88, IRAK, TRAF6 and TAK1 to its intracellular domains leading to the activation and phosphorylation of AKT 27, 29, 53. The phosphorylated AKT interacts with β‐catenin/Tcf4, which affects the expression of a multitude of downstream genes. We reported previously that IL‐1β acts via the FYN/MEK/ERK signaling pathway to induce hypomyelination in the PWM by inhibiting OPC differentiation in septic neonatal rats 65. When taken together with previous results, we conclude that C3a and IL‐1β on binding to their corresponding receptors, would activate the β‐catenin/Tcf4 pathway to inhibit the maturation and differentiation of OPCs in septic neonatal rats.
Conclusion
This study has shown that microglia and astrocytes are activated and generate a large amount of C3a in the PWM of neonatal brain after LPS injection. In this connection, C3aRa administration counters the effects of C3a and upregulates the expression of CNPase, PLP, MBP, MAG, CC1, Olig1, Olig2 and SOX10 and decreases the expression of NG2 in the PWM of septic neonatal rats induced by LPS injection. This was coupled with a reduction in number of immature OLs and an increase in number of mature OLs and myelin sheath. In vitro, C3a disturbs the differentiation and maturation of OPCs via Wnt/β‐catenin signaling pathway through its receptor C3aR. On the basis of the present in vivo and in vitro data, it is suggested that C3a is implicated in disorder of OPCs differentiation and maturation through activation of Wnt/β‐catenin signaling pathway (Figure 8). Moreover, this might contribute, at least in part, to axonal hypomyelination in the PWM of septic neonatal brain. This would make C3a as well as C3aR attractive therapeutic strategies for mitigation of PWMD induced by sepsis in the neonatal period.
Figure 8.
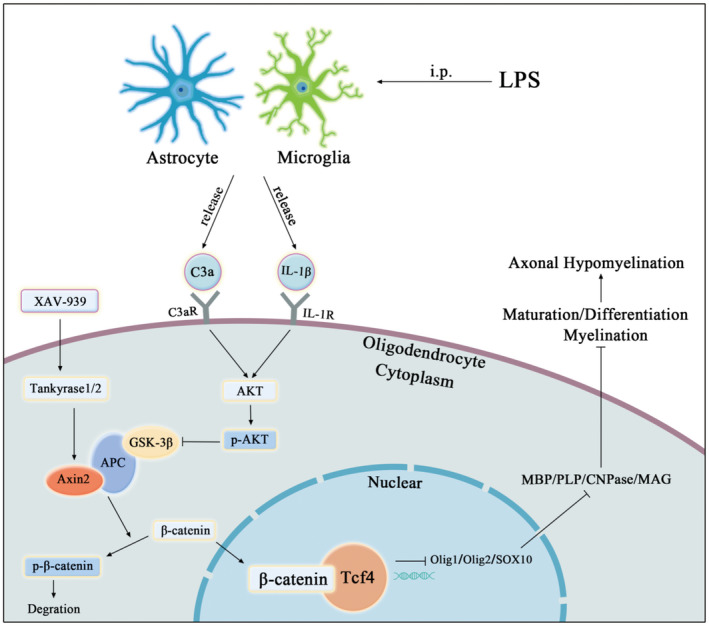
Table of Contents Image (TOCI): A sketch map demonstrates the cellular and molecular mechanism associated with PWMD in the septic neonatal rats. Microglia and astrocyte are activated in the PWM after intraperitoneal injection of LPS and release massive amounts of C3a and IL‐1β. Then they will bind to their receptors (C3aR and IL‐1R1) on the OLs and may activate AKT signaling pathway. It will inhibit GSK3β, allowing β‐catenin cytoplasmic accumulation and binding to TCF4 in the nucleus for nuclear translocation to active WNT/β‐catenin signaling pathway. It can lead to the delay of maturation or differentiation of OPCs through inhibiting the differentiation transcription factor of OPCs. This would contribute to axonal hypomyelination in the PWM in the experimental induced septic neonatal rats. XAV‐939, a selective inhibitor of WNT/β‐catenin signaling pathway, which may indirectly stabilize Axin2 through Tankyrase1/2, can promote the maturation or differentiation of OPCs in vitro.
Conflict of Interest
All authors read and approved the final manuscript. All authors have consented to publish the data in the manuscript. All authors also declared that there is no conflict of interest.
Author Contributions
Conceived and designed the experiments: Yiyu Deng. Performed the experiments and analyzed the data: Peixian Huang. Contributed reagents/materials/analysis tools: Peixian Huang. Wrote the paper: Peixian Huang, Yiyu Deng.
Supporting information
Figure S1. Activated microglia exhibit intense expression of C3a protein in the PWM of postnatal rats at 6, 24, 3 and 7 days after the LPS injection and their corresponding controls. Double immunofluorescence shows TMEM119 labeled (A, D, G, J, M, P green) and C3a (B, E, H, K, N, Q red) immunoreactive microglial cells in the PWM of postnatal rats at 24 h, 3 and 7 days after the LPS injection and their corresponding controls at the magnification of ×100 (n = 5). Note co‐localized expression of TMEM119 and C3a in microglia in C, F, I, L, O and R (n = 5). Panel S shows C3a protein expression level in the PWM of postnatal rats at 6, 24 h, 3 and 7 days after LPS injection and the corresponding controls with loading control of β‐actin (n = 6). Bar graph T depicts the optical density of C3a expression shown in S (n = 6). Bar graph in U shows a significant increase in number of C3a+/TMEM119+/DAPI+ in the PWM at 24 h, 3 and 7 days after LPS injection in comparison with their corresponding controls (n = 5). For statistical analysis, two‐way ANOVA with Tukey's post hoc test was used for T and U and presented as the mean ± standard error of measurement (SEM). Scale bars: 10 μm. *P < 0.05, **P < 0.01.
Figure S2. Activated astrocytes exhibit intense expression of C3a protein in the PWM of postnatal rats at 24 h, 3 and 7 days after the LPS injection and their corresponding controls. Double immunofluorescence shows GFAP‐labeled (A, D, G, J, M, P green) and C3a (B, E, H, K, N, Q red) immunoreactive astrocytes in the PWM of postnatal rats at 24 h, 3 and 7 days after the LPS injection and their corresponding controls at the magnification of ×100 (n = 5). Note co‐localized expression of GFAP and C3a in astrocytes in C, F, I, L, O and R (n = 5). Bar graph in S shows a significant increase in number of GFAP+/C3a+/DAPI+ in the PWM at 24 h, 3 and 7 days after LPS injection in comparison with their corresponding controls (n = 5). For statistical analysis, two‐way ANOVA with Tukey's post hoc test was used for S and presented as the mean ± standard error of measurement (SEM). Scale bars: 10 μm. *P < 0.05, **P < 0.01.
Figure S3. C3aR protein expression in the PWM of postnatal rats at 6, 24 h, 3 and 7 days after LPS injection and their corresponding controls. Double immunofluorescence shows the NG2 (A, D, G, J, M, P green) and C3aR (B, E, H, K, N, Q red) immunoreactive oligodendrocytes in the PWM of postnatal rats at 24 h, 3d and 7 days after the LPS injection and their corresponding controls at the magnification of ×60 (n = 5). Co‐localized expression of C3aR and NG2 in oligodendrocytes is evident in C, F, I and L, O, R. Panel S shows C3aR protein expression level in the PWM of postnatal rats at 6, 24 h, 3 and 7 days after LPS injection and the corresponding controls with loading control of β‐actin (n = 6). Bar graph T depicts the optical density of C3aR expression shown in S (n = 6). Bar graph in U shows a significant increase in number of C3aR+/NG2+/DAPI+ in the PWM at 24 h, 3 and 7 days after LPS injection in comparison with their corresponding controls (n = 5). For statistical analysis, two‐way ANOVA with Tukey's post hoc test was used for T and U and presented as the mean ± standard error of measurement (SEM). Scale bars: 20 μm. *P < 0.05, **P < 0.01.
Figure S4. Intense expression of C3aR co‐localizes in microglia cells in the PWM of postnatal rats at 24 h, 3 and 7 days after LPS injection and their corresponding controls. Double immunofluorescence shows the TMEM119 (A, D, G, J, M, P green) and C3aR (B, E, H, K, N, Q red) immunoreactive oligodendrocytes in the PWM of postnatal rats at 24 h, 3 and 7 days after the LPS injection and their corresponding controls at the magnification of ×60 (n = 5). Co‐localized expression of C3aR and TMEM119 in oligodendrocytes is evident in C, F, I and L, O, R. Bar graph in S shows a significant increase in number of C3aR+/TMEM119+/DAPI+ in the PWM at 24 h, 3 and 7 days after LPS injection in comparison with their corresponding controls (n = 5). For statistical analysis, two‐way ANOVA with Tukey's post hoc test was used for S and presented as the mean ± standard error of measurement (SEM). Scale bars: 20 μm. *P < 0.05, **P < 0.01.
Figure S5. C3aRa injection prevents impairment of OPCs maturation in the PWM of neonatal rats after LPS injection. Immunofluorescence staining shows NG2 immunoreactive oligodendrocytes (green) and DAPI (blue) in the PWM at 7 and 14 days after LPS injection (B, E), LPS + C3aRa injection (C, F) and their matching controls (A, D) at the magnification of ×40 (n = 5). Immunofluorescence staining shows CC1 immunoreactive oligodendrocytes (green) and DAPI (blue) in the PWM at 7 and 14 days after LPS injection (H, K), LPS + C3aRa injection (I, L) and their matching controls (G, J) (n = 5). Scale bar: 20 μm.
Figure S6. No significant change in the proliferation of OPCs in the PWM at 24 h, 3, 7, 14 and 28 days following LPS or LPS + C3aR injection when compared with their corresponding control. Immunofluorescence staining shows NG2 immunoreactive oligodendrocytes (green) and Ki‐67 (red) in the PWM at 24 h, 3, 7, 14 and 28 days after LPS injection (B, E, H, K, N), LPS + C3aRa injection (C, F, I, L, O) and their matching controls (A, D, G, J, M) at the magnification of ×60 (n = 5). Bar graph in M shows no significant change in the percentage of Ki‐67+/NG2+/DAPI+ cells in vivo at 3, 7, 14 and 28 days after LPS injection, LPS + C3aRa injection and their matching controls (n = 5). For statistical analysis, two‐way ANOVA with Tukey's post hoc test was used for M and presented as the mean ± standard error of measurement (SEM). Scale bar: 20 μm. # P > 0.05.
Figure S7. No significant change in the proliferation of primary OPCs in vitro following treatment with C3a, C3a + C3aRa, or C3aRa for 4 days. Immunofluorescence staining shows NG2 immunoreactive oligodendrocytes (green) and Ki‐67 (red) in the PWM at 4 days after the C3a, C3a + C3aRa and C3aRa treatment when compared with the corresponding control (A–L) at the magnification of ×60 (n = 5). Bar graph in M shows no significant change in the percentage of Ki‐67+/NG2+/DAPI+ OPCs in vitro following treatment with C3a, C3a + C3aRa, or C3aRa for 4 days (n = 5). For statistical analysis, one‐way ANOVA with Tukey's post hoc test was used for M and presented as the mean ± standard error of measurement (SEM). Scale bars: 20 μm. # P > 0.05.
Acknowledgments
The authors thank Research Department of Medical Sciences of Guangdong General Hospital for technical and facility supports.
This work was supported by National Natural Science Foundation of China (Grant numbers: 81271329 and 81471237), High‐level Hospital Construction Project (grant number: DFJH201804) and Natural Science Foundation of Guangdong Province (Grant number: 2019A1515010206).
Data Availability Statement
The datasets used and analyzed during the current study are available from the corresponding author on reasonable request.
References
- 1. Adams‐Chapman I, Bann CM, Das A, Goldberg RN, Stoll BJ, Walsh MC et al (2013) Neurodevelopmental outcome of extremely low birth weight infants with Candida infection. J Pediatr 163:961–967.e3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Adolf A, Rohrbeck A, Munster‐Wandowski A, Johansson M, Kuhn HG, Kopp MA et al (2019) Release of astroglial vimentin by extracellular vesicles: modulation of binding and internalization of C3 transferase in astrocytes and neurons. Glia 67:703–717. [DOI] [PubMed] [Google Scholar]
- 3. Albertsson AM, Bi D, Duan L, Zhang X, Leavenworth JW, Qiao L et al (2014) The immune response after hypoxia‐ischemia in a mouse model of preterm brain injury. J Neuroinflammation 11:153–166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Ames RS, Lee D, Foley JJ, Jurewicz AJ, Tornetta MA, Bautsch W et al (2001) Identification of a selective nonpeptide antagonist of the anaphylatoxin C3a Receptor that demonstrates antiinflammatory activity in animal models. J Immunol 166:6341–6348. [DOI] [PubMed] [Google Scholar]
- 5. Anblagan D, Pataky R, Evans MJ, Telford EJ, Serag A, Sparrow S et al (2016) Association between preterm brain injury and exposure to chorioamnionitis during fetal life. Sci Rep 6:37932. doi: 10.1038/srep37932 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Anderson SR, Zhang J, Steele MR, Romero CO, Kautzman AG, Schafer DP, Vetter ML (2019) Complement targets newborn retinal ganglion cells for phagocytic elimination by microglia. J Neurosci 39:2025–2040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Back SA (2017) White matter injury in the preterm infant: pathology and mechanisms. Acta Neuropathol 134:331–349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Barton M, Shen A, O'Brien K, Robinson JL, Davies HD, Simpson K et al (2017) Early‐onset invasive candidiasis in extremely low birth weight infants: perinatal acquisition predicts poor outcome. Clin Infect Dis 64:921–927. [DOI] [PubMed] [Google Scholar]
- 9. Basu S, Agarwal P, Anupurba S, Shukla R, Kumar A (2015) Elevated plasma and cerebrospinal fluid interleukin‐1 beta and tumor necrosis factor‐alpha concentration and combined outcome of death or abnormal neuroimaging in preterm neonates with early‐onset clinical sepsis. J Perinatol 35:855–861. [DOI] [PubMed] [Google Scholar]
- 10. Benard M, Gonzalez BJ, Schouft MT, Falluel‐Morel A, Vaudry D, Chan P et al (2004) Characterization of C3a and C5a receptors in rat cerebellar granule neurons during maturation. Neuroprotective effect of C5a against apoptotic cell death. J Biol Chem 279:43487–43496. [DOI] [PubMed] [Google Scholar]
- 11. Carty ML, Wixey JA, Reinebrant HE, Gobe G, Colditz PB, Buller KM (2011) Ibuprofen inhibits neuroinflammation and attenuates white matter damage following hypoxia‐ischemia in the immature rodent brain. Brain Res 1402:9–19. [DOI] [PubMed] [Google Scholar]
- 12. Chen YJ, Zhang JX, Shen L, Qi Q, Cheng XX, Zhong ZR et al (2015) Schwann cells induce Proliferation and Migration of Oligodendrocyte Precursor Cells Through Secretion of PDGF‐AA and FGF‐2. J Mol Neurosci 56:999–1008. [DOI] [PubMed] [Google Scholar]
- 13. Coppolino GT, Marangon D, Negri C, Menichetti G, Fumagalli M, Gelosa P et al (2018) Differential local tissue permissiveness influences the final fate of GPR17‐expressing oligodendrocyte precursors in two distinct models of demyelination. Glia 66:1118–1130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Crespo M, Vilar E, Tsai SY, Chang K, Amin S, Srinivasan T et al (2017) Colonic organoids derived from human induced pluripotent stem cells for modeling colorectal cancer and drug testing. Nat Med 23:878–884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Davoust N, Stahel PF, Ames R, Barnum SR (1999) Receptor for the C3a anaphylatoxin is expressed by neurons and glial cells. Glia 26:201–211. [DOI] [PubMed] [Google Scholar]
- 16. Dejanovic B, Huntley MA, De Maziere A, Meilandt WJ, Wu T, Srinivasan K et al (2018) Changes in the synaptic proteome in tauopathy and rescue of tau‐induced synapse loss by C1q antibodies. Neuron 100:1322–1336.e7. [DOI] [PubMed] [Google Scholar]
- 17. Deng YY, Lu J, Ling E‐A, Kaur C (2010) Microglia‐derived macrophage colony stimulating factor promotes generation of proinflammatory cytokines by astrocytes in the periventricular white matter in the hypoxic neonatal brain. Brain Pathol 20:909–925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Deng T, Postnikov Y, Zhang S, Garrett L, Becker L, Racz I et al (2017) Interplay between H1 and HMGN epigenetically regulates OLIG1&2 expression and oligodendrocyte differentiation. Nucleic Acids Res 45:3031–3045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Deverman BE, Patterson PH (2012) Exogenous leukemia inhibitory factor stimulates oligodendrocyte progenitor cell proliferation and enhances hippocampal remyelination. J Neurosci 32:2100–2109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Dionisio‐Santos DA, Olschowka JA, O'Banion MK (2019) Exploiting microglial and peripheral immune cell crosstalk to treat Alzheimer's disease. J Neuroinflammation 16:74–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Dohare P, Cheng B, Ahmed E, Yadala V, Singla P, Thomas S et al (2018) Glycogen synthase kinase‐3beta inhibition enhances myelination in preterm newborns with intraventricular hemorrhage, but not recombinant Wnt3A. Neurobiol Dis 118:22–39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Doyle LW, Cheong JLY (2019) Does bovine lactoferrin prevent late‐onset neonatal sepsis? Lancet 393:382–384. [DOI] [PubMed] [Google Scholar]
- 23. Duan T, Smith AJ, Verkman AS (2018) Complement‐dependent bystander injury to neurons in AQP4‐IgG seropositive neuromyelitis optica. J Neuroinflammation 15:294–304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Duncan ID, Radcliff AB, Heidari M, Kidd G, August BK, Wierenga LA (2018) The adult oligodendrocyte can participate in remyelination. Proc Natl Acad Sci U S A 115:E11807–E11816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Elbaz B, Popko B (2019) Molecular control of oligodendrocyte development. Trends Neurosci 42:263–277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Ferreira RC, Mello RR, Silva KS (2014) Neonatal sepsis as a risk factor for neurodevelopmental changes in preterm infants with very low birth weight. J Pediatr (Rio J) 90:293–299. [DOI] [PubMed] [Google Scholar]
- 27. Gao W, Wang W, Peng Y, Deng Z (2019) Antidepressive effects of kaempferol mediated by reduction of oxidative stress, proinflammatory cytokines and up‐regulation of AKT/beta‐catenin cascade. Metab Brain Dis 34:485–494. [DOI] [PubMed] [Google Scholar]
- 28. Gottle P, Manousi A, Kremer D, Reiche L, Hartung HP, Kury P (2018) Teriflunomide promotes oligodendroglial differentiation and myelination. J Neuroinflammation 15:76–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Gupta P, Srivastav S, Saha S, Das PK, Ukil A (2016) Leishmania donovani inhibits macrophage apoptosis and pro‐inflammatory response through AKT‐mediated regulation of beta‐catenin and FOXO‐1. Cell Death Differ 23(11):1815–1826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. de Haan TR, Beckers L, de Jonge RC, Spanjaard L, van Toledo L, Pajkrt D et al (2013) Neonatal gram negative and Candida sepsis survival and neurodevelopmental outcome at the corrected age of 24 months. PLoS ONE 8:e59214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Hack M, Costello DW (2008) Trends in the rates of cerebral palsy associated with neonatal intensive care of preterm children. Clin Obstet Gynecol 51:763–774. [DOI] [PubMed] [Google Scholar]
- 32. Hammond E, Lang J, Maeda Y, Pleasure D, Angus‐Hill M, Xu J et al (2015) The Wnt effector transcription factor 7‐like 2 positively regulates oligodendrocyte differentiation in a manner independent of Wnt/beta‐catenin signaling. J Neurosci 35:5007–5022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Han R, Hu S, Qin W, Shi J, Hou Q, Wang X et al (2019) C3a and suPAR drive versican V1 expression in tubular cells of focal segmental glomerulosclerosis. JCI Insight 4:e122912. doi: 10.1172/jci.insight.122912 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Han Q, Lin Q, Huang P, Chen M, Hu X, Fu H et al (2017) Microglia‐derived IL‐1beta contributes to axon development disorders and synaptic deficit through p38‐MAPK signal pathway in septic neonatal rats. J Neuroinflammation 14:52–69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Harada H, Tsuda Y, Yabuki K, Shiba E, Uchihashi K, Matsuyama A et al (2018) Inhibition of WNT/beta‐catenin signaling under serum starvation and hypoxia induces adipocytic transdifferentiation in human leiomyoma cells. Lab Invest 98:439–448. [DOI] [PubMed] [Google Scholar]
- 36. He D, Marie C, Zhao C, Kim B, Wang J, Deng Y et al (2016) Chd7 cooperates with Sox10 and regulates the onset of CNS myelination and remyelination. Nat Neurosci 19:678–689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Hentges CR, Silveira RC, Procianoy RS, Carvalho CG, Filipouski GR, Fuentefria RN et al (2014) Association of late‐onset neonatal sepsis with late neurodevelopment in the first two years of life of preterm infants with very low birth weight. J Pediatr (Rio J) 90:50–57. [DOI] [PubMed] [Google Scholar]
- 38. Hofer N, Muller W, Resch B. (2011) White matter damage and neonatal sepsis. Acta Paediatr 100:e1; author reply e1–e2. [DOI] [PubMed] [Google Scholar]
- 39. Hu J, Yang Y, Wang M, Yao Y, Chang Y, He Q et al (2019) Complement C3a receptor antagonist attenuates tau hyperphosphorylation via glycogen synthase kinase 3beta signaling pathways. Eur J Pharmacol 850:135–140. [DOI] [PubMed] [Google Scholar]
- 40. Hutamekalin P, Takeda K, Tani M, Tsuga Y, Ogawa N, Mizutani N, Yoshino S (2010) Effect of the C3a‐receptor antagonist SB 290157 on anti‐OVA polyclonal antibody‐induced arthritis. J Pharmacol Sci 112:56–63. [DOI] [PubMed] [Google Scholar]
- 41. Ingersoll SA, Martin CB, Barnum SR, Martin BK (2010) CNS‐specific expression of C3a and C5a exacerbate demyelination severity in the cuprizone model. Mol Immunol 48:219–230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Jurcoane A, Daamen M, Scheef L, Bauml JG, Meng C, Wohlschlager AM et al (2016) White matter alterations of the corticospinal tract in adults born very preterm and/or with very low birth weight. Hum Brain Mapp 37:289–299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Kim MY, Kim HY, Hong J, Kim D, Lee H, Cheong E et al (2016) CXXC5 plays a role as a transcription activator for myelin genes on oligodendrocyte differentiation. Glia 64:350–362. [DOI] [PubMed] [Google Scholar]
- 44. Li XG, Wang Z, Chen RQ, Fu HL, Gao CQ, Yan HC et al (2018) LGR5 and BMI1 increase pig intestinal epithelial cell proliferation by stimulating WNT/beta‐catenin signaling. Int J Mol Sci 19:1036. doi: 10.3390/ijms19041036 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Lian H, Yang L, Cole A, Sun L, Chiang AC, Fowler SW et al (2015) NFkappaB‐activated astroglial release of complement C3 compromises neuronal morphology and function associated with Alzheimer's disease. Neuron 85:101–115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Liddelow SA, Barres BA (2017) Reactive astrocytes: production, function, and therapeutic potential. Immunity 46:957–967. [DOI] [PubMed] [Google Scholar]
- 47. Liddelow SA, Guttenplan KA, Clarke LE, Bennett FC, Bohlen CJ, Schirmer L et al (2017) Neurotoxic reactive astrocytes are induced by activated microglia. Nature 541:481–487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Litvinchuk A, Wan YW, Swartzlander DB, Chen F, Cole A, Propson NE et al (2018) Complement C3aR inactivation attenuates tau pathology and reverses an immune network deregulated in tauopathy models and Alzheimer's Disease. Neuron 100:1337–1353.e5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Luchena C, Zuazo‐Ibarra J, Alberdi E, Matute C, Capetillo‐Zarate E (2018) Contribution of neurons and glial cells to complement‐mediated synapse removal during development, aging and in Alzheimer's Disease. Mediators Inflamm 2018:2530414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Martinez Sosa S, Smith KJ (2017) Understanding a role for hypoxia in lesion formation and location in the deep and periventricular white matter in small vessel disease and multiple sclerosis. Clin Sci (Lond) 131:2503–2524. [DOI] [PubMed] [Google Scholar]
- 51. McMullan RL, Gordon A (2018) Antibiotics at the time of removal of central venous catheter to reduce morbidity and mortality in newborn infants. Cochrane Database Syst Rev 3:CD012181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Parker SE, Hanton AM, Stefanou SN, Noakes PG, Woodruff TM, Lee JD (2019) Revisiting the role of the innate immune complement system in ALS. Neurobiol Dis 127:223–232. [DOI] [PubMed] [Google Scholar]
- 53. Perez‐Yepez EA, Ayala‐Sumuano JT, Lezama R, Meza I (2014) A novel beta‐catenin signaling pathway activated by IL‐1beta leads to the onset of epithelial‐mesenchymal transition in breast cancer cells. Cancer Lett 354:164–171. [DOI] [PubMed] [Google Scholar]
- 54. Popichak KA, Afzali MF, Kirkley KS, Tjalkens RB (2018) Glial‐neuronal signaling mechanisms underlying the neuroinflammatory effects of manganese. J Neuroinflammation 15:324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Preisner A, Albrecht S, Cui QL, Hucke S, Ghelman J, Hartmann C et al (2015) Non‐steroidal anti‐inflammatory drug indometacin enhances endogenous remyelination. Acta Neuropathol 130:247–261. [DOI] [PubMed] [Google Scholar]
- 56. Rocha‐Ferreira E, Kelen D, Faulkner S, Broad KD, Chandrasekaran M, Kerenyi A et al (2017) Systemic pro‐inflammatory cytokine status following therapeutic hypothermia in a piglet hypoxia‐ischemia model. J Neuroinflammation 14:44–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Rynkowski MA, Kim GH, Garrett MC, Zacharia BE, Otten ML, Sosunov SA et al (2009) C3a receptor antagonist attenuates brain injury after intracerebral hemorrhage. J Cereb Blood Flow Metab 29:98–107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Savard A, Brochu ME, Chevin M, Guiraut C, Grbic D, Sebire G (2015) Neuronal self‐injury mediated by IL‐1beta and MMP‐9 in a cerebral palsy model of severe neonatal encephalopathy induced by immune activation plus hypoxia‐ischemia. J Neuroinflammation 12:111–124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Simonsen KA, Anderson‐Berry AL, Delair SF, Davies HD (2014) Early‐onset neonatal sepsis. Clin Microbiol Rev 27:21–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Sripada K, Lohaugen GC, Eikenes L, Bjorlykke KM, Haberg AK, Skranes J, Rimol LM (2015) Visual‐motor deficits relate to altered gray and white matter in young adults born preterm with very low birth weight. NeuroImage 109:493–504. [DOI] [PubMed] [Google Scholar]
- 61. Vieira MS, Santos AK, Vasconcellos R, Goulart VAM, Parreira RC, Kihara AH et al (2018) Neural stem cell differentiation into mature neurons: mechanisms of regulation and biotechnological applications. Biotechnol Adv 36:1946–1970. [DOI] [PubMed] [Google Scholar]
- 62. Wang LW, Chang YC, Chen SJ, Tseng CH, Tu YF, Liao NS et al (2014) TNFR1‐JNK signaling is the shared pathway of neuroinflammation and neurovascular damage after LPS‐sensitized hypoxic‐ischemic injury in the immature brain. J Neuroinflammation 11:215–229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Wu T, Fan XP, Wang WY, Yuan TM (2014) Enterovirus infections are associated with white matter damage in neonates. J Paediatr Child Health 50:817–822. [DOI] [PubMed] [Google Scholar]
- 64. Wu F, Zou Q, Ding X, Shi D, Zhu X, Hu W et al (2016) Complement component C3a plays a critical role in endothelial activation and leukocyte recruitment into the brain. J Neuroinflammation 13:23–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Xie D, Shen F, He S, Chen M, Han Q, Fang M et al (2016) IL‐1beta induces hypomyelination in the periventricular white matter through inhibition of oligodendrocyte progenitor cell maturation via FYN/MEK/ERK signaling pathway in septic neonatal rats. Glia 64:583–602. [DOI] [PubMed] [Google Scholar]
- 66. Xiong A, Kundu S, Forsberg M, Xiong Y, Bergstrom T, Paavilainen T et al (2017) Heparanase confers a growth advantage to differentiating murine embryonic stem cells, and enhances oligodendrocyte formation. Matrix Biol 62:92–104. [DOI] [PubMed] [Google Scholar]
- 67. Yang Y, Cheng Z, Tang H, Jiao H, Sun X, Cui Q et al (2017) Neonatal maternal separation impairs prefrontal cortical myelination and cognitive functions in rats through activation of wnt signaling. Cereb Cortex 27:2871–2884. [DOI] [PubMed] [Google Scholar]
- 68. Yuan Y, Wu C, Ling E‐A (2019) Heterogeneity of microglia phenotypes: developmental, functional and some therapeutic considerations. Curr Pharm Des 25:1–19. [DOI] [PubMed] [Google Scholar]
- 69. Yuen TJ, Silbereis JC, Griveau A, Chang SM, Daneman R, Fancy SPJ et al (2014) Oligodendrocyte‐encoded HIF function couples postnatal myelination and white matter angiogenesis. Cell 158:383–396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Zhao C, Deng Y, Liu L, Yu K, Zhang L, Wang H et al (2016) Dual regulatory switch through interactions of Tcf7l2/Tcf4 with stage‐specific partners propels oligodendroglial maturation. Nat Commun 7:10883. doi: 10.1038/ncomms10883 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Zwarthoff SA, Berends ETM, Mol S, Ruyken M, Aerts PC, Jozsi M et al (2018) Functional characterization of alternative and classical pathway C3/C5 convertase activity and inhibition using purified models. Front Immunol 9:1691. doi: 10.3389/fimmu.2018.01691 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Figure S1. Activated microglia exhibit intense expression of C3a protein in the PWM of postnatal rats at 6, 24, 3 and 7 days after the LPS injection and their corresponding controls. Double immunofluorescence shows TMEM119 labeled (A, D, G, J, M, P green) and C3a (B, E, H, K, N, Q red) immunoreactive microglial cells in the PWM of postnatal rats at 24 h, 3 and 7 days after the LPS injection and their corresponding controls at the magnification of ×100 (n = 5). Note co‐localized expression of TMEM119 and C3a in microglia in C, F, I, L, O and R (n = 5). Panel S shows C3a protein expression level in the PWM of postnatal rats at 6, 24 h, 3 and 7 days after LPS injection and the corresponding controls with loading control of β‐actin (n = 6). Bar graph T depicts the optical density of C3a expression shown in S (n = 6). Bar graph in U shows a significant increase in number of C3a+/TMEM119+/DAPI+ in the PWM at 24 h, 3 and 7 days after LPS injection in comparison with their corresponding controls (n = 5). For statistical analysis, two‐way ANOVA with Tukey's post hoc test was used for T and U and presented as the mean ± standard error of measurement (SEM). Scale bars: 10 μm. *P < 0.05, **P < 0.01.
Figure S2. Activated astrocytes exhibit intense expression of C3a protein in the PWM of postnatal rats at 24 h, 3 and 7 days after the LPS injection and their corresponding controls. Double immunofluorescence shows GFAP‐labeled (A, D, G, J, M, P green) and C3a (B, E, H, K, N, Q red) immunoreactive astrocytes in the PWM of postnatal rats at 24 h, 3 and 7 days after the LPS injection and their corresponding controls at the magnification of ×100 (n = 5). Note co‐localized expression of GFAP and C3a in astrocytes in C, F, I, L, O and R (n = 5). Bar graph in S shows a significant increase in number of GFAP+/C3a+/DAPI+ in the PWM at 24 h, 3 and 7 days after LPS injection in comparison with their corresponding controls (n = 5). For statistical analysis, two‐way ANOVA with Tukey's post hoc test was used for S and presented as the mean ± standard error of measurement (SEM). Scale bars: 10 μm. *P < 0.05, **P < 0.01.
Figure S3. C3aR protein expression in the PWM of postnatal rats at 6, 24 h, 3 and 7 days after LPS injection and their corresponding controls. Double immunofluorescence shows the NG2 (A, D, G, J, M, P green) and C3aR (B, E, H, K, N, Q red) immunoreactive oligodendrocytes in the PWM of postnatal rats at 24 h, 3d and 7 days after the LPS injection and their corresponding controls at the magnification of ×60 (n = 5). Co‐localized expression of C3aR and NG2 in oligodendrocytes is evident in C, F, I and L, O, R. Panel S shows C3aR protein expression level in the PWM of postnatal rats at 6, 24 h, 3 and 7 days after LPS injection and the corresponding controls with loading control of β‐actin (n = 6). Bar graph T depicts the optical density of C3aR expression shown in S (n = 6). Bar graph in U shows a significant increase in number of C3aR+/NG2+/DAPI+ in the PWM at 24 h, 3 and 7 days after LPS injection in comparison with their corresponding controls (n = 5). For statistical analysis, two‐way ANOVA with Tukey's post hoc test was used for T and U and presented as the mean ± standard error of measurement (SEM). Scale bars: 20 μm. *P < 0.05, **P < 0.01.
Figure S4. Intense expression of C3aR co‐localizes in microglia cells in the PWM of postnatal rats at 24 h, 3 and 7 days after LPS injection and their corresponding controls. Double immunofluorescence shows the TMEM119 (A, D, G, J, M, P green) and C3aR (B, E, H, K, N, Q red) immunoreactive oligodendrocytes in the PWM of postnatal rats at 24 h, 3 and 7 days after the LPS injection and their corresponding controls at the magnification of ×60 (n = 5). Co‐localized expression of C3aR and TMEM119 in oligodendrocytes is evident in C, F, I and L, O, R. Bar graph in S shows a significant increase in number of C3aR+/TMEM119+/DAPI+ in the PWM at 24 h, 3 and 7 days after LPS injection in comparison with their corresponding controls (n = 5). For statistical analysis, two‐way ANOVA with Tukey's post hoc test was used for S and presented as the mean ± standard error of measurement (SEM). Scale bars: 20 μm. *P < 0.05, **P < 0.01.
Figure S5. C3aRa injection prevents impairment of OPCs maturation in the PWM of neonatal rats after LPS injection. Immunofluorescence staining shows NG2 immunoreactive oligodendrocytes (green) and DAPI (blue) in the PWM at 7 and 14 days after LPS injection (B, E), LPS + C3aRa injection (C, F) and their matching controls (A, D) at the magnification of ×40 (n = 5). Immunofluorescence staining shows CC1 immunoreactive oligodendrocytes (green) and DAPI (blue) in the PWM at 7 and 14 days after LPS injection (H, K), LPS + C3aRa injection (I, L) and their matching controls (G, J) (n = 5). Scale bar: 20 μm.
Figure S6. No significant change in the proliferation of OPCs in the PWM at 24 h, 3, 7, 14 and 28 days following LPS or LPS + C3aR injection when compared with their corresponding control. Immunofluorescence staining shows NG2 immunoreactive oligodendrocytes (green) and Ki‐67 (red) in the PWM at 24 h, 3, 7, 14 and 28 days after LPS injection (B, E, H, K, N), LPS + C3aRa injection (C, F, I, L, O) and their matching controls (A, D, G, J, M) at the magnification of ×60 (n = 5). Bar graph in M shows no significant change in the percentage of Ki‐67+/NG2+/DAPI+ cells in vivo at 3, 7, 14 and 28 days after LPS injection, LPS + C3aRa injection and their matching controls (n = 5). For statistical analysis, two‐way ANOVA with Tukey's post hoc test was used for M and presented as the mean ± standard error of measurement (SEM). Scale bar: 20 μm. # P > 0.05.
Figure S7. No significant change in the proliferation of primary OPCs in vitro following treatment with C3a, C3a + C3aRa, or C3aRa for 4 days. Immunofluorescence staining shows NG2 immunoreactive oligodendrocytes (green) and Ki‐67 (red) in the PWM at 4 days after the C3a, C3a + C3aRa and C3aRa treatment when compared with the corresponding control (A–L) at the magnification of ×60 (n = 5). Bar graph in M shows no significant change in the percentage of Ki‐67+/NG2+/DAPI+ OPCs in vitro following treatment with C3a, C3a + C3aRa, or C3aRa for 4 days (n = 5). For statistical analysis, one‐way ANOVA with Tukey's post hoc test was used for M and presented as the mean ± standard error of measurement (SEM). Scale bars: 20 μm. # P > 0.05.
Data Availability Statement
The datasets used and analyzed during the current study are available from the corresponding author on reasonable request.


