Monoclonal antibodies targeting CD38, such as daratumumab, have shown good therapeutic efficacy in multiple myeloma (MM), both alone1 and in combination with normal standard-of-care regimens.2,3 However, many patients eventually relapse because of resistance mechanisms, including FcγR-dependent downregulation of CD38 on tumor cells as well as inhibition of complement- dependent cytotoxicity, antibody-dependent cellmediated cytotoxicity and antibody-dependent cellular phagocytosis.4
Bispecific T-cell engaging (BiTE) antibodies belong to a new class of immunotherapeutic agents that can recognize, on the one hand, a specific antigen on the surface of the target cells (i.e., tumor antigen) and, on the other hand, the CD3e chain on T lymphocytes.5 By activating T cells via the CD3 complex and recruiting them in proximity of target cells, BiTE antibodies efficiently induce T-cell-mediated cytotoxicity.6 In MM, bispecific antibodies recognizing B-cell maturation antigen or FcRH5 (CD307) have been shown to eliminate tumor plasma cells in preclinical models. 7-9 However, FcRH5 expression is not limited to tumor plasma cells and B-cell maturation antigen is abundantly secreted in MM patients.10,11 These two features may limit the specificity or efficiency of these cognate bispecific antibodies in vivo. Recently, an anti-CD38 bispecific antibody, AMG424, was shown to eliminate MM cells in preclinical models, but also to trigger “off tumor” T-cell cytotoxicity on B, T and NK cells in vitro,12 Thus, development of an efficient and safer bispecific antibody could contribute to improve the treatment of MM.
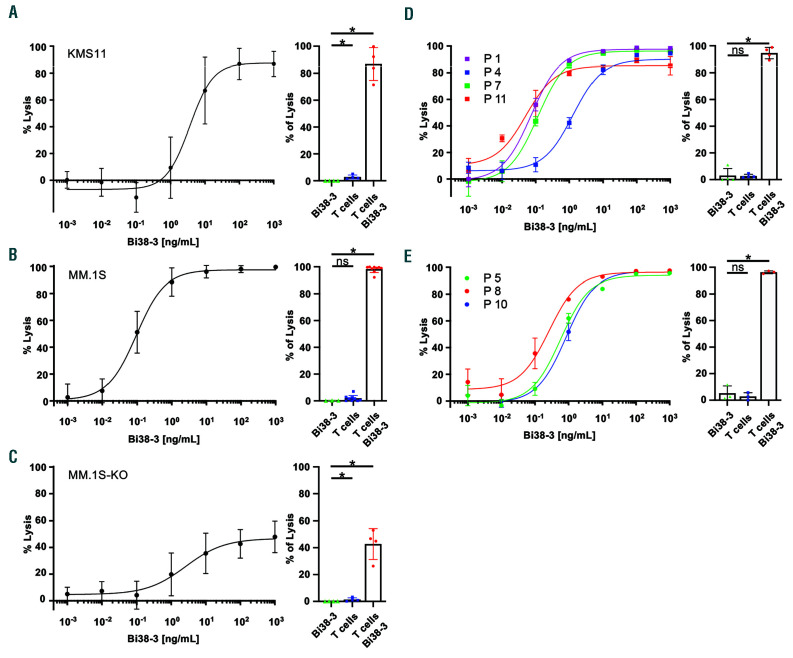
We have developed a new BiTE (Bi38-3) that consists of two single-chain variable fragments derived from mouse hybridomas, producing anti-human CD38 and CD3e (Online Supplementary Figure S1A and B). Purified Bi38-3 efficiently and specifically recognizes CD38 on MM cells and binds CD3e−expressing Jurkat T cells (Online Supplementary Figure S1C and D). To assess the effect of Bi38-3 on the cytotoxic activity of T cells, we performed co-culture assays with effector T cells, isolated from peripheral blood mononuclear cells of healthy donors, on firefly luciferase-expressing target KMS11 and MM.1S MM cell lines. We observed that T cells readily killed KMS11 cells (as measured by luciferase activity) in a Bi38-3 dose-dependent manner, with a half maximal effective concentration (EC50) around 5 ng/mL, the equivalent of 0.09 nM for this 55.6 Kda protein (Figure 1A). Bi38-3-mediated T-cell cytotoxic activity was also observed in co-culture with MM.1S cells (Figure 1B). However, in this cell line, which expresses heterogeneous levels of CD38, the EC50 was 10-fold lower (0.5 ng/mL), indicating that Bi38-3 also triggered strong T-cell cytotoxicity in MM cells with weaker CD38 expression. In line with this, stimulation of donor T cells with Bi38-3 in the presence of MM.1S cells led to robust proliferation, expression of activation markers CD25 and CD69, as well as production of interferon-γ, tumor necrosis factor- α and interleukin-2 in a Bi38-3 dose-dependent manner (Online Supplementary Figure S1E-H). The viability of MM.1S or KMS11 MM cells was not affected by co-culture with T cells or Bi38-3 alone (Figure 1A and B, right). In addition, Bi38-3 induced poor T-cell-mediated killing of CD38-deficient MM.1S cells (MM1.S-KO), with around half of CD38-deficient MM.1S cells surviving the co-culture even at the highest dose of Bi38-3 (1 mg/mL) (Figure 1C). These results indicate that, at lower doses, similar to those that are expected in patients, Bi38-3 directs efficient T-cell cytotoxic activity specifically towards CD38-expressing MM cells.
We next analyzed the potential of Bi38-3 to induce lysis of target tumor plasma cells, isolated from four patients at diagnosis, by autologous effector T cells. Fluorescence-activated cell sorting (FACS) analysis of overnight effector cell:target cell (E:T) co-cultures revealed that the numbers of viable CD138+ MM cells were reduced in a Bi38-3 dose-dependent manner, with the EC50 ranging from 0.028 to 1.29 ng/mL, depending on the patient (Figure 1D and Online Supplementary Figure S2). Importantly, in the absence of T cells, Bi38-3 exhibited no toxicity against fresh tumor cells. Bi38-3-induced cytotoxicity of autologous T cells was further investigated on tumor plasma cells from three MM patients at relapse and demonstrated similar efficacy, with EC50 values ranging from 0.2 to 0.86 ng/mL (Figure 1E). These results indicate that Bi38-3 triggered autologous T-cellmediated killing of tumor plasma cells from patients both at diagnosis and at relapse.
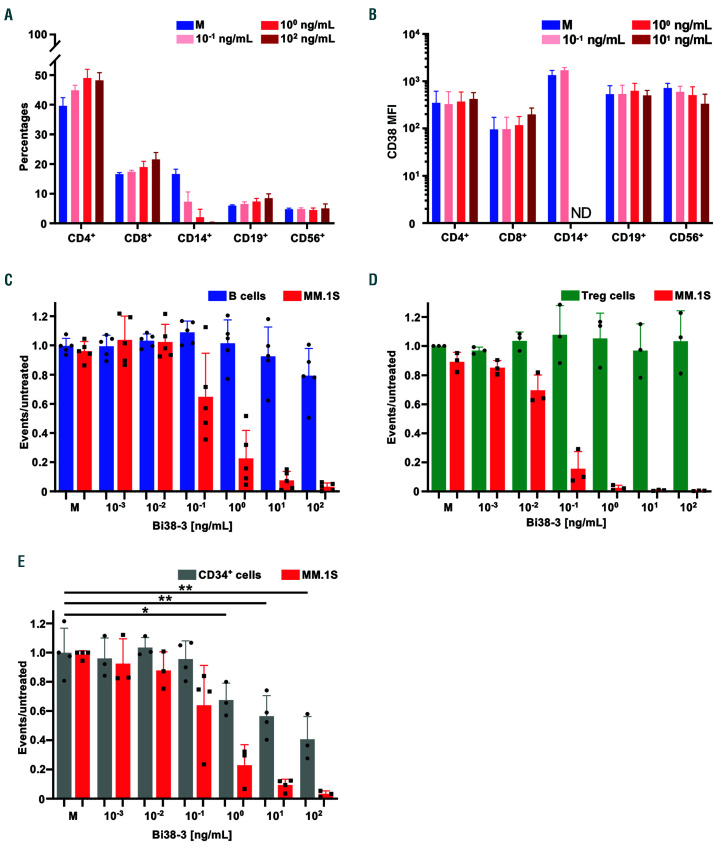
To investigate potential toxic effects of Bi38-3 on blood cells, peripheral blood mononuclear cells from donors were treated with various concentrations of Bi38-3 for 24 h and the mononuclear cell populations were individually analyzed by FACS (Online Supplementary Figure S3). We observed that the percentages of CD14-expressing monocytes included in the live gate were markedly reduced in a Bi38-3 dose-dependent manner (Figure 2A). In contrast, the percentages of CD4 and CD8 T lymphocytes, which together represented around 60% of total peripheral blood mononuclear cells, slightly increased in response to Bi38-3, probably due to the decrease in live CD14+ cells. Similarly, the B (CD19+) and NK (CD56+) cell populations remained at similar levels (around 10% and 5%, respectively), even at high concentrations of Bi38-3 (100 ng/mL) (Figure 2A).
Next, we investigated whether expression of CD38 at the surface of blood cells was downregulated by Bi38-3. FACS analysis indicated that CD38 mean fluorescence intensity on T, B and NK cells remained similar in cultures containing increasing doses of Bi38-3 (Figure 2B). In line with this, CD38 expression was not dramatically reduced on CD14+ monocytes. Of note, this analysis could not be performed at high doses because these cells, which express higher levels of CD38, were sensitive to elevated concentrations of Bi38-3 (above 1 ng/mL). To compare the activity of Bi38-3 on CD38high MM versus CD38int cells, we performed co-culture assays with MM.1S, expressing high levels of CD38, freshly isolated B cells, expressing intermediate levels of CD38 (Figure 2C) and autologous T cells. Following overnight culture, the percentages of viable CD20+ B cells and CD138+ MM.1S cells were analyzed by flow cytometry (Online Supplementary Figure S4A). We observed that the percentages of MM.1S cells dropped at Bi38-3 concentrations of 0.1 ng/mL and this reduction was more dramatic at higher doses (Figure 2C). In contrast, compared to untreated conditions, the percentages of viable CD20+ B cells remained unchanged even at high concentrations of Bi38-3 (Figure 2C).
We developed a similar autologous tri-culture assay to investigate potential toxic effects of Bi38-3 on CD34+ bone marrow hematopoietic progenitors and on regulatory T cells, which both express low levels of CD38. While Bi38-3 readily induced MM cell killing at low concentrations (10-2 ng/mL and above), we found that it did not trigger significant T-cell-mediated cytotoxicity on Foxp3+ regulatory T cells (Figure 2D). Similarly, there was no significant toxicity on CD34+ hematopoietic progenitors at concentrations below 10 ng/mL and moderate toxicity (>40% survival) at the highest concentrations (Figure 2E). Collectively, our results indicate that Bi38-3 does not impair the surface expression of CD38 and only triggers T-cell-mediated killing of cells expressing high levels of CD38 with no or limited toxicity against cells expressing intermediate levels of CD38, such as hematopoietic progenitors, B, T or NK cells.
Figure 1.
Bi38-3 induces CD38-dependent T-cell-mediated lysis of multiple myeloma cells in vitro. (A-C) KMS11-luc (A), MM.1S-luc (B) and CD38-deficient MM.1S-luc (MM.1S-KO-luc) (C) multiple myeloma cell lines were co-cultured with T cells, isolated from peripheral blood samples from healthy donors, at an effector: target (E:T) cell ratio of 5:1 with increasing concentrations of Bi38-3 for 24 h. Curves represent target cell lysis, monitored by luciferase activity and expressed as the percentage of the untreated condition (0% lysis). Data are the means of independent experiments with four (A), nine (B) and four (C) different donors. Standard deviations (SD) are shown for each concentration. Histograms on the left show target cell lysis induced by Bi38-3 alone, donor T cells alone and T cells with Bi38-3 (101 ng/mL) on KMS11-luc (A), MM.1S-luc (B) and MM1.S-KO-luc (C). (D and E) Fresh tumor plasma cells were collected from buffy coat of bone marrow aspirates from myeloma patients, then CD138+ cells were purified and co-cultured with autologous CD3+ T cells isolated from peripheral blood mononuclear cells at an E:T cell ratio of 5:1 for 24 h. Cultures were analyzed by fluorescence-activated cell sorting (FACS) to monitor the number of CD138+ cells falling into the live gate. The average percentages of lysis of CD138+ cells (relative to the untreated condition) in four different patients at diagnosis (D) and three different patients at relapse (E) are shown. The error bars indicate the SD. Histograms show the average effects of Bi38-3 alone, T cells alone and T cells with Bi38-3 (102 ng/mL) on tumor plasma cells from the same four patients at diagnosis (D) and three patients at relapse (E). SD are shown and P values were determined by a two-sided Mann–Whitney U-test (*P<0.05; **P<0.01; ***P<0.001).
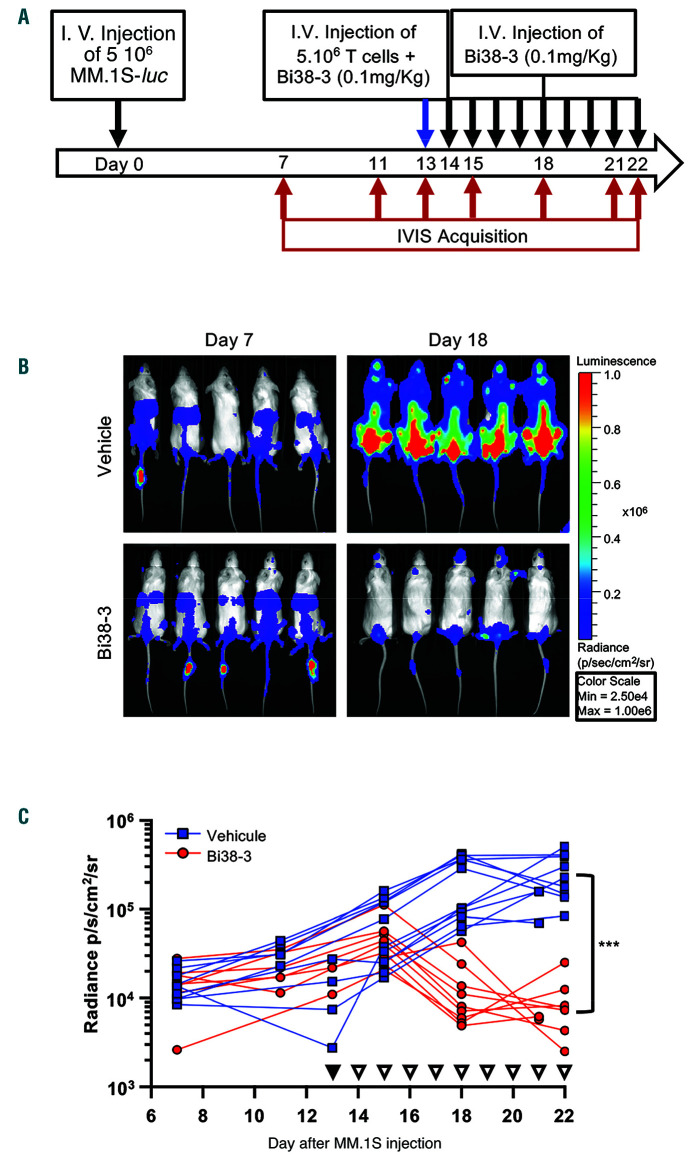
The antitumor activity of Bi38-3 was further assessed in vivo using a human MM xenograft mouse model. MM.1S cells expressing luciferase (MM.1Sluc) were injected into the tail vein of immunodeficient (NSG) mice and luciferase levels were measured using an IVIS Imaging System every 3 or 4 days. Thirteen days after MM.1S injection, purified human T cells were transplanted intravenously with or without Bi38-3 (0.1 mg/kg). Treatments with Bi38-3 or vehicle were repeated daily for 9 days (Figure 3A). Seven days after tumor cell injection, all mice showed similar levels of radiance (luciferase), indicating that MM cells had effectively engrafted in host animals prior to Bi38-3 treatment (Figure 3B). While control mice showed rapid tumor progression, all Bi38-3-treated animals displayed a marked reduction in tumor growth within the first 5 days of Bi38-3 treatment (Figure 3C). At the end of the treatments, the level of luciferase-expressing MM cells in Bi38-3-treated mice was only one tenth of the initial level and was on average 30-fold lower than that in untreated controls (Figure 3C). These results show that Bi38-3 is able to efficiently control MM tumor progression in vivo.
Figure 2.
Sensitivity of blood cells and bone marrow hematopoietic progenitors to Bi38-3. (A) Peripheral blood mononuclear cells (PBMC) from healthy donors (n=3) were cultured with medium (M) or various concentrations of Bi38-3 for 24 h and the percentages of live T, myeloid, B and NK cells were determined by fluorescence-activated cell sorting (FACS) analysis. Histograms show the average percentages of live CD4+ T cells, CD8+ T cells, CD14+ monocytes, CD19+ B cells and CD56+ NK cells in Bi38-3 cultures compared to untreated controls from three independent donors. The error bars indicate the standard deviation (SD). (B) Mean fluorescence intensity (MFI) was measured in MM.1S wild-type (WT) cells and in subsets of cells expressing CD4, CD8, CD14, CD19 and CD56, based on FACS analysis of CD38 expression levels in PBMC cell populations cultured with various concentrations of Bi38-3 as in (A). Histograms show average CD38 MFI of MM.1S cells and PBMC populations and error bars indicate the SD. ND: not determined because there were too few events. (C) Relative Bi38-3-mediated Tcell lysis of B versus MM.1S cells. Purified paired B and T cells from healthy donors (n=5) were co-cultured with increasing concentrations of Bi38-3 for 24 h in the presence of MM.1S cells. (D) Relative Bi38-3-mediated T-cell lysis of regulatory T cells (Treg) versus MM.1S cells. Purified T cells from healthy donors (n=3) were co-cultured with increasing concentrations of Bi38-3 for 24 h in the presence of MM.1S cells. (E) Relative Bi38-3-mediated T-cell lysis of CD34+ bone marrow hematopoietic progenitors versus MM.1S cells. Paired CD34+ hematopoietic progenitors and T cells purified from the bone marrow of healthy donors (taken during hip surgery) (n=4) were co-cultured with increasing concentrations of Bi38-3 for 24 h in the presence of MM.1S cells. In (C-E), the numbers of live CD20+ (B cells), FoxP3+ (Treg cells), CD34+(hematopoietic progenitors) and CD138+ (MM.1S cells) were calculated by FACS using counting beads and expressed as a ratio to untreated controls. Histograms show the ratios of B, Treg, CD34+ hematopoietic progenitor and MM.1S cells for each Bi38-3 concentrations and error bars indicate the SD. The normality of the CD34+ populations was established with a Shapiro-Wilk normality test and P-values were determined by an unpaired Student t test (*P<0.05; **P<0.01; ***P<0.001).
Figure 3.
In vivo activity of Bi38-3 in the MM.1Sluc xenograft mouse model. (A) Treatment schedule. Sixto 12-week-old NOD/SCID/IL-2Rγnull mice were inoculated with 5x106 MM.1SLuc cells by tail vein injection (i.v) at day 0, followed, 13 days later, by infusion of 5x106 purified human T cells, isolated from healthy donors, with (or without) Bi38-3 at a dose of 0.1 mg/kg (blue arrow). Treatment was initiated at day 13 when similar levels of luciferase-expressing MM cells were detected in all mice. Tail vein injections of Bi38-3 (0.1 mg/Kg) or phosphate-buffered saline (PBS, for controls) were repeated daily for 9 days (black arrows). Bioluminescence was measured with the IVIS Imaging System on days 7, 11, 13, 15, 18 and 21 (or 22) after tumor injection (red arrows). (B) Serial bioluminescence imaging was performed to assess myeloma progression/regression. Radiance was measured on the entire body of mice. Images on the left show bioluminescence at 7 days after inoculation with MM.1S myeloma cells and before the beginning of the treatment. Images on the right indicate bioluminescence 18 days after inoculation with MM.1S cells and 4 days after treatment with Bi38-3 (lower panel) or with vehicle (upper panel). The radiance color scale is represented on the right. (C) Longitudinal radiance levels of mice treated with vehicle (blue lines) or Bi38-3 (red lines). Red and blue curves represent groups of nine and 11 mice inoculated with MM.1SLuc and T cells and then treated with Bi38-3 (0.1 mg/kg) and PBS, respectively. Black filled triangles indicate the first injection of Bi38-3 or PBS with T cells and white filled triangles indicate Bi-38 or PBS injections every day for 9 days. These experiments were performed with T cells isolated from two independent donors. The normality of populations was established with the Shapiro-Wilk normality test, and P-values were calculated based on an unpaired Student t test (*P<0.05; **P<0.01; ***P<0.001).
We report here the development of Bi38-3, a new anti- CD38/CD3 BiTE, which triggers T-cell-mediated lysis of CD38+ MM cells in vitro, ex vivo and in vivo. Interestingly, Bi38-3 provokes no toxicity on B, T and NK cells in vitro and is, therefore, likely to have less "off tumor" effects than AMG424, a recently developed anti-CD38 bispecific antibody.12 Importantly, Bi38-3 efficiently triggers killing of MM cells from patients who are resistant to standard treatments. Furthermore, because it recognizes a specific epitope on CD38 and is devoid of the Fc region, Bi38-3 is expected to be efficient also in relapsed patients following daratumumab therapy. Collectively, the data presented in this study identifies Bi38-3 as a selective and efficient compound for the treatment of MM, which could be used as a front-line agent or at relapse (alone or in combination with other drugs), and which should be evaluated further in MM patients.
Supplementary Material
Acknowledgments
We thank M. Goodhardt, and D. Garrick for their critique of the manuscript. We are also grateful to the animal facility and to the pole of cytometry of the “Institut de Recherche Saint-Louis” for technical help.
Funding Statement
Funding: this work was supported by grants from the “Cancéropole Ile de France” and from the “Fondation Française pour la Recherche contre le Myélome et les Gammapathies monoclonales”. MF and CM-C were supported by fellowships from the French “Agence National pour la Recherche”, programme d’Investissements d’avenir (ANR-10-IDEX-03-02). MF was also supported by a fellowship from the “Société Française d’Hématologie”. AA was supported by a fellowship from the “Agence Nationale pour la Recherche Scientifique, CIFRE” in partnership with Servier.
References
- 1.Usmani SZ, Weiss BM, Plesner T, et al. Clinical efficacy of daratumumab monotherapy in patients with heavily pretreated relapsed or refractory multiple myeloma. Blood. 2016;128(1):37-44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Dimopoulos MA, Oriol A, Nahi H, et al. Daratumumab, lenalidomide, and dexamethasone for multiple myeloma. N Engl J Med. 2016;375(14):1319-1331. [DOI] [PubMed] [Google Scholar]
- 3.Palumbo A, Chanan-Khan A, Weisel K, et al. Daratumumab, bortezomib, and dexamethasone for multiple myeloma. N Engl J Med. 2016;375(8):754-766. [DOI] [PubMed] [Google Scholar]
- 4.van de Donk N, Usmani SZ. CD38 antibodies in multiple myeloma: mechanisms of action and modes of resistance. Front Immunol. 2018;9:2134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bannas P, Hambach J, Koch-Nolte F. Nanobodies and nanobodybased human heavy chain antibodies as antitumor therapeutics. Front Immunol. 2017;8:1603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Brischwein K, Schlereth B, Guller B, et al. MT110: a novel bispecific single-chain antibody construct with high efficacy in eradicating established tumors. Mol Immunol. 2006;43(8):1129-1143. [DOI] [PubMed] [Google Scholar]
- 7.Hipp S, Tai YT, Blanset D, et al. A novel BCMA/CD3 bispecific Tcell engager for the treatment of multiple myeloma induces selective lysis in vitro and in vivo. Leukemia. 2017;31(10):2278. [DOI] [PubMed] [Google Scholar]
- 8.Li J, Stagg NJ, Johnston J, et al. Membrane-proximal epitope facilitates efficient T cell synapse formation by anti-FcRH5/CD3 and Is a requirement for myeloma cell killing. Cancer Cell. 2017;31(3):383-395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Seckinger A, Delgado JA, Moser S, et al. Target expression, generation, preclinical activity, and pharmacokinetics of the BCMA-T cell bispecific antibody EM801 for multiple myeloma treatment. Cancer Cell. 2017;31(3):396-410. [DOI] [PubMed] [Google Scholar]
- 10.Ise T, Nagata S, Kreitman RJ, et al. Elevation of soluble CD307 (IRTA2/FcRH5) protein in the blood and expression on malignant cells of patients with multiple myeloma, chronic lymphocytic leukemia, and mantle cell lymphoma. Leukemia. 2007;21(1):169-174. [DOI] [PubMed] [Google Scholar]
- 11.Sanchez E, Li M, Kitto A, et al. Serum B-cell maturation antigen is elevated in multiple myeloma and correlates with disease status and survival. Br J Haematol. 2012;158(6):727-738. [DOI] [PubMed] [Google Scholar]
- 12.Zuch de Zafra CL, Fajardo F, Zhong W, et al. Targeting multiple myeloma with AMG 424, a novel anti-CD38/CD3 bispecific T-cellrecruiting antibody optimized for cytotoxicity and cytokine release. Clin Cancer Res. 2019;25(13):3921-3933. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.