Abstract
Objective:
To determine racial/ethnic differences in the use of conventional synthetic or biologic disease-modifying anti-rheumatic drugs (csDMARDs or bDMARDs, respectively) and long-term glucocorticoids (GC) or opioids among beneficiaries of the Social Security Disability Insurance (SSDI) with rheumatoid arthritis (RA) and <65 years old.
Methods:
Serial cross-sectional analyses of Centers for Medicare and Medicaid Services claims data (2007, 2011, and 2014) for individuals <65 years old with RA receiving SSDI Medicare and Medicaid, no longer working because they were considered disabled. Generalized estimating equation models were used to determine whether the proportion of patients who used csDMARD, bDMARD, long-term GC, and long-term opioids differed by race/ethnicity.
Results:
There were 12,931; 15,033; and 15,599 participants in 2007, 2011, and 2014, respectively. The overall use of csDMARD without bDMARD among beneficiaries of the SSDI were 31.1%, 30.3%, and 29.2%; 50.2%, 51.7%, and 53.8% used bDMARDs; 37.6%, 36.1%, and 34.4% used long-term GC; and 61.1%, 63.8%, and 63.7% used long-term opioids in years 2007, 2011, and 2014 respectively. The use of csDMARDs without bDMARDs was higher and the use of bDMARDs was lower among Blacks compared to Whites (adjusted absolute difference: +3.0%, +5.0%, and +3.3% for csDMARDs without bDMARDs and −4.6%, −5.7%, and −4.0% for bDMARDs in 2007, 2011, and 2014, respectively; all p<0.05). The use of bDMARDs was higher among Hispanics compared to Whites (adjusted absolute difference: +7.1%, +7.3%, and +7.5% in 2007, 2011, and 2014, respectively; all p<0.05). Long-term GC use was lower among Hispanics than among Whites only in year 2014 (absolute percentage point difference of −4.2%); no other difference in long-term GC use was identified. Whites were the patients with the highest use of long-term opioids (more than two third in each calendar year).
Conclusion:
Racial and ethnic differences exists in regards to the treatment of RA among beneficiaries of the SSDI. These findings suggest that this already vulnerable population of patients with RA can also have a racial and ethnic disparity that can contribute to additional disease burden and that should be examined in order to inform future interventions or even inform future policy changes to the SSDI.
Keywords: Rheumatoid arthritis, disability, biologics, disease modifying anti-rheumatic drugs, opioids
1. Introduction
Despite the increasing number of effective disease-modifying anti-rheumatic drugs (DMARDs) for rheumatoid arthritis (RA), disability remains pervasive among individuals with this disease.1 In a previous study, patients with disabilities between ages of 25-64 had higher odds of having unmet prescription medications when needed.2 Patients treated with biologic (b) DMARD with or without conventional synthetic (cs) DMARD were able to sustain low disease activity (LDA).3 That is why the current American College of Rheumatology RA treatment guidelines recommend escalating treatment until patients achieve either LDA or remission to preserve joint health, and improve outcomes. 4 However, it is not known the type of RA medications that patients with RA are prescribed once they begin to receive disability benefits before the age of 65, and whether racial disparities exist in the delivery of healthcare in this understudied population. 5–7 This is despite the intense focus on racial disparities across all medical fields while the cost of care is higher for disabled individuals younger than 65 years of age ($13,098 per capita under age 65, compared to $9,972 per capita over age 65).8
Racial and ethnic disparities have been reported extensively among patients with hypertension and lupus.9,10 There is limited data regarding racial/ethnic disparities in clinical outcomes of RA, including disability. Specifically, an analysis of 15 rheumatology practices in the United States (U.S.) found that among patients with RA, non-White patients were approximately twice as likely as White patients to be work-disabled.11 Additionally, a study of the Corrona RA registry during two different periods (2005-2007 and 2010-2012) revealed that disparities in disease activity and clinical outcomes persisted for minorities in the U.S., despite improvement of disease activity across all racial and ethnic groups.12 Several studies have reported differences in RA treatment by race and ethnicity. 13–15 These studies excluded those that were disabled before 65 years of age. Subsequently, there is a knowledge gap in understanding the type of treatment that patients with RA receive once they are unable to work before reaching 65 years of age. More recently, three studies also showed an increasing proportion of older patients with RA receiving chronic opioids.16–18 However, no study has assessed the use of these medications in an already vulnerable population, such as those disabled patients with RA.
The Centers for Medicare and Medicaid Services (CMS) Social Security Disability Insurance (SSDI) program provides medical insurance for individuals in the U.S. who are disabled and younger than 65 years of age.19 Social Security pays monthly benefits to individuals who have been deemed no longer able to work due to a significant illness or impairment. In June 2017, 8.9 million disabled workers received such benefits, accounting for roughly 15% of all Medicare beneficiaries.20 Therefore, CMS datasets provide valuable information on the disabled U.S. patient population that is sufficient to allow analysis of treatment differences by race once individuals receive SSDI benefits. The objectives of this study were to examine racial and ethnic differences in the use (defined as actual prescriptions filled at the pharmacy) of conventional synthetic DMARDs (csDMARDs), biologic DMARDs (bDMARDs), glucocorticoids (GC), and opioids among SSDI beneficiaries who have RA, are younger than 65 years of age, and received both Medicare and Medicaid (dual eligible) benefits.
2. Material and Methods
2.1. Study design and patients
We examined serial cross-sections of CMS data from 2007, 2011, and 2014, using data from beneficiaries with Medicare Part A (inpatient), B (outpatient), and D (drug) coverage. We included only those patients with RA (as defined below) with continuous enrollment in Medicare fee-for-service who were receiving benefits from SSDI and younger than 65 years of age. For inclusion, patients in each cross-sectional study had to be alive for the entire 12-month calendar year and be dually eligible for Medicare and Medicaid benefits, to ensure that all participants in the study had similar medication coverage.
RA was defined as two RA diagnoses (ICD9-714.xx) by a rheumatologist between 7 and 365 days apart (PPV 67%).21 DMARD were not used as part of the RA definition as these medications were the outcome. We excluded individuals with other inflammatory arthritis conditions (ankylosing spondylitis, psoriatic arthritis), psoriasis, systemic lupus erythematosus, inflammatory bowel disease, human immunodeficiency virus, organ transplants, end-stage renal disease, or cancer at any time during that calendar year. This study adhered to the Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) Initiative guidelines,22 as detailed in Supplemental Table 1.
2.2. Exposure and Outcomes
The main exposure for this study was race/ethnicity defined as White, Black (non-Hispanic), Hispanic, or Other (those who were Asian, Native Hawaiian, or Pacific Islander and those whose racial or ethnic status could not be defined). Race and ethnicity were self-reported. To identify Hispanics, CMS utilizes an algorithm created by Eicheldinger and Bonito to reclassify beneficiaries based on surname and language preference, which improves identification from 29.5%, using self-report alone, to 76.6% when using the algorithm among verified Hispanic beneficiaries.23
The outcomes were the proportion of the cohort using (defined as an actual prescription filled by the patient) csDMARDs with or without biologics, bDMARDs, long-term use of GC, or opioids by race/ethnicity. csDMARDs included methotrexate, leflunomide, hydroxychloroquine, sulfasalazine, or azathioprine. bDMARDs included adalimumab, etanercept, certolizumab, abatacept, tocilizumab, infliximab, rituximab, or golimumab. We subdivided bDMARDs as intravenous (IV; infliximab, rituximab, tocilizumab, abatacept, or golimumab), or subcutaneous (SQ; adalimumab, etanercept, certolizumab, golimumab, abatacept, or tocilizumab). We defined a filled prescription for a csDMARD or a bDMARD as a minimum of one filled prescription or infusion (for IV therapies) within each calendar year included in this study. We examined long-term GC use, defined as an annual cumulative prednisone-equivalent dose of 900 mg or more. The American College of Rheumatology glucocorticoid-induced osteoporosis (GIOP) guidelines and the FRAX scoring risk for osteoporotic fracture state that 5 mg of prednisone a day for at least 6 months is considered long-term use of glucocorticoids.24,25 The 900 cumulative prednisone-equivalent dose was the median cumulative dose of prednisone-equivalent across all three years. This would be the equivalent of using 5 mg daily for 6 months, which is in line with the GIOP definition of long-term use of glucocorticoids. Long-term opioid use was defined as 30-day opioid prescription filled at least three times or a 90-day prescription filled at least once.
2.3. Covariates
Age and sex were self-reported. We controlled for multiple comorbidities that could influence the prescription of bDMARD (e.g. heart failure) and the use of opioids (fibromyalgia). We controlled for U.S. region as previous studies have reported that there are heterogeneity in the application for SSDI benefits 26 and other common causes for disability (e.g. spinal cord injury and multiple sclerosis). Another important covariate included was the area deprivation index (ADI) to account as a measure for socio-economic status (SES). The ADI is composed of measures about education, employment, housing-quality, and poverty based on Census data and the American Community Survey (ACS) data.27 We also calculated the Charlson-Deyo Comorbidity Index (CDCI) as part of describing the cohorts.28 CDCI is a weighted index to predict risk of death within 1 year of hospitalization for patients with specific comorbid conditions. Because everyone in our cohort had RA, which earns a score of 1 in the CDCI 28, everyone had a score of at least 1. Mental illness was defined as having any combination of the following conditions: anxiety, depression, bipolar disorder, multiple personality disorder and/or schizophrenia.
2.4. Statistical Analysis
We calculated the absolute percentage point differences in the use of each medication for every racial/ethnic patient group compared with White patients. We then used generalized estimating equation (GEE) models to calculate adjusted differences in the csDMARD, bDMARD, long-term GC, or long-term opioid use between each racial/ethnic group and Whites for the calendar years 2007, 2011, and 2014.29 The models were adjusted for age, sex, U.S. region, ADI, and comorbidities. P values less than 0.05 were considered statistically significant. We conducted a sub-group analyses only among patients that were on any DMARD treatment because administrative claism data difinitions for RA commonly required the use of some RA medication (PPV = 89%).21 We then examined racial and ethnic differences in the use of advances treatment (e.g. bDMARD or tsDMARD) within this sub-group of patients who were on any DMARD at baseline.
3. Results
The overall number of CMS beneficiaries increased between 2007 and 2014, and the number of patients receiving SSDI benefits almost doubled from 332,456 patients in 2011 to 647,549 in 2014 (Supplemental Figure 1). The number of disabled patients with RA receiving SSDI beneficiaries who were younger than 65 years of age was 12,931 in 2007; 15,033 in 2011; and 15,599 in 2014. Most of these patients were White women between 51 and 60 years of age. Black patients had the largest burden of comorbidities, with 22.1%-26.8% having a CDCI score of ≥ 3 across the 3 years. The proportion of White and Hispanic patients having a CDCI ≥ 3 ranged from 19.6% to 21.9%, and 16.1% to 20.5%, respectively (Table 1). The proportion of patients with common conditions for which SSDI benefits were granted, did not exceed more than 10% of the population in each year (Table 1). These common conditions were hemiplegia, paraplegia, spinal cord injury, and multiple sclerosis. We excluded other common causes for disability from this study such as end-stage renal disease, cancer, and HIV. The overall use of csDMARD without bDMARD among beneficiaries of the SSDI were 31.1%, 30.3%, and 29.2%; 50.2%, 51.7%, and 53.8% used bDMARDs; 37.6%, 36.1%, and 34.4% used long-term GC; and 61.1%, 63.8%, and 63.7% used long-term opioids in years 2007, 2011, and 2014 respectively.
Table 1:
Characteristics of Patients with Rheumatoid Arthritis Disabled, Younger than 65 Years of Age, and Dually Eligible for Medicare and Medicaid
| Characteristic | 2007 N = 12,931 |
2011 N = 15,033 |
2014 N= 15,599 |
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| White | Black | Hispanic | Other | White | Black | Hispanic | Other | White | Black | Hispanic | Other | |
| Number | 7,631 | 2,721 | 2,101 | 478 | 8,830 | 3,400 | 2,150 | 653 | 9,152 | 3,309 | 2,504 | 634 |
| Female, % | 80.5 | 88.6 | 83.9 | 81.4 | 80.2 | 88.1 | 83.4 | 79.6 | 80.0 | 88.2 | 83.6 | 82.0 |
| Age Category, % | ||||||||||||
| under 30 years (y) | 2.9 | 2.1 | 2.8 | 3.1 | 2.6 | 2.3 | 2.7 | 3.2 | 2.3 | 2.1 | 2.6 | 3.5 |
| 31 - 40 | 8.2 | 7.5 | 8.2 | 11.3 | 8.2 | 6.7 | 8.0 | 9.5 | 7.4 | 7.1 | 8.5 | 7.4 |
| 41 - 50 | 26.0 | 26.1 | 22.6 | 23.6 | 24.0 | 22.8 | 21.2 | 19.8 | 21.7 | 20.2 | 19.3 | 18.6 |
| 51 - 60 | 43.1 | 45.0 | 46.3 | 44.8 | 45.2 | 47.8 | 45.4 | 43.6 | 49.3 | 49.2 | 48.2 | 48.7 |
| 61 - 64 | 19.8 | 19.2 | 20.2 | 17.2 | 19.9 | 20.4 | 22.7 | 23.9 | 19.2 | 21.5 | 21.4 | 21.8 |
| United States Region, % | ||||||||||||
| Northeast | 29.6 | 35.8 | 6.8 | 19.9 | 27.6 | 35.6 | 10.6 | 19.5 | 27.8 | 36.4 | 10.3 | 21.0 |
| Midwest | 16.5 | 11.2 | 7.0 | 11.7 | 19.0 | 14.9 | 9.5 | 14.1 | 18.6 | 15.7 | 8.5 | 12.9 |
| South | 32.8 | 35.8 | 71.2 | 46.2 | 30.4 | 33.2 | 61.6 | 45.6 | 31.6 | 32.0 | 64.3 | 43.4 |
| West | 12.1 | 8.8 | 6.5 | 9.2 | 12.5 | 8.0 | 8.7 | 8.6 | 13.0 | 9.1 | 8.1 | 9.8 |
| Outlying areas | 9.2 | 8.6 | 8.5 | 12.3 | 10.5 | 8.4 | 9.6 | 12.3 | 9.1 | 6.7 | 8.8 | 12.9 |
| Area Depravation Index | ||||||||||||
| Mean, (SD) | 61.7 (24.2) | 70.9 (24.7) | 59.5 (27.9) | 51.3 (28.3) | 61.6 (24.1) | 70.7 (24.6) | 56.1 (27.8) | 51.3 (28.9) | 60.7 (24.0) | 70.7 (24.3) | 61.1 (27.2) | 51.8 (29.0) |
| Chronic Conditions, % | ||||||||||||
| Myocardial infarction, or Stroke or Transient Ischemic Attack | 3.7 | 3.2 | 3.2 | 1.9 | 3.4 | 4.6 | 2.8 | 2.6 | 3.1 | 4.1 | 2.6 | 2.4 |
| Heart Failure | 6.1 | 8.9 | 5.6 | 3.6 | 5.7 | 9.5 | 4.4 | 4.0 | 5.2 | 8.4 | 4.5 | 2.8 |
| Diabetes mellitus | 22.9 | 30.1 | 33.9 | 24.5 | 23.7 | 32.6 | 32.4 | 29.7 | 23.7 | 31.6 | 31.9 | 28.4 |
| Diabetes Complications | 2.0 | 2.5 | 3.2 | 3.4 | 1.9 | 3.2 | 4.1 | 4.6 | 2.4 | 3.5 | 3.6 | 2.7 |
| PVD | 4.7 | 4.8 | 5.0 | 4.0 | 5.5 | 6.9 | 4.6 | 4.3 | 5.3 | 6.4 | 4.9 | 4.3 |
| Hemiplegia or paraplegia or SCI | 0.3 | 0.4 | 0.4 | 0.8 | 0.4 | 0.4 | 0.3 | 0.0 | 0.4 | 0.4 | 0.4 | 0.6 |
| Multiple Sclerosis | 0.9 | 0.7 | 0.6 | 0.0 | 0.9 | 1.1 | 0.4 | 0.6 | 0.9 | 0.8 | 0.4 | 0.5 |
| Dementias | 0.6 | 0.5 | 0.6 | 0.4 | 1.0 | 0.6 | 0.6 | 0.0 | 1.1 | 0.7 | 0.9 | 1.0 |
| COPD | 20.8 | 11.5 | 10.3 | 8.4 | 22.6 | 15.0 | 10.4 | 10.7 | 22.3 | 14.5 | 9.3 | 10.6 |
| Fibromyalgia | 25.9 | 20.8 | 29.3 | 19.3 | 37.4 | 32.8 | 31.8 | 28.6 | 44.2 | 38.3 | 39.9 | 33.6 |
| Mental Illness | 31.9 | 18.6 | 24.8 | 22.0 | 39.5 | 27.3 | 29.8 | 24.4 | 48.9 | 32.4 | 37.8 | 33.3 |
| Depression or Anxiety | 29.4 | 17.4 | 23.8 | 20.5 | 36.6 | 25.3 | 28.4 | 22.2 | 45.7 | 29.8 | 36.7 | 31.7 |
| Charlson-Deyo Comorbidity Index Score,% | ||||||||||||
| 1 | 50.8 | 49.9 | 50.0 | 55.6 | 48.4 | 44.7 | 47.4 | 49.5 | 48.1 | 44.1 | 49.3 | 52.2 |
| 2 | 29.6 | 28.0 | 29.9 | 28.2 | 29.6 | 28.5 | 32.2 | 30.0 | 30.0 | 29.0 | 30.3 | 29.3 |
| ≥3 | 19.6 | 22.1 | 20.1 | 16.1 | 22.0 | 26.8 | 20.4 | 20.5 | 21.9 | 26.8 | 20.4 | 18.5 |
| Any csDMARD, % | 66.0 | 66.7 | 69.4 | 67.6 | 65.8 | 67.7 | 67.9 | 66.6 | 63.5 | 66.4 | 65.6 | 64.7 |
| csDMARD no bDMARD,% | 31.4 | 34.5 | 26.6 | 27.8 | 30.0 | 35.3 | 25.1 | 26.8 | 29.2 | 32.7 | 24.8 | 27.4 |
| Any bDMARD,% | 49.7 | 45.4 | 56.7 | 56.1 | 51.7 | 46.1 | 59.0 | 57.3 | 53.3 | 49.3 | 60.9 | 57.9 |
| Any SQ bDMARD,% | 33.5 | 29.2 | 35.4 | 36.4 | 33.7 | 28.3 | 35.2 | 35.4 | 35.3 | 30.8 | 35.7 | 37.5 |
| Any IV bDMARD,% | 18.7 | 18.3 | 24.9 | 23.0 | 20.6 | 19.6 | 27.1 | 25.0 | 17.9 | 18.3 | 26.6 | 20.8 |
| Neither csDMARD or bDMARD, % | 18.9 | 20.1 | 16.7 | 16.1 | 18.3 | 18.7 | 12.7 | 15.9 | 17.5 | 18.0 | 14.3 | 14.7 |
| Glucocorticoids*, % | 37.8 | 38.3 | 35.8 | 37.9 | 36.1 | 36.6 | 34.5 | 38.0 | 34.7 | 36.3 | 30.2 | 36.3 |
| Opioids**,% | 66.0 | 58.9 | 48.8 | 48.5 | 67.8 | 64.0 | 49.5 | 55.0 | 66.7 | 64.6 | 54.8 | 50.2 |
csDMARD, conventional synthetic disease-modifying anti-rheumatic drug; bDMARD, biologic disease-modifying anti-rheumatic drug; SQ, subcutaneous; IV, intravenous. SCI = spinal cord injury; PVD = peripheral vascular disease; COPD = chronic obstructive pulmonary disease
csDMARDs included methotrexate, leflunomide, hydroxychloroquine, sulfasalazine, and azathioprine.
SQ bDMARDs included adalimumab, etanercept, certolizumab, golimumab, abatacept, and tocilizumab.
IV bDMARDs included infliximab, rituximab, tocilizumab, abatacept, and golimumab.
Long-term glucocorticoid treatment was defined as an annual cumulative prednisone-equivalent dose of 900 mg or more.
Long-term opioid use was defined as a 30-day prescription refilled for ≥ 2 additional months or a 90-day supply in a single prescription.
Mental illness was defined as any of the following conditions: anxiety, depression, bipolar disorder, multiple personality disorder or schizophrenia.
Notes: The higher the mean score of the ADI the highest the deprivation and the worse the socioeconomic status; Outlying areas include Alaska, Hawaii and U.S. territories.
After multivariable adjustment, the use of csDMARDs without biologics was higher among Blacks than among Whites each year, with an absolute percentage point difference of +2.2%, +4.6%, and +2.9% for years 2007, 2011, and 2014, respectively (all p<0.05; Figures 1A and 1B). Conversely, the use of csDMARDs without biologics was lower among Hispanics than among Whites each year, with an absolute percentage point difference of −4.7%, −4.7%, and −4.1% for years 2007, 2011, and 2014, respectively (all p<0.05; Figures 1A and 1B).
Figure 1.
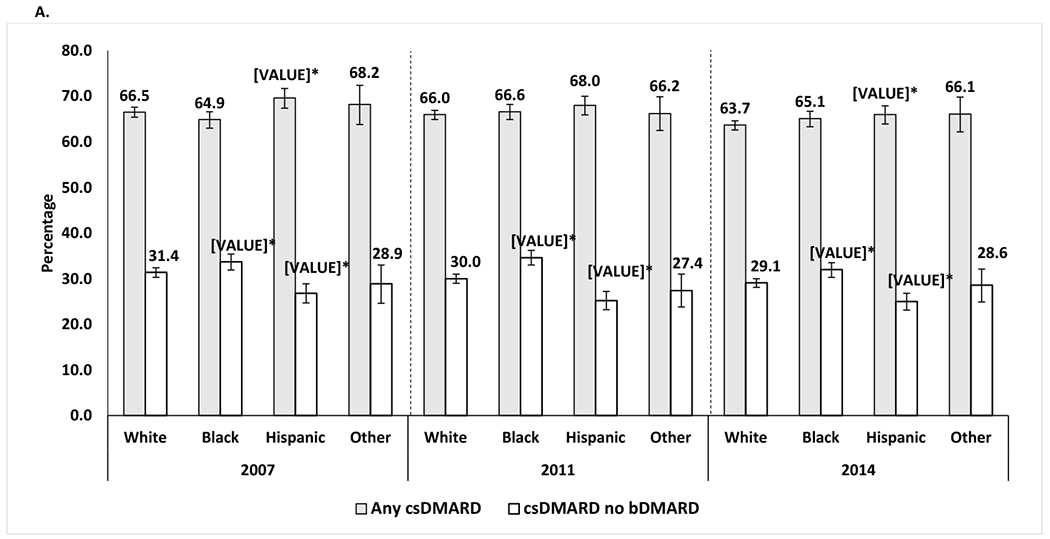
Differences in the use of csDMARD and long-term glucocorticoid by racial/ethnic groups among SSDI beneficiaries with RA who were disabled and younger than 65 years.
Percentage of patients who used csDMARDs with or without bDMARDs among all patients who used csDMARDs in each racial/ethnic group, by year, after adjustment for covariates.
The proportion of patients that were neither on csDMARD, bDMARD or tsDMARD was 18.7% in 2007, 17.9% in 2011 and 17.0% in 2014
*Statistically significant vs. the percentage for White patients
Adjusted for age, sex, Unites States region, heart failure, chronic obstructive pulmonary disease, area deprivation index, fibromyalgia, diabetes, diabetes complications, mental illnesses, transient ischemic attack, stroke or myocardial infarction, and peripheral vascular disease.
Mental illness was defined as any of the following conditions: anxiety, depression, bipolar disorder, multiple personality disorder or schizophrenia.
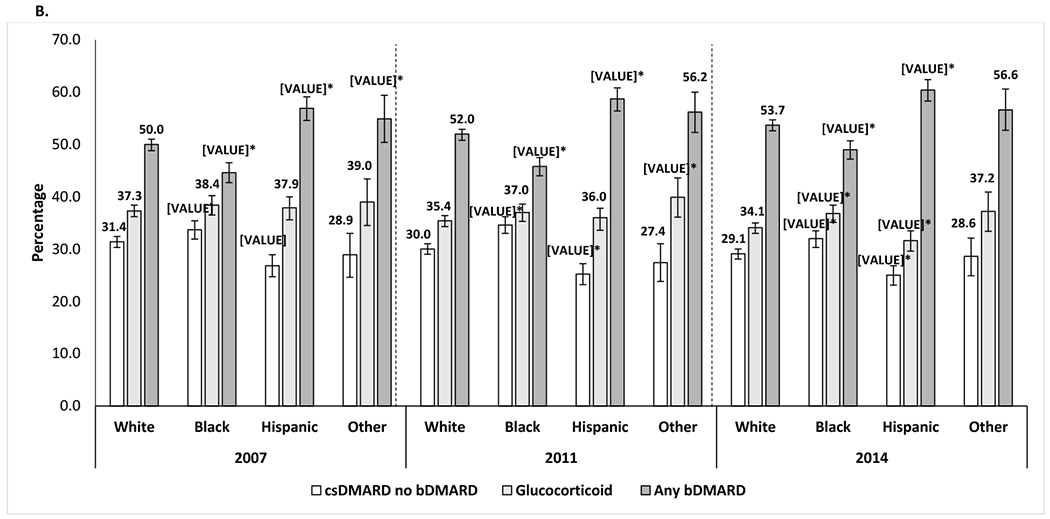
Percentage of all patients among each racial/ethnic group who used csDMARDs without biologics, long-term GCs.¥, or bDMARDs with or without csDMARD, by year, after adjustment for covariates. *, statistically significant vs. the percentage for Whites.
The proportion of patients that were neither on csDMARD, bDMARD or tsDMARD was 18.7% in 2007, 17.9% in 2011 and 17.0% in 2014.
¥Long-term glucocorticoid treatment was defined as an annual cumulative prednisone-equivalent dose of 900 mg or more.
Adjusted for age, sex, United States region, heart failure, chronic obstructive pulmonary disease, area deprivation index, fibromyalgia, diabetes, diabetes complications, mental illnesses, transient ischemic attack, stroke or myocardial infarction, and peripheral vascular disease.
Mental illness was defined as any of the following conditions: anxiety, depression, bipolar disorder, multiple personality disorder or schizophrenia.
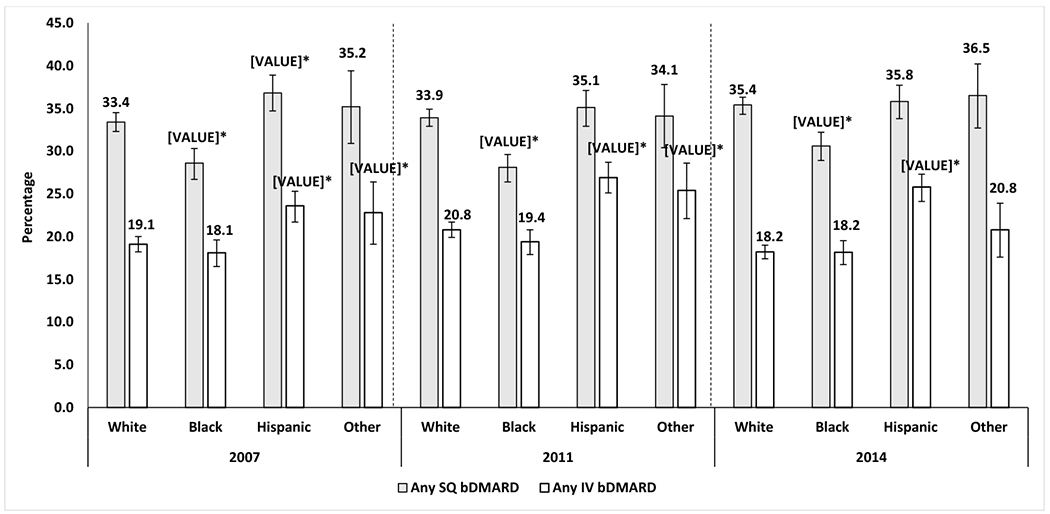
The use of bDMARDs, particularly SQ bDMARDs, was lower among Blacks than among Whites each year, with an absolute percentage difference in SQ bDMARD use of −4.7%, −5.7%, and −4.8% for years 2007, 2011, and 2014, respectively and after multivariable adjustment (all p<0.05; Figures 1B and 2). Conversely, the use of bDMARDs, particularly IV bDMARDs, was higher among Hispanics than among Whites each year, with an absolute percentage difference in IV bDMARD use of +4.6%, +6.1%, and +7.7% for years 2007, 2011, and 2014, respectively (Figures 2; all p<0.05). There were no differences in the use of csDMARD between the Other racial/ethnic group and Whites each year. The use of bDMARDs, was also higher among the Other racial/ethnic group than among Whites in 2007 and 2011, with an absolute percentage difference in any bDMARD use of +4.8%, +4.1%, respectively (Figures 1B; all p<0.05).
Figure 2:
Differences in the use of bDMARDs by racial/ethnic groups among SSDI beneficiaries with RA who were disabled and younger than 65 years.
Percentage of all patients among each racial/ethnic group who used SQ or IV bDMARDs, by year.
Adjusted for age, sex, United States region, heart failure, chronic obstructive pulmonary disease, area deprivation index, fibromyalgia, diabetes, diabetes complications, mental illnesses, transient ischemic attack, stroke or myocardial infarction, and peripheral vascular disease.
Mental illness was defined as any of the following conditions: anxiety, depression, bipolar disorder, multiple personality disorder or schizophrenia.
*Statistically significant vs. the percentage for White patients
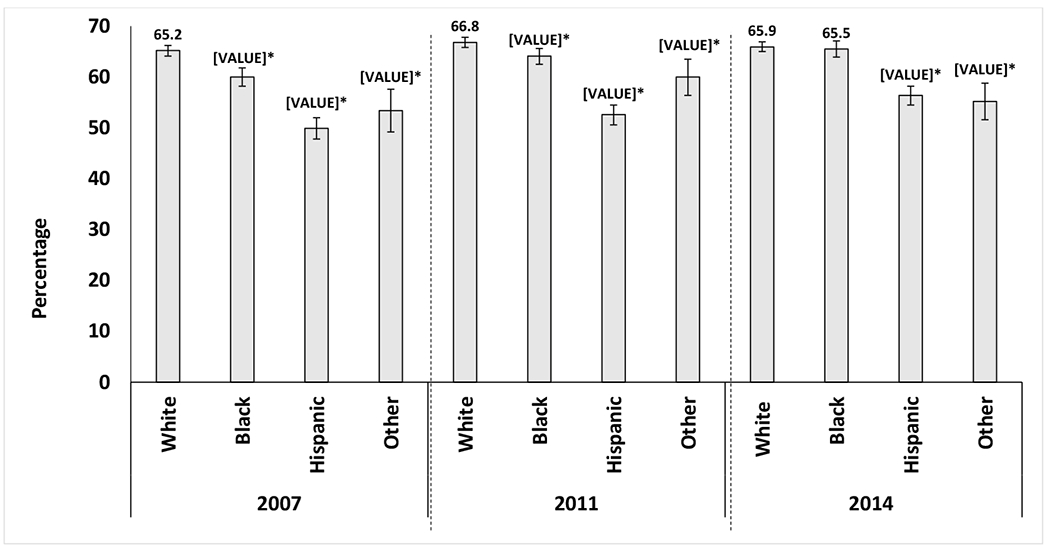
Long-term GC use was higher among the Other race/ethnicity group compared to Whites in 2011, with an absolute percentage point difference of +4.4, while Hispanics had lower use of GC compared to Whites in year 2014 only (absolute percentage point difference of −2.5%) (Figure 1B). Blacks had a higher use of GC compared to Whites in year 2014 only (+2.7 absolute percentage point difference). There were no other difference between racial/ethnic groups in long-term GC use across years. Long-term opioid use was recorded for more than two thirds (65.2%-66.8%) of the White patients in each calendar year (Figure 3). Long-term opioid use was lowest among Hispanic patients each year except 2014, when long-term opioid use was lowest among the Other racial/ethnic group (Figure 3). The long-term use of opioids increased over time among Blacks and Hispanics. The difference between Hispanics and Whites in long-term use of opioids narrowed from 2007 to 2014 (absolute percentage point difference: −15.4% in 2007 and −9.4% in 2014). The difference between Blacks and Whites (absolute percentage point difference: −5.2% in 2007) was not observed by 2014 when both groups had similar percentage points of long-term use of opioids (65.5% Blacks vs. 66.8% Whites).
Figure 3:
Differences in long-term opioid use by racial/ethnic groups among SSDI beneficiaries with RA who were disabled and younger than 65 years.
Percentage of all patients among each racial/ethnic group using long-term opioids, by year
Adjusted for age, sex, United States region of the United States, heart failure, chronic obstructive pulmonary disease, area deprivation index, fibromyalgia, diabetes, diabetes complications, mental illnesses, transient ischemic attack, stroke or myocardial infarction, and peripheral vascular disease.
¥Long-term opioid use was defined as a 30-day opioid prescription refilled for ≥ 2 additional months or a 90-day supply in a single prescription.
Mental illness was defined as: anxiety, depression, bipolar disorder, multiple personality disorder or schizophrenia.
*Statistically significant vs. the percentage for White patients.
3.1. Sub-group analyses results
The results from our sub-group analyses were conditional of being on any DMARD for RA (100% of the sample were on DMARD) at baseline. The differences in the proportion of patients that received bDMARD by race/ethnicity were −4.6 for Blacks and +6.5 for Hispanics in 2007 compared to Whites (p<0.005). In 2011 it was −6.5 for Blacks and +6.5 for Hispanics compared to Whites for the use of bDMARD (p<0.005). In 2014 it was −4.3 for Blacks and +5.9 for Hispanics for the use of either bDMARD or tsDMARD compared to Whites (p<0.005). The results from this sub-group analysis were slightly different in the magnitude of the racial and ethnic difference but remained the same statistical significance as those that included patients with and without any DMARD at baseline (Figures 1A and 1B).
4. Discussion
The results of this study highlight that, although small, there were significant differences by race and ethnicity in the use of csDMARDs, bDMARDs, long-term GC, and long-term opioids among SSDI beneficiaries younger than 65 years of age with RA in the United States. A high proportion of patients were treated with bDMARDs, with more than half of the patients in each racial/ethnic group treated with any bDMARD. Blacks had the highest use csDMARDs without bDMARDs, and the lowest use of any bDMARD. This contrasted with the pattern observed in Hispanics, who had the lowest long-term use opioids (except for 2014 where the Other racial/ethnic group had the lowest use of opioids), and csDMARDs without bDMARDs, but the highest use of bDMARDs among all groups. Opioid use was highest among Whites, with two thirds of these patients receiving long-term opioid medications in years 2007 and 2011, but the use of opioids was similar between Blacks and Whites by the year 2014.
Among these patients with RA who are SSDI beneficiaries and considered to be disabled, the use of bDMARDs, particularly SQ bDMARDs, was significantly lower among Blacks but significantly higher among Hispanics and the Other racial/ethnic group than among Whites.30–32 Multiple studies have shown that the rheumatologist’s treatment preference is an important determinant of the decision to initiate treatment with bDMARDs. 33,34 In a cross-sectional study comparing two clinic settings (university vs. public county hospital clinic), Barton et al. found significant racial and ethnic differences in RA activity such that patients who were non-White, non-English speaking, or immigrants had higher disease activity than U.S. born Whites by clinic site.15 This disparity occurred despite the fact that the same rheumatologists provided care at both clinic settings.15 In another study conducted using the National Ambulatory Care Medical Survey, Blacks were 30% less likely to be prescribed any DMARDs than Whites were.35 Hispanics were 29% less likely to receive anti-TNF biologic in a study conducted using the Corrona registry even after adjusting for age, sex, disease duration and disease activity.36 The high proportion of bDMARD prescriptions observed among Hispanics in our study is particularly difficult to explain or contextualize given the very few prior studies of Hispanics with RA. Still, none of these studies focused on disabled patients with RA, which is why our study among beneficiaries of the SSDI, provides insight of a possible issue of health disparity in those patients with RA who have now become beneficiaries of the SSDI. This is in line with studies that showed that racial and ethnic disparities in medical treatment remain even after adjustment for socioeconomic differences (i.e. ADI) and other healthcare access-related factors, which in the case of our study we took into consideration equal coverage (i.e. dual eligibility), there were still differences in RA treatment.37
Two small studies of patients with RA demonstrated that non-white racial and ethnic groups were less adherent to RA treatment or were less likely to fill a new prescription for RA medication.38,39 Another study also identified White individuals were 66% more likely to be prescribed a biologic compared to non-White.40 This suggests that the issue of health disparity is multifactorial and that despite differences in medication adherence that might exist across racial and ethnic groups, there is still a disparity in the care provided to patients with RA by race. The reasons for the health disparity observed in our study and others, either at the patient or the physician level, should be the next steps to study so that we can be better equipped to intervene and address this problem. The results of our study represent a signal of persistent racial and ethnic disparity in the medical treatment of already vulnerable populations such as those who are disabled and unable to work before the retirement age of 65 years old.
The results in our study about the use of opioids, have been the largest reported in any study of patients with RA. In a previous study using the Corrona registry, it was reported that the use of opioids increased from 7.4% in 2002 to 16.9% in 2015.17 In another study within Kaiser Permanente the reported use of opioids was 23%.18 Lastly, in a study among beneficiaries of Medicare older than 65 years of age, the average use of opioids was 40%.16 The findings of our study showed an astonishing high range of opioid use between 64.4% and 67.7%, the highest ever reported among patients with RA, and all of which were younger than 65 years of age and receiving disability benefits. This finding poses a large challenge for clinicians and policy makers regarding how opioid use could be a contributing factor for individuals filing for disability benefits. It is possible that at least for a time, these patients did not have adequate access to RA treatment before they were granted disability benefits, which made them rely on opioid medications to manage their pain. This led them to continue their use of opioids even though they now have extensive and affordable access to RA medications (i.e. after they become dual-eligible). Another possible explanation is that disabled patients have multiple comorbidities (i.e. multimorbidity), which made them more prone to long-term use of opioids than other populations of patients with RA who are not disabled. We accounted for such comorbidities in our analysis to avoid overestimating the use of these medications in this population.
Long-standing RA is another reason that could have led these patients to experience chronic debilitating pain. While we do not have information regarding RA disease duration, there is limited evidence that the age of incidence of RA is vastly different between racial and ethnic groups. The mean age of incidence of RA in the early RA registry of African American patients with RA, the Consortium for the Longitudinal Evaluations of African Americans with Early Rheumatoid Arthritis (CLEAR) Registry I, was reported to be 50 years.41 The incidence of RA among a predominantly White cohort from the Olmsted County was 55 years.42 Since almost half of the patients in our study were between 50-60 years of age it is unlikely that there might be differences across ages in terms of the disease duration by race, as average age of incidence of RA is relatively similar across racial and ethnic groups.
The high chronic use of opioids among SSDI beneficiaries have been found to increase the risk of overdose death, particularly among those with history of substance abuse, psychiatric disease and chronic pain.43 This merits the development of its own research agenda not only from the opioid use standpoint but to guarantee that patients with RA receive adequate, early care that prevents them from abandoning the workforce and developing chronic, debilitating pain that can make them at risk to rely on medications like opioids. It is also important to further study opioid use that we reported here to inform future policies and behavioral interventions for SSDI beneficiaries with RA.
This investigation is among the first to examine the prescription pattern of DMARD medications among patients with RA who are SSDI beneficiaries younger than 65 years of age. Strengths of this study included the large sample size, which increased our power to draw conclusions, and the use of SSDI data, which ensured that the patients in this study were indeed receiving CMS benefits because of disability. We also considered in our population those beneficiaries that were dual eligible (Medicare and Medicaid) to avoid heterogeneity in medical and pharmacy coverage, which would have limited comparisons between groups. We took into consideration socioeconomic status (SES) and U.S. regions as these could explain racial and ethnic differences. Accounting for them minimizes the risk of residual bias. SES and U.S. regions can also explain the differences in patients being granted disability benefits, which is another factor that adds to the robustness of our results. Limitations of our study include a lack of data regarding the reason for disability. While we assumed that disability was probably granted because the patient had RA, we believe this assumption is not particularly important, as RA is a disease that requires a high level of care and treatment. Regardless of the reason that SSDI benefits were granted, the patients had RA and were receiving DMARDs. Therefore, we were still able to examine whether racial and ethnic differences exist in RA treatment once patients with RA become beneficiaries of the SSDI and stop working before the retirement age of 65 years. We were also able to describe the use of DMARDs in this population. The proportion of individuals with the most common reasons for granting SSDI benefits was low (<5%) except for mental illness, which we controlled for in the models of our study to minimize bias. Another limitation of our study was that we only enrolled individuals who obtained SSDI benefits and thus represent only those who were willing to endure an extensive judiciary process to obtain disability benefits. Therefore, our analyses may not have captured patients who had significant RA-related functional limitations but did not pursue disability benefits. Another limitation was that the proportion of filled prescriptions may not directly correlate with usage (with the exception of IV administered medication), but adherence to medication was not an outcome of this study. Moreover, for medications not filled, we lacked information regarding the underlying reasons (e.g. serious adverse event or not responding to medication). Finally, we used a serial cross-sectional study design rather than a longitudinal study design. However, the multi-year cross sectional study allowed us to examine trends over time.
5. Conclusions
We observed significant differences by race and ethnicity in the proportion of prescriptions of csDMARDs, bDMARDs, long-term use of GC, and long-term use of opioids among patients disabled with RA who were younger than 65 years of age. Future studies should focus on examining potential reasons for these differences, such as physicians’ prescribing behaviors, patient preferences for RA medications, as well as the long-term clinical and economic outcomes of disabled RA patients.
Supplementary Material
Acknowledgments
Funding: This work was supported by INM’s IPCI award from Weill Cornell Medicine Division of General Internal Medicine. INM was supported by the K23AR068449 award from NIAMS. MMS was supported by K24 HL111154 from NHLBI. JC was supported by PCORI (PPRND-1507-32163).
Author Disclosures: JRC received research support and consulting fees from AbbVie, Amgen, BMS, Corrona, Eli Lilly, Janssen, Myriad, Pfizer, Regeneron, Roche, UCB. MMS received salary support from Amgen.
References:
- 1.Sokka T, Kautiainen H, Pincus T, et al. Work disability remains a major problem in rheumatoid arthritis in the 2000s: data from 32 countries in the QUEST-RA study. Arthritis Res Ther. 2010;12(2):R42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Mahmoudi E, Meade MA. Disparities in access to health care among adults with physical disabilities: Analysis of a representative national sample for a ten-year period. Disability and Health Journal. 2015;8(2):182–190. [DOI] [PubMed] [Google Scholar]
- 3.Kaltsonoudis E, Pelechas E, Voulgari PV, Drosos AA. Unmet needs in the treatment of rheumatoid arthritis. An observational study and a real-life experience from a single university center. Seminars in arthritis and rheumatism. 2019;48(4):597–602. [DOI] [PubMed] [Google Scholar]
- 4.Singh JA, Saag KG, Bridges SL Jr., et al. 2015 American College of Rheumatology Guideline for the Treatment of Rheumatoid Arthritis. Arthritis Care Res (Hoboken). 2016;68(1):1–25. [DOI] [PubMed] [Google Scholar]
- 5.Fiscella K, Sanders MR. Racial and Ethnic Disparities in the Quality of Health Care. Annual review of public health. 2016;37:375–394. [DOI] [PubMed] [Google Scholar]
- 6.Braveman PA, Cubbin C, Egerter S, et al. Socioeconomic status in health research: one size does not fit all. JAMA : the journal of the American Medical Association. 2005;294(22):2879–2888. [DOI] [PubMed] [Google Scholar]
- 7.Zsembik BA, Fennell D. Ethnic variation in health and the determinants of health among Latinos. Social science & medicine. 2005;61(1):53–63. [DOI] [PubMed] [Google Scholar]
- 8.Cubanski JNT, Damico A. Medicare’s Role for People Under Age 65 with Diabilities. The Henry J. Kaiser Family Foundation; 2016. [Google Scholar]
- 9.Lim SS, Helmick CG, Bao G, et al. Racial Disparities in Mortality Associated with Systemic Lupus Erythematosus - Fulton and DeKalb Counties, Georgia, 2002–2016. MMWR Morb Mortal Wkly Rep. 2019;68(18):419–422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Mills KT, Bundy JD, Kelly TN, et al. Global Disparities of Hypertension Prevalence and Control: A Systematic Analysis of Population-Based Studies From 90 Countries. Circulation. 2016;134(6):441–450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.De Roos AJ, Callahan LF. Differences by sex in correlates of work status in rheumatoid arthritis patients. Arthritis care and research : the official journal of the Arthritis Health Professions Association. 1999;12(6):381–391. [DOI] [PubMed] [Google Scholar]
- 12.Greenberg JD, Spruill TM, Shan Y, et al. Racial and ethnic disparities in disease activity in patients with rheumatoid arthritis. Am J Med. 2013;126(12):1089–1098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Chu LH, Portugal C, Kawatkar AA, Stohl W, Nichol MB. Racial/ethnic differences in the use of biologic disease-modifying antirheumatic drugs among California Medicaid rheumatoid arthritis patients. Arthritis Care Res (Hoboken). 2013;65(2):299–303. [DOI] [PubMed] [Google Scholar]
- 14.Kerr GS, Swearingen C, Mikuls TR, Yazici Y. Use of Biologic Therapy in Racial Minorities With Rheumatoid Arthritis From 2 US Health Care Systems. Journal of clinical rheumatology : practical reports on rheumatic & musculoskeletal diseases. 2017;23(1):12–18. [DOI] [PubMed] [Google Scholar]
- 15.Barton JL, Trupin L, Schillinger D, et al. Racial and ethnic disparities in disease activity and function among persons with rheumatoid arthritis from university-affiliated clinics. Arthritis Care Res (Hoboken). 2011;63(9):1238–1246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Curtis JR, Xie F, Smith C, et al. Changing Trends in Opioid Use among U.S. Rheumatoid Arthritis Patients. Arthritis & rheumatology. 2017. [DOI] [PubMed] [Google Scholar]
- 17.Lee YC, Kremer J, Guan H, Greenberg J, Solomon DH. Chronic Opioid Use in Rheumatoid Arthritis: Prevalence and Predictors. Arthritis & rheumatology. 2019;71(5):670–677. [DOI] [PubMed] [Google Scholar]
- 18.Herrinton LJ, Harrold L, Salman C, et al. Population Variations in Rheumatoid Arthritis Treatment and Outcomes, Northern California, 1998–2009. Perm J. 2016;20(1):4–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Administration SS. Medicare Information. https://www.ssa.gov/disabilityresearch/wi/medicare.htm. Accessed 1/14/2019.
- 20.The Board of Trustees FHIaFSMITF. 2018. ANNUAL REPORT OF THE BOARDS OF TRUSTEES OF THE FEDERAL HOSPITAL INSURANCE AND FEDERAL SUPPLEMENTARY MEDICAL INSURANCE TRUST FUNDS. Centers for Medicare and Medicaid Services2018. [Google Scholar]
- 21.Kim SY, Servi A, Polinski JM, et al. Validation of rheumatoid arthritis diagnoses in health care utilization data. Arthritis Res Ther. 2011;13(1):R32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Von Elm E, Altman DG, Egger M, et al. The Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) statement: guidelines for reporting observational studies. PLoS medicine. 2007;4(10):e296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Eicheldinger C, Bonito A. More accurate racial and ethnic codes for Medicare administrative data. Health Care Financ Rev. 2008;29(3):27–42. [PMC free article] [PubMed] [Google Scholar]
- 24.Kanis JA, Johansson H, Oden A, McCloskey EV. Guidance for the adjustment of FRAX according to the dose of glucocorticoids. Osteoporosis international : a journal established as result of cooperation between the European Foundation for Osteoporosis and the National Osteoporosis Foundation of the USA. 2011;22(3):809–816. [DOI] [PubMed] [Google Scholar]
- 25.Maricic M, Deal C, Dore R, Laster A. 2017 American College of Rheumatology Guideline for the Prevention and Treatment of Glucocorticoid-Induced Osteoporosis: Comment on the Article by Buckley et al. Arthritis Care Res (Hoboken). 2018;70(6):949–950. [DOI] [PubMed] [Google Scholar]
- 26.Coe N, Haverstick K, Munnell AH, Webb A. What Explains State Variation in SSDI Application Rates? Center for Retirement Research at Boston College Working Paper. 2011(2011-23). [Google Scholar]
- 27.Kind AJ, Jencks S, Brock J, et al. Neighborhood socioeconomic disadvantage and 30-day rehospitalization: a retrospective cohort study. Annals of internal medicine. 2014;161(11):765–774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Deyo RA, Cherkin DC, Ciol MA. Adapting a clinical comorbidity index for use with ICD-9-CM administrative databases. Journal of Clinical Epidemiology. 1992;45(6):613–619. [DOI] [PubMed] [Google Scholar]
- 29.Schmajuk G, Trivedi AN, Solomon DH, et al. Receipt of disease-modifying antirheumatic drugs among patients with rheumatoid arthritis in Medicare managed care plans. JAMA : the journal of the American Medical Association. 2011;305(5):480–486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Levine DA, Lewis CE, Williams OD, et al. Geographic and demographic variability in 20-year hypertension incidence: the CARDIA study. Hypertension. 2011;57(1):39–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Birns J, Morris R, Jarosz J, Markus H, Kalra L. Ethnic differences in the cerebrovascular impact of hypertension. Cerebrovascular Diseases. 2008;25(5):408–416. [DOI] [PubMed] [Google Scholar]
- 32.Fernandez M, Alarcon GS, Calvo-Alen J, et al. A multiethnic, multicenter cohort of patients with systemic lupus erythematosus (SLE) as a model for the study of ethnic disparities in SLE. Arthritis Rheum. 2007;57(4):576–584. [DOI] [PubMed] [Google Scholar]
- 33.Kalkan A, Husberg M, Hallert E, et al. Physician Preferences and Variations in Prescription of Biologic Drugs for Rheumatoid Arthritis: A Register-Based Study of 4,010 Patients in Sweden. Arthritis Care Res (Hoboken). 2015;67(12):1679–1685. [DOI] [PubMed] [Google Scholar]
- 34.Curtis JR, Chen L, Harrold LR, Narongroeknawin P, Reed G, Solomon DH. Physician preference motivates the use of anti-tumor necrosis factor therapy independent of clinical disease activity. Arthritis Care Res (Hoboken). 2010;62(1):101–107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Solomon DH, Ayanian JZ, Yelin E, Shaykevich T, Brookhart MA, Katz JN. Use of disease-modifying medications for rheumatoid arthritis by race and ethnicity in the National Ambulatory Medical Care Survey. Arthritis Care Res (Hoboken). 2012;64(2):184–189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Lee SJ, Kremer J, Kavanaugh A, America CoRRoN. Treatment disparity related to race/ethnicity and education in rheumatoid arthritis patients: Comment on the article by Constantinescu et al et al. Arthritis Care & Research. 2009;61(8):1141–1142. [DOI] [PubMed] [Google Scholar]
- 37.Staff IoM. Unequal treatment: Confronting racial and ethnic disparities in healthcare. National Academies Press; 2004. [PubMed] [Google Scholar]
- 38.Quinlan P, Price KO, Magid SK, Lyman S, Mandl LA, Stone PW. The relationship among health literacy, health knowledge, and adherence to treatment in patients with rheumatoid arthritis. HSS Journal®. 2013;9(1):42–49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Kan HJ, Dyagilev K, Schulam P, et al. Factors associated with physicians’ prescriptions for rheumatoid arthritis drugs not filled by patients. Arthritis Res Ther. 2018;20(1):79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Kerr GS, Swearingen C, Mikuls TR, Yazici Y. Use of Biologic Therapy in Racial Minorities With Rheumatoid Arthritis From 2 US Health Care Systems. JCR: Journal of Clinical Rheumatology. 2017;23(1). [DOI] [PubMed] [Google Scholar]
- 41.Bridges SL Jr., Causey ZL, Burgos PI, et al. Radiographic severity of rheumatoid arthritis in African Americans: results from a multicenter observational study. Arthritis Care Res (Hoboken). 2010;62(5):624–631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Myasoedova E, Crowson CS, Kremers HM, Therneau TM, Gabriel SE. Is the incidence of rheumatoid arthritis rising?: results from Olmsted County, Minnesota, 1955–2007. Arthritis Rheum. 2010;62(6):1576–1582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Kuo YF, Raji MA, Goodwin JS. Association of Disability With Mortality From Opioid Overdose Among US Medicare Adults. JAMA Netw Open. 2019;2(11):e1915638. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


