Abstract
The cyclin pathway may confer resistance to standard treatments but also offer novel therapeutic opportunities in prostate cancer. Herein, we analyzed prostate cancer samples (majority metastatic) using comprehensive genomic profiling performed by next‐generation sequencing (315 genes, >500× coverage) for alterations in activating and sensitizing cyclin genes (CDK4 amplification, CDK6 amplification, CCND1, CCND2, CCND3, CDKN2B [loss], CDKN2A [loss], SMARCB1), androgen receptor (AR) gene, and coalterations in genes leading to cyclin inhibitor therapeutic resistance (RB1 and CCNE1). Overall, cyclin sensitizing pathway genomic abnormalities were found in 9.7% of the 5,356 tumors. Frequent alterations included CCND1 amplification (4.2%) and CDKN2A and B loss (2.4% each). Alterations in possible resistance genes, RB1 and CCNE1, were detected in 9.7% (up to 54.6% in neuroendocrine) and 1.2% of cases, respectively, whereas AR alterations were seen in 20.9% of tumors (~27.3% in anaplastic). Cyclin sensitizing alterations were also more frequently associated with concomitant AR alterations.
Short abstract
Clinical trials with cyclin inhibitors for prostate cancer are ongoing; thus, characterization of the landscape of cyclin pathway genomic alterations is needed. To that end, this article analyzes prostate cancer samples using comprehensive genomic profiling performed by next‐generation sequencing.
Introduction
The cyclin pathway is crucial for cell cycle control. In cancer cells, deregulation can lead to uncontrolled cell division and progression. Preliminary data in prostate cancer suggest that the cyclin pathway plays an important role in the evolution to a castrate‐resistant state and demonstrates interplay with androgen signaling [1, 2]. A prior report indicates that next‐generation sequencing can be helpful in advanced prostate cancer, revealing alterations in genes from cyclin pathway [3]. Breast cancer is a hormone‐dependent cancer for which different cyclin inhibitors have been successfully approved. Clinical trials with cyclin inhibitors are ongoing in prostate cancer [4], and, thus, characterization of the landscape of cyclin pathway genomic alterations is needed. Herein, we analyzed prostate cancer samples (mostly metastatic) using comprehensive genomic profiling performed by next‐generation sequencing
Materials and Methods
We analyzed 5,356 anonymized patient prostate cancer samples (majority metastatic) at a Clinical Laboratory Improvement Amendments–certified, College of American Pathologists–accredited laboratory (Foundation Medicine).The proportion of samples from primary and metastatic sites in patients with prostate cancer in the database is approximately 48% and 52%, respectively [5]. Approval for the Foundation Medicine cohort, including a waiver of informed consent and Health Insurance Portability and Accountability Act waiver of authorization, was obtained from the Western Institutional Review Board (Protocol No. 20152817). Tissue diagnoses were designated according to the pathology report and further verified by a pathologist. Comprehensive genomic profiling was performed on hybridization‐captured, adaptor ligation‐based libraries (315 genes, >500× coverage). We described alterations in cyclin pathway sensitizing genes (8 genes, including CDK4 amplification, CDK6 amplification, CCND1, CCND2, CCND3, CDKN2B [loss], CDKN2A [loss], and SMARCB1) and genes related to resistance to cyclin inhibition (RB1 and CCNE1; supplemental online Table 1). Co‐occurrence analysis was performed matching cyclin‐pathway sensitizing genomic alterations with three different subsets of genomic alterations (resistance pathway [RB1 and CCNE1], cyclin‐related [SMAD3, CDKN1A, CDKN1B, CDKN2C], and androgen receptor [AR]). Statistical analysis was performed using GraphPad Prism, Python 2.7, and Anaconda V4 (Anaconda Software Distribution, Vers. 4–4.3.21; https://anaconda.com). Co‐occurrence analysis was performing matching cyclin‐pathway genomic alterations with three different subsets of genomic alterations (resistance pathway, cyclin‐related, and AR).
Results and Discussion
Alterations in any cyclin pathway sensitizing genes were found in 9.7% of the 5,356 tumors analyzed (the majority adenocarcinoma acinar [n = 4,897]), which is lower compared with other solid tumor types [6]. The most frequent type of alteration observed in cyclin sensitizing genes was copy number variation, except for SMARCB1 (single nucleotide change). Frequencies by gene were distributed according to Figure 1A. The most frequent alterations were CCND1 amplification (4.2%) and CDKN2A/CDKN2B loss (2.4% each). Histology was also important in regard to frequency of alterations. Ductal adenocarcinomas and anaplastic tumors were enriched for cyclin sensitizing alterations, especially CDKN2A/B loss. Both genes were also frequently altered in tumors with a mesenchymal component (sarcomas and carcinosarcomas) despite the low number of samples.
Figure 1.
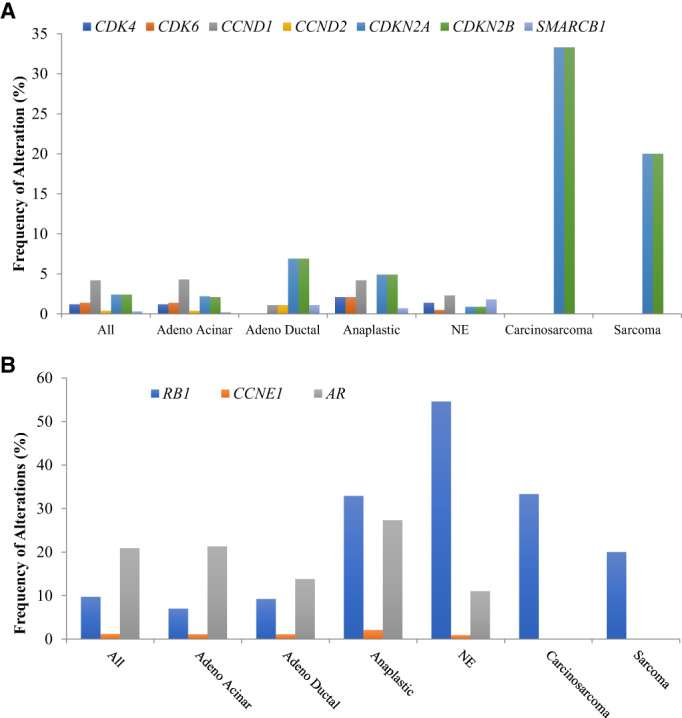
Frequency (percentage of patients) of each listed alteration in prostate cancer. (A): Cyclin pathway gene alterations in patients with prostate cancer alterations in (B). Alterations in putative cyclin resistance genes (RB1 and CCNE1) and AR Abbreviations: adeno, adenocarcinoma; AR, androgen receptor; NE, neuroendocrine.
Resistance to CDK inhibitors can be mediated by genomic alterations in genes such as RB1 and CCNE1 [7]. Overall, alterations in these genes were present in 9.7% and 1.2% of prostate cancer samples, respectively (Fig. 1B). Neuroendocrine tumors presented a high frequency of RB1 alterations (54.6%). We also analyzed the likelihood of co‐occurrence of a sensitizing alteration in the cyclin pathway and in a possible resistance pathway. A lower likelihood of co‐occurrence compared with an isolated alteration in cyclin sensitizing and resistance pathway genes was demonstrated (odd ratio [OR], 0.44; p < .001; Fig. 2; supplemental online Table 2), which suggests potential feasibility for activity of cyclin inhibitors.
Figure 2.
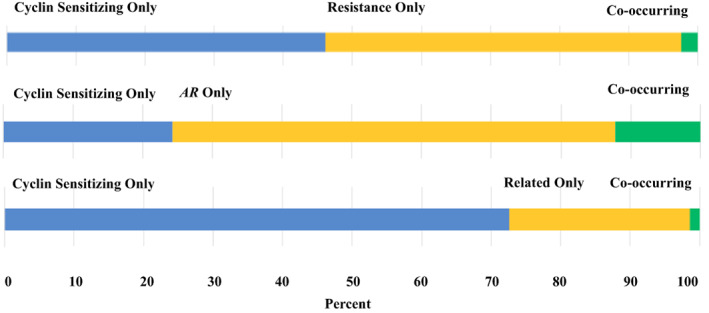
Co‐occurrence analysis of cyclin sensitizing (CDK4, CDK6, CCND1, CCND2, CCND3, CDKN2B, CDKN2A, and SMARCB1) and resistance genes (RB1 and CCNE1), AR, and cyclin‐related genes (SMAD3, CDKN1A, CDKN1B, CDKN2C). Percent refers to percentage of patients with an alteration. Patients with neither alteration are not included in this graphic, but the numbers are given in supplemental online Table 3. Abbreviations: AR, androgen receptor.
AR gene alterations can also occur in advanced prostate disease. In fact, 20.9% of all samples had AR alterations, with higher frequency in anaplastic tumors (27.3%). Overall, this frequency is lower compared with other genomic sequencing series, which described alterations in AR in approximately 60% of patients [8, 9, 10]. However, these prior series included only metastatic castration‐resistant prostate cancer, a state enriched for AR abnormalities. Our series included both primary and metastatic disease and was not restricted to castrate‐resistant advanced disease. Co‐occurrence analysis demonstrated a significant co‐occurrence between AR and sensitizing cyclin alterations (as compared with AR alterations in patients wild type for sensitizing cyclin alterations; OR, 1.79; p < .001; Fig. 2; supplemental online Table 2). In prostate cancer, the cyclin pathway may interplay with androgen signaling but may also mediate AR independence [11, 12]. A positive co‐occurrence of AR and cyclin sensitizing gene alterations might suggest the existence of a subset of patients with more intense resistance to monotherapy with next‐generation antiandrogens that could be addressed with cell cycle inhibitors as part of the therapeutic strategy. Interestingly, preclinical rationale suggests further testing of CDK4/6 inhibitors in this setting [13].
It is important to put our data from 5,356 patients with prostate cancer in perspective with other publications interrogating smaller numbers of patients (supplemental online Table 3). The Cancer Genome Atlas Program (available at http://www.cbioportal.org) included data from 494 prostate cancer samples (predominantly primary tumors) and described a lower frequency of cyclin sensitizing alterations. Other series with a mixture of primary and metastatic samples revealed frequencies that are more similar to our results (n = 1,013 samples analyzed) [14]. Taken together, we can hypothesize that cyclin sensitizing alterations are enriched in advanced tumors, perhaps as a result of therapeutic pressure or accumulation of genetic alterations during the course of disease. This study has several limitations, including the lack of clinical correlates, which limits possible associations of genomic alteration with prognosis and response to therapies in prostate cancer. These data support cyclin pathway alterations as relevant for the progression of prostate cancer and may inform opportunities for targeted therapy, especially for cyclin inhibitors alone or in combination with antiandrogens.
Disclosures
Denis L. Jardim: Roche, Janssen, Astellas, Merck Sharpy & Dohme, Bristol‐Myers Squibb, Libbs (Speaker fees), Janssen, Bristol‐Myers Squibb, Libbs (C/A); Sherri Millis: Foundation Medicine (E); Jeffrey Ross: Foundation Medicine (E); Michele Sue‐Ann Woo: Daiichi Sankyo, Foundation Medicine (E); Siraj M. Ali: EQRx Inc (E, equity), Foundation Medicine (E), In8bio, Elevation Oncology, Pillar Biosciences (SAB), Takeda, ArcherDX (C/A); Razelle Kurzrock: Genentech, Merck Serono, Pfizer, Boehringer Ingelheim, TopAlliance, Takeda, Incyte, Debiopharm, Medimmune, Sequenom, Foundation Medicine, Konica Minolta, Grifols, Omniseq, Guardant (RF), X‐Biotech, Neomed, Pfizer, Actuate Therapeutics, Roche, Turning Point Therapeutics, TD2/Volastra, Bicara Therapeutics, Inc., (C/A, Speaker fees), IDbyDNA and CureMatch Inc (Equity interest), CureMatch and CureMetrix (Board member), CureMatch (Cofounder).
(C/A) Consulting/advisory relationship; (RF) Research funding; (E) Employment; (ET) Expert testimony; (H) Honoraria received; (OI) Ownership interests; (IP) Intellectual property rights/inventor/patent holder; (SAB) Scientific advisory board
Supporting information
See http://www.TheOncologist.com for supplemental material available online.
Appendix S1. Supporting Information
Acknowledgments
Funded in part by National Cancer Institute grant P30 CA016672 and the Joan and Irwin Jacobs Fund philanthropic fund (all funds received by Razelle Kurzrock).
Disclosures of potential conflicts of interest may be found at the end of this article.
References
- 1. Drobnjak M, Osman I, Scher HI et al. Overexpression of cyclin D1 is associated with metastatic prostate cancer to bone. Clin Cancer Res 2000;6:1891–1895. [PubMed] [Google Scholar]
- 2. Xu Y, Chen SY, Ross KN et al. Androgens induce prostate cancer cell proliferation through mammalian target of rapamycin activation and post‐transcriptional increases in cyclin d proteins. Cancer Res 2006;66:7783–7792. [DOI] [PubMed] [Google Scholar]
- 3. Ikeda S, Elkin SK, Tomson BN et al. Next‐generation sequencing of prostate cancer: Genomic and pathway alterations, potential actionability patterns, and relative rate of use of clinical‐grade testing. Cancer Biol Ther 2019;20:219–226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Scheinberg T, Kench J, Stockler M et al. Pharmacodynamics effects of CDK4/6 inhibitor LEE011 (ribociclib) in high‐risk, localised prostate cancer: A study protocol for a randomised controlled phase II trial (LEEP STUDY: Lee011 in high‐risk, localised prostate cancer). BMJ Open 2020;10:e033667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Chung JH, Dewal N, Sokol E et al. Prospective comprehensive genomic profiling of primary and metastatic prostate tumors. JCO Precis Oncol 2019;3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Helsten T, Kato S, Schwaederle M et al. Cell‐cycle gene alterations in 4,864 tumors analyzed by next‐generation sequencing: Implications for targeted therapeutics. Mol Cancer Ther 2016;15:1682–1690. [DOI] [PubMed] [Google Scholar]
- 7. Pandey K, An HJ, Kim SK et al. Molecular mechanisms of resistance to CDK4/6 inhibitors in breast cancer: A review. Int J Cancer 2019;145:1179–1188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Robinson D, Van Allen EM, Wu YM et al. Integrative clinical genomics of advanced prostate cancer. Cell 2015;161:1215–1228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Quigley DA, Dang HX, Zhao SG et al. Genomic hallmarks and structural variation in metastatic prostate cancer. Cell 2018;174:758–769.e9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Abida W, Cyrta J, Heller G et al. Genomic correlates of clinical outcome in advanced prostate cancer. Proc Natl Acad Sci USA 2019;116:11428–11436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Blee AM, He Y, Yang Y et al. TMPRSS2‐ERG controls luminal epithelial lineage and antiandrogen sensitivity in PTEN and TP53‐mutated prostate cancer. Clin Cancer Res 2018;24:4551–4565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Handle F, Prekovic S, Helsen C et al. Drivers of AR indifferent anti‐androgen resistance in prostate cancer cells. Sci Rep 2019;9:13786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Stice JP, Wardell SE, Norris JD et al. CDK4/6 therapeutic intervention and viable alternative to taxanes in CRPC. Mol Cancer Res 2017;15:660–669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Armenia J, Wankowicz SAM, Liu D et al. The long tail of oncogenic drivers in prostate cancer. Nat Genet 2018;50:645–651. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
See http://www.TheOncologist.com for supplemental material available online.
Appendix S1. Supporting Information


