Abstract
Purpose
The noninterventional, prospective NIMES‐ROC phase IV study (NCT02825420) evaluated trabectedin plus pegylated liposomal doxorubicin (PLD) in real‐life clinical practice.
Patients and Methods
Eligible participants included adults with platinum‐sensitive recurrent ovarian cancer (PS‐ROC) who had received one or more cycles of trabectedin/PLD before inclusion according to the marketing authorization. The primary endpoint was progression‐free survival (PFS) according to investigator criteria.
Results
Two hundred eighteen patients from five European countries were evaluated, 72.5% of whom were pretreated with at least two prior chemotherapy lines and received a median of six cycles of trabectedin/PLD (range: 1–24). Median PFS was 9.46 months (95% confidence interval [CI], 7.9–10.9), and median overall survival (OS) was 23.56 months (95% CI, 18.1–34.1). Patients not pretreated with an antiangiogenic drug obtained larger median PFS (p < .007) and OS (p < .048), largely owning to differences between the two populations. Twenty‐four patients (11.0%) had a complete response, and 57 patients (26.1%) achieved a partial response for an objective response rate (ORR) of 37.2%. Fifty‐nine patients (27.1%) had disease stabilization for a disease control rate of 64.2%. No statistically significant difference in PFS, OS, or ORR was observed by BRCA1/2 status and platinum sensitivity. Most common grade 3/4 adverse events (AEs) were neutropenia (30.3%), anemia (6.4%), thrombocytopenia (5.5%), and asthenia (5.0%). No deaths attributed to treatment‐related AEs or unexpected AEs occurred.
Conclusion
The combination of trabectedin/PLD represents a clinically meaningful and safe option for patients with PS‐ROC regardless of prior treatment with an antiangiogenic drug, being comparable with previously observed outcomes in selected and less pretreated patients from clinical trials.
Implications for Practice
This noninterventional, prospective study, conducted in 57 reference sites across Europe, consistently confirmed that trabectedin plus pegylated liposomal doxorubicin (PLD) in routine clinical practice represents a clinically meaningful and safe option for women with platinum‐sensitive recurrent ovarian cancer. Although the study population represented a heterogeneous, older, and more pretreated population than those in prospective clinical trials, the combination of trabectedin plus PLD induced comparable clinical benefits, with a similar and manageable safety profile. Overall, these findings show that trabectedin in combination with PLD maintains antitumor activity when administered to heavily pretreated patients in real‐life clinical practice.
Keywords: Trabectedin, Noninterventional, Pegylated liposomal doxorubicin, Ovarian
Short abstract
This clinical trial evaluated the use of trabectedin plus pegylated liposomal doxorubicin (PLD) in patients with platinum‐sensitive recurrent ovarian cancer and its efficacy and safety in routine clinical practice in five European countries.
Introduction
Ovarian cancer is one of the most common gynecologic malignancies and the fifth most frequent cause of death by cancer in women [1]. Approximately 75% of patients with ovarian cancer present advanced stage of disease associated with poor outcome, and most cases (>80%) occur in women over 50 years of age [2]. The standard front‐line treatment for advanced disease consists of cytoreductive surgical debulking followed by platinum/taxane‐based chemotherapy. Despite the progress achieved in the last years with advances in surgery [3] and the introduction of new drug options, such as bevacizumab (first antiangiogenic) in 2011 and olaparib (first PARP inhibitor) in 2014, around 70%–80% of the patients with epithelial cancer relapse within 2 years after diagnosis [4, 5, 6]. Currently, it is an utmost clinical challenge of the scientific community to ameliorate the prognosis and to improve the quality of life of these patients, particularly by finding the right combinations and sequence of use of the available treatments together with the identification of new treatment options [7].
Trabectedin (Yondelis; PharmaMar, S.A., Madrid, Spain) is a semisynthetic drug originally isolated from the sea squirt Ecteinascidia turbinata (Anatomical Therapeutic Chemical code L01CX01). Trabectedin has pleiotropic mechanisms of action, as in addition to acting as a DNA‐binding agent, inducting direct growth inhibition and ultimately apoptosis, it has selective anti‐inflammatory and immunomodulatory effects on the tumor microenvironment prompted by the inhibition of factors that promote tumor growth, metastasis, and tumor‐promoted angiogenesis [8, 9, 10]. Since 2009, trabectedin in combination with pegylated liposomal doxorubicin (PLD) has been approved in the European Union and in approximately 70 other countries around the globe for the treatment of patients with platinum‐sensitive recurrent ovarian cancer (ROC). The approval is based on the results of the large randomized phase III OVA‐301 study that compared PLD alone with the nonplatinum combination of trabectedin plus PLD [11]. In the platinum‐sensitive population, OVA‐301 demonstrated that trabectedin plus PLD significantly improves progression‐free survival (PFS) over PLD alone (median PFS: 9.2 vs. 7.5 months; hazard ratio [HR], 0.73; 95% confidence interval [CI], 0.56–0.95; p = .0170) when given after failure of first‐line platinum‐based chemotherapy. An enhanced activity of trabectedin plus PLD was observed in patients with partially platinum‐sensitive disease, with a treatment‐free interval of platinum (TFIp) from 6 to 12 months, who also obtained significantly larger overall survival (OS) with the combination as compared with PLD alone (median OS, 22.4 vs. 16.4 months; HR, 0.64; 95% CI, 0.47–0.86; p = .0027) [12]. In addition, an exploratory analysis of the OVA‐301 results reported that platinum‐sensitive patients with BRCA1 mutations might be particularly sensitive to trabectedin plus PLD, as they obtained remarkably longer median PFS (13.6 vs. 5.5 months, p = .0001) and OS (27.4 vs. 18.7 months, p = .0093) than those treated with PLD alone [13]. In line with this analysis of OVA‐301, the prespecified analysis of another phase III study (OVC‐3006), which compared trabectedin plus PLD versus PLD alone in the third‐line setting in patients with platinum‐sensitive ROC, also reported significant survival advantage among BRCA1/2 mutation carriers (median OS, 34.2 vs. 20.9 months; HR, 0.54; 95% CI, 0.33–0.90; p = .016) [14] and a tendency to improve survival among patients with a TFIp of 6–12 months (24.8 vs. 17.4 months; HR, 0.69; 95% CI, 0.48–1.01; p = .056) [14].
Although controlled clinical trials are the cornerstone of medical evidence, their applicability and generalizability to daily clinical practice in a more diverse patient population need to be verified through postauthorization observational studies. Such observational studies can provide useful insights of the real‐world safety, efficacy, and management of patients who may be underrepresented in clinical trials because of more restrictive eligibility criteria. In that sense, three previous national studies [15, 16, 17] about the real‐life use of trabectedin plus PLD in patients with platinum‐sensitive ROC have reported that this combination confers clinically meaningful long‐term benefit to patients with platinum‐sensitive ROC, comparable to that reported in clinical trials. So far, no prospective, real‐life, pan‐European, noninterventional study with trabectedin plus PLD had been performed. Accordingly, we designed the prospective, noninterventional NIMES‐ROC study (ClinicalTrials.gov Identifier: NCT02825420) to evaluate the use of trabectedin plus PLD in patients with platinum‐sensitive ROC and its efficacy and safety in routine clinical practice in five European countries. In order to better understand the management of patients receiving trabectedin plus PLD after the approval of bevacizumab in 2011, all results of the combination were assessed considering the prior use of antiangiogenics.
Subjects, Materials, and Methods
Study Design
This prospective, noninterventional, multicenter, European phase IV NIMES‐ROC study evaluated the use of trabectedin plus PLD in adult women with platinum‐sensitive ROC in a routine clinical practice. In accordance with the noninterventional and observational nature of the study, no involvement with any treatment decisions or additional per protocol diagnostic or therapeutic procedures was required during the study. Eligible participants included adult women (≥18 years old) with platinum‐sensitive ROC, defined as disease relapse after a TFIp of ≥6 months after completion of last platinum‐containing therapy, who have received a minimum of one cycle of trabectedin and PLD before their inclusion in the study, and who signed an informed consent document.
The combination of trabectedin plus PLD was given regardless of prior use of antiangiogenics according to the terms of the marketing authorization (PLD 30 mg/m2 immediately followed by trabectedin 1.1 mg/m2, administered as an intravenous infusion over 3 hours every 3 weeks), standard local clinical practice, and the treating clinician's discretion. Any dose modifications and/or change in dosing interval was performed in accordance with the local marketing authorization and the treating clinician's best clinical judgment. There were no predefined limits to the number of administered cycles, and the treatment could continue as long as the patient had clinical benefit. The observational period of the study began the date of the first administration of trabectedin plus PLD after the signing of the informed consent and continued for up to 12 months after that or until treatment discontinuation, patient discontinuation for any reason, or patient's death. The follow‐up visit took place 1 month after the last on‐study administration. For patients who discontinued treatment before the end of the 12‐month treatment period, a follow‐up visit was performed 12 months after the first trabectedin plus PLD dose. After trabectedin plus PLD treatment discontinuation, patients could have been treated with subsequent anticancer therapies or supportive care as per the clinician's clinical judgment.
All study procedures were conducted in accordance with the ethical standards as laid down in the 1964 Declaration of Helsinki and its later amendments and with guidelines for Good Pharmacoepidemiology Practice and were approved by the institutional review boards of each participating center. Signed informed consents were obtained from all study participants prior to participation in the study.
Study Evaluations
The primary endpoint of this study was to assess PFS, preferably measured by RECIST version 1.0 or 1.1 [18, 19] as generally accepted standard criteria and/or according to clinical assessment. Secondary endpoints included the assessment of the objective response rate (ORR), defined as the percentage of patients with a complete response (CR) or partial response (PR); disease control rate (DCR), defined as the percentage of patients with an objective response and stable disease (SD); OS; and time to next treatment. Secondary endpoints also comprised an evaluation of treatment exposure and treatment duration and the safety of the combination. The PFS was defined as the time interval from the first administration of trabectedin plus PLD to the earliest date of disease progression or death, regardless of cause (whichever occurred first), whereas OS was defined as the time between the start of trabectedin and patient death from any cause. Patients without tumor progression or death at the time of the final analysis or considered lost to follow‐up were censored at the date of last contact/last date known alive.
The results of imaging and response evaluations were collected at baseline, prior to trabectedin plus PLD administration, or the earliest imaging study after the first treatment cycle and were repeated at the treating clinician's schedule from cycle 2 and onward. The final imaging was the assessment performed closest to the follow‐up visit and prior to initiation of any other chemotherapy treatment.
Adverse events (AEs) and serious adverse events (SAEs) were documented starting from the first application of trabectedin plus PLD and repeated at the treating clinician's typical schedule until 30 days after administration of the last dose. All AEs were coded using the Medical Dictionary for Regulatory Activities (MedDRA), version 17.0, and graded according to the last National Cancer Institute Common Terminology Criteria in force.
Statistical Analysis
All statistical analysis had an exploratory nature and was presented in a descriptive manner with no aim to confirm or reject predefined hypotheses, and for all p values the selected significance level was .05. The safety and efficacy analyses were based on the full analysis set (FAS), defined as all the enrolled patients into the study who received at least one dose of trabectedin plus PLD. All results were assessed in the overall population and according to prior administration of an antiangiogenic drug. PFS, OS, and response assessment were described by BRCA1/2 status (positive, negative, not tested) and by platinum sensitivity (fully sensitive with a TFIp: >12 months vs. partially sensitive with a TFIp: 6–12 months). Response assessment was also described by number of cycles of trabectedin (fewer than six vs. six or more cycles). Categorical variables were expressed as absolute and relative frequencies and continuous variables as the median, range (minimum to maximum), and 95% CI. Time‐to‐event endpoints (PFS and OS) and their fixed‐time estimations were estimated according to the Kaplan‐Meier method and were compared using the log‐rank test, whereas Cox regression models were performed for covariate analyses.
Results
Patient Disposition and Characteristics
From January 1, 2015, to September 18, 2019, 218 out of 220 enrolled patients from 57 European sites across Italy, Spain, Germany, France, and Belgium received trabectedin plus PLD and were included in the FAS. Two patients were excluded from the analysis set because of missing PLD treatment data. At study entry patients had a median age of 61.0 years (range: 39–86), most had high‐grade (n = 156, 71.6%) tumor at initial diagnosis, and papillary‐serous carcinoma (n = 157, 72.0%) was the most prevalent histological type (Table 1). BRCA mutation was tested in 135 patients (61.9%), and BRCA1/2 mutations were identified in 34 patients (15.6%). Eastern Cooperative Oncology Group (ECOG) performance status score of 0/1 was recorded in 164 patients (75.2%). All but one patient were pretreated with one up to eight prior lines of chemotherapy, and 72.5% of patients received at least two lines of chemotherapy prior to trabectedin plus PLD. The most frequently reported best responses to last prior therapy regimen were CR (n = 55, 25.2%), PR (n = 54, 24.8%), and SD (n = 48, 22.0%).
Table 1.
Patient and disease characteristics at baseline
| Demographics and baseline characteristics | Full analysis set (n = 218), n (%) |
|---|---|
| Age at study entry, median (range), years | 61.3 (39–86) |
| Age group (years) | |
| <65 years | 129 (59.2) |
| ≥65–74 years | 70 (32.1) |
| ≥75 years | 19 (8.7) |
| Tumor grade at diagnosis | |
| High | 156 (71.6) |
| Intermediate | 14 (6.4) |
| Low | 12 (5.5) |
| Not done/not reported/unknown | 36 (16.5) |
| Histopathology | |
| Papillary/serous | 157 (72.0) |
| Endometroid | 14 (6.4) |
| Clear cell carcinoma | 10 (4.6) |
| Peritoneal carcinoma | 9 (4.1) |
| Other | 15 (6.9) |
| Unknown | 13 (6.0) |
| Platinum sensitivity | |
| Partially platinum sensitive | 127 (58.3) |
| Fully platinum sensitive | 89 (40.8) |
| BRCA1/2 status | |
| Positive | 34 (15.6) |
| Negative | 100 (45.9) |
| Unknown | 1 (0.5) |
| Not done | 83 (38.1) |
| ECOG performance status | |
| 0 | 108 (49.5) |
| 1 | 56 (25.7) |
| 2 | 6 (2.8) |
| Missing | 48 (22.0) |
| Prior surgery | 199 (91.3) |
| Surgery residual disease | 84 (38.5) |
| Prior radiotherapy | 7 (3.2) |
| Prior chemotherapy: Prior platinum therapy | 217 (99.5) |
| No. of chemotherapy lines prior to trabectedin plus PLD | |
| None | 1 (0.5) |
| 1 prior line | 59 (27.1) |
| 2 prior lines | 71 (32.6) |
| 3 prior lines | 43 (19.7) |
| 4–8 prior lines | 44 (20.2) |
| Best response to last chemotherapy regimen | |
| CR | 55 (25.2) |
| PR | 54 (24.8) |
| SD | 48 (22.0) |
| PD | 32 (14.7) |
| NE | 29 (13.3) |
Abbreviations: CR, complete response; ECOG, Eastern Cooperative Oncology Group; NE, not evaluated; PD, progressive disease; PLD, pegylated liposomal doxorubicin; PR, partial response; SD, stable disease.
After stopping treatment with trabectedin plus PLD, subsequent therapy was given to 126 patients (57.8%) after a median time of 6.1 weeks (range, 2.1–61.1). An analysis of subsequent chemotherapies showed that most patients received carboplatin (n = 82; 37.6%), followed by paclitaxel (n = 56; 25.7%) and gemcitabine (n = 50; 22.9%).
Extent of Exposure
Patients received a median of six cycles of trabectedin plus PLD over a median treatment duration of 21.1 weeks (range: 3.0–74.0), with 126 (57.8%) patients receiving six or more cycles and up to a maximum of 24 cycles (Table 2). The number of patients treated on an outpatient basis (n = 139, 63.8%) was threefold higher than of those who received inpatient cancer treatment (n = 47, 21.6%). The most frequently (>5% overall) used prophylactic granulocyte colony‐stimulating factors were filgrastim (n = 39, 17.9%) and pegfilgrastim (n = 19, 8.7%).
Table 2.
Trabectedin plus PLD exposure
| Treatment delivery | Full analysis set (n = 218), n (%) |
|---|---|
| Number of cycles received per patient | |
| Median (range) | 6.0 (1.0–24.0) |
| Fewer than six cycles | 92 (42.2) |
| Six or more cycles | 126 (57.8) |
| Total number of cycles | 1,329.0 (100) |
| Cycle duration, median (range), days | 25.5 (19.3–51.3) |
| Time on treatment, median (range), weeks | 21.1 (3.0–74.0) |
| Total dose received per infusion, median (range), mg | |
| Trabectedin | 1.6 (0.9–2.3) |
| PLD | 45.4 (20.0–63.0) |
| Relative dose intensity, median (range), % | |
| Trabectedin | 74.3 (16.2–102.8) |
| PLD | 75.3 (16.2–102.5) |
| Type of treatment setting | |
| Outpatients | 139 (63.8) |
| Inpatients | 47 (21.6) |
| Both | 19 (8.7) |
| Missing | (6.0) |
Abbreviation: PLD, pegylated liposomal doxorubicin.
Cycle delays occurred in 126 patients (57.8%), commonly because of trabectedin plus PLD‐related AEs (n = 73; 33.5%) or scheduling conflict (n = 40; 18.3%). Dose reductions of trabectedin and PLD occurred in 47 patients (21.6%), mainly because of treatment‐related AEs (n = 42; 19.3%). Likewise, trabectedin and PLD dose was interrupted in four patients (1.8%), mainly because of treatment‐related AEs (n = 3; 1.4%). The most common causes leading to treatment discontinuation of 203 patients (93.1%) were disease progression (n = 106, 48.6%), completed treatment (n = 34, 15.6%), AEs (n = 28, 12.8%), and patient refusal (n = 14; 6.4%).
Primary Efficacy Endpoint
In the FAS, during the median follow‐up period of 13.4 months a total of 156 progression or death events (71.6% of patients) were recorded, whereas 62 patients (28.4%) who were alive or not assessed for disease progression at the time of this analysis were censored. Median PFS was 9.46 months (95% CI, 7.9–10.9), with 86.0%, 68.8%, and 40.5% of patients free from progression at 3, 6, and 12 months after treatment, respectively. No statistically significant differences were observed in an analysis of patients according to their platinum sensitivity (partially platinum‐sensitive vs. fully platinum‐sensitive; p = .62) and BRCA1/2 status (positive vs. negative vs. not tested; p = .58) (Fig. 1).
Figure 1.
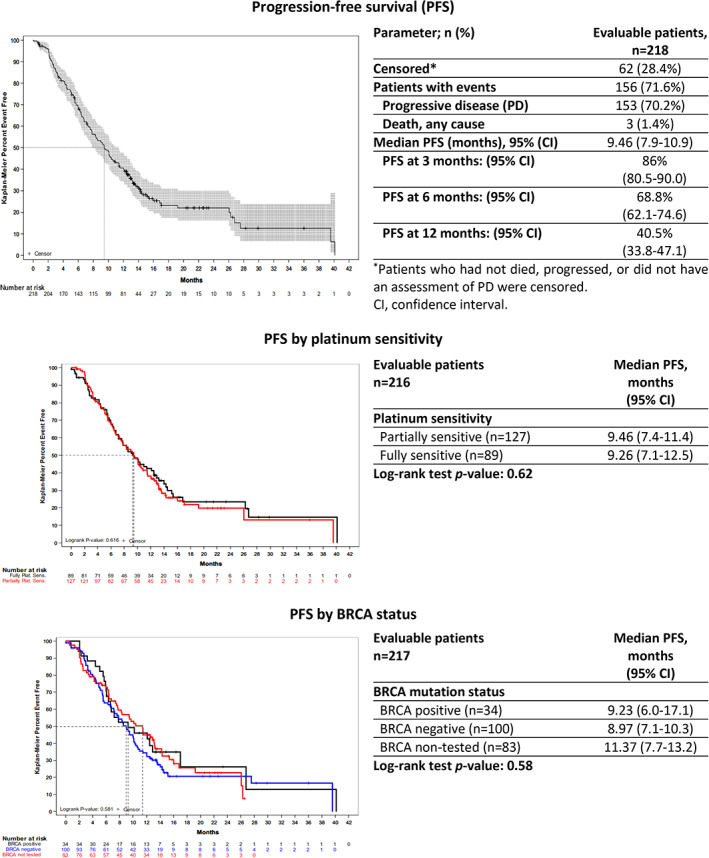
Kaplan‐Meier plots of progression‐free survival. Patients who had not died, progressed, or did not have an assessment of PD were censored.Abbreviations: CI, confidence interval; PD, progressive disease; PFS, progression‐free survival.
Other Efficacy Endpoints
The overall trabectedin plus PLD activity was commonly evaluated according to RECIST version 1.0 or 1.1 (n = 170; 78.0%). A total of 24 patients (11.0%) obtained a CR, and 57 patients (26.1%) achieved a PR response, reaching the ORR of 37.2% (95% CI, 30.7–43.9). Additionally, 59 patients (27.1%) had disease stabilization as a best response for a DCR of 64.2% (95% CI, 57.5–70.6). Patients who received six or more cycles of treatment obtained significantly better response (p < .001) than those who received fewer than six cycles. Similar to what was observed for PFS, no significant differences were observed in response according to patients' platinum sensitivity and BRCA1/2 status (Table 3).
Table 3.
Response assessment of trabectedin plus pegylated liposomal doxorubicin total population and by number of cycles of treatment, platinum sensitivity and BRCA status
| Best response to trabectedin (unconfirmed) | Number of cycles (n = 218), n (%) | Platinum sensitivity (n = 216), n (%) | BRCA status (n = 217), n (%) | Total (n = 218), n (%) | ||||
|---|---|---|---|---|---|---|---|---|
| Fewer than six cycles (n = 83) | Six or more cycles (n = 135) | Fully sensitive (n = 89) | Partially sensitive (n = 127) | Positive (n = 34) | Negative (n = 100) | Not tested (n = 83) | ||
| CR | 3 (3.6) | 21 (15.6) | 12 (13.5) | 12 (9.4) | 5 (14.7) | 11 (11.0) | 8 (9.6) | 24 (11.0) |
| PR | 15 (18.1) | 42 (31.1) | 28 (31.5) | 28 (22.0) | 11 (32.4) | 25 (25.0) | 20 (24.1) | 57 (26.1) |
| SD | 12 (14.5) | 47 (34.8) | 21 (23.6) | 37 (29.1) | 10 (29.4) | 27 (27.0) | 22 (26.5) | 59 (27.1) |
| PD | 43 (51.8) | 19 (14.1) | 24 (27.0) | 38 (29.9) | 7 (20.6) | 29 (29.0) | 26 (31.3) | 62 (28.4) |
| Not evaluable | 0 | 3 (2.2) | 1 (1.1) | 2 (1.6) | 0 | 1 (1.0) | 2 (2.4) | 3 (1.4) |
| Not done | 10 (12.0) | 3 (2.2) | 3 (3.4) | 10 (7.9) | 1 (2.9) | 7 (7.0) | 5 (6.0) | 13 (6.0) |
| Chi‐square p value | <.001 | .40 | .94 | |||||
Abbreviations: CR, complete response; PD, progressive disease; PR, partial response; SD, stable disease.
After 72 death events (33.0% of patients), treatment with trabectedin plus PLD resulted in a median OS of 23.56 months (95% CI, 18.1–34.1), with 91.4%, 78.9%, and 49.5% of patients alive 6, 12, and 24 months after treatment, respectively. No statistically significant differences were observed in OS by the grade of platinum sensitivity or patients' BRCA status (Fig. 2).
Figure 2.
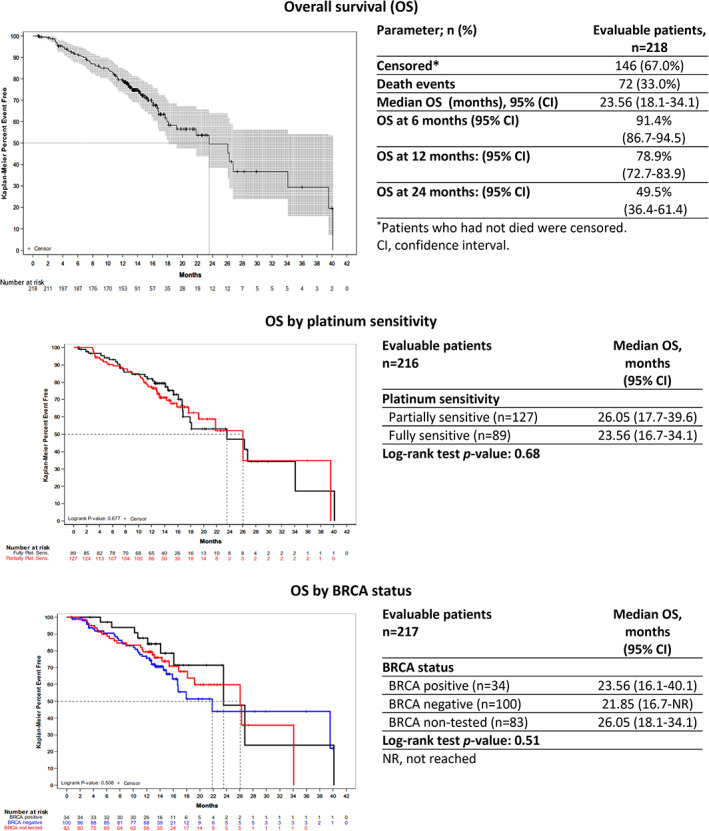
Kaplan‐Meier plots of overall survival. Patients who had not died were censored.Abbreviations: CI, confidence interval; NR, not reached; OS, overall survival.
Safety
Overall, 103 patients (47.2%) had at least one clinically significant laboratory test associated with an adverse reaction or serious adverse reaction. Most common (>2% overall) clinically significant laboratory tests were decreased absolute neutrophil count (n = 83, 38.1%), hemoglobin (n = 43, 19.7%), platelet count (n = 28; 12.8%), and alanine (n = 9; 4.1%) and aspartate aminotransferase (n = 5; 2.3%).
A total of 184 patients (84.4%) had at least one treatment‐related AE of any grade and 37 (17.0%) experienced SAEs, 10 patients (4.6%) and 11 patients (5.0%) had a AEs leading to trabectedin or PLD discontinuation, respectively. Grade 3/4 AEs were reported in 115 patients (52.7%), whereas two patients (0.9%) experienced grade 5 AEs assessed by the investigator as not related to the treatment. Most common grade 3/4 treatment‐related AEs (>5% of patients) were neutropenia (n = 66, 30.3%), anemia (n = 14, 6.4%), thrombocytopenia (n = 12, 5.5%), and asthenia (n = 11, 5.0%) (Table 4).
Table 4.
Treatment‐emergent adverse events of grade 3/4/5 by treatment group in at least ≥2% of patients (all treated patients) as reported by the investigators
| Grade 3/4/5 TEAEs as per NCI‐CTC; worst grade | Full analysis set (n = 218), n (%) |
|---|---|
| Patients with any grade 3/4/5 TEAE | |
| Any grade | 117 (53.7) |
| Grade 3 | 77 (35.3) |
| Grade 4 | 38 (17.4) |
| Grade 5 | 2 (0.9) |
| Hematologic | |
| Neutropenia | |
| Any grade | 66 (30.3) |
| Grade 3 | 37 (17.0) |
| Grade 4 | 29 (13.3) |
| Anemia | |
| Any grade | 14 (6.4) |
| Grade 3 | 14 (6.4) |
| Thrombocytopenia | |
| Any grade | 12 (5.5) |
| Grade 3 | 8 (3.7) |
| Grade 4 | 4 (1.8) |
| Leukopenia | |
| Any grade | 9 (4.1) |
| Grade 3 | 7 (3.2) |
| Grade 4 | 2 (0.9) |
| Febrile neutropenia | |
| Any grade | 8 (3.7) |
| Grade 3 | 5 (2.3) |
| Grade 4 | 3 (1.4) |
| Nonhematologic | |
| Vomiting | |
| Any grade | 9 (4.1) |
| Grade 3 | 8 (3.7) |
| Grade 4 | 0 |
| Grade 5 | 1 (0.5) |
| Asthenia | |
| Any grade | 11 (5.0) |
| Grade 3 | 11 (5.0) |
| Investigations | |
| Neutrophil count decreased | |
| Any grade | 10 (4.6) |
| Grade 3 | 7 (3.2) |
| Grade 4 | 3 (1.4) |
Abbreviations: NCI‐CTC, National Cancer Institute Common Terminology Criteria; TEAE, treatment‐emergent adverse event.
Over the whole study duration, 72 patients died (33.0%), mostly because of disease progression (n = 67; 30.7%). No deaths attributed to treatment‐related AEs or unexpected AEs occurred.
Analysis by Prior Use of Antiangiogenics
Out of 218 patients from the FAS, 129 (59.2%) were pretreated with an antiangiogenic drug, mostly bevacizumab (n = 125; 96.9%), whereas 89 (40.8%) did not receive prior antiangiogenic therapy. There were a number of statistically significant differences between both patient groups at baseline: among patients pretreated with an antiangiogenic drug, significantly more patients received three or more prior chemotherapy regimens (45.7% vs. 31.5%; p = .0361) and achieved worse response to the last prior chemotherapy regimen (i.e., with less CRs and more disease stabilizations and progressions; p = .0007), and significantly fewer patients were BRCA1/2 positive (18.1% vs. 37.3%, p = .0154) compared with non‐pretreated patients.
A statistically significant difference was observed by prior use of antiangiogenic drugs, with a significantly larger median time for PFS (12.45 vs. 7.59 months; p < .007) and OS (26.28 vs. 21.85 months; p < .048) in patients not pretreated with antiangiogenic drugs compared with pretreated patients. After a post hoc analysis of the impact of different patient prognostic variables (i.e., use of antiangiogenics, age, BRCA status, ECOG performance status, number of prior lines, and platinum sensitivity) on PFS and OS, only the nonantiangiogenic pretreatment showed a significantly positive effect on both PFS and OS (Fig. 3).
Figure 3.
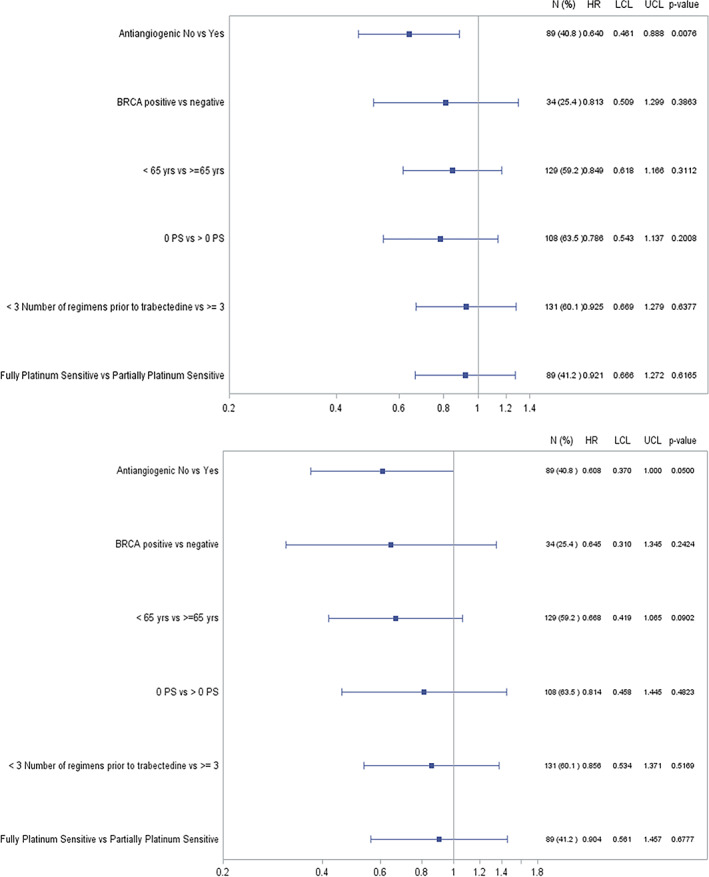
Forest plot of progression‐free survival and overall survival by prognostic variables (univariate Cox regression analyses).Abbreviations: HR, hazard ratio; LCL, lower confidence limit; OS, overall survival; PFS, progression‐free survival; UCL, upper confidence limit.
Moreover, better ORR (48.3% vs. 29.5%) and DCR (78.7% vs. 54.3%) were observed among non‐pretreated patients compared with antiangiogenic pretreated patients, who also experienced significantly less disease progressions (18.0% vs. 35.7%; p = .01). Concerning prior use of antiangiogenics, the safety profile between subgroups showed no differences as compared with that of the overall population.
Discussion
The NIMES‐ROC study is the first prospective observational study done in five European countries that evaluated the outcomes of trabectedin plus PLD in routine clinical practice in women with a platinum‐sensitive ROC. Despite the fact that the eligibility criteria of this study were less restrictive than those in prospective clinical trials and that our study population represented a heterogeneous and heavily pretreated population, the combination of trabectedin plus PLD induced responses consistent with the findings reported in the prior phase III clinical trials [11, 14]. Acknowledging that the results of NIMES‐ROC study cannot be considered representative of the whole group of ROC patients attended in Europe, they surely provide a good approximation to the European real‐life clinical practice, especially considering that we included data from 218 patients recruited in 57 sites in five European countries.
Patients characteristics at baseline in NIMES‐ROC study were in line with those observed in previous noninterventional studies [15, 16, 17], showing that the analyzed real‐life patient population was older (median age: 61 vs. 56 years) and more pretreated (72.5% of patients received at least two prior chemotherapy lines) than patients included in OVA‐301 study [11], where the inclusion criteria allowed only one previous line of treatment (Table 1). Despite the difference in patients' characteristics, the overall data observed in our study are consistent with those observed in the subgroup of platinum‐sensitive patients from the pivotal, randomized phase III OVA‐301 clinical trial [11] and are similar, or even, better than the those observed in previous real‐life studies [15, 16, 17]. The primary endpoint of the NIMES‐ROC resulted in a median time for PFS of 9.46 months. This is quite in line with PFS reported in OVA‐301 (i.e., 9.2 months). Similarly, the 23.6‐month median OS from this study is encouraging, as in OVA‐301 median OS was 27 months. As for PFS and OS, the ORR reported in this study compares with that reported in the subgroup of platinum‐sensitive patients from OVA‐301 (37.0% vs. 35.3%) [11]. Overall, those findings support that trabectedin in combination with PLD maintains antitumor activity when administered to heavily pretreated patients in real‐life clinical practice. The efficacy outcomes from NIMES‐ROC are particularly promising considering that it is more common to see a decline in efficiency when a regimen is applied in more advanced lines to the general population outside of a clinical trial. Our results are also in line with the recent findings from a randomized, phase III InovatYon study that compared trabectedin plus PLD followed by platinum at progression versus standard of care regimen with carboplatin plus PLD in patients with ovarian cancer progressing within 6–12 months to one or two prior platinum‐based therapies [20]. Although the study did not meet its primary endpoint of improving survival with the trabectedin combination, it reported comparable median OS between arms (trabectedin/PLD: 21.5 months vs. carboplatin/PLD: 21.3 months; HR, 1.10; 95% CI, 0.92–1.32; p = .284). There was a positive OS trend with the administration of trabectedin plus PLD to patients pretreated with two platinum‐based lines (HR, 0.87; 95% CI, 0.63–1.22; p = .426). Those results indicate a possible role for trabectedin plus PLD in patients pretreated with several prior platinum‐based chemotherapy regimens, who may need a longer recovery time from platinum‐related toxicities. Moreover, whereas median PFS was longer with carboplatin plus PLD (9.0 vs. 7.5 months; HR, 1.26; 95% CI, 1.07–1.49; p = .005) and similar in patients who had received two previous lines (HR, 1.03; 95% CI, 0.76–1.39; p = .863), median PFS after the subsequent line was in favor of trabectedin plus PLD, particularly when platinum was administered (7.6 vs. 5.7 months; HR, 0.80; 95% CI, 0.65–0.98; p = .028), suggesting that a resensitization to platinum may occur after trabectedin administration.
The median number of cycles of trabectedin plus PLD received per patient in NIMES‐ROC was the same as that reported in the pivotal OVA‐301 trial (median six cycles in OVA‐301) [11] and in real‐life prospective OVA‐YOND observational study conducted in patients with platinum‐sensitive ROC in Germany [17]. Of note, nearly 60% of patients received six or more cycles, suggesting an acceptable safety profile that allowed prolonged treatment, up to 24 cycles in NIMES‐ROC and 21 cycles in OVA‐YOND. In NIMES‐ROC, the safety profile for trabectedin plus PLD treatment was consistent with extensive prior experience and reports, with no new safety signals reported. Furthermore, patients who received six or more cycles of treatment obtained significantly better response (p < .001) than those who received fewer than six cycles, additionally supporting the use of trabectedin plus PLD until disease progression.
Some of the more important factors to predict the clinical outcomes in patients with ROC are closely related with the time to recurrence from previous chemotherapy, BRCA status, and residual toxicity or hypersensitivity reactions to prior chemotherapy lines. There is a growing body of evidence that patients with a BRCA1/2 mutation or PFS 6–12 months may have an enhanced response to trabectedin plus PLD compared with PLD alone [13, 21]. In contrast, NIMES‐ROC study did not demonstrate a statistically significant difference in PFS and OS during treatment with trabectedin plus PLD neither by BRCA1/2 status nor platinum sensitivity. Those outcomes are pretty in line with the data observed in another real‐life national survey of trabectedin plus PLD, which also did not observe significant differences in responses according to platinum sensitivity and BRCA status [16]. In our study, the nonsignificant outcomes by BRCA1/2 status could be explained, at least partially, by the high percentage of patients not tested for the BRCA mutation status (i.e., 38.1%). Even though it is recommended to test every new patient with ovarian cancer for genetic mutations, particularly BRCA1 and BRCA2, the testing rates in the real‐life treatment of ovarian cancer remains suboptimal [15, 16, 22, 23]. Recent results of a large retrospective analysis of real‐life clinical data from 1,921 patients with ovarian cancer showed a trend for increased rates of germline or somatic BRCA testing, with 25% of patients tested in 2011 to 69% in 2018, largely because of the targeted treatment opportunities with PARP inhibitors [22]. Thus, in the real‐life setting the universal testing of all patients with ovarian cancer remains the goal.
Trabectedin plus PLD was approved to be used in the treatment of patients with platinum‐sensitive ROC in 2009. At time when this study was designed, the antiangiogenics represented the new family of drugs approved for the treatment of ovarian cancer. Therefore, our understanding how prior use of antiangiogenics may influence the response to later treatment with trabectedin plus PLD was still very limited. Based on the results of our study, trabectedin plus PLD is effective in both pretreated and non‐pretreated with an antiangiogenic drug, although the benefit seems to be larger in non‐pretreated patients. Nevertheless, these results should be interpreted with caution, as enrolled patients pretreated with antiangiogenic drugs had a more severe disease at baseline, suggestive of worse prognosis as compared with non‐pretreated patients. Therefore, these results in patients pretreated versus not pretreated with antiangiogenic drugs could be linked to the differences observed in baseline parameters between the two patient populations. This it is not surprising considering that first approval for antiangiogenic drugs was in combination for the front‐line treatment of advanced (International Federation of Gynecology and Obstetrics stages IIIB, IIIC, and IV) ovarian cancer.
According with the noninterventional setting of this study, missing or unavailable data can be a limiting factor for the interpretation of the results. Moreover, the exact time points and method of response assessment were not previously fixed but were done according to the clinician's discretion with no central radiological review and response confirmation. Despite these limitations, this real‐life study complements the findings from the clinical trials with trabectedin plus PLD by providing valuable information on patient characteristics among unselected patients and treatment practices, necessary to guide treatment decisions.
Conclusion
The results of the noninterventional pan‐European NIMES‐ROC study consistently support that trabectedin plus PLD is active in pretreated patients with platinum‐sensitive ROC with an acceptable and manageable safety profile. Despite this real‐life patient population was older and more pretreated than patients included in in the pivotal, phase III OVA‐301 clinical trial, the overall data observed in our study are consistent with those observed in the subgroup of platinum‐sensitive patients and further support the use of trabectedin plus PLD for heavily pretreated patients with platinum‐sensitive ROC.
Author Contributions
Conception/design: Sandro Pignata, Giovanni Scambia, Luis Miguel de Sande
Provision of study material or patients: Sandro Pignata, Giovanni Scambia, Alessandro Villanucci, Emanuele Naglieri, Mikel Arruti Ibarbia, Federica Brusa, Hugues Bourgeois, Roberto Sorio, Antonio Casado, Dietmar Reichert, Catherine Dopchie, Luis Miguel de Sande
Collection and/or assembly of data: Sandro Pignata, Giovanni Scambia, Antonio Casado, Beatriz De Rivas
Data analysis and interpretation: Sandro Pignata, Antonio Casado, Beatriz De Rivas
Manuscript writing: Sandro Pignata, Antonio Casado, Beatriz De Rivas
Final approval of manuscript: Sandro Pignata, Giovanni Scambia, Alessandro Villanucci, Emanuele Naglieri, Mikel Arruti Ibarbia, Federica Brusa, Hugues Bourgeois, Roberto Sorio, Antonio Casado, Dietmar Reichert, Catherine Dopchie, Beatriz De Rivas, Luis Miguel de Sande
Disclosures
Sandro Pignata: Pharmamar, AstraZeneca, Merck Sharp & Dohme, Roche, Pfizer, Clovis, GlaxoSmithKline (H). The other authors indicated no financial relationships.
(C/A) Consulting/advisory relationship; (RF) Research funding; (E) Employment; (ET) Expert testimony; (H) Honoraria received; (OI) Ownership interests; (IP) Intellectual property rights/inventor/patent holder; (SAB) Scientific advisory board
Acknowledgments
This study was sponsored by PharmaMar, S.A., Madrid, Spain. The authors acknowledge Adnan Tanović for providing writing and editorial assistance for the manuscript (funded by Pharmamar, S.A.).
Disclosures of potential conflicts of interest may be found at the end of this article.
References
- 1. Torre LA, Bray F, Siegel RL et al. Global cancer statistics, 2012. CA Cancer J Clin 2015;65:87–108. [DOI] [PubMed] [Google Scholar]
- 2. Ledermann JA, Raja FA, Fotopoulou C et al. Newly diagnosed and relapsed epithelial ovarian carcinoma: ESMO clinical practice guidelines for diagnosis, treatment and follow‐up. Ann Oncol 2013;24(suppl 6):vi24–vi32. [DOI] [PubMed] [Google Scholar]
- 3. Lopez J, Banerjee S, Kaye SB. New developments in the treatment of ovarian cancer‐future perspectives. Ann Oncol 2013;24(suppl 10):x69–x76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Harter P, Hilpert F, Mahner S et al. Systemic therapy in recurrent ovarian cancer: Current treatment options and new drugs. Expert Rev Anticancer Ther 2010;10:81–88. [DOI] [PubMed] [Google Scholar]
- 5. Cannistra SA. Evaluating new regimens in recurrent ovarian cancer: How much evidence is good enough? J Clin Oncol 2010;28:3101–3103. [DOI] [PubMed] [Google Scholar]
- 6. Corrado G, Salutari V, Palluzzi E et al. Optimizing treatment in recurrent epithelial ovarian cancer. Expert Rev Anticancer Ther 2017;17:1147–1158. [DOI] [PubMed] [Google Scholar]
- 7. Pignata S, Pisano C, Di Napoli M et al. Treatment of recurrent epithelial ovarian cancer. Cancer 2019;125(suppl 24):4609–4615. [DOI] [PubMed] [Google Scholar]
- 8. Larsen AK, Galmarini CM, D'Incalci M. Unique features of trabectedin mechanism of action. Cancer Chemother Pharmacol 2016;77:663–671. [DOI] [PubMed] [Google Scholar]
- 9. D'Incalci M, Galmarini CM. A review of trabectedin (ET‐743): A unique mechanism of action. Mol Cancer Ther 2010;9:2157–2163. [DOI] [PubMed] [Google Scholar]
- 10. Allavena P, Germano G, Belgiovine C et al. Trabectedin: A drug from the sea that strikes tumor‐associated macrophages. Oncoimmunology 2013;2:e24614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Monk BJ, Herzog TJ, Kaye SB et al. Trabectedin plus pegylated liposomal doxorubicin in recurrent ovarian cancer. J Clin Oncol 2010;28:3107–3114. [DOI] [PubMed] [Google Scholar]
- 12. Monk BJ, Herzog TJ, Kaye SB et al. Trabectedin plus pegylated liposomal doxorubicin (PLD) versus PLD in recurrent ovarian cancer: Overall survival analysis. Eur J Cancer 2012;48:2361–2368. [DOI] [PubMed] [Google Scholar]
- 13. Monk BJ, Ghatage P, Parekh T et al. Effect of BRCA1 and XPG mutations on treatment response to trabectedin and pegylated liposomal doxorubicin in patients with advanced ovarian cancer: Exploratory analysis of the phase 3 OVA‐301 study. Ann Oncol 2015;26:914–920. [DOI] [PubMed] [Google Scholar]
- 14. Monk BJ, Herzog TJ, Wang G et al. A phase 3 randomized, open‐label, multicenter trial for safety and efficacy of combined trabectedin and pegylated liposomal doxorubicin therapy for recurrent ovarian cancer. Gynecol Oncol 2020;156:535–544. [DOI] [PubMed] [Google Scholar]
- 15. Nicoletto MO, Baldoni A, Casarin A et al. Trabectedin plus pegylated liposomal doxorubicin: Retrospective analysis in heavily pretreated platinum‐sensitive ovarian cancer. Tumori 2015;101:506–510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Romero I, Mallol P, Santaballa A et al. Multicenter retrospective study to evaluate the impact of trabectedin plus pegylated liposomal doxorubicin on the subsequent treatment in women with recurrent, platinum‐sensitive ovarian cancer. Anticancer Drugs 2019;30:628–635. [DOI] [PubMed] [Google Scholar]
- 17. Runnebaum IB, Reichert D, Ringsdorf U et al. Trabectedin plus pegylated liposomal doxorubicin (PLD) for patients with platinum‐sensitive recurrent ovarian cancer: A prospective, observational, multicenter study. J Cancer Res Clin Oncol 2018;144:1185–1195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Eisenhauer EA, Therasse P, Bogaerts J et al. New response evaluation criteria in solid tumours: Revised RECIST guideline (version 1.1). Eur J Cancer 2009;45:228–247. [DOI] [PubMed] [Google Scholar]
- 19. Therasse P, Arbuck SG, Eisenhauer EA et al. New guidelines to evaluate the response to treatment in solid tumors. European Organization for Research and Treatment of Cancer, National Cancer Institute of the United States, National Cancer Institute of Canada. J Natl Cancer Inst 2000;92:205–216. [DOI] [PubMed] [Google Scholar]
- 20. Colombo N, Gadducci A, Sehouli J et al. INOVATYON study: Randomized phase III international study comparing trabectedin/PLD followed by platinum at progression vs carboplatin/PLD in patients with recurrent ovarian cancer progressing within 6‐12 months after last platinum line. Ann Oncol 2020;31(suppl 4):LBA30a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Monk BJ, Lorusso D, Italiano A et al. Trabectedin as a chemotherapy option for patients with BRCA deficiency. Cancer Treat Rev 2016;50:175–182. [DOI] [PubMed] [Google Scholar]
- 22. Meyera L, Wrightb JD, Downerc MK et al. Patterns and adoption of BRCA testing in ovarian cancer in the real world: Observations from flatiron health. Presented at Society of Gynecologic Oncology (SGO) 2020 Annual Meeting on Women's Cancer; March 29, 2020; Abstract 113.
- 23. Dewdney S, Potter D, Haidle JL et al. Low rates of BRCA1 and BRCA2 testing for patients with ovarian cancer in ASCO's CancerLinQ, a real‐world database. J Clin Oncol 2020;38(suppl 15):6041a. [Google Scholar]


