Abstract
Lessons Learned
The overall safety profiles of ipilimumab 3 mg/kg and 10 mg/kg administered every 3 weeks, were consistent between Chinese patients with solid tumors in the current study and patients from previous U.S. ipilimumab monotherapy studies. No new safety signals were identified.
The mean systemic exposures to ipilimumab (assessed by first dose area under the curve during the dosing interval and maximum serum concentration) were numerically lower in the Chinese patient population than in U.S. patients for both 3 mg/kg and 10 mg/kg doses; however, the range of serum concentrations in the Chinese and U.S. populations overlapped (3 mg/kg and 10 mg/kg), suggesting that ipilimumab pharmacokinetics was ethnically insensitive in this study.
Background
This phase I, open‐label study assessed ipilimumab safety, tolerability, pharmacokinetics (PK), immunogenicity, and antitumor activity in Chinese patients with unresectable, metastatic, recurrent malignant melanoma (MM) or nasopharyngeal carcinoma (NPC).
Methods
Of 39 patients enrolled, 25 received ipilimumab (11 patients received 3 mg/kg, and 14 patients received 10 mg/kg). Reasons for not receiving treatment were withdrawal of consent (3 patients), no longer meeting the criteria (10 patients), and one recorded as “other.” During the induction phase, patients received ipilimumab (3 mg/kg, i.v.), on day 1 of a 3‐week cycle, to a maximum of four doses or progressive disease (PD). During the maintenance phase at week 24, patients received ipilimumab (3 mg/kg, i.v.) on day 1 of a 12‐week cycle, to a maximum of 3 years or PD. Considering the co‐primary safety and PK endpoints, the successive dosing required nine patients with two or fewer dose‐limiting toxicities during the 42‐day observation period to proceed with a new cohort of nine patients at 10 mg/kg.
Results
Ipilimumab safety and PK profiles were similar in Chinese and predominantly White populations. Ipilimumab was well tolerated. Most adverse events (AEs) were grades 1–2 and experienced by 11 patients treated with 3 mg/kg and 14 patients treated with 10 mg/kg. There were no new safety concerns. Incidence of anti‐ipilimumab antibodies was low (1 of 10 in the 3 mg/kg patients and 2 of 13 in the 10 mg/kg patients) and without safety implications. In the 3 mg/kg group, 8 of 11 patients had PD. In the 10 mg/kg group (all NPC, 0 MM patients), 11 of 14 patients had PD. Three patients had stable disease (one at 3 mg/kg and two at 10 mg/kg).
Conclusion
Ipilimumab was well tolerated in Chinese patients, showing similar safety and PK to previous studies in predominantly White populations.
Keywords: Ipilimumab, Melanoma, Nasopharyngeal carcinoma
Discussion
Ipilimumab is a human monoclonal antibody that binds CTLA‐4, a T‐cell negative regulator, preventing ligand interaction on antigen‐presenting cells [1]. It is approved treatment of advanced melanoma in >45 countries (3 mg/kg) and in the U.S. for melanoma adjuvant therapy (10 mg/kg) and unresectable or metastatic melanoma (3 mg/kg) [2]. Prognosis with metastatic/refractory solid tumors is poor, with an unmet medical need [3, 4]. Evidence for the safety, tolerability, and PK of ipilimumab is based on predominantly White population study data [5].
This phase I, open‐label study assessed the safety, tolerability, and PK of ipilimumab in Chinese patients with unresectable, metastatic, recurrent MM or NPC. Secondary objectives were immunogenicity and preliminary antitumor activity.
There were three study phases: screening (up to 28 days), treatment (induction and maintenance), and clinical follow‐up (90 days). During the induction phase, subjects received ipilimumab (i.v.) on day 1 of each cycle, every 3 weeks, up to four doses or until PD. During the maintenance phase, subjects received ipilimumab (i.v.) on day 1 of each cycle, every 12 weeks, starting at week 24 up to PD, for a maximum of 3 years from first dose.
Nine subjects were initially treated (3 mg/kg); no more than two subjects experienced dose‐limiting toxicities (DLTs) during the 42‐day observation period, so a new cohort of nine patients was treated at 10 mg/kg (this would have been increased to 18 patients if DLTs were experienced by three or more patients at 3 mg/kg). After determining the maximum tolerated/administered dose, additional patients were enrolled to have at least 10 per group with PK samples. Primary endpoints were AEs, serious AEs, AEs leading to discontinuation/death, and pharmacokinetic parameters of ipilimumab, including maximum serum concentration (Cmax), time to achieve Cmax (Tmax), trough concentration (Ctrough), and area under the curve from 0 to 21 days (AUC0–21 days). Secondary endpoints were best overall response, duration of response, progression‐free survival, and immunogenicity. Descriptive statistics were used to summarize continuous variables, and percentages/frequencies were used for categorical variables.
Safety profiles and cross‐dose comparisons were similar in Chinese patients (n = 25) and prior U.S. studies with ipilimumab (>94% White). There were no new safety signals, drug‐related deaths, or drug‐related serious adverse events (SAEs) during this study (Table 1: All 25 patients had AEs: 11 patients 3 mg/kg; 14 patients 10 mg/kg ipilimumab). Most AEs affected the skin, gastrointestinal tract, endocrine system, or liver and were grades 1–2. Eleven patients had adverse drug reactions (ADRs) in each group; Only one ADR led to discontinuation (3 mg/kg: hypothyroidism, NPC). Ipilimumab was well tolerated (one dose‐limiting toxicity: erythema; 10 mg/kg, NPC).
First dose area under the curve during the dosing interval and Cmax increased dose‐proportionally (Fig. 1A). Mean ipilimumab exposures for both doses were lower in Chinese versus U.S. patients. Serum concentrations in these populations overlapped, with both doses, indicating that ipilimumab PK was ethnically insensitive in this study (Fig. 1B and C).
Figure 1.
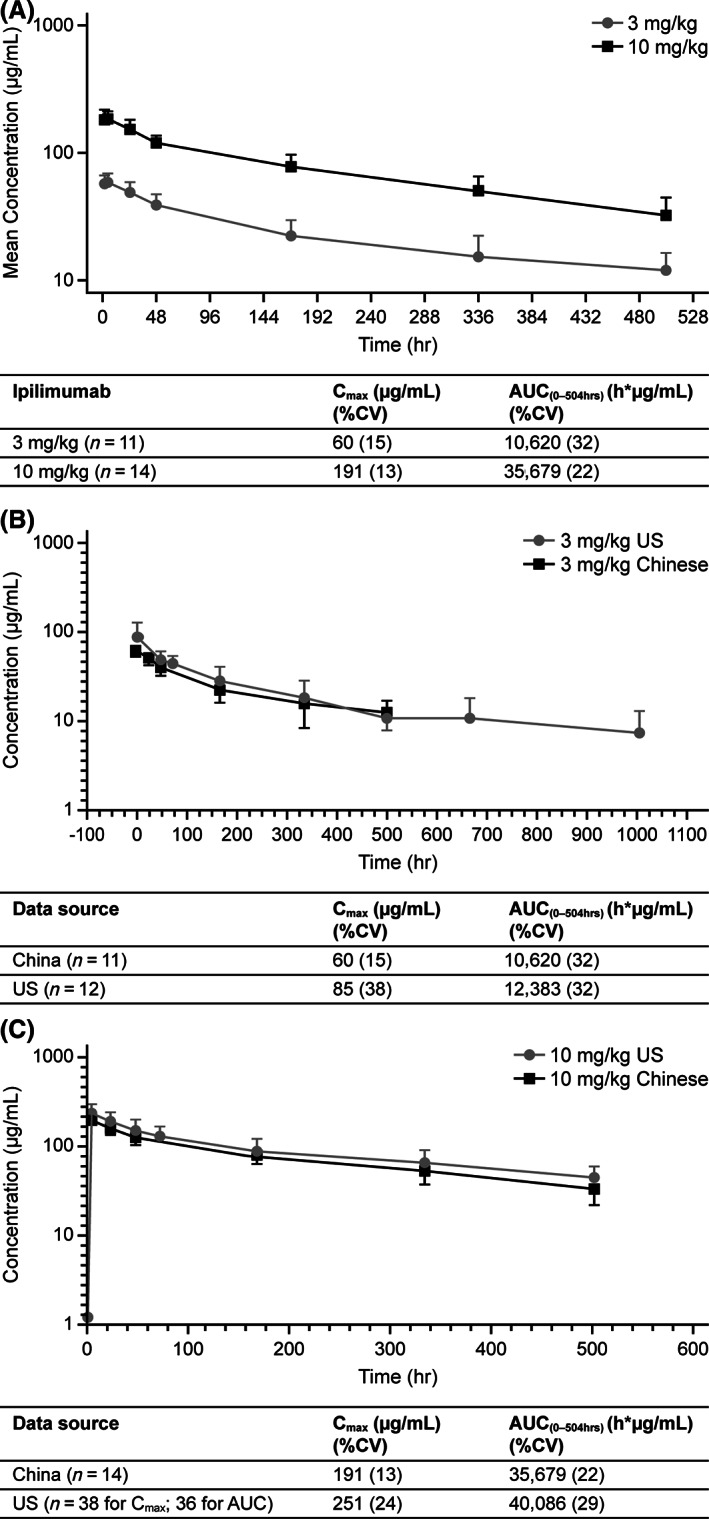
Comparison of ipilimumab concentration‐time profiles and pharmacokinetic parameters in Chinese and U.S. patients at 3 and 10 mg/kg. (A): Geometric mean ipilimumab serum concentration‐time profile and pharmacokinetic (PK) parameters: 3 mg/kg and 10 mg/kg. (B): Comparison of data obtained in Chinese and U.S. study participants: Mean ipilimumab serum concentration‐time profile and PK parameters after single dose (3 mg/kg). (C): Comparison of data obtained in Chinese and U.S. study participants: Mean ipilimumab serum concentration‐time profile and PK parameters after single dose (10mg/kg).
Abbreviations: AUC, area under the curve; Cmax, maximum concentration; CV, coefficient of variation.
Incidence of anti‐ipilimumab antibodies did not affect safety and was low (1/10 patients in the 3 mg/kg group; 2/13 patients in the 10 mg/kg group). In the 3 mg/kg group, five of six patients with MM had PD and one of six was not evaluable (NE), and three of five patients with NPC had PD, one of five was NE, and one of five had stable disease (SD). In the 10 mg/kg group (all with NPC, 0 MM), 11 of 14 patients had PD, 2 of 14 had SD, and 1 of 14 was NE.
Previous studies, conducted predominantly in White patients, have indicated that ipilimumab is safe and well tolerated. This study confirmed these findings in Chinese patients with MM/NPC and demonstrated similar PK between the study populations.
Trial Information
| Disease | Advanced cancer/solid tumors |
| Stage of Disease/Treatment | Metastatic/advanced or recurrent disease |
| Prior Therapy | No designated number of regimens |
| Type of Study | Phase I, dose evaluation and cohort expansion |
| Primary Endpoints | Safety, tolerability and pharmacokinetics |
| Secondary Endpoints | Immunogenicity and preliminary antitumor activity |
| Additional Details of Endpoints or Study Design | |
| Dose evaluation and cohort expansion phases: Based on clinical experience in a predominantly White population, ipilimumab safety, tolerability, and PK were assessed in Chinese patients with advanced/recurrent solid tumors. Patients started an induction phase of 3 mg/kg ipilimumab (i.v., day 1 of a 3‐week cycle, repeated up to four times or until progressive disease). Maintenance phase started at week 24 (i.v., day 1 of a 12‐week cycle), continuing up to 3 years from first dose, dependent on disease. The successive ascending dose (10 mg/kg) was administered by a 9 + 9 design. | |
| Investigator's Analysis | |
| Drug tolerable; efficacy was not fully evaluable due to limited patient numbers, warranting exploration in a larger study of patients with malignant melanoma and nasopharyngeal carcinoma. | |
Drug Information
| Generic/Working Name | Ipilimumab |
| Trade Name | Yervoy® |
| Company Name | Bristol‐Myers Squibb Pharmaceuticals Ltd. |
| Drug Type | Antibody |
| Drug Class | Immune therapy |
| Dose | Induction: 3 mg/kg every 3 weeks; maintenance: 3 mg/kg every 12 weeks, 10 mg/kg every 12 weeks milligrams (mg) per kilogram (kg) |
| Route | i.v. |
| Schedule of Administration | |
| Induction phase of 3 mg/kg ipilimumab (i.v., day 1 of a 3‐week cycle, repeated up to four times or until progressive disease). Maintenance phase started at week 24 (i.v., day 1 of a 12‐week cycle), continuing to 3 years from first dose, dependent on disease. The successive ascending dose (10 mg/kg) was administered by a 9 + 9 design. | |
Dose Escalation Table
| Dose level | Dose of drug | Number enrolled | Number evaluable for toxicity |
|---|---|---|---|
| Malignant melanoma | 3 mg/kg | 6 | 6 |
| Nasopharyngeal carcinoma | 3 mg/kg | 5 | 5 |
| Nasopharyngeal carcinoma | 10 mg/kg | 14 | 14 |
Dose Information – All Treated Patients
| Ipilimumab 3 mg/kg | Ipilimumab 10 mg/kg | |
|---|---|---|
| N | 11 | 14 |
| Malignant melanoma (n) | 6 | 0 |
| Number of doses | 4.0 (3–4) | 0 |
| Duration of therapy (weeks) | 12.1 (8.3–12.7) | 0 |
| Cumulative dose per patient (mg/kg) | 11.9 (9.0–12.3) | 0 |
| Nasopharyngeal carcinoma (n) | 5 | 14 |
| Number of doses | 4.0 (1–5) | 3.5 (1–6) |
| Duration of therapy (weeks) | 12.0 (3.0–32.1) | 10.5 (3.0–38.0) |
| Cumulative dose per patient (mg/kg) | 11.7 (3.0–15.3) | 35.1 (10.0–58.8) |
Data represent median (range).
Patient Characteristics: All Treated Patients
| Number of Patients, Male | 18 (72%) |
| Number of Patients, Female | 7 (28%) |
| Stage | Advanced (stage 3 or 4 at study entry) |
| Age | Median (range): 48 (23–68) years |
| Number of Prior Systemic Therapies | |
| Patients with malignant melanoma: One regimen: two patients (33.3%). Two regimens: one patient (16.7%). Three regimens: one patient (16.7%). Four or more regimens: two patients (33.3%). Patients with nasopharyngeal carcinoma: One regimen: two patients (10.5%). Two regimens: two patients (10.5%). Three regimens: five patients (26.3%). Four or more regimens: 10 patients (52.6%). | |
| Performance Status: ECOG |
0 : 11 patients 1 : 14 patients |
| Other | Race: Asian, 100%. Complete baseline demographic and disease characteristics are presented in Table 2. |
| Cancer Types or Histologic Subtypes | Malignant melanoma, 6 (24% of patients). Nasopharyngeal carcinoma, 19 (76% of patients). |
Table 2.
Baseline patient characteristics and prior cancer therapy
| Characteristics | Ipilimumab 3 mg/kg (n = 11), n (%) | Ipilimumab 10 mg/kg (n = 14), n (%) | Total (n = 25), n (%) |
|---|---|---|---|
| Age, median (range) | 44 (23, 68) | 48 (23, 57) | 48 (23, 68) |
| Male | 8 (73) | 10 (71) | 18 (72) |
| Race: Chinese | 11 (100) | 14 (100) | 25 (100) |
| Weight, kg, median (range) | 64.3 (47.9, 88.1) | 58.2 (44.1, 78.4) | 63.2 (44.1, 78.4) |
| ECOG status | |||
| 0 | 6 (55) | 5 (36) | 11 (44) |
| 1 | 5 (45) | 9 (64) | 14 (56) |
| Tumor type | |||
| MM | 6 (55) | 0 | 6 (24) |
| Stage 3 disease status at study entry | 1 (17) | 0 | 1 (17) |
| Stage 4 disease status at study entry | 5 (83) | 0 | 5 (83) |
| NPC | 5 (45) | 14 (100) | 19 (76) |
| Stage 3 disease status at study entry | 0 | 1 (7) | 1 (5) |
| Stage 4 disease status at study entry | 5 (100) | 13 (93) | 18 (95) |
| Prior surgery | |||
| MM | 6 (55) | 0 | 6 (55) |
| Yes | 6 (100) | 0 | 6 (100) |
| No | 0 | 0 | 0 |
| NPC | 5 (45) | 14 (100) | 19 (76) |
| Yes | 4 (80) | 13 (93) | 17 (90) |
| No | 1 (20) | 1 (7) | 2 (10) |
| Prior radiotherapy | |||
| MM | 6 (55) | 0 | 6 (24) |
| Yes | 3 (50) | 0 | 3 (50) |
| No | 3 (50) | 0 | 3 (50) |
| NPC | 5 (45) | 14 (100) | 19 (76) |
| Yes | 4 (80) | 12 (86) | 16 (84) |
| No | 1 (20) | 2 (14) | 3 (16) |
| Prior systemic therapy, number of regimens | |||
| MM | 6 (55) | 0 | 6 (55) |
| 1 | 2 (33) | 0 | 2 (33) |
| 2 | 1 (17) | 0 | 1 (17) |
| 3 | 1 (17) | 0 | 1 (17) |
| ≥4 | 2 (33) | 0 | 2 (33) |
| NPC | 5 (45) | 14 (100) | 19 (76) |
| 1 | 1 (20) | 1 (7) | 2 (11) |
| 2 | 1 (20) | 1 (7) | 2 (11) |
| 3 | 0 | 5 (36) | 5 (26) |
| ≥4 | 3 (60) | 7 (50) | 10 (53) |
Abbreviations: ECOG, Eastern Cooperative Oncology Group; MM, malignant melanoma; NPC, nasopharyngeal carcinoma.
Primary Assessment Method
| Title | Total Patient Population |
| Number of Patients Screened | 39 |
| Number of Patients Enrolled | 39 |
| Number of Patients Evaluable for Toxicity | 25 |
| Number of Patients Evaluated for Efficacy | 25 |
| Evaluation Method | RECIST version 1.1 |
| Response Assessment CR | n = 0 (0%) |
| Response Assessment PR | n = 0 (0%) |
| Response Assessment SD | n = 3 (12%) |
| Response Assessment PD | n = 19 (76%) |
| Response Assessment OTHER | n = 3 (12%) |
| Outcome Notes | |
| Objective response rate: n = 0 (0%). Disease control rate: n = 3 (12%). Enrolled and screened with signed informed consent within 28 days of first dose. Of the enrolled patients, 14 did not enter the treatment period: 10 no longer met the criteria, 3 withdrew informed consent, and 1 withdrew for other reasons; Other: Responses could not be determined for these patients. | |
Adverse Events of Special Interest, All Cycles
| Name | NC/NA, % | Grade 1, % | Grade 2, % | Grade 3, % | Grade 4, % | Grade 5, % | All grades, % |
|---|---|---|---|---|---|---|---|
| Pruritus | 55 | 27 | 18 | 0 | 0 | 0 | 45 |
| Rash | 55 | 27 | 18 | 0 | 0 | 0 | 45 |
| Aspartate aminotransferase increased | 64 | 27 | 9 | 0 | 0 | 0 | 36 |
| Nausea | 73 | 27 | 0 | 0 | 0 | 0 | 27 |
| Pyrexia | 82 | 18 | 0 | 0 | 0 | 0 | 18 |
| Vomiting | 82 | 18 | 0 | 0 | 0 | 0 | 18 |
| Dizziness | 91 | 9 | 0 | 0 | 0 | 0 | 9 |
| Emotional disorder | 91 | 0 | 9 | 0 | 0 | 0 | 9 |
| Fatigue | 91 | 9 | 0 | 0 | 0 | 0 | 9 |
| Flushing | 91 | 9 | 0 | 0 | 0 | 0 | 9 |
| Gastrointestinal disorder | 91 | 9 | 0 | 0 | 0 | 0 | 9 |
| Hyperthyroidism | 91 | 9 | 0 | 0 | 0 | 0 | 9 |
| Hypothyroidism | 91 | 0 | 9 | 0 | 0 | 0 | 9 |
| Pruritus generalized | 91 | 9 | 0 | 0 | 0 | 0 | 9 |
| Skin exfoliation | 91 | 0 | 9 | 0 | 0 | 0 | 9 |
| Somnolence | 91 | 9 | 0 | 0 | 0 | 0 | 9 |
| Thyroid disorder | 91 | 9 | 0 | 0 | 0 | 0 | 9 |
| Chest discomfort | 91 | 9 | 0 | 0 | 0 | 0 | 9 |
| Alanine aminotransferase increased | 91 | 0 | 9 | 0 | 0 | 0 | 9 |
| Pigmentation disorder | 91 | 0 | 9 | 0 | 0 | 0 | 9 |
| Asthenia | 82 | 18 | 0 | 0 | 0 | 0 | 18 |
| Pain | 82 | 9 | 9 | 0 | 0 | 0 | 18 |
| Mouth ulceration | 91 | 9 | 0 | 0 | 0 | 0 | 9 |
| Dreamy state | 91 | 9 | 0 | 0 | 0 | 0 | 9 |
| Adverse Events Legend | |
| Data represent number (%) of patients with all related adverse events by worst grade, occurring between the first dose and 90 days after the last dose, of study therapy. Adverse events were graded by the National Cancer Institute Common Terminology Criteria for Adverse Events version 3.0. | |
| Abbreviation: NC/NA, no change from baseline/no adverse event. |
All Cause Serious Adverse Events (3mg/kg group)
| Name | Grade | Attribution |
|---|---|---|
| Malignant neoplasm progression | 5 | Unrelated |
| Malignant neoplasm progression | 5 | Unrelated |
| Malignant neoplasm progression | 5 | Unrelated |
| Malignant neoplasm progression | 5 | Unrelated |
| Chest pain | 2 | Unrelated |
| Hyponatremia | 4 | Unrelated |
| Serious Adverse Events Legend | |
| Data represent number (%) of patients with all cause serious adverse events by worst grade, occurring between the first dose and 90 days after the last dose of study therapy. Adverse events were graded by the National Cancer Institute Common Terminology Criteria for Adverse Events version 3.0. |
All Related Adverse Events, All Cycles (10 mg/kg group)
| Name | NC/NA, % | Grade 1, % | Grade 2, % | Grade 3, % | Grade 4, % | Grade 5, % | All grades, % |
|---|---|---|---|---|---|---|---|
| Pruritus | 93 | 7 | 0 | 0 | 0 | 0 | 7 |
| Fatigue | 57 | 29 | 14 | 0 | 0 | 0 | 43 |
| Hypothyroidism | 72 | 21 | 7 | 0 | 0 | 0 | 28 |
| Hyperthyroidism | 93 | 7 | 0 | 0 | 0 | 0 | 7 |
| Hyponatremia | 93 | 7 | 0 | 0 | 0 | 0 | 7 |
| Productive cough | 93 | 7 | 0 | 0 | 0 | 0 | 7 |
| Pharyngitis | 93 | 7 | 0 | 0 | 0 | 0 | 7 |
| Vomiting | 93 | 7 | 0 | 0 | 0 | 0 | 7 |
| Pyrexia | 93 | 0 | 7 | 0 | 0 | 0 | 7 |
| Granulocytosis | 93 | 7 | 0 | 0 | 0 | 0 | 7 |
| Blister | 93 | 7 | 0 | 0 | 0 | 0 | 7 |
| Erythema | 93 | 0 | 0 | 7 | 0 | 0 | 7 |
| Leukocytosis | 93 | 7 | 0 | 0 | 0 | 0 | 7 |
| Rash | 93 | 0 | 7 | 0 | 0 | 0 | 7 |
| Adverse Events Legend | |
| Data represent number (%) of patients with all related adverse events by worst grade, occurring between the first dose and 90 days after the last dose, of study therapy. Adverse events were graded by the National Cancer Institute Common Terminology Criteria for Adverse Events version 3.0. | |
| Abbreviation: NC/NA, no change from baseline/no adverse event. |
All Cause Adverse Events (3 mg/kg)
| All phases, All cycles | Grade | Total | |||||
|---|---|---|---|---|---|---|---|
| NC/NA, % | 1 | 2 | 3 | 4 | 5 | ||
| Ipilimumab 3 mg/kg (n = 11), n (%) | |||||||
| Pruritus | 55 | 3 (27.3) | 2 (18.2) | 0 | 0 | 0 | 5 (45.5) |
| Rash | 55 | 3 (27.3) | 2 (18.2) | 0 | 0 | 0 | 5 (45.5) |
| Aspartate aminotransferase increased | 64 | 3 (27.3) | 1 (9.1) | 0 | 0 | 0 | 4 (36.4) |
| Constipation | 64 | 1 (9.1) | 3 (27.3) | 0 | 0 | 0 | 4 (36.4) |
| Cough | 64 | 3 (27.3) | 1 (9.1) | 0 | 0 | 0 | 4 (36.4) |
| Malignant neoplasm progression | 64 | 0 | 0 | 0 | 0 | 4 (36.4) | 4 (36.4) |
| Weight decreased | 64 | 2 (18.2) | 2 (18.2) | 0 | 0 | 0 | 4 (36.4) |
| Anemia | 73 | 0 | 3 (27.3) | 0 | 0 | 0 | 3 (27.3) |
| Decreased appetite | 73 | 2 (18.2) | 1 (9.1) | 0 | 0 | 0 | 3 (27.3) |
| Nausea | 73 | 3 (27.3) | 0 | 0 | 0 | 0 | 3 (27.3) |
| Asthenia | 82 | 2 (18.2) | 0 | 0 | 0 | 0 | 2 (18.2) |
| Blood lactate dehydrogenase | 82 | 0 | 2 (18.2) | 0 | 0 | 0 | 2 (18.2) |
| Dizziness | 82 | 2 (18.2) | 0 | 0 | 0 | 0 | 2 (18.2) |
| Hypokalemia | 82 | 0 | 0 | 2 (18.2) | 0 | 0 | 2 (18.2) |
| Hyponatremia | 82 | 0 | 0 | 1 (9.1) | 1 (9.1) | 0 | 2 (18.2) |
| Hypoproteinemia | 82 | 1 (9.1) | 1 (9.1) | 0 | 0 | 0 | 2 (18.2) |
| Pain | 82 | 1 (9.1) | 1 (9.1) | 0 | 0 | 0 | 2 (18.2) |
| Pyrexia | 82 | 2 (18.2) | 0 | 0 | 0 | 0 | 2 (18.2) |
| Vomiting | 82 | 1 (9.1) | 1 (9.1) | 0 | 0 | 0 | 2 (18.2) |
| Abdominal pain | 91 | 0 | 1 (9.1) | 0 | 0 | 0 | 1 (9.1) |
| Alanine aminotransferase increased | 91 | 0 | 1 (9.1) | 0 | 0 | 0 | 1 (9.1) |
| Blood alkaline phosphatase increased | 91 | 1 (9.1) | 0 | 0 | 0 | 0 | 1 (9.1) |
| Blood creatinine increased | 91 | 1 (9.1) | 0 | 0 | 0 | 0 | 1 (9.1) |
| Chest discomfort | 91 | 1 (9.1) | 0 | 0 | 0 | 0 | 1 (9.1) |
| Chest pain | 91 | 0 | 1 (9.1) | 0 | 0 | 0 | 1 (9.1) |
| Dreamy state | 91 | 1 (9.1) | 0 | 0 | 0 | 0 | 1 (9.1) |
| Electrocardiogram QT prolonged | 91 | 1 (9.1) | 0 | 0 | 0 | 0 | 1 (9.1) |
| Emotional disorder | 91 | 0 | 1 (9.1) | 0 | 0 | 0 | 1 (9.1) |
| Fatigue | 91 | 1 (9.1) | 0 | 0 | 0 | 0 | 1 (9.1) |
| Flushing | 91 | 1 (9.1) | 0 | 0 | 0 | 0 | 1 (9.1) |
| Gamma‐glutamyltransferase increased | 91 | 0 | 1 (9.1) | 0 | 0 | 0 | 1 (9.1) |
| Gastrointestinal disorder | 91 | 1 (9.1) | 0 | 0 | 0 | 0 | 1 (9.1) |
| Hyperkalemia | 91 | 0 | 1 (9.1) | 0 | 0 | 0 | 1 (9.1) |
| Hyperthyroidism | 91 | 1 (9.1) | 0 | 0 | 0 | 0 | 1 (9.1) |
| Hypothyroidism | 91 | 0 | 1 (9.1) | 0 | 0 | 0 | 1 (9.1) |
| Insomnia | 91 | 0 | 1 (9.1) | 0 | 0 | 0 | 1 (9.1) |
| Mouth ulceration | 91 | 1 (9.1) | 0 | 0 | 0 | 0 | 1 (9.1) |
| Neurotoxicity | 91 | 1 (9.1) | 0 | 0 | 0 | 0 | 1 (9.1) |
| Otitis media | 91 | 1 (9.1) | 0 | 0 | 0 | 0 | 1 (9.1) |
| Pain in extremity | 91 | 0 | 1 (9.1) | 0 | 0 | 0 | 1 (9.1) |
| Pigmentation disorder | 91 | 0 | 1 (9.1) | 0 | 0 | 0 | 1 (9.1) |
| Platelet count increased | 91 | 1 (9.1) | 0 | 0 | 0 | 0 | 1 (9.1) |
| Pneumonia | 91 | 0 | 1 (9.1) | 0 | 0 | 0 | 1 (9.1) |
| Productive cough | 91 | 1 (9.1) | 0 | 0 | 0 | 0 | 1 (9.1) |
| Pruritus generalised | 91 | 1 (9.1) | 0 | 0 | 0 | 0 | 1 (9.1) |
| Skin disorder | 91 | 0 | 1 (9.1) | 0 | 0 | 0 | 1 (9.1) |
| Skin exfoliation | 91 | 0 | 1 (9.1) | 0 | 0 | 0 | 1 (9.1) |
| Somnolence | 91 | 1 (9.1) | 0 | 0 | 0 | 0 | 1 (9.1) |
| Tachypnoea | 91 | 0 | 1 (9.1) | 0 | 0 | 0 | 1 (9.1) |
| Thyroid disorder | 91 | 1 (9.1) | 0 | 0 | 0 | 0 | 1 (9.1) |
| Urine abnormality | 91 | 0 | 1 (9.1) | 0 | 0 | 0 | 1 (9.1) |
All Cause Adverse Events All Cycles (10 mg/kg group)
| Name | NC/NA, % | Grade 1, % | Grade 2, % | Grade 3, % | Grade 4, % | Grade 5, % | All grades, % |
|---|---|---|---|---|---|---|---|
| Fatigue | 57 | 29 | 14 | 0 | 0 | 0 | 43 |
| Hypothyroidism | 72 | 14 | 14 | 0 | 0 | 0 | 28 |
| Anemia | 79 | 14 | 7 | 0 | 0 | 0 | 21 |
| Aspartate aminotransferase increased | 79 | 14 | 0 | 7 | 0 | 0 | 21 |
| Constipation | 79 | 21 | 0 | 0 | 0 | 0 | 21 |
| Hyponatremia | 79 | 7 | 0 | 14 | 0 | 0 | 21 |
| Weight decreased | 79 | 14 | 7 | 0 | 0 | 0 | 21 |
| Alanine aminotransferase increased | 86 | 14 | 0 | 0 | 0 | 0 | 14 |
| Back pain | 86 | 0 | 14 | 0 | 0 | 0 | 14 |
| Blood albumin decreased | 86 | 7 | 7 | 0 | 0 | 0 | 14 |
| Blood blister | 86 | 14 | 0 | 0 | 0 | 0 | 14 |
| C‐reactive protein increased | 86 | 14 | 0 | 0 | 0 | 0 | 14 |
| Hemangioma | 86 | 14 | 0 | 0 | 0 | 0 | 14 |
| Leukocytosis | 86 | 7 | 7 | 0 | 0 | 0 | 14 |
| Malignant neoplasm progression | 86 | 0 | 0 | 0 | 0 | 14 | 14 |
| Neutrophil count increased | 86 | 7 | 7 | 0 | 0 | 0 | 14 |
| Productive cough | 86 | 7 | 7 | 0 | 0 | 0 | 14 |
| Pruritus | 86 | 14 | 0 | 0 | 0 | 0 | 14 |
| Pyrexia | 86 | 7 | 7 | 0 | 0 | 0 | 14 |
| Rash | 86 | 7 | 7 | 0 | 0 | 0 | 14 |
| Vomiting | 86 | 7 | 7 | 0 | 0 | 0 | 14 |
| Arthralgia | 93 | 7 | 0 | 0 | 0 | 0 | 7 |
| Blister | 93 | 7 | 0 | 0 | 0 | 0 | 7 |
| Blood glucose increased | 93 | 7 | 0 | 0 | 0 | 0 | 7 |
| Blood lactate dehydrogenase increased | 93 | 0 | 7 | 0 | 0 | 0 | 7 |
| Capillary disorder | 93 | 7 | 0 | 0 | 0 | 0 | 7 |
| Chest pain | 93 | 0 | 7 | 0 | 0 | 0 | 7 |
| Decreased appetite | 93 | 0 | 7 | 0 | 0 | 0 | 7 |
| Dysphagia | 93 | 0 | 0 | 7 | 0 | 0 | 7 |
| Dyspnea exertional | 93 | 0 | 7 | 0 | 0 | 0 | 7 |
| Edema peripheral | 93 | 7 | 0 | 0 | 0 | 0 | 7 |
| Epistaxis | 93 | 7 | 0 | 0 | 0 | 0 | 7 |
| Erythema | 93 | 0 | 0 | 7 | 0 | 0 | 7 |
| Giingival bleeding | 93 | 7 | 0 | 0 | 0 | 0 | 7 |
| Granulocytosis | 93 | 7 | 0 | 0 | 0 | 0 | 7 |
| Hypercholesterolemia | 93 | 7 | 0 | 0 | 0 | 0 | 7 |
| Hyperthyroidism | 93 | 7 | 0 | 0 | 0 | 0 | 7 |
| Hyperuricemia | 93 | 7 | 0 | 0 | 0 | 0 | 7 |
| Hypoesthesia | 93 | 0 | 0 | 7 | 0 | 0 | 7 |
| Hypoalbuminemia | 93 | 7 | 0 | 0 | 0 | 0 | 7 |
| Increased viscosity of upper respiratory secretion | 93 | 0 | 7 | 0 | 0 | 0 | 7 |
| Lung infection | 93 | 0 | 7 | 0 | 0 | 0 | 7 |
| Lymphadenopathy | 93 | 0 | 7 | 0 | 0 | 0 | 7 |
| Muscle fatigue | 93 | 7 | 0 | 0 | 0 | 0 | 7 |
| Nausea | 93 | 0 | 7 | 0 | 0 | 0 | 7 |
| Otitis media | 93 | 0 | 7 | 0 | 0 | 0 | 7 |
| Pain | 93 | 0 | 7 | 0 | 0 | 0 | 7 |
| Paronychia | 93 | 0 | 7 | 0 | 0 | 0 | 7 |
| Peripheral sensorimotor neuropathy | 93 | 0 | 0 | 7 | 0 | 0 | 7 |
| Peripheral swelling | 93 | 7 | 0 | 0 | 0 | 0 | 7 |
| Pharyngitis | 93 | 7 | 0 | 0 | 0 | 0 | 7 |
| Pneumonitis | 93 | 0 | 7 | 0 | 0 | 0 | 7 |
| Pyelonephritis acute | 93 | 7 | 0 | 0 | 0 | 0 | 7 |
| Rhinorrhoea | 93 | 7 | 0 | 0 | 0 | 0 | 7 |
| Sensorimotor disorder | 93 | 0 | 0 | 7 | 0 | 0 | 7 |
| Sinusitis | 93 | 7 | 0 | 0 | 0 | 0 | 7 |
| Stomatitis | 93 | 7 | 0 | 0 | 0 | 0 | 7 |
| Adverse Events Legend | |
| Data represent number (%) of patients with all cause adverse events by worst grade, occurring between the first dose and 90 days after the last dose of study therapy. Adverse events were graded by the National Cancer Institute Common Terminology Criteria for Adverse Events version 3.0. | |
| Abbreviation: NC/NA, no change from baseline/no adverse event. |
All Cause Serious Adverse Events (10 mg/kg)
| Name | Grade | Attribution |
|---|---|---|
| Malignant neoplasm progression | 5 | Unrelated |
| Malignant neoplasm progression | 5 | Unrelated |
| Dysphagia | 3 | Unrelated |
| Lung infection | 2 | Unrelated |
| Pneumonia | 2 | Unrelated |
| Sensorimotor disorder | 3 | Unrelated |
| Serious Adverse Events Legend | |
| Data represent number (%) of patients with all serious cause adverse events by worst grade, occurring between the first dose and 90 days after the last dose of study therapy. Adverse events were graded by the National Cancer Institute Common Terminology Criteria for Adverse Events version 3.0. | |
| See also Tables 5, 6, and 7 for detailed summaries of all related AEs, all cause adverse events, and all cause SAEs. |
Table 5.
All related adverse events
| Adverse event | Grade, n (%) | Total, n (%) | ||||
|---|---|---|---|---|---|---|
| 1 | 2 | 3 | 4 | 5 | ||
| Ipilimumab 3 mg/kg (n = 11) | ||||||
| Pruritus | 3 (27.3) | 2 (18.2) | 0 | 0 | 0 | 5 (45.5) |
| Rash | 3 (27.3) | 2 (18.2) | 0 | 0 | 0 | 5 (45.5) |
| Aspartate aminotransferase increased | 3 (27.3) | 1 (9.1) | 0 | 0 | 0 | 4 (36.4) |
| Nausea | 3 (27.3) | 0 | 0 | 0 | 0 | 3 (27.3) |
| Asthenia | 2 (18.2) | 0 | 0 | 0 | 0 | 2 (18.2) |
| Pain | 1 (9.1) | 1 (9.1) | 0 | 0 | 0 | 2 (18.2) |
| Pyrexia | 2 (18.2) | 0 | 0 | 0 | 0 | 2 (18.2) |
| Vomiting | 2 (18.2) | 0 | 0 | 0 | 0 | 2 (18.2) |
| Alanine aminotransferase increased | 0 | 1 (9.1) | 0 | 0 | 0 | 1 (9.1) |
| Chest discomfort | 1 (9.1) | 0 | 0 | 0 | 0 | 1 (9.1) |
| Dizziness | 1 (9.1) | 0 | 0 | 0 | 0 | 1 (9.1) |
| Dreamy state | 1 (9.1) | 0 | 0 | 0 | 0 | 1 (9.1) |
| Emotional disorder | 0 | 1 (9.1) | 0 | 0 | 0 | 1 (9.1) |
| Fatigue | 1 (9.1) | 0 | 0 | 0 | 0 | 1 (9.1) |
| Flushing | 1 (9.1) | 0 | 0 | 0 | 0 | 1 (9.1) |
| Gastrointestinal disorder | 1 (9.1) | 0 | 0 | 0 | 0 | 1 (9.1) |
| Hyperthyroidism | 1 (9.1) | 0 | 0 | 0 | 0 | 1 (9.1) |
| Hypothyroidism | 0 | 1 (9.1) | 0 | 0 | 0 | 1 (9.1) |
| Mouth ulceration | 1 (9.1) | 0 | 0 | 0 | 0 | 1 (9.1) |
| Pigmentation disorder | 0 | 1 (9.1) | 0 | 0 | 0 | 1 (9.1) |
| Pruritus generalized | 1 (9.1) | 0 | 0 | 0 | 0 | 1 (9.1) |
| Skin exfoliation | 0 | 1 (9.1) | 0 | 0 | 0 | 1 (9.1) |
| Somnolence | 1 (9.1) | 0 | 0 | 0 | 0 | 1 (9.1) |
| Thyroid disorder | 1 (9.1) | 0 | 0 | 0 | 0 | 1 (9.1) |
| Ipilimumab 10 mg/kg (n = 14) | ||||||
| Fatigue | 4 (28.6) | 2 (14.3) | 0 | 0 | 0 | 6 (42.9) |
| Hypothyroidism | 3 (21.4) | 1 (7.1) | 0 | 0 | 0 | 4 (28.6) |
| Blister | 1 (7.1) | 0 | 0 | 0 | 0 | 1 (7.1) |
| Erythema | 0 | 0 | 1 (7.1) | 0 | 0 | 1 (7.1) |
| Granulocytosis | 1 (7.1) | 0 | 0 | 0 | 0 | 1 (7.1) |
| Hyperthyroidism | 1 (7.1) | 0 | 0 | 0 | 0 | 1 (7.1) |
| Hyponatremia | 1 (7.1) | 0 | 0 | 0 | 0 | 1 (7.1) |
| Leukocytosis | 1 (7.1) | 0 | 0 | 0 | 0 | 1 (7.1) |
| Pharyngitis | 1 (7.1) | 0 | 0 | 0 | 0 | 1 (7.1) |
| Productive cough | 1 (7.1) | 0 | 0 | 0 | 0 | 1 (7.1) |
| Pruritus | 1 (7.1) | 0 | 0 | 0 | 0 | 1 (7.1) |
| Pyrexia | 0 | 1 (7.1) | 0 | 0 | 0 | 1 (7.1) |
| Rash | 0 | 1 (7.1) | 0 | 0 | 0 | 1 (7.1) |
| Vomiting | 1 (7.1) | 0 | 0 | 0 | 0 | 1 (7.1) |
Data represent number (%) of patients with all cause adverse events by worst grade, occurring between the first dose and 90 days after the last dose, of study therapy. Adverse events were graded by the National Cancer Institute Common Terminology Criteria for Adverse Events version 3.0.
Table 6.
All cause adverse events
| Adverse event | Grade, n (%) | Total, n (%) | ||||
|---|---|---|---|---|---|---|
| 1 | 2 | 3 | 4 | 5 | ||
| Ipilimumab 3 mg/kg (n = 11) | ||||||
| Pruritus | 3 (27.3) | 2 (18.2) | 0 | 0 | 0 | 5 (45.5) |
| Rash | 3 (27.3) | 2 (18.2) | 0 | 0 | 0 | 5 (45.5) |
| Aspartate aminotransferase increased | 3 (27.3) | 1 (9.1) | 0 | 0 | 0 | 4 (36.4) |
| Constipation | 1 (9.1) | 3 (27.3) | 0 | 0 | 0 | 4 (36.4) |
| Cough | 3 (27.3) | 1 (9.1) | 0 | 0 | 0 | 4 (36.4) |
| Malignant neoplasm progression | 0 | 0 | 0 | 0 | 4 (36.4) | 4 (36.4) |
| Weight decreased | 2 (18.2) | 2 (18.2) | 0 | 0 | 0 | 4 (36.4) |
| Anemia | 0 | 3 (27.3) | 0 | 0 | 0 | 3 (27.3) |
| Decreased appetite | 2 (18.2) | 1 (9.1) | 0 | 0 | 0 | 3 (27.3) |
| Nausea | 3 (27.3) | 0 | 0 | 0 | 0 | 3 (27.3) |
| Asthenia | 2 (18.2) | 0 | 0 | 0 | 0 | 2 (18.2) |
| Blood lactate dehydrogenase | 0 | 2 (18.2) | 0 | 0 | 0 | 2 (18.2) |
| Dizziness | 2 (18.2) | 0 | 0 | 0 | 0 | 2 (18.2) |
| Hypokalemia | 0 | 0 | 2 (18.2) | 0 | 0 | 2 (18.2) |
| Hyponatremia | 0 | 0 | 1 (9.1) | 1 (9.1) | 0 | 2 (18.2) |
| Hypoproteinemia | 1 (9.1) | 1 (9.1) | 0 | 0 | 0 | 2 (18.2) |
| Pain | 1 (9.1) | 1 (9.1) | 0 | 0 | 0 | 2 (18.2) |
| Pyrexia | 2 (18.2) | 0 | 0 | 0 | 0 | 2 (18.2) |
| Vomiting | 1 (9.1) | 1 (9.1) | 0 | 0 | 0 | 2 (18.2) |
| Abdominal pain | 0 | 1 (9.1) | 0 | 0 | 0 | 1 (9.1) |
| Alanine aminotransferase increased | 0 | 1 (9.1) | 0 | 0 | 0 | 1 (9.1) |
| Blood alkaline phosphatase increased | 1 (9.1) | 0 | 0 | 0 | 0 | 1 (9.1) |
| Blood creatinine increased | 1 (9.1) | 0 | 0 | 0 | 0 | 1 (9.1) |
| Chest discomfort | 1 (9.1) | 0 | 0 | 0 | 0 | 1 (9.1) |
| Chest pain | 0 | 1 (9.1) | 0 | 0 | 0 | 1 (9.1) |
| Dreamy state | 1 (9.1) | 0 | 0 | 0 | 0 | 1 (9.1) |
| Electrocardiogram QT prolonged | 1 (9.1) | 0 | 0 | 0 | 0 | 1 (9.1) |
| Emotional disorder | 0 | 1 (9.1) | 0 | 0 | 0 | 1 (9.1) |
| Fatigue | 1 (9.1) | 0 | 0 | 0 | 0 | 1 (9.1) |
| Flushing | 1 (9.1) | 0 | 0 | 0 | 0 | 1 (9.1) |
| Gamma‐glutamyltransferase increased | 0 | 1 (9.1) | 0 | 0 | 0 | 1 (9.1) |
| Gastrointestinal disorder | 1 (9.1) | 0 | 0 | 0 | 0 | 1 (9.1) |
| Hyperkalemia | 0 | 1 (9.1) | 0 | 0 | 0 | 1 (9.1) |
| Hyperthyroidism | 1 (9.1) | 0 | 0 | 0 | 0 | 1 (9.1) |
| Hypothyroidism | 0 | 1 (9.1) | 0 | 0 | 0 | 1 (9.1) |
| Insomnia | 0 | 1 (9.1) | 0 | 0 | 0 | 1 (9.1) |
| Mouth ulceration | 1 (9.1) | 0 | 0 | 0 | 0 | 1 (9.1) |
| Neurotoxicity | 1 (9.1) | 0 | 0 | 0 | 0 | 1 (9.1) |
| Otitis media | 1 (9.1) | 0 | 0 | 0 | 0 | 1 (9.1) |
| Pain in extremity | 0 | 1 (9.1) | 0 | 0 | 0 | 1 (9.1) |
| Pigmentation disorder | 0 | 1 (9.1) | 0 | 0 | 0 | 1 (9.1) |
| Platelet count increased | 1 (9.1) | 0 | 0 | 0 | 0 | 1 (9.1) |
| Pneumonia | 0 | 1 (9.1) | 0 | 0 | 0 | 1 (9.1) |
| Productive cough | 1 (9.1) | 0 | 0 | 0 | 0 | 1 (9.1) |
| Pruritus generalized | 1 (9.1) | 0 | 0 | 0 | 0 | 1 (9.1) |
| Skin disorder | 0 | 1 (9.1) | 0 | 0 | 0 | 1 (9.1) |
| Skin exfoliation | 0 | 1 (9.1) | 0 | 0 | 0 | 1 (9.1) |
| Somnolence | 1 (9.1) | 0 | 0 | 0 | 0 | 1 (9.1) |
| Tachypnoea | 0 | 1 (9.1) | 0 | 0 | 0 | 1 (9.1) |
| Thyroid disorder | 1 (9.1) | 0 | 0 | 0 | 0 | 1 (9.1) |
| Urine abnormality | 0 | 1 (9.1) | 0 | 0 | 0 | 1 (9.1) |
| Ipilimumab 10 mg/kg (n = 14) | ||||||
| Fatigue | 4 (28.6) | 2 (14.3) | 0 | 0 | 0 | 6 (42.9) |
| Hypothyroidism | 2 (14.3) | 2 (14.3) | 0 | 0 | 0 | 4 (28.6) |
| Anemia | 2 (14.3) | 1 (7.1) | 0 | 0 | 0 | 3 (21.4) |
| Aspartate aminotransferase increased | 2 (14.3) | 0 | 1 (7.1) | 0 | 0 | 3 (21.4) |
| Constipation | 3 (21.4) | 0 | 0 | 0 | 0 | 3 (21.4) |
| Hyponatremia | 1 (7.1) | 0 | 2 (14.3) | 0 | 0 | 3 (21.4) |
| Weight decreased | 2 (14.3) | 1 (7.1) | 0 | 0 | 0 | 3 (21.4) |
| Alanine aminotransferase increased | 2 (14.3) | 0 | 0 | 0 | 0 | 2 (14.3) |
| Back pain | 0 | 2 (14.3) | 0 | 0 | 0 | 2 (14.3) |
| Blood albumin decreased | 1 (7.1) | 1 (7.1) | 0 | 0 | 0 | 2 (14.3) |
| Blood blister | 2 (14.3) | 0 | 0 | 0 | 0 | 2 (14.3) |
| C‐reactive protein increased | 2 (14.3) | 0 | 0 | 0 | 0 | 2 (14.3) |
| Hemangioma | 2 (14.3) | 0 | 0 | 0 | 0 | 2 (14.3) |
| Leukocytosis | 1 (7.1) | 1 (7.1) | 0 | 0 | 0 | 2 (14.3) |
| Malignant neoplasm progression | 0 | 0 | 0 | 0 | 2 (14.3) | 2 (14.3) |
| Neutrophil count increased | 1 (7.1) | 1 (7.1) | 0 | 0 | 0 | 2 (14.3) |
| Productive cough | 1 (7.1) | 1 (7.1) | 0 | 0 | 0 | 2 (14.3) |
| Pruritus | 2 (14.3) | 0 | 0 | 0 | 0 | 2 (14.3) |
| Pyrexia | 1 (7.1) | 1 (7.1) | 0 | 0 | 0 | 2 (14.3) |
| Rash | 1 (7.1) | 1 (7.1) | 0 | 0 | 0 | 2 (14.3) |
| Vomiting | 1 (7.1) | 1 (7.1) | 0 | 0 | 0 | 2 (14.3) |
| Arthralgia | 1 (7.1) | 0 | 0 | 0 | 0 | 1 (7.1) |
| Blister | 1 (7.1) | 0 | 0 | 0 | 0 | 1 (7.1) |
| Blood glucose increased | 1 (7.1) | 0 | 0 | 0 | 0 | 1 (7.1) |
| Blood lactate dehydrogenase increased | 0 | 1 (7.1) | 0 | 0 | 0 | 1 (7.1) |
| Capillary disorder | 1 (7.1) | 0 | 0 | 0 | 0 | 1 (7.1) |
| Chest pain | 0 | 1 (7.1) | 0 | 0 | 0 | 1 (7.1) |
| Decreased appetite | 0 | 1 (7.1) | 0 | 0 | 0 | 1 (7.1) |
| Dysphagia | 0 | 0 | 1 (7.1) | 0 | 0 | 1 (7.1) |
| Dyspnea exertional | 0 | 1 (7.1) | 0 | 0 | 0 | 1 (7.1) |
| Edema peripheral | 1 (7.1) | 0 | 0 | 0 | 0 | 1 (7.1) |
| Epistaxis | 1 (7.1) | 0 | 0 | 0 | 0 | 1 (7.1) |
| Erythema | 0 | 0 | 1 (7.1) | 0 | 0 | 1 (7.1) |
| Gingival bleeding | 1 (7.1) | 0 | 0 | 0 | 0 | 1 (7.1) |
| Granulocytosis | 1 (7.1) | 0 | 0 | 0 | 0 | 1 (7.1) |
| Hypercholesterolemia | 1 (7.1) | 0 | 0 | 0 | 0 | 1 (7.1) |
| Hyperthyroidism | 1 (7.1) | 0 | 0 | 0 | 0 | 1 (7.1) |
| Hyperuricemia | 1 (7.1) | 0 | 0 | 0 | 0 | 1 (7.1) |
| Hypoesthesia | 0 | 0 | 1 (7.1) | 0 | 0 | 1 (7.1) |
| Hypoalbuminemia | 1 (7.1) | 0 | 0 | 0 | 0 | 1 (7.1) |
| Increased viscosity of upper respiratory secretion | 0 | 1 (7.1) | 0 | 0 | 0 | 1 (7.1) |
| Lung infection | 0 | 1 (7.1) | 0 | 0 | 0 | 1 (7.1) |
| Lymphadenopathy | 0 | 1 (7.1) | 0 | 0 | 0 | 1 (7.1) |
| Muscle fatigue | 1 (7.1) | 0 | 0 | 0 | 0 | 1 (7.1) |
| Nausea | 0 | 1 (7.1) | 0 | 0 | 0 | 1 (7.1) |
| Otitis media | 0 | 1 (7.1) | 0 | 0 | 0 | 1 (7.1) |
| Pain | 0 | 1 (7.1) | 0 | 0 | 0 | 1 (7.1) |
| Paronychia | 0 | 1 (7.1) | 0 | 0 | 0 | 1 (7.1) |
| Peripheral sensorimotor neuropathy | 0 | 0 | 1 (7.1) | 0 | 0 | 1 (7.1) |
| Peripheral swelling | 1 (7.1) | 0 | 0 | 0 | 0 | 1 (7.1) |
| Pharyngitis | 1 (7.1) | 0 | 0 | 0 | 0 | 1 (7.1) |
| Pneumonia | 0 | 1 (7.1) | 0 | 0 | 0 | 1 (7.1) |
| Pyelonephritis acute | 1 (7.1) | 0 | 0 | 0 | 0 | 1 (7.1) |
| Rhinorrhea | 1 (7.1) | 0 | 0 | 0 | 0 | 1 (7.1) |
| Sensorimotor disorder | 0 | 0 | 1 (7.1) | 0 | 0 | 1 (7.1) |
| Sinusitis | 1 (7.1) | 0 | 0 | 0 | 0 | 1 (7.1) |
| Stomatitis | 1 (7.1) | 0 | 0 | 0 | 0 | 1 (7.1) |
Data represent number (%) of patients with all cause adverse events by worst grade, occurring between the first dose and 90 days after the last dose of study therapy. Adverse events were graded by the National Cancer Institute Common Terminology Criteria for Adverse Events version 3.0.
Table 7.
All cause serious adverse events
| Adverse event | Grade, n (%) | ||||
|---|---|---|---|---|---|
| 1 | 2 | 3 | 4 | 5 | |
| Ipilimumab 3 mg/kg (n = 11) | |||||
| Malignant neoplasm progression | 0 | 0 | 0 | 0 | 4 (36.4) |
| Chest pain | 0 | 1 (9.1) | 0 | 0 | 0 |
| Hyponatremia | 0 | 0 | 0 | 1 (9.1) | 0 |
| Ipilimumab 10 mg/kg (n = 14) | |||||
| Malignant neoplasm progression | 0 | 0 | 0 | 0 | 2 (14.3) |
| Dysphagia | 0 | 0 | 1 (7.1) | 0 | 0 |
| Lung infection | 0 | 1 (7.1) | 0 | 0 | 0 |
| Pneumonia | 0 | 1 (7.1) | 0 | 0 | 0 |
| Sensorimotor disorder | 0 | 0 | 1 (7.1) | 0 | 0 |
Data represent number (%) of patients with all cause adverse events by worst grade, occurring between the first dose and 90 days after the last dose of study therapy. Adverse events were graded by the National Cancer Institute Common Terminology Criteria for Adverse Events version 3.0.
Pharmacokinetics/Pharmacodynamics
| Dose level | Number enrolled | Cmax (μg/L) mean ± SD | AUC0–12 (h*12μg/L) mean ± SD |
|---|---|---|---|
| 3 mg/kg | 11 | 60 (15) | 10,620 (32) |
| 10 mg/kg | 14 | 191 (13) | 35,679 (22) |
Abbreviations: AUC, area under the curve; Cmax, maximum serum concentration.
Assessment, Analysis, and Discussion
| Completion | Study completed |
| Investigator's Assessment | Drug tolerable, efficacy was not fully evaluable due to limited patient numbers, it warrants exploration in a larger study of patients with malignant melanoma and nasopharyngeal carcinoma. |
Ipilimumab is a human monoclonal antibody that binds CTLA‐4, a negative regulator of T cells, preventing ligand interaction on antigen‐presenting cells [1]. Ipilimumab is approved for the treatment of advanced melanoma in >45 countries (3 mg/kg) and in the U.S. for unresectable or metastatic melanoma (10 mg/kg) [2]. Prognosis for patients with metastatic and refractory solid tumors is poor (median survival <10 months), and there is an unmet medical need to address this, in China and globally [3, 4].
The safety profile has been established in over 12,700 clinical study patients, with the most common adverse events (AEs) being medically manageable immune‐mediated events, consistent with the mechanism of action [6]. Existing knowledge of the safety, tolerability, and pharmacokinetics of ipilimumab is based on data from a predominantly White population [1, 5, 6].
This study (NCT02516527) assessed ipilimumab safety, tolerability, and pharmacokinetics (PK) in Chinese patients with advanced unresectable, metastatic, or recurrent malignant melanoma (MM), or nasopharyngeal carcinoma (NPC). Secondary objectives included assessing immunogenicity and preliminary antitumor activity.
The study was an open‐label, single‐center, phase 1b dose escalation study of ipilimumab in Chinese patients. There were three periods in the trial: screening (≤28 days), treatment (until disease progression or intolerable toxicity), and follow‐up (≥90 days). Within the induction phase of the treatment period, patients received 3 mg/kg ipilimumab every 3 weeks (i.v., day 1 of a 3‐week cycle, repeated up to four times or until progressive disease). The maintenance phase started at week 24 (i.v., day 1 of a 12‐week cycle), continuing to 3 years from first dose, dependent on disease. Patients received a last dose when there was disease progression, clinical deterioration, and the investigator assessed intolerability or unlikely further treatment benefit, or the study discontinuation criteria were met. Subsequently, patient follow‐up was initiated and continued for a minimum of 90 days. During follow‐up, AEs were categorized as resolved, returned to baseline, or considered irreversible. To assess the safety of ipilimumab, the successive ascending dose (10 mg/kg) was administered by a 9 + 9 design, taking into consideration the safety and PK co‐endpoints. Additional subjects were allowed to join during the enrollment phase, as this is a phase I open‐label study.
The study included Chinese adults (aged ≥18 years) with histologically or cytologically confirmed solid MM or NPC tumors that were advanced (unresectable, metastatic or recurrent), Eastern Cooperative Oncology Group performance status of 0 or 1, and with adequate organ function. Exclusion criteria included central nervous system metastases, prior malignancy (except for nonmelanoma or certain in situ cancers, or complete remission ≤2 years), autoimmune disease, active or latent tuberculosis infection, pregnancy, prior immunotherapy or immunosuppressive agent treatment, any anticancer therapy or radiation therapy within 4 weeks of study drug administration, use of traditional Chinese medicines within 2 weeks of study drug administration, and use of steroids at doses >10 mg/day.
PK samples were collected after the first dose to characterize ipilimumab exposure at baseline and at 1.5 (end of infusion), 4, 24, 48, 168, 336, and 504 hours after dose. After the first dose, the following were calculated: maximum serum concentration (Cmax), time to achieve Cmax (Tmax), and area under the curve during the dosing interval of 504 hours (AUCt). Additional trough samples were also collected at the predose timepoints and at weeks 7, 10, 24, and every 12 weeks thereafter, until discontinuation or a maximum of 3 years of study treatment.
Of the 39 patients enrolled, 25 patients received at least one dose of ipilimumab and were evaluable for safety (11 patients received the 3 mg/kg dose, and 14 patients received the 10 mg/kg dose; Table 1 and Fig. 2). The median patient age in the 3 mg/kg group was 44 years (range: 23–68 years) and 48 years (range: 23–57 years) in the 10 mg/kg group (Table 2). In both groups the majority (71–73%) were male (Table 2). In the 3 mg/kg treatment group, six patients were being treated for MM and five for NPC (Table 2). In the 10 mg/kg group all 14 patients were treated for NPC (Table 2). Most (84%) patients had received at least two prior lines of systemic anticancer therapy (Table 2). During the study all patients discontinued treatment, mostly due to disease progression (23 patients, 92%; Table 2). There was one event of drug‐related toxicity leading to discontinuation and one death (Table 2).
Table 1.
Summary of safety data
| Death or adverse event | 3 mg/kg treatment group (n = 11) | 10 mg/kg treatment group (n = 14) | ||||
|---|---|---|---|---|---|---|
| All grades (1–5) | Grade 3–4 a | Grade 5 | All grades (1–5) | Grade 3–4 a | Grade 5 | |
| Death | 4 (36.4) | 3 (21.4) | ||||
| Disease‐related death | 4 (36.4) | 3 (21.4) | ||||
| Drug‐related toxicity | 0 | 0 | ||||
| SAEs | ||||||
| All causality | 5 (45.5) | 0 | 4 (36.4) | 5 (35.7) | 2 (14.3) | 2 (14.3) |
| Drug‐related | 0 | 0 | 0 | 0 | 0 | 0 |
| SOC: Neoplasms benign, malignant, and unspecified | 4 (36.4) | 0 | 4 (36.4) | 2 (14.3) | 0 | 2 (14.3) |
| PT: Malignant neoplasm progression | 4 (36.4) | 0 | 4 (36.4) | 2 (14.3) | 0 | 2 (14.3) |
| SOC: General disorders and administration site conditions | 1 (9.1) | 0 | 0 | 0 | 0 | 0 |
| PT: Chest pain | 1 (9.1) | 0 | 0 | 0 | 0 | 0 |
| SOC: Metabolism and nutrition disorders | 1 (9.1) | 1 (9.1) | 0 | 0 | 0 | 0 |
| PT: Hyponatremia | 1 (9.1) | 1 (9.1) | 0 | 0 | 0 | 0 |
| SOC: Gastrointestinal disorders | 0 | 0 | 0 | 1 (7.1) | 1 (7.1) | 0 |
| PT: Dysphagia | 0 | 0 | 0 | 1 (7.1) | 1 (7.1) | 0 |
| SOC: Infections and infestations | 0 | 0 | 0 | 1 (7.1) | 0 | 0 |
| PT: Lung infections | 0 | 0 | 0 | 1 (7.1) | 0 | 0 |
| PT: Pneumonia | 0 | 0 | 0 | 1 (7.1) | 0 | 0 |
| SOC: Nervous system disorders | 0 | 0 | 0 | 1 (7.1) | 1 (7.1) | 0 |
| PT: Sensorimotor disorder | 0 | 0 | 0 | 1 (7.1) | 1 (7.1) | 0 |
| AEs | ||||||
| All causality | 11 (100.0) | 0 | 0 | 14 (100.0) | 4 (28.6) | 0 |
| Drug‐related (ADR) | 11 (100.0) | 0 | 0 | 11 (78.6) | 1 (7.1) | 0 |
| Leading to discontinuation (all causality) | 2 (18.2) | 0 | 0 | 2 (14.3) | 1 (7.1) | 0 |
| Drug‐related (ADR) | 1 (9.1) | 0 | 0 | 0 | 0 | 0 |
| SOC: Endocrine disorders | 1 (9.1) | 0 | 0 | 0 | 0 | 0 |
| PT: Hypothyroidism | 1 (9.1) | 0 | 0 | 0 | 0 | 0 |
| AEs by PT b | ||||||
| Pruritus | 5 (45.5) | 0 | 0 | 2 (14.3) | 0 | 0 |
| Rash | 5 (45.5) | 0 | 0 | 2 (14.3) | 0 | 0 |
| AST increased | 4 (36.4) | 0 | 0 | 3 (21.4) | 1 (7.1) | 0 |
| Constipation | 4 (36.4) | 0 | 0 | 3 (21.4) | 0 | 0 |
| Cough | 4 (36.4) | 0 | 0 | 2 (14.3) | 0 | 0 |
| Malignant neoplasm progression | 4 (36.4) | 0 | 0 | 2 (14.3) | 0 | 0 |
| Weight decreased | 4 (36.4) | 0 | 0 | 3 (21 .4) | 0 | 0 |
| Anemia | 3 (27.3) | 0 | 0 | 3 (21 .4) | 0 | 0 |
| Appetite decreased | 3 (27.3) | 0 | 0 | 1 (7.1) | 0 | 0 |
| Nausea | 3 (27.3) | 0 | 0 | 1 (7.1) | 0 | 0 |
| Fatigue | 1 (9.1) | 0 | 0 | 6 (42.9) | 0 | 0 |
| Hypothyroidism | 1 (9.1) | 0 | 0 | 4 (28.6) | 0 | 0 |
Most severe grade of events recorded.
Reported for ≥25% patients, in either group or of any grade.
Abbreviations: ADR, adverse drug reaction; AE, adverse event; AST, aspartate aminotransferase; PT, preferred term; SAE, serious adverse event; SOC, system organ class.
Figure 2.
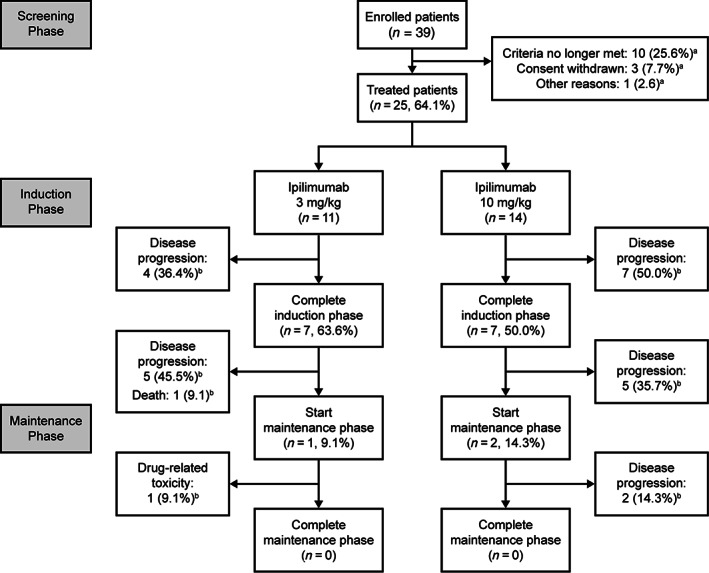
Patient disposition. aThe percentage refers to the proportion of patients entering the maintenance phase. bThe percentage refers to the proportion of patients treated in the dose group.
The median (range) of cumulative doses was 11.9 mg/kg (9.0–12.3) in patients with MM treated with 3 mg/kg ipilimumab, 11.7 mg/kg (3.0–15.3) in patients with NPC treated with 3 mg/kg ipilimumab, and 35.1 mg/kg (10.0–58.8) in patients with NPC treated with 10 mg/kg ipilimumab. The median duration (range) of treatment was 12.1 weeks (8.3–12.7) in patients with MM treated with 3 mg/kg ipilimumab, 12.0 weeks (3.0–32.1) in patients with NPC treated with 3 mg/kg ipilimumab, and 10.5 weeks (3.0–38.0) in patients with NPC treated with 10 mg/kg ipilimumab.
Safety profiles and cross‐dose comparisons observed in Chinese patients and prior U.S. clinical data (NCT00729950 and NCT00920907) were similar. There were no new safety signals, drug‐related deaths, or drug‐related serious adverse events. Most AEs affected the skin (14/25 patients), liver (7/25), endocrine system (6/25), or gastrointestinal tract (5/25) and were grades 1–2. There were 11 patients in each dose group with adverse drug reactions (ADRs), also most affecting skin, liver, endocrine system, and gastrointestinal tract, one ADR of hypothyroidism led to discontinuation (3 mg/kg), and there were no discontinuations from the 10 mg/kg group. Ipilimumab was tolerated, with only one dose‐limiting toxicity (erythema; 10 mg/kg treated patient with NPC). There were no hypersensitivity or infusion reactions.
First dose AUCt and Cmax increased dose‐proportionally (Fig. 1A). Ipilimumab exposure (assessed by AUCt and Cmax) was lower for both doses in Chinese than in U.S. patients. The serum concentration in these populations overlapped, suggesting that ipilimumab PK was ethnically insensitive in the populations compared (Fig. 1B and C). Monoclonal antibodies, such as ipilimumab, are cleared from the body by nonspecific mechanisms that do not show genetic polymorphic, ethnically influenced inhibition or induction [7, 8, 9]. During the induction phase, steady state was reached by approximately week 10 for both the 3 mg/kg and 10 mg/kg groups, based on trough concentrations (Fig. 3).
Figure 3.
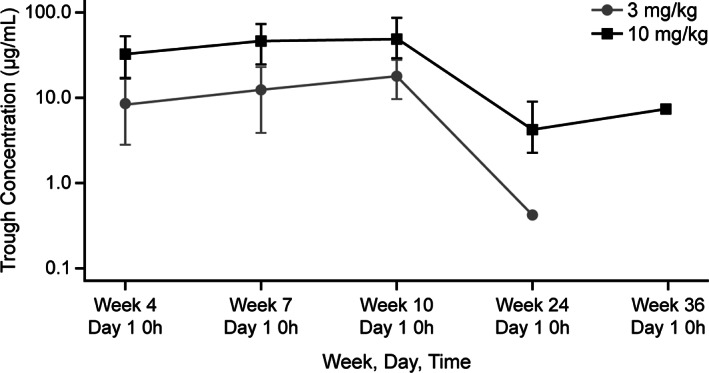
Geometric mean ipilimumab trough. (A): 3 mg/kg. (B): 10 mg/kg. Bars indicate 95% confidence intervals.
Immunogenicity was assessed by formation of antidrug antibodies (ADAs). In the 3 mg/kg group 10 of 11 patients (91%) and in the 10 mg/kg group 13 of 14 (93%) of patients were evaluable for ADAs, with baseline and at least one postbaseline ADA assessment (Table 3). No immunogenicity events affected the safety profile.
Table 3.
Ipilimumab treatment immunogenicity
| ADA status | Ipilimumab 3 mg/kg (n = 10), n (%) | Ipilimumab 10 mg/kg (n = 13), n (%) |
|---|---|---|
| ADA positive a | 1 (10) | 2 (15.4) |
| PP b | 0 | 0 |
| Not PP–last sample positive c | 1 (10) | 0 |
| Other positive d | 0 | 2 (15.4) |
| ADA negative | 9 (90) | 11 (84.6) |
At least one ADA positive (ADA+) sample relative to baseline after treatment initiation.
ADA+ samples at at least two consecutive timepoints.
Not PP, but ADA+ during the last sampling.
Not PP, some ADA+, with the last sampling being negative. Data shown are for patients with baseline ADA data and at least one postbaseline ADA assessment.
Abbreviations: ADA, antidrug antibody; PP, persistent positive.
Preliminary antitumor activity was assessed for all patients. The investigators assessed best overall response using RECIST version 1.1 criteria [10]. Of the six patients with MM (3 mg/kg), five patients had progressive disease (PD) and one was not evaluable (NE), and three of the five patients with NPC had PD, one was NE, and one had stable disease (SD). In the 10 mg/kg group (n = 14 patients with NPC), 11 had PD, 2 had SD, and 1 was NE. The disease control rates (DCRs) were 20% (a confidence interval was not applicable, as ≤10 patients) and 14.3% (95% confidence interval [CI]: 1.8–42.8) for the 3 mg/kg and 10 mg/kg ipilimumab treated patients with NPC, respectively (Table 4). The DCR was zero in the six patients with MM (Table 4), which was likely due to the advanced stage of disease at enrollment (Table 1) and the small sample size. For further exploration of the efficacy of ipilimumab in patients with MM or NPC, further studies with larger cohorts are warranted.
Table 4.
Antitumor responses
| Response | Ipilimumab 3 mg/kg | Ipilimumab 10 mg/kg | Total | |||
|---|---|---|---|---|---|---|
| MM (n = 6) | NPC (n = 5) | MM (n = 0) | NPC (n = 14) | MM (n = 6) | NPC (n = 14) | |
| Best overall response, n (%) | ||||||
| CR | 0 | 0 | 0 | 0 | 0 | 0 |
| PR | 0 | 0 | 0 | 0 | 0 | 0 |
| SD | 0 | 1 (20) | 0 | 2 (14) | 0 | 3 (16) |
| PD | 5 (83) | 3 (60) | 0 | 11 (79) | 5 (83) | 14 (74) |
| NE | 1 (17) | 1 (20) | 0 | 1 (7) | 1 (17) | 2 (11) |
| ORR a , n (% [95% CI]) b | 0 | 0 | 0 | 0 | 0 | |
| DCR c , n (% [95% CI]) b | 0 | 1 (20) | 0 | 2 (14 [2–43]) | 0 | 3 (16 [3–40]) |
The proportion of patients whose best overall response is either a CR or a PR.
Confidence intervals were computed for cohorts of 10 patients or more only.
The proportion of patients with a CR or PR or who achieved SD.
Abbreviations: CI, confidence interval; CR, complete response; DCR, disease control rate; MM, malignant melanoma; NE, not evaluable; NPC, nasopharyngeal carcinoma; ORR, objective response rate; PD, progressive disease; PR, partial response; SD, stable disease.
The median (range) follow‐up duration for patients treated with 3 mg/kg and 10 mg/kg ipilimumab was 4.9 (1.3–14.1) months and 4.8 (0.9–11.2) months, respectively. The median progression‐free survival (PFS) Kaplan‐Meier estimate was 10.1 (95% CI: 3.9–11.1) weeks in the patients with NPC treated with 10 mg/kg ipilimumab, and median overall survival Kaplan‐Meier estimate was not reached. For patients treated with 10 mg/kg ipilimumab, the PFS rate estimate at 24 weeks was 14.3% (95% CI: 2.3–36.6), and the overall survival rate estimate at 24 weeks was 76.6% (95% CI: 43.3–91.9).
Ipilimumab was well tolerated in Chinese patients, showing similar safety and pharmacokinetic profiles to previous studies in predominantly White patients. There were no new safety signals, hypersensitivity, or infusion reactions. In these pretreated patients with advanced or recurrent NPC, preliminary antitumor responses are considered worthy of further study in a larger patient population.
Disclosures
Sai Praneeth Bathena: Bristol‐Myers Squibb (E); Amol Tendolkar: Bristol‐Myers Squibb (E, OI); Jennifer Sheng: Bristol‐Myers Squibb (E, OI); Li Zhang: Bristol‐Myers Squibb, Roche, Pfizer, CTTQ Pharma, Hengrui Medicine, Innovent Bio (RF). The other authors indicated no financial relationships.
(C/A) Consulting/advisory relationship; (RF) Research funding; (E) Employment; (ET) Expert testimony; (H) Honoraria received; (OI) Ownership interests; (IP) Intellectual property rights/inventor/patent holder; (SAB) Scientific advisory board
Figures and Tables
Acknowledgments
Editorial assistance in manuscript preparation was provided by Rachael Profit, Tim Stentiford, and Aleksandra Erac‐Zganec of MediTech Media, Asia Pacific, and funded by Bristol‐Myers Squibb Pharmaceuticals Ltd., China.
No part of this article may be reproduced, stored, or transmitted in any form or for any means without the prior permission in writing from the copyright holder. For information on purchasing reprints contact commercialreprints@wiley.com. For permission information contact permissions@wiley.com.
Footnotes
- ClinicalTrials.gov Identifier: NCT02516527
- Sponsor: Bristol‐Myers Squibb Pharmaceuticals Ltd.
- Principal Investigator: Li Zhang, M.D.
- IRB Approved: Yes
Contributor Information
Jennifer Sheng, Email: jennifer.sheng@bms.com.
Li Zhang, Email: zhangli@sysucc.org.cn.
References
- 1. Ascierto PA, Del Vecchio M, Robert C et al. Ipilimumab 10 mg/kg versus ipilimumab 3 mg/kg in patients with unresectable or metastatic melanoma: A randomised, double‐blind, multicentre, phase 3 trial. Lancet Oncol 2017;18:611–622. [DOI] [PubMed] [Google Scholar]
- 2. Lamb LH, Lin SD, Sun J. Pharmacokinetics and pharmacodynamics of immunotherapy. In: Patel SP, Kurzrock R, eds. Early Phase Cancer Immunotherapy . Heidelberg, Germany: Springer, 2017:29–67. [Google Scholar]
- 3. Fanale D, Bronte G, Russo A. Targeted therapies in melanoma. In: Russo A, Rosell R, Rolfo C, eds. Targeted Therapies for Solid Tumors: A Handbook for Moving Toward New Frontiers in Cancer Treatment. Heidelberg, Germany: Springer, 2015:211–227. [Google Scholar]
- 4. Batus M, Waheed S, Ruby C et al. Optimal management of metastatic melanoma: Current strategies and future directions. Am J Clin Dermatol 2013;14:179–194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Weber J, Hamid O, Amin A et al. Randomized phase I pharmacokinetic study of ipilimumab with or without one of two different chemotherapy regimens in patients with untreated advanced melanoma. Cancer Immun 2013;13:7–16. [PMC free article] [PubMed] [Google Scholar]
- 6. Yervoy (ipilimumab) [package insert] . Princeton, NJ: Bristol‐Myers Squibb, 2020. Available at https://packageinserts.bms.com/pi/pi_yervoy.pdf. Accessed April 2020.
- 7. Ovacik M, Lin K. Tutorial on monoclonal antibody pharmacokinetics and its considerations in early development. Clin Transl Sci 2018;11:540–552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Lee JI, Zhang L, Men AY et al. CYP‐mediated therapeutic protein drug interactions: clinical findings, proposed mechanisms, and regulatory implications. Clin Pharmacokinet 2010;49:295–310. [DOI] [PubMed] [Google Scholar]
- 9. Keizer RJ, Huitema ADR, Schellens JHM et al. Clinical pharmacokinetics of therapeutic monoclonal antibodies. Clin Pharmacokinet 2010;49:493–507. [DOI] [PubMed] [Google Scholar]
- 10. Eisenhauer EA, Therasse P, Bogaerts J et al. New response evaluation criteria in solid tumours: Revised RECIST guideline (version 1.1). Eur J Cancer 2009;45:228–247. [DOI] [PubMed] [Google Scholar]


