Abstract
Background
Caregivers of adults with cancer often report a different understanding of the patient's prognosis than the oncologist. We examine the associations of caregiver–oncologist prognostic concordance with caregiver depressive symptoms, distress, and quality of life (QoL). We also explore whether these relationships differed by caregiver environment mastery, an individual's sense of control, and effectiveness in managing life situations.
Materials and Methods
We used data from a national geriatric assessment cluster‐randomized trial (URCC 13070) that recruited patients aged 70 years and older with incurable cancer considering any line of cancer treatment at community oncology practices, their caregivers, and their oncologists. At enrollment, caregivers and oncologists estimated the patient's prognosis (0–6 months, 7–12 months, 1–2 years, 2–5 years, and >5 years; identical responses were concordant). Caregivers completed the Ryff's environmental mastery at enrollment. At 4–6 weeks, caregivers completed the Patient Health Questionnaire‐2 (depressive symptoms), distress thermometer, and 12‐Item Short‐Form Health Survey (quality of life [QoL]). We used generalized estimating equations in models adjusted for covariates. We then assessed the moderation effect of caregiver mastery.
Results
Of 411 caregiver–oncologist dyads (mean age = 66.5 years), 369 provided responses and 28% were concordant. Prognostic concordance was associated with greater caregiver depressive symptoms (β = 0.30; p = .04) but not distress or QoL. A significant moderation effect for caregiver depressive symptoms was found between concordance and mastery (p = .01). Specifically, among caregivers with low mastery (below median), concordance was associated with greater depressive symptoms (β = 0.68; p = .003).
Conclusions
Caregiver–oncologist prognostic concordance was associated with caregiver depressive symptoms. We found a novel moderating effect of caregiver mastery on the relationship between concordance and caregiver depressive symptoms.
Implications for Practice
Caregiver–oncologist prognostic concordance is associated with greater caregiver depressive symptoms, particularly in those with low caregiver mastery. When discussing prognosis with caregivers, physicians should be aware that prognostic understanding may affect caregiver psychological health and should assess their depressive symptoms. In addition, while promoting accurate prognostic understanding, physicians should also identify strengths and build resilience among caregivers.
Keywords: Prognostic concordance, Environmental mastery, Depressive symptoms, Caregivers, Older patients
Short abstract
Patients and caregivers need an accurate understanding of prognosis to help them make informed treatment decisions and to prepare for the future. This article examines the association of caregiver–oncologist concordance in their estimates of patient length of life with caregiver depressive symptoms, distress, and quality of life.
Introduction
Caregivers play a crucial role in the care of older adults with cancer [1]. They assist patients with daily activities and help manage cancer‐ and treatment‐related symptoms [1, 2, 3]. Caregivers are often involved in patients' decision‐making throughout the cancer continuum from diagnosis to end‐of‐life, serving as important advocates [4, 5]. Therefore, it is imperative that caregivers have a good understanding of the patient's cancer prognosis.
Prior studies have demonstrated that 30%–55% of caregivers report a different understanding of the patient's treatment goal and prognosis compared with the patient's oncologist; most caregivers are more optimistic about the likelihood of cure than the oncologist [6, 7, 8]. Patients and caregivers need to have an accurate understanding of prognosis to help them make informed treatment decisions and prepare for the future [9, 10]. However, initial research in this area has shown that accurate prognostic understanding among patients with cancer correlates with worse patient‐reported outcomes, including greater depressive and anxiety symptoms and worse quality of life (QoL) [11, 12, 13, 14, 15]. Studies have also demonstrated that accurate caregiver prognostic understanding is associated with greater caregiver depressive and anxiety symptoms [13, 16].
Caregiving for older adults with cancer can be demanding and lead to significant caregiving burden, distress, depression, anxiety, and poor QoL [17]. Caregiver environmental mastery, which is an individual's sense of control and effectiveness in managing life situations, reflects a caregiver's ability to cope, adjust, and adapt to problems [18, 19]. Individuals who report poor mastery, meaning they do not feel their life circumstances are controllable, are likely to feel particularly burdened by caregiving and experience greater depressive symptoms [18, 20]. Among caregivers of patients with cancer, greater mastery correlates with better caregiver health and less depressive symptoms [21, 22]. Among patients with advanced cancer, prior work has demonstrated that the use of certain coping strategies (i.e., greater use of positive reframing and active coping) moderated the relationship between accurate prognostic understanding and greater depressive symptoms [12]. However, the moderating effects of caregiver mastery on the association between caregiver prognostic understanding and psychological health is currently unknown.
In this study, we examined the association of caregiver–oncologist concordance in their estimates of patient length of life with caregiver depressive symptoms, distress, and QoL. We also explored the moderating effects of caregiver mastery on these associations. We hypothesized that caregiver–oncologist concordance would be associated with greater caregiver depressive symptoms and distress, as well as lower QoL.
Materials and Methods
Study Design, Participants, and Setting
This was secondary analysis of a nationwide geriatric assessment cluster‐randomized controlled trial (URCC 13070, NCT02107443; Principal Investigator: Mohile). We have previously reported the details of the study [7, 23, 24, 25]. The primary study evaluated whether providing geriatric assessment (GA) and GA‐driven recommendations to patients, their caregivers, and their oncologists increased discussions about age‐related concerns and improved patient and caregiver satisfaction. Inclusion criteria for patients were (a) aged 70 or older with incurable cancer (per the determination of the treating oncologist at the time of enrollment); (b) considering and/or receiving any line of cancer treatment; (c) had one or more impaired domain on geriatric assessment (other than polypharmacy; geriatric assessment domains and definitions of impairment have been reported previously [7, 23, 24, 25]); and (d) able to provide informed consent. One caregiver could enroll with each patient if the patient wished. Patients were asked: “Is there a family member, partner, friend, or caregiver (age 21 or older) with whom you discuss or who can be helpful in health‐related matters.”
A total of 31 community oncology practices in the United States and within the University of Rochester National Cancer Institute Community Oncology Research Program (NCORP) participated in this study and enrolled patients and caregivers between October 2014 and April 2017. The institutional review boards at the University of Rochester and at all the individual NCORP affiliate sites approved the study prior to enrollment of participants.
Measures
Independent Variable: Caregiver–Oncologist Concordance in Estimates of Patient Length of Life
At enrollment, caregivers completed a paper questionnaire and were asked: “Considering the patient's health and underlying medical conditions, what would you estimate the patient's overall life expectancy to be?” Response options were 0–6 months, 7–12 months, 1–2 years, 2–5 years, and more than 5 years. We adapted this question from a previous study of seriously ill older patients (including those with cancer) and their caregivers [26]. Oncologists also completed a same assessment at enrollment. Caregivers and oncologists were considered concordant if they selected the same response option.
Dependent Variables: Caregiver Depressive Symptoms, Distress, and QoL
Four to six weeks following enrollment, caregivers completed the Patient Health Questionnaire (PHQ)‐2 depression screening tool [27] and distress thermometer [28], as well as the 12‐Item Short‐Form Health Survey (SF‐12), which assesses QoL [29]. For the PHQ‐2, caregivers were asked how often they have been bothered by the following problems: (a) little interest or pleasure in doing things and (b) feeling down, depressed, or hopeless. Each question was scored from 0 (not at all) to 3 (nearly every day), with a range of 0–6 and a higher score indicating greater depressive symptoms [27]. PHQ‐2 ≥ 2 was considered positive screening [27]. Caregiver distress was rated from 0 to 10, with a higher score indicating greater distress [28]. Distress ≥4 was considered moderate in severity [28]. QoL was measured with the SF‐12, which consists of physical and mental health subscales, with each subscale ranging from 0 to 100 and higher scores indicating better physical and mental health [29].
Moderation Variable: Caregiver Environmental Mastery
Caregivers completed the Ryff's environmental mastery at enrollment [19]. Environmental mastery measures the capacity to cope, adjust, and adapt to problems. The subscale consists of seven statements: (a) In general, I feel I am in charge of the situation in which I live; (b) the demands of everyday life often get me down; (c) I do not fit very well with the people and the community around me; (d) I am quite good at managing many responsibilities of my daily life; (e) I often feel overwhelmed by my responsibilities; (f) I have difficulty arranging my life in a way that is satisfying to me; and (g) I have been able to build a home and a lifestyle for myself that is much to my liking. Options were strongly disagree, disagree, undecided, agree, and strongly agree. The total score ranges from 7 to 35 (question 2, 3, 5, and 6 were reverse scored), with a higher score indicating greater mastery [19]. The Cronbach's α of Ryff's mastery was 0.76 in our study.
Covariates
Covariates included caregiver demographics, patient cancer type (gastrointestinal, genitourinary, lung, or other), and study arm (geriatric assessment or usual care). Caregiver demographics were age, gender, education (some college or above, high school graduate, or less than high school), race (non‐Hispanic white or other), annual household income (>$50,000, ≤$50,000, or decline to answer), and marital status (married or nonmarried).
Other Relevant Measures
Caregivers were asked the extent they have discussed the patient's prognosis with the oncologist. Patient overall survival was captured by clinical research associates at individual practices.
Statistical Analyses
We used descriptive analyses to summarize our study population and measures. We used a two‐sample t test to evaluate caregiver depressive symptoms, distress, and QoL among concordant versus discordant dyads. We then conducted generalized estimating equation models to evaluate the associations of baseline concordance with caregiver depressive symptoms, distress, and QoL at 4–6 weeks, adjusting for caregiver demographic, patient cancer type, study arm, and accounting for practice sites. Subsequently, we assessed the moderation effect of caregiver mastery on these associations. We created an interaction term (concordance [1, concordance; 0: discordance] X mastery [continuous variable]) and included this term, along with both concordance and mastery as independent variables in the model. If the interaction term was significant, we performed analyses within the subgroups (i.e., higher [median or higher] vs. lower [less than median] caregiver mastery).
For sensitivity analyses, we divided baseline discordance into caregivers estimated a longer patient length of life and oncologists estimated a longer patient length of life. We repeated the generalized estimating equation models (concordance vs. caregivers estimated a longer patient length of life and concordance vs. oncologist estimated a longer patient length of life).
A two‐sided p < .05 was considered to be statistically significant. We used SAS v.9.4 (SAS Institute Inc., Cary, NC) to perform all analyses.
Results
The primary study included 414 caregiver–oncologist dyads; 3 dyads were excluded because of missing all caregiver demographics (Fig. 1). Mean age of the 411 caregivers was 66.5 (SD, 12.5; range, 26–92) years. Three‐quarters of the caregivers were female, and 90% were White. Approximately 64% of caregivers had some college education or above, and 43% had an annual household income >$50,000. In terms of patient cancer type, lung cancer was the most common (26%), followed by gastrointestinal (23%), genitourinary (14%), and breast (11%). Other caregiver demographics are shown in Table 1.
Figure 1.
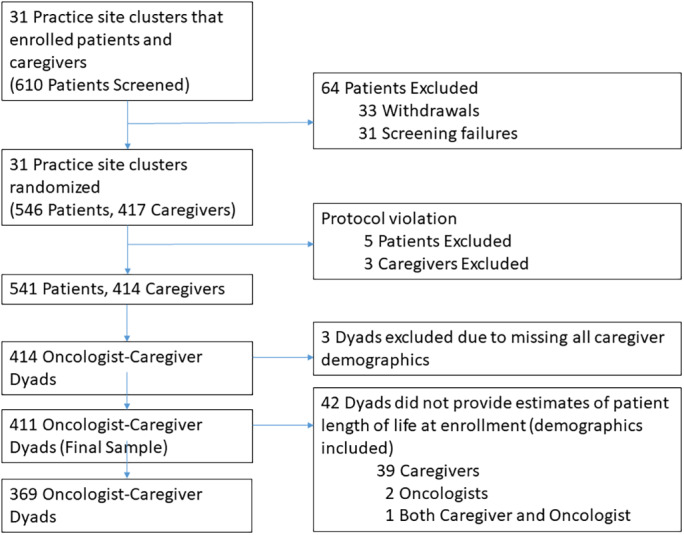
Flow diagram depicting the number of caregivers included in the secondary analysis.
Table 1.
Baseline characteristics of caregivers, patient cancer type, and study arm
| Variables | Caregivers (n = 411) |
|---|---|
| Age, mean (SD, range), yr | 66.5 (12.5–26–92) |
| Gender, % | |
| Male | 101 (25.6) |
| Female | 310 (75.4) |
| Marital status, a % | |
| Married | 335 (81.5) |
| Other | 76 (18.5) |
| Race, % | |
| Non‐Hispanic White | 369 (89.8) |
| Non‐White | 42 (10.2) |
| Education, % | |
| Some college or above | 263 (64.0) |
| High school graduate | 118 (28.7) |
| < High school | 30 (7.3) |
| Annual household income, a % | |
| > $50,000 | 178 (43.4) |
| ≤ $50,000 | 151 (36.8) |
| Decline to answer | 81 (19.7) |
| Patient cancer type a | |
| Breast | 44 (10.7) |
| Gastrointestinal | 94 (22.9) |
| Genitourinary | 58 (14.2) |
| Lung | 105 (25.6) |
| Other | 109 (26.6) |
| Study arm | |
| Intervention | 229 (55.7) |
| Usual care | 182 (44.3) |
One patient had missing data.
Caregiver–Oncologist Concordance in Estimates of Patient Length of Life
Among the 411 dyads, 371 caregivers and 408 oncologists provided estimates of patient length of life at enrollment (Fig. 2). A total of 369 caregiver–oncologist dyads provided estimates of patient length of life. Approximately 28% (n = 103) of the dyads were concordant in their estimates of patient length of life; 1% dyads estimated the length of life to be 0–6 months, 5% estimated it to be 7–12 months, 8% estimated it to be 1–2 years, 8% estimated it to be 2–5 years, and 7% it to be estimated >5 years. Among the discordant dyads (n = 266), 85% (n = 225) of caregivers estimated a longer patient length of life than the oncologists.
Figure 2.
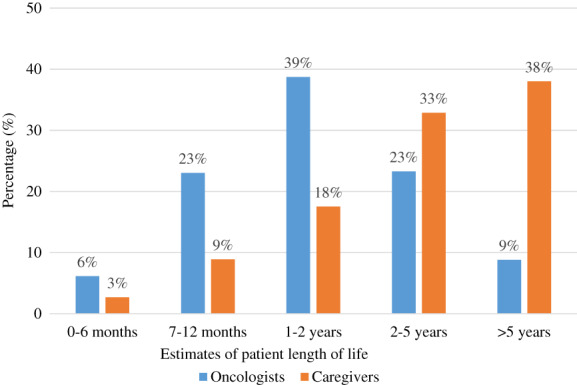
Distribution of caregiver and oncologist estimates of patient length of life.
Other Relevant Measures
Of the 396 caregivers that responded to the question “To what extent have you discussed the patient's prognosis with the cancer doctor,” 31% (n = 123) reported “completely,” 32% (n = 127) reported “mostly,” 28% reported (n = 112) reported “a little,” and 9% (n = 34) reported “not at all.”
In terms of actual survival of patients with a caregiver enrolled (n = 399), 24% died between 0–6 months after enrollment, 22% died between 7–12 months, and 54% were alive beyond 1 year.
Caregiver Environmental Mastery
At enrollment, the mean caregiver mastery score was 27.5 (SD, 4.6; range, 7–35; n = 395), higher than prior studies of cancer survivors [30] and caregivers of frail older adults [31]. Table 2 shows the distribution of the mastery subscale items. Approximately one quarter (22.4%) agreed or strongly agreed with the statement “the demands of everyday life often get me down.” Similarly, 21.8% agreed or strongly agreed with the statement “I often feel overwhelmed by my responsibilities.” Seventeen percent agreed or strongly agreed with the statement “I have difficulty arranging my life in a way that is satisfying to me.”
Table 2.
Distribution of the Ryff's environmental mastery subscale items
| Items | Strongly disagree, % | Disagree, % | Undecided, % | Agree, % | Strongly agree, % |
|---|---|---|---|---|---|
| In general, I feel I am in charge of the situation in which I live a | 3.6 | 5.6 | 11.7 | 47.7 | 31.5 |
| The demands of everyday life often get me down a | 24.9 | 38.8 | 14.0 | 21.1 | 1.3 |
| I do not fit very well with the people and the community around me b | 50.0 | 35.5 | 9.1 | 2.3 | 3.1 |
| I am quite good at managing many responsibilities of my daily life b | 3.0 | 2.8 | 3.8 | 48.6 | 41.8 |
| I often feel overwhelmed by my responsibilities b | 26.3 | 37.5 | 14.4 | 18.5 | 3.3 |
| I have difficulty arranging my life in a way that is satisfying to me c | 29.4 | 39.9 | 14.0 | 13.2 | 3.6 |
| I have been able to build a home and a lifestyle for myself that is much to my liking b | 4.1 | 5.1 | 11.9 | 46.7 | 32.2 |
17 caregivers had missing data.
16 caregivers had missing data.
1 patient had missing data.
Caregiver Depressive Symptoms, Distress, and QoL
Four to six weeks after enrollment, mean PHQ‐2 and distress scores were 0.65 (SD, 1.3; range, 0–6; n = 350) and 2.7 (SD, 2.6; range, 0–10; n = 347), respectively. Approximately 19% screened positive for depression (PHQ‐2 ≥ 2), and 32% reported moderate distress (distress ≥4). For SF‐12 (n = 349), mean physical health, mental health, and total scores were 46.7 (SD, 10.3; range, 15.7–64.7), 50.8 (SD, 10.0; range, 11.2–71.9), and 97.5 (SD, 14.1; range, 53.7–119.1), respectively.
Multivariate Analyses
On multivariate analyses, caregiver–oncologist concordance in their estimates of patient length of life was associated with greater depressive symptoms (β = 0.30; p = .04). The associations of concordance with distress (β = 0.47; p = .10), physical health (β = −1.43; p = .20), mental health (β = −1.66; p = .21), and QoL (β = −3.21; p = .07) were not statistically significant (Table 3).
Table 3.
Multivariate analyses evaluating associations of caregiver–oncologist concordance in estimates of patient length of life with depressive symptoms, distress, and quality of life
| Measures | Caregiver–oncologist concordance in estimates of patient length of life | p value a | p value for interaction | |
|---|---|---|---|---|
| Beta Estimate | SE | |||
| PHQ‐2 (n = 328) | 0.30 | 0.14 | .04 | .02 |
| Environmental mastery <28 (n = 146) | 0.68 | 0.23 | .003 | |
| Environmental mastery ≥28 (n = 181) | ‐0.07 | 0.14 | .63 | |
| Distress (n = 326) | 0.47 | 0.28 | .10 | .58 |
| Environmental mastery <28 (n = 146) | 0.49 | 0.35 | .16 | |
| Environmental mastery ≥28 (n = 179) | 0.24 | 0.31 | .44 | |
| SF‐12 (n = 327) | ‐3.21 | 0.90 | .07 | .65 |
| Environmental mastery <28 (n = 146) | ‐2.43 | 2.72 | .37 | |
| Environmental mastery ≥28 (n = 180) | ‐2.89 | 1.98 | .14 | |
| SF‐12 Physical Health (n = 327) | ‐1.43 | 1.10 | .20 | .27 |
| Environmental mastery <28 (n = 146) | ‐0.33 | 5.96 | .84 | |
| Environmental mastery ≥28 (n = 180) | ‐2.10 | 1.49 | .16 | |
| SF‐12 Mental Health (n = 327) | ‐1.66 | 1.31 | .21 | .70 |
| Environmental mastery <28 (n = 146) | ‐2.09 | 1.89 | .27 | |
| Environmental mastery ≥28 (n = 180) | ‐0.79 | 1.42 | .58 | |
All models were adjusted for caregiver age, gender, race, income, education, marital status, patient cancer type, and study arm, accounting for clustering at practice sites.
Interaction term was not included in the model.
Abbreviations: PHQ‐2, Patient Health Questionnaire‐2; SF‐12, 12‐Item Short‐Form Health Survey.
On sensitivity analyses, caregivers in the concordant dyads reported greater depressive symptoms compared with those who estimated a longer patient length of life than the oncologist (β = 0.32; p = .05). Depressive symptoms were not different between caregivers in the concordant dyads and those who estimated a shorter patient length of life than the oncologist (β = 0.26; p = .20).
Moderating Effect
A significant moderation effect was found between concordance and mastery for caregiver depressive symptoms (p = .02; Fig. 3). Among caregivers with low mastery (less than median), caregiver–oncologist concordance in estimates of patient length of life was associated with greater depressive symptoms (β = 0.68; p = .003). Among caregivers with high mastery (at or above median), concordance was not associated with depressive symptoms (β = −0.07; p = .63; Table 3). Mastery did not moderate the associations of concordance with distress and QoL.
Figure 3.
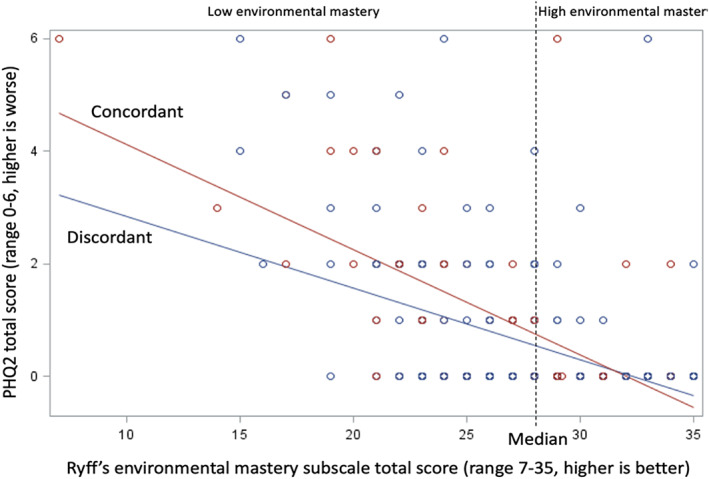
Moderation effect of mastery on the association between caregiver–oncologist concordance and caregiver depressive symptoms.
Discussion
In this secondary analysis of a nationwide geriatric assessment cluster‐randomized controlled trial, we found that caregiver–oncologist concordance in estimates of patient length of life was associated with greater caregiver depressive symptoms. We demonstrated that caregiver mastery moderated the association between caregiver–oncologist concordance and caregiver depressive symptoms. Specifically, we found that caregiver–oncologist concordance was associated with greater depressive symptoms among caregivers with low mastery but not among those with high mastery.
Prior studies have shown an association between accurate patient prognostic understanding and worse patient‐reported outcomes [11, 12, 13, 14, 15]. Data on these associations among caregivers, however, are limited. A prior cross‐sectional study of 167 caregivers of patients with advanced lung cancer in Japan showed that caregivers with an accurate understanding of prognosis had greater levels of anxiety and depression [13]. A separate longitudinal study by Loh et al. in the U.S. demonstrated that caregiver–oncologist concordance in beliefs about curability was associated with greater anxiety symptoms among 97 caregivers of adults with advanced cancers [16]. In this prior study, psychological health and QoL was assessed 7 months after the death of the patient, compared with the current study, which examined anxiety 4–6 weeks following enrollment of caregivers. Taken together, the findings from these studies emphasize the necessity of providing psychological and emotional support to caregivers, especially to those with an accurate understanding of the patient's poor prognosis. Of course, physicians should not be discouraged from disclosing accurate information regarding disease prognosis to patients and their caregivers. Prior studies have shown that most patients want to know about prognostic information [32], prognostic disclosures are not associated with worse patient–physician relationships [33], and end‐of‐life discussions are associated with less aggressive care, which in turn is associated with lower depressive symptoms in bereaved caregivers [34]. It is important that oncologists tailor the amount and type of prognostic information and communicate these information to both patients and caregivers at the appropriate timing. When prognosis is disclosed, it is also important that oncologists facilitate referral to mental health or social work services, when appropriate.
Several previous studies have demonstrated associations of mastery with caregiver outcomes [21, 22, 35]. For example, Yeh et al. found that mastery was positively correlated with the health of caregivers of patients with cancer [35]. Greater mastery also correlates with lower depressive symptoms among caregivers of patients with cancer, although none of these studies focused exclusively on older adults with cancer [21, 22]. Nijboer et al. showed that mastery moderated associations between caregiver experiences and caregiver depression, such that caregivers who perceived caregiving in a negative way and reported lower mastery were more likely to experience greater depressive symptoms [22]. Among caregivers of older adults, greater mastery has been shown to correlate with lower caregiver burden and less anxiety and depressive symptoms [18]. Caregiver involvement is often more substantial in the care of older adults [24, 36], and caregivers generally receive very little training [37, 38]. Therefore, understanding how mastery is associated with caregiver outcomes among those caring for vulnerable older adults with cancer is important.
To the best of our knowledge, this study is the first to demonstrate the moderation effect of caregiver mastery on the association between caregiver–oncologist concordance and caregiver depressive symptoms. Successful management by caregivers of environmental factors and activities (physically, by being able to provide the needs of their loved ones, and mentally, by regulating their emotional responses to caregiving) may decrease the negative impact of prognostic concordance on their psychological health [39, 40], although this needs to be investigated further. Examples of interventions that have been shown to improve mastery of caregivers of patients with other illnesses included the use of telemonitoring system for heart failure symptoms at home with follow‐up calls by an advance practice nurse and group programs focusing on problem‐solving techniques simulation for caregivers of patients with dementia [41, 42]. These interventions could potentially be adapted for caregivers of older patients with cancer. In fact, electronic symptom monitoring has been shown to improve patient outcomes [43, 44, 45] and could be further studied in caregivers of these patients. Our findings also have implications for the development of interventions targeting both prognostic understanding and mastery among caregivers. One such example is palliative care interventions for caregivers [46]. In one study, palliative care improved prognostic understanding among patients with cancer [39], potentially through improving their coping skills [12, 47]. Future studies should investigate if palliative care interventions could improve prognostic understanding and mastery among caregivers of older patients with cancer and thereby help decrease caregiver depressive symptoms.
The strengths of our study include its large number of caregivers of older adults with advanced cancer recruited from a national sample of community oncology practices. Our study has several limitations. First, our caregivers were mostly non‐Hispanic White and well‐educated, and therefore, our results may not be generalizable to caregivers of other races and ethnicities and with lower education levels. Second, we excluded approximately one‐eighth of the caregiver–oncologist dyads because they did not provide a response to the question on patient length of life estimates. Future studies should explore the characteristics of these individuals and reasons for why they refuse to answer the question on patient length of life estimates. Third, we lack information about the discussions of prognosis among the patients, caregivers, and oncologists in this study. We did not ask caregivers if it was helpful for them to discuss or know the patient's prognosis. Similarly, we did not ask oncologists if they had disclosed the patient's prognostic estimates to the patients or caregivers. Fourth, we cannot establish causality among prognostic understanding, mastery, and depressive symptoms. Finally, we did not adjust for multiple testing, given that this was an exploratory analysis.
Conclusion
We found that caregiver–oncologist concordance in patient length of life estimates was associated with greater caregiver depressive symptoms. Interestingly, we found that caregiver mastery moderated this relationship, such that caregiver–oncologist concordance was associated with greater depressive symptoms among those with lower mastery but not among those with higher mastery. These findings underscore a need for additional research to further investigate how prognostic understanding might lead to depression among caregivers of patients with cancer, particularly those with low caregiver mastery. When discussing prognosis with caregivers, physicians should be aware that prognostic understanding may affect caregiver psychological health and assess their depressive symptoms. In addition, while promoting accurate prognostic understanding, physicians should also identify strengths and build resilience among caregivers.
Author Contributions
Conception/design: Kah Poh Loh, Supriya G. Mohile
Provision of study material or patients: Arlene A Gayle, Alison Conlin, James Bearden III
Collection and/or assembly of data: Arlene A Gayle, Alison Conlin, James Bearden III
Data analysis and interpretation: Kah Poh Loh, Eva Culakova, Huiwen Xu
Manuscript writing: Kah Poh Loh, Mostafa R. Mohamed, Sindhuja Kadambi
Final approval of manuscript: Kah Poh Loh, Mostafa R. Mohamed, Sindhuja Kadambi, Eva Culakova, Huiwen Xu, Allison Magnuson, Marie Flannery, Paul R. Duberstein, Ronald M. Epstein, Colin McHugh, Ryan D. Nipp, Kelly M. Trevino, Chandrika Sanapala, Bianca A. Hall Beverly Canin Arlene A Gayle, Alison Conlin, James Bearden III, Supriya G. Mohile
Disclosures
Kah Poh Loh: Pfizer, Seattle Genetics (C/A); Supriya Mohile: Carevive (RF). The other authors indicated no financial relationships.
(C/A) Consulting/advisory relationship; (RF) Research funding; (E) Employment; (ET) Expert testimony; (H) Honoraria received; (OI) Ownership interests; (IP) Intellectual property rights/inventor/patent holder; (SAB) Scientific advisory board
Acknowledgments
We wish to acknowledge Dr. Susan Rosenthal, M.D., for her editorial assistance.
The work was supported by the Patient‐Centered Outcomes Research Institute (PCORI) Program contract (4634 to S.G.M.), the National Cancer Institute at the National Institutes of Health (UG1 CA189961; R01CA168387 to P.R.D.; K99CA237744 to K.P.L.), the National Institute of Aging at the National Institutes of Health (K24 AG056589 to S.G.M.; R33 AG059206 to S.G.M.; K76 AG064394 to A.M.), and the Wilmot Research Fellowship Award (grant number is not applicable; to K.P.L.). This work was made possible by the generous donors to the Wilmot Cancer Institute (WCI) geriatric oncology philanthropy fund. All statements in this report, including its findings and conclusions, are solely those of the authors, do not necessarily represent the official views of the funding agencies, and do not necessarily represent the views of the PCORI, its Board of Governors, or Methodology Committee.
The study was accepted for presentation as a poster and featured during the poster highlights session at the 2020 American Society of Clinical Oncology Quality Care Symposium.
Disclosures of potential conflicts of interest may be found at the end of this article.
No part of this article may be reproduced, stored, or transmitted in any form or for any means without the prior permission in writing from the copyright holder. For information on purchasing reprints contact commercialreprints@wiley.com. For permission information contact permissions@wiley.com.
References
- 1. Jayani R, Hurria A. Caregivers of older adults with cancer. Semin Oncol Nurs 2012;28:221–225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Kadambi S, Soto‐Perez‐de‐Celis E, Garg T et al. Social support for older adults with cancer: Young international society of geriatric oncology review paper. J Geriatr Oncol 2020;11:217–224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Ornstein KA, Liu B, Schwartz RM et al. Cancer in the context of aging: Health characteristics, function and caregiving needs prior to a new cancer diagnosis in a national sample of older adults. J Geriatr Oncol 2020;11:75–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Glajchen M. The emerging role and needs of family caregivers in cancer care. J Support Oncol 2004;2:145–155. [PubMed] [Google Scholar]
- 5. Dionne‐Odom JN, Ejem D, Wells R et al. How family caregivers of persons with advanced cancer assist with upstream healthcare decision‐making: A qualitative study. PLoS One 2019;14:e0212967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Shin DW, Cho J, Kim SY et al. Patients' and family caregivers' understanding of the cancer stage, treatment goal, and chance of cure: A study with patient‐caregiver‐physician triad. Psychooncology 2018;27:106–113. [DOI] [PubMed] [Google Scholar]
- 7. Loh KP, Mohile SG, Lund JL et al. Beliefs about advanced cancer curability in older patients, their caregivers, and oncologists. The Oncologist 2019;24:e292–e302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Malhotra K, Fenton JJ, Duberstein PR et al. Prognostic accuracy of patients, caregivers, and oncologists in advanced cancer. Cancer 2019;125:2684–2692. [DOI] [PubMed] [Google Scholar]
- 9. Cartwright LA, Dumenci L, Siminoff LA et al. Cancer patients' understanding of prognostic information. J Cancer Educ 2014;29:311–317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Sanders JJ, Curtis JR, Tulsky JA. Achieving goal‐concordant care: A conceptual model and approach to measuring serious illness communication and its impact. J Palliat Med 2018;21(suppl 2):S17–s27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. El‐Jawahri A, Traeger L, Kuzmuk K et al. Prognostic understanding, quality of life and mood in patients undergoing hematopoietic stem cell transplantation. Bone Marrow Transplant 2015;50:1119–1124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Nipp RD, Greer JA, El‐Jawahri A et al. Coping and prognostic awareness in patients with advanced cancer. J Clin Oncol 2017;35:2551–2557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Sato T, Soejima K, Fujisawa D et al. Prognostic understanding at diagnosis and associated factors in patients with advanced lung cancer and their caregivers. The Oncologist 2018;23:1218–1229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. El‐Jawahri A, Traeger L, Park ER et al. Associations among prognostic understanding, quality of life, and mood in patients with advanced cancer. Cancer 2014;120:278–285. [DOI] [PubMed] [Google Scholar]
- 15. Janssens A, Derijcke S, Galdermans D et al. Prognostic understanding and quality of life in patients with advanced lung cancer: A multicenter study. Clin Lung Cancer 2019;20:e369–e375. [DOI] [PubMed] [Google Scholar]
- 16. Loh KP, Xu H, Epstein RM et al. Associations of caregiver‐oncologist discordance in prognostic understanding with caregiver‐reported therapeutic alliance and anxiety. J Pain Symptom Manage 2020;60:20–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Kehoe LA, Xu H, Duberstein P et al. Quality of life of caregivers of older patients with advanced cancer. J Am Genet Soc 2019;67:969–977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Chan EY, Glass G, Chua KC et al. Relationship between mastery and caregiving competence in protecting against burden, anxiety and depression among caregivers of frail older adults. J Nutr Health Aging 2018;22:1238–1245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Ryff CD. Happiness is everything, or is it? Explorations on the meaning of psychological well‐being. J Pers Soc Psychol 1989;57:1069–1081. [Google Scholar]
- 20. Mausbach BT, Roepke SK, Chattillion EA et al. Multiple mediators of the relations between caregiving stress and depressive symptoms. Aging Ment Health 2012;16:27–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Sherwood PR, Given BA, Given CW et al. The influence of caregiver mastery on depressive symptoms. J Nurs Scholarsh 2007;39:249–255. [DOI] [PubMed] [Google Scholar]
- 22. Nijboer C, Tempelaar R, Triemstra M et al. The role of social and psychologic resources in caregiving of cancer patients. Cancer 2001;91:1029–1039. [PubMed] [Google Scholar]
- 23. Pandya C, Magnuson A, Flannery M et al. Association between symptom burden and physical function in older patients with cancer. J Am Geriatr Soc 2019;67:998–1004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Kehoe LA, Xu H, Duberstein P et al. Quality of life of caregivers of older patients with advanced cancer. J Am Geriatr Soc 2019;67:969–977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Loh KP, Mohile SG, Epstein RM et al. Willingness to bear adversity and beliefs about the curability of advanced cancer in older adults. Cancer 2019;125:2506–2513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Fried TR, Bradley EH, O'Leary J. Changes in prognostic awareness among seriously ill older persons and their caregivers. J Pallat Med 2006;9:61–69. [DOI] [PubMed] [Google Scholar]
- 27. Kroenke K, Spitzer RL, Williams JB. The Patient Health Questionnaire‐2: Validity of a two‐item depression screener. Med Care 2003;41:1284–1292. [DOI] [PubMed] [Google Scholar]
- 28. Hurria A, Li D, Hansen K et al. Distress in older patients with cancer. J Clin Oncol 2009;27:4346–4351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Ware J, Jr. , Kosinski M, Keller SD. A 12‐item Short‐Form Health Survey: Construction of scales and preliminary tests of reliability and validity. Med Care 1996;34:220–233. [DOI] [PubMed] [Google Scholar]
- 30. Costanzo ES, Ryff CD, Singer BH. Psychosocial adjustment among cancer survivors: Findings from a national survey of health and well‐being. Health Psychol 2009;28:147–156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Chan EY, Glass G, Chua KC et al. Relationship between mastery and caregiving competence in protecting against burden, anxiety and depression among caregivers of frail older adults. J Nutr Health Aging 2018;22:1238–1245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Hagerty RG, Butow PN, Ellis PA et al. Cancer patient preferences for communication of prognosis in the metastatic setting. J Clin Oncol 2004;22:1721–1730. [DOI] [PubMed] [Google Scholar]
- 33. Enzinger AC, Zhang B, Schrag D et al. Outcomes of prognostic disclosure: Associations with prognostic understanding, distress, and relationship with physician among patients with advanced cancer. J Clin Oncol 2015;33:3809–3816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Wright AA, Zhang B, Ray A et al. Associations between end‐of‐life discussions, patient mental health, medical care near death, and caregiver bereavement adjustment. JAMA 2008;300:1665–1673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Yeh PM, Wierenga ME, Yuan SC. Influences of psychological well‐being, quality of caregiver‐patient relationship, and family support on the health of family caregivers for cancer patients in Taiwan. Asian Nurs Res (Korean Soc Nurs Sci) 2009;3:154–166. [DOI] [PubMed] [Google Scholar]
- 36. Hsu T, Loscalzo M, Ramani R et al. Are disagreements in caregiver and patient assessment of patient health associated with increased caregiver burden in caregivers of older adults with cancer? The Oncologist 2017;22:1383–1391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Mollica MA, Litzelman K, Rowland JH et al. The role of medical/nursing skills training in caregiver confidence and burden: A canCORS study. Cancer 2017;123:4481–4487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Kadambi S, Loh KP, Dunne R et al. Older adults with cancer and their caregivers: Current landscape and future directions for clinical care. Nat Rev Clin Oncol 2020;17:742–755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Temel JS, Greer JA, Admane S et al. Longitudinal perceptions of prognosis and goals of therapy in patients with metastatic non‐small‐cell lung cancer: Results of a randomized study of early palliative care. J Clin Oncol 2011;29:2319–2326. [DOI] [PubMed] [Google Scholar]
- 40. Greer JA, Jacobs JM, El‐Jawahri A et al. Role of patient coping strategies in understanding the effects of early palliative care on quality of life and mood. J Clin Oncol 2018;36:53–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Schwarz KA, Mion LC, Hudock D et al. Telemonitoring of heart failure patients and their caregivers: A pilot randomized controlled trial. Prog Cardiovasc Nurs 2008;23:18–26. [DOI] [PubMed] [Google Scholar]
- 42. Chiu M, Wesson V, Sadavoy J. Improving caregiving competence, stress coping, and mental well‐being in informal dementia carers. World J Psychiatry 2013;3:65–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Basch E, Deal AM, Kris MG et al. Symptom monitoring with patient‐reported outcomes during routine cancer treatment: A randomized controlled trial. J Clin Oncol 2016;34:557–565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Nipp RD, Horick NK, Deal AM et al. Differential effects of an electronic symptom monitoring intervention based on the age of patients with advanced cancer. Ann Oncol 2020;31:123–130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Absolom K, Warrington L, Hudson E et al. Phase III randomized controlled trial of eRAPID: Ehealth intervention during chemotherapy. J Clin Oncol 2021:JCO2002015. [DOI] [PubMed] [Google Scholar]
- 46. Hendricks BA, Lofton C, Azuero A et al. The project ENABLE cornerstone randomized pilot trial: Protocol for lay navigator‐led early palliative care for african‐american and rural advanced cancer family caregivers. Contemp Clin Trials Commun 2019;16:100485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Dionne‐Odom JN, Ejem DB, Wells R et al. Effects of a telehealth early palliative care intervention for family caregivers of persons with advanced heart failure: The ENABLE CHF‐PC randomized clinical trial. JAMA Netw Open 2020;3:e202583. [DOI] [PMC free article] [PubMed] [Google Scholar]


