Abstract
Lessons Learned
Despite the initial optimism for using immune checkpoint inhibition in the treatment of multiple myeloma, subsequent clinical studies have been disappointing.
Preclinical studies have suggested that priming the immune system with various modalities in addition to checkpoint inhibition may overcome the relative T‐cell exhaustion or senescence; however, in this small data set, radiotherapy with checkpoint inhibition did not appear to activate the antitumor immune response.
Background
Extramedullary disease (EMD) is recognized as an aggressive subentity of multiple myeloma (MM) with a need for novel therapeutic approaches. We therefore designed a proof‐of‐principle pilot study to evaluate the synergy between the combination of the anti–PD‐L1, avelumab, and concomitant hypofractionated radiotherapy.
Methods
This was a single‐arm phase II Simon two‐stage single center study that was prematurely terminated because of the COVID‐19 pandemic after enrolling four patients. Key eligibility included patients with relapsed/refractory multiple myeloma (RRMM) who had exhausted or were not candidates for standard therapy and had at least one lesion amenable to radiotherapy. Patients received avelumab until progression or intolerable toxicity and hypofractionated radiotherapy to a focal lesion in cycle 2. Radiotherapy was delayed until cycle 2 to allow the avelumab to reach a study state, given the important observation from previous studies that concomitant therapy is needed for the abscopal effect.
Results
At a median potential follow‐up of 10.5 months, there were no objective responses, one minimal response, and two stable disease as best response. The median progression‐free survival (PFS) was 5.3 months (95% confidence interval [CI]: 2.5–7.1 months), and no deaths occurred. There were no grade ≥3 and five grade 1–2 treatment‐related adverse events.
Conclusion
Avelumab in combination with radiotherapy for patients with RRMM and EMD was associated with very modest systemic clinical benefit; however, patients did benefit as usual from local radiotherapy. Furthermore, the combination was very well tolerated compared with historical RRMM treatment regimens.
Keywords: Avelumab, Radiotherapy, Abscopal effect, Multiple myeloma
Discussion
Immunotherapy with PD‐1/L1 checkpoint inhibition has led to a revolution in the treatment of various malignancies—directly translating to longer patient survival. However, to date, PD‐1/L1 inhibition in the treatment of MM has not shown similar success. We hypothesized that radiotherapy might synergize with PD‐1/L1 inhibition and lead to more significant tumor responses via an abscopal effect. However, in this small study (Fig. 1), antitumor responses with the combination appeared modest. At the data cutoff date of November 1, 2020, all four enrolled patients had discontinued therapy because of progression. Three patients received 5 Gy of radiation to the EMD site for 5 days, and one patient received 2 Gy for 5 days because of a lesion on the skull. At a median potential follow‐up of 10.5 months, the overall response rate (ORR) was 0. One patient had a minimal response, and two patients had stable disease as their best response, resulting in a clinical benefit rate of 75.0% (95% CI: 19.4%–99.4%). The median PFS was 5.3 months (95% CI: 2.5–7.1 months) with a 6‐month PFS rate of 50% (95% CI: 5.8%–84.4%). At the time of this analysis, all patients were alive. Treatment was well tolerated with no grade ≥3 treatment‐related adverse events.
A major limitation of this study is the small sample size, and the question remains whether we would observe deep responses in a small subset of patients if we had enrolled more patients. Altogether, given our findings and those of previous studies, anti–PD‐1/L1 combination regimens (with immunomodulatory drugs or radiotherapy) do not appear to be synergistic in the clinical setting. However, one major issue still remains, namely, the ideal radiotherapy strategy in terms of not only dose, fractionation, timing, and duration, but also of whether multiple sites of radiation are needed to adequately prime the immune system and induce tumor‐associated antigens. Therefore, it is unknown whether, in the near future, PD‐1/L1 inhibitors will have a role in the treatment of MM.
Trial Information
| Disease | Multiple myeloma |
| Stage of Disease/Treatment | Metastatic/advanced |
| Prior Therapy | More than two prior regimens |
| Type of Study | Phase II, single arm |
| Primary Endpoint | Overall response rate |
| Secondary Endpoints | Complete response rate, progression‐free survival, overall survival, tolerability |
| Additional Details of Endpoints or Study Design | The statistical design incorporated a two‐stage Simon minimax design where the first stage would enroll 13 patients, and if one response occurred, the study would proceed to the second stage for a total enrollment of 27 patients. The study would be considered a success if four total responses occurred (14.7%), with an alpha of 5% and a power of 80%. |
| Response Definition: The 2014 International Myeloma Working Group (IMWG) response criteria for multiple myeloma was used. In brief, response criteria are as follows: | |
| Stringent complete response (CR): CR with normalization of serum‐free light chains | |
| CR: No detectable monoclonal protein by serum protein electrophoresis and immunofixation | |
| Very good partial response (PR): 90% improvement in M‐protein | |
| PR: 50% improvement in M‐protein | |
| Minimal response: 25% improvement in M‐protein | |
| Stable disease (SD): Neither PR nor progressive disease (PD) | |
| PD: 25% increase in M‐protein | |
| In terms of ORR, a PR or better is required. In cases with light chain–only disease, the difference between involved and uninvolved light chains is used to gauge response. | |
| Investigator's Analysis | Study prematurely terminated because of the COVID‐19 pandemic, with only modest activity in a small number of patients. |
Drug Information
| Generic Name | Avelumab |
| Trade Name | Bavencio |
| Company Name | EMD Serono, Inc. |
| Drug Type | Antibody |
| Drug Class | Immune therapy |
| Dose | 800 milligrams (mg) per flat dose |
| Route | i.v. |
| Schedule of Administration | Days 1 and 15 of every cycle (28‐day cycles) until progression or intolerable toxicity |
| Generic Name | Hypofractionated radiation therapy |
| Schedule of Administration | Patients received focal hypofractionated radiation therapy on cycle 2 days 1–5 at a goal strategy of 5 Gy daily for 5 days (adjusted at the radiation oncologist's discretion). |
Patient Characteristics
| Number of Patients, Male | 2 |
| Number of Patients, Female | 2 |
| Stage | Revised International Staging System for Myeloma: stage 1: 4 (100%) |
| Age | Median (range): 68 (62–83), years |
| Number of prior systemic therapies | Median (range): 3 (2–4) |
| Performance Status: ECOG |
0 —— 2 (50%) 1 —— 2 (50%) |
| Cytogenetics, n (%) |
Normal: 2 (50%) Hyperdiploidy: 1 (25%) Deletion 13q: 1 (25%) |
| Immunoglobulin isotype, n (%) |
IgG Kappa: 2 (50%) IgG Lambda: 1 (25%) IgD Kappa: 1 (25%) |
Primary Assessment Method
| Title | Response Rate |
| Number of Patients Screened | 9 |
| Number of Patients Enrolled | 4 |
| Number of Patients Evaluable for Toxicity | 4 |
| Number of Patients Evaluated for Efficacy | 4 |
| Evaluation Method | IMWG Response Criteria |
| Response Assessment SD | n = 2 (50%) |
| Response Assessment PD | n = 1 (25%) |
| Response Assessment OTHER | n = 1 (25%) |
| (Median) Duration Assessments PFS | 5.3 months, CI: 2.5–7.1 |
| Waterfall plot (Figure 2 ) | Waterfall plot of best percent change in M‐protein values and IMWG response. One patient had disease progression; two had stable disease, and one had a minimal response, based on M‐protein. There were no partial responses. |
| Outcome Notes | The 2014 IMWG response criteria for multiple myeloma were used. |
Figure 2.
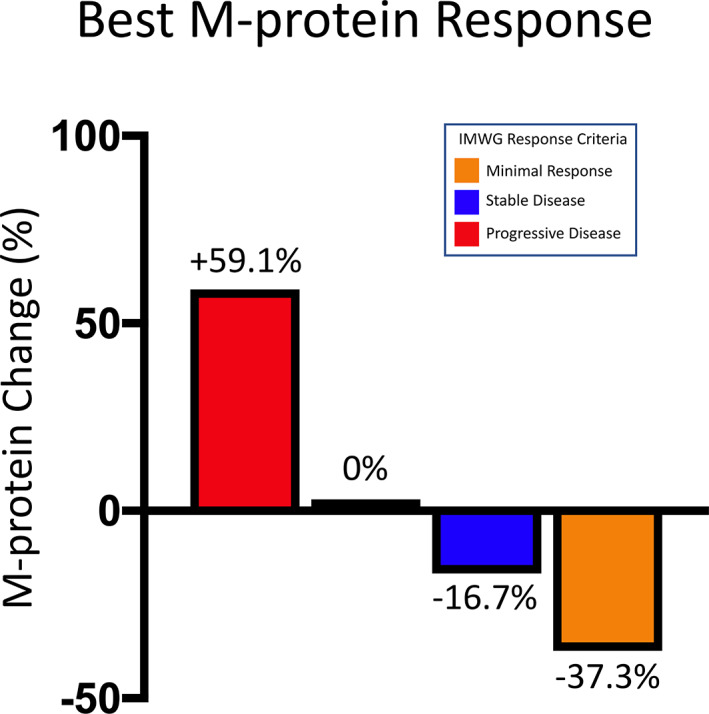
Waterfall plot of best percent change in M‐protein values and IMWG response. Abbreviation: IMWG, International Myeloma Working Group.
Adverse Events
| All Cycles | |||||||
|---|---|---|---|---|---|---|---|
| Name | NC/NA, % | Grade 1, % | Grade 2, % | Grade 3, % | Grade 4, % | Grade 5, % | All grades, % |
| Alanine aminotransferase increased | 75 | 0 | 25 | 0 | 0 | 0 | 25 |
| Aspartate aminotransferase increased | 75 | 0 | 25 | 0 | 0 | 0 | 25 |
| Pain | 75 | 25 | 0 | 0 | 0 | 0 | 25 |
| Arthralgia | 75 | 25 | 0 | 0 | 0 | 0 | 25 |
| Rash maculopapular | 75 | 25 | 0 | 0 | 0 | 0 | 25 |
| Adverse Events Legend | |||||||
| All‐grade treatment‐emergent adverse events occurring during any cycle | |||||||
Abbreviation: NC/NA, no change from baseline/no adverse event.
Assessment, Analysis, and Discussion
| Completion | Did not fully accrue; study terminated before completion |
| Investigator's Assessment | Study prematurely terminated because of the COVID‐19, with only modest activity in a small number of patients. |
Multiple myeloma (MM) remains incurable, with a median survival of 5–8 years [1]. Because of the recent therapeutic advances and development of highly efficacious MM regimens, patients more frequently develop extramedullary disease (EMD) during the longer disease course. EMD, now recognized as an aggressive subentity of MM, is characterized by the ability of a MM subclone to proliferate independently of the bone marrow microenvironment either by direct invasion from the medullary compartment disrupting the cortical bone or less commonly by hematogenous metastatic spread [2, 3]. Approximately 15% of patients newly diagnosed with MM present with EMD, which is associated with shorter progression‐free survival (PFS) and overall survival (OS) [4, 5]. Therefore, novel approaches to treatment are needed for this growing population of patients.
Pathways that inhibit antitumor T‐cell responses include the activation of the inhibitory programmed cell death 1 (PD‐1) and PD‐ligand 1 (PD‐L1) axis, allowing tumors to evade the immune system. Despite the success of monotherapy PD‐1/L1 inhibitors in solid tumors and promising preclinical results, antitumor activity in MM has been modest [6, 7, 8, 9]. Preclinical studies have shown that the combination of radiotherapy and PD‐1/L1 blockade may enhance antitumor activity by increasing interferon gamma, tumor antigen cross presentation, T‐cell receptor clonality, and PD‐L1 expression and reinvigorating tumor infiltrating lymphocytes while decreasing immunosuppressive myeloid derived suppressor and regulatory T cells [10]. Synergy between radiation and PD‐1 pathway inhibitors has been observed in breast, colon, melanoma, and glioma tumor models [11, 12, 13, 14]. We hypothesized that targeted radiation to a site of EMD may change the MM microenvironment niche to sensitize myeloma cells to PD‐1/L1 inhibition and activate systemic antitumor immune responses. We designed a proof‐of‐principle pilot clinical study to evaluate the combination of avelumab, an anti–PD‐L1 IgG1 monoclonal antibody, approved by the U.S. Food and Drug Administration for the treatment of Merkel cell, urothelial, and renal cell carcinomas, with concomitant hypofractionated radiotherapy.
Patients were enrolled in this single‐arm phase II single center study (NCT03910439) between April 10, 2019, and November 1, 2020. The study was prematurely terminated after enrolling four patients because of poor accrual in the face of the COVID‐19 pandemic. The study was approved by the National Cancer Institute Institutional Review Board, and patients provided written informed consent. Patients had documented relapsed/refractory MM and (a) had progressed on two or more prior lines of therapy and (b) had exhausted, or were not candidates for, additional MM therapy. Other key eligibility criteria included having (c) at least one extramedullary or lytic lesion deemed a candidate for radiotherapy by a radiation oncologist, (d) measurable disease, and (e) adequate organ function. Patients received a fixed dose of avelumab 800 mg i.v. on days 1 and 15 of every cycle (28‐day cycles) until progression or intolerable toxicity (Fig. 1). Additionally, patients received focal hypofractionated radiation therapy on cycle 2 days 1–5 at a goal strategy of 5 Gy daily for 5 days (adjusted at the radiation oncologist's discretion). The primary endpoint was to determine the overall response rate (ORR) according to the International Myeloma Working Group Response Criteria. Secondary endpoints included determination of the complete response rate, PFS, OS, and tolerability captured by National Cancer Institute Common Terminology for Adverse Events. Serum protein electrophoresis and immunofixation were assessed at baseline and at the start of every cycle.
Figure 1.
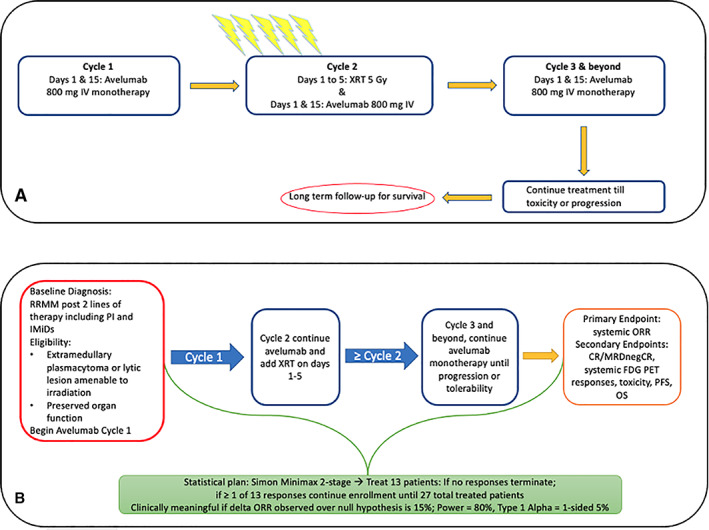
Avelumab in combination with radiation therapy in relapsed refractory multiple myeloma. (A): Study design. (B): Eligibility, endpoints, and statistical plan. Abbreviations: CR, complete response; FDG PET, fluorodeoxyglucose–positron emission tomography; IMiD, immunomodulatory drug; IV, intravenous; MRDnegCR, minimal residual disease negative complete response; ORR, overall response rate; OS, overall survival; PFS, progression‐free survival; PI, proteosome inhibitor; RRMM, relapsed/refractory multiple myeloma; XRT, radiation therapy.
At the data cutoff date of November 1, 2020, all four patients had discontinued therapy because of progression. All patients received avelumab as scheduled. Three patients received 5 Gy of radiation to the EMD site for 5 days, and one patient received 2 Gy for 5 days because of a lesion on the skull. At a median potential follow‐up of 10.5 months, the ORR was 0. One patient had a minimal response, and two patients had stable disease as their best response, resulting in a clinical benefit rate of 75.0% (95% confidence interval [CI]: 19.4%–99.4%). The median PFS was 5.3 months (95% CI: 2.5–7.1 months) with a 6‐month PFS rate of 50% (95% CI: 5.8%–84.4%). At the time of data cutoff, all patients were alive. Treatment was well tolerated with no grade ≥3 treatment‐related adverse events. In total, five grade 1–2 treatment‐related adverse events occurred in two of four patients and included alanine/aspartate aminotransferase elevation, arthralgia, general pain, and maculopapular rash.
Immunotherapy with PD‐1/L1 checkpoint inhibition has led to a revolution in the treatment of various malignancies—directly translating to longer patient survival. However, to date, PD‐1/L1 inhibition in the treatment of MM has not shown similar success. We hypothesized that radiotherapy might synergize with PD‐1/L1 inhibition and lead to more significant tumor responses via an abscopal effect. However, in this small study, antitumor responses with the combination appeared modest. No patients attained a partial response or better, and the median PFS was 5.3 months.
Recently, the abscopal effect and the use of radiotherapy as a combination partner “drug” has been of great interest with mixed conclusions, and more than 200 studies have been initiated [15]. Since the concept and implementation of our study, further results have been published regarding the abscopal effect, notably in solid cancers, as a potential mechanism of augmenting immune checkpoint inhibitor efficacy results. Interestingly, out of the vast number of studies, fewer than 10 studies have been noted to allow for delivery of radiotherapy to >1 lesion. As Brooks and Chang discuss, one focal site might not be sufficient to induce tumor‐associated antigens resulting in modest or no improvements observed with abscopal studies to date [15]. Response rates in radiotherapy combination arms have been similar to checkpoint inhibitor monotherapy arms. For example, McBride et al. compared anti–PD‐1 therapy (nivolumab) with and without stereotactic body radiotherapy in a randomized head and neck cancer study, but the combination did not improve outcomes [16]. Therefore, moving forward, perhaps a wiser strategy will be to irradiate as many lesions as possible in combination studies rather than a single focal site. Therefore, outside of a clinical trial, combination therapy to induce the abscopal effect should not be pursued unless patients have a clear indication (symptomatic or progressive lesions) for palliative radiotherapy [17]. Although our study allowed for irradiation of multiple sites as clinically indicated, the small sample size does not allow us to make any conclusions with our combination and single versus multiple sites of irradiation. In terms of relapsed/refractory MM specifically, irradiating all sites may be problematic, as patients with a heavy EMD burden may not be able to wait long enough for delayed immunotherapy responses. Furthermore, decreased bone marrow function and cytopenias from both MM and prior therapies may be problematic in this patient population and only exacerbated by multiple sites of irradiation.
A major limitation of this study is the small sample size, and the question remains whether we would observe deep responses in a small subset of patients if we had enrolled more patients. Given our and others’ findings, it is unknown whether immune checkpoint inhibitor combination regimens will synergize to produce meaningful clinical benefit. Therefore, excluding clinical trials, PD‐1/L1 inhibitors are unlikely to have a role in the MM clinic in the near future.
Disclosures
The authors indicated no financial relationships.
Acknowledgments
We acknowledge additional contributions by the following people: Christine Bryla (Office of Research Nursing); Yong Zhang, Ph.D. (Multiple Myeloma Program, Lymphoid Malignancies Branch); Jeffrey Schlom, Ph.D. (Laboratory of Tumor Immunology and Biology); James Gulley, M.D., Ph.D. (Genitourinary Malignancies Branch); Mark Roschewski, M.D. and Wyndham Wilson (Lymphoid Malignancies Branch); and Jane Trepel, Ph.D., Min‐Jung Lee, Ph.D., and Sunmin Lee, Ph.D. (Developmental Therapeutics Branch at the Center for Cancer Research, National Cancer Institute, National Institutes of Health, Bethesda, MD); and Crystal Lu, Pharm.D. (Department of Pharmacy) and Weixin Wang, Ph.D. (Hematology Service, Department of Laboratory Medicine) at the National Institutes of Health, Bethesda, MD. This work was supported by the National Institutes of Health, Intramural Research Program. This research was financially supported and avelumab was provided by Merck KGaA, Darmstadt, Germany, as part of an alliance between Merck KGaA and Pfizer. Merck KGaA, Darmstadt, Germany, and Pfizer reviewed the manuscript for medical accuracy only before journal submission. The authors are fully responsible for the content of this manuscript, and the views and opinions described in the publication reflect solely those of the authors.
No part of this article may be reproduced, stored, or transmitted in any form or for any means without the prior permission in writing from the copyright holder. For information on purchasing reprints contact commercialreprints@wiley.com.
Footnotes
- ClinicalTrials.gov Identifier: NCT03910439
- Sponsors: Center for Cancer Research, National Cancer Institute, NIH
- Principal Investigator: Dickran Kazandjian
- IRB Approved: Yes
References
- 1. Kazandjian D. Multiple myeloma epidemiology and survival: A unique malignancy. Semin Oncol 2016;43:676–681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Bladé J, Fernández de Larrea C, Rosiñol L et al. Soft‐tissue plasmacytomas in multiple myeloma: Incidence, mechanisms of extramedullary spread, and treatment approach. J Clin Oncol 2011;29:3805–3812. [DOI] [PubMed] [Google Scholar]
- 3. Bhutani M, Foureau DM, Atrash S et al. Extramedullary multiple myeloma. Leukemia 2020;34:1–20. [DOI] [PubMed] [Google Scholar]
- 4. Wu P, Davies FE, Boyd K et al. The impact of extramedullary disease at presentation on the outcome of myeloma. Leukemia Lymphoma 2009;50:230–235. [DOI] [PubMed] [Google Scholar]
- 5. Usmani SZ, Heuck C, Mitchell A et al. Extramedullary disease portends poor prognosis in multiple myeloma and is over‐represented in high‐risk disease even in the era of novel agents. Haematologica 2012;97:1761–1767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Lesokhin AM, Ansell SM, Armand P et al. Nivolumab in patients with relapsed or refractory hematologic malignancy: Preliminary results of a phase Ib Study. J Clin Oncol 2016;34:2698–2704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Takezako N, Kosugi H, Matsumoto M et al. Pembrolizumab plus lenalidomide and dexamethasone in treatment‐naive multiple myeloma (KEYNOTE‐185): Subgroup analysis in Japanese patients. Int J Hematol 2020;112:640–649. [DOI] [PubMed] [Google Scholar]
- 8. Manasanch EE, Han G, Mathur R et al. A pilot study of pembrolizumab in smoldering myeloma: Report of the clinical, immune, and genomic analysis. Blood Adv 2019;3:2400–2408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Pianko MJ, Liu Y, Bagchi S et al. Immune checkpoint blockade for hematologic malignancies: A review. Stem Cell Invest 2017;4:32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Gong J, Le TQ, Massarelli E et al. Radiation therapy and PD‐1/PD‐L1 blockade: The clinical development of an evolving anticancer combination. J Immunother Cancer 2018;6:46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Deng L, Liang H, Burnette B et al. Irradiation and anti–PD‐L1 treatment synergistically promote antitumor immunity in mice. J Clin Invest 2014;124:687–695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Dovedi SJ, Adlard AL, Lipowska‐Bhalla G et al. Acquired resistance to fractionated radiotherapy can be overcome by concurrent PD‐L1 blockade. Cancer Res 2014;74:5458–5468. [DOI] [PubMed] [Google Scholar]
- 13. Sharabi AB, Nirschl CJ, Kochel CM et al. Stereotactic radiation therapy augments antigen‐specific PD‐1–mediated antitumor immune responses via cross‐presentation of tumor antigen. Cancer Immunol Res 2015;3:345–355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Twyman‐Saint Victor C, Rech AJ, Maity A et al. Radiation and dual checkpoint blockade activate non‐redundant immune mechanisms in cancer. Nature 2015;520:373–377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Brooks E, Chang J. Time to abandon single‐site irradiation for inducing abscopal effects. Nat Rev Clin Oncol 2019;16:123–135. [DOI] [PubMed] [Google Scholar]
- 16. McBride S, Sherman E, Jillian Tsai C et al. Randomized phase II trial of nivolumab with stereotactic body radiotherapy versus nivolumab alone in metastatic head and neck squamous cell carcinoma. J Clin Oncol 2021;39:30–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Seiwert T, Kiess A. Time to debunk an urban myth? The “abscopal effect” with radiation and anti–PD‐1. J Clin Oncol 2021;39:1–3. [DOI] [PubMed] [Google Scholar]


