Abstract
On June 10, 2020, the U.S. Food and Drug Administration (FDA) approved nivolumab (OPDIVO; Bristol Myers Squibb, New York, NY) for the treatment of patients with unresectable advanced, recurrent, or metastatic esophageal squamous cell carcinoma (ESCC) after prior fluoropyrimidine‐ and platinum‐based chemotherapy. Approval was based on the results of a single, randomized, active‐control study (ATTRACTION‐3) that randomized patients to receive nivolumab or investigator's choice of taxane chemotherapy (docetaxel or paclitaxel). The study demonstrated a significant improvement in overall survival (OS; hazard ratio = 0.77; 95% confidence interval: 0.62–0.96; p = .0189) with an estimated median OS of 10.9 months in the nivolumab arm compared with 8.4 months in the chemotherapy arm. Overall, fewer patients in the nivolumab arm experienced treatment‐emergent adverse events (TEAEs) of any grade, grade 3–4 TEAEs, and serious adverse events compared with the control arm. The safety profile of nivolumab in patients with ESCC was generally similar to the known safety profile of nivolumab in other cancer types with the following exception: esophageal fistula was identified as a new, clinically significant risk in patients with ESCC treated with nivolumab. Additionally, the incidence of pneumonitis was higher in the ESCC population than in patients with other cancer types who are treated with nivolumab. This article summarizes the FDA review of the data supporting the approval of nivolumab for the treatment of ESCC.
Implications for Practice
The approval of nivolumab for the treatment of adult patients with unresectable advanced, recurrent, or metastatic esophageal squamous cell carcinoma (ESCC) after prior fluoropyrimidine‐ and platinum‐based chemotherapy was based on an overall survival (OS) benefit from a randomized, open‐label, active‐controlled study called ATTRACTION‐3. Prior to this study, no drug or combination regimen had demonstrated an OS benefit in a randomized study for patients with ESCC after prior fluoropyrimidine‐ and platinum‐based chemotherapy.
Keywords: Esophageal squamous cell carcinoma, Nivolumab
Short abstract
This article summarizes the FDA review of the data supporting the approval of nivolumab for the treatment of recurrent or metastatic esophageal squamous cell carcinoma.
Introduction
In the U.S., esophageal cancer is estimated to be the 11th leading cause of cancer‐related death in 2020 [1]. Risk factors for esophageal cancer include the following: smoking, alcohol consumption, Barrett's esophagus, gastric reflux, caustic injury to the esophagus, history of head and neck cancer, and a history of radiation therapy [2]. Approximately 18,400 new cases of esophageal cancer are expected to be diagnosed and approximately 16,000 deaths are expected in 2020. The estimated 5‐year survival rate for U.S. patients with esophageal cancer is approximately 20% [3], and the 5‐year survival rate of advanced unresectable or metastatic esophageal cancer is 3.4% [2]. Esophageal adenocarcinoma is more common in Western populations [4], and esophageal squamous cell carcinoma (ESCC) accounts for less than 30% of all esophageal cancers in the U.S. [1]. Standard first‐line systemic therapy for advanced, metastatic disease consists of a fluoropyrimidine‐ and platinum‐based regimen, and single‐agent taxanes such as docetaxel or paclitaxel are typically used in the second‐line setting. Pembrolizumab is approved for the treatment of refractory microsatellite‐high/mismatch repair–deficient (MSI‐H/dMMR) solid tumors including ESCC, and in the second‐line setting for the treatment of patients with ESCC whose tumors express Programmed death‐ligand 1 (PD‐L1) (Combined Positive Score [CPS] ≥10). Additionally, pembrolizumab was approved on June 16, 2020, for the treatment of unresectable or metastatic tumor mutational burden–high (TMB‐H; ≥10 mutations/megabase) solid tumors.
Nivolumab is a recombinant monoclonal antibody that binds to the programmed death receptor‐1 (PD‐1) and blocks its interaction with its ligands PD‐L1 and PD‐L2, releasing PD‐1 pathway–mediated inhibition of the antitumor immune response. Nivolumab is approved for the treatment of various cancers, including melanoma, non‐small and small cell lung cancer, advanced renal cell carcinoma, Hodgkin lymphoma, squamous cell carcinoma of the head and neck, urothelial carcinoma, hepatocellular carcinoma, and MSI‐H/dMMR colorectal cancer.
A single study (ATTRACTION‐3) was submitted in support of the approval of nivolumab for the treatment of patients with unresectable advanced, recurrent, or metastatic ESCC after prior fluoropyrimidine‐ and platinum‐based chemotherapy. The results of this study have been published [5]. This article summarizes the FDA's review of the data submitted in the supplemental Biologics Licensing Application (sBLA) and the basis for approval of nivolumab for this new indication.
The ATTRACTION‐3 Study
Study Design
ATTRACTION‐3 was a multicenter, randomized, open‐label, active‐controlled study that enrolled patients with unresectable, recurrent, or metastatic ESCC who were refractory or intolerant to at least one fluoropyrimidine‐ and platinum‐based regimen. A total of 419 patients from 90 study sites in eight countries (Japan, Korea, Taiwan, U.K., U.S., Germany, Italy, and Denmark) were randomly allocated 1:1 to receive nivolumab 240 mg by intravenous infusion over 30 minutes every 2 weeks or investigator's choice of taxane chemotherapy consisting of either docetaxel (75 mg/m2 intravenously every 3 weeks) or paclitaxel (100 mg/m2 intravenously once a week for 6 weeks followed by 1 week off). Randomization was stratified by region (Japan vs. the rest of the world), number of organs with metastases (≤1 vs. ≥2), and PD‐L1 status (≥1% vs. <1% or indeterminate).
The study enrolled patients regardless of PD‐L1 status, but tumor specimens were prospectively evaluated by a central laboratory using the PD‐L1 IHC 28‐8 pharmDx assay. The study excluded patients who were considered refractory to or intolerant to taxane therapy, had brain metastases that were symptomatic or required treatment, had autoimmune disease, used systemic corticosteroids or immunosuppressants, or had apparent tumor invasion of organs adjacent to the esophageal tumor or had stents in the esophagus or respiratory tract.
The primary endpoint was overall survival (OS) and secondary endpoints were overall response rate (ORR) and progression‐free survival (PFS) as assessed by the investigator using RECIST v1.1 and duration of response. Tumor assessments were conducted every 6 weeks for 1 year, and every 12 weeks thereafter. With a planned sample size of 390 patients with 331 events (deaths), the study had 90% power to detect a hazard ratio for OS of 0.70 using a log‐rank test (at a two‐sided significance level of .05 [median improvement vs. control of 2.8 months]).
Results
A total of 419 patients were randomized to receive nivolumab (n = 210) or chemotherapy (n = 209). A total of 209 patients actually received treatment in the nivolumab arm and 208 in the control arm (65 patients with docetaxel and 143 patients with paclitaxel). Baseline demographic and disease characteristics, summarized in Table 1, were generally similar between the nivolumab arm and the control arm; however, there were some minor differences noted including a lower percentage of patients with Eastern Cooperative Oncology Group (ECOG) performance status 1 in the control arm compared with the nivolumab arm (49% compared with 52%), a higher percentage of patients with stages I–III in the control arm compared with the nivolumab arm (9% compared with 5%), and a lower percentage of patients aged ≥75 years in the nivolumab arm compared with the control arm (7% compared with 13%).
Table 1.
Patient and tumor characteristics
| Characteristics | Study arm | |||
|---|---|---|---|---|
| Nivolumab (n = 210) | Chemotherapy control | |||
| Chemotherapy pooled (n = 209) | Docetaxel (n = 65) | Paclitaxel (n = 144) | ||
| Sex | ||||
| Male | 179 (85) | 185 (89) | 56 (86) | 129 (90) |
| Age, years | ||||
| Mean (SD) | 62.8 (8.9) | 64.9 (9.3) | 65.5 (8.6) | 64.6 (9.7) |
| Median (min, max) | 64.0 (37, 82) | 67.0 (33, 87) | 67.0 (48, 81) | 67.0 (33, 87) |
| Age group, years | ||||
| ≥65 | 98 (47) | 124 (59) | 44 (68) | 80 (56) |
| ≥75 | 14 (7) | 28 (13) | 7 (11) | 21 (15) |
| Race | ||||
| White | 9 (4) | 9 (4) | 4 (6) | 5 (3.5) |
| Asian | 201 (96) | 200 (96) | 61 (94) | 139 (96.5) |
| Geographic region a | ||||
| Japan | 136 (65) | 138 (66) | 44 (68) | 94 (65) |
| Rest of World | 74 (35) | 71 (34) | 21 (32) | 50 (35) |
| ECOG status | ||||
| 0 | 101 (48) | 107 (51) | 38 (58.5) | 69 (48) |
| 1 | 109 (52) | 102 (49) | 27 (41.5) | 75 (52) |
| Lesion site | ||||
| Cervical esophagus | 5 (2) | 7 (3) | 3 (5) | 4 (3) |
| Thoracic esophagus | 84 (40) | 93 (45) | 30 (46) | 63 (44) |
| Cervical and thoracic esophagus | 3 (1) | 7 (3) | 1 (2) | 6 (4) |
| Unknown | 118 (56) | 102 (49) | 31 (48) | 71 (49) |
| Recurrent | ||||
| Yes | 103 (49) | 89 (43) | 31 (48) | 58 (40) |
| Disease stage (TNM) | ||||
| I–III | 11 (5) | 18 (9) | 7 (11) | 11 (7.6) |
| IV | 172 (82) | 168 (80) | 49 (75) | 119 (82.6) |
| Unknown | 27 (13) | 23 (11) | 9 (14) | 14 (9.7) |
| Number of organs with metastases a | ||||
| ≤1 | 89 (42) | 91 (43.5) | 30 (46) | 61 (42) |
| ≥2 | 121 (58) | 118 (56.5) | 35 (54) | 83 (58) |
| PD‐L1 expression a | ||||
| <1% | 109 (52) | 107 (51) | 30 (46) | 77 (54) |
| ≥1% | 101 (48) | 102 (49) | 35 (54) | 67 (47) |
| <5% | 136 (65) | 137 (66) | 41 (63) | 96 (67) |
| ≥5% | 74 (35) | 72 (34) | 24 (37) | 48 (33) |
| <10% | 146 (69.5) | 152 (73) | 47 (72) | 105 (73) |
| ≥10% | 64 (30.5) | 57 (27) | 18 (28) | 39 (27) |
Data are shown as n (%) unless otherwise noted.
Stratification factor for randomization.
Abbreviations: ECOG, Eastern Cooperative Oncology Group; PD‐L1, Programmed death‐ligand 1; TNM, tumor, node, metastasis staging system.
Efficacy
Efficacy results are summarized in Figure 1 and Table 2 (primary endpoint) as well as in Table 3 (secondary endpoints). The ATTRACTION‐3 study demonstrated a statistically significant improvement in OS but not in ORR or PFS. The results of protocol‐specified subgroup analyses were generally supportive of the primary outcomes but were considered by FDA to be exploratory as no alpha was allocated to these analyses.
Figure 1.
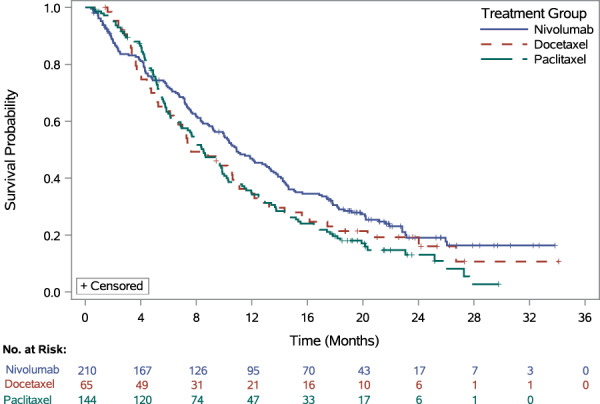
Kaplan‐Meier plot of overall survival (nivolumab): all randomized patients (intention to treat).
Table 2.
Overall survival: all randomized patients
| Efficacy parameter | All patients (ITT population) | |||
|---|---|---|---|---|
| Nivolumab | Control | |||
| Total | Docetaxel | Paclitaxel | ||
| n | 210 | 209 | 65 | 144 |
| OS | ||||
| Events, n (%) | 160 (76.2) | 173 (82.8) | 52 (80.0) | 121 (84.0) |
| Median (95% CI), months | 10.91 (9.23–13.34) | 8.38 (7.20–9.86) | 7.62 (6.11–10.68) | 8.51 (6.87–9.89) |
| HR (95% CI) | — | 0.77 a (0.62–0.96) | 0.78 b (0.56–1.07) | 0.76 c (0.60–0.97) |
| p value | — | .0189 d | — | — |
Stratified hazard ratio for nivolumab versus total control group.
Stratified hazard ratio for nivolumab versus docetaxel.
Stratified hazard ratio for nivolumab versus paclitaxel.
Two‐sided p‐value to test the difference between nivolumab and the total control group and compared with the significance level of .05.
Abbreviations: —,not applicable; CI, confidence interval; HR, hazard ratio; ITT, intention‐to‐treat; OS, overall survival.
Table 3.
Efficacy results: secondary endpoints
| Efficacy parameter | All patients (ITT) | |
|---|---|---|
| Nivolumab | Control | |
| RES population, n | 171 | 158 |
| Investigator ORR | ||
| Responders, n (%) | 33 (19.3) | 34 (21.5) |
| 95% CI | 13.7–26.0 | 15.4–28.8 |
| Odds ratio (95% CI) a | 0.88 (0.51–1.50) | |
| p value b | .6323 | |
| Investigator DoR | ||
| Median (95% CI), months | 6.93 (5.39–11.14) | 3.91 (2.79–4.17) |
| ITT population, n | 210 | 209 |
| Investigator PFS | ||
| Events, n (%) | 187 (89.0) | 176 (84.2) |
| Progression | 167 (79.5) | 162 (77.5) |
| Death | 20 (9.5) | 14 (6.7) |
| Median (95% CI), months | 1.68 (1.51–2.73) | 3.35 (2.99–4.21) |
| HR (95% CI) | 1.08 (0.87–1.34) | |
| 12‐month PFS rate (95% CI), % | 11.9 (7.8–16.8) | 7.2 (3.8–12.0) |
| 18‐month PFS rate (95% CI), % | 9.0 (5.5–13.6) | 4.0 (1.6–8.2) |
Stratified odds ratio.
p value based on stratified Cochran‐Mantel‐Haenszel test.
Abbreviations: CI, confidence interval; DoR, duration of response; HR, hazard ratio; ITT, intention‐to‐treat; ORR, overall response rate; PFS, progression‐free survival; RES, response‐evaluable set.
Safety
The primary safety population included 209 patients in the nivolumab arm and 208 patients in the chemotherapy control arm who received at least one dose of study drug in the ATTRACTION‐3 study. Fewer patients experienced treatment‐emergent adverse events (TEAEs), grade 3–4 TEAEs, treatment‐emergent serious adverse events, TEAEs leading to permanent discontinuation of study drug, and TEAEs leading to treatment delay in the nivolumab arm compared with the chemotherapy arm. Table 4 provides a summary of the TEAEs. The frequency of TEAEs leading to death was low and similar in both arms (5.3% in the nivolumab arm and 7.7% in the chemotherapy arm in the 100‐day window). The following fatal adverse reactions occurred in patients who received nivolumab up to 100 days after the last study dose: interstitial lung disease (ILD) or pneumonitis (1.4%), pneumonia (1.0%), sepsis or septic shock (1.0%), esophageal fistula (0.5%), gastrointestinal hemorrhage (0.5%), pulmonary embolism (0.5%), and sudden death (0.5%). The following fatal adverse reactions occurred during the 30‐day window from the last study dose in patients who received paclitaxel: pneumonia (1.4%), sepsis (0.7%), spinal abscess (0.7%), ILD (0.7%), tumor hemorrhage (0.7%), sudden death (0.7%), and hypercalcemia (0.7%); and in patients who received docetaxel: unknown (1.5%, narrative reported that the patient died during a hospitalization for grade 3 febrile neutropenia and grade 4 septic shock due to pneumonia). No new or unexpected adverse reactions were observed in patients who received nivolumab in the ATTRACTION‐3 study aside from esophageal fistula, which was not observed in studies supporting the approval of nivolumab for other cancers and may in part be related to the underlying disease. Overall, the incidence of immune‐mediated adverse reactions was similar to that seen in patients with other advanced solid tumors (e.g., colon, lung, and melanoma) treated with nivolumab as a single agent, but there was one exception: pneumonitis occurred at a higher incidence in patients with ESCC compared with the incidence reported in the Warning and Precautions section of the nivolumab U.S. Prescribing information (11% vs. 3%, respectively). To examine whether radiation therapy contributes to this observed difference, FDA performed an analysis of the incidence of pneumonitis by history of radiation therapy in patients in the nivolumab arm compared with the patients in the chemotherapy arm of the ATTRACTION‐3 study. In the ATTRACTION‐3 study, 72.7% and 68.3% of patients in the nivolumab and chemotherapy arm, respectively, had previously received radiation therapy; among this subgroup, the incidence of pneumonitis was 9.2% and 5.6% in the nivolumab and chemotherapy arms, respectively, compared with 7.0% and 7.6%, respectively, in the patients who did not receive radiation therapy.
Table 4.
Treatment‐emergent adverse events in ≥10% of patients receiving nivolumab a
| Adverse event | Nivolumab (n = 209) | Chemotherapy pooled (n = 208) | ||
|---|---|---|---|---|
| All grades, n (%) | Grades 3–4, n (%) | All grades, n (%) | Grades 3–4, n (%) | |
| Skin and subcutaneous tissue disorders | ||||
| Rash b | 48 (23) | 4 (2) | 58 (28) | 2 (1) |
| Pruritus | 25 (12) | 0 | 15 (7) | 0 |
| Gastrointestinal disorders | ||||
| Decreased appetite | 45 (22) | 5 (2) | 74 (36) | 12 (6) |
| Diarrhea c | 43 (21) | 5 (2) | 38 (18) | 4 (2) |
| Constipation | 37 (18) | 0 | 40 (19) | 0 |
| Hepatobiliary | 30 (14) | 15 (7) | 14 (7) | 6 (3) |
| Nausea | 23 (11) | 0 | 41 (20) | 1 (0.5) |
| Musculoskeletal and connective tissue disorders | ||||
| Musculoskeletal pain | 36 (17) | 1 (0.5) | 55 (26) | 3 (1) |
| Respiratory, thoracic, and mediastinal disorders | ||||
| Upper respiratory tract infection | 36 (17) | 2 (1) | 29 (14) | 0 |
| Cough | 35 (17) | 0 | 31 (15) | 1 (0.5) |
| Pneumonia d | 33 (15) e | 16 (8) | 49 (24) f | 30 (14) |
| Pneumonitis g | 22 (11) h | 5 (2) | 13 (6) i | 5 (2) |
| General disorders | ||||
| Pyrexia | 35 (17) | 1 (0.5) | 42 (20) | 1 (0.5) |
| Fatigue/asthenia | 26 (12) | 3 (1) | 57 (27) | 10 (5) |
| Blood and lymphatic system disorders | ||||
| Anemia | 29 (14) | 19 (9) | 63 (30) | 26 (13) |
| Endocrine disorders | ||||
| Hypothyroidism j | 23 (11) | 0 | 3 (1) | 0 |
Adverse events were collected between the start date of the first administration of the study drug and 28 days after the end of the treatment phase. Serious adverse events and immune‐mediated adverse events were collected during the treatment period and for 100 days following the last dose of study drug.
Includes urticaria, drug eruption, eczema, palmar‐plantar erythrodysesthesia syndrome, erythema, erythema multiforme, blister, skin exfoliation, Stevens‐Johnson syndrome, toxic skin eruption, dermatitis, dermatitis described as acneiform, bullous, or contact, and rash described as maculo‐papular, generalized, pustular, or pruritic.
Includes colitis and enterocolitis.
Includes pneumonia aspiration, pneumonia bacterial, lung infection, and Pneumocystis jirovecii.
Three patients (1%) died of pneumonia in the nivolumab group.
Seven patients (3%) died of pneumonia in the chemotherapy group during the 100‐day window. Five of the deaths were in the paclitaxel arm, and two deaths were in the docetaxel arm.
Includes interstitial lung disease and radiation pneumonitis.
Three patients (1%) died of pneumonitis in the nivolumab group during the 100‐day window, which is not an increase from the number of patients who died of pneumonitis in the nivolumab group during the 28‐day window.
One patient (0.5%) died of pneumonitis in the chemotherapy group during the 100‐day window, which is not an increase from the number of patients who died of pneumonitis in the chemotherapy group during the 28‐day window. This death occurred in the paclitaxel arm.
Includes blood thyroid‐stimulating hormone increased.
Discussion
The estimated 5‐year survival rate for U.S. patients with advanced unresectable or metastatic esophageal cancer is approximately 3.4% [2]. Aside from tumors with particular molecular characteristics (e.g., MSI‐H/dMMR or expressing PD‐L1 with a CPS ≥10), single‐agent taxanes are standard second‐line therapy for patients with advanced, metastatic disease. Prior to ATTRACTION‐3, no drug or combination regimen had demonstrated an overall survival benefit in a randomized study for patients with ESCC who had previously received a fluoropyrimidine‐ and platinum‐based regimen. Thus, this approval addresses an unmet medical need. The baseline demographic and disease characteristics generally reflect those expected for patients with advanced ESCC with minor differences across study arms (e.g., a lower percentage of patients with ECOG performance status 1 in the control arm compared with the nivolumab arm); FDA concluded that the magnitude of these differences did not likely impact the overall conclusions of this study. The data presented in this sBLA demonstrated a clinically meaningful and statistically significant improvement in overall survival in patients with ESCC randomized to receive nivolumab compared with those randomized to receive the investigator's choice of taxane chemotherapy (paclitaxel or docetaxel). As previously described, the discordance between the immunotherapy treatment arm and the control treatment arm at earlier timepoints for OS and PFS has been frequently observed in clinical studies investigating these agents versus chemotherapy. This is still an active area of research in the field, and one hypothesis postulated for this observation is the longer time to antitumor effect seen with immunotherapeutics compared with cytotoxic drugs [6]. Nivolumab was generally better tolerated than chemotherapy, but esophageal fistula is a clinically important safety event observed in this patient population. Additionally, the data suggest that the increased incidence of pneumonitis in patients receiving nivolumab is associated with prior receipt of radiation therapy for ESCC, but this relationship warrants further study because patients with ESCC may have other characteristics that may increase the risk pneumonitis (e.g., underlying lung disease, smoking history).
A key factor in our review of this supplemental application was the applicability of the study results to the U.S. population given that a majority of patients in the ATTRACTION‐3 study were enrolled in Japan, with only 18 Western patients (9 in each arm) enrolled. As noted above, ESCC is less common in the U.S., where esophageal adenocarcinoma predominates, compared with Asia. Although randomization was stratified by location (Japan vs. the rest of the world), subgroup analyses comparing the safety and effectiveness of nivolumab in Western or U.S. patients with ESCC with Asian patients with ESCC are of limited value. A review of the published literature suggests that there are similarities across regions (i.e., Western vs. Asia) with regard to disease features and treatment approaches for ESCC [7, 8, 9]. Additionally, data from studies of nivolumab with a broader representation of U.S. and Western patients in other disease settings do not suggest that regional differences lead to clinically meaningful differences in the efficacy of nivolumab across cancer types for which nivolumab is approved. Taken together, FDA considered that the evidence supported a conclusion that the underrepresentation of the U.S. population in the study did not compromise the applicability of study results to the U.S. population.
Another review issue is whether a subpopulation of patients defined by microsatellite or PD‐L1 status is driving the efficacy results. MSI‐H status and PD‐L1 expression are known predictors of response to immunotherapy in other clinical settings. In the ATTRACTION‐3 study, MSI status was available for approximately 30% of patients in the intention‐to‐treat set and all of these patients' tumors were microsatellite stable/mismatch repair proficient; thus, the role that MSI status may have on the efficacy of nivolumab in this setting was not directly evaluable. However, it is likely that the proportion of patients in this study who had MSI‐H tumors was overall very low given that none of the tested tumors were MSI‐H. Therefore, FDA concluded that there was a low likelihood that the results of the ATTRACTION‐3 study were driven by the presence of MSI‐H tumors. Regarding the potential impact of PD‐L1 expression on outcomes, randomization in ATTRACTION‐3 was stratified by PD‐L1 expression (≥1% vs. <1% or indeterminate). Exploratory subgroup analyses appeared to suggest a lower risk for death over time in patients with PD‐L1 expression ≥1%.
Conclusion
Treatment with nivolumab demonstrated a statistically significant and clinically meaningful improvement in overall survival in patients with unresectable advanced, recurrent, or metastatic ESCC after prior fluoropyrimidine‐ and platinum‐based chemotherapy. FDA's assessment of clinical meaningfulness is context dependent and generally considers factors such as study design, the clinical setting including the medical need for treatment options, and the benefit and risks of the treatment. The adverse reaction profile of nivolumab in patients with ESCC is generally consistent with the established safety profile for this biologic and was generally more tolerable compared with patients who received chemotherapy. The most important risks are immune‐mediated adverse reactions, which are largely manageable with patient surveillance, dose interruption, and supportive care. The risks of nivolumab are acceptable considering the life‐threatening nature of unresectable, recurrent, or metastatic ESCC in the second‐line setting. Taken together, FDA concluded that the benefit–risk assessment favored approval of nivolumab for this indication (Table 5).
Table 5.
Overall benefit–risk assessment
| Dimension | Evidence and uncertainties | Conclusions and reasons |
|---|---|---|
| Analysis of condition |
|
|
| Current treatment options |
|
|
| Benefit |
|
|
| Risk and risk management |
|
|
Abbreviations: CI, confidence interval; EAC, esophageal adenocarcinoma; ESCC, esophageal squamous cell carcinoma; HR, hazard ratio; OS, overall survival; PD‐L1, programmed death‐ligand 1.
Author Contributions
Conception/design: Lorraine Pelosof, May Tun Saung, Martha Donoghue, Sandra Casak, Sirisha Mushti, Joyce Cheng, Xiling Jiang, Jiang Liu, Hong Zhao, Maryam Khazraee, Kirsten B. Goldberg, Marc Theoret, Steven Lemery, Richard Pazdur, Lola Fashoyin‐Aje
Collection and/or assembly of data: Lorraine Pelosof, May Tun Saung, Martha Donoghue, Sandra Casak, Sirisha Mushti, Joyce Cheng, Xiling Jiang, Jiang Liu, Hong Zhao, Maryam Khazraee, Kirsten B. Goldberg, Marc Theoret, Steven Lemery, Richard Pazdur, Lola Fashoyin‐Aje
Data analysis and interpretation: Lorraine Pelosof, May Tun Saung, Martha Donoghue, Sandra Casak, Sirisha Mushti, Joyce Cheng, Xiling Jiang, Jiang Liu, Hong Zhao, Maryam Khazraee, Kirsten B. Goldberg, Marc Theoret, Steven Lemery, Richard Pazdur, Lola Fashoyin‐Aje
Manuscript writing: Lorraine Pelosof, May Tun Saung, Martha Donoghue, Sandra Casak, Sirisha Mushti, Joyce Cheng, Xiling Jiang, Jiang Liu, Hong Zhao, Maryam Khazraee, Kirsten B. Goldberg, Marc Theoret, Steven Lemery, Richard Pazdur, Lola Fashoyin‐Aje
Final approval of manuscript: Lorraine Pelosof, May Tun Saung, Martha Donoghue, Sandra Casak, Sirisha Mushti, Joyce Cheng, Xiling Jiang, Jiang Liu, Hong Zhao, Maryam Khazraee, Kirsten B. Goldberg, Marc Theoret, Steven Lemery, Richard Pazdur, Lola Fashoyin‐Aje
Disclosures
The authors indicated no financial relationships.
Disclosures of potential conflicts of interest may be found at the end of this article.
References
- 1. American Cancer Society . Cancer Statistics Center. Available at http://cancerstatisticscenter.cancer.org. Accessed May 18, 2020.
- 2. Zhang Y. Epidemiology of esophageal cancer. World J Gastroenterol 2013;19:5598–5606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Siegel RL, Miller KD, Jemal A. Cancer statistics, 2020. CA Cancer J Clin 2020;70:7–30. [DOI] [PubMed] [Google Scholar]
- 4. Torre LA, Siegel RL, Ward EM et al. Global cancer incidence and mortality rates and trends–An update. Cancer Epidemiol Biomarkers Prev 2016;25:16–27. [DOI] [PubMed] [Google Scholar]
- 5. Kato K, Cho BC, Takahashi M et al. Nivolumab versus chemotherapy in patients with advanced oesophageal squamous cell carcinoma refractory or intolerant to previous chemotherapy (ATTRACTION‐3): A multicentre, randomised, open‐label, phase 3 trial. Lancet Oncol 2019;20:1506–1517. [DOI] [PubMed] [Google Scholar]
- 6. Boland JL, Zhou Q, Martin M et al. Early disease progression and treatment discontinuation in patients with advanced ovarian cancer receiving immune checkpoint blockade. Gynecol Oncol 2019;152:251–258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Chen S, Zhou K, Yang L et al. Racial differences in esophageal squamous cell carcinoma: Incidence and molecular features. Biomed Res Int 2017;2017:1204082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Kato K, Tahara M, Hironaka S et al. A phase II study of paclitaxel by weekly 1‐h infusion for advanced or recurrent esophageal cancer in patients who had previously received platinum‐based chemotherapy. Cancer Chemother Pharmacol 2011;67:1265–1272. [DOI] [PubMed] [Google Scholar]
- 9. Mizota A, Shitara K, Kondo C et al. A retrospective comparison of docetaxel and paclitaxel for patients with advanced or recurrent esophageal cancer who previously received platinum‐based chemotherapy. Oncology 2011;81:237–242. [DOI] [PubMed] [Google Scholar]


