Abstract
The optimal management of advanced non‐small cell lung cancer (NSCLC) with noncanonical epidermal growth factor receptor (EGFR) mutations (i.e., exon 19 deletion and exon 21 L858R) is constrained by the heterogeneous behavior of individual uncommon mutations and limited prospective clinical data in this setting. Despite encouraging results with osimertinib from a recently published phase II trial from South Korea, afatinib remains the only currently approved drug for patients with tumors harboring uncommon EGFR mutations (i.e., S768I, L861Q, and/or G719X). When used at the standard dose of 40 mg daily, afatinib is associated with significant rates of treatment‐related adverse events, leading to frequent dose reductions and treatment discontinuations. We report a case of a woman with advanced NSCLC harboring EGFR‐G719A mutation treated with afatinib (at an off‐label pulse dose strategy that merits further evaluation in prospective studies) with sustained partial response for 20 months with manageable expected toxicities. Subsequent disease progression was mediated by off‐target pan‐EGFR inhibitor (including osimertinib)–resistant KRAS mutation and not by acquisition of EGFR‐T790M. We further present the current state of evidence in the literature behind use of first‐, second‐, and third‐generation tyrosine kinase inhibitors and summarize the evolving spectrum of activity ascribed to osimertinib (and newer EGFR inhibitors with a more favorable therapeutic window and intracranial penetration) in this population of patients with advanced NSCLC and uncommon EGFR mutations.
Key Points
Uncommon EGFR mutations characterize a heterogeneous group of patients with advanced non‐small cell lung cancer (NSCLC).
Afatinib is the only currently U.S. Food and Drug Administration–approved drug for management of advanced NSCLC with uncommon EGFR mutations (S768I, L861Q, and/or G719X).
Afatinib treatment at 40 mg daily is associated with high rates of adverse events and dose reductions; alternative strategies including pulse intermittent dosing should be evaluated prospectively.
Osimertinib (with favorable safety profile and intracranial penetration) has shown promising results in this population in a phase II trial from South Korea; additional trials are ongoing.
Short abstract
This article presents the case of patient with advanced non‐small cell lung cancer harboring an EGFR‐G719A mutation treated with afatinib and reviews the literature regarding the use of first‐, second‐, and third‐generation tyrosine kinase inhibitors in this patient population.
Introduction
The discovery of sensitizing mutations in the epidermal growth factor receptor (EGFR) gene and their antagonism with tyrosine kinase inhibitors (TKIs) has transformed the therapeutic landscape of advanced non‐small cell lung cancer (NSCLC) and kickstarted the era of precision oncology [1, 2]. First‐generation (gefitinib and erlotinib), second‐generation (afatinib and dacomitinib), and subsequently third‐generation (osimertinib) EGFR inhibitors have all been approved for first‐line treatment of advanced NSCLC harboring the two most common EGFR mutations (exon 19 deletion and exon 21 L858R), which account for 80% + of all EGFR‐positive lung cancers [3, 4]. Other less common but consistently occurring EGFR mutations in exons 18–21 are well established in NSCLC: exon 18 indels, G719X, exon 19 insertions, exon 20 S786I, exon 21 L861Q, exon 20 insertions, compound mutations, exon 18–25 kinase domain duplications, and rearrangements (EGFR‐RAD51 and EFFR‐PURB) (Fig. 1); however most clinical trials have excluded these subsets [3, 5].
Figure 1.
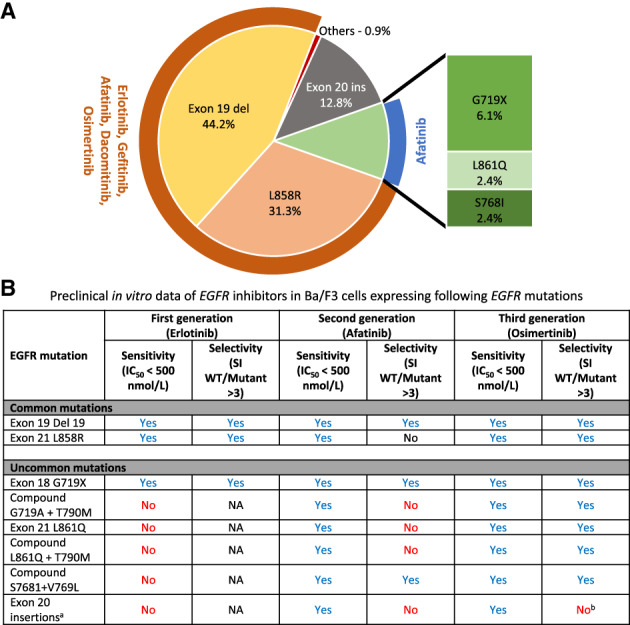
Uncommon EGFR mutations. (A): Frequency of individual mutations in EGFR‐mutated lung cancer calculated from [5]. Others include EGFR fusions, exon 19 insertion, and exon 18–25 kinase domain duplication. Note: Drugs listed in orange and blue are approved for classic and uncommon EGFR mutations, respectively. (B): Preclinical data on in vitro sensitivity of EGFR inhibitors in Ba/F3 cells expressing EGFR mutations. The content of the data is adapted from original research data of our group's prior publication, as detailed in reference [6].Abbreviations: del, deletion; IC50, half maximal inhibitory concentration; NA, not applicable; SI, selectivity index; WT, wild type.
Afatinib is currently the only U.S. Food and Drug Administration–approved agent for use against these noncanonical EGFR mutations (i.e., S768I, L861Q, and/or G719X). However, its use in real‐world settings is tempered by significant mucocutaneous toxicities, often necessitating dose reductions and/or treatment interruption/discontinuation at the approved dose of 40 mg daily. Here, we describe a case of a patient with advanced NSCLC with an uncommon EGFR mutation who was treated with pulse dose weekly afatinib with durable and tolerable disease control and review the relevant literature.
Patient Story
A 66‐year‐old woman of Chinese ethnicity with no history of tobacco exposure was found to have a right lower lobe (RLL) lung mass on a chest radiograph. She had intermittent dry cough but no other respiratory or systemic symptoms. Further evaluation with computed tomography (CT) of the chest and positron emission tomography (PET)/CT showed the RLL lung mass with right hilar adenopathy and additional pulmonary and bony metastases (Fig. 2). Magnetic resonance imaging of the brain showed no evidence of intracranial metastases. Fine needle aspiration of the RLL lung mass and level 7 lymph node showed adenocarcinoma of lung origin, also confirmed on left iliac biopsy and thus establishing stage IVB lung adenocarcinoma.
Figure 2.
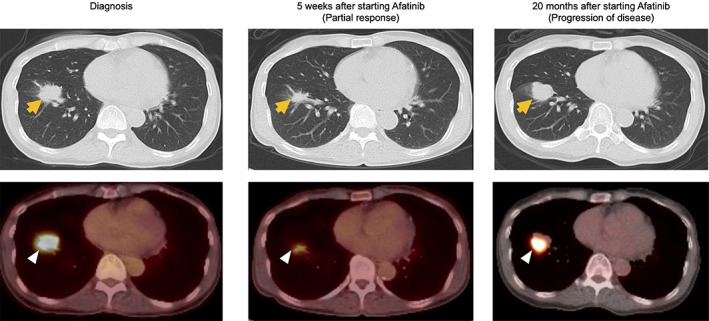
Radiographic findings from diagnosis, and response‐assessment at 5 weeks and 20 months after initiation of pulse dose afatinib. Yellow arrows represent lung findings on computed tomography chest and white arrowheads represent fluorodeoxyglucose‐avid areas on positron emission tomography scan.
Molecular Tumor Board
Comprehensive tumor genomic profiling (FoundationOne CDx, Foundation Medicine, Cambridge, MA) of the tumor showed presence of EGFR‐G719A and S720F mutations; additional noted alterations included TP53‐Q331* (pathogenic per the Catalogue of Somatic Mutations database); amplification of EGFR, NFKBIA, and NKX2‐1 genes; loss of CDKN2A, CDKN2B, and MTAP genes; microsatellite stable status; and tumor mutational burden of six mutations per megabase.
EGFR‐G719X mutation in exon 18 is one of the more frequent mutations in the diverse group of uncommon EGFR mutations seen in NSCLC (Fig. 1). It is a point mutation that results in substitution of the amino acid glycine at position 719 with other amino acids—alanine (G719A in our patient's case), serine (G719D), or cysteine (G719C)—leading to constitutive activation of the EGFR receptor. S720F is another deleterious mutation in exon 18 that leads to substitution of serine with phenylalanine at position 720. Evaluation in mostly observational studies has yielded inconsistent results regarding the clinical activity of first‐generation EGFR TKIs in these patients, at least in part because of the simultaneous grouping of patients with molecularly heterogeneous tumors (preclinical data by our group reviewed in Fig. 1 [6], major studies reviewed in Table 1 [7, 8] and Table 2 [7, 8, 9, 10], and additional studies reviewed in supplemental online Table 1 [11, 12, 13, 14, 15, 16, 17, 18, 19, 20, 21, 22, 23, 24, 25, 26, 27, 28]). Preclinical experiments and computational analysis by other groups have suggested augmented sensitivity of EGFR‐G719A to afatinib compared with first‐ and third‐generation TKIs [29, 30]. Similar preclinical results were reported for EGFR‐S768I and other exon 18 (E709K and exon 18 deletion) mutations, whereas L861Q mutations are sensitive to both afatinib and osimertinib [29, 31]. Retrospective clinical studies in patients with advanced NSCLC with uncommon EGFR mutations have suggested improved progression‐free survival (PFS) on treatment with afatinib compared with first‐generation TKIs (supplemental online Table 1) [32, 33, 34, 35].
Table 1.
Major studies of EGFR inhibitors in patients with advanced non‐small cell lung cancer with uncommon EGFR mutations a
| First generation | Second generation | Third generation | ||||
|---|---|---|---|---|---|---|
| EGFR‐TKIs | Gefitinib | Afatinib | Osimertinib | |||
| Type of study |
Prospective Phase II single arm [7] |
Prospective Post hoc analysis of phase III randomized trial [8] |
Prospective Post hoc pooled analysis of three (phase II + phase III) trials [36] |
Prospective Subgroup analysis of single‐arm phase IIIb trial [40] b |
Prospective Pooled data from clinical trials and real world [41] TKI naïve/pretreated |
Prospective Phase II single arm [49] |
| n | 7 | 5 | 38 | 67 | 110 / 32 | 36 |
| ORR (%) | 0% | 20% |
71.1% (95% CI, 54.1–84.6) |
Not reported | 60% / 25% |
50% (95% CI, 33–67) |
| Median PFS | Not reported |
2.2 months (range, 0.5–10.6) |
10.7 months (95% CI, 5.6–14.7) |
9.1 months (95% CI, 5.6–13.6) |
Not reported |
8.2 months (95% CI, 5.9–10.5) |
| Median OS | Not reported |
11.9 months (range, 5.8–22.6) |
19.4 months (95% CI, 16.4–26.9) |
Not reported | Not reported | Not reached |
Retrospective studies have been summarized in supplemental online Table 1.
Included patients with EGFR exon 20 insertions and T790M mutation.
Abbreviations: CI, confidence interval; ORR, objective response rate; OS, overall survival; PFS, progression‐free survival; TKI, tyrosine kinase inhibitor.
Table 2.
Major studies of EGFR inhibitors in patients with advanced non‐small cell lung cancer with selected uncommon EGFR mutations
| First generation | Second generation | Third generation | |||||
|---|---|---|---|---|---|---|---|
| EGFR‐TKIs | Erlotinib/gefitinib | Gefitinib | Afatinib | Neratinib | Osimertinib | ||
| Type of study | Retrospective pooled [9, 10] |
Prospective phase II single arm [7] |
Prospective Post hoc analysis of phase III randomized trial [8] |
Prospective Post hoc pooled analysis of three (phase II + phase III) trials [36] |
Prospective Pooled data from clinical trials and real world [41] TKI naïve/pretreated |
Prospective Subgroup analysis of single‐arm phase II trial [50] |
Prospective phase II single arm [49] |
| EGFR‐G719X a mutations | |||||||
| n | 142 [9] | 1 | 3 | 18 | 55 / 19 | 4 | 19 |
| ORR (%) | 35.2% | 0% | 0% |
77.8% (95% CI, 52.4–93.6) |
63.4% / 10.5% | 75% |
53% (95% CI, 28–77) |
| Median PFS | Not reported | Not reported |
1.8 months (range, 0.5–2.2) |
13.8 months (95% CI, 6.8–NE) |
Not reported |
52.7 weeks (90% CI, 25.6–57.0) |
8.2 months (95% CI, 6.2–10.2) |
| Median OS | Not reported | Not reported |
7.9 months (range, 5.8–11.9) |
26.9 months (95% CI, 16.4–NE) |
Not reported | Not reported | Not reported |
| EGFR‐L861Q a mutations | |||||||
| n | 70 [9] | 1 | 2 | 16 | 47 / 11 | 9 | |
| ORR (%) | 38.6% | 0% | 50% |
56.3% (95% CI, 29.9–80.2) |
59.6% / 45.5% |
78% (95% CI, 44–100) |
|
| Median PFS | Not reported | Not reported |
8.5 months (range, 6.4–10.6) |
8.2 months (95% CI, 4.5–16.6) |
Not reported |
15.2 months (95% CI, 1.3–29.1) |
|
| Median OS | Not reported | Not reported |
17.3 months (range, 12–22.6) |
17.1 months (95% CI, 15.3–21.6) |
Not reported | ||
| EGFR‐S786I a mutations | |||||||
| n | 33 [10] | 8 | 8 / 2 | 8 | |||
| ORR (%) | 45.4% b |
100% (95% CI, 63.1–100) |
62.5% / 50% |
38% (95% CI, 0–81) |
|||
| Median PFS | Not reported |
14.7 months (95% CI, 2.6–NE) |
Not reported |
12.3 months (95% CI, 0–28.8) |
|||
| Median OS | Not reported |
NE (95% CI, 3.4–NE) |
Not reported | Not reported | |||
Includes patients with compound EGFR mutations involving the particular mutation.
Calculated by excluding patients who received either afatinib or whose tumors had EGFR exon 19 deletion/L858R mutation in addition to S768I.
Abbreviations: CI, confidence interval; NE, not estimable; ORR, objective response rate; OS, overall survival; PFS, progression‐free survival; TKI, tyrosine kinase inhibitor.
Unlike most of the landmark trials that established the use of currently available EGFR TKIs, the LUX‐Lung clinical trials have allowed enrollment of patients with these less common EGFR mutations. A post hoc pooled analysis of the LUX‐Lung 2, LUX‐Lung 3, and LUX‐Lung 6 trials evaluated the clinical activity of afatinib in TKI‐naïve stage IIIB–IV lungadenocarcinomas with uncommon EGFR mutations [36]. Whereas LUX‐Lung 2 was a nonrandomized single‐arm phase II trial, LUX‐Lung 3 (global) and LUX‐Lung 6 (Asia) were randomized phase III trials that compared afatinib with chemotherapy control arms [37, 38, 39]. Thirty‐eight patients with noncanonical EGFR alterations were classified into one of the three groups: point mutations or duplications in in exons 18–21 (group 1); de novo T790M mutations alone or in combination with other mutations (group 2); or exon 20 insertions (group 3). Objective response rate (ORR) for group 1 was 71.1% (95% confidence interval [CI], 54.1–84.6), median PFS was 10.7 months (95% CI, 5.6–14.7), and median overall survival (OS) was 19.4 months (95% CI, 16.4–26.9) (Table 1). Analysis for individual mutations showed that ORR for patients with tumors harboring G719X mutation was 77.8% (95% CI, 52.4–93.6), median PFS was 13.8 months (95% CI, 6.8, not estimable), and median OS was 26.9 months (95% CI, 16.4, not estimable) (Table 2). In January 2018, this led to the approval of afatinib in advanced NSCLC harboring alterations in these less common subgroups (S768I, L861Q, and/or G719X). Further data supporting the activity of afatinib in these cohorts have also been published (Table 1, Table 2, supplemental online Table 1) [40, 41, 42, 43].
Daily dosing of afatinib at 40–50 mg in clinical trials has been associated with significant rates of treatment discontinuations and dose reductions because of treatment‐related adverse events (TRAEs). The most common toxicities involve the gastrointestinal tract (diarrhea), mucosa (oral mucositis), and skin (rash/acneiform dermatitis, dry skin, pruritus, paronychia) and are related to the simultaneous inhibition of wild‐type (WT) EGFR [44]. The rates of treatment discontinuation because of TRAEs have ranged from 3.8% to 12% across numerous studies [37, 38, 39, 40]. Dose reductions to less than 40 mg daily have been required in up to 50% of study participants in these trials. A combined analysis evaluating the impact of afatinib dose reductions in the LUX trials included 5.7% and 9.1% patients with uncommon EGFR mutations, respectively [45]. Although this study showed similar PFS for patients with dose reductions within the first 6 months compared with those without, it was limited by small subgroup sizes and the post hoc nature of the analysis.
Osimertinib is now the standard‐of‐care first‐line treatment option for patients with advanced NSCLC with common EGFR mutations in the U.S. and many other countries in view of brisk and durable systemic and intracranial efficacy with a favorable toxicity profile [46, 47]. In contrast to the first‐generation EGFR inhibitors, osimertinib has low avidity for the EGFR WT receptor [48]. The activity of osimertinib against patients with uncommon EGFR mutations was first observed in the phase I/II AURA study, in which two of ten patients had responses in their tumors after progression on a prior EGFR TKI [48]. A recently published multicenter, open‐label, single‐arm phase II trial from South Korea evaluated the clinical activity and safety of osimertinib in 36 EGFR TKI–naïve patients with metastatic or recurrent NSCLC harboring mutations other than exon 19 deletion, L858R/T790M mutations, and exon 20 insertions (Table 1) [49]. Nineteen (53%), nine (25%), eight (22%), and four (11%) patients had tumors with EGFR‐G719X, L861Q, S768I, and other mutations in the EGFR gene, respectively. Investigator‐assessed ORR was 50% (95% CI, 33–67), with median duration of response 11.2 months (95% CI, 7.7–14.7 months), disease control rate 89% (95% CI, 78–100), and median PFS 8.2 months (95% CI, 5.9–10.5); median OS was not reached. Intracranial ORR was 40%, with responses seen in two of five evaluable patients. Subgroup analysis revealed ORR of 53% (95% CI, 28–77) and median PFS of 8.2 months (95% CI, 6.2–10.2) in patients with G719X mutations (Table 2). The safety profile was as expected from prior studies, with rash, pruritus, anorexia, diarrhea, and dyspnea as the most common but nonsevere adverse events. Dose reduction was required in only one patient, whereas no patient (0%) discontinued therapy because of TRAEs. The shortcomings of cross‐trial comparisons notwithstanding, the comparative efficacy of osimertinib as compared with afatinib and neratinib is summarized in Table 1 (for all uncommon EGFR mutations) and Table 2 (for G719X, S768I, and L861Q mutations) [36, 40, 41, 49, 50].
In light of concern for high rates of adverse events and dose reductions with standard dose afatinib, we include discussions regarding an alternative off‐label strategy of intermittent pulsatile dosing of afatinib at 280 mg weekly while making shared management decisions with patients. This strategy was previously reported by our group in a small cohort of patients with ERBB2 exon 20 insertion–mutated lung adenocarcinoma [44]. In this study, partial responses were seen in two of three patients, with exceptional response in one; there was minimal diarrhea and no reported rash [44]. The biologic rationale behind this intermittent pulsatile dosing approach is to achieve adequate pharmacodynamic inhibition and intracranial penetration while avoiding toxicities incurred by daily continuous inhibition of WT EGFR, as evaluated previously with pulsatile administration of erlotinib [51, 52, 53].
Patient Update
After an informed discussion of known risks and benefits, the patient started on first‐line afatinib at an off‐label dose of 280 mg weekly (afatinib 40 mg × 7 tablets taken once weekly). She experienced Common Terminology Criteria for Adverse Events grade 2 oral mucositis, grade 1 diarrhea, grade 1 acneiform rash, and grade 2 paronychia. Topical dexamethasone use for oral mucositis was complicated by oral candidiasis and led to treatment interruption for 1 week. After resuming treatment, the patient had no recurrence of oral mucositis. Nonbloody diarrhea occurred predictably 3 days after each weekly dose and lasted for no more than 1 day. Cutaneous adverse events were managed successfully with topical steroids along with dermatology consultation. No dose reductions were needed.
PET/CT scan done 5 weeks after initiating afatinib showed a partial response using RECIST version 1.1 (Fig. 2) that lasted for 20 months. Ultimately, the patient developed new symptomatic bony lesions, intrathoracic progression, and new intracranial metastases (Fig. 2). Repeat tissue biopsy did not reveal histologic transformation to small cell carcinoma. Circulating tumor DNA evaluation showed a new KRAS‐G12V mutation and did not show new mutations in the EGFR gene in addition to the known G719A and S720F mutations, revealing acquired resistance without EGFR‐T790M or C797S. Her treatment was next transitioned to carboplatin and pemetrexed. However, because of rapid progression of systemic disease on chemotherapy after two cycles, treatment was switched to third‐line osimertinib (at 80 mg/day) that was associated with progressive disease as best response. Tissue biopsy while on osimertinib therapy confirmed the presence of the original EGFR mutation profile along with the KRAS‐G12V mutation, confirming the latter as the mechanism of resistance to EGFR‐targeted therapy. She, unfortunately, continued to clinically decline and died approximately 27 months from the start of first‐line afatinib treatment.
Conclusion
Patients with uncommon EGFR mutations represent a heterogeneous group with the possibility for benefit with existing TKIs. It is prudent to consider preclinical data, computational analysis, and available prospective/retrospective data in determining optimal therapies for these patients. Moreover, with expanding use of comprehensive genomic profiling platforms and potential for more sensitive detection of noncanonical EGFR alterations, it is becoming increasingly relevant to avoid exclusion of these patients from new and upcoming trials in this domain. We report here an off‐label strategy of intermittent pulse dose afatinib with clinically meaningful benefit and manageable toxicities. It is imperative to rigorously and prospectively evaluate such strategies vis‐à‐vis emerging data for EGFR‐T790M active and central nervous system–penetrant osimertinib (phase II trial from South Korea [49] and ongoing phase II clinical trial in the U.S., NCT03434418) for the optimal management of these patients. We believe that future reporting of on‐target and off‐target mechanisms of resistance to EGFR TKI monotherapy for EGFR‐G719X and other uncommon EGFR‐mutated NSCLC will help define unmet needs for future therapeutic advances.
Glossary of Genomic Terms and Nomenclature
- CDKN2A
cyclin dependent kinase inhibitor 2A
- CDKN2B
cyclin dependent kinase inhibitor 2B
- EGFR
epidermal growth factor receptor
- MTAP
methylthioadenosine phosphorylase
- NFKBIA
nuclear factor‐kappa‐B‐–inhibitor alpha
- NKX2‐1
NK2 homeobox 1
- TP53
tumor protein p53
Author Contributions
Conception/design: Kartik Sehgal, Daniel B. Costa
Provision of study material or patients: Kartik Sehgal, Deepa Rangachari, Paul A. VanderLaan, Susumu S. Kobayashi, Daniel B. Costa
Collection and/or assembly of data: Kartik Sehgal, Deepa Rangachari, Paul A. VanderLaan, Susumu S. Kobayashi, Daniel B. Costa
Data analysis and interpretation: Kartik Sehgal, Deepa Rangachari, Paul A. VanderLaan, Susumu S. Kobayashi, Daniel B. Costa
Manuscript writing: Kartik Sehgal, Deepa Rangachari, Daniel B. Costa
Final approval of manuscript: Kartik Sehgal, Deepa Rangachari, Paul A. VanderLaan, Susumu S. Kobayashi, Daniel B. Costa
Disclosures
Deepa Rangachari: DynaMed, Advance Medical/TelaDoc (C/A), Bristol‐Meyer Squibb, Novocure, Abvvie/Stemcentrx (RF); Paul A. VanderLaan: Gala Therapeutics, Foundation Medicine, Caris Life Sciences, Flatiron Health, Intuitive Surgical, Clearview Healthcare Partners (C/A); Daniel B. Costa: Takeda/Millennium Pharmaceuticals, AstraZeneca, Pfizer (C/A, H, RF—institutional), Merck Sharp & Dohme, Merrimack Pharmaceuticals, Bristol‐Myers Squibb, Clovis Oncology, Spectrum Pharmaceuticals, Tesaro (other—nonfinancial institutional research support). The other authors indicated no financial relationships.
(C/A) Consulting/advisory relationship; (RF) Research funding; (E) Employment; (ET) Expert testimony; (H) Honoraria received; (OI) Ownership interests; (IP) Intellectual property rights/inventor/patent holder; (SAB) Scientific advisory board
Supporting information
See http://www.TheOncologist.com for supplemental material available online.
Supplemental Table 1 Additional Studies of EGFR Inhibitors in Patients with Advanced Non‐Small Cell Lung Cancer with Uncommon (or Rare) EGFR Mutations
Acknowledgments
This work was supported in part by the National Institutes of Health/National Cancer Institute (grants R37CA218707 awarded to D.B.C. and grants R01CA169259 and CA240257 awarded to S.S.K.) and the Department of Defense (grant LC170223 awarded to S.S.K.). The funders/sponsors had no role in the design and conduct of the study; collection, management, analysis, and interpretation of the data; preparation, review, or approval of the manuscript; and decision to submit the manuscript for publication. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health or the Department of Defense.
Disclosures of potential conflicts of interest may be found at the end of this article.
No part of this article may be reproduced, stored, or transmitted in any form or for any means without the prior permission in writing from the copyright holder. For information on purchasing reprints contact Commercialreprints@wiley.com. For permission information contact permissions@wiley.com.
References
- 1. Riely GJ, Yu HA. EGFR: The paradigm of an oncogene‐driven lung cancer. Clin Cancer Res 2015;21:2221–2226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Lynch TJ, Bell DW, Sordella R et al. Activating mutations in the epidermal growth factor receptor underlying responsiveness of non‐small‐cell lung cancer to gefitinib. N Engl J Med 2004;350:2129–2139. [DOI] [PubMed] [Google Scholar]
- 3. Costa DB. Kinase inhibitor‐responsive genotypes in EGFR mutated lung adenocarcinomas: Moving past common point mutations or indels into uncommon kinase domain duplications and rearrangements. Transl Lung Cancer Res 2016;5:331–337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Castellanos E, Feld E, Horn L. Driven by mutations: The predictive value of mutation subtype in EGFR‐mutated non‐small cell lung cancer. J Thorac Oncol 2017;12:612–623. [DOI] [PubMed] [Google Scholar]
- 5. Konduri K, Gallant JN, Chae YK et al. EGFR fusions as novel therapeutic targets in lung cancer. Cancer Discov 2016;6:601–611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Udagawa H, Hasako S, Ohashi A et al. TAS6417/CLN‐081 is a pan‐mutation‐selective EGFR tyrosine kinase inhibitor with a broad spectrum of preclinical activity against clinically relevant EGFR mutations. Mol Cancer Res 2019;17:2233–2243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Sequist LV, Martins RG, Spigel D et al. First‐line gefitinib in patients with advanced non‐small‐cell lung cancer harboring somatic EGFR mutations. J Clin Oncol 2008;26:2442–2449. [DOI] [PubMed] [Google Scholar]
- 8. Watanabe S, Minegishi Y, Yoshizawa H et al. Effectiveness of gefitinib against non‐small‐cell lung cancer with the uncommon EGFR mutations G719X and L861Q. J Thorac Oncol 2014;9:189–194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Galli G, Corrao G, Imbimbo M et al. Uncommon mutations in epidermal growth factor receptor and response to first and second generation tyrosine kinase inhibitors: A case series and literature review. Lung Cancer 2018;115:135–142. [DOI] [PubMed] [Google Scholar]
- 10. Zhu X, Bai Q, Lu Y et al. Response to tyrosine kinase inhibitors in lung adenocarcinoma with the rare epidermal growth factor receptor mutation S768I: A retrospective analysis and literature review. Target Oncol 2017;12:81–88. [DOI] [PubMed] [Google Scholar]
- 11. Wu JY, Yu CJ, Chang YC et al. Effectiveness of tyrosine kinase inhibitors on “uncommon” epidermal growth factor receptor mutations of unknown clinical significance in non‐small cell lung cancer. Clin Cancer Res 2011;17:3812–3821. [DOI] [PubMed] [Google Scholar]
- 12. De Pas T, Toffalorio F, Manzotti M et al. Activity of epidermal growth factor receptor‐tyrosine kinase inhibitors in patients with non‐small cell lung cancer harboring rare epidermal growth factor receptor mutations. J Thorac Oncol 2011;6:1895–1901. [DOI] [PubMed] [Google Scholar]
- 13. Beau‐Faller M, Prim N, Ruppert AM et al. Rare EGFR exon 18 and exon 20 mutations in non‐small‐cell lung cancer on 10 117 patients: A multicentre observational study by the French ERMETIC‐IFCT network. Ann Oncol 2014;25:126–131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Keam B, Kim DW, Park JH et al. Rare and complex mutations of epidermal growth factor receptor, and efficacy of tyrosine kinase inhibitor in patients with non‐small cell lung cancer. Int J Clin Oncol 2014;19:594–600. [DOI] [PubMed] [Google Scholar]
- 15. Chiu CH, Yang CT, Shih JY et al. Epidermal growth factor receptor tyrosine kinase inhibitor treatment response in advanced lung adenocarcinomas with G719X/L861Q/S768I mutations. J Thorac Oncol 2015;10:793–799. [DOI] [PubMed] [Google Scholar]
- 16. Lohinai Z, Hoda MA, Fabian K et al. Distinct epidemiology and clinical consequence of classic versus rare EGFR mutations in lung adenocarcinoma. J Thorac Oncol 2015;10:738–746. [DOI] [PubMed] [Google Scholar]
- 17. Baek JH, Sun JM, Min YJ et al. Efficacy of EGFR tyrosine kinase inhibitors in patients with EGFR‐mutated non‐small cell lung cancer except both exon 19 deletion and exon 21 L858R: A retrospective analysis in Korea. Lung Cancer 2015;87:148–154. [DOI] [PubMed] [Google Scholar]
- 18. Oyaert M, Demedts I, Boone E et al. Antitumoral activity of tyrosine kinase inhibitors in patients with non‐small cell lung cancer harbouring rare epidermal growth factor receptor mutations. Mol Diagn Ther 2015;19:267–272. [DOI] [PubMed] [Google Scholar]
- 19. Chen D, Song Z, Cheng G. Clinical efficacy of first‐generation EGFR‐TKIs in patients with advanced non‐small‐cell lung cancer harboring EGFR exon 20 mutations. Onco Targets Ther 2016;9:4181–4186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Xu J, Jin B, Chu T et al. EGFR tyrosine kinase inhibitor (TKI) in patients with advanced non‐small cell lung cancer (NSCLC) harboring uncommon EGFR mutations: A real‐world study in China. Lung Cancer 2016;96:87–92. [DOI] [PubMed] [Google Scholar]
- 21. Shi J, Yang H, Jiang T et al. Uncommon EGFR mutations in a cohort of Chinese NSCLC patients and outcomes of first‐line EGFR‐TKIs and platinum‐based chemotherapy. Chin J Cancer Res 2017;29:543–552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Pilotto S, Rossi A, Vavala T et al. Outcomes of first‐generation EGFR‐TKIs against non‐small‐cell lung cancer harboring uncommon EGFR mutations: A post hoc analysis of the BE‐POSITIVE study. Clin Lung Cancer 2018;19:93–104. [DOI] [PubMed] [Google Scholar]
- 23. Li H, Wang C, Wang Z et al. Efficacy and long‐term survival of advanced lung adenocarcinoma patients with uncommon EGFR mutations treated with 1st generation EGFR‐TKIs compared with chemotherapy as first‐line therapy. Lung Cancer 2019;130:42–49. [DOI] [PubMed] [Google Scholar]
- 24. Kate S, Chougule A, Joshi A et al. Outcome of uncommon EGFR mutation positive newly diagnosed advanced non‐small cell lung cancer patients: A single center retrospective analysis. Lung Cancer (Auckl) 2019;10:1–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Arrieta O, Cardona AF, Corrales L et al. The impact of common and rare EGFR mutations in response to EGFR tyrosine kinase inhibitors and platinum‐based chemotherapy in patients with non‐small cell lung cancer. Lung Cancer 2015;87:169–175. [DOI] [PubMed] [Google Scholar]
- 26. Krawczyk P, Kowalski DM, Ramlau R et al. Comparison of the effectiveness of erlotinib, gefitinib, and afatinib for treatment of non‐small cell lung cancer in patients with common and rare EGFR gene mutations. Oncol Lett 2017;13:4433–4444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Hunter S, Nickless G, El‐Shakankery K et al. Treatment outcomes with tyrosine kinase inhibitors in patients with uncommon EGFR mutations in non‐small cell lung cancer: A multi‐centre retrospective analysis. J Clin Oncol 2017;35(suppl 15):e20678a. [Google Scholar]
- 28. Passaro A, Prelaj A, Bonanno L et al. Activity of EGFR TKIs in Caucasian patients with NSCLC harboring potentially sensitive uncommon EGFR mutations. Clin Lung Cancer 2019;20:e186–e194. [DOI] [PubMed] [Google Scholar]
- 29. Kobayashi Y, Togashi Y, Yatabe Y et al. EGFR exon 18 mutations in lung cancer: Molecular predictors of augmented sensitivity to afatinib or neratinib as compared with first‐ or third‐generation TKIs. Clin Cancer Res 2015;21:5305–5313. [DOI] [PubMed] [Google Scholar]
- 30. Akula S, Kamasani S, Sivan SK et al. Computational analysis of epidermal growth factor receptor mutations predicts differential drug sensitivity profiles toward kinase inhibitors. J Thorac Oncol 2018;13:721–726. [DOI] [PubMed] [Google Scholar]
- 31. Banno E, Togashi Y, Nakamura Y et al. Sensitivities to various epidermal growth factor receptor‐tyrosine kinase inhibitors of uncommon epidermal growth factor receptor mutations L861Q and S768I: What is the optimal epidermal growth factor receptor‐tyrosine kinase inhibitor? Cancer Sci 2016;107:1134–1140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Shen YC, Tseng GC, Tu CY et al. Comparing the effects of afatinib with gefitinib or erlotinib in patients with advanced‐stage lung adenocarcinoma harboring non‐classical epidermal growth factor receptor mutations. Lung Cancer 2017;110:56–62. [DOI] [PubMed] [Google Scholar]
- 33. Kim Y, Lee SH, Ahn JS et al. Efficacy and safety of afatinib for EGFR‐mutant non‐small cell lung cancer, compared with gefitinib or erlotinib. Cancer Res Treat 2019;51:502–509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Lau SC, Chooback N, Ho C et al. Outcome differences between first‐ and second‐generation EGFR inhibitors in advanced EGFR mutated NSCLC in a large population‐based cohort. Clin Lung Cancer 2019;20:e576–e583. [DOI] [PubMed] [Google Scholar]
- 35. Chang LC, Lim CK, Chang LY et al. Non‐small cell lung cancer harbouring non‐resistant uncommon EGFR mutations: Mutation patterns, effectiveness of epidermal growth factor receptor‐tyrosine kinase inhibitors and prognostic factors. Eur J Cancer 2019;119:77–86. [DOI] [PubMed] [Google Scholar]
- 36. Yang JC, Sequist LV, Geater SL et al. Clinical activity of afatinib in patients with advanced non‐small‐cell lung cancer harbouring uncommon EGFR mutations: A combined post‐hoc analysis of LUX‐Lung 2, LUX‐Lung 3, and LUX‐Lung 6. Lancet Oncol 2015;16:830–838. [DOI] [PubMed] [Google Scholar]
- 37. Yang JC, Shih JY, Su WC et al. Afatinib for patients with lung adenocarcinoma and epidermal growth factor receptor mutations (LUX‐Lung 2): A phase 2 trial. Lancet Oncol 2012;13:539–548. [DOI] [PubMed] [Google Scholar]
- 38. Sequist LV, Yang JC, Yamamoto N et al. Phase III study of afatinib or cisplatin plus pemetrexed in patients with metastatic lung adenocarcinoma with EGFR mutations. J Clin Oncol 2013;31:3327–3334. [DOI] [PubMed] [Google Scholar]
- 39. Wu YL, Zhou C, Hu CP et al. Afatinib versus cisplatin plus gemcitabine for first‐line treatment of Asian patients with advanced non‐small‐cell lung cancer harbouring EGFR mutations (LUX‐Lung 6): An open‐label, randomised phase 3 trial. Lancet Oncol 2014;15:213–222. [DOI] [PubMed] [Google Scholar]
- 40. Wu Y, Tu H, Feng J et al. A phase IIIb open‐label, single‐arm study of afatinib in EGFR TKI‐naive patients with EGFRM+ NSCLC: An interim analysis. J Thorac Oncol 2017;12(11 suppl 2):P3.01‐036a. [Google Scholar]
- 41. Chih‐Hsin Yang J, Schuler M, Popat S et al. Afatinib for the treatment of non‐small cell lung cancer harboring uncommon EGFR mutations: A database of 693 cases. J Thorac Oncol 2020;15:803–815. [DOI] [PubMed] [Google Scholar]
- 42. Heigener DF, Schumann C, Sebastian M et al. Afatinib in non‐small cell lung cancer harboring uncommon EGFR mutations pretreated with reversible EGFR inhibitors. The Oncologist 2015;20:1167–1174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Moran T, Taus A, Arriola E et al. Clinical activity of afatinib in patients with non‐small cell lung cancer harboring uncommon EGFR mutations: A Spanish retrospective multicenter study. Clinical Lung Cancer 2020;21:428–436.e2. [DOI] [PubMed] [Google Scholar]
- 44. Costa DB, Jorge SE, Moran JP et al. Pulse afatinib for ERBB2 exon 20 insertion‐mutated lung adenocarcinomas. J Thorac Oncol 2016;11:918–923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Yang JC, Sequist LV, Zhou C et al. Effect of dose adjustment on the safety and efficacy of afatinib for EGFR mutation‐positive lung adenocarcinoma: Post hoc analyses of the randomized LUX‐Lung 3 and 6 trials. Ann Oncol 2016;27:2103–2110. [DOI] [PubMed] [Google Scholar]
- 46. Ramalingam SS, Vansteenkiste J, Planchard D et al. Overall survival with osimertinib in untreated, EGFR‐mutated advanced NSCLC. N Engl J Med 2020;382:41–50. [DOI] [PubMed] [Google Scholar]
- 47. Arbour KC, Riely GJ. Systemic therapy for locally advanced and metastatic non‐small cell lung cancer: A review. JAMA 2019;322:764–774. [DOI] [PubMed] [Google Scholar]
- 48. Janne PA, Yang JC, Kim DW et al. AZD9291 in EGFR inhibitor‐resistant non‐small‐cell lung cancer. N Engl J Med 2015;372:1689–1699. [DOI] [PubMed] [Google Scholar]
- 49. Cho JH, Lim SH, An HJ et al. Osimertinib for patients with non‐small‐cell lung cancer harboring uncommon EGFR mutations: A multicenter, open‐label, phase II trial (KCSG‐LU15‐09). J Clin Oncol 2020;38:488–495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Sequist LV, Besse B, Lynch TJ et al. Neratinib, an irreversible pan‐ERBB receptor tyrosine kinase inhibitor: Results of a phase II trial in patients with advanced non‐small‐cell lung cancer. J Clin Oncol 2010;28:3076–3083. [DOI] [PubMed] [Google Scholar]
- 51. Grommes C, Oxnard GR, Kris MG et al. “Pulsatile” high‐dose weekly erlotinib for CNS metastases from EGFR mutant non‐small cell lung cancer. Neuro Oncol 2011;13:1364–1369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Clarke JL, Pao W, Wu N et al. High dose weekly erlotinib achieves therapeutic concentrations in CSF and is effective in leptomeningeal metastases from epidermal growth factor receptor mutant lung cancer. J Neurooncol 2010;99:283–286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Dhruva N, Socinski MA. Carcinomatous meningitis in non‐small‐cell lung cancer: Response to high‐dose erlotinib. J Clin Oncol 2009;27:e31–e32. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
See http://www.TheOncologist.com for supplemental material available online.
Supplemental Table 1 Additional Studies of EGFR Inhibitors in Patients with Advanced Non‐Small Cell Lung Cancer with Uncommon (or Rare) EGFR Mutations


