Abstract
Background
We examined overall survival (OS) outcomes based on plasma 25‐hydroxyvitamin D [25(OH)D] levels in this post hoc analysis of the phase III MPACT trial of metastatic pancreatic cancer.
Materials and Methods
Patients were subdivided based on 25(OH)D level: sufficient (≥30 ng/mL), relatively insufficient (20–<30 ng/mL), or insufficient (<20 ng/mL).
Results
Of 861 patients randomized in MPACT, 422 were included in this analysis. In the all‐patients group, the median OS among those with insufficient, relatively insufficient, and sufficient 25(OH)D levels was 7.9, 9.4, and 7.8 months, respectively. No statistically significant OS difference was observed with relatively insufficient (p = .227) or sufficient (p = .740) versus insufficient 25(OH)D levels or with sufficient vs relatively insufficient (p = .301) 25(OH)D levels.
Conclusion
No association was observed between plasma 25(OH)D levels and survival. Further investigations are needed to understand any role of vitamin D in pancreatic cancer. Clinical trial identification number. NCT00844649.
Short abstract
Studies have suggested a possible survival benefit for patients with pancreatic cancer and sufficient vitamin D levels. This article examines potential correlations between plasma 25‐hydroxyvitamin D levels and overall survival outcomes in patients from the MPACT trial.
Introduction
The use of biomarkers has allowed for the identification of patients who may experience improved efficacy with certain treatments; however, few such biomarkers exist for pancreatic cancer. On the basis of the PRODIGE 4/ACCORD 11 and MPACT trials, folinic acid, fluorouracil, irinotecan, oxaliplatin (FOLFIRINOX) and nab‐paclitaxel plus gemcitabine are preferred category 1 regimens per National Comprehensive Cancer Network guidelines for the treatment of patients with metastatic pancreatic cancer [1]. For patients with metastatic disease and BRCA1/2 or PALB2 mutations, FOLFIRINOX (category 1) as well as modified FOLFIRINOX and cisplatin plus gemcitabine (category 2A) are options [1]. Data from the POLO trial support the National Comprehensive Cancer Network recommendation for maintenance olaparib in patients with germline BRCA1/2 mutations; however, germline BRCA1/2 mutations are fairly rare among patients with pancreatic cancer [1, 2]. Pembrolizumab may be used as a second‐line therapy for metastatic pancreatic cancer but only for patients with high microsatellite instability or mismatch repair–deficient tumors [1, 3]. Clearly, a need exists to identify a biomarker with broader applicability in pancreatic cancer management.
Studies have suggested a possible survival benefit for patients with pancreatic cancer and sufficient vitamin D levels. A study of patients with pancreatic cancer from five prospective U.S. cohorts revealed a significantly longer survival among patients with sufficient levels of plasma 25‐hydroxyvitamin D [25(OH)D] versus those with insufficient levels [4]. A meta‐analysis demonstrated a significant association between high plasma 25(OH)D levels and reduction in pancreatic cancer mortality [5]. In this analysis, we examined potential correlations between plasma 25(OH)D levels and overall survival (OS) outcomes in patients from the MPACT trial to test the hypothesis that plasma 25(OH)D sufficiency may be a marker of survival advantage in patients with metastatic pancreatic cancer.
Materials and Methods
Study Oversight
The MPACT trial was conducted in accordance with the International Council on Harmonisation E6 requirements for Good Clinical Practice and with the ethical principles of the Declaration of Helsinki. The independent ethics committee at each participating institution approved the study. Written informed consent was provided by all patients before study initiation.
Patients and Treatments
Patients included in this post hoc exploratory analysis were enrolled in the international, multicenter, open‐label, randomized, phase III MPACT trial (NCT00844649); eligibility criteria have been published previously [6]. Patients were randomly assigned 1:1 to receive nab‐paclitaxel 125 mg/m2 plus gemcitabine 1,000 mg/m2 on days 1, 8, 15, 29, 36, and 43 of an 8‐week cycle or gemcitabine 1,000 mg/m2 alone (weekly for 7 of 8 weeks) for cycle 1; in subsequent cycles, all patients were treated on days 1, 8, and 15 every 4 weeks. Treatment continued until disease progression or unacceptable adverse events.
Statistical Analysis
Median OS was estimated using the Kaplan‐Meier method, and the associated hazard ratios (HRs) and two‐sided 95% confidence intervals (CIs) were calculated using the Cox proportional hazards model. The p values were based on a log‐rank test. Patients were subdivided into three groups based on plasma 25(OH)D levels: sufficient (≥30 ng/mL), relatively insufficient (20–<30 ng/mL), or insufficient (<20 ng/mL) [7]. Plasma 25(OH)D levels were determined at various time points (pretreatment and post‐treatment) throughout the trial. Levels of 25(OH)D2 and 25(OH)D3 were quantified by liquid chromatography–tandem mass spectrometry using multiple reaction monitoring and reported individually and as a sum with a clinical reference range attached to the sum.
Results
Demographics
Of the 861 patients randomized in the MPACT trial, 422 had adequate‐to‐measure plasma 25(OH)D levels (supplemental online Fig. 1). Demographic and baseline clinical characteristics between the two treatment groups were generally balanced (Table 1).
Figure 1.
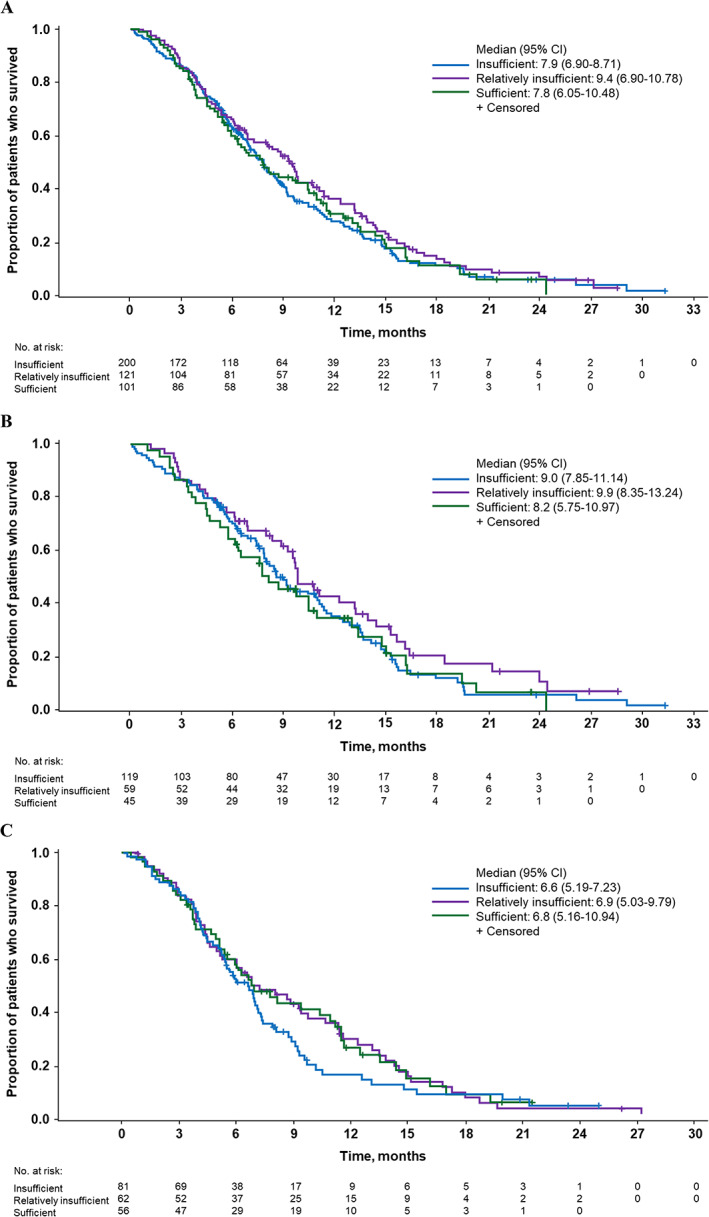
Kaplan‐Meier curves for overall survival in (A) all patients, (B) patients treated with nab‐paclitaxel plus gemcitabine, and (C) patients treated with gemcitabine alone (intent‐to‐treat population).Abbreviation: CI, confidence interval.
Table 1.
Patient demographics and baseline clinical characteristics for all patients and by treatment arm (intent‐to‐treat population)
| Characteristic | All patients | nab‐Paclitaxel + gemcitabine | Gemcitabine | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 25(OH)D level | Total (n = 422) | |||||||||||
| Insufficient (<20 ng/mL), (n = 200) | Relatively insufficient (20 to <30 ng/mL), (n = 121) | Sufficient (≥30 ng/mL), (n = 101) | Insufficient (<20 ng/mL) (n = 119) | Relatively insufficient (20 to <30 ng/mL) (n = 59) | Sufficient (≥30 ng/mL) (n = 45) | Total (n = 223) | Insufficient (<20 ng/mL) (n = 81) | Relatively insufficient (20 to <30 ng/mL) (n = 62) | Sufficient (≥30 ng/mL) (n = 56) | Total (n = 199) | ||
| 25(OH)D, median (range), ng/mL | 13 (6–19) | 24 (20–29) | 35 (30–74) | 21 (6–74) | 12 (6–19) | 25 (20–29) | 35 (30–51) | 19 (6–51) | 14 (6–19) | 24 (20–29) | 35 (30–74) | 23 (6–74) |
| Age | 63 (27–86) | |||||||||||
| Median (range), yr | 61 (32–82) | 64 (27–82) | 66 (37–86) | 242 (57) | 59 (34–81) | 63 (27–82) | 63 (37–83) | 61 (27–83) | 63 (32–82) | 66 (42–80) | 66 (42–86) | 64 (32–86) |
| <65 yr, n (%) | 131 (66) | 62 (51) | 49 (49) | 180 (43) | 82 (69) | 34 (58) | 24 (53) | 140 (63) | 49 (61) | 28 (45) | 25 (45) | 102 (51) |
| ≥65 yr, n (%) | 69 (35) | 59 (49) | 52 (52) | 37 (31) | 25 (42) | 21 (47) | 83 (37) | 32 (40) | 34 (55) | 31 (55) | 97 (49) | |
| Sex, n (%) | ||||||||||||
| Female | 85 (43) | 46 (38) | 51 (51) | 182 (43) | 54 (45) | 25 (42) | 26 (58) | 105 (47) | 31 (38) | 21 (34) | 25 (45) | 77 (39) |
| Male | 115 (58) | 75 (62) | 50 (50) | 240 (57) | 65 (55) | 34 (58) | 19 (42) | 118 (53) | 50 (62) | 41 (66) | 31 (55) | 122 (61) |
| BMI category, n (%) | ||||||||||||
| <25.0 kg/m2 | 103 (52) | 55 (46) | 52 (52) | 210 (50) | 67 (56) | 26 (44) | 22 (49) | 115 (52) | 36 (44) | 29 (47) | 30 (54) | 95 (48) |
| 25.0–29.9 kg/m2 | 60 (30) | 51 (42) | 29 (29) | 140 (33) | 28 (24) | 24 (41) | 14 (31) | 66 (30) | 32 (40) | 27 (44) | 15 (27) | 74 (37) |
| ≥30.0 kg/m2 | 37 (19) | 15 (12) | 20 (20) | 72 (17) | 24 (20) | 9 (15) | 9 (20) | 42 (19) | 13 (16) | 6 (10) | 11 (20) | 30 (15) |
| Geographic region, n (%) | ||||||||||||
| Australia | 27 (14) | 20 (17) | 14 (14) | 61 (15) | 17 (14) | 10 (17) | 5 (11) | 32 (14) | 10 (12) | 10 (16) | 9 (16) | 29 (15) |
| Eastern Europe | 54 (27) | 15 (12) | 6 (6) | 75 (18) | 33 (28) | 4 (7) | 3 (7) | 40 (18) | 21 (26) | 11 (18) | 3 (5) | 35 (18) |
| Western Europe | 30 (15) | 10 (8) | 3 (3) | 43 (10) | 19 (16) | 4 (7) | 1 (2) | 24 (11) | 11 (14) | 6 (10) | 2 (4) | 19 (10) |
| North America | 89 (45) | 76 (63) | 78 (78) | 243 (58) | 50 (42) | 41 (70) | 36 (80) | 127 (57) | 39 (48) | 35 (57) | 42 (75) | 116 (58) |
| KPS, n (%) | ||||||||||||
| 90–100 | 118 (59) | 78 (65) | 60 (59) | 256 (61) | 68 (57) | 38 (64) | 26 (58) | 132 (59) | 50 (62) | 40 (65) | 34 (61) | 124 (62) |
| 70–80 | 82 (41) | 43 (36) | 40 (40) | 165 (39) | 51 (43) | 21 (36) | 18 (40) | 90 (40) | 31 (38) | 22 (36) | 22 (39) | 75 (38) |
| <70 | 0 | 0 | 1 (1) | 1 (< 1) | 0 | 0 | 1 (2) | 1 (< 1) | 0 | 0 | 0 | 0 |
| Primary pancreatic cancer location, n (%) | ||||||||||||
| Head | 102 (51) | 52 (43) | 40 (40) | 194 (46) | 61 (51) | 23 (39) | 20 (44) | 104 (47) | 41 (51) | 29 (47) | 20 (36) | 90 (45) |
| Other | 98 (49) | 69 (57) | 61 (60) | 228 (54) | 58 (49) | 36 (61) | 25 (56) | 119 (53) | 40 (49) | 33 (53) | 36 (64) | 109 (55) |
| Site of metastatic disease, n (%) | ||||||||||||
| Hepatic/liver | 169 (85) | 101 (84) | 86 (85) | 356 (84) | 102 (86) | 51 (86) | 36 (80) | 189 (85) | 67 (83) | 50 (81) | 50 (89) | 167 (84) |
| Lung/thoracic | 82 (41) | 52 (43) | 38 (38) | 172 (41) | 43 (36) | 22 (37) | 20 (44) | 85 (38) | 39 (48) | 30 (48) | 18 (32) | 87 (44) |
| Abdomen/peritoneum | 175 (88) | 104 (86) | 93 (92) | 372 (88) | 100 (84) | 48 (81) | 42 (93) | 190 (85) | 75 (93) | 56 (90) | 51 (91) | 182 (92) |
| Disease stage at diagnosis, n | ||||||||||||
| I–III | 30 (15) | 17 (14) | 10 (10) | 57 (14) | 23 (19) | 10 (17) | 4 (9) | 37 (17) | 7 (9) | 7 (11) | 6 (11) | 20 (10) |
| IV | 156 (78) | 101 (84) | 83 (82) | 340 (81) | 91 (77) | 48 (81) | 37 (82) | 176 (79) | 65 (80) | 53 (86) | 46 (82) | 164 (82) |
| Unknown | 14 (7) | 3 (3) | 8 (8) | 25 (6) | 5 (4) | 1 (2) | 4 (9) | 10 (5) | 9 (11) | 2 (3) | 4 (7) | 15 (8) |
| No. of metastatic sites, n (%) | ||||||||||||
| 1 | 14 (7) | 8 (7) | 2 (2) | 24 (6) | 10 (8) | 4 (7) | 2 (4) | 16 (7) | 4 (5) | 4 (7) | 0 | 8 (4) |
| 2 | 91 (46) | 53 (44) | 56 (55) | 200 (47) | 59 (50) | 27 (46) | 20 (44) | 106 (48) | 32 (40) | 26 (42) | 36 (64) | 94 (47) |
| 3 | 68 (34) | 44 (36) | 29 (29) | 141 (33) | 35 (30) | 21 (36) | 15 (33) | 71 (32) | 33 (41) | 23 (37) | 14 (25) | 70 (35) |
| >3 | 27 (14) | 16 (13) | 14 (14) | 57 (14) | 15 (13) | 7 (12) | 8 (18) | 30 (14) | 12 (15) | 9 (15) | 6 (11) | 27 (14) |
| CA19‐9 level, n (%) | ||||||||||||
| Normal | 34 (17) | 17 (14) | 10 (10) | 61 (15) | 22 (19) | 8 (14) | 5 (11) | 35 (16) | 12 (15) | 9 (15) | 5 (9) | 26 (13) |
| ULN to <59 × ULN | 60 (30) | 32 (26) | 27 (27) | 119 (28) | 34 (29) | 15 (25) | 15 (33) | 64 (29) | 26 (32) | 17 (27) | 12 (21) | 55 (28) |
| ≥ 59 × ULN | 90 (45) | 56 (46) | 55 (55) | 201 (48) | 50 (42) | 26 (44) | 22 (49) | 98 (44) | 40 (49) | 30 (48) | 33 (59) | 103 (52) |
| CA19‐9 | ||||||||||||
| n | 184 | 105 | 92 | 381 | 106 | 49 | 42 | 197 | 78 | 56 | 50 | 184 |
| Median (range), U/mL | 1,842 (0.3–1,161,996) | 2,216 (3.3–973,209) | 3,844 (2.6–2,564,235) | 2,392 (0.3–2,564,235) | 1,755 (2.9–1,161,996) | 2,176 (4.4–671,110) | 3,746 (4.2–2,564,235) | 1924 (2.9–2,564,235) | 2,599 (0.3–1,138,680) | 2,564 (3.3–973,209) | 5,408 (2.6–597,568) | 3,508 (0.3–1,138,680) |
| Previous therapy, n (%) | ||||||||||||
| Whipple procedure | 18 (9) | 12 (10) | 3 (3) | 33 (8) | 14 (12) | 6 (10) | 2 (4) | 22 (10) | 4 (5) | 6 (10) | 1 (2) | 11 (6) |
| Biliary stent | 33 (17) | 26 (22) | 17 (17) | 76 (18) | 18 (15) | 14 (24) | 11 (24) | 43 (19) | 15 (19) | 12 (19) | 6 (11) | 33 (17) |
| Serum albumin | ||||||||||||
| n | 199 | 121 | 101 | 421 | 118 | 59 | 45 | 222 | 81 | 62 | 56 | 199 |
| Median (range), g/dL | 4.2 (2.7–5.3) | 4.2 (3.1–5.0) | 4.3 (3.3–5.1) | 4.2 (2.7–5.3) | 4.2 (2.7–5.0) | 4.2 (3.1–5.0) | 4.2 (3.6–5.1) | 4.2 (2.7–5.1) | 4.2 (3.2–5.3) | 4.1 (3.1–4.8) | 4.3 (3.3–4.9) | 4.2 (3.1–5.3) |
Abbreviations: 25(OH)D, 25‐hydroxyvitamin D; BMI, body mass index; CA19‐9, carbohydrate antigen 19‐9; KPS, Karnofsky performance status; ULN, upper limit of normal.
Survival Outcomes
In the all‐patients group, the median OS was 7.9, 9.4, and 7.8 months in patients with insufficient, relatively insufficient, and sufficient 25(OH)D levels, respectively (Fig. 1A). Compared with patients with insufficient plasma 25(OH)D levels, no statistically significant differences in OS were observed in patients with relatively insufficient (HR, 0.85; 95% CI, 0.66–1.10; p = .227) or sufficient (HR, 0.99; 95% CI, 0.76–1.29; p = .740) levels. No significant OS difference was observed among patients with sufficient versus relatively insufficient 25(OH)D levels (HR, 1.17; 95% CI, 0.87–1.57; p = .301).
In the nab‐paclitaxel plus gemcitabine group, median OS in patients with insufficient, relatively insufficient, and sufficient plasma 25(OH)D levels was 9.0, 9.9, and 8.2 months, respectively; no statistically significant difference was noted by comparing any cohorts (Fig. 1B).
In the gemcitabine group, median OS in patients with insufficient, relatively insufficient, and sufficient plasma 25(OH)D levels was 6.6, 6.9, and 6.8 months, respectively; no statistically significant difference was noted by comparing any cohorts (Fig. 1C).
Discussion
In this analysis of the MPACT trial, we found no statistically significant differences in median OS based on degree of plasma 25(OH)D sufficiency. The MPACT trial was not designed to compare survival outcomes based on levels of plasma 25(OH)D. As such, plasma 25(OH)D levels were quantified at varying time points throughout the trial, and measurable samples were available for only 49% of the randomized patients from the MPACT trial. The relatively low number of patients with measurable plasma 25(OH)D levels was a limitation. However, despite a low availability of measurable plasma 25(OH)D levels, the availability was generally balanced between treatment arms. In addition, subgroup analyses by ethnicity were not conducted because the vast majority (87%) of patients in the MPACT trial were White. [6].
Although cross‐study comparisons are problematic and the underlying reasons for divergent outcomes with prior studies of different design are indiscernible, <50% of patients [41%–47% across plasma 25(OH)D cohorts] in the aforementioned five‐cohort U.S. study had metastatic disease [4], far lower than the >99% of patients in our study. Additionally, although the meta‐analysis by Zhang et al. showed a significant reduction in pancreatic cancer mortality for patients with high versus low plasma 25(OH)D levels, a subgroup analysis of patients with locally advanced or metastatic disease demonstrated a trend in favor of high plasma 25(OH)D levels, but did not reach statistical significance [5]. Thus, it is possible that stage of disease could be a contributing factor to associations between plasma 25(OH)D levels and survival.
Conclusion
Overall, our results call into question the role, if any, of plasma 25(OH)D as a biomarker in pancreatic cancer. However, prospective investigation of vitamin D in pancreatic cancer may be warranted. Recent data suggest that vitamin D or its analogs may act as therapeutic tools in pancreatic cancer treatment. The vitamin D receptor (VDR) has been reported to be involved in modulating immune responses, and VDR agonists are currently under investigation in combination with immune checkpoint inhibitors. It has been hypothesized that VDR agonists may facilitate delivery of chemotherapeutic agents to pancreatic tumors, enhance immune checkpoint inhibition and reduce immune‐related adverse events [8, 9]. Additionally, a phase II clinical trial is currently investigating paricalcitol, a vitamin D2 analog, combined with pembrolizumab for maintenance therapy in patients with metastatic pancreatic cancer [10]. Results from these studies should facilitate a deeper understanding of the role of vitamin D in pancreatic cancer tumor biology.
Disclosures
Daniel D. Von Hoff: Honor Health (Other‐grants, nonfinancial support), Celgene, a Bristol Myers Squibb Company (Other‐grants), Medtronic, CerRx, SynDevRx, United Health Care, Anthem Inc, Stromatis Pharma, Systems Oncology (OI), DNAtrix, Esperance Pharmaceuticals, Five Prime Therapeutics, Imaging Endpoints, Immodulon Therapeutics, Medical Prognosis Institute, Senhwa Biosciences, Tolero Pharmaceuticals, Trovagene, Alpha Cancer Technologies, Arvinas, Bellicum Pharmaceuticals, CanBas, Horizon Discovery, Lixte Biotechnology, Oncolyze, RenovoRx, TD2, AADi, Aptose Biosciences, BiolineRx, CV6 Therapeutics, EMD Serono, Evelo Therapeutics, Fujifilm, Intezyne Technologies, Kalos Therapeutics, Kura, Phosplatin Therapeutics, SOTIO, Strategia Therapeutics, Sun Biopharma, Synergene, 7 Hills Pharma, Actinium Pharmaceuticals, Cancer Prevention Pharmaceuticals, Geistlich Pharma, HUYA Bioscience International, Immunophotonics, Novocure, Genzada Pharmaceuticals, LEAF Pharmaceuticals, Oncology Venture, Reflexion Medical, TP Therapeutics, Verily, Athenex, Fate Therapeutics, Jounce Therapeutics, Samus Therapeutics, Aegela Biotherapeutics, 2X Oncology, Novita Pharmaceuticals, NuCana BioMed, Ipsen, Vicus Therapeutics, Codiak Biosciences, Decoy Biosystems, Agenus, Globe Life Science, Kelun ‐ Klus Pharma, RadImmune, Samumed, SOBI, Adicet Bio, BioXCel therapeutics, Bryologyx, Üelgene, Helix BioPharma, Sirnaomics, AiMed Bio, Boston Scientific, Corcept Therapeutics, Erimos Pharma, Gimbal, Amunix, Pfizer, Apeiron, GiraFpharma, Axis Therapeutics, DrugCendR, ImmuneOncia, Orphagen Pharmaceuticals, Array BioPharma, MaveriX Oncology, Northern Biologics, Viracta Therapeutics, Varian Biopharma (C/A), Eli Lilly & Co, Genentech, Celgene, a Bristol‐Myers Squibb Company, Incyte, Merrimack, Plexxikon, Minneamrita Therapeutics, Abbvie, Aduro Biotech, Cleave Biosciences, CytRx Corporation, Daiichi Sankyo, Deciphera, Endocyte, Exelixis, Five Prime Therapeutics, Gilead Sciences, Merck, Pfizer, Pharmacyclics, Phoenix Biotech, Samumed, Strategia, Halozyme (RF [Institution]), Intramedullary Catheter, Methods of Human Prostate Cancer, Use of 5,6‐Dihydro‐5‐Azacytidine in the Treatment of Prostate Cancer, Targeting Site‐2 Protease (S2P) for the Treatment of Pancreatic Cancer (pending), Targeting Ecto‐5‐Nucleotidase (Cd73) for the Treatment of Pancreatic Cancer, Targeting a Protein Tyrosine Phosphotase‐PRL‐1 for the Treatment of Pancreatic Cancer (pending), Targeting a Protein PRC1 for the Treatment of Pancreatic Cancer (pending), Targeting Ecto‐5‐Nucleotidase (CD73) for the Treatment of Pancreatic Cancer (pending), Protein Kinase Inhibitors (pending), Methods, Compounds and Compositions with Genotype Selective Anticancer Activity (pending), Methods and Kits to Predict Therapeutic Outcome of BTK Inhibitors (pending), Muscle Fatigue Substance Cytokines and Methods of Inhibiting Tumor Growth Therewith (pending), 2‐aryl‐pyridylazoles for the Treatment of Solid Tumors such as Pancreatic Cancer (pending) (IP); Haiyong Han: Celgene, a Bristol‐Myers Squibb Company (RF); Michael S. Ondovik: Bristol‐Myers Squibb Company (OI, E); Chrystal U. Louis: Bristol‐Myers Squibb Company (E). The other authors indicated no financial relationships.
(C/A) Consulting/advisory relationship; (RF) Research funding; (E) Employment; (ET) Expert testimony; (H) Honoraria received; (OI) Ownership interests; (IP) Intellectual property rights/inventor/patent holder; (SAB) Scientific advisory board
Supporting information
See http://www.TheOncologist.com for supplemental material available online.
Supplemental Figure 1. CONSORT diagram of patients in the current analysis. a One patient randomized to gemcitabine (Gem) was treated with nab‐paclitaxel (nab‐P) plus Gem. In the intent‐to‐treat analysis, this patient was categorized as randomized. In all analyses of the treated population, this patient was categorized as treated. 25(OH)D, 25‐hydroxyvitamin D.
Acknowledgments
We thank the patients who participated in the phase III MPACT trial and their families. Medical writing assistance was provided by Brilee Smith, Ph.D., of MediTech Media, Ltd, and funded by Bristol Myers Squibb Company. The authors are fully responsible for all content and editorial decisions for this manuscript.
This work was supported by a grant from the Bristol Myers Squibb Company and from the Stand Up To Cancer Dream Team (to D.D.V.H.).
Disclosures of potential conflicts of interest may be found at the end of this article.
No part of this article may be reproduced, stored, or transmitted in any form or for any means without the prior permission in writing from the copyright holder. For information on purchasing reprints contact commercialreprints@wiley.com. For permission information contact permissions@wiley.com.
References
- 1. National Comprehensive Cancer Network . Pancreatic Adenocarcinoma (Version 1.2021). Available at https://www.nccn.org/professionals/physician_gls/pdf/pancreatic.pdf. Accessed Dec 29, 2020.
- 2. Golan T, Hammel P, Reni M, et al. Maintenance olaparib for germline BRCA‐mutated metastatic pancreatic cancer. N Engl J Med 2019;381:317–327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Le DT, Uram JN, Wang H et al. PD‐1 blockade in tumors with mismatch‐repair deficiency. N Engl J Med 2015;372:2509–2520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Yuan C, Qian ZR, Babic A et al. Prediagnostic plasma 25‐hydroxyvitamin D and pancreatic cancer survival. J Clin Oncol 2016;34:2899–2905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Zhang X, Huang XZ, Chen WJ et al. Plasma 25‐hydroxyvitamin D levels, vitamin D intake, and pancreatic cancer risk or mortality: A meta‐analysis. Oncotarget 2017;8:64395–64406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Von Hoff DD, Ervin T, Arena FP et al. Increased survival in pancreatic cancer with nab‐paclitaxel plus gemcitabine. N Engl J Med 2013;369:1691–1703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Gallagher JC, Sai AJ. Vitamin D insufficiency, deficiency, and bone health. J Clin Endocrinol Metab 2010;95:2630–2633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Sherman MH, Yu RT, Engle DD et al. Vitamin D receptor‐mediated stromal reprogramming suppresses pancreatitis and enhances pancreatic cancer therapy. Cell 2014;159:80–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Palmer MT, Lee YK, Maynard CL et al. Lineage‐specific effects of 1,25‐dihydroxyvitamin D(3) on the development of effector CD4 T cells. J Biol Chem 2011;286:997–1004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Chung VM, Borazanci EH, Jameson GS et al. A SU2C catalyst randomized phase II trial of pembrolizumab with or without paricalcitol in patients with stage IV pancreatic cancer who have been placed in best possible response. J Clin Oncol 2018;36:TPS4154a. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
See http://www.TheOncologist.com for supplemental material available online.
Supplemental Figure 1. CONSORT diagram of patients in the current analysis. a One patient randomized to gemcitabine (Gem) was treated with nab‐paclitaxel (nab‐P) plus Gem. In the intent‐to‐treat analysis, this patient was categorized as randomized. In all analyses of the treated population, this patient was categorized as treated. 25(OH)D, 25‐hydroxyvitamin D.


