Abstract
Whether the immune suppressive action of glucocorticoid steroids, such as dexamethasone, might reduce the benefits of cancer immunotherapy has long been a concern. Observations that established tumor regressions in response to immune checkpoint inhibitors (ICIs) often persist, despite the use of steroids to mitigate ICI‐related autoimmune breakthrough, are not sufficiently reassuring, because these observations do not address the potential blunting of immune priming at the initiation of ICI therapy. With increasing indications for ICI in combination with chemotherapy, this issue merits reconsideration. Professional society guidance advises that dexamethasone should be used as first‐line prophylaxis for nausea and vomiting in patients receiving ICI and highly emetogenic chemotherapy combination regimens. Here, we review the availability of data on this subject and propose an alternative approach focused on the adoption of steroid minimization or sparing for prophylaxis of nausea until the underlying immune biology is better understood.
Short abstract
This commentary considers clinical decision‐making for the prescribing of dexamethasone as an antiemetic for patients receiving chemotherapy in combination with immune checkpoint inhibitor therapy.
Introduction
Glucocorticoids (GCs), such as dexamethasone, are used clinically as both potent antiemetics and immune suppressants. Chemotherapy‐induced nausea and vomiting (CINV) can be a debilitating side effect of common chemotherapy regimens, with a significant impact on patient quality of life [1] and high associated health care costs [2]. Dexamethasone is given in combination with 5‐hydroxytryptamine (5‐HT3) and neurokinin‐1 (NK1) receptor antagonists as prophylaxis [3, 4] for patients receiving highly emetogenic chemotherapy (HEC), such as carboplatin [5], and is a commonly used “as‐needed” prescription for nausea complicating HEC and other chemotherapy after treatment. HEC is now indicated in combination with immune checkpoint inhibitors (ICIs) for cancers such as non‐small‐cell lung cancer (NSCLC), small‐cell lung cancer (SCLC), and head and neck squamous cell carcinoma (HNSCC) [6, 7]. ICIs and other less emetogenic chemotherapy are also garnering increasing regulatory approval [8, 9], and it is highly likely that additional combination immunotherapy regimens will be licensed in the future, including novel immunotherapy targets [10, 11]. As a result, dexamethasone is likely to be coadministered with ICIs [4], posing the question of whether its immunosuppressive effects may lessen the efficacy of ICI. Wide national variation in adoption of antiemesis guidelines [12] has prompted the U.S. Centers for Medicare and Medicaid Services to implement 30‐day postchemotherapy acute care as a new performance measure [13]. In this perspective, we discuss considerations for clinical decision‐making around antiemetic prescribing in patients receiving combination ICI treatments.
Biological Function and Pharmacology of Glucocorticoids
GCs are a type of steroid hormone derived from cholesterol that bind to and then activate the glucocorticoid receptor (GR), which is expressed in almost all cells. The main endogenous GC in humans, cortisol, was first isolated from the adrenal cortex in 1938 [14]. The immunosuppressive role of cortisol was discovered in 1948, with the finding that injections of cortisol could treat rheumatoid arthritis (RA) [15], leading to the Nobel Prize in Medicine in 1950. Cortisol is essential for life [16] and is released according to a circadian rhythm, with peaks before waking and troughs around midnight [17]. Synthetic GCs have been developed through chemical modification of cortisol's molecular structure; examples include prednisolone, methylprednisolone and dexamethasone [18]. The major differences between GC derivatives relate to potency, duration of action, and mineralocorticoid receptor affinity. For example, dexamethasone has been modified to prolong its duration of action (using cortisol suppression as a surrogate marker) from approxiamtely 8 to 36 hours and increase its anti‐inflammatory potency 20‐fold [19]. Despite having a similar plasma half‐life to prednisolone (3–4 hours) [20], dexamethasone has a markedly longer duration of action [21], suggesting the possibility of altered binding kinetics with GR [22]. GC‐mediated activation of GR causes homodimerization and translocation to the nucleus, where the GR drives a complex transcriptional program affecting up to 20% of all genes [23], with pleiotropic effects on multiple immune subsets [24]. The dominant mechanism by which GCs drive immune suppression remains poorly understood. Recently, GR signaling was shown to promote gene expression signatures reflecting T‐cell dysfunction, associated with increased expression of inhibitory checkpoint receptors such as programmed cell death protein‐1 and increased interleukin‐10 production [25].
Antiemetic Effects of Glucocorticoids
Exogenous GCs, in particular dexamethasone, are routinely used for the preventative treatment of CINV [26], and benefit is enhanced in combination with 5‐HT3 receptor antagonists such as granisetron and NK1 receptor antagonists such as aprepitant [27, 28]. This is consistent with evidence that CINV is mediated by abdominal vagal afferents and neurons in the area postrema, both of which express 5‐HT3 and NK1 receptors [29]. Although dexamethasone is the most common exogenous GC used for antiemesis, response rates to methylprednisolone are broadly comparable [30, 31]. The antiemetic mechanism of action of GC is poorly understood and may involve reduction prostaglandin production [32], direct noncompetitive modulation of the 5‐HT3 receptor [33] or compensation for acute adrenal hypofunction induced by platinum‐based chemotherapy [34]. In comparison with other synthetic GCs, dexamethasone has a prolonged duration of action, which could partly explain its efficacy for CINV.
Immune Suppressive Effects of Glucocorticoids
Endogenous GC secretion is inversely related to inflammation [35, 36]. Exogenous GCs are prescribed routinely for their immunosuppressive effects, across a range of indications including transplantation, RA, systemic lupus erythematosus, asthma, eczema, and septic shock. Prednisolone treatment is sufficient to prevent rejection of a genetically distinct (allogenic) transplant [37]. It has been recently demonstrated that the transcriptional immune profile of tumors responding to cancer immunotherapy shares the same activation profile and integrated immune response as rejecting renal allografts, including accumulation of T, natural killer, and B cells and the formation of tertiary lymphoid structures [38]. Furthermore, ICI treatment can promote both allograft rejection and tumor regression in the same patient [39], consistent with convergent immunological responses. Considering that, like allografts, tumors express non‐self antigens that derive from accumulation of coding genetic alterations (neoantigens) [40], it is conceivable that GC signaling would promote immunological tolerance to tumors and so curb the effectiveness of ICI. Consistent with this hypothesis, moderate elevations in systemic GCs are sufficient to cause suppression of T‐cell activation signatures and failure of anti–programmed death ligand‐1 antibody and CXCR4 antagonist treatment in an in vivo spontaneous genetically engineered model of pancreatic cancer [41].
Clinical Studies Relating to Coadministration of Glucocorticoids and Immunotherapy
There are three frequent situations in which GCs could be coadministered with ICIs (Fig. 1): baseline (for example, patients with chronic autoimmune conditions), antiemetic prophylaxis for HEC, and secondary immune modulation in patients with immune‐related adverse effects (irAEs), such as colitis [42]. irAEs are strongly associated with beneficial responses [43, 44, 45, 46], likely reflecting systemic immune competence [47], and in this subset there is minimal evidence that exogenous GCs blunt response to treatment. In contrast, baseline administration of supraphysiological doses of GCs is associated with adverse clinical outcomes in melanoma, NSCLC, and glioblastoma [48, 49, 50]. For example, Arbour et al. reported that baseline GC equivalent to more than 10 mg prednisolone per day was associated with significantly poorer overall survival on multivariate analysis in patients with NSCLC (hazard ratio, 1.66; p < .001) [48]. Genetic variants partly explain the risk of autoimmune conditions that are treated with systemic steroids, such as RA [51], and in turn, such variants are associated with enhanced responsiveness to ICI [52]. This potentially confounding issue may partly explain contradictory findings regarding the association between baseline GC and ICI response, which has not been consistently demonstrated in all patient cohorts [53]. Consistent with this, an analysis of ICI‐treated patients with NSCLC found that the subset of patients taking GCs for underlying cancer‐unrelated indications, such as RA, had noninferior survival outcomes [54].
Figure 1.
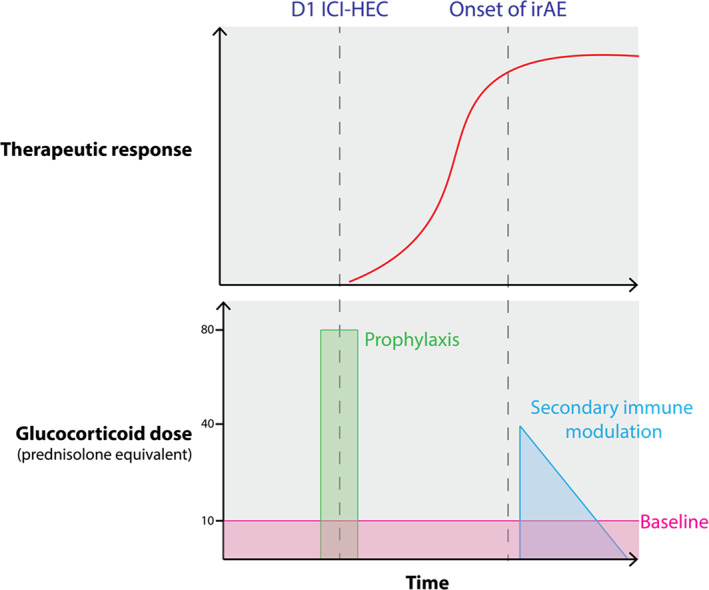
Coadministration of glucocorticoids (GCs) with ICIs can be classified into three phases: prophylaxis coinciding with day 1 for patients treated in combination with HEC drugs such as carboplatin, secondary immune modulation coinciding with irAEs or baseline chronic treatment in patients with chronic autoimmune disease or organ transplants. irAEs are associated with an active therapeutic response, with unmasking of preexisting anti‐tumoral immunity by ICIs. Approximate doses are shown as prednisolone equivalents in milligrams, with 12 mg dexamethasone approximately equivalent to 80 mg prednisolone.Abbreviations: HEC, highly emetogenic chemotherapy; ICI, immune checkpoint inhibitor; irAE, immune‐related adverse effect.
Approximately 40% of ICI clinical trials specifically preclude the use of baseline exogenous GC [55]. This includes trials demonstrating the benefit of combination ICI regimens in NSCLC, SCLC, and HNSCC [8, 56, 57, 58, 59, 60, 61], making it difficult to extrapolate these findings to patients on long‐term GC treatment. There is a paucity of data relating the implications of short‐term dexamethasone as antiemetic prophylaxis in patients receiving ICI in combination with HEC. Trials examining ICI‐HEC combinations vary between recommending [56], permitting [59], or explicitly advising against dexamethasone‐based prophylaxis [11, 57, 58, 60, 61]. Consequently, from the available data, it is not possible to assess what proportion of patients in these trials received dexamethasone prophylaxis and whether it was associated with inferior clinical outcomes. In clinical trials of agonistic CD40 monoclonal antibodies as immune therapy of cancer, biomarkers of immune response at 2–3 days after infusion are highly blunted if the patient receives dexamethasone either prophylactically or as needed for monoclonal antibody infusional reactions [62]. Altogether, the totality of molecular, preclinical, and clinical data suggest that supraphysiological GC treatment preceding ICI may well reduce effectiveness. It is likely that these findings can be extrapolated to dexamethasone‐based emesis prophylaxis, despite absence of specific data.
Conclusion and Perspectives
Available evidence supports a model in which steroids, akin to their role in transplant medicine, can drive immunological tolerance to the tumor and so undermine the action of ICIs. When ICIs were first approved as single‐agents this issue was less concerning, but with increasing number of indications of ICI and chemotherapy, it becomes important to better understand. We raise the question of whether the clinical benefits of ICI and chemotherapy may be suboptimal in the setting of GCs given prior or during chemotherapy administration. Recent guidance from the American Society of Clinical Oncology (ASCO), however, states that “there is no clinical evidence to warrant omission of dexamethasone from guideline‐compliant prophylactic antiemetic regimens when…[ICIs] are administered to adults in combination with chemotherapy” [4]. We propose consideration of an alternative approach focused on the adoption of dexamethasone‐sparing prophylaxis, with the aim of administering the minimum dose of dexamethasone that is sufficient to relieve symptoms.
Current national guidelines from National Comprehensive Cancer Network and ASCO recommend 8–12 mg dexamethasone once daily on days 1–4 of each cycle of HEC [4, 63]. In light of the prolonged duration of action of dexamethasone (>32 hours), it is likely that supraphysiological GR signaling may persist for days or even weeks after a 4‐day course of dexamethasone. Indeed, a 4‐day course of dexamethasone is at least as effective as a 4‐week course of prednisolone for primary immune thrombocytopenia [64], whereas the efficacy of a single dose of dexamethasone is broadly comparable to a 4‐day course of prednisolone for acute asthma exacerbations [65, 66]. Recently, a randomized controlled trial (RCT) in patients receiving HEC demonstrated that, when in combination with 5‐HT3 and NK1 antagonists, a single dose of 8 mg dexamethasone on day 1 is noninferior to a 3‐day course [67]. A recent RCT in cisplatin‐treated patients demonstrated the addition of 5 mg olanzapine once daily on days 1–4 to standard emesis prophylaxis (including dexamethasone) substantially increased the proportion of patients without vomiting from 66% to 79% [68].
We fully acknowledge the importance of minimizing side‐effects from treatments for patients and, therefore, of high‐quality prophylaxis of nausea and vomiting during HEC treatment. However, we and other colleagues [69] advocate for consideration of alternatives to high doses of dexamethasone, until more is understood regarding potentially negative effects of dexamethasone with ICI and chemotherapy. We recommend the lowest effective steroid dose, tailored to the patient, and suggest reconsideration of the broad use of standard operating procedures in oncology infusion suites that call for high‐dose dexamethasone administration as prophylaxis.
Disclosures
Robert H. Vonderheide: Medimmune, Verastem (C/A, H) Fibrogen, Janssen, Eli Lilly & Co(RF), inventor on a licensed patent relating to cancer cellular immunotherapy and cancer vaccines (IP), Children's Hospital Boston (Other‐royalties for a licensed research‐only monoclonal antibody). The other authors indicated no financial relationships.
(C/A) Consulting/advisory relationship; (RF) Research funding; (E) Employment; (ET) Expert testimony; (H) Honoraria received; (OI) Ownership interests; (IP) Intellectual property rights/inventor/patent holder; (SAB) Scientific advisory board
Acknowledgments
This work was supported, in part, by Developmental Funds from the Cold Spring Harbor Laboratory (CSHL) Cancer Center Support grant 5P30CA045508. Funding is acknowledged from CSHL (to T. Janowitz and S. Kleeman), from the Pershing Square Foundation, and the Mark Foundation for Cancer Research (to T. Janowitz), Funding is acknowledge from the NIH P30 CA016520 (BV).
Disclosures of potential conflicts of interest may be found at the end of this article.
Editor's Note: See the related articles, “Avoidable Acute Care Use Associated with Nausea and Vomiting Among Patients Receiving Highly Emetogenic Chemotherapy or Oxaliplatin,” by Rudolph M. Navari, Kathryn J. Ruddy, Thomas W. LeBlanc, et al., on page 325 and “Emergency Department Visits for Emesis Following Chemotherapy: Guideline Nonadherence, OP‐35, and a Path Back to the Future,” by Alfred I. Neugut and Susan E. Bates, on page 274 of this issue.
No part of this article may be reproduced, stored, or transmitted in any form or for any means without the prior permission in writing from the copyright holder. For information on purchasing reprints contact commercialreprints@wiley.com. For permission information contact permissions@wiley.com.
References
- 1. De Boer‐Dennert M, De Wit R, Schmitz PIM et al. Patient perceptions of the side‐effects of chemotherapy: The influence of 5HT3 antagonists. Br J Cancer 1997;76:1055–1061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Burke TA, Wisniewski T, Ernst FR. Resource utilization and costs associated with chemotherapy‐induced nausea and vomiting (CINV) following highly or moderately emetogenic chemotherapy administered in the US outpatient hospital setting. Support Care Cancer 2011;19:131–140. [DOI] [PubMed] [Google Scholar]
- 3. Roila F, Molassiotis A, Herrstedt J et al. 2016. MASCC and ESMO guideline update for the prevention of chemotherapy‐ and radiotherapy‐induced nausea and vomiting and of nausea and vomiting in advanced cancer patients. Ann Oncol 2016;27(suppl 5):v119–v133. [DOI] [PubMed] [Google Scholar]
- 4. Hesketh PJ, Kris MG, Basch E et al. Antiemetics: ASCO guideline update. J Clin Oncol 2020;38:2782–1797. [DOI] [PubMed] [Google Scholar]
- 5. Grunberg SM, Warr D, Gralla RJ et al. Evaluation of new antiemetic agents and definition of antineoplastic agent emetogenicity ‐ State of the art. Support Care Cancer 2011;19 suppl 1:S43–S47. [DOI] [PubMed] [Google Scholar]
- 6. KEYTRUDA (pembrolizumab) injection, for intravenous use. Initial U.S. approval 2014. U.S. Food and Drug Administration; 2020. [Google Scholar]
- 7. TECENTRIQ (atezolizumab) injection, for intravenous use. Initial U.S. approval 2016. U.S. Food and Drug Administration; 2020. [Google Scholar]
- 8. Paz‐Ares L, Luft A, Vicente D et al. Pembrolizumab plus chemotherapy for squamous non–small‐cell lung cancer. N Engl J Med 2018;379:2040–2051. [DOI] [PubMed] [Google Scholar]
- 9. Schmid P, Adams S, Rugo HS et al. Atezolizumab and nab‐paclitaxel in advanced triple‐negative breast cancer. N Engl J Med 2018;379:2108–2121. [DOI] [PubMed] [Google Scholar]
- 10. Beatty GL, Chiorean EG, Fishman MP et al. CD40 agonists alter tumor stroma and show efficacy against pancreatic carcinoma in mice and humans. Science 2011;331:1612–1616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. O'Hara MH, O'Reilly EM, Varadhachary G et al. An open‐label, multicenter, phase 1b study evaluating the safety and efficacy of CD40 Agonistic monoclonal antibody APX005M and chemotherapy, with or without nivolumab, for the treatment of metastatic pancreatic adenocarcinoma. Lancet Oncol 2020;22:118–131. [DOI] [PubMed] [Google Scholar]
- 12. Navari RM, Ruddy KJ, LeBlanc TW et al. Avoidable acute care use associated with nausea and vomiting among patients receiving highly emetogenic chemotherapy or oxaliplatin. The Oncologist 2020. (Epub ahead of print). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Centers for Medicare & Medicaid Services . CMS proposes hospital outpatient prospective payment changes for 2017. July 6, 2016. Available at www.cms.gov/newsroom/fact‐sheets/cms‐proposes‐hospital‐outpatient‐prospective‐payment‐changes‐2017. Accessed 11, 29, 2020.
- 14. Mason HL, Hoehn WM, Kendal EC. Chemical studies of supra‐renal cortex iv structure of compounds C‐D‐E‐F and G. J Biol Chem 1938;124:459–474. [Google Scholar]
- 15. Hench PS, Kendall EC. The effect of a hormone of the adrenal cortex (17‐hydroxy‐11‐dehydrocorticosterone; compound E) and of pituitary adrenocorticotropic hormone on rheumatoid arthritis. Proc Staff Meet Mayo Clin 1949;24:181–197. [PubMed] [Google Scholar]
- 16. Oelkers W. Adrenal insufficiency. N Engl J Med 1996;335:1206–1212. [DOI] [PubMed] [Google Scholar]
- 17. Weitzman ED, Fukushima D, Nogeire C et al. Twenty‐four hour pattern of the episodic secretion of cortisol in normal subjects. J Clin Endocrinol Metab 1971;33:14–22. [DOI] [PubMed] [Google Scholar]
- 18. Hardy RS, Raza K, Cooper MS. Therapeutic glucocorticoids: Mechanisms of actions in rheumatic diseases. Nat Rev Rheumatol 2020;16:133–144. [DOI] [PubMed] [Google Scholar]
- 19. Goodman LS, Hardman JG, Limbird LE et al. Goodman and Gilman's the Pharmacological Basis of Therapeutics. New York: McGraw‐Hill; 1996. [Google Scholar]
- 20. Czock D, Keller F, Rasche FM et al. Pharmacokinetics and pharmacodynamics of systemically administered glucocorticoids. Clin Pharmacokinet 2005;44:61–98. [DOI] [PubMed] [Google Scholar]
- 21. Mager DE, Lin SX, Blum RA et al. Dose equivalency evaluation of major corticosteroids: Pharmacokinetics and cell trafficking and cortisol dynamics. J Clin Pharmacol 2003;43:1216–1227. [DOI] [PubMed] [Google Scholar]
- 22. Koopmans RP, Braat MCP, Oosterhuis B et al. Time‐dependent effects of dexamethasone administration on the suppression of plasma hydrocortisone, assessed with a pharmacokinetic model. J Pharmacol Exp Ther 1992;262:503–508. [PubMed] [Google Scholar]
- 23. Galon J, Franchimont D, Hiroi N et al. Gene profiling reveals unknown enhancing and suppressive actions of glucocorticoids on immune cells. FASEB J 2002;16:61–71. [DOI] [PubMed] [Google Scholar]
- 24. Chrousos GP. The hypothalamic–pituitary–adrenal axis and immune‐mediated inflammation. N Engl J Med 1995;332:1351–1362. [DOI] [PubMed] [Google Scholar]
- 25. Acharya N, Madi A, Zhang H et al. Endogenous glucocorticoid signaling regulates CD8+ T cell differentiation and development of dysfunction in the tumor microenvironment. immunity 2020;53:658–671.e6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Aapro MS, Alberts DS. High‐dose dexamethasone for prevention of cis‐platin‐induced vomiting. Cancer Chemother Pharmacol 1981;7:11–14. [DOI] [PubMed] [Google Scholar]
- 27. Schmitt T, Goldschmidt H, Neben K et al. Aprepitant, granisetron, and dexamethasone for prevention of chemotherapy‐induced nausea and vomiting after high‐dose melphalan in autologous transplantation for multiple myeloma: Results of a randomized, placebo‐controlled phase III Trial. J Clin Oncol 2014;32:3413–3420. [DOI] [PubMed] [Google Scholar]
- 28. The Italian Group for Antiemetic Research . Dexamethasone, granisetron, or both for the prevention of nausea and vomiting during chemotherapy for cancer. N Engl J Med 1995;332:1–5. [DOI] [PubMed] [Google Scholar]
- 29. Hesketh PJ. Chemotherapy‐induced nausea and vomiting. N Engl J Med 2008;358:2482–2494. [DOI] [PubMed] [Google Scholar]
- 30. Chiara S, Campora E, Lionetto R et al. Methylprednisolone for the control of CMF‐induced emesis. Am J Clin Oncol Cancer Clin Trials 1987;10:264–267. [DOI] [PubMed] [Google Scholar]
- 31. Lee BJ. Methylprednisolone as an Antiemetic. N Engl J Med 1981;304:486. [DOI] [PubMed] [Google Scholar]
- 32. Chu CC, Hsing CH, Shieh JP et al. The cellular mechanisms of the antiemetic action of dexamethasone and related glucocorticoids against vomiting. Eur J Pharmacol 2014;722:48–54. [DOI] [PubMed] [Google Scholar]
- 33. Suzuki T, Sugimoto M, Koyama H et al. Inhibitory effect of glucocorticoids on human‐cloned 5‐ hydroxytryptamine3A receptor expressed in xenopus oocytes. Anesthesiology 2004;101:660–605. [DOI] [PubMed] [Google Scholar]
- 34. Morrow GR, Hickok JT, Andrews PLR et al. Reduction in serum cortisol after platinum based chemotherapy for cancer: A role for the HPA axis in treatment‐related nausea? Psychophysiology 2002;39:491–495. [DOI] [PubMed] [Google Scholar]
- 35. Cove‐Smith JR, Kabler P, Pownall R et al. Circadian variation in an immune response in man. Br Med J 1978;2:253–254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Harkness JAL, Richter MB, Panayi GS et al. Circadian variation in disease activity in rheumatoid arthritis. Br Med J (Clin Res Ed) 1982;284:551–554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Starzl TE, Marchioro TL, Waddell WR. THE reversal of rejection in human renal homografts with subsequent development of homograft tolerance. Surg Gynecol Obstet 1963;117:385–395. [PMC free article] [PubMed] [Google Scholar]
- 38. Biasci D, Smoragiewicz M, Connell CM et al. CXCR4 inhibition in human pancreatic and colorectal cancers induces an integrated immune response. Proc Natl Acad Sci USA 2020;117:28960–28970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Lipson EJ, Bagnasco SM, Moore J et al. Tumor regression and allograft rejection after administration of anti–PD‐1. N Engl J Med 2016;374:896–898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. McGranahan N, Furness AJS, Rosenthal R et al. Clonal neoantigens elicit T cell immunoreactivity and sensitivity to immune checkpoint blockade. Science 2016;351:1463–1369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Flint TR, Janowitz T, Connell CM et al. Tumor‐induced IL‐6 reprograms host metabolism to suppress anti‐tumor immunity. Cell Metab 2016;24:672–684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Brahmer JR, Lacchetti C, Schneider BJ et al. Management of immune‐related adverse events in patients treated with immune checkpoint inhibitor therapy: American society of clinical oncology clinical practice guideline. J Clin Oncol 2018;36:1714–1768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Ellen Maher V, Fernandes LL, Weinstock C et al. Analysis of the association between adverse events and outcome in patients receiving a programmed death protein 1 or programmed death ligand 1 antibody. J Clin Oncol 2019;37:2730–2737. [DOI] [PubMed] [Google Scholar]
- 44. Eggermont AMM, Kicinski M, Blank CU et al. Association between immune‐related adverse events and recurrence‐free survival among patients with stage III melanoma randomized to receive pembrolizumab or placebo: A secondary analysis of a randomized clinical trial. JAMA Oncol 2020;6:519–527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Tawagi K, Maslov D, Simenson V et al. Cumulative steroid doses and response rates to immune checkpoint inhibitors in metastatic cancer. J Clin Oncol 2020;38:e15133a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Elias R, Yan F, Singla N et al. Immune‐related adverse events are associated with improved outcomes in ICI‐treated renal cell carcinoma patients. J Clin Oncol 2019;37:64a. [Google Scholar]
- 47. Postow MA, Sidlow R, Hellmann MD. Immune‐related adverse events associated with immune checkpoint blockade. N Engl J Med 2018;378:158–168. [DOI] [PubMed] [Google Scholar]
- 48. Arbour KC, Mezquita L, Long N et al. Impact of baseline steroids on efficacy of programmed cell death‐1 and programmed death‐ligand 1 blockade in patients with non–small‐cell lung cancer. J Clin Oncol 2018;36:2872–2878. [DOI] [PubMed] [Google Scholar]
- 49. Tokunaga A, Sugiyama D, Maeda Y et al. Selective inhibition of low‐affinity memory CD8+ T cells by corticosteroids. J Exp Med 2019;216:2701–2713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Iorgulescu JB, Gokhale PC, Speranza MC et al. Concurrent dexamethasone limits the clinical benefit of immune checkpoint blockade in glioblastoma. Clin Cancer Res 2020;27:276–287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Stahl EA, Wegmann D, Trynka G et al. Bayesian inference analyses of the polygenic architecture of rheumatoid arthritis. Nat Genet 2012;44:483–489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Khan Z, Di Nucci F, Kwan A et al. Polygenic risk for skin autoimmunity impacts immune checkpoint blockade in bladder cancer. Proc Natl Acad Sci USA 2020;117:12288–12294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Garant A, Guilbault C, Ekmekjian T et al. Concomitant use of corticosteroids and immune checkpoint inhibitors in patients with hematologic or solid neoplasms: A systematic review. Crit Rev Oncol Hematol 2017;120:.86–92. [DOI] [PubMed] [Google Scholar]
- 54. Ricciuti B, Dahlberg SE, Adeni A et al. Immune checkpoint inhibitor outcomes for patients with non‐small‐cell lung cancer receiving baseline corticosteroids for palliative versus nonpalliative indications. J Clin Oncol 2019;37:1927–1934. [DOI] [PubMed] [Google Scholar]
- 55. Connell CM, Raby S, Beh I et al. Cancer immunotherapy trial registrations increase exponentially but chronic immunosuppressive glucocorticoid therapy may compromise outcomes. Ann Oncol 2017;1678–1679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Gandhi L, Rodríguez‐Abreu D, Gadgeel S et al. Pembrolizumab plus chemotherapy in metastatic non–small‐cell lung cancer. N Engl J Med 2018;378:2078–2092. [DOI] [PubMed] [Google Scholar]
- 57. Burtness B, Harrington KJ, Greil R et al. Pembrolizumab alone or with chemotherapy versus cetuximab with chemotherapy for recurrent or metastatic squamous cell carcinoma of the head and neck (KEYNOTE‐048): A randomised, open‐label, phase 3 study. Lancet 2019;394:1915–1928. [DOI] [PubMed] [Google Scholar]
- 58. Horn L, Mansfield AS, Szczęsna A et al. First‐line atezolizumab plus chemotherapy in extensive‐stage small‐cell lung cancer. N Engl J Med 2018;379:2220–2229. [DOI] [PubMed] [Google Scholar]
- 59. Paz‐Ares L, Dvorkin M, Chen Y et al. Durvalumab plus platinum–etoposide versus platinum–etoposide in first‐line treatment of extensive‐stage small‐cell lung cancer (CASPIAN): A randomised, controlled, open‐label, phase 3 trial. Lancet 2019;394:1929–1939. [DOI] [PubMed] [Google Scholar]
- 60. Socinski MA, Jotte RM, Cappuzzo F et al. Atezolizumab for first‐line treatment of metastatic nonsquamous nsclc. N Engl J Med 2018;378:2288–2301. [DOI] [PubMed] [Google Scholar]
- 61. West H, McCleod M, Hussein M et al. Atezolizumab in combination with carboplatin plus nab‐paclitaxel chemotherapy compared with chemotherapy alone as first‐line treatment for metastatic non‐squamous non‐small‐cell lung cancer (IMpower130): A multicentre, randomised, open‐label, phase 3 trial. Lancet Oncol 2019;20:924–937. [DOI] [PubMed] [Google Scholar]
- 62. Vonderheide RH. CD40 agonist antibodies in cancer immunotherapy. Annu Rev Med 2020;27:47–58. [DOI] [PubMed] [Google Scholar]
- 63. Berger MJ, Ettinger DS, Aston J et al. NCCN Guidelines Insights: Antiemesis, Version 2.2017. J Natl Compr Cancer Netw 2017;15:883–893. [DOI] [PubMed] [Google Scholar]
- 64. Wei Y, Bin Ji X, Wang YW et al. High‐dose dexamethasone vs prednisone for treatment of adult immune thrombocytopenia: A prospective multicenter randomized trial. Blood 2016;127:296–302; quiz 372. [DOI] [PubMed] [Google Scholar]
- 65. Rehrer MW, Liu B, Rodriguez M et al. A Randomized controlled noninferiority trial of single dose of oral dexamethasone versus 5 days of oral prednisone in acute adult asthma. Ann Emerg Med 2016;68:608–613. [DOI] [PubMed] [Google Scholar]
- 66. Altamimi S, Robertson G, Jastaniah W et al. Single‐dose oral dexamethasone in the emergency management of children with exacerbations of mild to moderate asthma. Pediatr Emerg Care 2006;22:786–793. [DOI] [PubMed] [Google Scholar]
- 67. Ito Y, Tsuda T, Minatogawa H et al. Placebo‐controlled, double‐blinded phase III study comparing dexamethasone on day 1 with dexamethasone on days 1 to 3 with combined neurokinin‐1 receptor antagonist and palonosetron in high‐emetogenic chemotherapy. J Clin Oncol 2018;36:1000–1006. [DOI] [PubMed] [Google Scholar]
- 68. Hashimoto H, Abe M, Tokuyama O et al. Olanzapine 5 mg plus standard antiemetic therapy for the prevention of chemotherapy‐induced nausea and vomiting (J‐FORCE): A multicentre, randomised, double‐blind, placebo‐controlled, phase 3 trial. Lancet Oncol 2020;21:242–249. [DOI] [PubMed] [Google Scholar]
- 69. Neugut AI, Bates SE. Emergency department visits for emesis following chemotherapy: Guideline nonadherence, OP‐35, and a path back to the future. The Oncologist 2020. (Epub ahead of print). [DOI] [PMC free article] [PubMed] [Google Scholar]


