ABSTRACT
A long-term follow-up (LTFU) of the nine-valent human papillomavirus (9vHPV) vaccine efficacy study in young women aged 16–26 years was initiated to evaluate if vaccine effectiveness for up to 14 years post-vaccination will remain above 90%. Vaccine effectiveness is measured as percent reduction in the incidence of HPV16/18/31/33/45/52/58-related high-grade cervical dysplasia in the LTFU cohort relative to expected incidence in a similar unvaccinated cohort. We report an interim analysis 8 years post-vaccination. Overall, 2029 participants from Denmark, Norway, and Sweden who received the 9vHPV vaccine during the clinical efficacy study continued into the LTFU study. National health registries were used to identify screening attendance and cervical pre-cancer/cancer diagnoses. Tissue samples were retrieved for HPV testing by PCR and pathology diagnosis adjudication. A control chart method was used to detect signals indicative of vaccine effectiveness waning below 90%. No new cases of HPV16/18/31/33/45/52/58-related high-grade cervical dysplasia were observed during the LTFU study period over 4084.2 person-years’ follow-up (per-protocol effectiveness population; n = 1448). Thus, there were no signals indicative of vaccine effectiveness waning below 90%. These observations show that the 9vHPV vaccine provides continued statistically significant protection through at least 6 years, with indications of continued effectiveness through 8 years.
Trial registration
Clinicaltrials.gov: NCT00543543, NCT02653118.
KEYWORDS: Cervical intraepithelial neoplasia, effectiveness, human papillomavirus, long-term follow-up, vaccine, nine-valent human papillomavirus vaccine
The nine-valent human papillomavirus (9vHPV) vaccine was developed to prevent persistent infection and disease related to the four HPV types covered by the quadrivalent HPV (qHPV; HPV6/11/16/18) vaccine as well as the next five most common oncogenic HPV types (HPV31/33/45/52/58). In the pivotal efficacy study in young women (Study V503-001; NCT00543543), the 9vHPV vaccine demonstrated efficacy against persistent infection and cervical, vulvar, and vaginal disease related to the HPV types covered by the vaccine.1–3 Robust antibody responses to the nine HPV types persisted through 5 years in individuals who received the 9vHPV vaccine.1
A long-term follow-up (LTFU) extension study of the pivotal efficacy study (base study) was initiated to assess the effectiveness of the 9vHPV vaccine for a total of 14 years from the start of vaccination (i.e., approximately 4 years in the base study plus 10 years in the LTFU) in the Scandinavian countries of Denmark, Norway, and Sweden.4 These countries have implemented nationwide cervical-cancer screening programs with routine centralized administration and registration, allowing the LTFU study to leverage national health registries to assess the incidence of cervical pre-cancers and cancers due to vaccine HPV types in a registry-based effectiveness follow-up. Pre-specified interim analyses are conducted every 2 years to promptly detect any evidence of waning efficacy during the LTFU study period. We report data from an interim analysis conducted 8 years after the third 9vHPV vaccine dose.
The base-study design and results have been described in detail elsewhere.1,2,5,6 Briefly, young women 16 to 26 years of age from 18 countries (n = 14,215) were randomized 1:1 to receive a three-dose regimen of the 9vHPV or qHPV vaccine, at Day 1, Month 2, and Month 6. Participants were followed for efficacy, immunogenicity, and safety at scheduled visits approximately every 6 months through to the Month 54 visit (Figure 1).1,2,5 The median follow-up in the base study was 4 years (maximum 6 years).
Figure 1.
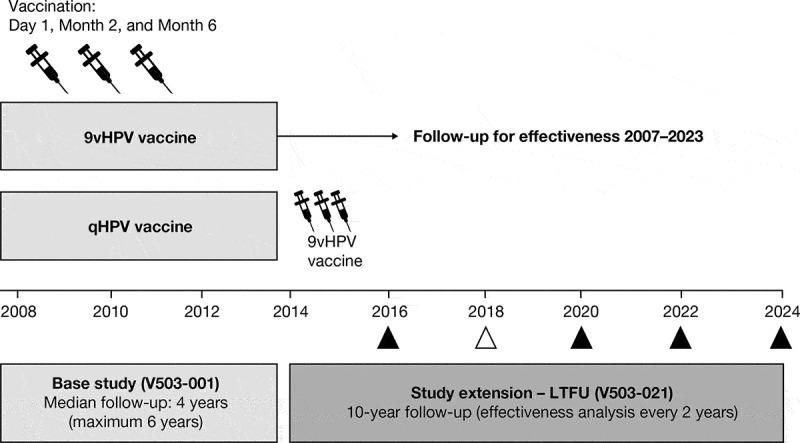
Study design. In the base study, participants received the 9vHPV vaccine or qHPV vaccine (control) at Day 1, Month 2, and Month 6, and were followed for efficacy every 6 months thereafter up to the Month 54 visit. After their last visit in the base study, participants from Scandinavian countries who received 9vHPV in the base study and provided consent continued for effectiveness follow-up in the study extension (LTFU study). Follow-up in the study extension begins for each participant after their last visit in the base study to ensure continuous follow-up between the base study and the study extension. In the study extension, follow-up for effectiveness is based on a search of national health registries; analyses of data are conducted every 2 years. The timing of each analysis is shown as a triangle; the timing of the current analysis is shown as an empty triangle. 9vHPV, nine-valent human papillomavirus; LTFU, long-term follow-up; qHPV, quadrivalent human papillomavirus
Base-study participants from Denmark, Norway, and Sweden continued into the LTFU study (Protocol V503-021; NCT02653118), which was initiated on January 1, 2014 and is ongoing at the time of this report. The LTFU study design has been described elsewhere.4 Key information relevant to this interim report is summarized below and in Figure 1. We report effectiveness data through Year 4 of the LTFU study (data cutoff date: January 1, 2018) from the cohort of participants who received the 9vHPV vaccine in the base study and provided consent for the LTFU study. Registry-based follow-up for effectiveness in the LTFU study began the day after a participant’s final visit in the base study. Participants who withdrew early from the base study were eligible for enrollment in the LTFU study; LTFU effectiveness follow-up for such participants started after their last visit in the base study. The study is being conducted in accordance with principles of Good Clinical Practice, and was approved by the respective ethics committees and/or regulatory agencies, as appropriate. The participants provided informed consent at the start of the base study and for registry-based follow-up in the LTFU study.
The primary objective of the LTFU study is to ascertain that the long-term effectiveness of the 9vHPV vaccine against the combined incidence of cervical pre-cancers (cervical intraepithelial neoplasia [CIN]2, CIN3, adenocarcinoma in situ [AIS]) and cervical cancers, referred to as “CIN2 or worse”, related to HPV types 16, 18, 31, 33, 45, 52, and 58 in women vaccinated with the 9vHPV vaccine will remain at least 90% for up to 14 years post-vaccination. Ascertainment of long-term effectiveness against the composite incidence of CIN (any grade), AIS, cervical cancer, vulvar cancer (in situ or invasive), and vaginal cancer (in situ or invasive) related to HPV types 6, 11, 16, 18, 31, 33, 45, 52, and 58 is a secondary objective.
National Registry Study Centers (NRSCs) were established in participating countries to perform regular linkages between the study participants and national screening registries.4 The NRSCs retrieved information from the registries about the population, screening, and follow-up, including details of pathology diagnoses, endocervical curettage, and definitive therapy. Based on identification in the registries, the relevant tissue specimens were obtained from hospital archives for adjudication of pathology diagnosis for determination of study endpoints by the Nordic Pathology Panel (NPP) and tested for HPV DNA by polymerase chain reaction (PCR). An outcome case related to a given HPV type occurred if (1) the NPP provided a consensus diagnosis, and (2) PCR testing detected the relevant HPV type in an adjacent section from the same tissue block.4 The specimen adjudication process was the same as in the base study.5 HPV DNA was detected in tissue samples using the same multiplex PCR assay as used in the base study and qHPV vaccine clinical development program.1,4,7,8
Participants who received the qHPV vaccine in the base study could not be used as controls in the LTFU study since they were offered the 9vHPV vaccination at the end of the base study.4 Therefore, vaccine effectiveness against the primary endpoint of HPV-related CIN2 or worse was calculated as the percent reduction in the incidence of HPV-related CIN2 or worse in the LTFU cohort relative to the expected incidence in an unvaccinated cohort of similar age and risk exposure. The expected incidence in an unvaccinated cohort of similar age and risk exposure was estimated using data from both an historical registry and a large survey study performed in each participating country. Information from Nordic registries (Denmark, Iceland, Norway, and Sweden) prior to the introduction of the qHPV vaccine accurately measured the incidence of HPV-related disease in the entire population in an unvaccinated state. The Concomitant Cohort Study surveyed upwards of 70,000 women across four Nordic countries prior to the introduction of HPV vaccination (2004–2005) and collected information on lifestyle, including their sexual habits.9 Based on a combination of these sources and accounting for the proportions of LTFU-study participants from each country, the incidence of HPV-related CIN2 or worse among participants 23–29 years of age with one to six sexual partners were estimated as 548 per 100,000 person-years.4 As HPV types 16, 18, 31, 33, 45, 52, and 58 are associated with approximately 80% of HPV-related CIN2 or worse lesions in Europe,10 the incidence of HPV16/18/31/33/45/52/58-related CIN2 or worse was estimated to be 438 per 100,000 person-years. An adapted Poisson Shewhart-based control chart method was used for the primary analysis of monitoring breakthrough disease and waning vaccine effectiveness, as previously described.4 Upper monitoring bounds (or upper control limits) were used to indicate whether the incidence of breakthrough disease in the LTFU cohort exceeds 43.8/100,000 person-years, which is indicative of effectiveness falling below 90%, by plotting the observed numbers of endpoint cases in comparison with the upper control limits within 2-year intervals. The analyses were conducted in the per-protocol effectiveness (PPE) population, which included participants who received all three doses of the 9vHPV vaccine within 1 year, were seronegative at Day 1 and PCR-negative from Day 1 to Month 7 of the base study for the HPV type being analyzed, and had no protocol violations that could affect the evaluation of vaccine prophylactic efficacy.
A total of 2223 participants from Denmark, Norway, and Sweden received at least one dose of the 9vHPV vaccine in the base study, of whom 2029 consented to being included in registry-based searches for long-term effectiveness analyses. For this interim analysis, participants were followed for effectiveness for up to 9.5 years post-Dose 3 (median: 6.3 years) or 10.0 years post-Dose 1 (median: 6.8 years). Most (n = 1699/2029, 83.7%) participants had at least one cervical cytology/HPV screening visit during the LTFU period covered by this analysis (Denmark: n = 1416/1653, 85.7%; Norway: n = 249/318, 78.3%; Sweden: n = 34/58, 58.6%).
No cases of HPV16/18/31/33/45/52/58-related CIN2 or worse were observed during the LTFU period, indicating a vaccine effectiveness of 100% (95% confidence interval: 79.4–100) during the LTFU period (Table 1). Based on the 4084.2 person-years of follow-up time accrued during the LTFU study period and an estimated background incidence rate of 438 per 100,000 person-years in an unvaccinated population, at most two cases of HPV16/18/31/33/45/52/58-related CIN2 or worse were expected if vaccine effectiveness was maintained at ≥90%.
Table 1.
Analysis of 9vHPV vaccine effectiveness against HPV16/18/31/33/45/52/58-related CIN2, CIN3, AIS, and cervical cancer by time since 9vHPV vaccination, HPV type, and lesion type (PPE population).a.
| |
Young women 16–26 years of age (N = 2029) |
|
||
|---|---|---|---|---|
| Cases/n | Person-years’ follow-up | Rate per 100,000 person-years (95% CI) | Vaccine effectiveness,b % (95% CI) | |
| From the start of the LTFU study | ||||
| HPV16/18/31/33/45/52/58-related CIN2 or worsec | 0/1448 | 4084.2 | 0.0 (0.0–90.3) | 100 (79.4–100) |
| By time since start of the LTFU study | ||||
| >0 to 2 yearsd | 0/1448 | 2682.5 | 0.0 (0.0–137.5) | |
| >2 to 4 yearsd | 0/1094 | 1351.0 | 0.0 (0.0–273.1) | |
| >4 to 6 yearsd | 0/194 | 50.8 | 0.0 (0.0–7266.3) | |
| From the start of the base study | ||||
| HPV16/18/31/33/45/52/58-related CIN2 or worsee | 1/1783 | 10,303.1 | 9.7 (0.2–54.1) | |
| By time since 9vHPV vaccine Dose 1 | ||||
| ≤4 yearsf | 1/1783 | 5938.6 | 16.8 (0.4–93.8) | |
| >4 to 6 yearsd | 0/1586 | 2767.0 | 0.0 (0.0–133.3) | |
| >6 to 8 yearsd | 0/1147 | 1488.0 | 0.0 (0.0–247.9) | |
| >8 to 10 yearsd | 0/271 | 109.5 | 0.0 (0.0–3370.0) | |
| By HPV type | ||||
| HPV16-related | 0/1391 | 8128.6 | 0.0 (0.0–45.4) | |
| HPV18-related | 1/1564 | 9059.2 | 11.0 (0.3–61.5) | |
| HPV31-related | 0/1541 | 8981.7 | 0.0 (0.0–41.1) | |
| HPV33-related | 0/1604 | 9338.6 | 0.0 (0.0–39.5) | |
| HPV45-related | 0/1685 | 9752.9 | 0.0 (0.0–37.8) | |
| HPV52-related | 0/1583 | 9156.9 | 0.0 (0.0–40.3) | |
| HPV58-related | 0/1627 | 9464.0 | 0.0 (0.0–39.0) | |
| By lesion type | ||||
| CIN2 or CIN3 | 1/1783 | 10,302.9 | 9.7 (0.2–54.1) | |
| CIN2 | 1/1783 | 10,291.6 | 9.7 (0.2–54.1) | |
| CIN3 | 0/1783 | 10,301.2 | 0.0 (0.0–35.8) | |
| AIS | 0/1783 | 10,303.5 | 0.0 (0.0–35.8) | |
| Cervical cancer | 0/1783 | 10,303.5 | 0.0 (0.0–35.8) | |
N = number of participants who received at least one dose of the 9vHPV vaccine and consented to effectiveness follow-up.
n = number of PPE-eligible participants who have at least one follow-up visit. During the LTFU study, a follow-up visit represents retrieval of a cervical cytology or cervical, vulvar, or vaginal tissue sample collection record from the relevant national health registry.
aThe PPE population included participants who received all three doses of vaccine within 1 year, were seronegative at Day 1 and PCR-negative from Day 1 to Month 7 of the base study for the HPV type being analyzed, and had no protocol violations that could affect the evaluation of vaccine prophylactic efficacy.
bVaccine effectiveness measures the relative reduction of the disease incidence in vaccine recipients compared with a background incidence rate of 438 per 100,000 person-years, based on the incidence in an unvaccinated cohort.
cFor an individual participant, total person-years’ follow-up was calculated as the number of years starting from the beginning of the LTFU study (either the date when the participant reached the base study Year 4 or exited from the base study, whichever was later) through the date of the participant’s latest cervical cytology or cervical, vulvar, or vaginal tissue sample collection record obtained from the relevant national health registry.
dFor an individual participant with cervical cytology or cervical, vulvar, or vaginal tissue sample collection record obtained from the relevant national health registry within the indicated time interval, total person-years’ follow-up was calculated as the number of years starting from the date when the participant reached the beginning of the indicated time interval through either the date when the participant reached the end of the indicated time interval or the date of the participant’s latest cervical cytology or cervical, vulvar, or vaginal tissue sample collection record obtained from the relevant national health registry, whichever is earlier.
eFor an individual participant, total person-years’ follow-up was calculated as the number of years starting from the date when the participant reached Month 7 of the base study (the case counting start time in the per-protocol efficacy population) through the date of the participant’s latest cervical cytology or cervical, vulvar, or vaginal tissue sample collection record obtained from the relevant national health registry.
fThis time interval covers the base study period. For an individual participant, total person-years’ follow-up was calculated as the number of years starting from the date when the participant reached Month 7 of the base study through either the date when the participant reached the base study Year 4 or exited the base study, whichever is earlier.
9vHPV, nine-valent human papillomavirus; AIS, adenocarcinoma in situ; CI, confidence interval; CIN, cervical intraepithelial neoplasia; HPV, human papillomavirus;LTFU, long-term follow-up; PCR, polymerase chain reaction; PPE, per-protocol effectiveness.
The attribution of statistical significance of the observed vaccine effectiveness over time and detection of signals of waning vaccine effectiveness through the control chart method have been described previously.4,11 At least 60% of the total expected person-years of follow-up time (2140 person-years of follow-up) is necessary in any given 2-year interval of time since the start of the LTFU study in order to draw firm conclusions from the results of this analysis based on the statistical method.4,11 A total of 2683 person-years have accrued over the period from 0 to 2 years following the start of the LTFU, which is a sufficient amount of follow-up time to conclude that the 9vHPV vaccine continued to be effective through at least 6 years (Table 1). As can be seen in Figure 2, no points on the graph cross the pre-specified 1.83- or 2.75-sigma control limits during the evaluable time period. Therefore, there was no signal of decreased vaccine effectiveness in the PPE population through at least 6 years post-vaccination. The same pattern was seen in the interval up to 8 years, indicating a trend of continued effectiveness through 8 years. However, there is insufficient follow-up time in the 6- to 8-year interval to make a conclusive claim of effectiveness beyond 6 years.
Figure 2.
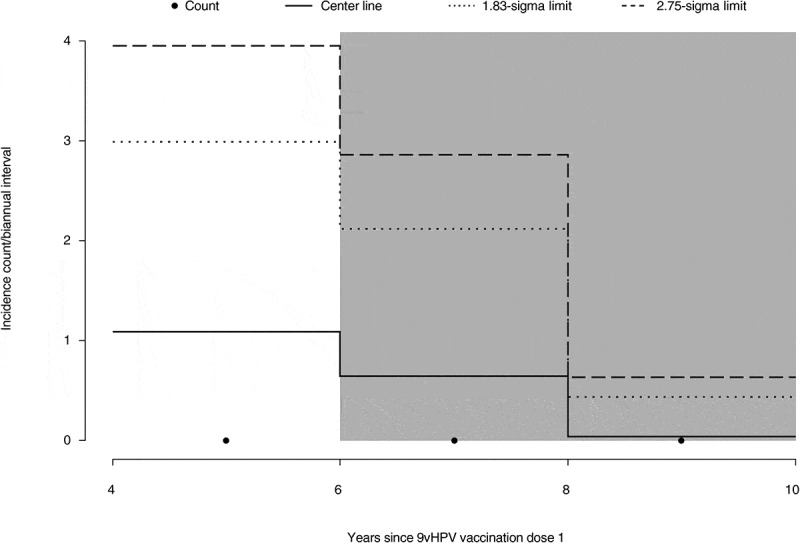
Control chart analysis of the effectiveness of the 9vHPV vaccine against HPV16/18/31/33/45/52/58-related CIN2, CIN3, AIS, and cervical cancer in the PPE population. The incidence of HPV-related disease was evaluated at 2-year intervals during the LTFU period and, if plotted incidences crossed the 1.83- and 2.75-sigma control limits of the control chart, an inference was made that the accumulating data were indicative of waning effectiveness. Shaded intervals indicate intervals with insufficient follow-up time to declare statistical significance. The center line indicates the expected count in each interval. 9vHPV, nine-valent human papillomavirus; AIS, adenocarcinoma in situ; CIN, cervical intraepithelial neoplasia; HPV, human papillomavirus; LTFU, long-term follow-up; PPE, per-protocol effectiveness
No new cases of the secondary endpoint of HPV6/11/16/18/31/33/45/52/58-related CIN (any grade), AIS, cervical cancer, vulvar cancer, or vaginal cancer were reported from the start of the LTFU study (Table 2).
Table 2.
Incidence of HPV6/11/16/18/31/33/45/52/58-related CIN (any grade), AIS, cervical cancer, vulvar cancer, and vaginal cancer by time since 9vHPV vaccination, HPV type, and lesion type (PPE population).a.
| Young women 16–26 years of age (N = 2029) |
|||
|---|---|---|---|
| Cases/n | Person-years’ follow-up | Rate per 100,000 person-years (95% CI) | |
| From the start of the base study | |||
| HPV6/11/16/18/31/33/45/52/58-related CIN (any grade), AIS, cervical cancer, vulvar cancer, or vaginal cancerb | 2/1797 | 10,730.8 | 18.6 (2.3–67.3) |
| By time since 9vHPV vaccine Dose 1 | |||
| ≤4 yearsc | 2/1797 | 6089.6 | 32.8 (4.0–118.6) |
| >4 to 6 yearsd | 0/1658 | 2920.0 | 0.0 (0.0–126.3) |
| >6 to 8 yearsd | 0/1219 | 1597.1 | 0.0 (0.0–231.0) |
| >8 to 10 yearsd | 0/307 | 124.1 | 0.0 (0.0–2971.7) |
| By HPV type | |||
| HPV6-related | 0/1413 | 8446.3 | 0.0 (0.0–43.7) |
| HPV11-related | 0/1413 | 8446.3 | 0.0 (0.0–43.7) |
| HPV16-related | 0/1395 | 8285.4 | 0.0 (0.0–44.5) |
| HPV18-related | 1/1572 | 9351.1 | 10.7 (0.3–59.6) |
| HPV31-related | 1/1549 | 9266.0 | 10.8 (0.3–60.1) |
| HPV33-related | 0/1616 | 9667.3 | 0.0 (0.0–38.2) |
| HPV45-related | 0/1699 | 10,156.4 | 0.0 (0.0–36.3) |
| HPV52-related | 0/1593 | 9484.4 | 0.0 (0.0–38.9) |
| HPV58-related | 0/1641 | 9810.4 | 0.0 (0.0–37.6) |
| By lesion type | |||
| CIN1 | 1/1783 | 10,268.7 | 9.7 (0.2–54.3) |
| CIN2 or CIN3 | 1/1783 | 10,302.9 | 9.7 (0.2–54.1) |
| CIN2 | 1/1783 | 10,291.6 | 9.7 (0.2–54.1) |
| CIN3 | 0/1783 | 10,301.2 | 0.0 (0.0–35.8) |
| AIS | 0/1783 | 10,303.5 | 0.0 (0.0–35.8) |
| Cervical cancer | 0/1783 | 10,303.5 | 0.0 (0.0–35.8) |
| Vulvar cancer | 0/1797 | 10,738.1 | 0.0 (0.0–34.4) |
| Vaginal cancer | 0/1797 | 10,738.1 | 0.0 (0.0–34.4) |
N = number of participants who received at least one dose of the 9vHPV vaccine and consented to effectiveness follow-up.
n = number of PPE-eligible participants who have at least one follow-up visit. During the LTFU study, a follow-up visit represents retrieval of a cervical cytology or cervical, vulvar, or vaginal tissue sample collection record from the relevant national health registry.
aThe PPE population included participants who received all three doses of vaccine within 1 year, were seronegative at Day 1 and PCR-negative from Day 1 to Month 7 of the base study for the HPV type being analyzed, and had no protocol violations that could affect the evaluation of vaccine prophylactic efficacy.
bFor an individual participant, total person-years’ follow-up was calculated as the number of years starting from the date when the participant reached Month 7 of the base study through the date of the participant’s latest cervical cytology or cervical, vulvar, or vaginal tissue sample collection record obtained from the relevant national health registry.
cThis time interval covers the base study period. For an individual participant, total person-years’ follow-up was calculated as the number of years starting from the date when the participant reached Month 7 of the base study through either the date when the participant reached the base study Year 4 or exited the base study, whichever is earlier.
dFor an individual participant with cervical cytology or cervical, vulvar, or vaginal tissue sample collection record obtained from the relevant national health registry within the indicated time interval, total person-years’ follow-up was calculated as the number of years starting from the date when the participant reached the beginning of the indicated time interval through either the date when the participant reached the end of the indicated time interval or the date of the participant’s latest cervical cytology or cervical, vulvar, or vaginal tissue sample collection record obtained from the relevant national health registry, whichever is earlier.
9vHPV, nine-valent human papillomavirus; AIS, adenocarcinoma in situ; CI, confidence interval; CIN, cervical intraepithelial neoplasia; HPV, human papillomavirus; LTFU, long-term follow-up; PCR, polymerase chain reaction; PPE, per-protocol effectiveness.
During the base study, one case of HPV18-related CIN2 and one case of HPV31-related CIN1 were observed (Tables 1 and 2). The participant with the CIN2 case was diagnosed at Month 24 (cervical biopsy tested positive for HPV58; endocervical curettage tested positive for HPV18 and HPV58). She had positive results with HPV58 at baseline and at all study visits until the diagnosis; she was positive for HPV18 only at the Month 12 visit and at the time of the diagnosis. Therefore, HPV18 was unlikely to have caused the lesion. The participant with the CIN1 case was diagnosed at Month 7 (cervical definitive therapy tested positive for HPV31 and 51). The cervical definitive therapy was performed following a diagnosis of CIN2 that tested positive for HPV51. The participant tested positive for HPV51 at all visits from Months 7 to 24. She was positive for HPV31 only with the cervical definitive therapy sample at Month 7. Therefore, HPV31 was unlikely to have caused the lesion.
The World Health Organization has determined that LTFU studies assessing efficacy, immunogenicity, and safety should be an integral part of prophylactic HPV-vaccine development.12 Because the risk of HPV exposure can be lifelong, durable protection from new infections is required to maximize the benefit of vaccination. LTFU studies are therefore an integral part of the 9vHPV vaccine clinical development program.4,13 Of note, in a similarly designed registry-based follow-up study of qHPV vaccine, vaccine effectiveness was ascertained through 14 years post-vaccination in Nordic young women.11 The long-term effectiveness that was demonstrated with the qHPV vaccine is likely to be applicable to the 9vHPV vaccine since the two vaccines are manufactured similarly, share antigens for four HPV types, and have similar efficacy and immunogenicity profiles.13 Further follow-up of this long-term effectiveness study of the 9vHPV vaccine will test this presumption.
The participants in this study represent a sentinel cohort who received the 9vHPV vaccine at least 5 years before the vaccine became commercially available. Should evidence of waning vaccine effectiveness be observed in the study, this would allow public health decisions (e.g., the necessity for a booster vaccination) to be implemented in advance of the period of lower protection in the general population.4 Results of the monitoring of vaccine effectiveness over time using the control chart method during the LTFU study have ascertained statistically significant vaccine effectiveness of at least 90% through at least 6 years post-vaccination, with indications of continuing effectiveness through up to 8 years. Note that we used conservative estimates of expected HPV incidence based on survey data from 2004 to 2005, and more recent data suggest that HPV exposure has increased since then, which further strengthens our observation of high vaccine effectiveness.14
The following limitations of this study are noted. The LTFU study does not have a control arm since participants who received the qHPV vaccine in the base study were offered the 9vHPV vaccination at the end of the base study.4 Therefore, the effectiveness of the 9vHPV vaccine was determined relative to estimated incidence in an unvaccinated population. The high compliance to cervical screening programs in Scandinavian countries, and the rigorous statistical methods and analyses used in the study, allowed the design and conduct of a hypothesis-driven LTFU study, so that rigorous conclusions could be drawn. Because the study is based on routine cervical screening, the assessment did not include non-cervical HPV disease endpoints. However, it is reasonable to assume that effectiveness results for cervical disease would be applicable to non-cervical disease considering that the natural history, pathophysiology, and mechanisms of protection elicited by HPV vaccination are similar across different anatomic sites.
In summary, the effectiveness data presented herein indicate that the 9vHPV vaccine induces durable protection against vaccine HPV type-related disease in young women through at least 6 years following vaccination. There were no new cases of HPV6/11/16/18/31/33/45/52/58-related CIN, AIS, cervical cancer, vulvar cancer, or vaginal cancer in the PPE population of Scandinavian young women during the 4-year LTFU study period covered by this report. Even though the vaccine is highly effective, women need to continue to attend screening for cervical cancer.15,16 The study will continue to assess persistence of vaccine effectiveness for at least 6 more years, to give a cumulative follow-up of at least 14 years since the start of the base study.
Supplementary Material
Acknowledgments
The authors would like to thank the study participants, investigators, and study-site personnel.
Medical writing assistance, under the direction of the authors, was provided by Erin Bekes, PhD, of CMC AFFINITY, McCann Health Medical Communications, in accordance with Good Publication Practice (GPP3) guidelines. This assistance was funded by Merck Sharp & Dohme Corp., a subsidiary of Merck & Co., Inc., Kenilworth, NJ, USA.
Funding Statement
Funding for this research was provided by Merck Sharp & Dohme Corp., a subsidiary of Merck & Co., Inc., Kenilworth, NJ, USA.
Disclosure of potential conflicts of interest
Susanne K. Kjaer reports grants through her affiliating institute from Merck Sharp & Dohme during the conduct of the study, and grants and personal fees from Merck Sharp & Dohme outside the submitted work.
Mari Nygård reports research grants from Merck Sharp & Dohme Norway through her affiliating institute during the conduct of the study and outside the scope of this study.
Karin Sundström reports research grants from Merck Sharp & Dohme to her institution for the present work on HPV vaccination in Sweden and research grants to her institution for other register-based studies on HPV vaccination in Sweden.
Christian Munk reports that his host institution received research grants from Merck Sharp & Dohme during the conduct of the study.
Sophie Berger reports that her host institution received research grants from Merck Sharp & Dohme Norway during the conduct of the study.
Mensur Dzabic and Katrin Elisabeth Fridrich have nothing to disclose.
Marianne Waldstrøm reports that her host institution received research grants from Merck Sharp & Dohme for pathology review of biopsies during the conduct of the study.
Sveinung Wergeland Sørbye has received grants from Merck Sharp & Dohme for the pathology review of biopsies during the conduct of the study.
Oliver Bautista, Thomas Group, and Alain Luxembourg are employees of Merck Sharp & Dohme and may own stock or stock options in Merck & Co., Inc., Kenilworth, NJ, USA.
Abbreviations
- 9vHPV
nine-valent human papillomavirus
- AIS
adenocarcinoma in situ
- CI
confidence interval
- CIN
cervical intraepithelial neoplasia
- HPV
human papillomavirus
- LTFU
long-term follow-up
- NPP
Nordic Pathology Panel
- NRSC
National Registry Study Centers
- PCR
polymerase chain reaction
- PPE
per-protocol effectiveness
- qHPV
quadrivalent human papillomavirus
Supplementary material
Supplemental data for this article can be accessed online at http://dx.doi.org/10.1080/21645515.2020.1839292.
References
- 1.Huh WK, Joura EA, Giuliano AR, Iversen OE, de Andrade RP, Ault KA, Bartholomew D, Cestero RM, Fedrizzi EN, Hirschberg AL, et al. Final efficacy, immunogenicity, and safety analyses of a nine-valent human papillomavirus vaccine in women aged 16-26 years: a randomised, double-blind trial. Lancet. 2017;390:2143–59. [DOI] [PubMed] [Google Scholar]
- 2.Joura EA, Giuliano AR, Iversen O-E, Bouchard C, Mao C, Mehlsen J, Moreira ED, Ngan Y, Petersen LK, Lazcano-Ponce E, et al. A 9-valent HPV vaccine against infection and intraepithelial neoplasia in women. N Engl J Med. 2015;372(8):711–23. doi: 10.1056/NEJMoa1405044. [DOI] [PubMed] [Google Scholar]
- 3.Giuliano AR, Joura EA, Garland SM, Huh WK, Iversen O-E, Kjaer SK, Ferenczy A, Kurman RJ, Ronnett BM, Stoler MH, et al. Nine-valent HPV vaccine efficacy against related diseases and definitive therapy: comparison with historic placebo population. Gynecol Oncol. 2019;154(1):110–17. doi: 10.1016/j.ygyno.2019.03.253. [DOI] [PubMed] [Google Scholar]
- 4.Luxembourg A, Kjaer SK, Nygard M, Ellison MC, Group T, Marshall JB, Radley D, Saah A.. Design of a long-term follow-up effectiveness, immunogenicity and safety study of women who received the 9-valent human papillomavirus vaccine. Contemp Clin Trials. 2017;52:54–61. doi: 10.1016/j.cct.2016.10.006. [DOI] [PubMed] [Google Scholar]
- 5.Luxembourg A, Bautista O, Moeller E, Ritter M, Chen J. Design of a large outcome trial for a multivalent human papillomavirus L1 virus-like particle vaccine. Contemp Clin Trials. 2015;42:18–25. doi: 10.1016/j.cct.2015.02.009. [DOI] [PubMed] [Google Scholar]
- 6.Chen YH, Gesser R, Luxembourg A. A seamless phase IIB/III adaptive outcome trial: design rationale and implementation challenges. Clin Trials. 2015;12(1):84–90. doi: 10.1177/1740774514552110. [DOI] [PubMed] [Google Scholar]
- 7.Else EA, Swoyer R, Zhang Y, Taddeo FJ, Bryan JT, Lawson J, Van Hyfte I, Roberts CC. Comparison of real-time multiplex human papillomavirus (HPV) PCR assays with INNO-LiPA HPV genotyping extra assay. J Clin Microbiol. 2011;49(5):1907–12. doi: 10.1128/JCM.00236-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Roberts CC, Swoyer R, Bryan JT, Taddeo FJ. Comparison of real-time multiplex human papillomavirus (HPV) PCR assays with the linear array HPV genotyping PCR assay and influence of DNA extraction method on HPV detection. J Clin Microbiol. 2011;49:1899–906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kjaer SK, Tran TN, Sparen P, Tryggvadottir L, Munk C, Dasbach E, Liaw K-L, Nygård J, Nygård M. The burden of genital warts: a study of nearly 70,000 women from the general female population in the 4 Nordic countries. J Infect Dis. 2007;196(10):1447–54. doi: 10.1086/522863. [DOI] [PubMed] [Google Scholar]
- 10.Castellsagué X, Ault KA, Bosch FX, Brown D, Cuzick J, Ferris DG, Joura EA, Garland SM, Giuliano AR, Hernandez-Avila M, et al. Human papillomavirus detection in cervical neoplasia attributed to 12 high-risk human papillomavirus genotypes by region. Papillomavirus Res. 2016;2:61–69. doi: 10.1016/j.pvr.2016.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kjaer SK, Nygård M, Sundström K, Dillner J, Tryggvadottir L, Munk C, Berger S, Enerly E, Hortlund M, Ágústsson ÁI, et al. Final analysis of a 14-year long-term follow-up study of the effectiveness and immunogenicity of the quadrivalent human papillomavirus vaccine in women from four Nordic countries. EClinicalMedicine. 2020;23:100401. doi: 10.1016/j.eclinm.2020.100401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.World Health Organization (WHO) . Guidelines to assure the quality, safety and efficacy of recombinant HPV virus-like particle vaccines. 2006. [Accessed 2020 June30]. http://screening.iarc.fr/doc/WHO_vaccine_guidelines_2006.pdf.
- 13.Luxembourg A, Moeller E. 9-Valent human papillomavirus vaccine: a review of the clinical development program. Expert Rev Vaccines. 2017;16:1119–39. doi: 10.1080/14760584.2017.1383158. [DOI] [PubMed] [Google Scholar]
- 14.Orumaa M, Leinonen MK, Campbell S, Møller B, Myklebust TÅ, Nygård M. Recent increase in incidence of cervical precancerous lesions in Norway: nationwide study from 1992 to 2016. Int J Cancer. 2019;145:2629–38. doi: 10.1002/ijc.32195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Burger EA, Smith MA, Killen J, Sy S, Simms KT, Canfell K, Kim JJ. Projected time to elimination of cervical cancer in the USA: a comparative modelling study. Lancet Public Health. 2020;5(4):e213–e222. doi: 10.1016/S2468-2667(20)30006-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Pedersen K, Burger EA, Nygard M, Kristiansen IS, Kim JJ. Adapting cervical cancer screening for women vaccinated against human papillomavirus infections: the value of stratifying guidelines. Eur J Cancer. 2018;91:68–75. doi: 10.1016/j.ejca.2017.12.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


