ABSTRACT
Ischemic stroke is a common cerebrovascular disease with the main cause considered to be cerebral ischemia and reperfusion (I/R), which exerts irreparable injury on nerve cells. Thus, the development of neuroprotective drugs is an urgent concern. Curcumin, a known antioxidant, has been found to have neuroprotective effects. To determine the protective mechanism of curcumin in ischemic stroke, oxygen and glucose deprivation/reoxygenation (OGD/R) was used to treat PC12 cells to mimic the cerebral I/R cell model. Curcumin (20 μM) was applied to OGD/R PC12 cells, followed by Ca2+ concentration, transepithelial electrical resistance (TEER), and cell permeability measurements. The results showed that OGD/R injury induced a decrease in TEER and increases in Ca2+ concentration and cell permeability. In contrast, curcumin alleviated these effects. The protein kinase C θ (PKC-θ) was associated with the protective function of curcumin in the OGD/R cell model. Moreover, the middle cerebral artery occlusion and reperfusion model (MCAO/R) was applied to simulate the I/R rat model. Our results demonstrated that curcumin could reverse the MCAO/R-induced increase in Ca2+ concentration and blood–brain barrier (BBB) disruption. Our study demonstrates the mechanisms by which curcumin exhibited a protective function against cerebral I/R through PKC-θ signaling by reducing BBB dysfunction.
KEYWORDS: Curcumin, OGD/R, PKC-θ
Introduction
Ischemic stroke is a severe cerebrovascular disease that causes approximately 5 million deaths worldwide and contributes to a high degree of disability, making it a primary cause of death and disability in today’s world [1]. Considering that the main cause of ischemic stroke is insufficient blood and oxygen supply, restoring blood and oxygen levels to normal by re-opening the occluded cerebral vessels in ischemic brain tissue emerges as the primary treatment. However, this treatment often produces cerebral ischemia/reperfusion (I/R) injury, including hemorrhagic transformation and blood–brain barrier (BBB) destruction [1–3]. Thus, lowering the detrimental outcomes induced by cerebral I/R is very important in clinical treatments. So, research has shown that apoptosis, oxidative stress, Ca2+ overload, energy metabolism disorders, glutamate toxicity, and excessive NO synthesis are all involved in cerebral I/R injury [4]. Nonetheless, few strategies have been explored to reduce cerebral I/R injury.
The protein kinase C (PKC) family is composed of three subfamilies: conventional PKC (α, β, and γ), novel PKC (δ, ɛ, η, and θ), and atypical PKC (ζ and ι/κ) [5]. PKC isozymes are of great importance in many cellular processes, including adhesion, activation, survival, motility, and differentiation [6]. PKC isoenzymes have been revealed to play a crucial role in cerebral I/R injury [7]. PKC-ɛ confers beneficial effects on neuronal mitochondrial functions during cerebral I/R injury [8]. In addition, the cardioprotective effects of PKC-ɛ; has been found in rat I/R injury [9]. PKC-θ, a novel PKC isoenzyme, limits store-independent Ca2+ entry during glycoprotein VI signaling [10]. Furthermore, PKC-θ plays a role in immune response by regulating T-cell receptor signaling [11]. While PKC-ɛ and PKC-θ belong to the novel PKC subfamilies, little information is available on these PKCs, specifically the function of PKC-θ in cerebral I/R injury.
Curcumin, a natural phenolic component isolated from Curcuma longa Linn. (turmeric), is involved in many biological mechanisms, including anti-inflammation [12], anti-apoptosis [13], anti-tumor [14], and anti-oxidation [15] activities. The protective role of curcumin has been demonstrated to increase mitochondrial biogenesis in cerebral I/R injury [16] and through the PI3K/Akt/mTOR pathway [15]. Moreover, curcumin has been suggested to protect the blood–brain barrier during cerebral I/R injury [17]. However, much more information is needed to establish the protective mechanism of curcumin during cerebral I/R injury.
In the present study, oxygen and glucose deprivation/reoxygenation (OGD/R) was applied to PC12 cells to mimic the cerebral I/R cell model. Curcumin was used to treat OGD/R PC12 cells to explore the effects of curcumin. We demonstrated that curcumin alleviated the injury generated by I/R and promoted cell survival through the PKC-θ signaling pathway.
Materials and methods
Reagents
Anti-PKC-θ (#2058, 1:1000), anti-p-PKC-θ (#9374, 1:800), and anti-GAPDH (#5174, 1:3000) were obtained from Cell Signaling Technology (Danvers, MA, USA). Anti-ZO-1 (Ab96587, 1:800) and anti-occludin-1 (Ab167161, 1:800) were purchased from Abcam (St. Louis, MO, USA). Anti-Orai1 (13130-1-AP, 1:1000) was purchased from Proteintech (Rosemont, IL, USA). Curcumin was purchased from Sigma (St. Louis, MO, USA. Cat. C1386) and PKC-θ inhibitor, AS2521780, was purchased from MedChemExpress (Monmouth Junction, NJ, USA. Cat. 1214726–89-2).
Cells and cell culture
Rat PC12 cell line was purchased from the American Type Culture Collection (Manassas, VA, USA) and cultured with Dulbecco’s Modified Eagle Medium (DMEM) containing 10% (v/v) heat-inactivated horse serum (Gibco, Carlsbad, CA, USA) and 5% (v/v) fetal bovine serum (Gibco) at 37°C in 5% CO2.
OGD/R cell model
The OGD/R cell model was established as previously described [18]. For the OGD/R group, glucose-free DMEM was used to wash PC12 cells. Then the cells were cultured with glucose-free DMEM under 1% O2, 5% CO2, and 95% N2 at 37°C for 4 h. After that, PC12 cells were incubated with DMEM in a normoxic incubator (5% CO2 at 37°C) for 4 h, followed by treatment with different concentrations of curcumin or AS2521780.
Cell proliferation
The Cell Proliferation Assay Cell Counting Kit (CCK-8; Beyotime, Shanghai, China) was used to assess the proliferation of PC12 cells. The CCK-8 working solution (CCK-8: DMEM = 1:10) was prepared beforehand. The cells were cultured with 100 μL CCK-8 working solution at 37°C for 1 h, and the optical density was recorded at 450 nm using an automatic microplate reader (Bio-Rad Laboratories, Inc., Hercules, CA, USA). Each sample was measured in triplicate.
Quantitative real-time polymerase chain reaction (PCR)
The total RNA of treated cells was obtained using TRIzol (Invitrogen, Carlsbad, CA), and random nucleotides were primed for cDNA synthesis (Invitrogen). The relative expression level of Orai1 (Primer F 5ʹ CGTCCACAACCTCAACTC 3ʹ/Primer R 5ʹ GCCAGGAAAAGCAGCGTC 3ʹ) was measured using the SYBR kit (Thermo Fisher Scientific Inc., Grand Island, NY, USA) on an ABI7400 producer (Foster City, CA, USA). GAPDH (Primer F 5ʹ GCAAGTTCAACGGCACAG 3ʹ/Primer R 5ʹ CCCCATTTGATGTTAGCG 3ʹ) was used as an internal control and all the samples were analyzed in triplicate.
Western blot analysis
Treated cells and the brain tissues were collected and lysed in ice-cold RIPA lysis buffer (Bio-Rad Laboratories,). After centrifugation at 12,000 g for 15 min, the supernatant was collected and measured using a bicinchoninic acid assay kit (Bio-Rad Laboratories, Inc.) for protein concentrations. Subsequently, equal amounts of protein were separated on a SDS polyacrylamide gel and transferred onto polyvinylidene difluoride membranes (Millipore Corp., Bedford, MA, USA). Afterward, the membranes were blocked with 5% nonfat milk and incubated with primary antibodies at 4°C overnight and with secondary antibodies (1:3000) at room temperature for 1 h. Finally, the protein bands were detected using a Bio-Rad imaging system.
Transepithelial electrical resistance (TEER) assay
The TEER of PC12 cells was analyzed using a MERSST × 01 Electrode (EMD Millipore Corporation, Bedford, MA, USA). PC12 cells were seeded at 5 × 105 on 0.4 μm fibronectin-coated transwell filters and cultured to full confluence. The TEER values of samples were detected using the MERSST × 01 Electrode.
Cell permeability analysis
Cell permeability was determined using FITC-Dextrans (Sigma, St. Louis, MO, USA) as described previously [19]. Briefly, 1 × 105 of PC12 cells were seeded on 0.4 μm transwell filters and cultured. Before measurement, cells were cultured with serum and phenol red-free DMEM at 37°C for 4 h. Next, 100 μL of FITC-Dextran (1 mg/mL) was added to the upper chamber and cultured at 37°C for 1 h. After that, 200 μL of lower chamber samples were collected and measured on a fluorescent plate reader (Bio-Rad Laboratories; excitation 490 nm, emission 520 nm). The FITC-dextran concentrations were calculated according to standard curves.
Measurement of intracellular Ca2+
The Fluo-3-AM kit (Thermo Fisher Scientific Inc.) was used to detect intracellular Ca2+ concentration. Treated PC12 cells were collected and rinsed three times with PBS and subsequently incubated with Fluo-3-AM at 37°C for 1 h. The samples were centrifugated and rinsed three times with PBS. Finally, PBS without Ca2+ was used to resuspend the cells. Fluorescence at 488 nm was monitored using a flow cytometer (BD Biosciences, San Jose, CA, USA).
Rat model of I/R
Male Sprague–Dawley (SD) rats (weight range 240–270 g) were purchased from Shanghai SLAC Laboratory Animal Co., Ltd. (Shanghai, China) and housed at 19°C–22°C with a 12 h light/dark cycle and free access to food and water. This study was performed in accordance with the Declaration of Helsinki. All the experiments were approved by the Shanghai University of Medicine & Health Sciences Affiliated Zhoupu Hospital.
To induce transient focal cerebral ischemia, the right middle cerebral artery occlusion and reperfusion model (MCAO/R) was performed as previously described [20]. In brief, the rats were anesthetized with 10% chloral hydrate (3.5 mL/kg) via intraperitoneal injection. A silicone-coated 8–0 monofilament was introduced into the left internal carotid artery to occlude the middle cerebral artery. After 90 min MCAO, the filament was withdrawn to complete reperfusion.
All the rats were divided into 4 groups (n = 6): Control group, the rats were subjected to sham surgery; MCAO/R group, the rats were subjected to MCAO/R; AS2521780 group, the rats were subjected to MCAO/R and treated with 3.5 mg/kg AS2521780 via intraperitoneal injection twice a day for 2 days (starting on the day before operation); curcumin group, the rats were pretreated with 100 mg/kg/d curcumin via intraperitoneal injection twice (24 h and 1 h before operation) and then subjected to MCAO/R; curcumin+AS2521780 group: the rats were pretreated with 100 mg/kg/d curcumin and 3 mg/kg AS2521780, then subjected to MCAO/R;. The control and MCAO/R groups were given an equal volume of physiological saline via intraperitoneal injection.
Hematoxylin-eosin (H&E) staining
After neurological score assessment, all the rats were anesthetized with 10% chloral hydrate, PBS (0.1 M) was used to perfuse rats through the heart for 2 min. After that, PBS was replaced with 4% paraformaldehyde, and perfusion was performed for an additional 5 min. Finally, the intact brain was carefully removed and fixed in 4% formaldehyde fixative for H&E staining and immunofluorescence (IF) staining.
Following 24 h fixation, the brain samples were embedded in paraffin, sliced into 5 μm specimens, and stained using an H&E kit (Bio-Rad Laboratories) according to manufacturer’s instructions. A light microscope (NIKON, Japan) was used to collect images of the stained slides.
Immunohistochemistry (IHC) assay
Brain section placed on cover slides were incubated with cleaved caspase 3 and TNF-α antibody at 4°C for 12 h, and then incubated with a secondary antibody (D-3004, Long Island Biotech, China) at 25°C for 30 min. Positive cells stained using a 3,3ʹ-Diaminobenzidine Substrate Kit (Long Island Biotech) and hematoxylin (714094, BASO) were presented as brown-yellow particles.
Neurological score assessment
Neurological deficits were evaluated using Bederson’s neurological scale [21]: Score 0, no neurologic deficit; Score 1, failure to fully extend right forepaw; Score 2, reduced resistance to right lateral push when mouse was held by the tail; Score 3, spontaneous circling to the right; and Score 4, did not walk spontaneously or unconsciousness.
Statistical analysis
All the data were presented as mean ± SD. One-way analysis of variance was used to compare the differences among groups. P < 0.05 was considered statistically significant.
Results
Levels of cell proliferation, TEER, Ca2+, and cell permeability in PC12 cells after OGD/R
The cell proliferation of PC12 cell was measured by CCK-8 after OGD/R. As shown in Figure 1(a), OGD/R treatment significantly decreased cell proliferation in PC12 cells at all three time points (12, 24, and 48 h). In contrast, incubation with 5, 10, and 20 μM of curcumin after OGD/R markedly increased cell proliferation at all three time points (12, 24, and 48 h) in comparison with the OGD/R group. However, 30 and 40 μM curcumin seemed to be toxic to cells. Thus, 20 μM of curcumin was selected to treat PC12 cell in subsequent experiments.
Figure 1.
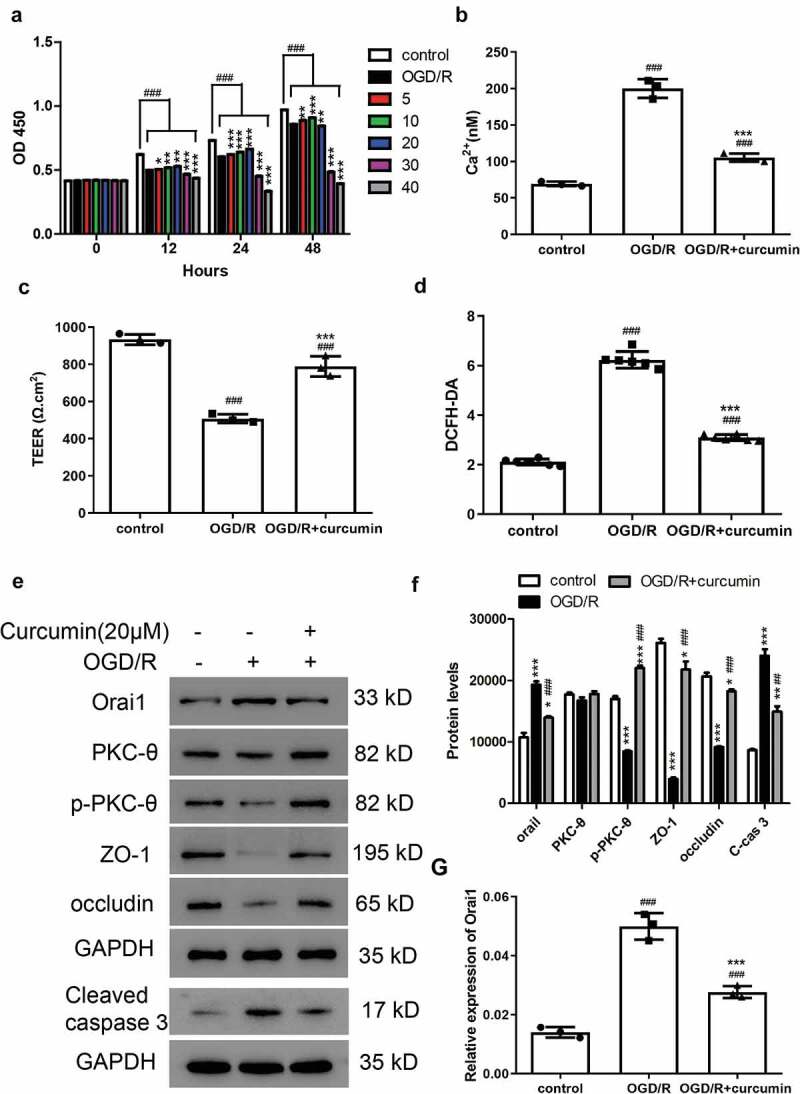
Cell proliferation, TEER, Ca2+ concentrations, and cell permeability in PC12 cells after OGD/R. (a) CCK-8 assay was used to measure cell proliferation after treatment with OGD/R and curcumin (0, 5, 10, 20, 30, and 40 μM) in PC12 cells. ###P < 0.001 vs control; *P < 0.05, **P < 0.01, and ***P < 0.001 vs OGD/R. (b–d) PC12 cells were treated with OGD/R and/or 20 μM curcumin, then (b) Ca2+ concentration, (c) TEER, and (d) cell permeability were analyzed. Control, PC12 cells; OGD/R, PC12 cells treated with OGD/R; OGD/R+ curcumin, PC12 cells treated with OGD/R and 20 μM curcumin. ###P < 0.001 vs control; ***P < 0.001 vs OGD/R. (e) Western blot assay was performed to detect Orai1, PKC-θ, p- PKC-θ, ZO-1, occludin, and cleaved caspase 3 levels. (f) Analysis of protein levels. *P < 0.05, **P < 0.01, and ***P < 0.001 vs control; ##P < 0.01 and ###P < 0.001 vs OGD/R. (g) Orai1 mRNA levels were measured by real-time PCR. ###P < 0.001 vs control; ***P < 0.001 vs OGD/R
To explore the role of curcumin in cerebral I/R injury, PC12 cells were subjected to OGD/R and/or curcumin treatment. Ca2+ overload is known to play a key role in cerebral I/R [22]. We measured the cellular Ca2+ concentrations and found that Ca2+concentration increased after OGD/R in comparison with the control group (Figure 1(b)). In addition, cellular Ca2+ concentration was significantly decreased in the curcumin (20 μM) treatment group. These results indicated that cellular Ca2+ accumulated after OGD/R in PC12 cells, and this effect can be alleviated by curcumin (Figure 2(b)). Furthermore, BBB disruption has been reported to be one of the contributing factors to cerebral ischemia [23]. In the present study, TEER and cell permeability analyses were performed to measure BBB disruption. As shown in Figure 1(c,d), the TEER value decreased and cell permeability promoted in the OGD/R group. Compared with the OGD/R group, the TEER value increased and cell permeability decreased in the OGD/R+ curcumin group. These results demonstrated that OGD/R-induced BBB disruption, and curcumin alleviated this effect. Subsequently, we analyzed the protein levels of cell junction markers including ZO-1 and occludin. As shown in Figure 1(e), ZO-1 and occludin were downregulated in the OGD/R group compared with the control group but were upregulated in the OGD/R+ curcumin group compared with the OGD/R group. The p-PKC-θ protein level was lower in the OGD/R+ curcumin group than in the control group, but higher than in the OGD/R group. Furthermore, cleaved caspase 3 was found upregulated in the OGD/R group and was alleviated by curcumin (Figure 1(e,f)). Calcium release-activated calcium channel protein 1 (Orai1) has been found to mediate intracellular Ca2+ concentration [24]. Thus, we measured Orai1 levels and found that the Orai1 protein and mRNA levels were higher in the OGD/R+ curcumin group than in the control group, but lower than in the OGD/R group (Figure 1(e,g)). These results suggested that curcumin may play a protective role against OGD/R in vivo through the PKC-θ signaling pathway.
Figure 2.
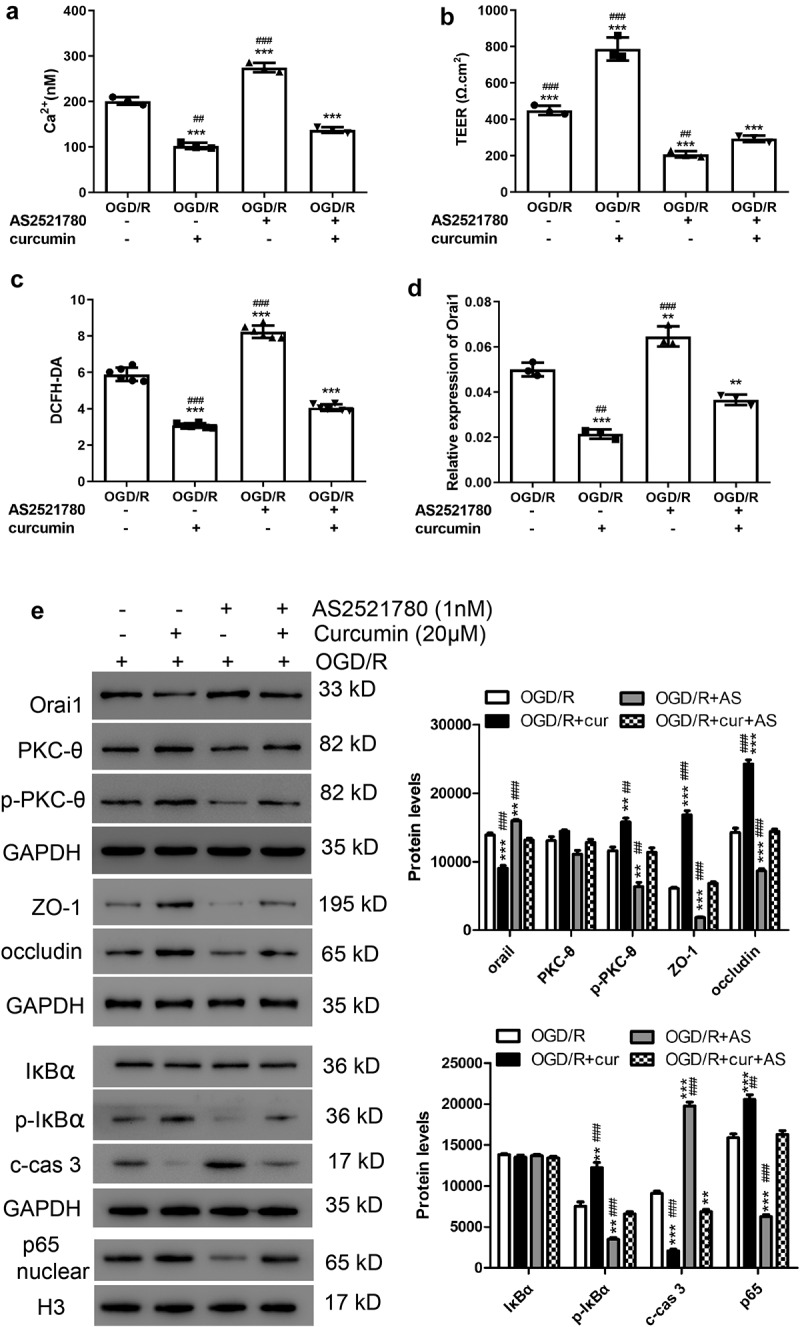
Curcumin alleviated OGD/R-induced increase in TEER and decrease in Ca2+ concentration and cell permeability through the PKC-θ signaling pathway. After OGD/R treatment, PC12 cells were treated with 1 nM AS2521780 and/or 20 μM curcumin. (a) Ca2+ concentrations, (b) TEER, and (c) cell permeability were analyzed. **P < 0.01 and ***P < 0.001 vs OGD/R; ##P < 0.01 and ###P < 0.001 vs curcumin+AS2521780. (d) Orai1 mRNA levels were measured by real-time PCR. **P < 0.01 and ***P < 0.001 vs OGD/R; ##P < 0.01 and ###P < 0.001 vs curcumin+AS2521780. (e) Western blot assay was performed to measure Orai1, PKC-θ, p- PKC-θ, ZO-1, occludin, IκBα, p-IκBα cleaved caspase 3, and nuclear p65 levels. **P < 0.01 and ***P < 0.001 vs OGD/R; ##P < 0.01 and ###P < 0.001 vs curcumin+AS2521780
Curcumin alleviated OGD/R-induced increase of TEER and decrease of Ca2+ concentration and cell permeability through the PKC-θ signaling pathway
To confirm whether the PKC-θ signaling pathway is involved, 1 nM of AS2521780 was applied to OGD/R PC12 cells together with curcumin. As shown in Figure 2(a), after OGD/R treatment, the increase in Ca2+ concentration induced by AS2521780 could be alleviated by curcumin. In addition, we found that AS2521780 decreased TEER and increased cell permeability in OGD/R PC12 cells, but these effects were blocked by curcumin (Figure 2(b,c)). As shown in Figure 2(e), ZO-1 and occludin were downregulated in the curcumin + AS2521780 group compared with the curcumin only group but were upregulated compared with the AS2521780 only group. Furthermore, the p-PKC-θ, p-IκBα, and nuclear p65 protein levels were downregulated after AS2521780 treatment, which was also alleviated by curcumin (Figure 2(e)). Meanwhile, the Orai1 protein and mRNA levels were lower in the curcumin+AS2521780 group than in the AS2521780 group, but still higher than in the curcumin group (Figure 2(e,d)). In addition, cleaved caspase 3 was observed to be downregulated after AS2521780 treatment, and this effect was alleviated by curcumin (Figure 2(e)). These results indicated a protective effect exhibited in vivo by curcumin against OGD/R through the PKC-θ signaling pathway.
Neurological scores, TEER, Ca2+ levels, and cell permeability in MCAO injury rat model
To assess the effects of curcumin on cerebral I/R injury, SD rats were subjected to 90 min of MCAO. Neurological scores were measured according to Bederson’s neurological scale [21]. The rats in the control group were in good condition, but all the other groups showed different degrees of neurological disorder. The rats in the MCAO and AS2521780 groups exhibited severe neurological disorder, which was significantly ameliorated by curcumin treatment (Figure 3(a)). Ca2+ concentrations increased after MCAO, and the AS2521780 group showed the highest Ca2+ levels. In contrast, curcumin inhibited the increase in Ca2+ concentration induced by MCAO and AS2521780 (Figure 3(b)). Furthermore, MCAO and AS2521780 demonstrably inhibited TEER and promoted cell permeability, which were both inhibited by curcumin (Figure 3(c,d)). As shown in Figure 3(e), the ZO-1 and occludin protein levels were significantly downregulated after MCAO, with the AS2521780 group showing the lowest levels. However, curcumin alleviated these effects. Moreover, the results indicated that MCAO and AS2521780 decreased the p-PKC-θ, p-IκBα, and nuclear p65 protein levels but increased those of Orai1, all of which were blocked by curcumin (Figure 3(e)). In addition, cleaved caspase 3 was observed to be downregulated after MCAO and AS2521780, which was alleviated by curcumin (Figure 3(e)).
Figure 3.
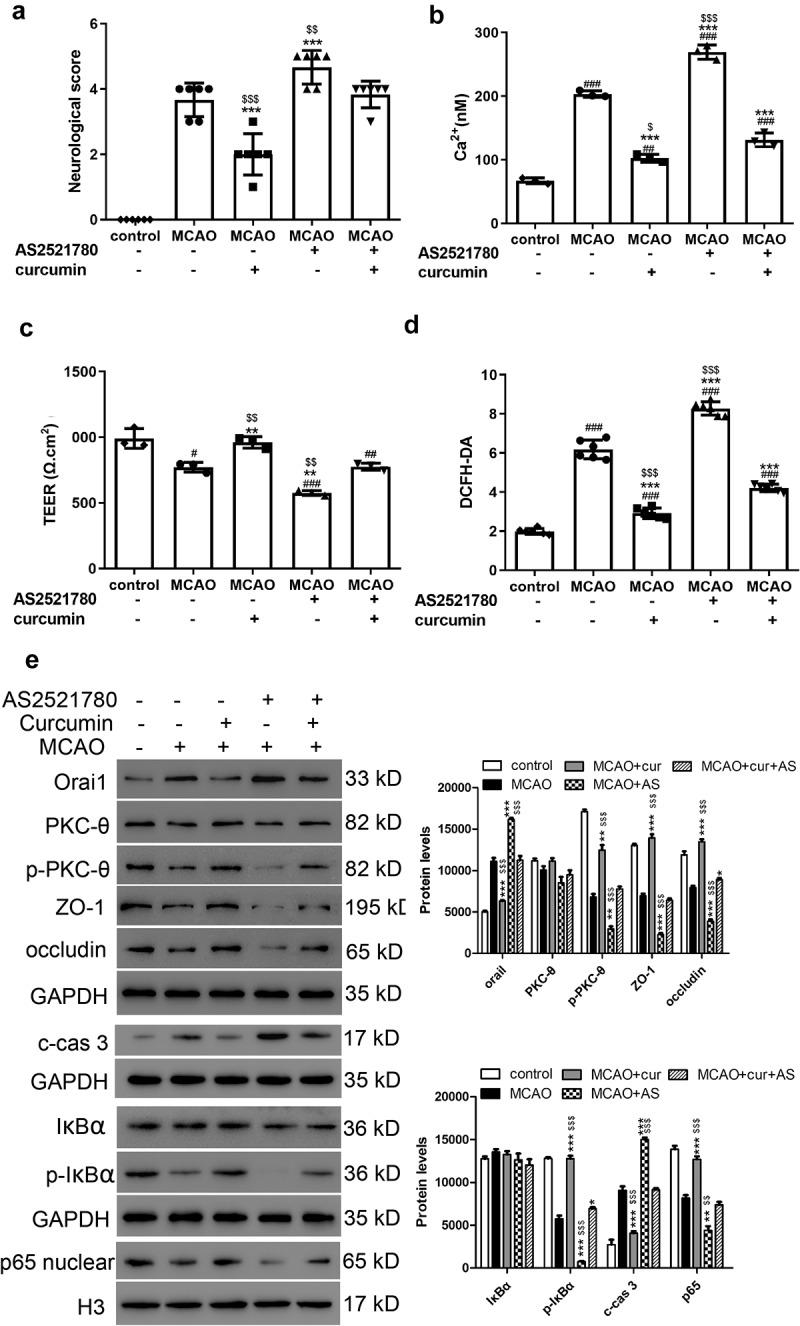
Neurological scores, TEER, Ca2+ concentrations, and cell permeability in MCAO injury rat model. (a) Neurological scores of the rats in each group. ***P < 0.001 vs MCAO; $$P < 0.01 and $$$P < 0.001 vs curcumin+AS2521780. (b) Ca2+ concentration, (c) TEER, and (d) cell permeability were analyzed. #P < 0.05, ##P < 0.01, and ###P < 0.001 vs control; **P < 0.01 and ***P < 0.001 vs MCAO; $P < 0.05, $$P < 0.01, and $$$P < 0.001 vs curcumin+AS2521780. (e) Western blot assay was performed to measure Orai1, PKC-θ, p- PKC-θ, ZO-1, occludin, IκBα, p-IκBα cleaved caspase 3, and nuclear p65 levels. *P < 0.05, **P < 0.01, and ***P < 0.001 vs MCAO; $P < 0.05, $$P < 0.01, and $$$P < 0.001 vs curcumin+AS2521780
H&E staining revealed that the nerve cells in the control group exhibited a normal condition with a clear cell outline and nuclear and synaptic structure. However, edema and necrosis were observed in nerve cells from the MCAO and AS2521780 groups (Figure 4(a)). Compared with the MCAO group, edema and necrosis were less severe in the curcumin group. These results indicated that curcumin buffered the MCAO damage to nerve cells. Moreover, IHC results showed that MCAO and AS2521780 promoted cleaved caspase 3 and TNF-α and curcumin was demonstrated to block these effects (Figure 4(b,c)). In summary, curcumin could ameliorate the harmful effects induced by MCAO, specifically by reducing BBB disruption and the PKC-θ signaling pathway.
Figure 4.
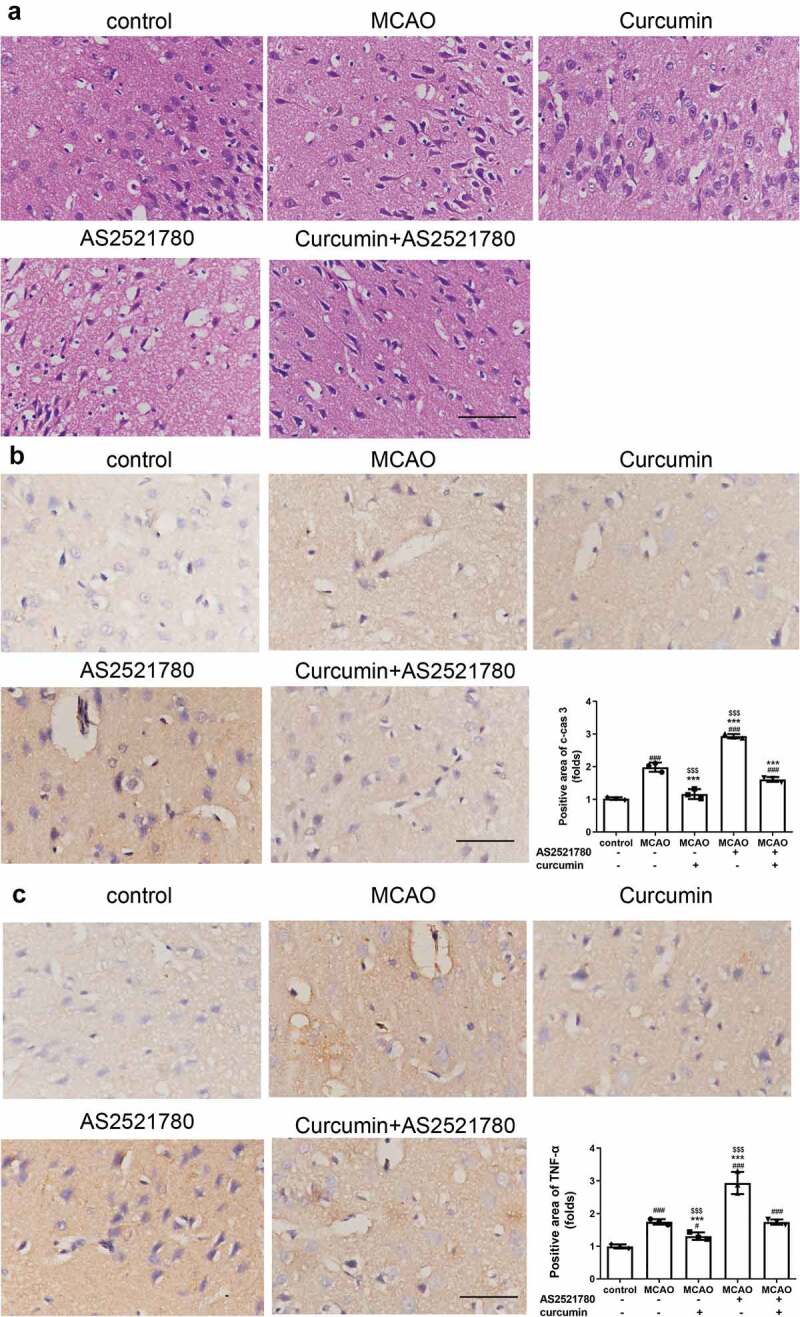
HE staining and IHC of brain samples from I/R rats. (a) Representative images of HE staining. Scale bar, 100 μm. (b-c) IHC assay was performed to detect cleaved caspase 3 (b) and TNF-α (c). Scale bar, 100 μm. ###P < 0.001 vs control; ***P < 0.001 vs MCAO; $$$P < 0.001 vs curcumin+AS2521780
Discussion
Our study demonstrated that the protective effects of curcumin in the OGD/R injury cell model involved lessening BBB disruption and reducing Ca2+ concentrations, which was likewise demonstrated using the MCAO rat model. Moreover, we found that the PKC-θ signaling pathway was involved in curcumin-mediated neuroprotection in both the OGD/R injury cell and MCAO rat models.
Curcumin, a known antioxidant, has been found to protect neurons from ischemic injury through the Akt/Nrf2 pathway [25]. Curcumin also confers a protective effect against cerebral I/R injury by activating the JAK2/STAT3 signaling pathway [26]. In this study, the CCK-8 assay showed that cell survival was markedly increased when curcumin was applied to OGD/R PC12 cells. The OGD/R-induced increase in cleaved caspase 3 was alleviated by curcumin. Moreover, a decrease in p-PKC-θ, p-IκBα and nuclear p65 protein levels induced by OGD/R was reversed by curcumin (Figure 2). We also found that curcumin inhibited the mRNA and protein levels of Orai1. PKC-θ belongs to the novel PKC subtype, which is activated by diacylglycerol, but not Ca2+. Ca2+ overload is considered to be the most important ion imbalance in neuronal injury, and OGD/R exposure is known to increase intracellular Ca2+ concentration [27,28]. Orai1, an essential membrane protein for the activation of store-operated calcium entry, has been found to mediate intracellular Ca2+ concentration [24]. In the current study, the increase in Ca2+ concentration and Orai1 levels were observed in OGD/R PC12 cells, and AS2521780 enhanced these effects. The data indicated that PKC-θ may play a role in the mediation of Ca2+ concentration by Orai1.
Intracellular Ca2+ signaling has been found to mediate BBB impairment [29]. Since an intact BBB is essential for maintaining a normal brain condition, and less BBB disruption always presents a better survival in cerebral I/R injury [30], we hypothesized that curcumin treatment could reduce BBB disruption. The results of our study showed that curcumin inhibited I/R-induced BBB disruption by increasing TEER, decreasing cell permeability and ZO-1 and occluding levels in vitro and in vivo. Curcumin has consistently demonstrated a protective effect on BBB against cerebral I/R Injury [17]. A decrease in BBB disruption has been reported to contribute to neuronal survival in cerebral I/R by the inhibition of S6K1 [23]. Moreover, PKC-θ is associated with interleukin-1β-induced barrier dysfunction in the microvascular endothelium of the human brain [31]. Our results showed that the inhibition of PKC-θ aggravated the BBB disruption induced by I/R, thereby decreasing TEER and increasing cell permeability and ZO-1 and occludin levels.
Conclusion
Curcumin exhibits a protective effect in cerebral I/R injury by regulating PKC-θ and Orai1, which in turn mediate Ca2+ concentrations that affect BBB integrity.
Supplementary Material
Funding Statement
This study was supported by the Key Disciplines Construction Foundation of Health Commission of Shanghai Pudong New District of China [No.PWZxk2017-25] and the Natural Science Foundation of Shanghai of China [No.17ZR1425800].
Disclosure statement
The authors have no conflicts of interest to declare.
Supplementary material
Supplemental data for this article can be accessed here.
References
- [1].Eltzschig HK, Eckle T.. Ischemia and reperfusion–from mechanism to translation. Nat Med. 2011;17:1391–1401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Turley KR, Toledo-Pereyra LH, Kothari RU, et al. Molecular mechanisms in the pathogenesis and treatment of acute ischemic stroke. J Invest Surg. 2005;18:207–218. [DOI] [PubMed] [Google Scholar]
- [3].Zhang H, Park JH, Maharjan S, et al. Sac-1004, a vascular leakage blocker, reduces cerebral ischemia-reperfusion injury by suppressing blood-brain barrier disruption and inflammation. J Neuroinflammation. 2017;14:122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Meng X, Xie W, Xu Q, et al. Neuroprotective effects of radix scrophulariae on cerebral ischemia and reperfusion injury via MAPK pathways. Molecules. 2018;23:2401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Kawasaki T, Ueyama T, Lange I, et al. Protein kinase C-induced phosphorylation of Orai1 regulates the intracellular Ca2+ level via the store-operated Ca2+ channel. J Biol Chem. 2010;285:25720–25730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Fauconnier M, Bourigault M-L, Meme S, et al. Protein kinase C-theta is required for development of experimental cerebral malaria. Am J Pathol. 2011;178:212–221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Bright R, Mochly-Rosen D.. The role of protein kinase C in cerebral ischemic and reperfusion injury. Stroke. 2005;36:2781–2790. [DOI] [PubMed] [Google Scholar]
- [8].Bright R, Sun G-H, Yenari MA, et al. epsilonPKC confers acute tolerance to cerebral ischemic reperfusion injury. Neurosci Lett. 2008;441:120–124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Teng JC, Kay H, Chen Q, et al. Mechanisms related to the cardioprotective effects of protein kinase C epsilon (PKC epsilon) peptide activator or inhibitor in rat ischemia/reperfusion injury. Naunyn-Schmiedeberg’s Arch Pharmacol. 2008;378:1–15. [DOI] [PubMed] [Google Scholar]
- [10].Harper MT, Poole AW. Protein kinase Ctheta negatively regulates store-independent Ca2+ entry and phosphatidylserine exposure downstream of glycoprotein VI in platelets. J Biol Chem. 2010;285:19865–19873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Chaudhary D, Kasaian M. PKCtheta: a potential therapeutic target for T-cell-mediated diseases. Curr Opin Invest Drugs. 2006;7:432–437. [PubMed] [Google Scholar]
- [12].Ferreira VH, Nazli A, Dizzell SE, et al. The anti-inflammatory activity of curcumin protects the genital mucosal epithelial barrier from disruption and blocks replication of HIV-1 and HSV-2. PloS One. 2015;10:e0124903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Yu L, Fan Y, Ye G, et al. Curcumin inhibits apoptosis and brain edema induced by hypoxia-hypercapnia brain damage in rat models. Am J Med Sci. 2015;349:521–525. [DOI] [PubMed] [Google Scholar]
- [14].Dhandapani KM, Mahesh VB, Brann DW, et al. Curcumin suppresses growth and chemoresistance of human glioblastoma cells via AP-1 and NFkappaB transcription factors. J Neurochem. 2007;102:522–538. [DOI] [PubMed] [Google Scholar]
- [15].Das L, Vinayak M. Long term effect of curcumin in restoration of tumour suppressor p53 and phase-II antioxidant enzymes via activation of Nrf2 signalling and modulation of inflammation in prevention of cancer. PloS One. 2015;10:e0124000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Liu L, Zhang W, Wang L, et al. Curcumin prevents cerebral ischemia reperfusion injury via increase of mitochondrial biogenesis. Neurochem Res. 2014;39:1322–1331. [DOI] [PubMed] [Google Scholar]
- [17].Wang Y, Luo J, Li S-Y, et al. Nano-curcumin simultaneously protects the blood-brain barrier and reduces M1 microglial activation during cerebral ischemia-reperfusion injury. ACS Appl Mater Interfaces. 2019;11:3763–3770. [DOI] [PubMed] [Google Scholar]
- [18].Hu Z, Yang B, Mo X, et al. HspB8 mediates neuroprotection against OGD/R in N2A cells through the phosphoinositide 3-kinase/Akt pathway. Brain Res. 2016;1644:15–21. [DOI] [PubMed] [Google Scholar]
- [19].Hawkins BT, Gu Y-H, Izawa Y, et al. Dabigatran abrogates brain endothelial cell permeability in response to thrombin. J Cereb Blood Flow Metab. 2015;35:985–992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Wang S, Wang M, Wang M, et al. Bavachinin induces oxidative damage in HepaRG cells through p38/JNK MAPK pathways. Toxins (Basel). 2018;10. DOI: 10.3390/toxins10040154 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Gong L, Tang Y, An R, et al. RTN1-C mediates cerebral ischemia/reperfusion injury via ER stress and mitochondria-associated apoptosis pathways. Cell Death Dis. 2017;8:e3080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Matsuda T, Arakawa N, Takuma K, et al. SEA0400, a novel and selective inhibitor of the Na+-Ca2+ exchanger, attenuates reperfusion injury in the in vitro and in vivo cerebral ischemic models. J Pharmacol Exp Ther. 2001;298:249–256. [PubMed] [Google Scholar]
- [23].Chi OZ, Kiss GK, Mellender SJ, et al. Inhibition of p70 ribosomal S6 kinase 1 (S6K1) by PF-4708671 decreased infarct size in early cerebral ischemia-reperfusion with decreased BBB permeability. Eur J Pharmacol. 2019;855:202–207. [DOI] [PubMed] [Google Scholar]
- [24].Zhao X, Moloughney JG, Zhang S, et al. Orai1 mediates exacerbated Ca(2+) entry in dystrophic skeletal muscle. PloS One. 2012;7:e49862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Wu J, Li Q, Wang X, et al. Neuroprotection by curcumin in ischemic brain injury involves the Akt/Nrf2 pathway. PloS One. 2013;8:e59843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Li L, Li H, Li M, et al. Curcumin protects against cerebral ischemia-reperfusion injury by activating JAK2/STAT3 signaling pathway in rats. Int J Clin Exp Med. 2015;8:14985–14991. [PMC free article] [PubMed] [Google Scholar]
- [27].Sun B, Ou H, Ren F, et al. Propofol inhibited autophagy through Ca(2+)/CaMKKbeta/AMPK/mTOR pathway in OGD/R-induced neuron injury. Mol Med. 2018;24:58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Yang S, Wu P, Xiao J, et al. Overexpression of COX6B1 protects against I/Rinduced neuronal injury in rat hippocampal neurons. Mol Med Rep. 2019;19:4852–4862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Hawkins BT, Davis TP. The blood-brain barrier/neurovascular unit in health and disease. Pharmacol Rev. 2005;57:173–185. [DOI] [PubMed] [Google Scholar]
- [30].Tan F, FU W, CHENG N, et al. Ligustrazine reduces blood-brain barrier permeability in a rat model of focal cerebral ischemia and reperfusion. Exp Ther Med. 2015;9:1757–1762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].Rigor RR, Beard RS, Litovka OP, et al. Interleukin-1beta-induced barrier dysfunction is signaled through PKC-theta in human brain microvascular endothelium. Am J Physiol Cell Physiol. 2012;302:C1513–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


