ABSTRACT
The World Health Organization (WHO) recommends antenatal influenza vaccination (AIV) for pregnant women at any stage of pregnancy. This study assessed fundamental aspects of AIV acceptance and demand among key stakeholders in urban Pune, India. Semi-structured interviews for rapid ethnographic assessment of AIV-related awareness, priorities, and practices were used to study clinicians and their communities of practice. A qualitative survey was conducted among 16 private clinicians providing antenatal care (ANC) in slum and middle-class areas of Pune. Following the survey, clinicians were informed about authoritative AIV recommendations. A qualitative community survey was also conducted with 60 women aged 20–35 years and 30 spouses from the same slum and middle-class practice areas of the ANC providers. Subsequently, a second clinician survey was conducted to assess changes in clinicians’ awareness, priority, and vaccination practice. After this interview, clinicians were informed of community survey findings. Most community respondents were unaware of AIV, in contrast with well-known and widely used antenatal tetanus vaccination. They expressed confidence in vaccines and trust in the clinicians. Clinicians’ advice was reportedly the most important determinant of community vaccine acceptance. Clinicians were confident of the safety of AIV and they anticipated patients’ acceptance if recommended. The second clinician interview showed increased awareness of AIV policy, but clinicians were more skeptical about the severity of maternal influenza in their practice. Our findings indicate community acceptance though not demand for AIV. We recommend five essential elements for vaccination program strategies to improve coverage with AIV and other ANC vaccines.
KEYWORDS: Antenatal influenza vaccination, community vaccine recipients, India, vaccination stakeholders, vaccine acceptance, vaccine demand, vaccine priority
Background
Pregnant women are at high risk of greater morbidity and mortality from seasonal and pandemic influenza infections owing to physiological and immunological effects of pregnancy that compromise pulmonary function, cardiac output, and immune function. If infected, hospitalization, and adverse pregnancy and birth outcomes are more likely. Their newborns are more likely to be premature or have low birthweight.1-7 Vaccination of pregnant women is the most effective preventive measure. Antenatal influenza vaccination (AIV) is safe and effective for protecting them and their newborns for up to 6 months after delivery.8-12 The World Health Organization (WHO) recommends seasonal influenza vaccination globally for pregnant women at any stage of pregnancy.13,14 Nevertheless, in many low- and middle-income countries (LMICs), including India, effective strategies to improve AIV coverage are lacking.15 Seasonal influenza vaccination is recommended but not mandatory for pregnant women in India. This recommendation for AIV was introduced in 2012 in the aftermath of the H1N1 pandemic.16 Studies in various parts of India, however, have found low uptake of influenza vaccination among pregnant women (i.e. < 4%).17-19
Research suggests that influenza vaccine acceptance and demand among pregnant women are determined by various factors, including availability, access, and cost of vaccines. Cultural and religious community beliefs may also play a role.20-22 The term vaccine hesitancy, originally defined as “delay in acceptance or refusal of vaccines despite availability” is now applied broadly to a range of social, cultural, and behavioral factors discouraging vaccination.23 Although vaccine hesitancy in the community of potential vaccine recipients clearly limits influenza vaccine acceptance in Western countries,24 clinician hesitancy appears to play a greater role in LMICs.25,26 A literature review of 45 studies in 10 countries, however, indicated that despite limited awareness of the risk of influenza, the value of vaccination and concerns about safety, if clinicians recommend it, pregnant women are likely to accept vaccination.20 Studies in different settings of various high-income countries (HICs) showed that clinicians recommending AIV and providing information were the most important drivers for their vaccine uptake.27-33 Studies in India, including our previous work in Pune district in the western part of the country, also indicated that low influenza vaccination coverage following the H1N1 pandemic in 2009 is better explained as a failure of providers to recommend and vaccinate than by community hesitancy or lack of confidence limiting acceptance.17,18,34 Clinician vaccination practices may be the most important factor explaining whether or not pregnant women are vaccinated.
The WHO Strategic Advisory Group of Experts (SAGE) on immunization identified three domains of vaccine hesitancy; namely (i) contextual influences, including the role of policymakers and government authorities; (ii) individual/social-group factors that influence community perceptions and experience of vaccines; and (iii) vaccine-specific issues, such as vaccine access, cost, and the influence of healthcare professionals.23,24 Although recent studies of vaccine acceptance and demand have investigated the role of community determinants of vaccination coverage in HICs, further research is needed in LMICs to explain how clinicians affect community AIV acceptance. A recent relevant study in Switzerland35 highlights the value of a multi-stakeholder framework – considering complementary roles of clinicians, communities, and policymakers – to identify strengths and weaknesses and what makes vaccination programs more effective.
Our study was developed to examine the role of clinician and community stakeholder views affecting AIV. It employs a framework considering the role of awareness, priorities, and practices of both stakeholder groups. Research on this topic is lacking in India. Recognizing the impact of wealth and poverty, we planned to study AIV in both middle-class and low-income slum communities. Although we also recognize the distinctive features of government and private antenatal care (ANC) health services, we have limited the scope of this preliminary study to private-sector ANC providers in urban Pune, India. A community component in the practice areas of study clinicians involves the study of women of reproductive age and their spouses. Specific aims were to (i) assess community awareness, priorities, and practices regarding childhood and antenatal vaccinations generally; (ii) determine private clinicians’ awareness, priorities, and practices regarding influenza vaccination for pregnant women; and (iii) compare community and clinicians’ views on influenza vaccination for pregnant women.
Materials and methods
Setting
The study was conducted in three middle-class and three slum communities (recognized by Pune Municipal Corporation (PMC)) from Aundh, Ghole Road, Kothrud, and Karve Nagar areas in urban Pune, Maharashtra, India, from July 2015 to May 2016. The term slum is an official designation of the PMC for low-income communities; it is widely used and not intended as pejorative. Pune is the sixth largest city in India with approximately 3 million inhabitants, of which more than 40% live in slum areas. During the 2009 influenza pandemic, Pune city was the epicenter of the country with a substantial number of reported cases and further outbreaks in the following years. The research team had previously studied use of the influenza vaccine developed by the Serum Institute in Pune for the H1N1 pandemic of 2009. Our experience in the earlier study informed the development of our study plan, and the diverse and representative features of the city as a major urban center in India informed selection of Pune for the current study. The study sites were selected randomly among the administrative wards of PMC in Pune city. Within these selected sites, private clinics were identified and approached for participation. Interviews within the population were conducted in communities neighboring on the participating clinics.
Study design
Clinicians providing ANC in their private clinics of these communities, and women aged 20–35 years and their spouses were recruited.36 Qualitative survey instruments were developed for a rapid ethnographic assessment to ascertain the respective roles of community and clinician stakeholders likely to affect AIV coverage in these middle-class and low-resource communities. The rapid ethnographic assessment consisted of interviews with clinicians and community respondents to assess relevant aspects of both nonspecific vaccine-related and AIV-specific awareness, priorities, and practices.37
As a rapid ethnographic qualitative survey, our study was nested in a comparison of ANC vaccination practices among clinicians at urban community sites. The number of clinicians was determined by identifying all ANC clinicians at a manageable number of urban field sites and assigning them to study or control groups; study group clinicians were interviewed, while control group clinicians were not.38 The community sample comprised 60 women with varied status of prior, current and no pregnancy experience, and 30 male spouses. This sample size is consistent with accepted ethnographic interviewing practices, suggesting approximately 30–50 participants, typically sufficient to assess qualitative research questions and distinctive features of the subgroups.39
The first of two clinician interactions assessed clinicians’ awareness of AIV recommendations, priority of AIV and prescribing practices. After the interview, information on authoritative AIV policy recommendations of WHO’s SAGE and the Federation of Obstetric and Gynecological Societies of India (FOGSI) was provided to the clinicians. The community assessment framework was planned to enable rapid analysis and feedback to participating-clinicians for improving vaccine coverage in their clinical practice. The second clinician interaction repeated questions in the first interaction to assess change, and findings were presented from the community survey. Results on intended effects of these two clinician interactions on improving coverage with AIV have been published elsewhere.38
Community interviews
We recruited 60 women aged 20–35 years from communities served by the participating clinics. Purposive sampling was facilitated by the help of local community leaders and health workers. Equal numbers (20 each) of previously but not currently pregnant women, currently pregnant women, and women who had never been pregnant were selected. Thirty spouses, who were available at the time of the household visit, were additionally interviewed after obtaining informed consent. Ten male spouses were selected from each of the three groups of women.
A semi-structured community interview with questions to elicit open narrative and codable categorical responses was developed. Questions addressed sociodemographic characteristics (e.g. age, education, occupation, household income, pregnancy, and childbearing history), healthcare experience, and essential features of vaccine acceptance and demand (viz. awareness, priorities, and practice). Questions on awareness of existing childhood and antenatal vaccines, and access were included. Questions on priority assessed experienced and perceived vaccination-related problems and benefits, and persons who may have influenced vaccine acceptance and priority. Questions on vaccination practices addressed actual use based on recall and anticipated vaccination experience for both antenatal and childhood vaccinations. A version of the qualitative survey instrument was adapted for use with male spouses, and ANC-related questions focussed on their female spouse.
Clinician interviews
Private clinicians routinely providing ANC were identified using the Practo web-based search engine,40 including specialist obstetricians and gynecologists or general practice physicians. Clinicians in middle-class and slum areas were identified for recruitment to participate in two qualitative survey interviews and informational interactions. The first interaction with clinicians (Clin-1) occurred in September 2015 and the second one (Clin-2) in December 2015.
A semi-structured clinician interview with questions to elicit open narrative and codable categorical responses was developed. Questions addressed sociodemographic characteristics; professional training and experience; and AIV awareness, priorities, and vaccination practices. Questions on awareness included clinicians’ understanding of AIV recommendations. Questions on priority considered the severity and impact of influenza on pregnant women and newborns, including risks, vulnerabilities, and benefits of vaccination. The role of access, cost, and views of patients’ hesitancy and confidence were also addressed among considerations of priority. Questions of practice referred to AIV and tetanus in their clinical ANC practice. As detailed in the design, clinicians were informed of authoritative AIV recommendations at the end of Clin-1, and of community views about AIV at the end of Clin-2.
Data collection and management
The six research assistants (RAs) who conducted the interviews each had a master’s degree in either health or social sciences, and they were native Marathi speakers. The investigators in Pune trained them to administer the qualitative interviews and to manage data according to the study plan. Both clinician and community interviews were administered in Marathi using Android tablet devices running Open Data Kit (ODK) software. This eliminated the need for subsequent data entry and enabled audio recording during the interviews. Clinicians were interviewed at their clinics and the community interviews were conducted at participants’ households. The average lengths of the first and second clinician interviews and of the community interviews were 26, 22, and 45 minutes, respectively. Clinician and community respondents were cooperative and engaged.
Digital interview data were uploaded daily to a central ODK aggregate server. All the interviews were downloaded from the server and processed in Excel spreadsheets. Narratives of the clinician and community interviews were transcribed from Marathi audio recordings and translated into English by two of the RAs. Qualitative analysis including thematic coding was conducted with MAXQDA software version 11.0 (Verbi GmbH; Berlin, Germany).
Approach to analysis
Descriptive statistical analysis summarized the categorical responses of clinician interviews and the community survey. Clinician responses from the first and the second interviews were compared with findings visualized in bar charts, stratifying between middle-class and slum clinics. All statistical analysis was done using Stata/SE version 14.2 (StataCorp; College Station, Texas, USA). Quantitative variables were also used in MAXQDA as selection variables for relevant qualitative comparisons based on categorical responses, employing an integrative approach for analysis of quantitative and qualitative data.
Initial deductive coding was based on questions of the interviews, which were structured according to a framework of awareness, priority, and practice. With further consideration in a process of familiarization with the narratives, a second level of coding was undertaken inductively. Thematic analysis based on our awareness-priority-practice framework, common to both clinician and community data, addressed the first two study aims concerning community and clinician surveys, respectively. The framework also facilitated comparison of clinician- and community data relevant for comparative interests of objective 3.
Results
Sample characteristics
Community sample
We conducted 90 interviews with 60 women and 30 men with equal numbers in the slum and middle-class communities (Table 1). Among the 60 women, 51 (85%) were aged 20–29 years. Nearly 90% (53 of 60) reported ≥10 years of education, but about two-thirds of the women (39 of 60, 65%) were unemployed. Among the 30 male spouses, all but one reported ≥10 years of education, and all were employed at the time of the interview. Most respondents, 72 of 90 (80%), were satisfied with general healthcare services routinely available for them. Nearly all, 27 of 28 women (96%), were satisfied with their ANC during their last pregnancy.
Table 1.
Summary of community sample characteristics
| Sample characteristics | Women (n = 60) | Men (n = 30) |
|---|---|---|
| Pregnancy status* | ||
| Currently and previously | 8 | 3 |
| Currently but not previously | 12 | 7 |
| Previously but not currently | 20 | 10 |
| Neither previously nor currently | 20 | 10 |
| Age (in years) | ||
| Mean (SD) | 25.3 (3.6) | 28.8 (4.3) |
| Minimum | 20 | 21 |
| Maximum | 35 | 40 |
| Site | ||
| Slum area | 30 | 15 |
| Middle-class area | 30 | 15 |
| Educational attainment | ||
| No education | 1 | 0 |
| ≤Secondary school | 13 | 8 |
| Higher secondary school | 14 | 5 |
| Graduation | 17 | 10 |
| Post-graduation | 15 | 7 |
| Occupation | ||
| Housewife | 39 | NA |
| Employed | 21 | 29 |
| Retired | 0 | 1 |
| Household income per month (INR) | ||
| No Income | 0 | 4 |
| ≤20,000 | 22 | 7 |
| 20,001–60,000 | 21 | 14 |
| >60,001 | 12 | 3 |
| Cannot say | 5 | 2 |
| Children ≥1 | ||
| Yes | 26 | 12 |
| No | 34 | 18 |
SD: Standard deviation, NA: Not applicable, * Male respondents were categorized based on the respective status of their wife.
Clinician sample
Among the 16 respondent clinicians, 11 were practicing in slum areas and the remaining 5 in middle-class communities. Twelve clinicians had training in obstetrics and gynecology and 4 were general practitioners. Ten clinicians had more than 15 years of professional experience.
Community views on vaccine acceptance and demand generally and for pregnant women
Awareness of vaccinations
All respondents were aware of the Government of India’s Universal Immunization Programme for vaccinating children in the first 5 years after birth. Over 90% of the 90 respondents were aware that vaccines are useful for preventing diseases in children. A typical response from a woman in a middle-class site explained, “Vaccines are used as a preventive measure, which means a vaccine is taken to avoid the infection in future.”
The following vaccines were reported most frequently (number and proportion) by these community respondents: polio (59, 66%), Bacillus Calmette–Guérin (BCG) (30, 32%), measles (21, 23%), diphtheria, pertussis, and tetanus (DPT) (16, 18%) and influenza (13, 14%). Other vaccines mentioned less frequently included hepatitis A, hepatitis B, and tetanus toxoid (TT). Respondents generally provided accurate accounts of the vaccines that they identified. For example, a woman from the slum area used the term “triple” for DPT vaccination.
Only four of 28 women with pregnancy experience were aware of vaccines prescribed to pregnant women against flu-like illness (FLI). A woman from a slum site, who was pregnant when interviewed, elaborated on information about vaccines for FLI: “To avoid the occurrence of swine flu and all, this has been told. Despite that, I did not take the vaccine.” Only one of 13 men with a previously pregnant wife indicated awareness of a vaccine for FLI during pregnancy. Of 49 respondents with no personal or spousal previous pregnancy experience (32 women and 17 men), six women and two men reported that they were aware of a vaccine against FLI. One woman who was aware of a vaccine for FLI, however, said that she thought it was not for pregnant women: “There is a normal influenza vaccine. But I think doctors don’t recommend it to pregnant women routinely, as far as I have heard.”
Community priority of vaccinations
Over 95% (86 of 90) of respondents considered childhood vaccines useful. Regarding vaccination-related problems, 32 of 90 (36%) respondents reported awareness of possible mild adverse events such as mild fever, redness and pain at the injection site. Respondents with a child (women, n = 26; men, n = 12) were also asked whether influential advisors affected their decision to get their child vaccinated, and 25 of 38 respondents referred to their doctor as the main advisor, and nearly half of the women respondents also said their husbands’ views were important.
Most respondents (76 of 90, 84%) said that vaccines for pregnant women were useful and beneficial, with a higher proportion for men (28 of 30, 93%) than for women (49 of 60, 82%). Analysis of narratives showed that most of them (70 of 90, 78%) considered antenatal vaccination protective for both the mother and her newborn. A woman from a slum site with no pregnancy experience said, “Vaccination is better for pregnant women and for their baby also. Both will be strong.” But they regarded effects of vaccinating pregnant women mainly as benefiting the newborn. A currently pregnant woman from a middle-class site explained, “If a pregnant woman doesn’t take the vaccine, then the baby will be in danger. So, it is better if a pregnant woman gets vaccinated, so that baby will be safe.” A man from a middle-class site highlighted the cost-effectiveness of antenatal vaccination: “If she is vaccinated prior and her immunity increases, then she won’t suffer from these diseases. That means it will not increase the financial burden for healthcare, and patient’s expenses are saved.”
Most respondents reported they had not heard of any problems during pregnancy from vaccines, including 47 of 60 (78%) women and 27 of 30 (90%) men. A woman from a middle-class site with no pregnancy experience said, “I have not heard any news about any problem effects, like termination of pregnancy, because of this vaccination.” Overall, 13 of 90 (14%) respondents indicated possible concerns, but they did not elaborate with concrete examples.
Figure 1 summarizes responses of 28 women with past pregnancy experience, including 8 currently pregnant women and 13 of their male spouses, whom they identified as their key advisors influencing decisions about accepting antenatal vaccination. Over half of the respondents reported that the doctor’s advice was important in the decision-making process. A currently pregnant woman from the slum site said, “If a doctor recommends, then I take that on my own. I don’t ask anyone else.” A man from the slum site noted, “One should ask the doctor and take the vaccine if it is beneficial and the doctor knows about it. So, the vaccine must be taken with their due consultation.” However, more than half of the women respondents said that they would also take advice from their husbands. A previously pregnant woman from a slum site responded, “Mainly my husband’s advice, but it is a collective decision of family members. I decide with the help of my family members’ advice.” Some respondents mentioned that advice from elders at home with prior vaccination experience during pregnancy was influential and motivating. A woman from a slum site explained, “At my home, my sister-in-law … and her advice is good because she had prior experience.”
Figure 1.
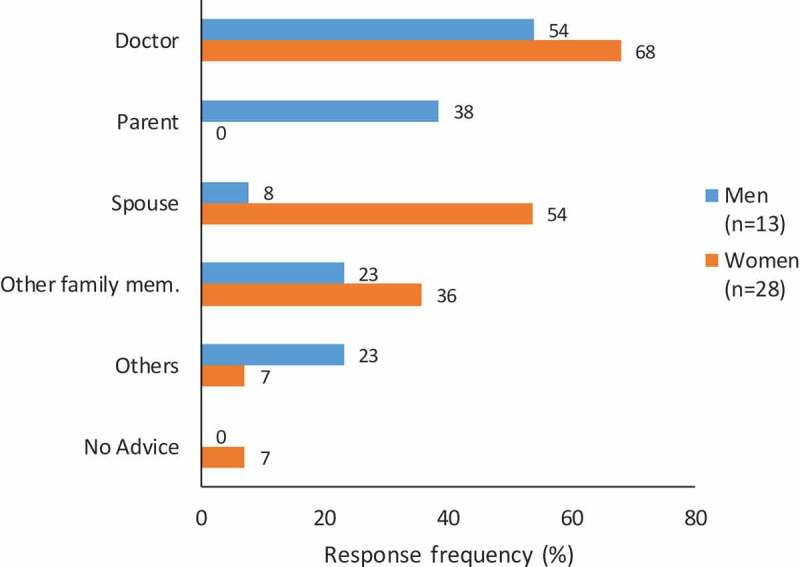
Community views about influential advisors for vaccination during pregnancy. Responses to question for all previously pregnant women (n = 20) and both previously and currently pregnant women (n = 8) respondents: “Whose advice was most important in deciding that you should get the vaccine during pregnancy?” (Multiple answers possible)
Question for male-spouse respondents of previously pregnant women: “Whose advice was most important in deciding that your wife should get the vaccine during pregnancy?” (Multiple answers possible). The response category, “Other family mem.” includes parents-in-law and siblings-in-law. “Others” includes colleagues, neighbors, and friends.
Community practice of vaccinations
All women respondents with at least one child (n = 26) reported that their children received basic vaccinations before they reached the age of 5 years. The following vaccines were identified by the indicated number and proportion of these women: BCG (20, 77%), polio (19, 73%), DPT (18, 69%), hepatitis B (16, 62%), measles (11, 42%), and influenza (5, 19%).
Of 28 previously pregnant women, 20 (71%) said that they recalled receiving a TT vaccine. None of these 28 women, however, recalled getting an influenza vaccine during their last pregnancy. A few respondents noted the availability of free vaccines for FLI in government ANC facilities, but that payment was required at private ANC clinics. A currently pregnant woman from a middle-class site said, “Now swine flu vaccination is free in government hospitals for a pregnant woman.” Two women from the slum site felt that information regarding antenatal vaccines was not properly conveyed to them at public hospitals, and they thought that they would get more information about vaccines at private clinics because they pay at private clinics. A woman from the slum area criticized government health services, “In Government hospital, they don’t tell anything. In a private hospital, it is like we give money; therefore, they tell us.”
Clinicians’ awareness, priorities and practices for AIV
Awareness of AIV policy
During the Clin-1 interviews, 9 of 16 (56%) clinicians reported that they were unaware of any existing policy to vaccinate pregnant women routinely against influenza. The 7 (44%) clinicians who were aware of AIV recommendations referred to the WHO and national medical societies, e.g. FOGSI, as their information sources. Clinicians in slum areas (4 of 11) were less likely to be aware of AIV recommendations than the clinicians in middle-class areas (3 of 5). Changes in awareness of AIV policy from the Clin-1 to Clin-2 interview in both settings are detailed in Figure 2. In Clin-2 interviews, 13 (81%) clinicians indicated awareness of AIV policy recommending influenza vaccination during pregnancy. The three clinicians who were still not clearly aware of the existence of any AIV policy in Clin-2 interviews were all practicing in slum settings.
Figure 2.
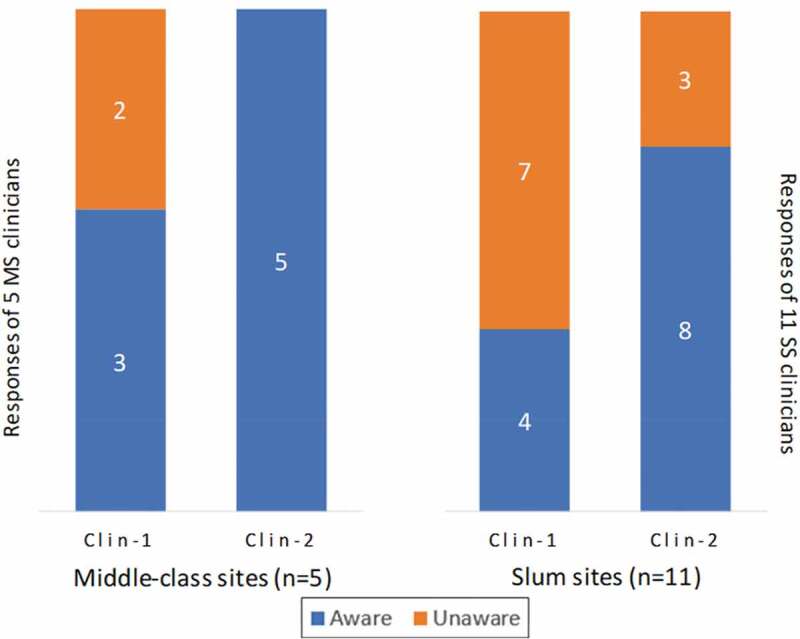
Clinicians’ awareness of recommended antenatal influenza vaccination policy in baseline and follow-up interviews. Clinicians were interviewed in middle-class and slum settings. After the baseline interview (Clin-1), a follow-up interview (Clin-2) was conducted 3 months later. They were asked to respond to a question about awareness of AIV policy: “What is your understanding of the public health policy recommendation for vaccinating pregnant women for influenza?” The figure distinguishes the number of clinicians in each setting aware or unaware of AIV policy recommendations in each of the two interviews
Clinicians’ priority of AIV
The priority of AIV among clinicians was assessed with reference to the seriousness of influenza illness, benefits of AIV, and consideration of any risks. Except for one clinician in a slum area, all clinicians acknowledged that influenza is a serious problem for pregnant women and/or newborns. In the narrative responses, two clinicians explained it was a serious problem that may require hospitalization, and another clinician thought it could lead to secondary infections. A clinician in a slum site said, “Now (in this season) flu is spreading vigorously. If symptoms are not regressing after 5–6 days, then it is turning into a fatal case.”
In the Clin-2 interviews, respondents indicated a wider range of opinions about the seriousness and clinical significance of influenza affecting their patients. Eight clinicians were concerned about its serious clinical impact, referring both to higher risk of hospitalization and potential stillbirth or low birth weight of newborns. Two were less concerned, and six did not regard influenza as a serious problem, dismissing it as a seasonal infection. A clinician in a middle-class area reported having observed no cases of influenza-related illness among her pregnant patients once the flu season is over. “It is not that frequent now. The rainy season is over. In that way flu cases have also decreased.”
AIV benefits
Over 90% of the clinicians reported that AIV provides clear benefits, and there was no difference between clinicians in slum and middle-class sites. Many clinicians indicated that influenza vaccination during pregnancy could also benefit the newborn child, as illustrated by the following comment, “Influenza vaccine is beneficial if pregnant women receive it … It protects the mother as well as newborn.” A clinician in a slum site, however, expressed uncertainty about the value of the vaccine, explaining, “I haven’t observed influenza infection among people who were not vaccinated against influenza H1N1.”
AIV risks
Eleven clinicians in slum and middle-class sites reported that they had never observed any adverse events after AIV in their own practice, nor had they heard of any such events. In response to our question about that, a slum-based clinician reverted to a restatement of benefits: “Influenza vaccine is beneficial. I never noticed any side effects in my practice, and it is safe.” Two clinicians acknowledged the possibility of adverse events but also noted that these were not severe. Three clinicians in middle-class and slum sites were uncertain about any risks associated with AIV during pregnancy.
Figure 3 summarizes the change in responses from Clin-1 to Clin-2 interviews regarding clinicians’ views on AIV benefits. Clinicians’ regard for the benefits of AIV remained mostly unchanged, similarly acknowledging protection of newborns from AIV in both interviews. During Clin-2 interviews clinicians reaffirmed there were no adverse events from AIV affecting the pregnancy, and they considered influenza vaccination to be safe for pregnant women.
Figure 3.
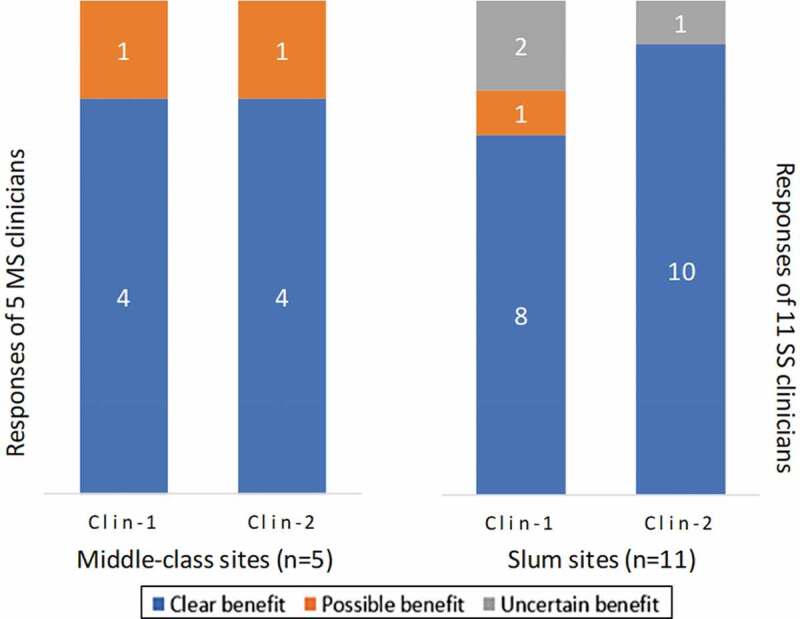
Clinicians’ views on benefits of antenatal influenza vaccination for pregnant women. Clinicians were interviewed in middle-class and slum settings (SS). After the baseline interview (Clin-1), a follow-up interview (Clin-2) was conducted 3 months later. They were asked to respond to a question about the benefits of AIV: “Do you think vaccinating pregnant women against influenza is beneficial for them and their newborns?” The figure presents the number of clinicians asserting clear benefit, possible benefit, or uncertain benefit
Clinicians’ AIV practices
Only one clinician in a middle-class and two in slum-site settings reported a clear policy for administering AIV in their clinics; all other clinicians said they had no such mandatory policy at their clinics. Notwithstanding current practice, all clinicians in both middle-class and slum sites endorsed implementing such a policy in their clinics. A middle-class-setting clinician explained that such a policy could protect pregnant women from severe influenza infections. She elaborated, “We should implement it; this is the only policy to protect pregnant women from severe types of influenza in their immune-compromised state.” Apart from two clinicians in slum sites, all other clinicians said that influenza vaccines were available from vaccine manufacturers, vaccine stocklists, medical retailers and distributors, and from the local chemists. Two clinicians, one each in a middle-class and slum site, reported that they were routinely vaccinating pregnant women against influenza. One middle-class and five slum-site clinicians said that they occasionally administered AIV. Their narrative responses referred to demand from their patients and influenza outbreaks as factors motivating them to vaccinate. The remaining eight clinicians reported that they were not vaccinating.
Figure 4 summarizes the change in reported AIV practice from Clin-1 to Clin-2. The number of influenza-vaccinating middle-class-site clinicians increased from Clin-1 to Clin-2 interviews but decreased among slum-site clinicians. In Clin-2 interviews, all middle-class-site clinicians reported they were vaccinating either routinely (3 of 5) or sometimes (2 of 5), but 7 of 11 slum-site clinicians reported they were never vaccinating pregnant women against influenza. The acknowledged priority of AIV, however, was inconsistent with changes in practice from Clin-1 to Clin-2 interviews at middle-class sites. All clinicians of both sites affirmed in Clin-1 interviews that implementing AIV was a high priority, but in Clin-2 fewer affirmed high priority (3 of 5 in middle-class sites, and 9 of 11 in slum sites). In middle-class sites, two clinicians said AIV was okay but not highly recommended; one slum-based clinician considered it unnecessary and one as not recommended.
Figure 4.
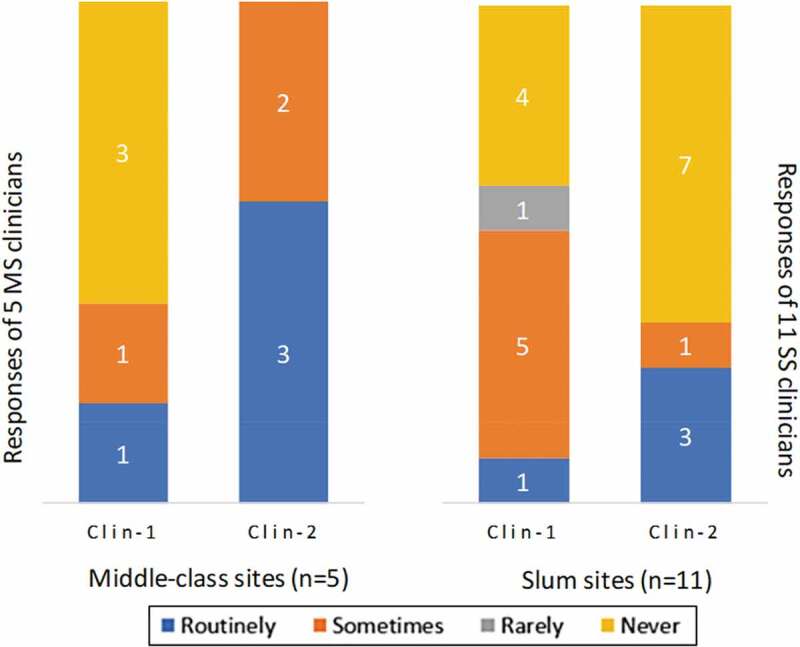
Clinicians’ antenatal influenza vaccination practices. Clinicians were interviewed in middle-class and slum settings. After the baseline interview (Clin-1), a follow-up interview (Clin-2) was conducted three months later. They were asked to respond to a question about their vaccination practices: “Do you prescribe or give influenza vaccines to pregnant women in your practice? If so, how often?” The figure presents the number of clinicians responding routinely, sometimes, rarely, or never
Clinicians’ perception of community acceptance and demand for influenza vaccination
When asked about community views concerning AIV, clinicians explained that many of their pregnant patients are unaware of influenza vaccination during pregnancy, but that most of them know about TT vaccination. A middle-class-site clinician elaborated: “Ninety percent of the ladies are aware of tetanus. In fact, many times they ask us. Now the influenza vaccine has come. About 30% of patients ask us about the influenza vaccine on their own, and we need to tell the remaining 70% cases.” Hence, these clinicians indicated no need to provide further information about TT vaccination, but for the less-known influenza vaccine, patients need more information. Such information, they said, should explain vaccine benefits, risks, and cost. Clinicians identified sources of information for those pregnant women who were aware of influenza vaccines as digital media, information brochures at health facilities, or interactions with other pregnant women who had been vaccinated.
In Clin-1 interviews, 13 clinicians reported that although pregnant women may not demand an influenza vaccine on their own, they will accept it if clinicians recommend it. In Clin-2 interviews, four clinicians said that a pregnant woman who is aware of the availability of the influenza vaccine will demand it. A middle-class vaccinating clinician highlighted patients’ desire for AIV, “Usually pregnant women ask for it. I mean out of 100, maybe 90 to 95 ask me for the vaccination.” More generally, even without such high levels of demand, clinicians reported that patients’ vaccination-hesitancy was not a relevant barrier for AIV.
Clinicians who were recommending AIV indicated that pregnant women trust their advice, consider it mandatory and accept vaccines without any doubt. Most clinicians in slum sites, however, emphasized cost of the vaccine remains a barrier for most pregnant women in their practice. Clinicians in middle-class sites, on the other hand, had mixed opinions about the impact of vaccine cost as a reason for refusal. A vaccinating clinician from a middle-class site explained, “For the most part, I have not seen the problem of patients refusing the vaccine because of the cost. No one has refused it for this reason.” And she also explained that perceived low value of the vaccine was a reason for the refusal only in very few cases: “One or two cases who refused it, said to me that we don’t want to take it; it doesn’t make much difference.” Another clinician from a middle-class site, however, indicated that the cost of the vaccine was indeed a reason for refusal: “Some of the pregnant patients refuse the vaccine because of the cost. Suppose the vaccine costs 800 Indian rupees, then some patients refuse it.”
Comparison of community and clinicians’ views
Narratives from community interviews were notable for lack of awareness of AIV in contrast to widespread awareness of TT vaccination during pregnancy. A remark of a previously pregnant woman from a slum site is illustrative: “During my pregnancy, there was only tetanus vaccination, that’s all. I don’t know any other special vaccines.” Consistent with and analogous to community awareness, clinicians noted that influenza vaccination generally is not mandatory according to the national vaccination schedule, but most were less aware of AIV policies – strikingly so in comparison with TT. A slum-based clinician exemplifies that point: “According to the antenatal immunization chart provided by the government, TT is compulsory. There is a doubt about influenza vaccination because it is not listed in the chart.”
Community respondents commonly reported that any vaccination during pregnancy is not useful just for pregnant women, and its main benefit was protecting their newborns from illness. They emphasized this latter point. A currently pregnant woman noted, “Vaccination is useful, because if she does not take the vaccine, then the baby will also be in danger. So, it is better if a pregnant woman gets vaccinated so that the baby will also be safe” (29-year-old woman at middle-class site). Similar responses from clinicians, though relatively more attentive to risk for their pregnant women patients, characterized views about influenza vaccination during pregnancy: “The vaccine is a lifesaving measure; it is useful for both mother and newborn, and thus it should be taken.”
None of the community respondents who received any vaccination during a previous pregnancy recalled any serious adverse events from their vaccination. Clinicians who reported they were vaccinating pregnant women against influenza similarly indicated no adverse events affecting the pregnant mother or their newborn. Remarks of a clinician from a middle-class site reflected the prevailing view, “I started and have been giving influenza vaccination for 6 months now. I haven’t seen a single problem from this vaccination, not after delivery or in the course of pregnancy, and not affecting newborns.”
Only one woman among community respondents questioned maternal vaccination in a manner indicating vaccination hesitancy. Others indicated acceptance of authoritative advice. Clinicians who were vaccinating against influenza also said that if they recommend it, most of their pregnant women patients will accept influenza vaccination. However, they also highlighted the importance of actively explaining their recommendation to pregnant women to ensure vaccination acceptance. A middle-class-site clinician explained, “I have to recommend and only 2 out of 10 will take it. But for the remaining 8, I must motivate them to take this. Eighty percent of them will accept and the remaining 20% would go for the second opinion.” Another clinician said, “If I recommend, then they will accept, but some extra counseling is needed. Because influenza is not a routine vaccination.”
Discussion
This study assessed the views of vaccine-providing clinicians and vaccine-receiving community stakeholders about AIV acceptance and demand in urban Pune, India. The findings from community interviews indicated that respondents lacked awareness of AIV even though they typically were aware of routine childhood and antenatal TT vaccinations. Community respondents expressed their confidence in vaccines in general and for antenatal vaccines during pregnancy. Women community respondents tended to regard the antenatal vaccines as primarily benefiting their newborns. Clinician interviews were notable for limited awareness of AIV policy recommendations and lack of a clear mandate to vaccinate at their private clinics. Study clinicians reported almost no indication of vaccination hesitancy among their pregnant patients for antenatal vaccinations. Some noted that the cost may be a barrier to influenza vaccination in private clinics, especially in slum-sites but also in some middle-class practice settings.
A clear finding from our community and clinician interviews that clinicians’ advice and recommendations are drivers for pregnant women’s acceptance of influenza vaccination is notably important. Although not presented in our results, a comparison of awareness of the previously pregnant (n = 28) and never pregnant (n = 20) women in our sample shows that a higher proportion of previously pregnant women were aware of childhood vaccines for measles (46% vs 15%), BCG (43% vs 30%), DPT (36% vs 10%), and influenza (18% vs 5%), which may be interpreted as an indication of the significance of clinical advice, either from their ANC or pediatric care of their children. There was, however, no such difference in awareness of polio (61% vs 65%), which has been much more widely publicized in the context of a massive national and global eradication program.
Regarding maternal influenza, both community and clinician respondents expressed their confidence in AIV. Clinicians indicated demand for it among their pregnant patients, and when aware of the vaccine, community respondents also expressed their desire for AIV. These findings show a clear need to better inform both clinicians and communities of the benefits and importance of AIV. Strategies for that are likely to be facilitated by referring to better-known TT vaccination policy and practices. Although cost is a factor, a policy to regularize, monitor, and promote AIV is supported by study findings indicating acceptance and demand responsive to advocacy and information.
This finding is consistent with other research, notably studies in Australia of vaccination facilitators and barriers during pregnancy.41,42 The important role of both clinicians’ and communities’ priorities demonstrated in our study and those of other studies of AIV also indicate the utility of a stakeholder framework acknowledging determinants of vaccination acceptance and demand beyond an earlier narrow focus on community hesitancy. The relative importance of community and clinician stakeholders may also vary across cultural settings and for other vaccines, such as the HPV vaccine. Setting-specific features of vaccination hesitancy have become more widely appreciated.43,44 We had anticipated that the community focus of previous Euro-American studies of vaccine hesitancy would be less of a deterrent to vaccination in India. That view is supported not only by our findings but also by a shifting emphasis in the field. A review of 185 articles extended the concept of vaccine hesitancy to healthcare providers, underlining the influence of their own vaccine confidence on their practice recommendations to their patients. It concluded that health care providers “remain the most trusted advisor[s] and influencer[s] of vaccination decisions”45
In the United States of America, analysis of vaccination monitoring data showed that provider recommendations increased coverage nearly six-fold during the 2010–2011 influenza season.46 A study in Korea indicated that providing professional information to obstetricians affected their priorities and prescribing practices and improved vaccination coverage for pregnant women.47 A South Asian study in Karachi, Pakistan, also emphasized the role of clinician authority and family advisors for AIV acceptance.48 Though women acknowledged the role of spouses and family elders in decision-making for accepting vaccines, male spouses emphasized their own role, as they did in our study, in which male spouses commonly said, “I decide for her.”
It was notable that in our Clin-1 baseline interviews, clinicians acknowledged influenza illness among pregnant women as a serious concern, but they indicated more ambivalence about that in their Clin-2 follow-up interviews. This result, which was contrary to the expected impact of presenting authoritative policy at the end of Clin-1 interview, may reflect a professional desirability bias, comparable with social desirability bias in community studies, which may have shaped responses of clinicians who had agreed to participate in a study of AIV. Over the course of regular interactions with the study team regularly monitoring their vaccination practices, they may perhaps have become more comfortable to speak more frankly about reservations in their Clin-2 responses.
Other factors have also been suggested in the literature to explain clinician vaccination practices. Failure to appreciate the seriousness of influenza illness has been shown to affect vaccine prescribing practices. A study in the United States of America emphasized the role of clinicians’ experience with serious sequelae from influenza-like illness, which was an important factor affecting vaccination of pregnant women in the 2009 pandemic.49 As noted, a clinician expressed doubts about the value of AIV because, as he said, “I haven’t observed influenza infection among people who were not vaccinated against influenza H1N1.” Clinical case-based experience and familiarity with relevant medical literature affect the priority of AIV, and our finding of clinicians’ reduced priority of serious influenza affecting pregnant women in our Clin-2 interviews shows that further attention to this issue is required.
Our study identified vaccine cost as a possible barrier, mainly in slum communities. Financial concerns limiting AIV have also been emphasized elsewhere, even in HICs, including the United States of America.50 A study in North India, however, like ours, emphasizes other factors with greater impact than financial concerns.18 Insights from our clinician interviews suggest that although cost is a relevant consideration, efforts to inform clinicians and regularize AIV policy are more critical for improving influenza vaccine coverage for pregnant women, consistent also with other research in the field.25,43,51 Common themes identified in both community and clinician interviews highlighted the fact that AIV was neither mandatory nor promoted as a regular feature of ANC in most clinics, and community information about AIV was lacking.
Although clinicians and community respondents acknowledged the safety and efficacy of AIV in our study, that is not the case everywhere. Although WHO recommends administering AIV at any stage of pregnancy, in practice there is widespread reluctance to vaccinate in the first trimester. This is not necessarily a serious issue, however, if it is routinely provided in a later ANC visit. But in some settings clinicians’ concerns about potential adverse events to the fetus have been deterrents, as shown from research in Georgia, in central Asia.52 Investigators in that study also highlighted the need for education and outreach to physicians.
Dissemination activities
The need for information and improved outreach to clinicians and community was notable over the course of our study. We had planned dissemination activities for the professional groups and communities participating in our study to provide such feedback, and the information needs identified in the study indicated how important that feedback might be. Consequently, we conducted two sets of dissemination activities, one for policymakers and clinical ANC providers, and another for interested community participants at the study sites. In these meetings, we presented and discussed the research experience, findings, and implications at a level appropriate for each group.
Three meetings were arranged in Pune in August 2016. Two of them were conducted in the middle-class and in the slum sites with a public invitation to community study participants. The third meeting was held for professionals – including clinicians, health policymakers from the state and municipal governments, and interested public health and academic researchers. A brochure for community residents and a policy brief for policymakers were prepared, distributed, and discussed at these events. During these dissemination meetings, we solicited feedback from the participants – clinicians in professional meetings and interested laypersons in the community meetings – and took note of their suggestions for future action to improve maternal influenza vaccination coverage. In the dissemination meetings with health professionals, which included policymakers and government health officials, we also emphasized the need for comparable research among government ANC providers, acknowledging distinctive issues confronting policy and practice in public and private sectors.
Limitations and strengths of the study
Although some of our findings may be applicable in comparable urban settings in India, we recognize that generalizability of our study findings is limited, and they are embedded in the cultural and the political, economic, and structural features of society and the health system.
Our exclusive focus on private clinicians addresses only part of the story, and an extension of this research to government healthcare settings is needed. Many pregnant women in slum settings rely on government health services for influenza vaccination and may even prefer them. The priority of AIV in government programs, however, varies widely across India, especially in Maharashtra, where it has spiked and sputtered in the aftermath of the 2009 H1N1 pandemic.53 Additional considerations of AIV in rural areas where access is more limited also apply, but research on the topic there requires prior consideration of access. We did not include policymakers, apart from their inclusion in dissemination activities. Their role in shaping the structure of health systems, funding, the priorities, and operations of programs, and enabling access to vaccines is of course critical, though beyond the scope of this study.
The use of a rapid ethnographic assessment methodology enabled the prompt collection of data on community views of vaccination, which were needed for briefing clinicians in their second scheduled interview. By including two interviews with clinicians in the study design, we were able to mitigate a social (or professional) desirability bias. After interacting with the research team over a period of months, some spoke more freely about their lower priority for problems of clinical influenza and AIV in the second interview, contrary to expectations that it would be higher. Our use of tablet interviewing devices and software for qualitative analysis enabled rapid processing to derive results that we could use in the Clin-2 interviews to inform clinicians about local community views on antenatal vaccines. The use of semi-structured interviews with open- and closed-ended questions enabled us to capture quantitative data enhanced by relevant narratives. The community sample was drawn from the clinicians’ practice communities, but excluding their patients to minimize the social desirability response bias. Purposive sampling of community respondents among three different pregnancy-status groups was intended to enhance diversity of relevant opinions.
To our knowledge, this study is the first to jointly assess community and clinicians’ views on acceptance and demand for antenatal seasonal influenza vaccination in an urban area of India. Although the generalizability of specific findings may be limited, they are relevant for consideration in other settings. Our stakeholder framework, which is extendable to policymakers and other relevant stakeholders, is applicable not only in other areas of India and LMICs but also in HICs. Attending to awareness, priorities, and practices provides a widely applicable lens through which to examine and interpret relevant views in urban middle-class and slum community settings.
Conclusion
This study clarified vaccination awareness, priorities, and practices among key stakeholders to guide efforts to improve AIV coverage. Stakeholder study groups included women of reproductive age and their spouses, and ANC providers in urban communities of Pune, India. Community residents expressed confidence in vaccines and trust in their clinicians. Clinicians’ advice and recommendations were found to be the major determinants of influenza vaccination of pregnant women. Our study findings suggest a comprehensive program strategy comprising the following elements: (i) a mandate for promoting, supporting, and monitoring AIV; (ii) professional workshops for healthcare providers and healthcare workers to provide information on AIV efficacy, safety and benefits, and policy recommendations; (iii) ethnographic assessment for community engagement to promote vaccination awareness through coordinated national and local activities (e.g. campaigns, publicity, and mass media messaging); (iv) better access for vaccination with subsidized vaccines at healthcare facilities; and (v) further efforts to enhance public and private health-sector collaboration for coordinated provision of AIV in urban and rural areas. Involvement of policymakers and further research are needed to implement this 5-point strategy for improved vaccination programs for AIV and other ANC vaccinations.
Acknowledgments
The authors would like to thank community residents and clinicians for participating in this study. We also thank our team of research assistants, namely, Ms. Jyoti Gaikwad, Ms. Mugdha Phutane, Ms. Arati Waghmare, Ms. Pranali Kulkarni, Ms. Seema Mohite, Mr. Abhijit Bhalerao, and Mr. Govind Bhosale at the Maharashtra Association of Anthropological Sciences for their assistance in collecting, transcribing, and translating the data.
Funding Statement
This study was funded by a grant from the World Health Organization’s Initiative for Vaccine Research. The authors would like to acknowledge the contributions of the Centers for Disease Control and Prevention (CDC), which provides financial support to the World Health Organization Initiative for Vaccine Research (U50CK000431). Joseph G. Giduthuri was the recipient of a Swiss Government Excellence Fellowship, granted by the State Secretariat for Education, Research and Innovation (ESKAS, grant no. 2016.0408). The funding agencies had no role in the study design, data collection, analysis, interpretation, and/or writing the manuscript.
List of abbreviations
- AIV
Antenatal influenza vaccination
- ANC
Antenatal care
- BCG
Bacillus Calmette–Guérin
- Clin-1
First clinician interaction
- Clin-2
Second clinician interaction
- DPT
Diphtheria, pertussis and tetanus
- FLI
Flu-like-illness
- FOGSI
Federation of Obstetric and Gynaecological Societies of India
- HICs
High-income countries
- LMICs
Low- and middle-income countries
- ODK
Open Data Kit
- PMC
Pune Municipal Corporation
- RAs
Research assistants
- SAGE
Strategic Advisory Group of Experts
- TT
Tetanus toxoid
- WHO
World Health Organization
Disclosure of potential conflicts of interest
The authors declare that they have no competing interests.
Authors’ contributions
Study concept and design: JGG, VP, AK, CS, and MGW; Acquisition of data: JGG and VP; Analysis and interpretation of data: JGG, CS, and MGW; Drafting of the manuscript: JGG, VP, CS, and MGW; Critical revision of the manuscript for important intellectual content: JGG, VP, AK, JU, CS, and MGW; All authors read and approved the final manuscript.
Ethics
The Institutional Ethics Committee of the Maharashtra Association of Anthropological Sciences, the Ethics Commission of Northwest and Central Switzerland (EKNZ) and the WHO Ethics Review Committee (WHO reference no. 2015/571608 2015/571608 0) independently approved this study. Written informed consent was obtained from all clinicians and community participants.
References
- 1.Neuzil KM, Reed GW, Mitchel EF, Simonsen L, Griffin MR.. Impact of influenza on acute cardiopulmonary hospitalizations in pregnant women. Am J Epidemiol. 1998;148:1094–102. doi: 10.1093/oxfordjournals.aje.a009587. [DOI] [PubMed] [Google Scholar]
- 2.Cox S, Posner SF, McPheeters M, Jamieson DJ, Kourtis AP, Meikle S. Hospitalizations with respiratory illness among pregnant women during influenza season. Obstet Gynecol. 2006;107:1315–22. doi: 10.1097/01.AOG.0000218702.92005.bb. [DOI] [PubMed] [Google Scholar]
- 3.Mak TK, Mangtani P, Leese J, Watson JM, Pfeifer D. Influenza vaccination in pregnancy: current evidence and selected national policies. Lancet Infect Dis. 2008;8:44–52. doi: 10.1016/S1473-3099(07)70311-0. [DOI] [PubMed] [Google Scholar]
- 4.Anzic Influenza Investigators . AMOSS. Critical illness due to 2009 A/H1N1 influenza in pregnant and postpartum women: population based cohort study. BMJ. 2010;340:c1279. doi: 10.1136/bmj.c1279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Mosby LG, Rasmussen SA, Jamieson DJ. 2009 pandemic influenza A (H1N1) in pregnancy: a systematic review of the literature. Am J Obstet Gynecol. 2011;205:10–18. doi: 10.1016/j.ajog.2010.12.033. [DOI] [PubMed] [Google Scholar]
- 6.Bhalerao-Gandhi A, Chhabra P, Arya S, Simmerman JM. Influenza and pregnancy: a review of the literature from India. Infect Dis Obstet Gynecol . 2015:867587. [accessed 2016 Apr 27]. http://www.ncbi.nlm.nih.gov/pmc/articles/PMC4355110/. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Rasmussen SA, Jamieson DJ, Uyeki TM. Effects of influenza on pregnant women and infants. Am J Obstet Gynecol. 2012;207:S3–8. doi: 10.1016/j.ajog.2012.06.068. [DOI] [PubMed] [Google Scholar]
- 8.Zaman K, Roy E, Arifeen SE, Rahman M, Raqib R, Wilson E, Omer SB, Shahid NS, Breiman RF, Steinhoff MC. Effectiveness of maternal influenza immunization in mothers and infants. N Engl J Med. 2008;359:1555–64. doi: 10.1056/NEJMoa0708630. [DOI] [PubMed] [Google Scholar]
- 9.Tamma PD, Ault KA, Del Rio C, Steinhoff MC, Halsey NA, Omer SB. Safety of influenza vaccination during pregnancy. Am J Obstet Gynecol. 2009;201:547–52. doi: 10.1016/j.ajog.2009.09.034. [DOI] [PubMed] [Google Scholar]
- 10.Moro PL, Broder K, Zheteyeva Y, Walton K, Rohan P, Sutherland A, Guh A, Haber P, Destefano F, Vellozzi C. Adverse events in pregnant women following administration of trivalent inactivated influenza vaccine and live attenuated influenza vaccine in the vaccine adverse event reporting system, 1990–2009. Am J Obstet Gynecol. 2011;204:146.e1–7. doi: 10.1016/j.ajog.2010.08.050. [DOI] [PubMed] [Google Scholar]
- 11.Omer SB, Goodman D, Steinhoff MC, Rochat R, Klugman KP, Stoll BJ, Ramakrishnan U. Maternal influenza immunization and reduced likelihood of prematurity and small for gestational age births: a retrospective cohort study. PLoS Med. 2011;8:e1000441. doi: 10.1371/journal.pmed.1000441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Håberg SE, Trogstad L, Gunnes N, Wilcox AJ, Gjessing HK, Samuelsen SO, Skrondal A, Cappelen I, Engeland A, Aavitsland P, et al. Risk of fetal death after pandemic influenza virus infection or vaccination. N Engl J Med. 2013;368:333–40. doi: 10.1056/NEJMoa1207210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.WHO. Vaccines against influenza WHO position paper – November 2012. Wkly Epidemiol Rec. 2012;87:461–76. [PubMed] [Google Scholar]
- 14.WHO . WHO recommends seasonal influenza vaccination to pregnant women as the highest priority [Internet]. WHO; [accessed 2018 January9]. http://www.who.int/immunization/newsroom/newsstory_seasonal_influenza_vaccination_pregnancy/en/
- 15.Ortiz JR, Perut M, Dumolard L, Wijesinghe PR, Jorgensen P, Ropero AM, Danovaro-Holliday MC, Heffelfinger JD, Tevi-Benissan C, Teleb NA, et al. A global review of national influenza immunization policies: analysis of the 2014 WHO/UNICEF joint reporting form on immunization. Vaccine. 2016;34:5400–05. doi: 10.1016/j.vaccine.2016.07.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.National Immunization Schedule (NIS) for infants, children and pregnant women. [Internet]. [accessed 2019 February1]. https://mohfw.gov.in/sites/default/files/245453521061489663873.pdf
- 17.Bhaskar E, Thobias S, Anthony S, Kumar V, Navaneethan. Vaccination rates for pandemic influenza among pregnant women: an early observation from Chennai, South India. Lung India. 2012;29:232–35. doi: 10.4103/0970-2113.99105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Koul PA, Bali NK, Ali S, Ahmad SJ, Bhat MA, Mir H, Akram S, Khan UH. Poor uptake of influenza vaccination in pregnancy in northern India. Int J Gynecol Obstet. 2014;127:234–37. doi: 10.1016/j.ijgo.2014.05.021. [DOI] [PubMed] [Google Scholar]
- 19.Hirve S, Lambach P, Paget J, Vandemaele K, Fitzner J, Zhang W. Seasonal influenza vaccine policy, use and effectiveness in the tropics and subtropics - a systematic literature review. Influenza Other Respir Viruses. 2016;10:254–67. doi: 10.1111/irv.12374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Yuen CYS, Tarrant M. Determinants of uptake of influenza vaccination among pregnant women – asystematic review. Vaccine. 2014;32:4602–13. doi: 10.1016/j.vaccine.2014.06.067. [DOI] [PubMed] [Google Scholar]
- 21.Wilson RJ, Paterson P, Jarrett C, Larson HJ. Understanding factors influencing vaccination acceptance during pregnancy globally: aliterature review. Vaccine. 2015;33:6420–29. doi: 10.1016/j.vaccine.2015.08.046. [DOI] [PubMed] [Google Scholar]
- 22.Schmid P, Rauber D, Betsch C, Lidolt G, Denker M-L. Barriers of influenza vaccination intention and behavior - a systematic review of influenza vaccine hesitancy, 2005–2016. PLoS One. 2017;12:e0170550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.WHO . Report of the SAGE working group on vaccine hesitancy [Internet]. [accessed 2019 February12]. https://www.who.int/immunization/sage/meetings/2014/october/1_Report_WORKING_GROUP_vaccine_hesitancy_final.pdf
- 24.Larson HJ, Jarrett C, Eckersberger E, Smith DMD, Paterson P. Understanding vaccine hesitancy around vaccines and vaccination from a global perspective: asystematic review of published literature, 2007–2012. Vaccine. 2014;32:2150–59. doi: 10.1016/j.vaccine.2014.01.081. [DOI] [PubMed] [Google Scholar]
- 25.Meharry PM, Cusson RM, Stiller R, Vázquez M. Maternal influenza vaccination: evaluation of a patient-centered pamphlet designed to increase uptake in pregnancy. Matern Child Health J. 2014;18:1205–14. doi: 10.1007/s10995-013-1352-4. [DOI] [PubMed] [Google Scholar]
- 26.Praphasiri P, Ditsungneon D, Greenbaum A, Dawood FS, Yoocharoen P, Stone DM, Olsen SJ, Lindblade KA, Muangchana C. Do Thai physicians recommend seasonal influenza vaccines to pregnant women? A cross-sectional survey of physicians’ perspectives and practices in Thailand. PLos One. 2017;12:e0169221. doi: 10.1371/journal.pone.0169221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Tong A, Biringer A, Ofner-Agostini M, Upshur R, McGeer A. A cross-sectional study of maternity care providers’ and women’s knowledge, attitudes, and behaviours towards influenza vaccination during pregnancy. J Obstet Gynaecol Can. 2008;30:404–10. doi: 10.1016/S1701-2163(16)32825-0. [DOI] [PubMed] [Google Scholar]
- 28.Blanchard-Rohner G, Meier S, Ryser J, Schaller D, Combescure C, Yudin MH, Burton-Jeangros C, de Tejada BM, Siegrist C-A. Acceptability of maternal immunization against influenza: the critical role of obstetricians. J Matern Fetal Neonatal Med. 2012;25:1800–09. [DOI] [PubMed] [Google Scholar]
- 29.Wiley KE, Massey PD, Cooper SC, Wood NJ, Ho J, Quinn HE, Leask J. Uptake of influenza vaccine by pregnant women: a cross-sectional survey. Med J Aust. 2013;198:373–75. doi: 10.5694/mja12.11849. [DOI] [PubMed] [Google Scholar]
- 30.Mak DB, Regan AK, Joyce S, Gibbs R, Effler PV. Antenatal care provider’s advice is the key determinant of influenza vaccination uptake in pregnant women. Aust N Z J Obstet Gynaecol. 2015;55:131–37. doi: 10.1111/ajo.12292. [DOI] [PubMed] [Google Scholar]
- 31.Bödeker B, Betsch C, Wichmann O. Skewed risk perceptions in pregnant women: the case of influenza vaccination. BMC Public Health. 2015; 16:1308 doi: 10.1186/s12889-015-2621-5. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC4785668/. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ding H, Black CL, Ball S, Donahue S, Fink RV, Williams WW, Kennedy ED, Bridges CB, Lu P-J, Kahn KE, et al. Influenza vaccination coverage among pregnant women–United States, 2014-15 influenza season. MMWR Morb Mortal Wkly Rep. 2015;64:1000–05. doi: 10.15585/mmwr.mm6436a2. [DOI] [PubMed] [Google Scholar]
- 33.Lotter K, Regan AK, Thomas T, Effler PV, Mak DB. Antenatal influenza and pertussis vaccine uptake among aboriginal mothers in Western Australia. Aust N Z J Obstet Gynaecol. 2018;58:417–24. doi: 10.1111/ajo.12739. [DOI] [PubMed] [Google Scholar]
- 34.Sundaram N, Purohit V, Schaetti C, Kudale A, Joseph S, Weiss MG. Community awareness, use and preference for pandemic influenza vaccines in Pune, India. Hum Vaccines Immunother. 2015;11:2376–88. doi: 10.1080/21645515.2015.1062956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Masserey Spicher V, Weiss MG. Policy and socio-cultural differences between cantons in Switzerland with high and low adolescent vaccination coverage for hepatitis B and HPV. Vaccine. 2019;37:7539–46. doi: 10.1016/j.vaccine.2019.09.085. [DOI] [PubMed] [Google Scholar]
- 36.Project protocol to assess awareness and acceptance of maternal influenza vaccination in low-resource settings [Internet]. [accessed 2017 June19]. http://www.who.int/immunization/research/development/Project_Protocol_Annexures.pdf?ua=1
- 37.Coreil J, Augustin A, Holt E, Halsey NA. Use of ethnographic research for instrument development in a case-control study of immunization use in Haiti. Int J Epidemiol. 1989;18:S33–37. doi: 10.1093/ije/18.Supplement_2.S33. [DOI] [PubMed] [Google Scholar]
- 38.Giduthuri JG, Purohit V, Maire N, Kudale A, Utzinger J, Schindler C, Weiss MG. Influenza vaccination of pregnant women: engaging clinicians to reduce missed opportunities for vaccination. Vaccine. 2019;37:1910–17. doi: 10.1016/j.vaccine.2019.02.035. [DOI] [PubMed] [Google Scholar]
- 39.Morse JM. Designing funded qualitative research. In: Denzin NK, Lincoln YS, editors. Handbook of qualitative research. Thousand Oaks (CA): Sage Publications, Inc; 1994. p. 220–35. [Google Scholar]
- 40.Your home for health | Practo [Internet]. [accessed 2018 September12]. https://www.practo.com/
- 41.Krishnaswamy S, Cheng AC, Wallace EM, Buttery J, Giles ML. Understanding the barriers to uptake of antenatal vaccination by women from culturally and linguistically diverse backgrounds: a cross-sectional study. Hum Vaccines Immunother. 2018;14:1591–98. doi: 10.1080/21645515.2018.1445455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Collins J, Alona I, Tooher R, Marshall H. Increased awareness and health care provider endorsement is required to encourage pregnant women to be vaccinated. Hum Vaccines Immunother. 2014;10:2922–29. doi: 10.4161/21645515.2014.971606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Ogburn T, Espey EL, Contreras V, Arroyo P. Impact of clinic interventions on the rate of influenza vaccination in pregnant women. J Reprod Med Obstet Gynecol. 2007;52:753–56. [PubMed] [Google Scholar]
- 44.Wong VWY, Lok KYW, Tarrant M. Interventions to increase the uptake of seasonal influenza vaccination among pregnant women: asystematic review. Vaccine. 2016;34:20–32. doi: 10.1016/j.vaccine.2015.11.020. [DOI] [PubMed] [Google Scholar]
- 45.Paterson P, Meurice F, Stanberry LR, Glismann S, Rosenthal SL, Larson HJ. Vaccine hesitancy and healthcare providers. Vaccine. 2016;34:6700–06. doi: 10.1016/j.vaccine.2016.10.042. [DOI] [PubMed] [Google Scholar]
- 46.Kennedy ED, Ahluwalia IB, Ding H, Lu P-J, Singleton JA, Bridges CB. Monitoring seasonal influenza vaccination coverage among pregnant women in the United States. Am J Obstet Gynecol. 2012;207:S9–16. doi: 10.1016/j.ajog.2012.06.069. [DOI] [PubMed] [Google Scholar]
- 47.Noh JY, Seo YB, Song JY, Choi WS, Lee J, Jung E, Kang S, Choi MJ, Jun J, Yoon JG, et al. Perception and attitudes of Korean obstetricians about maternal influenza vaccination. J Korean Med Sci. 2016;31:1063–68. doi: 10.3346/jkms.2016.31.7.1063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Khan AA, Varan AK, Esteves-Jaramillo A, Siddiqui M, Sultana S, Ali AS, Zaidi AKM, Omer SB. Influenza vaccine acceptance among pregnant women in urban slum areas, Karachi, Pakistan. Vaccine. 2015;33:5103–09. doi: 10.1016/j.vaccine.2015.08.014. [DOI] [PubMed] [Google Scholar]
- 49.Kissin DM, Power ML, Kahn EB, Williams JL, Jamieson DJ, MacFarlane K, Schulkin J, Zhang Y, Callaghan WM. Attitudes and practices of obstetrician–gynecologists regarding influenza vaccination in pregnancy. Obstet Gynecol. 2011;118:1074–80. doi: 10.1097/AOG.0b013e3182329681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Power ML, Leddy MA, Anderson BL, Gall SA, Gonik B, Schulkin J. Obstetrician–gynecologists’ practices and perceived knowledge regarding immunization. Am J Prev Med. 2009;37:231–34. doi: 10.1016/j.amepre.2009.05.019. [DOI] [PubMed] [Google Scholar]
- 51.Li R, Xie R, Yang C, Rainey J, Song Y, Greene C. Identifying ways to increase seasonal influenza vaccine uptake among pregnant women in China: aqualitative investigation of pregnant women and their obstetricians. Vaccine. 2018;36:3315–22. doi: 10.1016/j.vaccine.2018.04.060. [DOI] [PubMed] [Google Scholar]
- 52.Dvalishvili M, Mesxishvili D, Butsashvili M, Kamkamidze G, McFarland D, Bednarczyk RA. Knowledge, attitudes, and practices of healthcare providers in the country of Georgia regarding influenza vaccinations for pregnant women. Vaccine. 2016;34:5907–11. doi: 10.1016/j.vaccine.2016.10.033. [DOI] [PubMed] [Google Scholar]
- 53.Purohit V, Kudale A, Sundaram N, Joseph S, Schaetti C, Weiss MG. Public health policy and experience of the 2009 H1N1 influenza pandemic in Pune, India. Int J Health Policy Manag. 2017;7:154–66. doi: 10.15171/ijhpm.2017.54. [DOI] [PMC free article] [PubMed] [Google Scholar]


