ABSTRACT
This study aimed to assess the global mapping risk of human islet isolation, using a failure mode and effect analysis (FMEA), and highlight the impact of quality assurance procedures on the risk level of criticality. Risks were scored using the risk priority number (RPN) scoring method. The risk level of criticality was made based on RPN and led to risk classification (low to critical). A raw risk analysis and a risk control analysis (with control means and quality assurance performance) were undertaken. The process of human islet isolation was divided into 11 steps, and 230 risks were identified. Analysis of the highest RPN of each of the 11 steps showed that the 4 highest risks were related to the pancreas digestion and islet purification stages. After implementation of reduction measures and controls, critical and severe risks were reduced by 3-fold and by 2-fold, respectively, so that 90% of risks could be considered as low to moderate. FMEA has proven to be a powerful approach for the identification of weaknesses in the islet isolation processes. The results demonstrated the importance of staff qualification and continuous training and supported the contribution of the quality assurance system to risk reduction.
KEYWORDS: Pancreatic islet isolation, risk mapping, manufacturing procedure, risk criticality, human islet, quality subject
Introduction
Islet transplantation is a recognized treatment of type 1 diabetes.1–3 The technique for human pancreatic islet isolation developed more than 30 years ago, consists of different crucial steps including pancreas digestion and islet purification.4 Despite improvements of the isolation procedure, its outcome, including yield, purity, function, and safety of isolated islets, is still uncertain and widely variable. Numerous parameters influence successful isolation outcome, including donor characteristics,5–9 organ procurement,10 ischemia time, preservation solution,10,11 digestion enzymes,12–14 purification techniques15,16 and methods of islet maintenance before transplantation.17–19 Furthermore, human islet isolation is a long, complex and expensive process that requires a cleanroom facility, specific-maintained and qualified materials, but especially trained and qualified personnel.20,21 The personnel, working in a sterile environment monitored for particle and microbiological levels, must be able to work several hours in special gowning conditions and perform many various-specialized tasks, sometimes simultaneously, in a sterile manner. Therefore, in every step of human islet isolation there are potential risks of technical and human errors that can sometimes lead to the isolation failure or affect patient safety. It is therefore of great importance to establish standardized procedures for the different stages of the process so that the result of the isolation becomes independent of the isolation team. In addition, in order to meet regulatory requirements, a quality assurance policy must be enforced, including a risk management system.
There are various tools available for risk analysis and management to enhance the result of a process and patient safety, such as failure mode and effect analysis (FMEA). FMEA is a methodology designed to identify, analyze, and quantify risk on a process or a product.22 FMEA was first applied in high-risk systems or industries such as transportation, nuclear power, the military and the aerospace industries but, in the 1990s, the pharmaceutical industry began using this method as well as public health care systems to prevent medication errors. As a part of management review, FMEA although being a preventive method, could be implemented at any time during the process.
To our knowledge there are no published studies available on the application of FMEA on human islet isolation, whether from the patient safety or the process perspective. This study aimed to assess the global mapping risk of human islet isolation and transplantation processes, and to highlight the impact of the quality assurance procedure and control measures on risk criticality.
Results
The FMEA was performed over a 6-month period between January and June 2019. The process of human islet isolation was divided into 10 major steps from raw materials to islet transportation, plus a more general step to include all risks that could arise throughout the process (Figure 1). The investigative team identified a total of 230 risks, distributed between the different steps, with a mean of approximately 20 risks identified per step (Table 1). The risk scoring matrix (Table 2) and the risk level of criticality (Table 3) were defined by the same team (see methods for more details). Regarding severity of risks, 9.1% were about patient safety and others were about isolation process. Some risks identified with a maximum severity of 10 regard errors in donor selection such as the acceptance of an organ with out-of-specification criteria for donor serology, glycated hemoglobin or ABO group compatibility between the donor and recipient.
Figure 1.
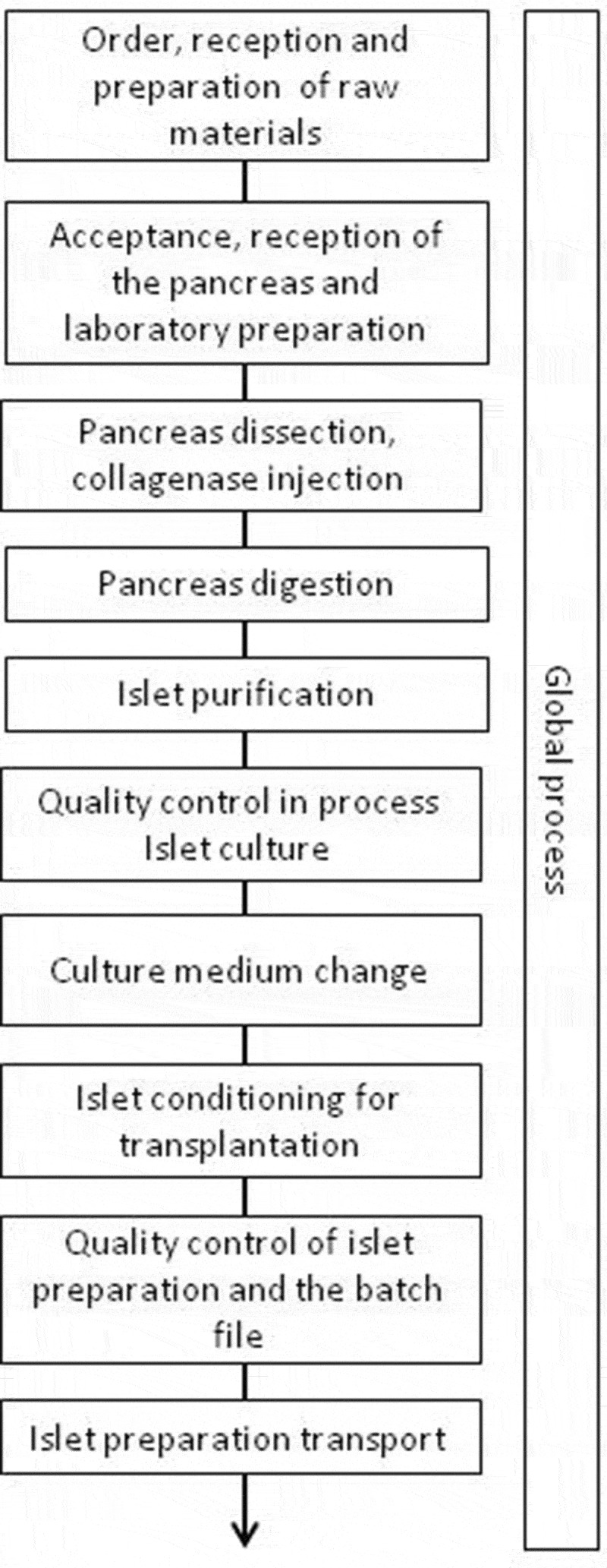
Process mapping of human islet isolation and conditioning
Table 1.
Repartition of risks according to the process of isolation and conditioning
| Major steps in the process | Number of risks |
|---|---|
| Order, reception and preparation of raw materials | 32 |
| Acceptance, reception of the pancreas and laboratory preparation |
26 |
| Pancreas dissection, collagenase injection | 23 |
| Pancreas digestion | 19 |
| Islet purification | 10 |
| Quality control in process Islet culture |
23 |
| Culture medium change | 14 |
| Islet conditioning for transplantation | 18 |
| Quality control of islet preparation batch record | 12 |
| Islet preparation transport | 16 |
| Global process | 36 |
| Total | 230 |
Table 2.
Rating scale of occurrence, severity and detectability
| Score | Occurrence | Severity |
Detectability | |
|---|---|---|---|---|
| Impact on patient safety | Impact on process | |||
| 1 | Non-existent | No impairment, would not be noticeable by the patient, would not affect the provision of care | No/little impact on the isolation procedure | Very easy to detect Quality assurance in place |
| 2 | Occurred 1 time | |||
| 3 | Occurred several times in the past 5 years | Mild impairment, increase in the level of required care the event may be noticeable by the patient | Light boredom The process is lengthened without causing additional delay in patient care Loss of islets (100–9000 IEQ) |
Easily detectable May not be detected in the absence of verification |
| 4 | 1 time per year | |||
| 5 | Several times per year | Moderate involvement, may cause injury or increase the level of required care; clearly visible by the patient | Disorganized process Significant loss of islets (10.000–100.000 IEQ) |
Detectable with verification |
| 6 | 20% of isolation | Moderate difficulty in detecting | ||
| 7 | 40% of isolation | |||
| 8 | 60% of isolation | Serious injury | Very disorganized process Very significant loss of islets (> 50%) |
Significant difficulty in detecting |
| 9 | 80% of isolation | |||
| 10 | 100% of isolation | Death | Destruction of islets Isolation failure |
Impossible to detect No quality assurance in place |
Table 3.
Matrix of level of criticality according to the risk mapping
| Severity |
|||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 | ||
| Occurence | 1 | 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 |
| 2 | 2 | 4 | 6 | 8 | 10 | 12 | 14 | 16 | 18 | 20 | |
| 3 | 3 | 6 | 9 | 12 | 15 | 18 | 21 | 24 | 27 | 30 | |
| 4 | 4 | 8 | 12 | 16 | 20 | 24 | 28 | 32 | 36 | 40 | |
| 5 | 5 | 10 | 15 | 20 | 25 | 30 | 35 | 40 | 45 | 50 | |
| 6 | 6 | 12 | 18 | 24 | 30 | 36 | 42 | 48 | 54 | 60 | |
| 7 | 7 | 14 | 21 | 28 | 35 | 42 | 49 | 56 | 63 | 70 | |
| 8 | 8 | 16 | 24 | 32 | 40 | 48 | 56 | 64 | 72 | 80 | |
| 9 | 9 | 18 | 27 | 36 | 45 | 54 | 63 | 72 | 81 | 90 | |
| 10 | 10 | 20 | 30 | 40 | 50 | 60 | 70 | 80 | 90 | 100 | |
The RPN is the product of severity, occurrence, and detectability scoring of the risk.
In this table where only 2 attributes appear, the level of criticality is presented as follows:
Low risk: Moderate risk: Severe risk: Critical risk.
The score assessed for detectability does not change the risk classification.
In this study, we detailed the risks with the highest RPN for each of the 11 steps of the islet isolation process (Table 4), with raw RPN scores ranging from 50 to 392. Among the 11 main highlighted risks, 3 are related to the digestion stage with the preparation of a wrong concentration of digestion enzymes, the poor perfusion of the pancreas with the enzyme solution, and under-digestion of the pancreas. However, over digestion of the pancreas also represents a high risk with a RPN of 245 (risk severity of 5); over digestion of islets was not good either, especially with regard to their function. The risk with the highest RPN regards the purification step, with a wrong preparation of the gradient of density.
Table 4.
Highest scoring failure mode of different steps in islet isolation process
| Raw evaluation |
Post FMEA Evaluation |
||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Isolation Steps | Risk description | Risk consequences | O | D | S | RPN | Risk reduction measure | O | D | S | RPN |
| Preparation of raw materials | Reversal of 2 products during the preparation of aliquot | Adding an unwanted product in a medium | 4 | 5 | 9 | 180 | • Operator training • Aliquots of similar products or with similar packaging are not made at the same time • Control of aliquot manufacturing forms |
2 | 5 | 9 | 90 |
| Laboratory preparation | During the preparation of solution, wrong concentration of digestion enzymes | Poor digestion of the pancreas | 4 | 7 | 8 | 224 | • Operator training • Each batch of collagenase is tested before use • Use of the entire bottle of collagenase or neutral protease • Number of vials used to be manually noted on the batch file |
2 | 5 | 8 | 80 |
| Collagenase injection | Poor perfusion of the pancreas with digestion enzymes | Poor digestion Impact on the success of isolation |
5 | 2 | 6 | 60 | • Perfusion by double cannulation by peristaltic pump • Controlled and constant infusion pressure • Manual syringe infusion as a last resort |
3 | 1 | 6 | 18 |
| Pancreas digestion | Under digestion of the pancreas | Poor digestion Inadequate number of isolated islets and/or embedded islets |
7 | 7 | 7 | 343 | • Control of the digestion by regular sampling in the digestion chamber • Operator training |
3 | 3 | 7 | 63 |
| Islet purification | Poor mixing of the vials to make the concentration gradient | Ineffective purification Redo the step Time waste |
7 | 8 | 7 | 392 | • Both bottles to be mixed (Biocoll and Belzer) are labeled with the same wording • All the vials necessary for a purification are gathered in a the same box (purification kit) |
2 | 5 | 7 | 70 |
| Quality control in process | Insufficient number of IEQs for transplantation | Isolation without transplant Destruction of the preparation |
9 | 1 | 10 | 90 | • Change in donor selection parameters • Improvement of pancreas digestion • Improvement of purification technique • External factors |
7 | 1 | 10 | 70 |
Based on these findings, we determined the effect of implementing a quality assurance policy on these same risks. The quality assurance policy began in 2001 and the implementation has been continuously since this date. The same team established a new score by including in the rating 1) the establishment of standard operating procedures (SOPs), 2) the initial and continuous training of personnel and 3) the risk management resources implemented. Table 4 lists the recommended risk reduction measures and post-FMEA RPNs for the 11 risks previously identified. Overall, the RPN scores were reduced by about 4 after FMEA, with post-FMEA RPN scores ranging from 18 to 250. The largest reduction in RNP was observed for risks attributed to human mistakes, such as forgetting to add heparin or mixing 2 preparations, with a risk reduction by 10.7- and 8-fold respectively. On the contrary, the smallest decrease in RPN was observed for risks that were specific to the intrinsic quality of the organ, i.e. quality control in process and final quality control steps with a risk reduction of 1.3- and 1.7-fold, respectively. Reduction of these 2 risks was only due occurrence decreased. The initial rate of successful isolation was 24% in 1999 (8 successes and 25 failures) vs 61% in 2019 (28 successes and 18 failures).
Regarding the overall RPN scores for the 230 risks, raw RPN ranged from a minimum of 2 to a maximum of 450 vs 1 to 250 for post-FMEA RPN. This analysis revealed that 72% of identified risks were related to human mistakes. Fifteen risks had a severity of 10 indicating that the consequences of these hazards may be failure of isolation or patient death. Before implementing the quality assurance policy (in 1999), 13 risks occurred in at least 60% of isolation procedures (score ≥ 8) vs 5% after implementation (in 2019), and 22 risks could not be detected (score ≥ 8) vs only 3 after quality assurance policy implementation. With the raw scoring and from the level of criticality, 52, 26, 16, and 6% of the risks were graded as low, moderate, severe, and critical, respectively (Figure 2). After the implementation of reduction measures and controls, critical risks decreased by 3-fold (2%) severe risks 2-fold (8%). The percentage of low risk increased from 52% to 70%. Consequently, 90% of identified risks in the process of human pancreatic islet isolation became considered as low to moderate (vs 78%) (Figure 2).
Figure 2.
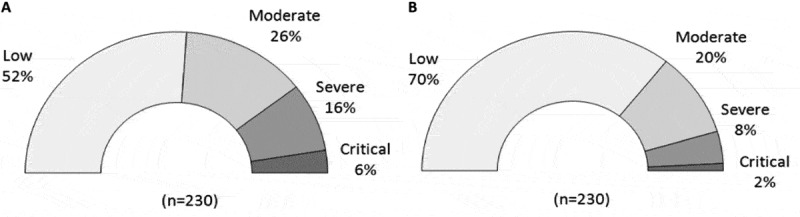
Distribution of risk level of criticality. Distribution of risk level for the 230 identified risks during the raw evaluation analysis (a) and after new evaluation post FMEA (b) during which the means of control and quality assurance procedures implemented were taken into account
Discussion
The islet isolation and transplantation center of the University of Geneva Hospitals participates to one of the most active clinical islet transplantation programs worldwide. Our center serves as the centralized islet production facility for the GRAGIL network, a Swiss-French multicenter consortium.23
FMEA is a risk management tool that can proactively identify and assess the causes and consequences of potential failures with the purpose to avoid such occurrences.22 It was important to differentiate the quantity of risks from their severity, which is why FMEA is a substantial tool.
Although the FMEA method is meant to be a prospective approach, we used it to assess the overall risk analysis of human islet isolation process. We focused on all factors that may cause errors in the islet transplantation procedure, including islet isolation technology, quality control, shipment of islets and logistics of this activity, such as ordering of raw materials, aliquot, and equipment preparation.
Two types of expression of the risk were used, the RPN and the level of criticality (low to critical). With RPN, the entire risk treatment method takes into account all criteria of the risk: occurrence, severity, and detectability. The RPN of the risk can be decreased by acting on risk detection or by reducing its occurrence. This approach allowed the identification of 230 risks, of which 72% were attributable to human errors such as using wrong products, volumes, or concentrations or mishandling of specialized equipment. These issues can be avoided if the personnel is initially well trained, qualified through practical tests and regularly retrained. The qualification of personnel should also be implemented by other risk reduction measures such as acquisition of bar code technology for stock management, labeling and automated reading of all products used with an alert system when an out-of-date or incorrect products are used.24
Of the 11 main risks highlighted in this study, 4 are related to the digestion and the purification steps. This result is not surprising given that these two essential steps in the success of islet isolation require the preparation of critical solutions and a specific expertise.25,26 The reduction of certain risks can be obtained through the implementation of a solid quality assurance policy that works in the drafting of SOPs, on which staff can rely, and in the introduction of additional controls such as double-checking when the error can have serious consequences. A check of the aliquot preparation forms is carried out to verify the validity and adequacy of the prepared products. Before batch release, the same type of check is carried out on the batch record by a third party who also checks compliance with the specifications. A high-risk situation is encountered when two islet isolation procedures are carried out successively and the resulting islets destined to different recipients have to be stored in the same incubator. A cross-contamination can occur during culture medium changes or the harvesting of islets before transplantation. Transplantation of an ABO-incompatible preparation could have serious consequences for the patient such as acute hemolysis and immune rejection.27,28 The measures introduced to avoid this risk have resulted in a reduction by 8 of the RPN.
Risk criticality analysis provides a global view of the distribution of risks and facilitates the comparison of this distribution before (raw evaluation) and after the implementation of quality assurance (post-FMEA). As a result, we also demonstrated that quality assurance system, including SOPs, regular training, and monitoring, which sometimes seem restrictive and burdensome, have a real positive effect on the safety of the product. Indeed, when all controls are included, the critical risk (near-destruction of the islet preparation) was divided by 3.
On the other hand, the risks related to quality controls are difficult to reduce. Islet count (IEQ), purity, tissue volume, viability, and sterility are the quality controls performed on the islet preparation. To avoid errors in quality controls, the different techniques used were validated and the personnel trained and qualified in all these techniques. The validation of control quality techniques is a prerogative of the production manager to prevent false positive or negative results, particularly in the evaluation of the sterility of islet preparations. Indeed, sterility of the islet preparation impacts on patient health, unlike other quality controls. As a result, we conducted checkpoints from organ procurement, isolation to islet infusion to ensure the clinical safety of islet transplantation. The search for bacterial or fungal contamination was determined by the BACT/ALERT® system,29 Gram staining30 and endotoxin assay.31 The last 2 methods provide an immediate result (within 30 min) unlike the BACT/ALERT® system which requires several days of incubation. The organ preservation medium is often contaminated, but nonetheless pancreas washing with decontamination solutions before pancreas digestion and the further washing steps of digested tissue and islets reduce the risk of microbial contamination of the final islet preparation.32 The decontamination protocol we used allowed decreasing contamination rates from 64.4% (contaminated preservation liquid or medium) to 6.0% after islet isolation and culture.33 The principal cause of islet preparation contamination was the persistence of germs present in the pancreas preservation medium. The only way to reduce the risk of contamination of islet preparations is to reduce occurrence of this risk. However, the removal of the pancreas is an independent step in our process and is performed by surgeons in procurement hospitals.
The other major risk related to isolation failure was an insufficient number of islets to comply with the release criteria. In our center, this accounts for about 40% of all islet isolations; in other centers, this value may be higher or lower.34,35 In addition to staff training improved success rate within 20 years could be due to several factors including improvement in organ preservation fluid and harvesting, improvement in perfusion of the pancreas with enzyme by a control of the pressure using peristaltic pump, improvement in enzyme quality used in digestion of the pancreas and improvement in a well-defined gradient used in the purification step.
However, it is difficult to reduce more this failure rate because it is not fully dependent on the process. Indeed, isolated islet yields remain unpredictable and vary considerably according to a multiplicity of parameters, including donor and pancreas characteristics. Despite strict donor selection criteria, attempts to reduce ischemia time and the respect of all steps of the isolation procedure, the rate of failure is still quite high.
Using the FMEA tool, we have successfully assessed a global risk analysis of human islet isolation and conditioning processes. We also developed strategies to prevent or decrease the occurrence of some failures. This method could be easily spread to other centers that can use the same scale of occurrence, severity, and detectability. The risks described here and the preventive measures presented to reduce the risk can be easily used and implemented by those who want to open a new islet isolation center. The results of this quality project demonstrate the importance of staff qualification and continuous training and support the impact of the quality assurance system on risk reduction. It should be considered that the main task of risk management, related to the success and quality of the islet preparation is the protection of the patients. Following the ancient saying “better safe than sorry,” assessment of the main risks allows for preventive, rather than reactive action.
Methods
We performed a FMEA in three steps comprising 1) mapping of the process and risk identification, 2) raw risk analysis and evaluation without control means and 3) risk control analysis with all means of verification and quality assurance procedure. Participants included laboratory director, production manager, quality assurance manager, and involved operators performing islet isolation and conditioning (i.e., MDs, PhDs, and lab technicians). According to a consensus achieved during a round table discussion, a detailed process map was created from raw material order to human islet transportation (Figure 1). Using discussion, brainstorming, analysis of deviations/events already recorded, direct observation, and opinion experience of each staff involved in islet isolation, the team identified potential risks of failure or hazards for every step of the process. Then, they defined the risk scoring matrix composed of 3 criteria: occurrence, severity, and difficulty of detection (Table 2). This initial scoring was obtained during a voting session of each participant. Severity evaluation was performed regarding patient safety or the process perspective. Each of these 3 criteria was evaluated on a scale from 1 to 10. The risk scoring matrix from Williams et al36 was adapted for the human islet isolation process regarding occurrence. About 40 to 60 isolations per year were performed at our islet isolation facility. Quantification of islet loss level was used to edit the severity scale concerning success of process. For isolation failure or destruction of islet preparation, severity level was considered to be at 10 (Table 2). In our center, successful isolation is defined by obtaining transplantable islet preparations that meet release criteria, including an IEQ number > 4000 IEQ/kg recipient weight, a purity >30% and a sterile preparation.
Once the risks were evaluated as rating scores for each criterion, quantification of the risk was defined by its risk priority number (RPN). RPN was estimated by multiplying severity, occurrence and detection ratings. Thereafter, the lowest and highest RPNs range from 1 (1 × 1 × 1) for the best score to 1ʹ000 (10 × 10 × 10) for the worst.
The risk level of criticality was assessed based on calculated RPNs and led to risk classification in 4 categories: low, moderate, severe, and critical (Table 3). The risk level of criticality takes into a count only the occurrence and the severity of the risk.
Using the same risk rating matrix, current RPNs and levels of criticality were assessed in a new evaluation post-FMEA (based on 2019 activity), during which the means of control and quality assurance procedures implemented for about 20 years were taken into account. For each mean of control a consensus about implementation of risk reduction was obtained during the same round table 5 discussion described above. Risks with high current RPNs were used for developing new control strategies.
Table 5.
Highest scoring failure mode of different steps in islet isolation process
| Raw evaluation |
Post FMEA Evaluation |
||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Isolation Steps | Risk description | Risk consequences | O | D | S | RPN | Risk reduction measure | O | D | S | RPN |
| Culture medium change | Cross-contamination in case of simultaneous storage of 2 different islet preparations | Mixture of 2 preparations for 2 different patients | 4 | 8 | 8 | 256 | • The 2 preparations are identified with different colors and a different pancreas number • Placed on different shelves in incubators • The number of culture flasks used is recounted • Two isolation are never done simultaneously, but successively |
1 | 4 | 8 | 32 |
| Conditioning of islets for transplantation | Forgetting to add heparin to the tube containing the islet preparation before bagging | Possible Thrombosis Risk in the Patient Recipient | 4 | 8 | 8 | 256 | • Double-checking at the time of heparin addition | 1 | 3 | 8 | 24 |
| Quality control of islet preparation | Positive microbiological control by gram staining of the islet preparation | No transplantation of islet Destruction of the preparation |
5 | 1 | 10 | 50 | • Training of operators at work in sterile condition • Antibiotics present in all culture media before transplantation |
3 | 1 | 10 | 30 |
| Islet preparation transport | Missing infusion lines without filter or sending a line with filter | No transplantation | 4 | 5 | 7 | 140 | • Check list of the composition of the transport container • Double the infusion line in the transport container |
1 | 3 | 7 | 21 |
| Global process | Wrong pressure differential (<10 Pa) in the production room | Risk of contamination of the working environment and of islet preparation | 10 | 5 | 5 | 250 | • Check pressure differential before to start isolation • Adding of environmental controls during the process in case of bad pressure differential • Work under laminar flow hood/ceiling or in close system during isolation |
10 | 5 | 5 | 250 |
O: Occurrence, D: non detectability, S: severity, RPN: Risk priority numb.
Acknowledgments
The author thanks Joana Cruz, David Matthey-Doret, Lisa Perez, Caroline Rouget, and Corinne Sinigaglia for their excellent technical work (Geneva University Hospitals and Department of Surgery, University of Geneva, Geneva, Switzerland).
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
Abbreviations
- FMEA Failure mode and effect analysis
- RPN Risk priority number
- SOPs Standard operating procedures
Authorship
DB, TB, QP, GP, VL: participated in research design and in the writing of the paper.
QP, GP, VL, NP: participated in data analysis.
All authors participated in the performance of the research and approved the final paper.
Ethical approval
Ethical Approval is not applicable for this article.
Statement of human and animal rights
This article does not contain any studies with human or animal subjects.
Statement of informed consent
There are no human subjects in this article and informed consent is not applicable.
References
- 1.Lablanche S, Borot S, Wojtusciszyn A, Bayle F, Tétaz R, Badet L, Thivolet C, Morelon E, Frimat L, Penfornis A, et al. Five-year metabolic, functional, and safety results of patients with Type 1 diabetes transplanted with allogenic islets within the Swiss-French GRAGIL network. Diabetes Care. 2015;38(9):1714–1722. doi: 10.2337/dc15-0094. [DOI] [PubMed] [Google Scholar]
- 2.Bartlett ST, Markmann JF, Johnson P, Korsgren O, Hering BJ, Scharp D, Kay TWH, Bromberg J, Odorico JS, Weir GC, et al. Report from IPITA-TTS opinion leaders meeting on the future of β-cell replacement. Transplantation. 2016;100(Suppl 2):S1–44. doi: 10.1097/TP.0000000000001055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Shapiro AMJ, Pokrywczynska M, Ricordi C.. Clinical pancreatic islet transplantation. Nat Rev Endocrinol. 2017;13(5):268–277. doi: 10.1038/nrendo.2016.178. [DOI] [PubMed] [Google Scholar]
- 4.Ricordi C, Lacy PE, Scharp DW.. Automated islet isolation from human pancreas. Diabetes. 1989;38(Suppl 1):140–142. doi: 10.2337/diab.38.1.s140. [DOI] [PubMed] [Google Scholar]
- 5.Yeh -C-C, Wang L-J, Mcgarrigle JJ, Wang Y, Liao CC, Omami M, Khan A, Nourmohammadzadeh M, Mendoza-Elias J, McCracken B, et al. Effect of manufacturing procedures on human islet isolation from donor pancreata standardized by the North American Islet donor score. Cell Transplant. 2017;26(1):33–44. doi: 10.3727/096368916X692834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Matsumoto S, Zhang G, Qualley S, Clever J, Tombrello Y, Strong DM, Reems JA.. Analysis of donor factors affecting human islet isolation with current isolation protocol. Transplant Proc. 2004;36(4):1034–1036. doi: 10.1016/j.transproceed.2004.04.013. [DOI] [PubMed] [Google Scholar]
- 7.Gołębiewska JE, Bachul PJ, Wang L, Matosz S, Basto L, Kijek MR, Fillman N, Gołąb K, Tibudan M, Dębska-Ślizień A, et al. Validation of a new North American Islet donor score for donor pancreas selection and successful islet isolation in a medium-volume islet transplant center. Cell Transplant. 2019;28(2):185–194. doi: 10.1177/0963689718816989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Nano R, Clissi B, Melzi R, Calori G, Maffi P, Antonioli B, Marzorati S, Aldrighetti L, Freschi M, Grochowiecki T, et al. Islet isolation for allotransplantation: variables associated with successful islet yield and graft function. Diabetologia. 2005;48(5):906–912. doi: 10.1007/s00125-005-1725-3. [DOI] [PubMed] [Google Scholar]
- 9.Ponte GM, Pileggi A, Messinger S, Alejandro A, Ichii H, Baidal DA, Khan A, Ricordi C, Goss JA, Alejandro R. Toward maximizing the success rates of human islet isolation: influence of donor and isolation factors. Cell Transplant. 2007;16(6):595–607. doi: 10.3727/000000007783465082. [DOI] [PubMed] [Google Scholar]
- 10.Andres A, Kin T, O’Gorman D, Bigam D, Kneteman N, Senior P, Shapiro AJ. Impact of adverse pancreatic injury at surgical procurement upon islet isolation outcome. Transpl Int Off J Eur Soc Organ Transplant. 2014;27(11):1135–1142. doi: 10.1111/tri.12392. [DOI] [PubMed] [Google Scholar]
- 11.Niclauss N, Wojtusciszyn A, Morel P, Demuylder-Mischler S, Brault C, Parnaud G, Ris F, Bosco D, Badet L, Benhamou P-Y, et al. Comparative impact on islet isolation and transplant outcome of the preservation solutions Institut Georges Lopez-1, University of Wisconsin, and Celsior. Transplantation. 2012;93(7):703–708. doi: 10.1097/TP.0b013e3182476cc8. [DOI] [PubMed] [Google Scholar]
- 12.O’Gorman D, Kin T, Pawlick R, Imes S, Senior PA, Shapiro AJ. Clinical islet isolation outcomes with a highly purified neutral protease for pancreas dissociation. Islets. 2013;5(3):111–115. doi: 10.4161/isl.25222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Brandhorst H, Johnson PR, Mönch J, Kurfürst M, Korsgren O, Brandhorst D. Comparison of clostripain and neutral protease as supplementary enzymes for human islet isolation. Cell Transplant. 2019;28(2):176–184. doi: 10.1177/0963689718811614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Brandhorst D, Brandhorst H, Johnson PRV. Enzyme development for human islet isolation: five decades of progress or stagnation? Rev Diabetes Stud RDS. 2017;14(1):22–38. doi: 10.1900/RDS.2017.14.22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Chadwick DR, Robertson GS, Contractor H, Swift S, Rose S, Thirdborough ST, Chamberlain R, James RFL, Bell PRF, London NJM, et al. Human islet purification: a prospective comparison of Euro-Ficoll and bovine serum albumin density gradients. Acta Diabetol. 1993;30(1):57–59. doi: 10.1007/bf00572876. [DOI] [PubMed] [Google Scholar]
- 16.Shimoda M, Itoh T, Iwahashi S, Takita M, Sugimoto K, Kanak MA, Chujo D, Naziruddin B, Levy MF, Grayburn PA, et al. An effective purification method using large bottles for human pancreatic islet isolation. Islets. 2012;4(6):398–404. doi: 10.4161/isl.23008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Noguchi H, Miyagi-Shiohira C, Kurima K, Kobayashi N, Saitoh I, Watanabe M, Noguchi Y, Matsushita M. Islet culture/preservation before islet transplantation. Cell Med. 2015;8(1–2):25–29. doi: 10.3727/215517915X689047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Berney T. Islet culture and counter-culture. Commentary on: effect of short-term culture on functional and stress-related parameters in isolated human islets by Ihm et al. Transpl Int Off J Eur Soc Organ Transplant. 2009;22(5):531–533. doi: 10.1111/j.1432-2277.2008.00794.x. [DOI] [PubMed] [Google Scholar]
- 19.Murdoch TB, McGhee-Wilson D, Shapiro AMJ, Lakey JRT. Methods of human islet culture for transplantation. Cell Transplant. 2004;13(6):605–618. doi: 10.3727/000000004783983602. [DOI] [PubMed] [Google Scholar]
- 20.Rheinheimer J, Bauer AC, Silveiro SP, Estivalet AAF, Bouças AP, Rosa AR, Souza BMD, Oliveira FSD, Cruz LA, Brondani LA, et al. Human pancreatic islet transplantation: an update and description of the establishment of a pancreatic islet isolation laboratory. Arch Endocrinol Metab. 2015;59(2):161–170. doi: 10.1590/2359-3997000000030. [DOI] [PubMed] [Google Scholar]
- 21.Guignard AP, Oberholzer J, Benhamou P-Y, Touzet S, Bucher P, Penfornis A, Bayle F, Kessler L, Thivolet C, Badet L, et al. Cost analysis of human islet transplantation for the treatment of type 1 diabetes in the Swiss-French Consortium GRAGIL. Diabetes Care. 2004;27(4):895–900. doi: 10.2337/diacare.27.4.895. [DOI] [PubMed] [Google Scholar]
- 22.Paparella S. Failure mode and effects analysis: a useful tool for risk identification and injury prevention. J Emerg Nurs. 2007;33(4):367–371. doi: 10.1016/j.jen.2007.03.009. [DOI] [PubMed] [Google Scholar]
- 23.Kempf M-C, Andres A, Morel P, Benhamou P-Y, Bayle F, Kessler L, Badet L, Thivolet C, Penfornis A, Renoult E, et al. Logistics and transplant coordination activity in the GRAGIL Swiss-French multicenter network of islet transplantation. Transplantation. 2005;79(9):1200–1205. doi: 10.1097/01.tp.0000161224.67535.41. [DOI] [PubMed] [Google Scholar]
- 24.Truitt E, Thompson R, Blazey-Martin D, NiSai D, Salem D. Effect of the implementation of Barcode technology and an electronic medication administration record on adverse drug events. Hosp Pharm. 2016;51(6):474–483. doi: 10.1310/hpj5106-474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kin T, Johnson PRV, Shapiro AMJ, Lakey JRT. Factors influencing the collagenase digestion phase of human islet isolation. Transplantation. 2007;83(1):7–12. doi: 10.1097/01.tp.0000243169.09644.e6. [DOI] [PubMed] [Google Scholar]
- 26.Qi M, Barbaro B, Wang S, Wang Y, Hansen M, Oberholzer J. Human pancreatic islet isolation: Part II: purification and culture of human islets. J Vis Exp JoVE. 2009:27. doi: 10.3791/1343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ekdahl KN, Hong J, Hamad OA, Larsson R, Nilsson B. Evaluation of the blood compatibility of materials, cells, and tissues: basic concepts, test models, and practical guidelines. Adv Exp Med Biol. 2013;735:257–270. doi: 10.1007/978-1-4614-4118-2_18. [DOI] [PubMed] [Google Scholar]
- 28.Mohanakumar T, Narayanan K, Desai N, Ramachandran S, Shenoy S, Jendrisak M, Susskind BM, Olack B, Benshoff N, Phelan DL, et al. A significant role for histocompatibility in human islet transplantation. Transplantation. 2006;82(2):180–187. doi: 10.1097/01.tp.0000226161.82581.b2. [DOI] [PubMed] [Google Scholar]
- 29.Thorpe TC, Wilson ML, Turner JE, DiGuiseppi JL, Willert M, Mirrett S, Reller LB. BacT/Alert: an automated colorimetric microbial detection system. J Clin Microbiol. 1990;28(7):1608–1612. doi: 10.1128/JCM.28.7.1608-1612.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Coico R. Gram staining. Curr Protoc Microbiol. 2005. Appendix 3:Appendix3C. doi: 10.1002/9780471729259.mca03cs00. [DOI] [PubMed] [Google Scholar]
- 31.Gee AP, Sumstad D, Stanson J, Watson P, Proctor J, Kadidlo D, Koch E, Sprague J, Wood D, Styers D, et al. A multicenter comparison study between the Endosafe PTS rapid-release testing system and traditional methods for detecting endotoxin in cell-therapy products. Cytotherapy. 2008;10(4):427–435. doi: 10.1080/14653240802075476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Bucher P, Oberholzer J, Bosco D, Mathe Z, Toso C, Bühler LH, Berney T, Morel P. Microbial surveillance during human pancreatic islet isolation. Transpl Int Off J Eur Soc Organ Transplant. 2005;18(5):584–589. doi: 10.1111/j.1432-2277.2005.00092.x. [DOI] [PubMed] [Google Scholar]
- 33.Meier RPH, Andrey DO, Sun P, Niclauss N, Bédat B, Demuylder–Mischler S, Borot S, Benhamou PY, Wojtusciszyn A, Buron F, et al. Pancreas preservation fluid microbial contamination is associated with poor islet isolation outcomes - a multi-centre cohort study. Transpl Int Off J Eur Soc Organ Transplant. 2018;31(8):917–929. doi: 10.1111/tri.13159. [DOI] [PubMed] [Google Scholar]
- 34.Ricordi C, Goldstein JS, Balamurugan AN, Szot GL, Kin T, Liu C, Czarniecki CW, Barbaro B, Bridges ND, Cano J, et al. National institutes of health–sponsored clinical islet transplantation consortium Phase 3 trial: manufacture of a complex cellular product at eight processing facilities. Diabetes. 2016;65(11):3418–3428. doi: 10.2337/db16-0234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Nano R, Kerr-Conte J, Scholz H, Engelse M, Karlsson M, Saudek F, Bosco D, Antonioli B, Bertuzzi F, Johnson PRV, et al. Heterogeneity of human pancreatic islet isolation around Europe: results of a survey study. Transplantation. 2020;104(1):190–196. doi: 10.1097/TP.0000000000002777. [DOI] [PubMed] [Google Scholar]
- 36.Williams E, Talley R. The use of failure mode effect and criticality analysis in a medication error subcommittee. Hosp Pharm. 1994;29:331–332, 334–336, 339. [PubMed] [Google Scholar]


