ABSTRACT
High-risk human papillomaviruses (HPV) can be present and cooperate with Epstein–Barr virus (EBV) to promote the onset and/or progression of various cancers including cervical, breast, head and neck as well as colorectal. In this investigation, we explored the co-prevalence of high-risk HPV and EBV in 74 breast cancer tissues from Qatari women using polymerase chain reaction. We found that high-risk HPV and EBV are present in 48/74 (65%) and 36/74 (49%) of the cases, respectively. While we noted that the presence of HPV presence is associated with triple-negative breast cancer (TNBC) (p = .008), however, the presence of EBV did not correlate with any breast cancer subgroup. Moreover, our data revealed that high-risk HPV and EBV are co-present in 35/74 (47%) of the samples and their co-presence is significantly associated with tumor grade (p = .04) and tumor stage (p = .04). These data indicate that HPV and EBV are commonly co-present in breast cancer and their association could be linked with a more aggressive tumor phenotype. Thus, further investigations are essential to understand the underlying mechanisms of HPV and EBV cooperation in breast carcinogenesis.
KEYWORDS: HPV, EBV, breast cancer, tumor stage, tumor grade, Qatari population
Introduction
Breast cancer, is the most commonly diagnosed type of malignancy among women worldwide including the Middle East (ME) region and Qatar, accounting for around 1/4th of all cancer cases.1 In comparison to breast cancer cases in the West, the ME region has the highest incidence where women are affected at a relatively younger age (<50 years) and usually present to the clinic with advanced stage disease and aggressive phenotype.2–4 Along with genetic and environmental factors, it is estimated that around 20% of human cancers are associated with infectious agents including oncoviruses especially high-risk human papillomaviruses (HPVs) and Epstein-Barr virus (EBV) which could initiate the onset and progression of different types of human carcinomas.5–8
Today, it well-known that high-risk HPVs are linked with the development and progression of several cancers including cervical, colorectal, head and neck (HN) in addition to breast cancers.9–12 More interestingly, it was pointed out that the presence of high-risk HPVs is linked with vascular invasion, lymph node metastases and tumor phenotype in different types of human carcinomas including cervical, HN and breast.11,13–16 In this context, it has been reported that E6/E7 oncoproteins of high-risk HPV type 16, which is the most frequent HPV type, can converts noninvasive and non-metastatic breast cancer cells into invasive and metastatic form.17 On the other hand, EBV infection is also associated with several types of epithelial carcinomas including nasopharyngeal (NPC), breast, cervical and gastric cancer.18 EBNA1 and LMP1 are oncogenic proteins of EBV that provoke cellular proliferation and motility, inhibit apoptosis, promote cellular motility and angiogenesis, thus, indicating the role of EBV in carcinogenesis.19–21
A study showed that E6/E7 oncoproteins of HPV interact with Nucleophosmin, a nucleolar protein, to enhance proliferation and inhibition of differentiation of E6/E7 expressing cells.22 Likewise, infection with a single type of high-risk HPV alone is not enough to incite neoplastic transformation; high-risk HPV-infected cells either have to endure genetic transformation and/or co-infection with another oncoviruses for complete cellular transformation resulting in the onset and development of tumor.23–29 Earlier studies demonstrated that high-risk HPVs and EBV are co- present in human breast cancer worldwide including the ME; and their incidence is generally associated with an aggressive phenotype.30,31 While, a few studies failed to detect high-risk HPVs in human breast cancer as well as normal mammary tissues.32,33 Based on previous studies and our work on the role of HPV and EBV in the initiation of various cancers including HN, colorectal, cervical and breast,26,29,34–36 we explored the co-presence of high-risk HPVs and EBV in breast cancer in Qatari women. Our data revealed that 47% of the examined samples are positive for both HPV and EBV and their co-presence is significantly associated with tumor grade and stage in comparison with HPV and EBV alone.
Materials and methods
Sample collection and DNA extraction
Breast cancer samples from a total of 505 Qatari female patients were collected over a 12-year period (January 2008-December 2019), out of which 9 patients had received neoadjuvant chemotherapy prior to their breast surgery. Seventy-four patients received surgical treatment at Hamad General Hospital, Qatar, which were included in the study. All the tumors were graded according to the Nottingham histological grade (modified Scarff-Bloom-Richardson grade).37 The tumors were considered positive for estrogen (ER) and progesterone receptors (PR) if nuclear positivity was observed in >1% of tumor cells.38 HER2 positivity (score 3+) was defined as intense, complete, circumferential membranous expression in >10% tumor cells.39 Equivocal (score 2+) was defined as a weak, complete membranous staining in >10% of tumor cells. The tumor cells with scores 0–1+ were considered negative for HER2. The proliferation index was assessed using Ki-67 proliferation marker (MIB1 antibody). The tumors were considered highly proliferative if Ki-67 > 20% of tumor cells. Tumor samples were classified into four molecular subtypes (Luminal A, luminal B, HER2-positive and triple-negative) based on the status of ER, PR, HER2 and Ki-67.
All samples (punch samples of 2 mm thickness) were taken from formalin-fixed paraffin-embedded (FFPE) tissues from surgically removed and pathologically confirmed breast carcinomas. The samples were retrieved from Qatar University (QU) and the pathology archive of the Department of Laboratory Medicine and Pathology, Hamad General Hospital (HMC), Qatar, after the approval of the Ethical Committees of QU & HMC (QUCG-CMED-2018\2019-3; HMC:24-2-2019, Doha, Qatar).
Exclusion criteria included other nationalities, Qatari males, slides and blocks of patients that received surgical intervention outside Hamad General Hospital and presented to the histopathology lab for second opinion, breast core/true-cut/stereotactic biopsy, and lymph node biopsy/skin biopsy diagnosed with metastatic breast carcinoma, repeated excisions from the same patient, specimens of patients post chemotherapy with complete resolution of the cancer and microscopic tumors less than 1 cm in addition to the tumors that are presented within one paraffin block.
The Thermo Scientific GeneJET FFPE DNA Purification Kit was used to extract DNA from FFPE tissues according to the manufacturer’s instructions (ThermoFisher Scientific, USA). Briefly, FFPE sections underwent enzymatic digestion (200 µL of Digestion Buffer) and lysis using 20 µL Proteinase K solution to release genomic DNA. The released DNA was de-crosslinked by heat incubation at 90°C for 40 minutes. The resulting solution was then centrifuged and the supernatant containing DNA was mixed with 200 µL Binding Buffer. After addition of ethanol (96%), the lysate was added into the purification column. The adsorbed DNA was washed to remove contaminants and then eluted with 60 µL Elution Buffer.
HPV and EBV detection by PCR
Genotyping and detection of the presence of HPV and EBV was done using specific primers for high-risk HPV types (16, 18, 31, 33, 35, 39, 45, 51, 52, 56 and 58) of E6/E7 region and for EBV genes, EBNA1 and LMP1 as previously described.40,41 GAPDH was used as an internal control (Figures 1 and 2). Analysis was performed as previously described by our group.40,41
Figure 1.
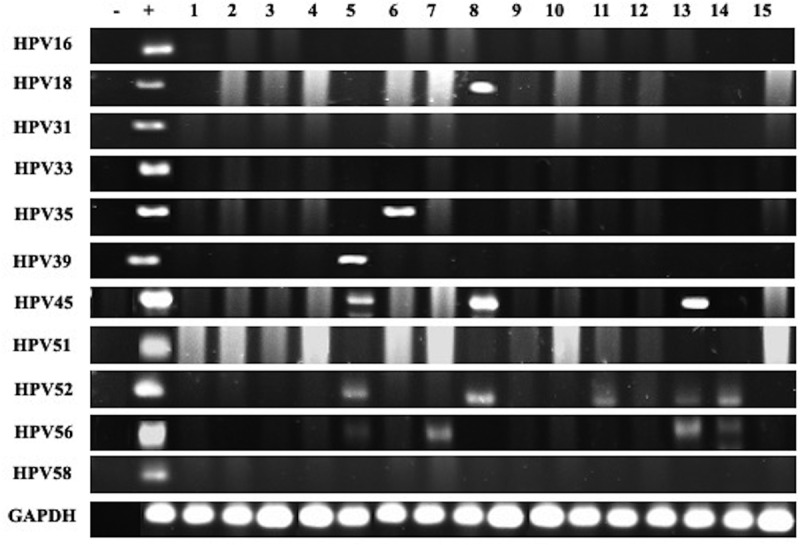
Representative PCR reactions for high-risk HPV-subtypes in 15 different breast cancer samples
Figure 2.
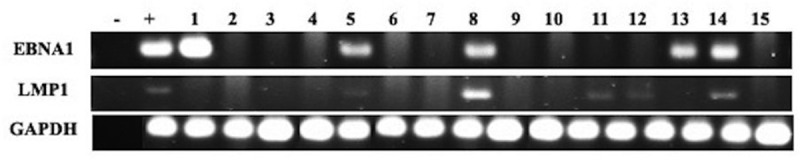
Representative PCR reactions for EBV (EBNA1 and LMP1) in 15 different breast cancer samples
PCR was performed using the Invitrogen Platinum II Hot-Start Green PCR Master Mix (2X) (ThermoFisher Scientific, USA). HPV and EBV genes were amplified for an initial denaturation at 94°C for 2 mins followed by 40 cycles of 94°C for 30s, annealing at temperatures ranging from 50 to 62°C for 30s depending on each primer’s melting temperature as previously described,40,41 and 72°C for 30s with a final incubation for 10 mins at 72°C. The PCR product from each exon was resolved using 1.5% agarose gel electrophoresis and visualized using iBrightCL1000 Imaging System (ThermoFisher). In each experiment, negative control (instead of DNA, MDA-MB-453 cell line42 and sterile water) and positive control (Hela cell line for L1 region43 and normal oral epithelial (NOE) cell line transfected with E6/E7 of HPV type 16 for E6/E7 region23) were used.
Statistical analysis
Statistical analysis was performed using IBM Statistical Package for the Social Sciences (version 25). Data were calculated as non-parametric files. To assess the significance of HPV and EBV association we utilized χ2 test with Yates correction. Further, we utilized χ2 test with Yates correction to assess the significance of the association between clinic-pathological data (patient’s age, Nottingham histological grade and tumor stage) in correlation with the presence/co-presence of HPVs and EBV. Statistical significance was achieved at p < .05.
Results
Clinicopathological characteristics of the cohort
The clinicopathological characteristics of the cohort are summarized in Table 1. The mean age of all patients is 55.3 (standard deviation (SD), ±12.4) years. Most of the patients (66%) are aged >50 years. Axillary lymph nodes were involved in 41% of patients (Table 1).
Table 1.
Clinicopathological characteristics of patients with breast cancer
| Characteristic | Categories | Number (%) |
|---|---|---|
| Age | ≤50 | 25 (34) |
| >50 | 49 (66) | |
| Histopathological Subtypes of Breast Cancer | Invasive Ductal Carcinoma | 60 (81) |
| Invasive Lobular Carcinoma | 8 (11) | |
| Mucinous Carcinoma | 5 (7) | |
| Unknown | 1 (1) | |
| Nottingham Histological Grade | I | 14 (19) |
| II | 40 (54) | |
| III | 18 (24) | |
| Unknown | 2 (3) | |
| Tumor (pT) Stage | pT1 | 18 (24) |
| pT2 | 21 (28) | |
| pT3 | 6 (8) | |
| pT4 | 2 (3) | |
| Unknown | 27 (37) | |
| Lymph Node Involvement | Positive | 30 (40) |
| Negative | 37 (50) | |
| Unknown | 7 (10) | |
| Estrogen Receptor (ER) Status | ER+ | 59 (80) |
| ER- | 13 (17) | |
| Unknown | 2 (3) | |
| Progesterone Receptor (PR) Status | PR+ | 54 (73) |
| PR- | 18 (24) | |
| Unknown | 2 (3) | |
| HER2 Status | Positive (3+) | 8 (11) |
| Equivocal (2+) | 8 (11) | |
| Negative (0–1+) | 56 (76) | |
| Unknown | 2 (3) | |
| Ki-67 Proliferative Index (PI) | Low (<10%) PI | 19 (26) |
| Intermediate (10–20%) PI | 10 (13) | |
| High (>20%) PI | 23 (31) | |
| Unknown | 22 (30) | |
| Molecular Classification of Breast Cancer | Luminal A | 32 (43) |
| Luminal B | 28 (38) | |
| HER2+ | 2 (3) | |
| Triple Negative | 10 (13) | |
| Unknown | 2 (3) | |
| HPV Expression | Invasive Ductal Carcinoma | 38 (51) |
| Invasive Lobular Carcinoma | 5 (7) | |
| Mucinous Carcinoma | 4 (5) | |
| EBV Expression | Invasive Ductal Carcinoma | 21 (28) |
| Invasive Lobular Carcinoma | 1 (1) | |
| Mucinous Carcinoma | 3 (4) |
The majority of patients had invasive ductal carcinomas (IDC), no special type (NST) (60 cases, 81%) while 8 patients (11%) were diagnosed with invasive lobular carcinoma (ILC) and five patients (7%) with mucinous carcinomas. The hormone receptor status was available for 72 patients; 59 (80%) and 54 (73%) of the patients expressed estrogen and progesterone receptors, respectively. With reference to HER2 status, it was available for 72 patients of which 8 (11%) had overexpression (score 3+), 8 (11%) had equivocal expression (score 2+) and 56 (76%) lacked HER2 expression (scores 0–1+). The status for Ki-67 proliferative index was available for 52 patients, 19 of which had low proliferation rate (<10%), 10 had intermediate (10–20%) and the remaining 23 had a high (>20%) proliferation rate. All cases were categorized into four molecular subtypes: Luminal A (32 cases, 43%), Luminal B (28 cases, 38%), HER2+ (2 cases, 3%) and triple-negative (10 cases, 14%) (Table 1).
The status of high-risk HPV and EBV and their association with clinicopathological characteristics
Forty-eight of the 74 samples in our cohort are positive for high-risk HPVs (64.8%) (Table 2); the most commonly present high-risk HPVs are HPV52 (51%) followed by HPV56 (44%), HPV45 (22%), HPV58 (15%), HPV18 (11%), HPV35 (3%) and HPV39 (1%) (Figure 3). HPV types 16, 31, 33 and 51 were not detected in our examined samples (Figure 3). On the other hand, we found that 36/74 of the samples are positive for EBV (49%) (Table 2); of these 36 cases, 33/74 (45%) and 28/74 (38%) are positive for EBNA1 and LMP1 of EBV, respectively. Meanwhile, we noted a significant correlation between EBV and various HPV types: HPV18 (p = .03), HPV35 (p = .03), HPV52 (p = .03) and HPV56 (p = .003) (Table 3). Furthermore, our data revealed that 14/74 (19%) cases are positive for two HPV subtypes; the most commonly present combination is HPV52/56 (24/74, 32%), followed by HPV52/45 (13/74, 18%), HPV52/58 (8.74, 11%), HPV52/18 (7/74, 9%) and HPV52/35 (1/74, 1%) (Figure 4). Finally, we observed that, 18/74 (24%) of the samples are positive for more than two HPV subtypes; and the most frequent combinations are: HPV52/56/45 (11/74, 15%), HPV52/56/58 (7/74, 9%), HPV52/56/18 (6/74, 8%) and HPV52/56/59 (1/74, 1%) (Figure 4).
Table 2.
Prevalence of high-risk HPVs and EBV in Qatari breast cancer patients
| Single Infection |
Multiple Infection |
|
|---|---|---|
| HPV | EBV | HPV + and EBV+ |
| 65% | 49% | 47% |
Figure 3.
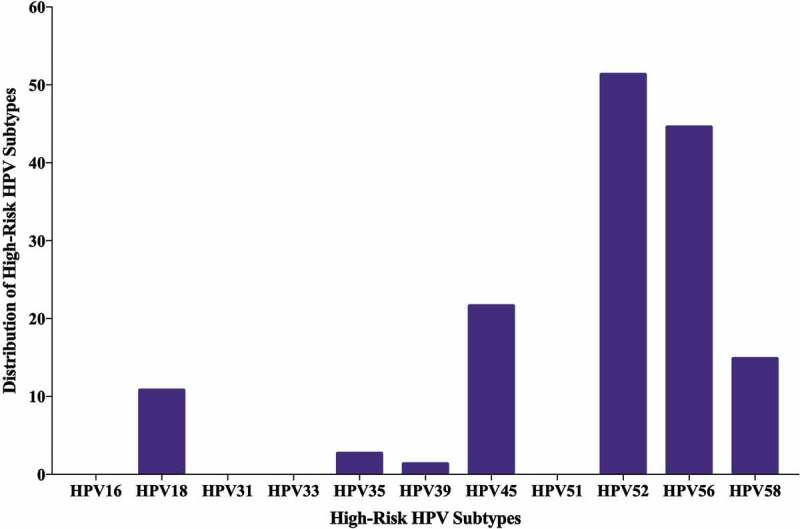
Distribution of each high-risk HPV subtype in Qatari breast cancer samples. PCR analysis included 74 breast cancer samples revealing that the most frequent HPV subtypes are 52, 56 and 45
Table 3.
Presence of EBV and HPV-subtypes in Qatari breast cancer patients
| Samples | No. of Cases | High-Risk HPV Types |
||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 16 | 18 | 31 | 33 | 35 | 39 | 45 | 51 | 52 | 56 | 58 | ||
| EBV (+) | 36 | 0 | 7 | 0 | 0 | 1 | 0 | 15 | 0 | 27 | 24 | 10 |
| EBV (-) | 38 | 0 | 1 | 0 | 0 | 1 | 0 | 1 | 0 | 11 | 9 | 1 |
| Total | 74 | 0 | 8 | 0 | 0 | 2 | 0 | 16 | 0 | 38 | 33 | 11 |
| p-value | N/A | 0.02* | N/A | N/A | 0.98 | N/A | 0.0001* | N/A | 0.0001* | 0.0005* | 0.007* | |
Comparison was made between presence/absence of EBV (EBV+/EBV-) with high-risk HPV subtypes.
*indicates significant p-values (<0.05).
N/A denotes Not Applicable as χ2 test is invalid in these cases, since value is 0.
Figure 4.
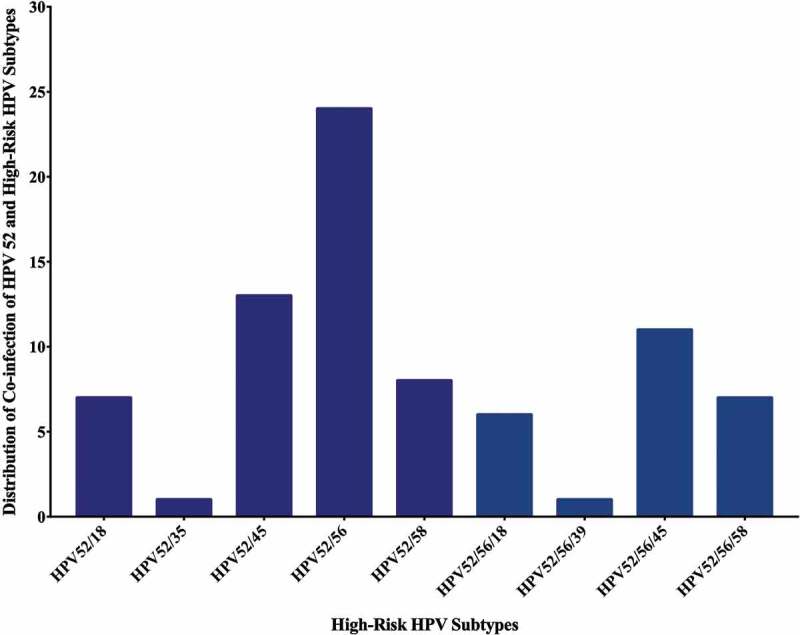
Distribution of HPVs co-infection in Qatari breast cancer cohort (n = 74). The graph illustrates that the two most common high-risk HPVs are HPV52/56 (24/74 cases) while co-infection with more than two HPVs is HPV52/56/45 (11/74 cases)
More significantly, our data revealed that the co-presence of EBV and high-risk HPV is detected in 47% (35/74) of breast cancer cases (Table 2); and there is a significant correlation between the coincidence of EBV with various HPV types (HPVs- 18, 45, 52, 56 and 58) in the 74 breast cancer samples (p < .001).
Regarding the clinicopathological characteristics and their association with high-risk HPV/EBV co-presence, we noted that high-risk HPVs positivity (HPV+/EBV-) significantly correlates with triple-negative breast cancer subtype (p = .008) (Table 4); while, EBV positivity alone (HPV-/EBV+) as well as lack of both HPV and EBV (HPV-/EBV-) did not correlate with any clinicopathological feature. More importantly, our data revealed that the co-presence of high-risk HPVs and EBV (HPV+/EBV+) is associated with luminal A (p = .02) subtype in addition to tumor grade (p = .04) and tumor stage (p = .04) (Table 5); nevertheless, there is no association with lymph node involvement (p = .85).
Table 4.
Correlation between clinicopathological characteristics and HPV positivity
| Molecular Subtypes of Breast Cancer | |||
|---|---|---|---|
| Subtypes | HPV Positive (%) | HPV Negative (%) | p-value |
| Luminal A | 23 (32) | 9 (12) | p = .103 |
| Luminal B | 18 (25) | 10 (14) | p = .476 |
| HER2-Positive | 2 (3) | 0 (0) | p = .140 |
| Triple Negative | 3 (4) | 7 (10) | p = .008* |
| Total | 46 (64) | 26 (36) | |
| Nottingham Histological Grade | |||
| Grade | |||
| I | 11 (15) | 3 (4) | p = .354 |
| II | 23 (32) | 17 (24) | |
| III | 12 (17) | 6 (8) | |
| Total | 46 (64) | 26 (36) | |
| Tumor Stage | |||
| Stage | |||
| Early Stage (I–II) | 26 (55) | 13 (28) | p = .251 |
| Advanced Stage (III–IV) | 3 (7) | 5 (10) | |
| Total | 29 (62) | 18 (38) | |
Comparison was made between presence/absence of HPV (HPV+/HPV-) and clinicopathological characteristics.
*indicates significant p-values (p < 0.05).
Table 5.
The correlation between clinicopathological characteristics and EBV/HPV status (the HPV/EBV positive group was compared with other subgroups of breast cancer with various HPV/EBV status)
| Molecular Subtypes of Breast Cancer | |||
|---|---|---|---|
| Subtypes | HPV±/EBV± (%) | HPV+/EBV+ (%) | p-value |
| Luminal A | 19 (26.3) | 13 (18) | p = .02* |
| Luminal B | 10 (13.9) | 18 (0.3) | p = .08 |
| HER2-Positive | 2 (2.8) | 0 (0) | p = .06 |
| Triple Negative | 2 (2.8) | 8 (11.1) | p = .04* |
| Total | 33 (45.8) | 39 (54.2) | |
| Nottingham Histological Grade | |||
| Grade | |||
| I | 4 (5.5) | 10 (13.9) | p = .04* |
| II | 27 (37.5) | 13 (18.1) | |
| III | 10 (13.9) | 8 (11.1) | |
| Total | 41 (56.9) | 31 (43.1) | |
| Tumor Stage | |||
| Stages | |||
| Early Stage (I–II) | 20 (42.5) | 19 (40.4) | p = .04* |
| Advanced Stage (III–IV) | 1 (2.1) | 7 (14.9) | |
| Total | 21 (44.6) | 26 (55.3) | |
| Lymph Node Involvement | |||
| Status | |||
| Positive | 16 (23.9) | 14 (20.9) | p = .85 |
| Negative | 20 (29.8) | 17 (25.3) | |
| Total | 36 (53.7) | 31 (46.2) | |
| Tumor Type | |||
| IDC | 27 (37) | 32 (43.8) | p = .53 |
| ILC | 2 (2.7) | 4 (5.5) | |
| Others | 5 (6.9) | 3 (4.1) | |
| Total | 34 (46.6) | 39 (53.4) | |
*indicates significant p-values (<0.05).
HPV+/EBV+ denotes a co-presence of HPV and EBV. HPV±/EBV± indicates a combination of HPV+/EBV- (HPV presence/EBV absence), HPV-/EBV+ (HPV absence/EBV presence) and HPV-/EBV- (lack of both HPV and EBV).
Discussion
To the best of our knowledge, this is the first study on the presence/co-presence of high-risk HPVs and EBV in human breast cancer and its association with tumor grade and stage in the Gulf region. It is well known that oncogenic proteins of high-risk HPV can stimulate inflammation, making it a plausible candidate for the onset and progression of different types of human carcinomas including breast.44 In this study, we analyzed 74 breast cancer samples for the presence of high-risk HPVs, we found that 65% of our samples are positive for these oncoviruses. Several studies reported the presence of high-risk HPVs in human breast cancer patients worldwide including the ME region, with prevalence ranging from 4 to 86%.11,42,45–48 More specifically, our data is supported by several investigations in the ME region including one from Syria and another one from Turkey, where a high frequency of high-risk HPVs of 61% and 74%, respectively, were reported.11,49 Moreover, one investigation from Iraq, showed that HPVs are present in 46% of breast cancer samples from Iraqi women.50 Additionally, a few studies from Iran showed varying HPV prevalence in breast cancer (14–49%) in Iranian women.45,51–53 On the other hand, it is important to highlight that a study on cervical samples in Qatari women found HPV prevalence of 55%, which also supports our data.54
Indeed, one of the key findings in our study is the predominance of HPV genotypes – 52 and −56 in breast cancer from Qatari women. Accordingly, a study using cervical samples revealed that high-risk HPV type 56 is one of the most frequent type in Qatari women.55 More interestingly, two studies one in Bahraini women and another one in Omani women with cervical cancer, reported that HPV52 is the most prevalent subtype in Bahrain and Oman.56 However, studies on cervical cancer samples from other Gulf countries showed that high-risk HPV types 68 and 73 are the most prevalent in the Kingdom of Saudi Arabia,57 while in Kuwait HPVs 16, 66 and 33 are common.58 Although, it is important to emphasize that HPV types 16 and 18 are the most frequently expressed genotypes in cancers worldwide;46,47 in our study we did not detect HPV type 16, while HPV type 18 had a low prevalence of 11%. Other studies also had similar data where, HPV type18 is present in around 12% of breast cancer cases.16,46 A study in the Chinese population did not detect the presence of HPV16 and 18 in breast cancer samples.59 Moreover, a study in Australia performed PCR on breast cancer samples and identified HPV18 as the most prevalent subtype in breast cancer specimens.60 Therefore, the difference in HPV prevalence and genotype distribution can be attributed to geographical location, sample size as well as methodological differences.47,61 Regarding breast cancer subtypes and their association with high-risk HPVs, our data show high prevalence of HPV DNA in Luminal A and B cancer tissues showing high levels of Ki67 expression, which is supported by De Carolis et al., (2019).62 Furthermore, according to previous reports and concordant with our data, HPV presence is significantly associated with TNBC.48,62–64 In this context, it is important to emphasize that TNBC form a highly invasive breast cancer subgroup; thus, we have demonstrated that E6/E7 oncoproteins of high-risk HPVs converts noninvasive and non-metastatic breast cancer cells into invasive and metastatic ones.17 Thus, our new finding concurs with our previous work regarding the role of E6/E7 in breast cancer cells.
Vis-a vis the presence of EBV in human breast cancer, it has been revealed that EBV is present in (30–50%) of this cancer cases worldwide.65–69 Studies in Turkey and Syria reported 58%70 and 52%71 EBV positivity in breast cancer, respectively. While in Egypt, 45% of breast cancer cases were positive for EBV.72 Additionally, Tunisia and Iraq reported EBV presence in breast cancer in 27%73 and 28%72 of the examined cases, respectively. Recently, studies in breast cancer from Iran reported 27% of EBV DNA in their samples.74,75 In our study, we report that EBV is present in approximately 49% of breast cancer cases in Qatari women. Therefore, our data indicate the prevalence of EBV in breast cancer tissues in Qatar is similar to the incidence present worldwide including the ME region.
Intriguingly, based on previous works, including ours, co-presence of high-risk HPV and EBV can play an important role in the onset and progression of different cancers including oral, colorectal, cervical as well as breast.11,26,27,29,35,76 In concordance, in our present investigation, we report that 47% of breast cancer cases, from Qatari women, are co-infected with both HPVs and EBV. Our previous work in Syrian samples pointed out that high-risk HPVs and EBV are co-present in 32% of breast cancer samples from Syrian women.26 While an earlier study from Pakistan revealed that HPVs and EBV are co-present in approximately 9% of breast cancer samples from Pakistani women.77 On the other hand, it is important to highlight that the presence of EBV alone is not shown to be associated with tumor stage, histological grade, molecular subtypes or nodal status; as previously reported by other investigations.78 However, HPVs and EBV have been considered as risk factors for TNBC;79 we found a significant association between HPVs and EBV co-presence and TNBC as well as luminal A subtypes. Moreover, studies showed co-presence of HPVs and EBV to be correlated with advanced breast Nottingham histological grade65 and aggressive phenotype.80 Likewise, we herein demonstrate for the first time that the co-presence of high-risk HPVs and EBV is associated with advanced tumor stage and grade (p = .04); indicating the possible cooperative role of high-risk HPVs and EBV oncoproteins in the initiation and/or progression of certain subtypes of human breast cancer as previously reported by several studies.65,77,81,82 We have pointed out earlier that oncoproteins of HPVs (E5 and E6/E7) and EBV (LMP1 and/or EBNA1) can interact and cooperate in the initiation and/or progression of human oral carcinomas via the EMT event;25 thus indicating a similar mechanism in the pathogenesis of human breast malignancy. Based on HPVs and EBV role in the pathogenesis of cancer as discussed above, we postulate that oncoproteins of HPVs can interact with those of EBV (LMP1 and/or EBNA1) and result in progression and metastasis by enhancing the EMT event of different types of cancers including breast, as recently demonstrated in other types of cancers, such as colorectal and HN.18,35,83
The current study has several limitations. Firstly, we did not have a proper control group for the study (e.g. reduction mammoplasty samples); however, using the same methodology, we recently reported the prevalence of EBV and HPV in a large cohort of healthy blood donors from Qatar.40 Although Qatari females were under-represented, we found a similar prevalence of both viruses. Secondly, access to breast cancer tissue samples was restricted, which led us to apply only one method (PCR-based) for EBV and HPV assessment. Of note, our previous studies exploring HPV and EBV in different types of cancers using PCR and IHC yielded comparable results.29,41,84,85
Conclusions
In conclusion, although the sample size used in the study is relatively small, this report indicates the co-presence of HPVs and EBV in breast cancer in this region and implies that it is associated with tumor grade and stage. Our investigation suggests that HPVs and future EBV vaccines can be used to prevent the development and progression of certain subtypes of breast cancer in the population of the Gulf region including Qatar. However, it is important to take into consideration the most frequent HPV types in this region in order to select the suitable HPV vaccine. Meanwhile, we believe that future investigations with a larger cohort from different countries of the ME region including Qatar are needed to confirm the co-presence and HPV subtypes of these oncoviruses in breast cancer and therefore to elucidate the exact role of EBV and high-risk HPVs cooperation in breast carcinogenesis.
Acknowledgments
We would like to thank Mrs. A. Kassab for her critical reading of the manuscript. Open Access funding provided by the Qatar National Library.
Funding Statement
The current study was supported by the grants from Qatar University [QUCG-CMED-2018/2019-3, QUHI-CMED-19/20-1 and QUCG-CMED-20/21-2].
Disclosure of potential conflicts of interest
No potential conflicts of interest were disclosed.
References
- 1.Ferlay J, Colombet M, Soerjomataram I, Mathers C, Parkin DM, Piñeros M, Znaor A, Bray F.. Estimating the global cancer incidence and mortality in 2018: GLOBOCAN sources and methods. Int J Cancer. 2019;144(8):1941–53. doi: 10.1002/ijc.31937. [DOI] [PubMed] [Google Scholar]
- 2.Narayan AK, Al-Naemi H, Aly A, Kharita MH, Khera RD, Hajaj M, Rehani MM. Breast cancer detection in Qatar: evaluation of mammography image quality using a standardized assessment tool. Eur J Breast Health. 2020;16(2):124–28. doi: 10.5152/ejbh.2020.5115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Donnelly TT, Al Khater A-H, Al Kuwari MG, Al-Bader SB, Al-Meer N, Abdulmalik M, Singh R, Chaudhry S, Fung T. Do socioeconomic factors influence breast cancer screening practices among Arab women in Qatar? BMJ Open. 2015;5(1):e005596–e005596. doi: 10.1136/bmjopen-2014-005596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bener A, Ayub H, Kakil R, Ibrahim W. Patterns of cancer incidence among the population of Qatar: a worldwide comparative study. Asian Pac J Cancer Prev. 2008. April 29;9(1):19–24. [PubMed] [Google Scholar]
- 5.Venuti ABG, Rizzo C, Mafera B, Rahimi S, Vigili M. Presence of HPV in head and neck tumours: high prevalence in tonsillar localization. J Exp Clin Cancer Res. 2004;23:561–66. [PubMed] [Google Scholar]
- 6.Daling JRMM, Johnson LG, Schwartz SM, Shera KA, Wurscher MA, Carter JJ, Porter PL, Galloway DA, McDougall JK. Human papillomavirus, smoking, and sexual practices in the etiology of anal cancer. Cancer. 2004;101:270–80. doi: 10.1002/cncr.20365. [DOI] [PubMed] [Google Scholar]
- 7.Ragin C, Taioli E. Survival of squamous cell carcinoma of the head and neck in relation to human papillomavirus infection: review and meta-analysis. Int J Cancer. 2007;121:1813–20. doi: 10.1002/ijc.22851. [DOI] [PubMed] [Google Scholar]
- 8.Shi Y, Peng S-L, Yang L-F, Chen X, Tao Y-G, Cao Y. Co-infection of Epstein-Barr virus and human papillomavirus in human tumorigenesis. Chin J Cancer. 2016;35:16–16. doi: 10.1186/s40880-016-0079-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Smith JS, Lindsay L, Hoots B, Keys J, Franceschi S, Winer R, Clifford GM. Human papillomavirus type distribution in invasive cervical cancer and high-grade cervical lesions: A meta-analysis update. Int J Cancer. 2007;121(3):621–32. doi: 10.1002/ijc.22527. [DOI] [PubMed] [Google Scholar]
- 10.Syrjänen KJ. HPV infections and oesophageal cancer. J Clin Pathol. 2002;55(10):721–28. doi: 10.1136/jcp.55.10.721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Akil N, Yasmeen A, Kassab A, Ghabreau L, Darnel AD, Al Moustafa AE. High-risk human papillomavirus infections in breast cancer in Syrian women and their association with Id-1 expression: a tissue microarray study. Br J Cancer. 2008;99(3):404–07. doi: 10.1038/sj.bjc.6604503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Delgado-García S, Martínez-Escoriza JC, Alba A, Martín-Bayón T-A, Ballester-Galiana H, Peiró G, Caballero P, Ponce-Lorenzo J. Presence of human papillomavirus DNA in breast cancer: a Spanish case-control study. BMC Cancer. 2017;17(1):320. doi: 10.1186/s12885-017-3308-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Graflund MSB, Sigurdardóttir S, Karlsson M. HPV-DNA, vascular space invasion, and their impact on the clinical outcome in early-stage cervical carcinomas. Int J Gynecol Cancer. 2004;14:896–902. doi: 10.1111/j.1048-891X.2004.014527.x. [DOI] [PubMed] [Google Scholar]
- 14.Umudum H, Rezanko T, Dag F, Dogruluk T. Human papillomavirus genome detection by in situ hybridization in fine-needle aspirates of metastatic lesions from head and neck squamous cell carcinomas. Cancer. 2005;105:171–77. doi: 10.1002/cncr.21027. [DOI] [PubMed] [Google Scholar]
- 15.Zuna R, Allen RA, Moore WE, Mattu R, Dunn ST. Comparison of human papillomavirus genotypes in high-grade squamous intraepithelial lesions and invasive cervical carcinoma: evidence for differences in biologic potential of precursor lesions. Mod Pathol. 2004;17:1314–22. doi: 10.1038/modpathol.3800223. [DOI] [PubMed] [Google Scholar]
- 16.Salman NA, Davies G, Majidy F, Shakir F, Akinrinade H, Perumal D, Ashrafi GH. Association of high risk human papillomavirus and breast cancer: a UK based study. Sci Rep. 2017;7(1):43591. doi: 10.1038/srep43591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Yasmeen A, Bismar TA, Kandouz M, Foulkes WD, Desprez P-Y, Al Moustafa A-E. E6/E7 of HPV type 16 promotes cell invasion and metastasis of human breast cancer cells. Cell Cycle. 2007;6(16):2038–42. doi: 10.4161/cc.6.16.4555. [DOI] [PubMed] [Google Scholar]
- 18.Cyprian FS, Al-Farsi HF, Vranic S, Akhtar S, Al Moustafa A-E. Epstein-barr virus and human papillomaviruses interactions and their roles in the initiation of epithelial-mesenchymal transition and cancer progression. Front Oncol. 2018;8:111–111. doi: 10.3389/fonc.2018.00111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Shimakage M, Horii K, Tempaku A, Kakudo K, Shirasaka T, Sasagawa T. Association of Epstein-Barr virus with oral cancers. Hum Pathol. 2002;33(6):608–14. doi: 10.1053/hupa.2002.129786. [DOI] [PubMed] [Google Scholar]
- 20.Horiuchi K, Mishima K, Ichijima K, Sugimura M, Ishida T, Kirita T. Epstein-Barr virus in the proliferative diseases of squamous epithelium in the oral cavity. Oral Sur Oral Med Oral Pathol Oral Radiol Endodontol. 1995;79(1):57–63. doi: 10.1016/S1079-2104(05)80075-7. [DOI] [PubMed] [Google Scholar]
- 21.Boudreault S, Armero VES, Scott MS, Perreault J-P, Bisaillon M. The Epstein-Barr virus EBNA1 protein modulates the alternative splicing of cellular genes. Virol J. 2019;16(1):29. doi: 10.1186/s12985-019-1137-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.McCloskey R, Menges C, Friedman A, Patel D, McCance DJ. Human papillomavirus type 16 E6/E7 upregulation of nucleophosmin is important for proliferation and inhibition of differentiation. J Virol. 2010;84(10):5131–39. doi: 10.1128/jvi.01965-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Al Moustafa A, Foulkes WD, Benlimame N, Wong A, Yen L, Bergeron J, Batist G, Alpert L, Alaoui-Jamali MA. E6/E7 proteins of HPV type 16 and ErbB-2 cooperate to induce neoplastic transformation of primary normal oral epithelial cells. Oncogene. 2004;23:350–58. doi: 10.1038/sj.onc.1207148. [DOI] [PubMed] [Google Scholar]
- 24.Al Moustafa A, Kassab A, Darnel A, Yasmeen A. High-risk HPV/ErbB-2 interaction on E-cadherin/catenin regulation in human carcinogenesis. Curr Pharm Des. 2008;14:2159–72. doi: 10.2174/138161208785740216. [DOI] [PubMed] [Google Scholar]
- 25.Al Moustafa A-E. E5 and E6/E7 of high-risk HPVs cooperate to enhance cancer progression through EMT initiation. Cell Adh Migr. 2015;9(5):392–93. doi: 10.1080/19336918.2015.1042197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Al Moustafa A-E, Al-Antary N, Aboulkassim T, Akil N, Batist G, Yasmeen A. Co-prevalence of Epstein-Barr virus and high-risk human papillomaviruses in Syrian women with breast cancer. Hum Vaccin Immunother. 2016;12(7):1936–39. doi: 10.1080/21645515.2016.1139255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Al Moustafa A-E, Chen D, Ghabreau L, Akil N. Association between human papillomavirus and Epstein-Barr virus infections in human oral carcinogenesis. Med Hypotheses. 2009;73(2):184–86. doi: 10.1016/j.mehy.2009.02.025. [DOI] [PubMed] [Google Scholar]
- 28.Al Moustafa A-E, Cyprian FS, Al-Antary N, Yasmeen A. High-risk human papillomaviruses and epstein-barr virus presence and crosstalk in human oral carcinogenesis. In: Al Moustafa A-E, editor. Development of oral cancer: risk factors and prevention strategies. Cham, Switzerland: Springer International Publishing; 2017. p. 83–94. [Google Scholar]
- 29.Al-Thawadi H, Ghabreau L, Aboulkassim T, Yasmeen A, Vranic S, Batist G, Al Moustafa A-E. Co-incidence of epstein-barr virus and high-risk human papillomaviruses in cervical cancer of syrian women. Front Oncol. 2018;8:250–250. doi: 10.3389/fonc.2018.00250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Al Moustafa A-E, Al-Awadhi R, Missaoui N, Adam I, Durusoy R, Ghabreau L, Akil N, Ahmed HG, Yasmeen A, Alsbeih G, et al. Human papillomaviruses-related cancers. Presence and prevention strategies in the Middle East and North African regions. Hum Vaccin Immunother. 2014;10(7):1812–21. doi: 10.4161/hv.28742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Al Moustafa A-E. Role of high-risk human papillomaviruses in breast carcinogenesis. In: Gupta S, editor. Breast carcinogenesis; Oncoviruses and their inhibitors. Boca Raton, Florida: CRC: Taylor and Francis Group; 2014. p. 245–62. [Google Scholar]
- 32.de Cremoux P, Thioux M, Lebigot I, Sigal-Zafrani B, Salmon R, Sastre-Garau X. No evidence of human papillomavirus DNA sequences in invasive breast carcinoma. Breast Cancer Res Treat. 2008;109(1):55–58. doi: 10.1007/s10549-007-9626-4. [DOI] [PubMed] [Google Scholar]
- 33.Hedau S, Kumar U, Hussain S, Shukla S, Pande S, Jain N, Tyagi A, Deshpande T, Bhat D, Mir MM, et al. Breast cancer and human papillomavirus infection: no evidence of HPV etiology of breast cancer in Indian women. BMC Cancer. 2011;11(1):27. doi: 10.1186/1471-2407-11-27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Brumbaugh J, Ferris RL, Hu S. HPV and EBV in head and neck cancer. In: Bernier J, editor. Head and neck cancer: multimodality management. Cham, Switzerland: Springer International Publishing; 2016. p. 163–79. [Google Scholar]
- 35.Malki MI, Gupta I, Fernandes Q, Aboulkassim T, Yasmeen A, Vranic S, Al Moustafa A-E, Al-Thawadi HA. Co-presence of Epstein-Barr virus and high-risk human papillomaviruses in Syrian colorectal cancer samples. Hum Vaccin Immunother. 2020:1–5. doi: 10.1080/21645515.2020.1726680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Al-Thawadi H, Gupta I, Jabeen A, Skenderi F, Aboulkassim T, Yasmeen A, Malki MI, Batist G, Vranic S, Al Moustafa A-E. Co-presence of human papillomaviruses and Epstein–Barr virus is linked with advanced tumor stage: a tissue microarray study in head and neck cancer patients. Cancer Cell Int. 2020;20:1–13. doi: 10.1186/s12935-020-01348-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Elston CW, Ellis IO. Pathological prognostic factors in breast cancer. I. The value of histological grade in breast cancer: experience from a large study with long-term follow-up. Histopathology. 1991;19(5):403–10. doi: 10.1111/j.1365-2559.1991.tb00229.x. [DOI] [PubMed] [Google Scholar]
- 38.Allison KH, Hammond MEH, Dowsett M, McKernin SE, Carey LA, Fitzgibbons PL, Hayes DF, Lakhani SR, Chavez-MacGregor M, Perlmutter J, et al. Estrogen and progesterone receptor testing in breast cancer: ASCO/CAP guideline update. J Clin Oncol. 2020;38(12):1346–66. doi: 10.1200/jco.19.02309. [DOI] [PubMed] [Google Scholar]
- 39.Wolff AC, Hammond MEH, Allison KH, Harvey BE, Mangu PB, Bartlett JMS, Bilous M, Ellis IO, Fitzgibbons P, Hanna W, et al. Human epidermal growth factor receptor 2 testing in breast cancer: American society of clinical oncology/college of American pathologists clinical practice guideline focused update. Arch Pathol Lab Med. 2018;142(11):1364–82. doi: 10.5858/arpa.2018-0902-SA. [DOI] [PubMed] [Google Scholar]
- 40.Gupta I, Nasrallah GK, Sharma A, Jabeen A, Smatti MK, Al-Thawadi HA, Sultan AA, Alkhalaf M, Vranic S, Moustafa AA. Co-prevalence of human papillomaviruses (HPV) and Epstein-Barr virus (EBV) in healthy blood donors from diverse nationalities in Qatar. Cancer Cell Int. 2020;20:107–107. doi: 10.1186/s12935-020-01190-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Gupta I, Al Farsi H, Jabeen A, Skenderi F, Al-Thawadi H, AlAhmad YM, Abdelhafez I, Al Moustafa A-E, Vranic S. High-risk human papillomaviruses and Epstein–Barr virus in colorectal cancer and their association with clinicopathological status. Pathogens. 2020;9(6):452. doi: 10.3390/pathogens9060452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Heng B, Glenn WK, Ye Y, Tran B, Delprado W, Lutze-Mann L, Whitaker NJ, Lawson JS. Human papilloma virus is associated with breast cancer. Br J Cancer. 2009;101(8):1345–50. doi: 10.1038/sj.bjc.6605282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Xiao C-Y, Fu -B-B, Li Z-Y, Mushtaq G, Kamal MA, Li J-H, Tang G-C, Xiao -S-S. Observations on the expression of human papillomavirus major capsid protein in HeLa cells. Cancer Cell Int. 2015;15:53–53. doi: 10.1186/s12935-015-0206-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Zur Hausen H. Papillomaviruses in the causation of human cancers — a brief historical account. Virology. 2009;384(2):260–65. doi: 10.1016/j.virol.2008.11.046. [DOI] [PubMed] [Google Scholar]
- 45.Khodabandehlou N, Mostafaei S, Etemadi A, Ghasemi A, Payandeh M, Hadifar S, Norooznezhad AH, Kazemnejad A, Moghoofei M. Human papilloma virus and breast cancer: the role of inflammation and viral expressed proteins. BMC Cancer. 2019;19(1):61. doi: 10.1186/s12885-019-5286-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Herrera-Goepfert R, Vela-Chávez T, Carrillo-García A, Lizano-Soberón M, Amador-Molina A, Oñate-Ocaña LF, Hallmann RSR. High-risk human papillomavirus (HPV) DNA sequences in metaplastic breast carcinomas of Mexican women. BMC Cancer. 2013;13(1):445. doi: 10.1186/1471-2407-13-445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Khan NA, Castillo A, Koriyama C, Kijima Y, Umekita Y, Ohi Y, Higashi M, Sagara Y, Yoshinaka H, Tsuji T, et al. Human papillomavirus detected in female breast carcinomas in Japan. Br J Cancer. 2008;99(3):408–14. doi: 10.1038/sj.bjc.6604502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Fernandes A, Bianchi G, Feltri AP, Pérez M, Correnti M. Presence of human papillomavirus in breast cancer and its association with prognostic factors. Ecancermedicalscience. 2015;9:548. doi: 10.3332/ecancer.2015.548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Gumus M, Yumuk PF, Salepci T, Aliustaoglu M, Dane F, Ekenel M, Basaran G, Kaya H, Barisik N, Turhal NS, et al. HPV DNA frequency and subset analysis in human breast cancer patients’ normal and tumoral tissue samples. J Exp Clin Cancer Res. 2006. January 22;25(4):515–21. [PubMed] [Google Scholar]
- 50.Ali SH, Al-Alwan NA, Al-Alwany SH. Detection and genotyping of human papillomavirus in breast cancer tissues from Iraqi patients. East Mediterr Health J. 2014. June 25;20(6):372–77. doi: 10.26719/2014.20.6.372. [DOI] [PubMed] [Google Scholar]
- 51.Sigaroodi A, Nadji SA, Naghshvar F, Nategh R, Emami H, Velayati AA. Human papillomavirus is associated with breast cancer in the north part of Iran. Sci World J. 2012;2012:837191–837191. doi: 10.1100/2012/837191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Ahangar-Oskouee M, Shahmahmoodi S, Jalilvand S, Mahmoodi M, Ziaee AA, Esmaeili H-A, Keshtvarz M, Pishraft-Sabet L, Yousefi M, Mollaei-Kandelous Y, et al. No detection of ‘high-risk’ human papillomaviruses in a group of Iranian women with breast cancer. Asian Pac J Cancer Prev. 2014;15(9):4061–65. doi: 10.7314/apjcp.2014.15.9.4061. [DOI] [PubMed] [Google Scholar]
- 53.Manzouri L, Salehi R, Shariatpanahi S, Rezaie P. Prevalence of human papilloma virus among women with breast cancer since 2005–2009 in Isfahan. Adv Biomed Res. 2014;3:75. doi: 10.4103/2277-9175.125873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Elmi A, Bansal D, Al-Thani A, Al-Ansari A, Mohamed N, Sultan A. Molecular epidemiology of human papillomavirus among Arab women in Qatar. Qatar Foundation Annual Research Forum Proceedings. 2012;2012(1). doi: 10.5339/qfarf.2012.BMP6. [DOI] [Google Scholar]
- 55.Bansal D, Elmi AA, Skariah S, Haddad P, Abu-Raddad LJ, Al Hamadi AH, Mohamed-Nady N, Affifi NM, Ghedira R, Hassen E, et al. Molecular epidemiology and genotype distribution of human papillomavirus (HPV) among Arab women in the state of Qatar. J Transl Med. 2014;12(1):300. doi: 10.1186/s12967-014-0300-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Moosa K, Alsayyad AS, Quint W, Gopala K, DeAntonio R. An epidemiological study assessing the prevalence of human papillomavirus types in women in the Kingdom of Bahrain. BMC Cancer. 2014;14:905–905. doi: 10.1186/1471-2407-14-905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.AlObaid A, Al-Badawi IA, Al-Kadri H, Gopala K, Kandeil W, Quint W, Al-Aker M, DeAntonio R. Human papillomavirus prevalence and type distribution among women attending routine gynecological examinations in Saudi Arabia. BMC Infect Dis. 2014;14(1):643. doi: 10.1186/s12879-014-0643-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Al-Awadhi R, Chehadeh W, Jaragh M, Al-Shaheen A, Sharma P, Kapila K. Distribution of human papillomavirus among women with abnormal cervical cytology in Kuwait. Diagn Cytopathol. 2013;41(2):107–14. doi: 10.1002/dc.21778. [DOI] [PubMed] [Google Scholar]
- 59.Yu Y, Morimoto T, Sasa M, Okazaki K, Harada Y, Fujiwara T, Irie Y, Takahashi E-I, Tanigami A, Izumi K, et al. Human papillomavirus type 33 DNA in breast cancer in Chinese. Breast Cancer. 2000;7(1):33–36. doi: 10.1007/BF02967185. [DOI] [PubMed] [Google Scholar]
- 60.Lawson JS, Glenn WK, Salyakina D, Delprado W, Clay R, Antonsson A, Heng B, Miyauchi S, Tran DD, Ngan CC, et al. Human papilloma viruses and breast cancer. Front Oncol. 2015;5:277–277. doi: 10.3389/fonc.2015.00277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Wang T, Chang P, Wang L, Yao Q, Guo W, Chen J, Yan T, Cao C. The role of human papillomavirus infection in breast cancer. Med Oncol. 2012;29(1):48–55. doi: 10.1007/s12032-010-9812-9. [DOI] [PubMed] [Google Scholar]
- 62.De Carolis S, Storci G, Ceccarelli C, Savini C, Gallucci L, Sansone P, Santini D, Seracchioli R, Taffurelli M, Fabbri F, et al. HPV DNA associates with breast cancer malignancy and it is transferred to breast cancer stromal cells by extracellular vesicles. Front Oncol. 2019;9(860). doi: 10.3389/fonc.2019.00860 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Corbex M, Bouzbid S, Traverse-Glehen A, Aouras H, McKay-Chopin S, Carreira C, Lankar A, Tommasino M, Gheit T. Prevalence of papillomaviruses, polyomaviruses, and herpesviruses in triple-negative and inflammatory breast tumors from algeria compared with other types of breast cancer tumors. Plos One. 2014;9(12):e114559. doi: 10.1371/journal.pone.0114559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Piana AF, Sotgiu G, Muroni MR, Cossu-Rocca P, Castiglia P, De Miglio MR. HPV infection and triple-negative breast cancers: an Italian case-control study. Virol J. 2014;11(1):190. doi: 10.1186/s12985-014-0190-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Glenn WK, Heng B, Delprado W, Iacopetta B, Whitaker NJ, Lawson JS. Epstein-Barr virus, human papillomavirus and mouse mammary tumour virus as multiple viruses in breast cancer. PloS One. 2012;7(11):e48788–e48788. doi: 10.1371/journal.pone.0048788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Joshi D, Quadri M, Gangane N, Joshi R, Gangane N. Association of Epstein Barr virus infection (EBV) with breast cancer in rural Indian women. PloS One. 2009;4(12):e8180–e8180. doi: 10.1371/journal.pone.0008180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Lorenzetti MA, De Matteo E, Gass H, Martinez Vazquez P, Lara J, Gonzalez P, Preciado MV, Chabay PA. Characterization of Epstein Barr virus latency pattern in Argentine breast carcinoma. PloS One. 2010;5(10):e13603–e13603. doi: 10.1371/journal.pone.0013603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Fawzy S, Sallam M, Awad NM. Detection of Epstein-Barr virus in breast carcinoma in Egyptian women. Clin Biochem. 2008;41(7–8):486–92. doi: 10.1016/j.clinbiochem.2007.12.017. [DOI] [PubMed] [Google Scholar]
- 69.Xue SA, Lampert IA, Haldane JS, Bridger JE, Griffin BE. Epstein-Barr virus gene expression in human breast cancer: protagonist or passenger? Br J Cancer. 2003;89(1):113–19. doi: 10.1038/sj.bjc.6601027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Kalkan A, Ozdarendeli A, Bulut Y, Yekeler H, Cobanoglu B, Doymaz MZ. Investigation of Epstein-Barr virus DNA in formalin-fixed and paraffin- embedded breast cancer tissues. Med Princ Pract. 2005;14(4):268–71. doi: 10.1159/000085748. [DOI] [PubMed] [Google Scholar]
- 71.Aboulkassim T, Yasmeen A, Akil N, Batist G, Al Moustafa A-E. Incidence of Epstein-Barr virus in Syrian women with breast cancer: A tissue microarray study. Hum Vaccin Immunother. 2015;11(4):951–55. doi: 10.1080/21645515.2015.1009342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Zekri A-RN, Bahnassy AA, Mohamed WS, El-Kassem FA, El-Khalidi SJ, Hafez MM, Hassan ZK. Epstein-Barr virus and breast cancer: epidemiological and molecular study on Egyptian and Iraqi women. J Egypt Natl Cancer Inst. 2012;24(3):123–31. doi: 10.1016/j.jnci.2012.06.001. [DOI] [PubMed] [Google Scholar]
- 73.Hachana M, Amara K, Ziadi S, Romdhane E, Gacem RB, Trimeche M. Investigation of Epstein-Barr virus in breast carcinomas in Tunisia. Pathol Res Pract. 2011;207(11):695–700. doi: 10.1016/j.prp.2011.09.007. [DOI] [PubMed] [Google Scholar]
- 74.Sharifpour C, Makvandi M, Samarbafzadeh A, Talaei-Zadeh A, Ranjbari N, Nisi N, Azaran A, Jalilian S, Varnaseri M, Pirmoradi R, et al. Frequency of Epstein–Barr virus DNA in formalin-fixed paraffin-embedded tissue of patients with ductal breast carcinoma. Asian Pac J Cancer Prev. 2019;20(3):687–92. doi: 10.31557/apjcp.2019.20.3.687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Farahmand M, Monavari SH, Shoja Z, Ghaffari H, Tavakoli M, Tavakoli A. Epstein-Barr virus and risk of breast cancer: a systematic review and meta-analysis. Future Oncol. 2019;15(24):2873–85. doi: 10.2217/fon-2019-0232. [DOI] [PubMed] [Google Scholar]
- 76.Lawson JS, Glenn WK. Multiple oncogenic viruses are present in human breast tissues before development of virus associated breast cancer. Infect Agent Cancer. 2017;12(1):55. doi: 10.1186/s13027-017-0165-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Naushad W, Surriya O, Sadia H. Prevalence of EBV, HPV and MMTV in Pakistani breast cancer patients: A possible etiological role of viruses in breast cancer. Infec Genet Evol. 2017;54:230–37. doi: 10.1016/j.meegid.2017.07.010. [DOI] [PubMed] [Google Scholar]
- 78.Fina F, Romain S, Ouafik L, Palmari J, Ayed FB, Benharkat S, Bonnier P, Spyratos F, Foekens JA, Rose C, et al. Frequency and genome load of Epstein-Barr virus in 509 breast cancers from different geographical areas. Br J Cancer. 2001;84(6):783–90. doi: 10.1054/bjoc.2000.1672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Horakova D, Bouchalova K, Cwiertka K, Stepanek L, Vlckova J, Kollarova H. Risks and protective factors for triple negative breast cancer with a focus on micronutrients and infections. Biomedical Papers. 2018;162(2):83–89. doi: 10.5507/bp.2018.014. [DOI] [PubMed] [Google Scholar]
- 80.Corbex M, Bouzbid S, Boffetta P. Features of breast cancer in developing countries, examples from North-Africa. Eur J Cancer. 2014;50(10):1808–18. doi: 10.1016/j.ejca.2014.03.016. [DOI] [PubMed] [Google Scholar]
- 81.Lawson JS, Salmons B, Glenn WK. Oncogenic viruses and breast cancer: mouse mammary tumor virus (MMTV), bovine leukemia virus (BLV), human papilloma virus (HPV), and Epstein-Barr virus (EBV). Front Oncol. 2018;8:1–1. doi: 10.3389/fonc.2018.00001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Aguayo F, Khan N, Koriyama C, González C, Ampuero S, Padilla O, Solís L, Eizuru Y, Corvalán A, Akiba S, et al. Human papillomavirus and Epstein-Barr virus infections in breast cancer from chile. Infect Agent Cancer. 2011;6(1):7–7. doi: 10.1186/1750-9378-6-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Gupta I, Al Farsi H, Jabeen A, Skendri F, Al-Thawadi H, AlAhmad YM, Al Moustafa AE, Vranic S. High-risk human papillomaviruses and Epstein-Barr virus in colorectal cancer and their association with clinicopathological status. Pathogens. 2020;9:(In Press). doi: 10.3390/pathogens9060452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Gupta I, Jabeen A, Skenderi F, Malki MI, Al-Thawadi H, Al Moustafa AE, Vranic S. High-risk human papillomaviruses (HPV) and Epstein - Barr virus (EBV) are commonly present in rectal cancer. Mod Pathol. 2020;33:676.31673084 [Google Scholar]
- 85.Gupta I, Ghabreau L, Al-Thawadi H, Yasmeen A, Vranic S, Al Moustafa AE, Malki MI. Co-incidence of human papillomaviruses and Epstein-Barr virus is associated with high to intermediate tumor grade in human head and neck cancer in Syria. Front Oncol. 2020. doi: 10.3389/fonc.2020.01016. [DOI] [PMC free article] [PubMed] [Google Scholar]


