ABSTRACT
Tertiary lymphoid structures (TLS) are ectopic cellular aggregates that resemble secondary lymphoid organs in their composition and structural organization. In contrast to secondary lymphoid organs, TLS are not imprinted during embryogenesis but are formed in non-lymphoid tissues in response to local inflammation. TLS structures exhibiting a variable degree of maturation are found in solid tumors. They are composed of various immune cell types including dendritic cells and antigen-specific B and T lymphocytes, that together, actively drive the immune response against tumor development and progression. This review highlights the successive steps leading to tumor TLS formation and its association with clinical outcomes. We discuss the role played by tumor-infiltrating B lymphocytes and plasma cells, their prognostic value in solid tumors and immunotherapeutic responses and their potential for future targeting.
KEYWORDS: Tertiary lymphoid structures, b lymphocytes, plasma cells, cancer, immunotherapy
Introduction
The composition and quality of tumor infiltrating immune cells directly dictates patient’s outcomes and therapeutic efficiencies.1 Strong anti-tumor immune responses are achieved through the interplay between innate and adaptive immune cells which drive the expansion and activation of tumor antigen-specific cytotoxic T cells and the production of antibodies by plasmablasts and plasma cells (collectively termed antibody-secreting cells (ASCs)).2 However, the latter has not been extensively investigated in the tumor context until recently.3–5 At steady-state, secondary lymphoid organs (SLO) act as central hubs where dynamic immune cell interactions can occur continuously between sentinel cells, such as dendritic cells, which migrate to the lymph nodes through dedicated vessels, called high endothelial venules (HEV),6 and lymph node-resident immune lymphocytes (B and T cells). These innate–adaptive immune cell interactions allow screening of the body surfaces for detection of, and appropriate response to, potential immune threats. The coordination of optimal cell positioning to enable these interactions depends on chemokines that control the migratory patterns of these cells.7 When a threat is detected, such as an epithelial cell transformation that may lead to tumor development, the local immune response may be insufficient, and the generation of an efficient adaptive immune response then relies on the capacity of activated dendritic cells to migrate to the closest draining lymph node and to present MHC-antigen-derived peptide complexes to CD4+ and CD8+ T cells leading to antigen-specific T cell expansion and activation.8 In addition, the production of antibodies results from the activation of antigen-specific B cells following cognate interactions with CD4+ T cells to help drive B cell expansion, germinal center formation and ASC differentiation.9–11
In cancer, these effector cells then egress the regional lymph node to infiltrate the tumor microenvironment where they can pinpoint and eradicate cancer cells. Several groups have revealed that, in addition to this pathway, in some tumors, an adaptive immune response is generated in situ that mirrors the pattern normally associated with SLO. This happens within spatially well-organized structures called tertiary lymphoid structures (TLS). De novo TLS formation in tumors requires optimal cytokine and chemokine concentration and specialized immune cell types.12 TLS formation can occur at both the margins and in the core of tumors. Similar to SLOs, mature TLS are composed of T and B cell zones and germinal centers. These compartmentalized structures contain innate immune cells and adaptive lymphocytes and may include dendritic cells, neutrophils, macrophages, helper CD4+ and cytotoxic CD8+ T lymphocytes, B cells, plasmablasts and plasma cells.11,13 In addition, HEV often colocalizes with TLS in tumors allowing the initial immune cell recruitment, but also the egress of activated immune cells from the TLS to the circulation.14,15
Our current understanding of the local anti-tumor immune response is still fairly limited. For example, in melanoma, it is not unusual to observe partial or even complete spontaneous regression of primary tumors indicative of a potent ongoing endogenous anti-tumor immune response. However, regression of metastatic melanoma lesions is extremely rare, suggesting that the emergence of antigen-loss tumor variants that have invaded distant organs overwhelms the initial anti-tumor immune response.16 Thus, we can ask how is such a potent outcome achieved early during tumor formation? What are the local cellular and molecular events that lead to primary tumor eradication? Does a tumor disappearance correlate with specific structures, such as TLS, that are engendered by tumor development? Furthermore, what are the exact mechanisms involved? and, most importantly, can we increase or induce effective anti-tumor immune responses early in a response to drive stronger, or complete, tumor eradication before the spread of disease to distant organs? Successful cooperation between tumor-infiltrating innate and adaptive immune cells within TLS is certainly key to fruitful anti-tumor responses. Such outcomes appear likely to be achievable in the near future given our increased understanding of local and systemic immune responses through the use of cutting-edge technologies, sophisticated models and the development of innovative immunotherapies.
In this review, we explore the cellular and molecular requirements for TLS formation and highlight the role and impact of these ectopic lymphoid structures in cancer. As tumor B lymphocyte infiltration is closely linked to the presence of intra-tumoral TLS, we further detail the prognostic and therapeutic predictive value of B cells and ASCs in tumors, particularly in light with the latest findings in melanoma, renal cell carcinoma and sarcoma tumors.3–5 We will discuss the possibility to further enhance anti-tumor immune responses by increasing TLS formation and targeting B cells and the antibody response in tumors.
1. Tertiary lymphoid structures – formation and composition
TLS formation – paralleling SLO development
TLS formation necessitates the cooperation of stromal cells with innate and adaptive immune cells, that together are positioned within the tissues to induce TLS neogenesis.12 Similar to SLO formation, TLS generation might requires the local accumulation of CXCL13, RANKL and interleukin(IL)-7, which together recruit and activate lymphoid tissue-inducer cells (LTi)17–20 (Figure 1). LTi cells interact with stromal cells through the pairing of lymphotoxin (LT) α1β2, expressed on the surface of LTi cells, with its receptor (LTβR) expressed on stromal cells. Interleukin (IL)-17 production by LTi cells21 together with LTα1β2-LTR interaction induce de novo secretion of chemokines, angiogenic growth factors and the expression of adhesive molecules by IL-17R+ stromal cells.22 The expression of some of these factors is further amplified by additional interactions with other cell types, such as dendritic cells, CD8+ T cells or natural killer (NK) cells, culminating in the secretion of VEGFA, VEGFC, essential for HEV formation, the production of CXCL12, CXCL13, CCL19 and CCL21, for immune cell recruitment and the expression of ICAM1, VCAM1 and MADCAM1 for cell retention18,23–30 (Figure 1). Of note, mice deficient for Rorγt (which fail to develop LTi cells), Cxcl13 and IL-7 Rα (Cxc13−/- × Il7ra−/- mice) or lymphotoxin α (Lta−/- mice) expression, lack SLO formation, 20,31,32 although some lymphoid tissues such as the nasal-associated tissue (NALT)33 or the tear duct-associated lymphoid tissue (TALT)34 develop in the absence of Rorγt, LTβR or IL-7 R signaling. Collectively, these studies demonstrate that while SLO formation relies on critical cellular and molecular pathways during embryogenesis for their formation, post-natal development of lymphoid structures such as NALT or TALT does not follow these rules. This raises the question whether TLS development, which occurs after birth in chronic inflammatory responses, also depends on these pathways or alternatively, may use different cellular and molecular mechanisms for their initiation and formation.
Figure 1.
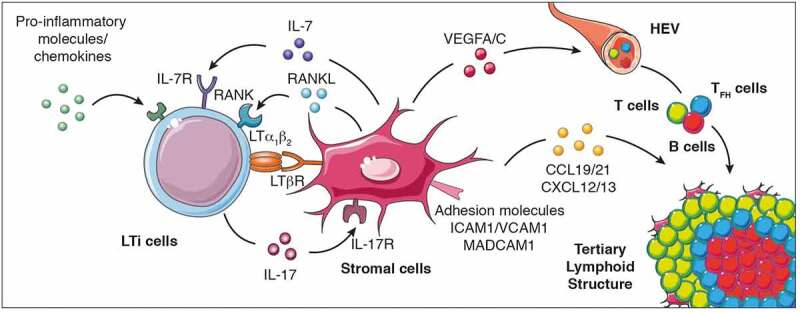
Cellular and molecular signals that control TLS formation. The local accumulation of pro-inflammatory molecules and chemokines promotes the recruitment of LTi cells to the inflammed site promoting their interaction with stromal cells to initiate TLS genesis and cytokine (IL-7, IL-17, RANKL, and LTα1β2) and cytokine receptor (IL-7R, IL-17R, RANK and LTβR) expression. When LTi cells are absent, other immune cells such as macrophages, B lymphocytes and Th17 cells can also interact with stromal cells to induce TLS formation. This interaction culminates in the production of chemokines (CCL19, CCL21, CXCL12 and CXCL13), pro-angiogenic molecules (vascular endothelial growth factors VEGFA and VEGFC) and the expression of adhesion molecules which facilitate the recruitment of additional immune cell types, their retention and organization into the nascent TLS. LTi cells, lymphoid tissue-inducer cells; Th17 cells, T helper cells secreting IL-17; LT, lymphotoxin; RANK, receptor activator of nuclear factor-κB; ICAM, intercellular adhesion molecule 1; VCAM1, Vascular adhesion molecule 1; MADCAM, mucosal vascular addressin cell adhesion molecule 1; VEGFC, vascular endothelial growth factor A/C; IL, interleukin; CCL19: C-C motif chemokine ligand 19; CXCL13, C-X-C motif chemokine ligand 13; HEV, high endothelial venules
Is SLO the right model?
In various inflammatory contexts, B cells,35 macrophages36 or IL-17 expressing T cells37 can induce de novo TLS formation even when mice are deficient for LTi cells and have defective SLO. In mucosal tissues such as the gut, a fine balance between pro- and anti-inflammatory immune cells and signaling molecules is necessary to control microbiota diversity and composition and to maintain tissue homeostasis. Rorγt-deficient mice have impaired intestinal SLO development resulting in the absence of mesenteric lymph nodes and Peyer’s and colonic patches, but also have defective cryptopatches and isolated lymphoid follicles.32,38 While these lymphoid structures are critical to mount appropriate immune responses, LTi-deficient mice are still able to preserve their barrier integrity and to maintain intestinal homeostasis, as TLS development occurs in the colon of Rorγt-deficient mice during inflammation and seems to be dependent on the gut microbiota.39 Similarly, inducible bronchus-associated lymphoid tissue (iBALT) develops in lungs after LPS exposure or influenza-infection in mice lacking SLO.37,40 In influenza-infected Lta−/- mice, local CXCL13 and CCL21 expression colocalizes with the B cell-rich zone and PNAd-expressing HEVs, respectively, in iBALT.40 Similarly, Rorc−/− and Id2−/- mice exposed to LPS and infected with influenza also form iBALT.37 Together, these results demonstrate that local inflammation is a principal trigger of TLS formation, and similar mechanisms are likely to occur in tumors.41 These vascular structures together with local chemotactic factors allow the recruitment and accumulation of B and T cells sustaining the initial formation and the assembly of the nascent TLS.25,42 Overall, while a parallel between SLO and TLS formation occurs, additional molecular and cellular events principally dependent on sustained local inflammation trigger TLS neogenesis.
Temporal development and recruitment of cells in TLS
The temporal development and recruitment of cells to intratumoral TLS are, to date, largely unknown. Recently, Meylan and colleagues examined TLS formation in preneoplastic hepatic lesions from cirrhotic livers to determine whether TLS neogenesis was induced in early hepatic lesions and its associated immune profile.43 This group found that a quarter of these preneoplastic hepatic lesions display TLS in cirrhotic nodules.43 The presence of these structures was associated with increased densities of T cells, B cells and mature dendritic cells but lack CD21+ follicular dendritic cells, indicating that most TLS consisted of immature lymphocytic aggregates rather than fully developed follicles.43 They further identified the presence of immunosuppressive genes in lesions where TLS were found suggesting that inhibitory signals that emerge within cirrhotic nodules potentially inhibit ongoing immune responses.43 However, for ethical and practical reasons, it was impossible to correlate patient clinical outcomes with the presence of these TLS in preneoplastic hepatic lesions. Nevertheless, this study has been critical in pinpointing that TLS formation is likely to occur very early during tumor development, in parallel with the early development of inflammation.
TLS maturation, composition and diversity
TLS composition encompasses both innate and adaptive immune cells that are surrounded by HEV. Using immunohistochemistry, early studies in melanoma44 and non-small lung cancer45 found tumor-associated structures resembling SLO that are composed of T cells (CD3+), mature DCs (DC-LAMP+) and follicular B cells (CD20+) (Figure 2). The immune cell composition of these structures differs, ranging from disorganized cellular aggregates, often referred as immature or early TLS, to well-organized and structured organs containing follicles, mirroring SLO and populated by germinal centers and tumor antigen-specific T and B cells (Figure 2). Recent analyses used additional markers to better characterize the immune composition of TLS and follicles. Studies identified proliferative (Ki67+) germinal center B cells (CD23+) expressing AID, involved in somatic hypermutations and class switch recombination, as well as Bcl6, a transcription factor involved in germinal center B cell maturation (Figure 2).46–48 In addition, follicular dendritic cells (CD21+ or CD23+) were detected within germinal centers with macrophages (CD68+), CD4+ and CD8+ T cells (CD3+), follicular CD4+ T cells (Bcl6+PD-1+ICOS+IL-21+) and plasma cells (CD38+CD138+) often surrounding germinal centers indicative of an ongoing humoral and cytotoxic immune responses46,49 (Figure 2).
Figure 2.
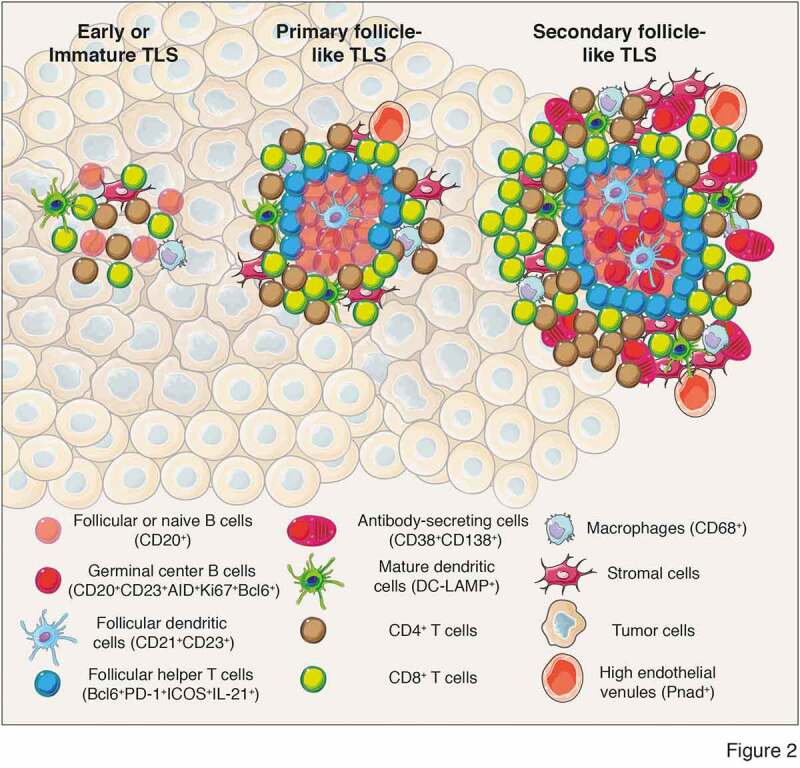
Different levels of TLS maturation and their composition. Tumor-associated TLS are heterogenous and range from poorly-organized cellular aggregates (Early or immature TLS) to well-organized structures forming primary follicles or secondary follicles containing germinal centers surrounded by specific vessels called high endothelial venules (PNad+). Their cellular composition include stromal cells, innate and adaptive immune cells. In most of the analyses performed, cellular composition has been determined using immunofluorescence or immunochemistry analyses and relies on the expression of cell-specific markers to identify the cell types that form TLS. Mature secondary follicle-like TLS harbor a germinal center composed of proliferating mature germinal center B lymphocytes (CD20+CD23+AID+Ki67+Bcl6+) and follicular dendritic cells (CD21+CD23+) surrounded by naïve or follicular B cells (CD20+) and bordered by follicular helper T cells (Bcl6+PD-1+ICOS+IL-21+). In addition, TLS are formed of CD4+ and CD8+ T cells (CD3+), plasma cells (CD38+CD138+), mature dendritic cells (DC-LAMP+) and macrophages (CD68+). CD, cluster of differenciation; DC-LAMP, dendritic cell lysosomal associated membrane glycoprotein; PD-1, programmed cell death 1; ICOS, Inducible costimulator; AID, activation-induced deaminase; Bcl6, B cell lymphoma 6 protein; PNad, peripheral node addressin
2. Tertiary lymphoid structures in cancer – prognostic and predictive values
TLS have been detected in numerous tumor types using immunohistochemistry or chemokine gene signatures which tightly correlated with the presence of TLS identified by immunochemistry.41,50–52 In particular, a 12-chemokine gene signature was correlated to increased survival in melanoma,50 colorectal51,52 and breast53 cancers. In addition, other gene signatures containing 8 and 19 genes related to the presence of T follicular helper cells, type 1 helper CD4+ T cells and B cells were reported in breast54 and gastric55 cancers. Collectively, this has led to the detection of TLS in lung,41,45,56,57 oral squamous cell carcinoma,58 breast,54,59–61 colon,51,62 stomach,55,63,64 liver,65 sarcoma,3,66,67 bladder,68 clear cell renal cell carcinoma,4 ovarian cancer46,69 and melanoma4,5,15,44,50,70 (Table 1). TLS formation and densities vary between tumor types, and between patients. While the presence of TLS are largely associated with favorable outcomes, other studies do not see this positive association between TLS and patient prognosis (Table 1). These inconsistencies within a tumor type might be explained by TLS location (tumor core versus stroma or invasive margin), tumor stage (primary versus metastatic lesions), tumor subtype (e.g., highly or lowly mutated tumors), patient treatment history which may influence immune cell infiltration (e.g., immunogenic chemotherapy) or TLS immune cell composition diversity, particularly in T and B cell subsets71 (Table 1). In addition, several studies have reported the positive association between the presence of TLS and B lymphocytes, and therapy responses. Particularly, three seminal studies demonstrated the beneficial impact of TLS and B lymphocytes in melanoma, renal cell carcinoma and sarcoma tumors with response to immune checkpoint blockers.3–5 This observation has been recently extended to patients with advanced urothelial cancer who received combination of anti-PD-1 and anti-CTLA-4 antibodies before tumor resection.72 In this setting, while no correlation was observed between the presence of TLS at baseline and therapy response, all patients who experienced pathological complete responses had enriched TLS after treatment.72 Thus, TLS could be induced during immune checkpoint treatment and favored the generation of local anti-tumor immune responses.
Table 1.
The prognostic value of TLS in cancers
| Cancer type | Tumor subtype | Tumor localization | Treatment history | TLS localization | TLS composition | Prognostic value | Predictive value | Refs |
|---|---|---|---|---|---|---|---|---|
| Non-small cell lung | Adenocarcinoma, squamous cell carcinoma and large cell carcinoma | Primary tumors | N.A. | Ti-BALT | CD4+ and CD8+ T cells, FDC (CD21+), mature DC (DC-Lamp+) and Plasma cells (CD138+) | N.D. | N.A. | 41 |
| Non-small cell lung | Adenocarcinoma, squamous cell carcinoma | Primary tumors | Naive | Ti-BALT | B cells (CD20+), mature DC (DC-Lamp+), CD3+ T cells and FDC (CD21+) | Favorable (DC-Lamp high); No impact of other markers | N.A. | 45 |
| Non-small cell lung | Adenocarcinoma, squamous cell carcinoma and other subtypes | Primary tumors(2 cohorts) | Naive and neo-adjuvant chemotherapy | Ti-BALT | mature DC (DC-Lamp+), CD3+ T cells, macrophages (CD68+), B cells (CD20+) and plasma cells (CD138+) – germinal centers (CD23+IgD−AID+Bcl6+) | Favorable in both cohorts (DC-Lamp+ and CD20+) | N.A. | 56 |
| Non-small cell lung | Adenocarcinoma, squamous cell carcinoma and other subtypes | Primary tumors | Naive | Tumor | H&E staining – TLS and germinal centers – CD3+ T cells and B cells (CD20+) | Patients with higher numbers of TLS or B cells in their tumors have increased overall survival | N.A. | 57 |
| Oralsquamous cell carcinoma | Tongue, bucca, gingiva and other locations | Primary tumors | Naive | Tumor | H&E staining – TLS and germinal centers – CD3+ T cells, B cells (CD20+), mature DC (DC-Lamp+) and PNAd+ HEV like vessels | Favorable | N.A. | 58 |
| Breast | ER−HER2−; HER2+; ER+HER2+ | Primary tumors | 2 cohorts – one treated with neoadjuvant chemotherapy | Tumor | Tfh cell signature and CXCL13 gene expression | Favorable | Associated with a higher rate of pathological complete response | 54 |
| Breast | HER2+ | Primary tumors | Chemotherpay and trastuzumab | Peri-tumoral tissue | H&E staining – TLS in adjacent tissue and germinal centers | N.D. | No impact of TLS. Increased disease free-survival with moderate to abundant tumor infiltrating lymphocytes | 59 |
| Breast | ER, PR and HER2± | Primary tumors | Naive | Tumor and peri-tumoral tissue | CD3+ CD4+ and CD8+ T cells, B cells (CD20+), FDC (CD21+) – germinal centers (Bcl6+), PNAd+ HEV like vessels | Associated with aggressive tumors | N.A. | 60 |
| Breast | TNBC | Primary tumors | Naive | Tumor and peri-tumoral tissue | H&E staining – PNAd+ HEV like vessels | Favorable | N.A. | 61 |
| Colon | Left and right segments, rectum and sigmoid. MSI-H and MSS | Primary tumors | Naive and treated patients | Tumor and peri-tumoral tissue | CD3+ T cells, B cells (CD20+ and CD79a+), FDC (CD21+) and gene signature | Favorable | N.A. | 51 |
| Colon | Colon and rectum, MSI-H and MSS | Primary tumors | Naive | Invasive margin | CD3+ T cells, B cells (CD20+), FDC (CD21+), CCL21 and CXCL13, PNAd+ HEV like vessels and Lyve-1+ lymphatic vessels | Favorable for Stage II patients (without lymph node metastases). No impact for Stage II patients | N.A. | 62 |
| Stomach | Intestinal type (Lauren class) and H. Pylori ±. | Primary tumors | N.A. | Tumor | CD3+ T cells, B cells (CD20+) and gene signature (CXCL13, CCL19 and CCL21) | Associated with advanced disease | N.A. | 63 |
| Stomach | Intestinal, mixte and Signet ring cells | Primary tumors | Naive and neoadjuvant chemotherapy | Tumor and invasive margin | CD3+ T cells, B cells (CD20+), mature DC (DC-Lamp+) and PNAd+ HEV like vessels | Tbet CD20high associated with a favorable outcome | N.A. | 55 |
| Stomach | Differentiated and undifferentiated | Primary tumors | Naive | Tumor and invasive margin | CD3+ T cells, B cells (CD20+), plasma cells (CD138+), and FDC (CD21+). | Favorable | N.A. | 64 |
| Liver | Hepatocellular carcinoma | Primary tumors | Naive | Tumor | Hematein-eosin-saffron stained slides and genes signature | Presence of intr-tumoral TLS associated with a lower risk of early relapse | N.A. | 65 |
| Sarcoma | Six different histology types (DDLPS, LMS, UPS, Synovial sarcoma, Myxoid liposarcoma and GIST) | Primary tumors | N.A. | Tumor | Immunofluorescence and genes signature |
B cells and TLS are both associated with favorable outcomes | Patients in Class E (high density of B cells and presence of TLS) exhibited the highest response rate to PD-1 blockade treatment | 3 |
| Sarcoma | Three different histology types (UPS, ERMS and ARMS) | Primary tumors and metastases | Naive and pretreated patients | Tumor | CD3+ CD8+ and Foxp3+ T cells, CD163+ macrophages, CD31+ endothelial cells and B cells (CD20+) | No impact of T cell and macrophage densities or the presence of TLS with clinical outcomes | N.A. | 66 |
| Sarcoma | GIST | Primary tumors | Naive | Tumor | Multiplex immunohistochemistry | Presence of TLS associated with smaller tumor size, reduced relapse and increased survival | Presence of TLS are associated with reduced probability of future development of imatinib resistances | 67 |
| Kidney | Various histological subtypes | Primary tumors andmetastases | Naive and pretreated patients | Tumor | Genes signature | No impact of B cell-lineage | B cell signature presence of TLS associated with response to immunotherapy | 4 |
| Bladder | pT1 bladder cancer | Primary tumors | Naive | Tumor and peri-tumoral tisue | CD3+ and CD8+ T cells | Associated with advanced disease and poor prognosis | N.A. | 68 |
| Ovarian | High-grade serous ovarian cancer | Primary tumors | Naive | Tumor stroma | CD3+ and CD8+ T cells, CD20+ B cells, CD21+ FDC and CD38+CD138+CD79a+ plasma cells | High tumor-infiltration with plasma cells are associated with increased survival | N.A. | 46 |
| Ovarian | High-grade serous ovarian cancer | Primary tumors | Naive | Tumor stroma | B cells (CD20+) and mature DC (DC-Lamp+) | Favorable | N.A. | |
| Skin | Various histological subtypes | Metastases | Naive and pretreated patients | Tumor and peri-tumoral tissue | Immunofluorescence and genes signature | B cell-linage high tumors associated with favorable outcomes | B cell signature and presence of TLS associated with response to immunotherapy. | 4 |
| Skin | Various histological subtypes | Primary tumors and metastases | Naive and pretreated patients | Tumor and peri-tumoral tissue | CD3+ and CD8+ T cells, CD20+ B cells, Ki67+ proliferative cells | Favorable | TLS-derived genes signature from pretreatment samples are associated with increased survival in patients treated with CTLA-4 and PD-1 blockade therapies | 5 |
| Skin | Various histological subtypes | Primary tumors | Naive | N.A. | HEV detection (MECA-79+) | High HEV density in lower tumor stages | N.A. | 15 |
| Skin | N.A. | Metastases | Naive and pretreated patients | Tumor and peri-tumoral tissue | 12 genes signature and immunohistochemistry: CD3+ and CD8+ T cells, CD20+ B cells and CD86+ antigen presenting cells | Favorable | N.A. | 50 |
| Skin | Various histological subtypes | Primary tumors | Naive | Tumor and peri-tumoral tissues | OX40+ and CD25+ T cells, CD1a+ DC and mature DC (DC-Lamp+) | Favorable | N.A. | 44 |
| Skin | N.A. | Metastases | Pretreated patients | Tumor and peri-tumoral tissues | CD20+ B cells, CD138+ plasma cells, CD21+ FDC, MECA-79+ and Pnad+ HEV | N.A. | N.A. | 70 |
N.A., not available; Ti-BALT, tumor-induced bronchus-associated lymphoid tissue; FDC, follicular dendritic cells; MSI-H, colorectal cancer with high levels of microsatellite instability; MSS, colorectal cancer with low microsatellite instability; DDLPS, dedifferentiated liposarcoma; LMS, leiomyosarcoma; UPS, Undifferentiated pleomorphic sarcoma; GIST, gastrointestinal stromal tumors; EMRS, embryonal rhabdomyosarcomas; AMRS, alveolar rhabdomyosarcomas.
3. B lymphocytes take the center stage in anti-tumor immune responses
Unlike their T cell kin, the contribution of the B lymphocytes (B cells and their progeny, the ASC) to the antitumor immune response has only been acknowledged recently as their presence has been increasingly reported in a wide variety of tumors11 (Table 2). Unexpectedly, both beneficial and detrimental roles were described, and their exact function thus remains unclear (Table 2). This complexity stems from the failure to appreciate the diversity of the B cell lineage, and the often limited characterization of the tumor-associated B lymphocytes. Accumulating evidence now points to substantial heterogeneity among B lymphocytes found within the tumor infiltrates, particularly in TLS, both in terms of maturation status and effector functions. B lymphocytes are the sole producers of antibodies making them critical to humoral immunity, but they also influence other immune and nonimmune cell subsets through the production of various cytokines and cellular mediators in the tumor microenvironment. For example, they secrete interferon (IFN)-γ and IL-1293 which may promote cytotoxic CD8+ T cell responses. In contrast, particularly in tumors, B lymphocytes may produce immunosuppressive molecules triggering regulatory T cell development and differentiation of myeloid-derived suppressor cells (MDSCs).121,122 An emerging model posits that the divergent prognostic outcomes linked B cells could be reconciled by delineating subpopulations and clonality with pro- and anti-tumor roles.
Table 2.
The prognostic value of B lymphocytes in cancer
| Cancer type | Tumor subtype | Treatment history | Cell type | Identification markers | Prognostic value | Refs |
|---|---|---|---|---|---|---|
| Bladder cancer | Muscle-invasive bladder cancer | Adjuvant platinium based chemotherapy | B cells | CD19 | Favorable | 73 |
| Urothelial bladder cancer | IgA1 | Associated with advanced disease | 74 | |||
| Breast cancer | Primary operable invasive ductal breast cancer | B cells | CD20 | Neutral | 75 | |
| Favorable | 76 | |||||
| Plasma cells | CD138 | Negative | 75,77 | |||
| Invasive breast carcinoma | B cells | CD20 | Favorable | 78,79 | ||
| Primary triple- negative breast cancer | B cells | CD20 | Favorable | 80,81 | ||
| Stage II or III | Neoadjuvant chemotherapy | B cells | CD20 | Favorable | 82 | |
| Colorectal cancer | B cells | CD20 | Favorable | 83–86 | ||
| Plasma cells | CD138 | Favorable | 83 | |||
| Esophageal adenocarcinoma | Adenocarcinoma of esophagogastric junction | B cells | CD20 | Favorable | 87 | |
| Plasma cells | CD138 | Favorable | 87 | |||
| B cells | IgKC | Favorable | 88 | |||
| Plasma cells | CD138 | Favorable | 88 | |||
| Neoadjuvant chemo-radiotherapy | Plasma cells | IgG4 | Favorable when associated with ulcer | 89 | ||
| Gastric cancer | Plasma cells | CD138 | Favorable | 88 | ||
| B cells | CD20 | Favorable for disease free interval | 55,90 | |||
| Plasma cells | IgG4 | Negative | 91 | |||
| Hepatocellular carcinoma | B cells | CD20 | Favorable when associated with high CD3+ count | 92 | ||
| Favorable when located in tumor margin | 93,94 | |||||
| Plasma cells | IgA PDL1 | Negative, associated with advanced disease | 95 | |||
| Renal cell carcinoma | Renal clear cell carcinoma | B cells | CD20, RNAseq B cell signature | Negative | 96–98 | |
| Metastatic | Immune checkpoint therapy | RNAseq B cell signature | Favorable, associated with response to treatment | 4 | ||
| Non-small cell lung cancer | Lung adenocarcinoma | B cells | CD20, RNAseq B cell signature | Favorable | 96,98–101 | |
| Plasma cells | CD138 | Favorable | 99,100,102 | |||
| IgG | Favorable | 96,103 | ||||
| Squamous cell carcinoma | B cells | RNAseq activated/memory signature | Negative | 98,102 | ||
| IgG4 | Favorable | 104 | ||||
| Large cell carcinoma | Plasma cells | Negative | 102 | |||
| Melanoma | B cells | CD20, RNAseq B cell signature | Favorable | 96,98,105,106 | ||
| Stage III/IV | Neoadjuvant treatment with immune checkpoint blockade | B cells | RNAseq B cell signature | Favorable, associated with response to treatment | 4 | |
| Plasma cells | CD138 | Favorable | 105,107 | |||
| IgG | Favorable | 96,108 | ||||
| IgA | Negative | 108,109 | ||||
| Plasma cells | IgA | Negative | 107 | |||
| Mesothelioma | B cells | CD20 | Favorable | 110,111 | ||
| Serous ovarian cancer | Plasma cells | CD138 | Negative | 112 | ||
| B cell, IgG signature | Favorable | 113 | ||||
| High grade serous ovarian cancer | B cells | CD20 | Favorable | 114,115 | ||
| Pancreatic adenocarcinoma | B cells | CD20 | Favorable | 116,117 | ||
| Plasma cells | IgG4 | Negative | 118 | |||
| Prostate cancer | Metastatic or therapy-resistant | Plasma cells | IgA | Negative | 119 | |
| Soft tissue sarcoma | B cells | RNAseq B cell signature | Favorable | 3 | ||
| Squamous cell carcinoma | B cells | CD19 | Favorable | 120 |
B lymphocyte properties associated with anti-tumor responses
From the perspective of antitumor responses, the IgG1 antibody subclass exerts the most efficient effector activity. It fixes complement efficiently, possesses a high affinity to the Fcγ receptors expressed at the surface of myeloid and NK cells, and its binding triggers antibody-dependent cell cytotoxicity (ADCC), phagocytosis and tumor antigen uptake by antigen-presenting cells. Furthermore, IgG+ memory B cells can drive direct cytotoxic activity against tumor cells through the expression of TRAIL or the release of granzyme B93 (Figure 3). They also secrete IFN-γ, which drives Th1 and cytotoxic response by polarizing macrophages, CD4+ and CD8+ T cells (Figure 3). Hence, IgG+ ASCs and memory B cells make a valuable contribution to the antitumor response, and their presence is positively correlated to increased survival in patients with high-grade serious ovarian cancer,46,114 hepatocellular carcinoma,93 melanoma,105,123 KRAS-mutant lung carcinoma,103 pancreatic,124,125 gastric,64,126 breast80,113,127 and non-small cell lung cancers.96,103
Figure 3.
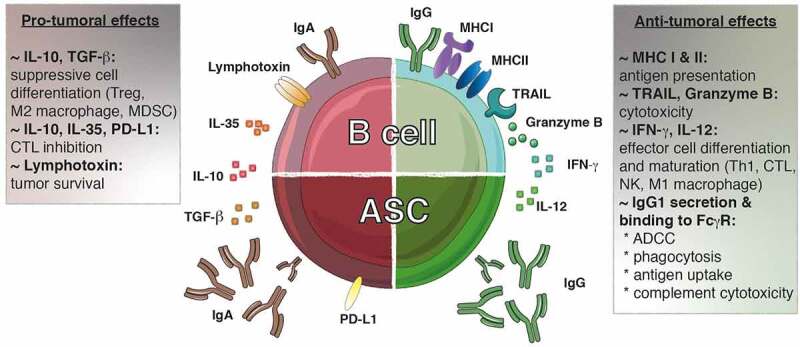
The dichotomy of tumor-infiltrating B lymphocytes. Both pro- and anti-tumoral roles can be attributed to B lymphocytes. IgG1+ B cells promote the anti-tumoral response by presenting antigens to T cells and secreting cytokines (IFN-γ, IL-12) that polarizing the response toward an optimal Th1/CTL composition. These B cells can exert direct cytotoxic functions through the expression of TRAIL and granzyme B. Furthermore, the IgG1 antibodies secreted by the ASC can bind to the FcγR at the surface of NK cells, macrophages and dendritic cells allowing induction of ADCC, phagocytosis and antigen uptake, respectively. Furthermore, IgG1 antibodies fix complement to trigger its cytotoxic cascade. In contrast, IgA+ cells are associated with the secretion of inhibitory cytokines (IL-10, IL-35, TGF-β) that create a suppressive environment favoring the emergence of Treg, M2 macrophages and MDSC while repressing the function of the effector cells. In addition, the expression of lymphotoxin by B cells supports tumor cell survival. ADCC, antibody-dependent cell-mediated cytotoxicity; ASC, antibody secreting cell; CTL, cytotoxic T lymphocyte; FcγR, Fc gamma receptor; Ig, immunoglobulin; IFN-γ, interferon gamma; MDSC, myeloid-derived suppressor cells; MHC: major histocompatibility complex; PD-L1: progammed cell death ligand 1; TGF-β, transforming growth factor-beta; TRAIL, tumor-necrosis-factor related apoptosis-inducing ligand; Treg, regulatory T cell
Features of pro-tumor or regulatory B lymphocytes
In contrast to the IgG antibody subclass, both IgA and IgE isotypes have been associated with poor prognosis for patients with hepatocellular carcinoma,95 melanoma,107 KRAS-mutant lung carcinoma,103 prostate119 and bladder74 cancers. These isotypes do not fix complement, or mediate the ADCC by NK cells. IgA in particular is a neutralizing antibody that does not trigger inflammatory responses. IgA+ ASCs, expressing suppressive molecules such as progammed cell death ligand 1 (also known as PD-L1) and IL-10, were described in both patient samples and mouse models of hepatocellular and prostate cancer.95,119 These cells inhibited the cytotoxic T cell response and were involved in resistance to chemotherapy and checkpoint inhibitor therapy. Like regulatory T cells, regulatory B cells can secrete IL-10, transforming growth factor (TGF)-β or IL-35 that will favor switching to IgA and the generation of new suppressive immune cells.121 In addition, the secretion of lymphotoxin by B cells was shown to favor tumor cell survival in prostate cancers128 (Figure 3). Although under different designations and defined by various markers, suppressive B lymphocytes have been described in a wide variety of tumor types where they hinder antitumor immune responses. These include hepatocellular carcinoma,129,130 gastric tumors, 131 squamous cell carcinoma,132melanoma133 and pancreatictumors.134
4. Strategies to enhance TLS formation and/or anti-tumor B cell accumulation in cancers
The modulation of TLS or TLS-forming immune cells such as B lymphocytes is an attractive option to (i) induce de novo local antitumor immunity in poorly immunogenic tumors, (ii) increase endogenous immune responses, and (iii) redirect a suppressive immune microenvironment toward effective antitumor immunity. Anti-cancer treatments, including chemo- and radio-therapy, or targeted therapies such as immune checkpoint inhibitors, all stimulate the immune system to fight cancer cells. In various cancer types, the presence of TLS has been associated with increased disease free- and overall survival of patients treated with immune checkpoint inhibitors,4,5,72,135,136 adjuvant trastuzumab (anti-HER2 antibody)59 or adjuvant and neoadjuvant chemotherapeutic regimens.81,137,138 These observations indicate that enhancing the formation, and/or maturation, of TLS in tumors could further augment therapy responses and increase the prognosis of cancer patients.
Several approaches have been successful in inducing TLS formation associated with anti-tumor immunity. Vaccination of HPV-driven cervical cancer patients against E6 and E7 proteins139 or pancreatic carcinoma patients using irradiated allogenic GM-CSF-secreting pancreatic tumor vaccine140 have been shown to induce TLS formation. In preclinical models, the overexpression of LTα under the rat insulin promotor in non-tumor bearing mice induced TLS formation in the pancreas and kidneys of transgenic animals.141 This was exacerbated in the pancreas of mice overexpressing both LTα and LTβ under the rat insulin promotor II22 and drove T cells, B cells and follicular dendritic cell recruitment which are structurally organized and mimic the configuration of SLO. More recently, the targeting of LIGHT, a member of the TNF superfamily which binds to the lymphotoxin receptor (encoded by TNFSF14), to tumor vessels via a vascular targeting peptide (LIGHT-VTP) was reported to induce TLS formation and vasculature remodeling in mouse tumors.142 Importantly, the combination of LIGHT-VTP with immune checkpoint inhibitors increased anti-tumor responses and survival in mice by inducing the recruitment of a large number of effector and memory T cells into tumors.142 Different approaches were also studied and notably, the injection of dendritic cells engineered to deliver the cytokine IL-36,143 or the chemokine CCL21,144–147 were both shown to promote intratumoral TLS formation.
The dichotomy between pro- and anti-tumor immune cell populations such as B and T lymphocytes suggests that patients could be better stratified based on the characterization of TLS-forming cells rather than simply assessing the presence or absence of TLS in tumors. One potential approach is the eradication of the suppressive B cells with an anti-CD20 antibody. However, the use of an anti-CD20 antibody in melanoma increased the development of melanoma metastases in lungs, and the growth of B16 primary melanoma tumors when injected subcutaneously.148–151 Thus, the tumor context matters, and such strategies need to be evaluated in every tumor type. ASCs, which no longer express CD20 are more complicated to target. Proteasome inhibitors, BAFF/APRIL antagonists (e.g., tacicept or tabalumab) or specific inhibitors of the pro-survival molecules Mcl1/Bcl2 all constitute logical strategies that need to be evaluated. However, for the later, the targeting would also need to be highly specific, depleting suppressive cells and leaving anti-tumor immune subsets including cytotoxic CD8+ T cells or NK cells intact. The use of such approaches could be combined with strategies to impair suppressive functions such as checkpoint inhibitors to target PD-L1. Alternately, it could be combined with engineered bifunctional fusion antibodies such Bintrafusp Alfa (also known as M7824) that simultaneously neutralizes PD-L1 and TGF-β and results in the activation of both innate and adaptive immune systems thereby conferring potent anti-tumor immunity152 and long-term anti-tumor protection153 Alternatively, the reduction of accessory suppressive populations, such as regulatory T cells in TLS154 could divert the microenvironment away from pro-tumor signals leading to tumor eradication.
Future perspectives and outstanding questions
Collectively, TLS and the associated T and B lymphocytes might serve as biomarkers useful to select patients who might better respond to immunotherapy. However, there are still many questions that remain to be answered before they can be incorporated into clinical practice as prognostic tools.155
Do immature and mature TLS differentially impact a patient’s prognosis?
It is still unclear whether the degree of TLS maturation impacts a patient’s prognosis or treatment efficacy. Indeed, whether immature and disorganized TLS with sparse cellular aggregates and no evidence of effective conventional adaptive immunity convey similar prognostic value as mature and structurally well-defined TLS harboring follicles and germinal centers remains unclear. Recently, Li and colleagues (2020) endeavored to examine this issue in oral squamous cell carcinoma. They found that the presence of TLS was associated with increased 5 years overall- and relapse-free survival, and importantly, both immature and mature TLS conveyed equally positive outcomes.58 In contrast, Posch et al. (2018) delineated that TLS in colorectal tumors exhibited different degrees of maturation which were associated with differential prognostic values.47 In particular, mature TLS containing germinal centers had a more positive prognostic outcome compared with immature TLS.47 Thus, evaluation of TLS maturation status in every tumor type would bring TLS into focus as an accurate prognostic tool for cancer treatment.
Can patient survival and response to treatment be predicted based on prospective evaluation of TLS ?
In most cases, the presence of TLS in tumors and their correlation with patient outcomes have been evaluated retrospectively. Given the consistent positive correlation of TLS with the anti-tumor immune response, prognosis and immunotherapeutic responses, prospective studies are warranted to determine the utility of measuring TLS presence, composition and density as prognostic tools or predictive markers of therapy efficacy.
Does TLS composition differently impact patient prognosis?
While the presence of TLS often positively impacts clinical outcomes, TLS composition itself might dictate treatment efficacy, tumor recurrence and patient survival. Indeed, Yamaguchi and colleagues156 classified TLS into five categories based on their immune cell composition and found that TLS enriched in helper T cells were associated with disease relapse in advanced colorectal cancer. Another example is the diversity found among ASC where IgA producing cells are almost exclusively associated with a poor prognosis, while IgG+ secreting cells frequently correlated with increased patient survival. Thus, better understanding of the composition of the immune infiltrate and function of TLS-forming cells, such as the isotype of ASC may be important.
Therapeutic intervention – can we specifically induce or enhance TLS formation in tumors?
Strategies augmenting de novo TLS formation in patient tumors could potentiate antitumor treatments leading to an increase in therapy response rates and patient progression- and overall-survival. While a number of preclinical studies have demonstrated the potential value of such treatments, additional studies and clinical trials are necessary to determine the therapeutic value conveyed by combining TLS- or B lymphocyte-specific targeting with current immunotherapeutic treatments.
The development of new technologies that enable the interrogation of more than 50 cellular markers simultaneously allows the precise characterization of the cellular composition, function and localization within tumors.157–159 Similar approaches could be used to investigate in detail TLS composition and function. Recently, Schurch and colleagues160 elegantly identified nine conserved distinct components characteristic of colorectal cancer immune microenvironment – described as ‘cellular neighbourhoods’ – which differentially impact a patient’s survival. The expansion of our capabilities to study a large number of parameters simultaneously might increase our understanding of the tumor microenvironment. This is likely to shed light on the cellular and molecular events associated with TLS formation and intratumoral T and B lymphocytes function associated with successful anti-tumor immunity and therapy responses.
Acknowledgments
This work was supported by grants (1122277 GTB, 1160830 SLN) and fellowships (1135898 GTB, 1155342 SLN) from the National Health and Medical Research Council (NHMRC), and support from the Cure Cancer Australia and Cancer Australia Cancer Australia Priority-driven Cancer Research Scheme (1163990 NJ). This work was supported through Victorian State Government Operational Infrastructure Support and Australian Government NHMRC IRIIS.
Funding Statement
This work was supported by the Cure Cancer Australia Foundation [1163990]; National Health and Medical Research Council [1135898]; National Health and Medical Research Council [1122277]; National Health and Medical Research Council [1160830]; National Health and Medical Research Council [1155342].
Disclosure of interests
The authors have no potential competing interests to disclose.
Disclosure statement
No potential conflict of interest was reported by the authors.
References
- 1.Fridman WH, Zitvogel L, Sautes-Fridman C, Kroemer G.. The immune contexture in cancer prognosis and treatment. Nat Rev Clin Oncol. 2017;14(12):717–16. doi: 10.1038/nrclinonc.2017.101. [DOI] [PubMed] [Google Scholar]
- 2.Vesely MD, Kershaw MH, Schreiber RD, Smyth MJ. Natural innate and adaptive immunity to cancer. Annu Rev Immunol. 2011;29(1):235–271. doi: 10.1146/annurev-immunol-031210-101324. [DOI] [PubMed] [Google Scholar]
- 3.Petitprez F. B cells are associated with survival and immunotherapy response in sarcoma. Nature. 2020;577(7791):556–560. doi: 10.1038/s41586-019-1906-8. [DOI] [PubMed] [Google Scholar]
- 4.Helmink BA. B cells and tertiary lymphoid structures promote immunotherapy response. Nature. 2020;577(7791):549–555. doi: 10.1038/s41586-019-1922-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Cabrita R. Tertiary lymphoid structures improve immunotherapy and survival in melanoma. Nature. 2020;577(7791):561–565. doi: 10.1038/s41586-019-1914-8. [DOI] [PubMed] [Google Scholar]
- 6.Girard JP, Moussion C, Forster R. HEVs, lymphatics and homeostatic immune cell trafficking in lymph nodes. Nat Rev Immunol. 2012;12(11):762–773. doi: 10.1038/nri3298. [DOI] [PubMed] [Google Scholar]
- 7.Griffith JW, Sokol CL, Luster AD. Chemokines and chemokine receptors: positioning cells for host defense and immunity. Annu Rev Immunol. 2014;32(1):659–702. doi: 10.1146/annurev-immunol-032713-120145. [DOI] [PubMed] [Google Scholar]
- 8.Lian J, Luster AD. Chemokine-guided cell positioning in the lymph node orchestrates the generation of adaptive immune responses. Curr Opin Cell Biol. 2015;36:1–6. doi: 10.1016/j.ceb.2015.05.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Tellier J, Nutt SL. Plasma cells: the programming of an antibody-secreting machine. Eur J Immunol. 2019;49(1):30–37. doi: 10.1002/eji.201847517. [DOI] [PubMed] [Google Scholar]
- 10.Nutt SL, Hodgkin PD, Tarlinton DM, Corcoran LM. The generation of antibody-secreting plasma cells. Nat Rev Immunol. 2015;15(3):160–171. doi: 10.1038/nri3795. [DOI] [PubMed] [Google Scholar]
- 11.Sharonov GV, Serebrovskaya EO, Yuzhakova DV, Britanova OV, Chudakov DM. B cells, plasma cells and antibody repertoires in the tumour microenvironment. Nat Rev Immunol. 2020;20(5):294–307. doi: 10.1038/s41577-019-0257-x. [DOI] [PubMed] [Google Scholar]
- 12.Pitzalis C, Jones GW, Bombardieri M, Jones SA. Ectopic lymphoid-like structures in infection, cancer and autoimmunity. Nat Rev Immunol. 2014;14(7):447–462. doi: 10.1038/nri3700. [DOI] [PubMed] [Google Scholar]
- 13.Sautes-Fridman C, Petitprez F, Calderaro J, Fridman WH. Tertiary lymphoid structures in the era of cancer immunotherapy. Nat Rev Cancer. 2019;19(6):307–325. doi: 10.1038/s41568-019-0144-6. [DOI] [PubMed] [Google Scholar]
- 14.Martinet L. Human solid tumors contain high endothelial venules: association with T- and B-lymphocyte infiltration and favorable prognosis in breast cancer. Cancer Res. 2011;71(17):5678–5687. doi: 10.1158/0008-5472.CAN-11-0431. [DOI] [PubMed] [Google Scholar]
- 15.Martinet L. High endothelial venules (HEVs) in human melanoma lesions: major gateways for tumor-infiltrating lymphocytes. Oncoimmunology. 2012;1(6):829–839. doi: 10.4161/onci.20492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kalialis LV, Drzewiecki KT, Klyver H. Spontaneous regression of metastases from melanoma: review of the literature. Melanoma Res. 2009;19(5):275–282. doi: 10.1097/CMR.0b013e32832eabd5. [DOI] [PubMed] [Google Scholar]
- 17.Meier D. Ectopic lymphoid-organ development occurs through interleukin 7-mediated enhanced survival of lymphoid-tissue-inducer cells. Immunity. 2007;26(5):643–654. doi: 10.1016/j.immuni.2007.04.009. [DOI] [PubMed] [Google Scholar]
- 18.Hess E. RANKL induces organized lymph node growth by stromal cell proliferation. J Immunol. 2012;188(3):1245–1254. doi: 10.4049/jimmunol.1101513. [DOI] [PubMed] [Google Scholar]
- 19.Yoshida H. Different cytokines induce surface lymphotoxin-alphabeta on IL-7 receptor-alpha cells that differentially engender lymph nodes and peyer’s patches. Immunity. 2002;17(6):823–833. doi: 10.1016/S1074-7613(02)00479-X. [DOI] [PubMed] [Google Scholar]
- 20.Luther SA, Ansel KM, Cyster JG. Overlapping roles of CXCL13, interleukin 7 receptor alpha, and CCR7 ligands in lymph node development. J Exp Med. 2003;197(9):1191–1198. doi: 10.1084/jem.20021294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Takatori H. Lymphoid tissue inducer-like cells are an innate source of IL-17 and IL-22. J Exp Med. 2009;206(1):35–41. doi: 10.1084/jem.20072713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Drayton DL, Ying X, Lee J, Lesslauer W, Ruddle NH. Ectopic LT alpha beta directs lymphoid organ neogenesis with concomitant expression of peripheral node addressin and a HEV-restricted sulfotransferase. J Exp Med. 2003;197(9):1153–1163. doi: 10.1084/jem.20021761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Mueller CG, Hess E. Emerging functions of RANKL in lymphoid tissues. Front Immunol. 2012;3:261. doi: 10.3389/fimmu.2012.00261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Cupedo T. Human fetal lymphoid tissue-inducer cells are interleukin 17-producing precursors to RORC+ CD127+ natural killer-like cells. Nat Immunol. 2009;10(1):66–74. doi: 10.1038/ni.1668. [DOI] [PubMed] [Google Scholar]
- 25.Luther SA. Differing activities of homeostatic chemokines CCL19, CCL21, and CXCL12 in lymphocyte and dendritic cell recruitment and lymphoid neogenesis. J Immunol. 2002;169:424–433. [DOI] [PubMed] [Google Scholar]
- 26.Chyou S. Fibroblast-type reticular stromal cells regulate the lymph node vasculature. J Immunol. 2008;181(6):3887–3896. doi: 10.4049/jimmunol.181.6.3887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Webster B. Regulation of lymph node vascular growth by dendritic cells. J Exp Med. 2006;203(8):1903–1913. doi: 10.1084/jem.20052272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ager A. High endothelial venules and other blood vessels: critical regulators of lymphoid organ development and function. Front Immunol. 2017;8:45. doi: 10.3389/fimmu.2017.00045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Chyou S. Coordinated regulation of lymph node vascular-stromal growth first by CD11c+ cells and then by T and B cells. J Immunol. 2011;187:5558–5567. doi: 10.4049/jimmunol.1101724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Peske JD. Effector lymphocyte-induced lymph node-like vasculature enables naive T-cell entry into tumours and enhanced anti-tumour immunity. Nat Commun. 2015;6(1):7114. doi: 10.1038/ncomms8114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.De Togni P. Abnormal development of peripheral lymphoid organs in mice deficient in lymphotoxin. Science. 1994;264(5159):703–707. doi: 10.1126/science.8171322. [DOI] [PubMed] [Google Scholar]
- 32.Eberl G. An essential function for the nuclear receptor RORgamma(t) in the generation of fetal lymphoid tissue inducer cells. Nat Immunol. 2004;5(1):64–73. doi: 10.1038/ni1022. [DOI] [PubMed] [Google Scholar]
- 33.Kiyono H, Fukuyama S. NALT- versus peyer’s-patch-mediated mucosal immunity. Nat Rev Immunol. 2004;4(9):699–710. doi: 10.1038/nri1439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Nagatake T. Id2-, RORgammat-, and LTbetaR-independent initiation of lymphoid organogenesis in ocular immunity. J Exp Med. 2009;206(11):2351–2364. doi: 10.1084/jem.20091436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Lochner M. Microbiota-induced tertiary lymphoid tissues aggravate inflammatory disease in the absence of RORgamma t and LTi cells. J Exp Med. 2011;208(1):125–134. doi: 10.1084/jem.20100052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Furtado GC. TNFalpha-dependent development of lymphoid tissue in the absence of RORgammat(+) lymphoid tissue inducer cells. Mucosal Immunol. 2014;7(3):602–614. doi: 10.1038/mi.2013.79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Rangel-Moreno J. The development of inducible bronchus-associated lymphoid tissue depends on IL-17. Nat Immunol. 2011;12(7):639–646. doi: 10.1038/ni.2053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Tsuji M. Requirement for lymphoid tissue-inducer cells in isolated follicle formation and T cell-independent immunoglobulin A generation in the gut. Immunity. 2008;29(2):261–271. doi: 10.1016/j.immuni.2008.05.014. [DOI] [PubMed] [Google Scholar]
- 39.Lochner M. Tertiary lymphoid tissues in the colon: friend and foe. Gut Microbes. 2011;2(3):193–197. doi: 10.4161/gmic.2.3.16732. [DOI] [PubMed] [Google Scholar]
- 40.Moyron-Quiroz JE. Role of inducible bronchus associated lymphoid tissue (iBALT) in respiratory immunity. Nat Med. 2004;10(9):927–934. doi: 10.1038/nm1091. [DOI] [PubMed] [Google Scholar]
- 41.De Chaisemartin L. Characterization of chemokines and adhesion molecules associated with T cell presence in tertiary lymphoid structures in human lung cancer. Cancer Res. 2011;71(20):6391–6399. doi: 10.1158/0008-5472.CAN-11-0952. [DOI] [PubMed] [Google Scholar]
- 42.Ansel KM. A chemokine-driven positive feedback loop organizes lymphoid follicles. Nature. 2000;406(6793):309–314. doi: 10.1038/35018581. [DOI] [PubMed] [Google Scholar]
- 43.Meylan M. Early hepatic lesions display immature tertiary lymphoid structures and show elevated expression of immune inhibitory and immunosuppressive molecules. Clin Cancer Res. 2020;26(16):4381–4389. doi: 10.1158/1078-0432.CCR-19-2929. [DOI] [PubMed] [Google Scholar]
- 44.Ladanyi A. Density of DC-LAMP(+) mature dendritic cells in combination with activated T lymphocytes infiltrating primary cutaneous melanoma is a strong independent prognostic factor. Cancer Immunol Immunother. 2007;56(9):1459–1469. doi: 10.1007/s00262-007-0286-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Dieu-Nosjean MC. Long-term survival for patients with non-small-cell lung cancer with intratumoral lymphoid structures. J Clin Oncol. 2008;26(27):4410–4417. doi: 10.1200/JCO.2007.15.0284. [DOI] [PubMed] [Google Scholar]
- 46.Kroeger DR, Milne K, Nelson BH. Tumor-infiltrating plasma cells are associated with tertiary lymphoid structures, cytolytic T-Cell responses, and superior prognosis in ovarian cancer. Clin Cancer Res. 2016;22(12):3005–3015. doi: 10.1158/1078-0432.CCR-15-2762. [DOI] [PubMed] [Google Scholar]
- 47.Posch F. Maturation of tertiary lymphoid structures and recurrence of stage II and III colorectal cancer. Oncoimmunology. 2018;7(2):e1378844. doi: 10.1080/2162402X.2017.1378844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Silina K. Germinal centers determine the prognostic relevance of tertiary lymphoid structures and are impaired by corticosteroids in lung squamous cell carcinoma. Cancer Res. 2018;78(5):1308–1320. doi: 10.1158/0008-5472.CAN-17-1987. [DOI] [PubMed] [Google Scholar]
- 49.Gu-Trantien C. CXCL13-producing TFH cells link immune suppression and adaptive memory in human breast cancer. JCI Insight. 2017;2(11). doi: 10.1172/jci.insight.91487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Messina JL. 12-Chemokine gene signature identifies lymph node-like structures in melanoma: potential for patient selection for immunotherapy?. Sci Rep. 2012;2(1):765. doi: 10.1038/srep00765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Coppola D. Unique ectopic lymph node-like structures present in human primary colorectal carcinoma are identified by immune gene array profiling. Am J Pathol. 2011;179(1):37–45. doi: 10.1016/j.ajpath.2011.03.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Tokunaga R. 12-Chemokine signature, a predictor of tumor recurrence in colorectal cancer. Int J Cancer. 2020;147(2):532–541. doi: 10.1002/ijc.32982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Prabhakaran S. Evaluation of invasive breast cancer samples using a 12-chemokine gene expression score: correlation with clinical outcomes. Breast Cancer Res. 2017;19(1):71. doi: 10.1186/s13058-017-0864-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Gu-Trantien C. CD4(+) follicular helper T cell infiltration predicts breast cancer survival. J Clin Invest. 2013;123(7):2873–2892. doi: 10.1172/JCI67428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Hennequin A. Tumor infiltration by Tbet+ effector T cells and CD20+ B cells is associated with survival in gastric cancer patients. Oncoimmunology. 2016;5(2):e1054598. doi: 10.1080/2162402X.2015.1054598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Germain C. Presence of B cells in tertiary lymphoid structures is associated with a protective immunity in patients with lung cancer. Am J Respir Crit Care Med. 2014;189(7):832–844. doi: 10.1164/rccm.201309-1611OC. [DOI] [PubMed] [Google Scholar]
- 57.Tang J. B Cells and tertiary lymphoid structures influence survival in lung cancer patients with resectable tumors. Cancers (Basel). 2020;12(9). doi: 10.3390/cancers12092644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Li Q. Prognostic value of tertiary lymphoid structure and tumour infiltrating lymphocytes in oral squamous cell carcinoma. Int J Oral Sci. 2020;12(1):24. doi: 10.1038/s41368-020-00092-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Lee HJ. Prognostic significance of tumor-infiltrating lymphocytes and the tertiary lymphoid structures in HER2-positive breast cancer treated with adjuvant trastuzumab. Am J Clin Pathol. 2015;144(2):278–288. doi: 10.1309/AJCPIXUYDVZ0RZ3G. [DOI] [PubMed] [Google Scholar]
- 60.Figenschau SL, Fismen S, Fenton KA, Fenton C, Mortensen ES. Tertiary lymphoid structures are associated with higher tumor grade in primary operable breast cancer patients. BMC Cancer. 2015;15(1):101. doi: 10.1186/s12885-015-1116-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Lee HJ. Tertiary lymphoid structures: prognostic significance and relationship with tumour-infiltrating lymphocytes in triple-negative breast cancer. J Clin Pathol. 2016;69(5):422–430. doi: 10.1136/jclinpath-2015-203089. [DOI] [PubMed] [Google Scholar]
- 62.Di Caro G. Occurrence of tertiary lymphoid tissue is associated with T-cell infiltration and predicts better prognosis in early-stage colorectal cancers. Clin Cancer Res. 2014;20(8):2147–2158. doi: 10.1158/1078-0432.CCR-13-2590. [DOI] [PubMed] [Google Scholar]
- 63.Hill DG. Hyperactive gp130/STAT3-driven gastric tumourigenesis promotes submucosal tertiary lymphoid structure development. Int J Cancer. 2018;143(1):167–178. doi: 10.1002/ijc.31298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Yamakoshi Y. Immunological potential of tertiary lymphoid structures surrounding the primary tumor in gastric cancer. Int J Oncol. 2020;57(1):171–182. doi: 10.3892/ijo.2020.5042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Calderaro J. Intra-tumoral tertiary lymphoid structures are associated with a low risk of early recurrence of hepatocellular carcinoma. J Hepatol. 2019;70(1):58–65. doi: 10.1016/j.jhep.2018.09.003. [DOI] [PubMed] [Google Scholar]
- 66.Chen L. The immunosuppressive niche of soft tissue sarcomas is sustained by tumor associated macrophages and characterized by intratumoral tertiary lymphoid structures. Clin Cancer Res. 2020;15. doi: 10.1158/1078-0432.CCR-19-3416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Lin Q. Tumor-associated tertiary lymphoid structure predicts postoperative outcomes in patients with primary gastrointestinal stromal tumors. Oncoimmunology. 2020;9(1):1747339. doi: 10.1080/2162402X.2020.1747339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Hulsen S. High stroma T-Cell infiltration is associated with better survival in stage pT1 bladder cancer. Int J Mol Sci. 2020;21(21). doi: 10.3390/ijms21218407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Truxova I. Mature dendritic cells correlate with favorable immune infiltrate and improved prognosis in ovarian carcinoma patients. J Immunother Cancer. 2018;6(1):139. doi: 10.1186/s40425-018-0446-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Cipponi A. Neogenesis of lymphoid structures and antibody responses occur in human melanoma metastases. Cancer Res. 2012;72(16):3997–4007. doi: 10.1158/0008-5472.CAN-12-1377. [DOI] [PubMed] [Google Scholar]
- 71.Goc J. Dendritic cells in tumor-associated tertiary lymphoid structures signal a Th1 cytotoxic immune contexture and license the positive prognostic value of infiltrating CD8+ T Cells. Cancer Res. 2014;74(3):705–715. doi: 10.1158/0008-5472.CAN-13-1342. [DOI] [PubMed] [Google Scholar]
- 72.Van Dijk N. Preoperative ipilimumab plus nivolumab in locoregionally advanced urothelial cancer: the NABUCCO trial. Nat Med. 2020;12. doi: 10.1038/s41591-020-1085-z. [DOI] [PubMed] [Google Scholar]
- 73.Jiang Q. CD19(+) tumor-infiltrating B-cells prime CD4(+) T-cell immunity and predict platinum-based chemotherapy efficacy in muscle-invasive bladder cancer. Cancer Immunol Immunother. 2019;68(1):45–56. doi: 10.1007/s00262-018-2250-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Welinder C. Intra-tumour IgA1 is common in cancer and is correlated with poor prognosis in bladder cancer. Heliyon. 2016;2(8):e00143. doi: 10.1016/j.heliyon.2016.e00143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Mohammed ZM, Going JJ, Edwards J, Elsberger B, McMillan DC. The relationship between lymphocyte subsets and clinico-pathological determinants of survival in patients with primary operable invasive ductal breast cancer. Br J Cancer. 2013;109(6):1676–1684. doi: 10.1038/bjc.2013.493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Xu Y, Lan S, Zheng Q. Prognostic significance of infiltrating immune cell subtypes in invasive ductal carcinoma of the breast. Tumori. 2018;104(3):196–201. doi: 10.5301/tj.5000624. [DOI] [PubMed] [Google Scholar]
- 77.Mohammed ZM. The relationship between components of tumour inflammatory cell infiltrate and clinicopathological factors and survival in patients with primary operable invasive ductal breast cancer. Br J Cancer. 2012;107(5):864–873. doi: 10.1038/bjc.2012.347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Mahmoud SM. The prognostic significance of B lymphocytes in invasive carcinoma of the breast. Breast Cancer Res Treat. 2012;132(2):545–553. doi: 10.1007/s10549-011-1620-1. [DOI] [PubMed] [Google Scholar]
- 79.Brown JR. Multiplexed quantitative analysis of CD3, CD8, and CD20 predicts response to neoadjuvant chemotherapy in breast cancer. Clin Cancer Res. 2014;20(23):5995–6005. doi: 10.1158/1078-0432.CCR-14-1622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Garaud S. Tumor infiltrating B-cells signal functional humoral immune responses in breast cancer. JCI Insight. 2019;5(18):e129641. doi: 10.1172/jci.insight.129641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Song IH. Predictive value of tertiary lymphoid structures assessed by high endothelial venule counts in the neoadjuvant setting of triple-negative breast cancer. Cancer Res Treat. 2017;49(2):399–407. doi: 10.4143/crt.2016.215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Garcia-Martinez E. Tumor-infiltrating immune cell profiles and their change after neoadjuvant chemotherapy predict response and prognosis of breast cancer. Breast Cancer Res. 2014;16(6):488. doi: 10.1186/s13058-014-0488-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Berntsson J, Nodin B, Eberhard J, Micke P, Jirstrom K. Prognostic impact of tumour-infiltrating B cells and plasma cells in colorectal cancer. Int J Cancer. 2016;139(5):1129–1139. doi: 10.1002/ijc.30138. [DOI] [PubMed] [Google Scholar]
- 84.Kasajima A. Down-regulation of the antigen processing machinery is linked to a loss of inflammatory response in colorectal cancer. Hum Pathol. 2010;41(12):1758–1769. doi: 10.1016/j.humpath.2010.05.014. [DOI] [PubMed] [Google Scholar]
- 85.Mlecnik B. Comprehensive intrametastatic immune quantification and major impact of Immunoscore on survival. J Natl Cancer Inst. 2018;110(1). doi: 10.1093/jnci/djx123. [DOI] [PubMed] [Google Scholar]
- 86.Meshcheryakova A. B cells and ectopic follicular structures: novel players in anti-tumor programming with prognostic power for patients with metastatic colorectal cancer. PLoS One. 2014;9(6):e99008. doi: 10.1371/journal.pone.0099008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Knief J. High density of tumor-infiltrating B-Lymphocytes and plasma cells signifies prolonged overall survival in adenocarcinoma of the esophagogastric junction. Anticancer Res. 2016;36(10):5339–5345. doi: 10.21873/anticanres.11107. [DOI] [PubMed] [Google Scholar]
- 88.Fristedt R. Prognostic impact of tumour-associated B cells and plasma cells in oesophageal and gastric adenocarcinoma. J Gastrointest Oncol. 2016;7(6):848–859. doi: 10.21037/jgo.2016.11.07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Yakirevich E. Prognostic significance of IgG4+ plasma cell infiltrates following neoadjuvant chemoradiation therapy for esophageal adenocarcinoma. Hum Pathol. 2017;66:126–135. doi: 10.1016/j.humpath.2017.06.009. [DOI] [PubMed] [Google Scholar]
- 90.Zheng X. Prognostic role of tumor-infiltrating lymphocytes in gastric cancer: a meta-analysis. Oncotarget. 2017;8(34):57386–57398. doi: 10.18632/oncotarget.18065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Miyatani K. A high number of IgG4-positive cells in gastric cancer tissue is associated with tumor progression and poor prognosis. Virchows Arch. 2016;468(5):549–557. doi: 10.1007/s00428-016-1914-0. [DOI] [PubMed] [Google Scholar]
- 92.Garnelo M. Interaction between tumour-infiltrating B cells and T cells controls the progression of hepatocellular carcinoma. Gut. 2017;66(2):342–351. doi: 10.1136/gutjnl-2015-310814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Shi JY. Margin-infiltrating CD20+ B Cells display an atypical memory phenotype and correlate with favorable prognosis in hepatocellular carcinoma. Clin Cancer Res. 2013;19(21):5994–6005. doi: 10.1158/1078-0432.CCR-12-3497. [DOI] [PubMed] [Google Scholar]
- 94.Brunner SM. Tumor-infiltrating B cells producing antitumor active immunoglobulins in resected HCC prolong patient survival. Oncotarget. 2017;8(41):71002–71011. doi: 10.18632/oncotarget.20238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Shalapour S. Inflammation-induced IgA+ cells dismantle anti-liver cancer immunity. Nature. 2017;551(7680):340–345. doi: 10.1038/nature24302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Iglesia MD. Genomic analysis of immune cell infiltrates across 11 tumor types. J Natl Cancer Inst. 2016;108(11). doi: 10.1093/jnci/djw144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Sjoberg E. A minority-group of renal cell cancer patients with high infiltration of CD20+B-cells is associated with poor prognosis. Br J Cancer. 2018;119(7):840–846. doi: 10.1038/s41416-018-0266-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Charoentong P. Pan-cancer immunogenomic analyses reveal genotype-immunophenotype relationships and predictors of response to checkpoint blockade. Cell Rep. 2017;18(1):248–262. doi: 10.1016/j.celrep.2016.12.019. [DOI] [PubMed] [Google Scholar]
- 99.Kurebayashi Y. Comprehensive immune profiling of lung adenocarcinomas reveals four immunosubtypes with plasma cell subtype a negative indicator. Cancer Immunol Res. 2016;4(3):234–247. doi: 10.1158/2326-6066.CIR-15-0214. [DOI] [PubMed] [Google Scholar]
- 100.Lohr M. The prognostic relevance of tumour-infiltrating plasma cells and immunoglobulin kappa C indicates an important role of the humoral immune response in non-small cell lung cancer. Cancer Lett. 2013;333(2):222–228. doi: 10.1016/j.canlet.2013.01.036. [DOI] [PubMed] [Google Scholar]
- 101.Schmidt M. A comprehensive analysis of human gene expression profiles identifies stromal immunoglobulin kappa C as a compatible prognostic marker in human solid tumors. Clin Cancer Res. 2012;18(9):2695–2703. doi: 10.1158/1078-0432.CCR-11-2210. [DOI] [PubMed] [Google Scholar]
- 102.Gentles AJ. The prognostic landscape of genes and infiltrating immune cells across human cancers. Nat Med. 2015;21(8):938–945. doi: 10.1038/nm.3909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Isaeva OI. Intratumoral immunoglobulin isotypes predict survival in lung adenocarcinoma subtypes. J Immunother Cancer. 2019;7(1):279. doi: 10.1186/s40425-019-0747-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Fujimoto M. Stromal plasma cells expressing immunoglobulin G4 subclass in non-small cell lung cancer. Hum Pathol. 2013;44(8):1569–1576. doi: 10.1016/j.humpath.2013.01.002. [DOI] [PubMed] [Google Scholar]
- 105.Erdag G. Immunotype and immunohistologic characteristics of tumor-infiltrating immune cells are associated with clinical outcome in metastatic melanoma. Cancer Res. 2012;72(5):1070–1080. doi: 10.1158/0008-5472.CAN-11-3218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Garg K. Tumor-associated B cells in cutaneous primary melanoma and improved clinical outcome. Hum Pathol. 2016;54:157–164. doi: 10.1016/j.humpath.2016.03.022. [DOI] [PubMed] [Google Scholar]
- 107.Bosisio FM. Plasma cells in primary melanoma. Prognostic significance and possible role of IgA. Mod Pathol. 2016;29(4):347–358. doi: 10.1038/modpathol.2016.28. [DOI] [PubMed] [Google Scholar]
- 108.Mose LE. Assembly-based inference of B-cell receptor repertoires from short read RNA sequencing data with V’DJer. Bioinformatics. 2016;32(24):3729–3734. doi: 10.1093/bioinformatics/btw526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Bolotin DA. Antigen receptor repertoire profiling from RNA-seq data. Nat Biotechnol. 2017;35(10):908–911. doi: 10.1038/nbt.3979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Chee SJ. Evaluating the effect of immune cells on the outcome of patients with mesothelioma. Br J Cancer. 2017;117(9):1341–1348. doi: 10.1038/bjc.2017.269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Ujiie H. The tumoral and stromal immune microenvironment in malignant pleural mesothelioma: a comprehensive analysis reveals prognostic immune markers. Oncoimmunology. 2015;4(6):e1009285. doi: 10.1080/2162402X.2015.1009285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Lundgren S, Berntsson J, Nodin B, Micke P, Jirstrom K. Prognostic impact of tumour-associated B cells and plasma cells in epithelial ovarian cancer. J Ovarian Res. 2016;9(1):21. doi: 10.1186/s13048-016-0232-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Iglesia MD. Prognostic B-cell signatures using mRNA-seq in patients with subtype-specific breast and ovarian cancer. Clin Cancer Res. 2014;20(14):3818–3829. doi: 10.1158/1078-0432.CCR-13-3368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Nielsen JS. CD20+ tumor-infiltrating lymphocytes have an atypical CD27- memory phenotype and together with CD8+ T cells promote favorable prognosis in ovarian cancer. Clin Cancer Res. 2012;18:3281–3292. doi: 10.1158/1078-0432.CCR-12-0234. [DOI] [PubMed] [Google Scholar]
- 115.Milne K. Systematic analysis of immune infiltrates in high-grade serous ovarian cancer reveals CD20, FoxP3 and TIA-1 as positive prognostic factors. PLoS One. 2009;4(7):e6412. doi: 10.1371/journal.pone.0006412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Tewari N. The presence of tumour-associated lymphocytes confers a good prognosis in pancreatic ductal adenocarcinoma: an immunohistochemical study of tissue microarrays. BMC Cancer. 2013;13(1):436. doi: 10.1186/1471-2407-13-436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Miksch RC. Prognostic impact of tumor-infiltrating lymphocytes and neutrophils on survival of patients with upfront resection of pancreatic cancer. Cancers (Basel). 2019;11(1). doi: 10.3390/cancers11010039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Liu Q. Immunoglobulin G4 (IgG4)-positive plasma cell infiltration is associated with the clinicopathologic traits and prognosis of pancreatic cancer after curative resection. Cancer Immunol Immunother. 2016;65(8):931–940. doi: 10.1007/s00262-016-1853-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Shalapour S. Immunosuppressive plasma cells impede T-cell-dependent immunogenic chemotherapy. Nature. 2015;521(7550):94–98. doi: 10.1038/nature14395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Lao XM. Distribution and significance of interstitial fibrosis and stroma-infiltrating B cells in tongue squamous cell carcinoma. Oncol Lett. 2016;11(3):2027–2034. doi: 10.3892/ol.2016.4184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Zhang Y. Mammary-tumor-educated B cells acquire LAP/TGF-beta and PD-L1 expression and suppress anti-tumor immune responses. Int Immunol. 2016;28(9):423–433. doi: 10.1093/intimm/dxw007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Sarvaria A, Madrigal JA, Saudemont A. B cell regulation in cancer and anti-tumor immunity. Cell Mol Immunol. 2017;14(8):662–674. doi: 10.1038/cmi.2017.35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Ladanyi A. Prognostic impact of B-cell density in cutaneous melanoma. Cancer Immunol Immunother. 2011;60(12):1729–1738. doi: 10.1007/s00262-011-1071-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Castino GF. Spatial distribution of B cells predicts prognosis in human pancreatic adenocarcinoma. Oncoimmunology. 2016;5(4):e1085147. doi: 10.1080/2162402X.2015.1085147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Hamanaka Y. Circulating anti-MUC1 IgG antibodies as a favorable prognostic factor for pancreatic cancer. Int J Cancer. 2003;103(1):97–100. doi: 10.1002/ijc.10801. [DOI] [PubMed] [Google Scholar]
- 126.Kurtenkov O. Humoral immune response to MUC1 and to the thomsen-friedenreich (TF) glycotope in patients with gastric cancer: relation to survival. Acta Oncol. 2007;46(3):316–323. doi: 10.1080/02841860601055441. [DOI] [PubMed] [Google Scholar]
- 127.Schmidt M. The humoral immune system has a key prognostic impact in node-negative breast cancer. Cancer Res. 2008;68(13):5405–5413. doi: 10.1158/0008-5472.CAN-07-5206. [DOI] [PubMed] [Google Scholar]
- 128.Ammirante M, Luo JL, Grivennikov S, Nedospasov S, Karin M. B-cell-derived lymphotoxin promotes castration-resistant prostate cancer. Nature. 2010;464(7286):302–305. doi: 10.1038/nature08782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Xiao X. PD-1hi identifies a novel regulatory B-cell population in human hepatoma that promotes disease progression. Cancer Discov. 2016;6:546–559. doi: 10.1158/2159-8290.CD-15-1408. [DOI] [PubMed] [Google Scholar]
- 130.Ye L. Tumor-derived exosomal HMGB1 fosters hepatocellular carcinoma immune evasion by promoting TIM-1(+) regulatory B cell expansion. J Immunother Cancer. 2018;6(1):145. doi: 10.1186/s40425-018-0451-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Murakami Y. Increased regulatory B cells are involved in immune evasion in patients with gastric cancer. Sci Rep. 2019;9(1):13083. doi: 10.1038/s41598-019-49581-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Zhou X, Su YX, Lao XM, Liang YJ, Liao GQ. CD19(+)IL-10(+) regulatory B cells affect survival of tongue squamous cell carcinoma patients and induce resting CD4(+) T cells to CD4(+)Foxp3(+) regulatory T cells. Oral Oncol. 2016;53:27–35. doi: 10.1016/j.oraloncology.2015.11.003. [DOI] [PubMed] [Google Scholar]
- 133.Wu H. PD-L1(+) regulatory B cells act as a T cell suppressor in a PD-L1-dependent manner in melanoma patients with bone metastasis. Mol Immunol. 2020;119:83–91. doi: 10.1016/j.molimm.2020.01.008. [DOI] [PubMed] [Google Scholar]
- 134.Das S, Bar-Sagi D. BTK signaling drives CD1d(hi)CD5(+) regulatory B-cell differentiation to promote pancreatic carcinogenesis. Oncogene. 2019;38(17):3316–3324. doi: 10.1038/s41388-018-0668-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Cottrell TR. Pathologic features of response to neoadjuvant anti-PD-1 in resected non-small-cell lung carcinoma: a proposal for quantitative immune-related pathologic response criteria (irPRC). Ann Oncol. 2018;29(8):1853–1860. doi: 10.1093/annonc/mdy218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Lin Z. Pan-cancer analysis of genomic properties and clinical outcome associated with tumor tertiary lymphoid structure. Sci Rep. 2020;10(1):21530. doi: 10.1038/s41598-020-78560-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Remark R. Immune contexture and histological response after neoadjuvant chemotherapy predict clinical outcome of lung cancer patients. Oncoimmunology. 2016;5(12):e1255394. doi: 10.1080/2162402X.2016.1255394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Franz L. Postoperative radiotherapy for laryngeal cancer. the prognostic role of programmed death-ligand 1: an immune microenvironment-based cluster analysis. Pathol Res Pract. 2020;216(9):153120. doi: 10.1016/j.prp.2020.153120. [DOI] [PubMed] [Google Scholar]
- 139.Maldonado L. Intramuscular therapeutic vaccination targeting HPV16 induces T cell responses that localize in mucosal lesions. Sci Transl Med. 2014;6(221):221ra213. doi: 10.1126/scitranslmed.3007323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Lutz ER. Immunotherapy converts nonimmunogenic pancreatic tumors into immunogenic foci of immune regulation. Cancer Immunol Res. 2014;2(7):616–631. doi: 10.1158/2326-6066.CIR-14-0027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Kratz A, Campos-Neto A, Hanson MS, Ruddle NH. Chronic inflammation caused by lymphotoxin is lymphoid neogenesis. J Exp Med. 1996;183(4):1461–1472. doi: 10.1084/jem.183.4.1461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Johansson-Percival A. De novo induction of intratumoral lymphoid structures and vessel normalization enhances immunotherapy in resistant tumors. Nat Immunol. 2017;18(11):1207–1217. doi: 10.1038/ni.3836. [DOI] [PubMed] [Google Scholar]
- 143.Weinstein AM. Tbet and IL-36gamma cooperate in therapeutic DC-mediated promotion of ectopic lymphoid organogenesis in the tumor microenvironment. Oncoimmunology. 2017;6(6):e1322238. doi: 10.1080/2162402X.2017.1322238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Kirk CJ, Hartigan-O’Connor D, Mule JJ. The dynamics of the T-cell antitumor response: chemokine-secreting dendritic cells can prime tumor-reactive T cells extranodally. Cancer Res. 2001;61:8794–8802. [PubMed] [Google Scholar]
- 145.Kirk CJ. T cell-dependent antitumor immunity mediated by secondary lymphoid tissue chemokine: augmentation of dendritic cell-based immunotherapy. Cancer Res. 2001;61:2062–2070. [PubMed] [Google Scholar]
- 146.Yang SC. Intrapulmonary administration of CCL21 gene-modified dendritic cells reduces tumor burden in spontaneous murine bronchoalveolar cell carcinoma. Cancer Res. 2006;66(6):3205–3213. doi: 10.1158/0008-5472.CAN-05-3619. [DOI] [PubMed] [Google Scholar]
- 147.Lee JM. Phase I trial of intratumoral injection of CCL21 gene-modified dendritic cells in lung cancer elicits tumor-specific immune responses and CD8(+) T-cell infiltration. Clin Cancer Res. 2017;23:4556–4568. doi: 10.1158/1078-0432.CCR-16-2821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.DiLillo DJ, Yanaba K, Tedder TF. B Cells are required for optimal CD4 + and CD8 + T Cell tumor immunity: therapeutic B Cell depletion enhances B16 melanoma growth in mice. J Immunol. 2010;184(7):4006–4016. doi: 10.4049/jimmunol.0903009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.David JM. A novel bifunctional anti-PD-L1/TGF-beta trap fusion protein (M7824) efficiently reverts mesenchymalization of human lung cancer cells. Oncoimmunology. 2017;6(10):e1349589. doi: 10.1080/2162402X.2017.1349589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Strauss J. Phase I trial of M7824 (MSB0011359C), a bifunctional fusion protein targeting PD-L1 and TGFbeta, in advanced solid tumors. Clin Cancer Res. 2018;24(6):1287–1295. doi: 10.1158/1078-0432.CCR-17-2653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Lind H. Dual targeting of TGF-beta and PD-L1 via a bifunctional anti-PD-L1/TGF-betaRII agent: status of preclinical and clinical advances. J Immunother Cancer. 2020;8(1). doi: 10.1136/jitc-2019-000433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Knudson KM. M7824, a novel bifunctional anti-PD-L1/TGFbeta trap fusion protein, promotes anti-tumor efficacy as monotherapy and in combination with vaccine. Oncoimmunology. 2018;7(5):e1426519. doi: 10.1080/2162402X.2018.1426519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Lan Y. Enhanced preclinical antitumor activity of M7824, a bifunctional fusion protein simultaneously targeting PD-L1 and TGF-beta. Sci Transl Med. 2018;10(424). doi: 10.1126/scitranslmed.aan5488. [DOI] [PubMed] [Google Scholar]
- 154.Joshi NS. Regulatory T Cells in tumor-associated tertiary lymphoid structures suppress anti-tumor T Cell responses. Immunity. 2015;43(3):579–590. doi: 10.1016/j.immuni.2015.08.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Munoz-Erazo L, Rhodes JL, Marion VC, Kemp RA. Tertiary lymphoid structures in cancer - considerations for patient prognosis. Cell Mol Immunol. 2020;17(6):570–575. doi: 10.1038/s41423-020-0457-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Yamaguchi K. Helper T cell-dominant tertiary lymphoid structures are associated with disease relapse of advanced colorectal cancer. Oncoimmunology. 2020;9(1):1724763. doi: 10.1080/2162402X.2020.1724763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Hofman P. Multiplexed immunohistochemistry for molecular and immune profiling in lung cancer-just about ready for prime-time?. Cancers (Basel). 2019;11(3). doi: 10.3390/cancers11030283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Parra ER, Francisco-Cruz A, Wistuba II. State-of-the-art of profiling immune contexture in the era of multiplexed staining and digital analysis to study paraffin tumor tissues. Cancers (Basel). 2019;11(2). doi: 10.3390/cancers11020247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Lin JR. Highly multiplexed immunofluorescence imaging of human tissues and tumors using t-CyCIF and conventional optical microscopes. Elife. 2018;7. doi: 10.7554/eLife.31657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Schurch CM. Coordinated cellular neighborhoods orchestrate antitumoral immunity at the colorectal cancer invasive front. Cell. 2020;182(5):1341–1359 e1319. doi: 10.1016/j.cell.2020.07.005. [DOI] [PMC free article] [PubMed] [Google Scholar]


