ABSTRACT
Down syndrome (DS) is an independent risk factor for severe respiratory syncytial virus (RSV) infection. Palivizumab – passive immunization for RSV – is the only pharmacological measure for preventing severe disease. In most countries, palivizumab is indicated in young children with congenital heart disease, premature birth, and chronic lung disease. In Japan, since 2013, children with DS, but without such “standard” risk factors, have been able to receive insurance-covered palivizumab prophylaxis, but its effectiveness of policy is unknown. From a nationwide database, we extracted data of children with DS who hospitalized for RSV-related lower respiratory infections (LRTIs), from April 2010 to January 2019. Using an interrupted time-series design, we analyzed data from before and after the introduction of the universal palivizumab prophylaxis program for DS children in Japan. As a result, we identified a total of 152 RSV-related LRTIs in 147 children hospitalized with DS. With time-series analysis, we did not observe a significant change in both level (−1.07, P = .11) and slope (0.26 per 12 months, P = .30), before and after 2013. In summary, the expansion of the palivizumab prophylaxis program to all children with DS in Japan was not associated with a reduction in RSV-related hospitalization in these children.
KEYWORDS: Respiratory syncytial virus, lower respiratory infection, hospitalization, a quasi-experimental study design, passive immunization
Respiratory syncytial virus (RSV) is one of the most common respiratory pathogens in young children worldwide.1 Palivizumab – passive immunization for RSV – is thus far the only pharmacological measure for preventing severe RSV disease. The standard indication of palivizumab is a high-risk condition in young children such as prematurity, congenital heart disease, and chronic lung disease.2 In Japan, palivizumab was licensed in 2002 for those standard indications.
In addition to standard indications, Down syndrome (DS) is an independent risk factor for severe RSV infection, with a six-fold higher risk of hospitalization in children with DS – including both children with and without congenital heart defects – as compared with children without DS.3 In August 2013, Japan introduced a new expanded program of palivizumab prophylaxis for children with DS aged ≤24 months, such that they could receive insurance-covered palivizumab even when they lack the standard indication and other medical problems.4 Starting in 2013, in Japan up to 90% of DS children with various RSV risk levels receive palivizumab prophylaxis.5 Previously, using mixed-effects logistic regression models and multivariate analysis of the commercial claims database in Japan demonstrated a statistically significant decrease in RSV-related hospital admissions, but neither RSV infection nor mortality was associated with the expanded palivizumab prophylaxis program. This study aimed to assess whether this expanded program reduced hospitalization due to RSV-related lower respiratory infection (LRTI) using a sophisticated statistical technique – interrupted time-series (ITS) analysis.
Before conducting the study, we obtained the approval from the Institutional Review Board of Kyoto University, with the approved number of R0878-2.
This study used an inpatient database provided by Medical Data Vision (MDV) Co., Ltd. (Tokyo, Japan). The details of the MDV database are described elsewhere.6 In brief, MDV collected inpatient data, including patient characteristics (e.g., sex and age), diagnosis (according to International Statistical Classification of Diseases and Related Health Problems, 10th revision [ICD-10] codes), dates of admission, outcome at discharge, procedures, and medications, from hospitals providing acute care in Japan. For 2015, the MDV database included 13 million records of inpatients of all ages from 230 hospitals. The size of the MDV database has increased over time because of the increased number of hospitals submitting data to MDV (https://www.mdv.co.jp/press/2020/detail_1339.html. [in Japanese]). The MDV database does not indicate whether a child received palivizumab prophylaxis.
From the MDV database, data from children with DS who were hospitalized for RSV-related LRTI from April 2010 to January 2019 were extracted. Only children aged ≤24 months in September each year were eligible because this is the approved indication of palivizumab immunoprophylaxis for children with DS. For identification of DS and RSV-related LRTI, we relied on ICD10 codes: Q90 for DS and J121, J201, and J205 for RSV-related LRTI.
ITS is a quasi-experimental design suited to evaluate public health interventions at a population level rather than at the individual patient level.7 In the ITS analysis, we modeled both the level and slope change to evaluate whether the baseline number and the trend changed, respectively, before and after the implementation of expanded palivizumab use in children with DS. We applied the analysis while accounting for seasonality and the MDV database size in each year; for the latter adjustment, we used a total number of all-cause hospitalizations among children aged ≤2 years in the database each year as a proxy of the MDV database size. The Poisson distribution was used for the analysis, and we confirmed that there was no overdispersion by model checking.
All analyses were performed with the R statistical software package, version 3.61 (https://cran.r-project.org/). A p-value <0.05 indicated statistical significance.
Of all approximately 810,000 inpatient records among children aged ≤2 years, we identified a total of 152 RSV-related LRTI hospitalizations in 147 children with DS (Table 1). Of 147 children, 23.1% had ≥one standard indication making them eligible for palivizumab prophylaxis. We found six cases requiring respiratory support with their median age of 4.5 months (interquartile range: 1.25–7.25 months). No deceased cases were reported in 152 admissions.
Table 1.
Demographic and clinical features of the patients (n = 147)
| Variable | |
|---|---|
| Sex (male) | 64.6% (95/147) |
| Age (median, IQR) | 18 (9–25) months |
| Standard indication | |
| CHD | 21.8% (32/147) |
| Premature birth | 1.4% (2/147) |
| CLD | 6.8% (11/147) |
| Any of the above | 23.1% (34/147) |
IQR: interquartile range, CHD: congenital heart disease, CLD: chronic lung disease.
The ITS analysis revealed a nonsignificant change in both level (−1.07, 95% confidence interval: −2.37 to 0.23, P = .11) and slope (0.26 per 12 months, 95% confidence interval: −0.23 to 0.75, P = .30) before and after August 2013 (Figure 1).
Figure 1.
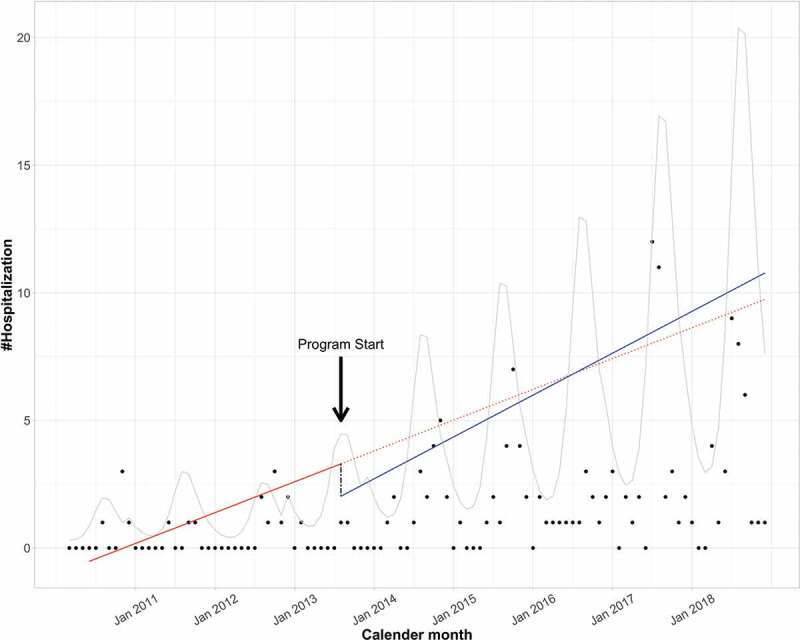
Interrupted time series with level and slope change
Dot: crude number of hospitalized casesCurve: predicted trend of hospitalized cases accounting for seasonality; Solid line: observed linear trend; Dotted line: hypothetical trend without intervention; Dashed line: level change before and after the intervention.
We quantified the impact of the expanded palivizumab program for children with DS who lacked the standard indication. By ITS analysis, there was no clear evidence that the universal palivizumab prophylaxis program reduced LRTI-related hospitalization due to RSV in children with DS.
Children with DS are also at risk of respiratory diseases due to pathogens other than RSV. The causes are multifactorial, including anomalies in upper and lower respiratory organs, immune dysregulations, and hypotonia prone to respiratory distress.8 Although palivizumab has been approved for children with the standard indications, because of these multiple risk factors, it is not guaranteed whether passive immunization against RSV by palivizumab is effective as well for children with DS.9
Because the mean age of children hospitalized with RSV who did have DS is significantly older than the age of those without DS, the American Academy of Pediatrics guideline issued in 2014 does not recommend palivizumab immunoprophylaxis for children with DS unless they have a qualified standard indication.2 Thereafter, some observational studies were published, consistently reporting the effectiveness of palivizumab for children with DS.10-11 However, in these studies, the comparator group comprised children without DS receiving palivizumab for other risks or untreated DSchildren in different countries with different healthcare systems. The interpretation of such comparisons is rather complicated, leaving uncertainty regarding the effectiveness of a universal palivizumab prophylaxis program for children with DS10-12.
There are three possible reasons that we failed to detect the effectiveness of the new palivizumab policy. First, approximately 40–50% of children with DS have a standard indication, such as congenital heart disease. Accordingly, the residual effect of the expanded program could be marginal. Second, the sample size was small (n = 152), although our study captured, thus far, the largest cohort of hospitalized patients with DS and RSV-related LRTIs. Study power calculations are difficult in ITS analyses,7 and thus, a power analysis was not conducted in this study. Last, DS is a well-established risk for severe RSV disease, and care providers could lower the threshold for hospitalization even when the patient was the recipient of palivizumab. If this occurred, such a practice would have biased the program effect (i.e., preventing hospitalization) toward the null.
Here, we mention two limitations of the study design. First, it is unknown whether each hospitalized case received palivizumab before hospitalization. However, according to another report, the adherence for palivizumab was generally high among children with indication.5 Second, the accuracy of ICD-10 coding practice for DS or RSV disease has not been validated in most databases in Japan, including the MDV database. There is no reason, however, that we assume that the coding practice differed before and after the implementation of expanded palivizumab prophylaxis for children with DS. Finally, access to healthcare services and thresholds for hospitalization can differ among countries; thus, generalizability to other settings is unknown.
Palivizumab is highly costly and is considered as cost-effective only when it is indicated to the children with the highest risk, rather than high-risk.13 Given that also in mind, this study implicates that prospective evidence is essential for evaluating the effectiveness of a universal palivizumab program for children with DS without a standard indication in areas where such new programs are under consideration. Ideally, a randomized controlled trial should be conducted, but the practical issue is the sample size; 896 participants would be needed to show a 50% relative risk reduction.14 In such a situation, an adaptive trial design might be helpful to reduce the sample size required.15 If this is not feasible, the laboratory confirmation of RSV infection is a minimal requirement in the prospective observational studies, as experienced in the case of cystic fibrosis, another condition at risk for severe RSV disease but not included in the standard indication.16
In summary, our ITS analysis did not find that in Japan there was a population-level benefit in terms of reduced hospitalizations of an expanded program of palivizumab prophylaxis for children with DS without a standard indication. Since access to healthcare services and thresholds for hospitalization can differ among countries, the generalizability of this conclusion to settings other than Japan is unknown. Prospective studies are warranted in areas where such new programs are under consideration for public health policy that efficiently works.
Acknowledgments
We thank AJE (https://www.aje.com/) for editing our research manuscript.
Funding Statement
This research was partially supported by Grant-in-Aid for Scientific Research from Japan Society for the Promotion of Science (grant number: 18K14950 and 20H03941).
Disclosure of potential conflicts of interest
There is no financial interest or benefit that has arisen from the direct applications of the present research.
References
- 1.Shi T, McAllister DA, O’Brien KL, Simoes EAF, Madhi SA, Gessner BD, Polack FP, Balsells E, Acacio S, Aguayo C, et al.; RSV Global Epidemiology Network . Global, regional, and national disease burden estimates of acute lower respiratory infections due to respiratory syncytial virus in young children in 2015: a systematic review and modelling study. Lancet. 2017;390:946–58. doi: 10.1016/S0140-6736(17)30938-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.American Academy of Pediatrics Committee on Infectious Diseases; American Academy of Pediatrics Bronchiolitis Guidelines Committee . Updated guidance for palivizumab prophylaxis among infants and young children at increased risk of hospitalization for respiratory syncytial virus infection. Pediatrics. 2014;134:e620–638. doi: 10.1542/peds.2014-1666. [DOI] [PubMed] [Google Scholar]
- 3.Mitra S, El Azrak M, McCord H, Paes BA.. Hospitalization for respiratory syncytial virus in children with Down syndrome less than 2 years of age: a systematic review and meta-analysis. J Pediatr. 2018;203:92–100.e3. doi: 10.1016/j.jpeds.2018.08.006. [DOI] [PubMed] [Google Scholar]
- 4.Mori M, Morio T, Ito S, Morimoto A, Ota S, Mizuta K, Iwata T, Hara T, Saji T. Risks and prevention of severe RS virus infection among children with immunodeficiency and Down’s syndrome. J Infect Chemother. 2014;20:455–59. doi: 10.1016/j.jiac.2014.05.001. [DOI] [PubMed] [Google Scholar]
- 5.Kimura T, Takeuchi M, Kawakami K. Utilization and efficacy of palivizumab for children with Down syndrome. Pediatr Int. 2020;62(6):677–82. [Epub ahead of print]. doi: 10.1111/ped.14157. [DOI] [PubMed] [Google Scholar]
- 6.Takeuchi M, Ito S, Nakamura M, Kawakami K. Changes in hemoglobin concentrations post-immunoglobulin therapy in patients with Kawasaki disease: a population-based study using a claims database in Japan. Paediatr Drugs. 2018;20:585–91. doi: 10.1007/s40272-018-0316-y. [DOI] [PubMed] [Google Scholar]
- 7.Bernal JL, Cummins S, Gasparrini A. Interrupted time series regression for the evaluation of public health interventions: a tutorial. Int J Epidemiol. 2017;46:348–55. doi: 10.1093/ije/dyw098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Alsubie HS, Rosen D. The evaluation and management of respiratory disease in children with Down syndrome (DS). Paediatr Respir Rev. 2018;26:49–54. doi: 10.1016/j.prrv.2017.07.003. [DOI] [PubMed] [Google Scholar]
- 9.Bloemers BL, Broers CJ, Bont L, Weijerman ME, Gemke RJ, van Furth AM. Increased risk of respiratory tract infections in children with Down syndrome: the consequence of an altered immune system. Microbes Infect. 2010;12:799–808. doi: 10.1016/j.micinf.2010.05.007. [DOI] [PubMed] [Google Scholar]
- 10.Paes B, Mitchell I, Yi H, Li A, Lanctôt KL; CARESS Investigators . Hospitalization for respiratory syncytial virus illness in Down syndrome following prophylaxis with palivizumab. Pediatr Infect Dis J. 2014;33:e29–33. doi: 10.1097/INF.0000000000000019. [DOI] [PubMed] [Google Scholar]
- 11.Simon A, Gehrmann S, Wagenpfeil G, Wagenpfeil S. Palivizumab use in infants with Down syndrome-report from the German Synagis™ registry 2009–2016. Eur J Pediatr. 2018;177:903–11. doi: 10.1007/s00431-018-3142-x. [DOI] [PubMed] [Google Scholar]
- 12.Yi H, Lanctôt KL, Bont L, Bloemers BL, Weijerman M, Broers C; CARESS investigators . Respiratory syncytial virus prophylaxis in Down syndrome: a prospective cohort study. Pediatrics. 2014;133:1031–37. [DOI] [PubMed] [Google Scholar]
- 13.Resch B. Product review on the monoclonal antibody palivizumab for prevention of respiratory syncytial virus infection. Hum Vaccin Immunother. 2017;13:2138–49. doi: 10.1080/21645515.2017.1337614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Yi H, Lanctôt KL, Bont L, Bloemers BLP, Weijerman M, Broers C, Li A, Kiss A, Mitchell I, Paes B. Respiratory syncytial virus prophylaxis in Down syndrome: a prospective cohort study. Pediatrics. 2014;133:1031–37. doi: 10.1542/peds.2013-3916. [DOI] [PubMed] [Google Scholar]
- 15.Cui L, Zhang L. On the efficiency of adaptive sample size design. Stat Med. 2019;38:933–44. doi: 10.1002/sim.8034. [DOI] [PubMed] [Google Scholar]
- 16.Bjornson C, Chan P, Li A, Paes B, Lanctôt KL, Mitchell I. Palivizumab prophylaxis for respiratory syncytial virus in infants with cystic fibrosis: is there a need? Eur J Clin Microbiol Infect Dis. 2018;37:1113–18. doi: 10.1007/s10096-018-3225-7. [DOI] [PubMed] [Google Scholar]


