ABSTRACT
The glycolytic enzyme PGAM1 is overexpressed in gliomas where it efficiently facilitates the repair of DNA damage. Mechanistically, PGAM1 prevents inactivation of the ataxia-telangiectasia mutated (ATM) signaling pathway by sequestering the wild-type p53-induced phosphatase 1 (WIP1) in the cytoplasm. Genetic inhibition of PGAM1 expression subsequently sensitizes glioma cells against irradiation and chemotherapy-induced DNA damage.
KEYWORDS: Glioma, PGAM1, TMZ, IR, treatment resistance, WIP1, DNA damage repair
Gliomas are the most common of all malignant primary brain tumor in adults.1 Among these, glioblastoma (GBM; WHO astrocytoma grade IV) is the most aggressive and carries the worst prognosis. Despite multimodal therapy including surgery, irradiation (IR), and chemotherapy with the alkylating agent temozolomide (TMZ), the outcome for GBM patients remains poor with median survival and 5-year survival rate of around 14 months and 7%, respectively.2 New therapeutic strategies are urgently warranted as the development of resistance toward the current standard of care inevitably occurs. Identifying the underlying molecular basis of treatment resistance in GBMs may yield important clues at which such novel approaches should be directed. Several independent DNA repair mechanisms contributing to drug resistance have already been identified in GBMs,3 but none have so far successfully been translated into novel clinical treatment strategies.
In normal cells, phosphoglycerate mutase 1 (PGAM1) converts 3-phosphoglycerate (3-PG) into 2-phosphoglycerate (2-PG) as part of glycolysis to eventually obtain pyruvate from glucose. Several glycolytic enzymes, including PGAM1, are overexpressed in a variety of cancer forms.4–6 In a recently published study,7 we found that PGAM1 is consistently overexpressed up to five-fold in human glioma tissue of all histologic malignancy grades when compared to normal human brain tissue. This increased PGAM1 expression corresponded well with increased PGAM1 enzymatic activity in these tumor samples.
Importantly, it is being increasingly recognized that PGAM1 is a moonlighting enzyme that possesses additional functions independently of its metabolic enzymatic activity8 and can modulate DNA damage repair through homologous recombination (HR)9 and promote cancer invasion and metastasis.10 In our recently published study, we aimed to elucidate the role of overexpressed PGAM1 in gliomas by genetically inhibiting PGAM1 expression in several established and primary glioma stem cell (GSC) line using two different PGAM1 shRNA lentiviral constructs. Loss of PGAM1 in itself did not impact cell proliferation or colony formation in soft agar, but PGAM1 deficient cells were significantly more susceptible to IR and TMZ as they underwent apoptosis to a higher extent compared to their PGAM1 proficient corresponding controls. In contrast, blockade of PGAM1 enzyme activity by pharmacological inhibitors did not lead to improved IR and TMZ sensitivity, which would, in fact, suggest that the non-enzymatic part of PGAM1 was crucial in maintaining increased DNA repair efficiency and that loss of the entire protein therefore would be required.
As genetic inhibition of PGAM1 sensitizes glioma cells toward IR and TMZ, we subsequently investigated whether DNA repair activity was compromised in PGAM1 deficient cells. Notably, DNA damage following IR and TMZ was left unrepaired in PGAM1 deficient cells, and this was shown to occur independently of any changes in established DNA repair pathways such as homologous recombination (HR) or non-homologous end joining (NHEJ). Our implication of PGAM1 as a regulator of the DNA damage response was further corroborated by findings showing that PGAM1 expression was required for activation of ATM signaling in response to IR and TMZ. Specifically, this activation was enabled by PGAM1 structurally binding to WIP1, a phosphatase known to regulate phosphorylation of DNA damage response proteins, thereby inhibiting translocation of WIP1 from the cytoplasm to the nucleus. As a result, PGAM1 loss resulted in WIP1-mediated dephosphorylation of key components of the ATM signaling pathway, thus reducing DNA repair activity and increasing IR and TMZ sensitivity in GBM cells (Figure 1). To further substantiate the functional association between PGAM1 and WIP1, we showed that silencing of WIP1 in PGAM1 deficient cells significantly reversed the inactivation of the DNA damage response and restored cellular survivability in response to IR and TMZ. Importantly, PGAM1 deficient intracranial GBM xenografts were considerably more responsive to IR and TMZ, which was demonstrated by significantly decreased tumor growth and increased apoptosis as well as extended survival in tumor-bearing mice.
Figure 1.
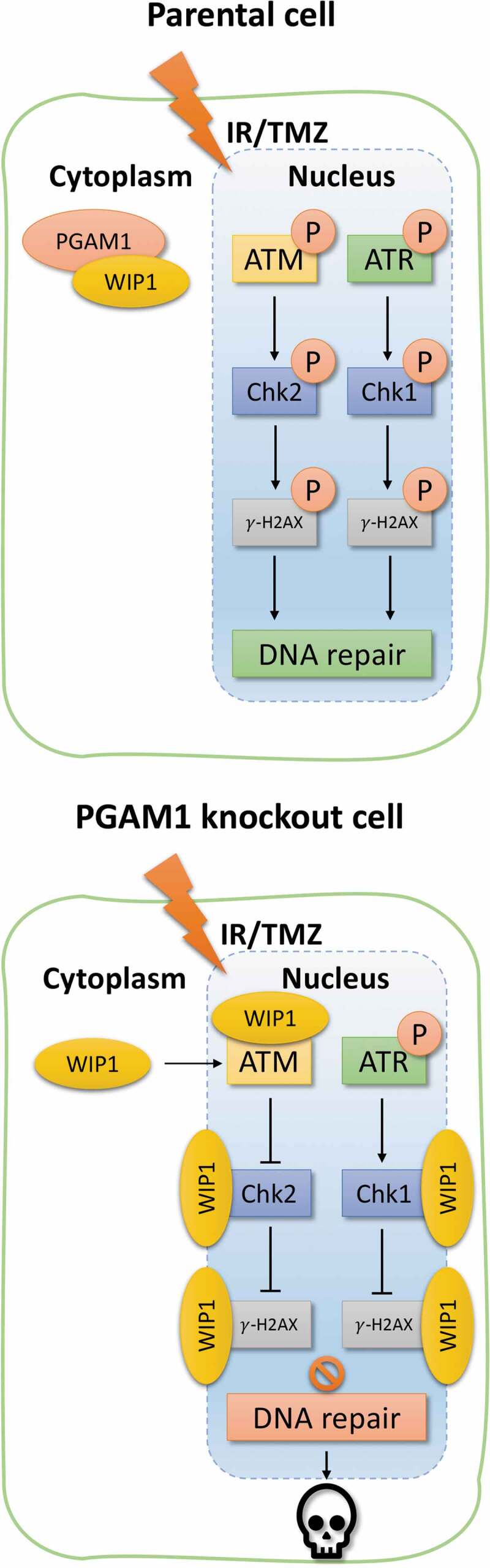
Potential mechanism on phosphoglycerate mutase 1 (PGAM1)-mediated DNA damage repair regulation
Schematic overview of the proposed mechanism on PGAM1-mediated DNA damage repair pathway regulation through wild-type p53-induced phosphatase 1 (WIP1) binding in the cytoplasm. Overexpressed PGAM1 in gliomas binds to the cytoplasmic phosphatase WIP1 and counteracts the latter’s translocation to the cell nucleus where it would normally dephosphorylate the ataxia-telangiectasia mutated (ATM) signaling pathway. When PGAM1 expression is genetically inhibited, WIP1 is allowed to enter the nucleus and deactivate the ATM signaling pathway which in turn increases cellular sensitivity to irradiation (IR) and temozolomide (TMZ) chemotherapy.
In agreement with our initial observation that pharmacological inhibition of PGAM1 enzyme activity was not sufficient to increase IR and TMZ sensitivity in a similar manner as genetic PGAM1 loss, we found that PGAM1 enzymatic activity was not required for WIP1 binding and subsequent regulation of the DNA damage response. In fact, introduction of kinase-dead mutant forms of PGAM1 (H186R and Y92F) in PGAM1 deficient GBM cells effectively restored WIP1 sequestering in the cytoplasm and rendered GBM xenografts equally resistant to IR and TMZ as parental PGAM1 tumors.
Overall, our study in GBM cells shows how PGAM1 significantly blocks the deactivation of the DNA damage response pathway independently of its metabolic activity by physically interacting with the WIP1 phosphatase. As a result, the activated DNA damage response pathway significantly contributes to resistance against clinically established treatment modalities such as IR and TMZ chemotherapy. These results, therefore, define PGAM1 as a therapeutically important target that can be exploited to increase glioma treatment sensitivity.
Disclosure of potential conflicts of interest
No potential conflicts of interest were disclosed.
References
- 1.Louis DN, Perry A, Reifenberger G, von Deimling A, Figarella‐Branger D, Cavenee WK, Ohgaki H, Wiestler OD, Kleihues P, David WE.. The 2016 world health organization classification of tumors of the central nervous system: a summary. Acta Neuropathol. 2016;131(6):803–20. [DOI] [PubMed] [Google Scholar]
- 2.Ostrom QT, Cioffi G, Gittleman H, Patil N, Waite K, Kruchko C, Barnholtz-Sloan JS. CBTRUS statistical report: primary brain and other central nervous system tumors diagnosed in the United States in 2012-2016. Neuro Oncol. 2019;21:1–3. doi: 10.1093/neuonc/noz150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Johannessen TC. Bjerkvig R: molecular mechanisms of temozolomide resistance in glioblastoma multiforme. Expert Rev Anticancer Ther. 2012;12:635–642. doi: 10.1586/era.12.37. [DOI] [PubMed] [Google Scholar]
- 4.Vander Heiden MG, Locasale JW, Swanson KD, Sharfi H, Heffron GJ, Amador-Noguez D, Christofk HR, Wagner G, Rabinowitz JD, Asara JM, et al. Evidence for an alternative glycolytic pathway in rapidly proliferating cells. Science. 2010;329:1492–1499. doi: 10.1126/science.1188015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Wolf A, Agnihotri S, Micallef J, Mukherjee J, Sabha N, Cairns R, Hawkins C, Guha A. Hexokinase 2 is a key mediator of aerobic glycolysis and promotes tumor growth in human glioblastoma multiforme. J Exp Med. 2011;14;208(2):313–326. doi: 10.1016/0006-2952(75)90011-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Mukherjee J, Phillips JJ, Zheng S, Wiencke J, Ronen SM, Pieper RO. Pyruvate kinase M2 expression, but not pyruvate kinase activity, is up-regulated in a grade-specific manner in human glioma. PLoS One. 2013;8:e57610. doi: 10.1371/journal.pone.0057610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ohba S, Johannessen TA, Chatla K, Yang X, Pieper RO, Mukherjee J. Phosphoglycerate mutase 1 activates DNA damage repair via regulation of WIP1 activity. Cell Rep. 2020;31:107518. doi: 10.1016/j.celrep.2020.03.082. [DOI] [PubMed] [Google Scholar]
- 8.Hitosugi T, Zhou L, Elf S, Fan J, Kang HB, Seo JH, Shan C, Dai Q, Zhang L, Xie J, et al. Phosphoglycerate mutase 1 coordinates glycolysis and biosynthesis to promote tumor growth. Cancer Cell. 2012;13;22(5):585–600. doi: 10.1016/j.ccr.2012.09.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Qu J, Sun W, Zhong J, Lv H, Zhu M, Xu J, Jin N, Xie Z, Tan M, Lin SH, et al. Phosphoglycerate mutase 1 regulates dNTP pool and promotes homologous recombination repair in cancer cells. J Cell Biol. 2017;216(2):409–424. doi: 10.1083/jcb.201607008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Zhang D, Jin N, Sun W, Li X, Liu B, Xie Z, Qu J, Xu J, Yang X, Su Y, et al. Phosphoglycerate mutase 1 promotes cancer cell migration independent of its metabolic activity. Oncogene. 2017;18;36(20):2900–2909. doi: 10.1038/onc.2016.446. [DOI] [PubMed] [Google Scholar]


