Abstract
BACKGROUND:
The Sarcopenia Definitions and Outcomes Consortium (SDOC) sought to identify cut-points for muscle strength and body composition measures derived from dual-energy x-ray absorptiometry (DXA) that discriminate older adults with slow walking speed. This paper presents the core analyses used to guide the SDOC position statements.
DESIGN:
Cross-sectional data analyses of pooled data.
SETTING:
University-based research assessment centers
PARTICIPANTS:
Community dwelling Men (n=13,652) and Women: (n=5,115) with information on lean mass by DXA, grip strength and walking speed.
MEASUREMENTS:
Thirty-five candidate sarcopenia variables were entered into sex-stratified classification and regression trees (CART) models to agnostically choose variables and cut-points that discriminate slow-walkers (<0.80 m/sec). Models with alternative walking speed outcomes were also evaluated (<0.60, <1.0 m/sec and walking speed treated continuously).
RESULTS:
CART models identified grip strength (GR)/body mass index (GRBMI) and GR/total body fat (GRTBF) as the primary discriminating variables for slowness in men and women, respectively. Men with GRBMI≤1.05 kg/kg/m2 were about 4 times more likely to be slow walkers than those with GRBMI>1.05 kg/kg/m2. Women with GRTBF<0.65 kg/kg were twice as likely to be slow walkers than women with GRTBF≥0.65 kg/kg. Models with alternative walking speed outcomes selected only functions of GR as primary discriminators of slowness in both men and women. DXA-derived lean mass measures did not consistently discriminate slow walkers.
CONCLUSION:
Grip strength with and without adjustments for body size and composition consistently discriminated older adults with slowness. CART models did not select DXA-based lean mass as primary discriminators of slowness. These results were presented to a SDOC Consensus Panel who used them and other information to develop the SDOC Position Statements.
Keywords: disability, dynapenia, weakness
Introduction
There has been considerable effort to operationally define sarcopenia – the age-related loss of muscle mass and/or strength. Over the past 20 years, both public and private entities have partnered to better characterize the sarcopenia phenotype for identifying the most appropriate at-risk population for evaluating a growing number of interventions available to promote muscle health in older adults. The first approach put forth by Baumgartner in 1998 operationally defined sarcopenia as the occurrence of appendicular lean mass two standard deviations below the mean for healthy young adults that is analogous to T-scores for osteoporosis diagnosis. Since then, European Working Group on Sarcopenia in Older People (EWGSOP) in 2010,1 Foundation for the National Institutes of Health (FNIH) Sarcopenia Project in 20142, International Working Group on Sarcopenia in 2011,3 Asian Working Group for Sarcopenia in 2013,4 and the Society of Sarcopenia, Cachexia and Wasting Disorders in 20145 have proposed various conceptual and operational definitions of sarcopenia. In 2019, the EWGSOP issued a revision of their 2010 operational definition.6 Most of these definitions were based on expert opinion and existing knowledge in the literature. However, the FNIH Sarcopenia Project identified dual-energy x-ray absorptiometry (DXA)-based lean mass and grip strength cut-points based on an a-priori conceptual model on a pooled sample of relatively healthy older adults.
As described previously,7 in 2016, the National Institute on Aging and the FNIH funded the Sarcopenia Definitions and Outcomes Consortium (SDOC) to develop an evidence-based definition of sarcopenia. The primary aim of the SDOC project was to develop evidence-based diagnostic cut-points for lean mass and/or muscle strength that identify people who are more likely to have slowness.. The effort also extends the work of the FNIH Sarcopenia Project by enhancing the racial/ethnic and geographic diversity of the analytic sample and to include a wider range of self-reported mobility limitations. Slow walking speed (<0.80 m/sec) served as an anchor to operationally define sarcopenia because muscle and neuromuscular function are important components of ambulatory movement.8, 9 It is also strongly associated with a myriad of adverse health consequences in older adults.10–12 Its use as an outcome for SDOC ensures the cut-points are relevant to a geriatric-centric health condition, but should not be conceived as a replacement of walking speed as a viable clinical measure. Additionally, as discussed in other linked manuscripts, the SDOC aimed to validate cut-points in clinical populations and against incident clinical outcomes such as mortality, falls, and fractures, and assessed their performance characteristics (sensitivity, specificity, and predictive value) in population-based studies.13
This manuscript describes the analytical approach and results of the SDOC analyses to establish cut-points for lean mass and strength variables to ultimately define a sarcopenia phenotype. The approach is akin to formulating a diagnostic model whereby predictors are combined to estimate the probability that a certain condition or disease is currently present (or absent). Such an approach follows guidelines set forth by the “Transparent Reporting of a multivariable prediction model for Individual Prognosis Or Diagnosis” or otherwise known as the TRIPOD Initiative.14 The SDOC used a combination of receiver operator characteristic (ROC) curves and empirical non-parametric machine-learning methods to select specific variables and respective cut-points from a comprehensive pool of available sarcopenia variables as potential discriminators of slowness. Variables included age, anthropometrics, grip strength, DXA-derived body composition and their respective combinations. This is a major departure from previous heuristic consensus approaches.3, 5, 6 It differs from the FNIH Sarcopenia Project that followed a conceptual model of muscle weakness originating from low lean mass and then applying decision analytics to determine relevant cut-points. The SDOC approach allows muscle related variables to directly compete and interact with each other to discriminate those with and without slowness. This analytic approach empirically determines which subgroups, defined by a specific muscle related variable or combination of variables, are most discriminative of slowness. As a result, the approach considers DXA lean mass and grip strength by themselves and their interactions to comprehensively evaluate an operational definition for sarcopenia. The information herein, and the relation of the cut-points to clinical outcomes reported separately,13 represent the core analyses that informed the SDOC position statements and recommendations for an operational definition of sarcopenia.15
Methods
Study population
We used data from 8 cohort studies of community-dwelling older adults (Supplemental Table S1). These studies have been described elsewhere.7 Analyses were limited to those age 65+ years with data on DXA-derived body composition, grip strength, walking speed and reported mobility function. Data from the baseline visit of each study was used, except for Health ABC where the Year 7 (visit 8) was used to enrich the sample with reports of mobility limitations; and the Study of Osteoporotic Fractures (SOF) year 10 (visit 6); and the Cardiovascular Health Study (CHS) Year 6 data where measures of interest were conducted simultaneously.
Grip strength
Grip strength was assessed with handheld dynamometry; protocol details for each cohort are summarized in Supplementary Table S2. The maximum value from either hand was used in the analysis.
Walking speed
Walking speed was assessed as the participant’s usual pace. Five cohorts used in the analyses performed the walk speed test over 6 m and others at different distances as described in Supplementary Table S2 (range 2.4 to 20 m). To account for slower walking speeds over shorter distances due to less acceleration when initiating movement, walking speed on the 2.4 m course was converted to an equivalent speed that would have been observed over 4–6 meters using equations previously published by Guralnik.16 Slowness was defined as walking speed <0.80 m/sec because of its strong association with health outcomes in older persons and its historical use in the literature.16–19 The SDOC investigators also considered other walking speed cut-points of <1.0 m/sec and <0.60 m/sec. Walking speed was also treated as continuous variable. These were performed as sensitivity analyses and to address the variability of walk speed cut-points noted in the literature.17, 20, 21
Body size and composition
Body height and weight were measured using standardized procedures in a clinic for all cohorts and body mass index (BMI) was calculated as weight (kg)/height2 (m2). Body surface area was calculated with the following equation: 0.20247 × height^0.725 × weight^0.425.22 Lean mass and fat mass of the total body, arms (both right and left) and legs (both right and left) were determined by DXA. Appendicular body composition was calculated by summing the right and left arms and legs.
It is known that even within the same manufacturer, different scanners and software could influence “raw” body composition values across studies. To examine this influence, we adjusted data from the scanner and software versions from each cohort to a common standard using a normative dataset from the National Health and Nutrition Examination Survey (the “NHANES” standard). Sufficient information for harmonization was available from six of the eight cohorts (18,767 participants, 13,652 men and 5,115 women). Following harmonization, we re-estimated statistical models for discrimination of slowness (gait speed <0.8 m/s) as a function of lean mass and/or strength cutpoints to provide an empirical assessment of the influence of standardization. Harmonization to the NHANES standard reassigned approximately 5% of lean mass and/or bone mineral content estimates to fat mass compartment. Predictive models developed to assess association between lean mass and strength measures and slow gait were not meaningfully affected by harmonization. Since results were robust to harmonization, analyses on original non-harmonized values are therefore presented in this manuscript and the series of manuscripts, in order to preserve correspondence to cohort-specific results previously published.
Statistical Analysis
Cross-sectional analyses were stratified by sex because there are clear sex differences in walking speed, strength and body size. First, we computed receiver operating characteristic (ROC) curves for each of the candidate sarcopenia variables on walking speed <0.8 m/s. This approach characterizes the discriminant capability of each variable entered into the CART analyses. We calculated the area under the ROC curve and Youden’s Index(sensitivity + specificity – 1), which provides an optimal cut-point value for each candidate variable,23 which summarizes each candidate’s ability to discriminate slowness. The Youden index ranges from 0 (poor diagnostic accuracy) to 1.0 (perfect accuracy) and was used to evaluate the global test performance as it equally weights sensitivity and specificity.24 Geometrically speaking, Youden’s index is the maximum vertical distance from the ROC curve to the 45° diagonal line. It represents a specific cut-point that optimizes sensitivity and specificity. This process was repeated for walking speed cut-points <0.60 m/sec and <1.0 m/sec. The following are reported from these analyses: the ROC area under the curve; Youden’s index (0–1.0); and the cut-point that simultaneously maximizes both sensitivity and specificity based on Youden’s index.
The SDOC chose CART because it is empirical, non-parametric, and produces interpretable results. CART uses recursive partitioning to reduce a large number of candidate variables (possibly collinear) and identify interactions that can improve discrimination 25. As such, the method has multiple advantages for identifying potential target areas for additional confirmation studies and/or pursuing future interventions. Sex-stratified CART models were performed with 10-fold cross-validation to choose variables and specific cut-points that optimally discriminated those with and without slowness. Thirty-five candidate predictors listed in Supplementary Table S3 were included simultaneously. These predictors included measures of body size (height, weight, BMI); body composition (total fat, percent fat); total lean mass, appendicular lean mass (leg and/or arm lean mass); lean mass variables standardized to measures of body size or fat; and grip strength standardized to measures of body size, fat and arm lean mass. All analyses were repeated for alternative walk speed outcomes (<0.6 m/s and <1.0 m/s). We also evaluated CART models with walk speed as a continuous measure that was not possible for ROC and Youden analyses. Analyses were performed in SAS Version 9.4, and CART models in R software (version 3.1.2) using the command rpart.
Results
Baseline characteristics of the pooled cohorts
The pooled SDOC cohorts included n=13,652 men and n=5,115 women. Characteristics of the participants by slowness group (<0.80 m/sec) are reported in Table 1. Women (28.2%) had higher rates of slowness than men (9.2%). Slowness was more common in Blacks (22.6% in men; 40.8% in women) and less common in women of Asian descent (20.7% vs. 28.2% overall). Both men and women with slowness had slightly larger body sizes than those without slowness— they had higher average body mass index, total fat mass and percent fat. However, those with slowness had lower grip strength than those without slowness.
Table 1.
Characteristics of participants with and without slowness in men and women.
| Men (N=13,652) | Women (N=5,115) | |||||
|---|---|---|---|---|---|---|
| Characteristic | < 0.8 m/s (N= 1251) | ≥ 0.8 m/s (N= 12401) | P value | < 0.8 m/s (N= 1445) | >0.80 m/sec (N= 3670) | P value |
| Age, yrs | 78.06 ± 6.08 | 74.25 ± 5.19 | <0.001 | 77.05 ± 5.8 | 74.85 ± 5.45 | <0.001 |
| Height, m | 1.68 ± 0.08 | 1.72 ± 0.08 | <0.001 | 1.55 ± 0.07 | 1.55 ± 0.07 | 0.6351 |
| Total body weight, kg | 76.03 ± 15.11 | 78.98 ± 14.12 | <0.001 | 66.95 ± 16.73 | 62.38 ± 13.41 | <0.001 |
| Body mass index, kg/m2 | 26.9 ± 4.45 | 26.56 ± 3.85 | 0.0097 | 27.68 ± 5.95 | 25.72 ± 4.66 | <0.001 |
| Body surface area, m2 | 1.85 ± 0.2 | 1.92 ± 0.19 | <0.001 | 1.65 ± 0.21 | 1.61 ± 0.18 | <0.001 |
| Total fat mass, kg | 21.71 ± 8.28 | 21.05 ± 7.31 | 0.0067 | 26.39 ± 11.05 | 23.41 ± 8.57 | <0.001 |
| Total percent fat mass, % | 28.22 ± 6.52 | 26.46 ± 5.75 | <0.001 | 38.38 ± 7.93 | 36.82 ± 6.72 | <0.001 |
| Appendicular lean mass, kg | 21.88 ± 3.95 | 23.55 ± 3.58 | <0.001 | 16.34 ± 3.71 | 15.63 ± 2.85 | <0.001 |
| Arm lean mass, kg | 5.84 ± 1.36 | 6.27 ± 1.12 | <0.001 | 4.12 ± 1.13 | 3.87 ± 0.83 | <0.001 |
| Appendicular lean mass/height2, kg/m2 | 7.72 ± 1.14 | 7.91 ± 0.92 | <0.001 | 6.74 ± 1.29 | 6.45 ± 0.98 | <0.001 |
| Grip strength, kg | 32.94 ± 8.34 | 40.38 ± 8.52 | <0.001 | 20.97 ± 5.57 | 22.63 ± 5.26 | <0.001 |
| Grip strength/BMI, kg/kg/m2 | 1.25 ± 0.33 | 1.54 ± 0.35 | <0.001 | 0.78 ± 0.23 | 0.9 ± 0.24 | <0.001 |
| Usual paced walk speed, m/s | 0.68 ± 0.11 | 1.21 ± 0.22 | <0.001 | 0.64 ± 0.13 | 1.03 ± 0.15 | <0.001 |
| Race, n (%) | ||||||
| White | 881 (70.42) | 9814 (79.14) | <0.001 | 554 (38.34) | 1389 (37.85) | <0.001 |
| Black | 130 (10.39) | 446 (3.6) | <0.001 | 476 (32.94) | 691 (18.83) | <0.001 |
| Asian | 234 (18.71) | 1951 (15.73) | <0.001 | 414 (28.65) | 1588 (43.27) | <0.001 |
| Other | 6 (0.48) | 190 (1.53) | <0.001 | 1 (0.07) | 2 (0.05) | <0.001 |
| Leg lean mass, kg | 16.04 ± 2.77 | 17.28 ± 2.6 | <0.001 | 12.22 ± 2.72 | 11.76 ± 2.12 | <0.001 |
| Appendicular fat mass, kg | 8.67 ± 3.57 | 8.16 ± 3.08 | <0.001 | 13.34 ± 6.21 | 11.47 ± 4.78 | <0.001 |
| Grip strength/weight, kg/kg | 0.44 ± 0.11 | 0.52 ± 0.11 | <0.001 | 0.33 ± 0.1 | 0.37 ± 0.1 | <0.001 |
Values are pooled means ± SD, unless otherwise noted, from cohorts listed in supplementary table S2. Variable units are presented without cancellation for clarity.
In Table 2, ROC AUC and Youden index for slowness (<0.80 m/sec) are presented for each candidate variable. For men, the AUCs ranged from 0.68 to 0.76 indicating a “sufficient” to “good” diagnostic accuracy. The AUCs for measures of ALM and body composition performed only slightly better than or similar to the AUC for age alone (AUC=0.68). In contrast, AUCs for measures of grip strength were approximately 5–10% higher than the AUC for age alone. For women, the AUC’s ranged from 0.61 to 0.67, which were numerically lower than men. As with men, AUC’s for body composition variables were only marginally better than the AUC for age alone and while grip strength measures provided a 5–8% higher AUC. The Youden’s index was consistently low for both men (average=0.10) and women (average=0.31). Optimal cut-points generated by Youden’s index are presented in Table 2. Supplemental tables S4 and S5 contain ROC AUC’s and Youden’s indexes for slowness defined as <0.60 m/sec and <1.0 m/sec, respectively.
Table 2.
Area under the receiver operating characteristic curve and Youden’s index for slowness (walk speed < 0.80 m/sec) according to measures of body anthropometry, DXA lean mass and grip strength in men and women.
| Men | Women | |||||
|---|---|---|---|---|---|---|
| Variable | Area under the curve | Youden’s Index | Optimal cutpoint from Youden | Area under the curve | Youden’s Index | Optimal cutpoint from Youden |
| Age, yrs | 0.68 | 0.13 | 78.90 | 0.61 | 0.33 | 79.00 |
| Body mass index (BMI), kg/m2 | 0.68 | 0.10 | 30.06 | 0.64 | 0.30 | 27.55 |
| Total body fat (TBF), kg | 0.68 | 0.09 | 23.72 | 0.64 | 0.32 | 30.21 |
| Appendicular fat mass (AFM), kg | 0.69 | 0.10 | 9.53 | 0.64 | 0.33 | 15.93 |
| Arm lean mass (ARMLM), kg | 0.69 | 0.10 | 5.80 | 0.63 | 0.32 | 4.71 |
| Appendicular lean mass (ALM) | ||||||
| ALM, kg | 0.70 | 0.10 | 22.36 | 0.62 | 0.31 | 18.01 |
| ALM/Height, kg/m | 0.69 | 0.10 | 13.04 | 0.63 | 0.32 | 11.55 |
| ALM/BMI, kg/kg/m2 | 0.73 | 0.10 | 0.84 | 0.62 | 0.30 | 0.56 |
| ALM/TBF, kg/kg | 0.68 | 0.10 | 1.02 | 0.61 | 0.30 | 0.55 |
| ALM/Weight, kg/kg | 0.69 | 0.11 | 0.28 | 0.61 | 0.30 | 0.24 |
| ALM/Height2, kg/m2 | 0.68 | 0.10 | 7.44 | 0.63 | 0.31 | 7.14 |
| ALM/Body Surface Area, kg/m2 | 0.69 | 0.10 | 11.64 | 0.61 | 0.30 | 10.45 |
| ALM/Percent Fat, kg/% | 0.71 | 0.11 | 0.80 | 0.61 | 0.29 | 0.50 |
| ALM/AFM, kg/kg | 0.69 | 0.10 | 2.75 | 0.61 | 0.30 | 1.27 |
| Leg lean mass (LLM) | ||||||
| LLM, kg | 0.70 | 0.10 | 16.09 | 0.62 | 0.31 | 13.27 |
| LLM/Weight, kg/kg | 0.69 | 0.10 | 0.21 | 0.62 | 0.29 | 0.18 |
| LLM/Height, kg/m | 0.69 | 0.09 | 9.71 | 0.62 | 0.30 | 8.22 |
| LLM/ Height2, kg/m2 | 0.68 | 0.10 | 5.52 | 0.63 | 0.32 | 5.53 |
| LLM/BMI, kg/kg/m2 | 0.73 | 0.10 | 0.62 | 0.62 | 0.31 | 0.41 |
| LLM/TBF, kg/kg | 0.69 | 0.10 | 0.72 | 0.61 | 0.30 | 0.43 |
| LLM/Body Surface Area, kg/m2 | 0.70 | 0.10 | 8.66 | 0.61 | 0.29 | 7.98 |
| Maximum grip strength (GR) | ||||||
| GR, kg | 0.76 | 0.12 | 34.00 | 0.63 | 0.32 | 19.00 |
| GS/ALM, kg/kg | 0.73 | 0.10 | 1.60 | 0.65 | 0.29 | 1.36 |
| GR/weight, kg/kg | 0.74 | 0.11 | 0.45 | 0.66 | 0.30 | 0.34 |
| GR/BMI, kg/kg/m2 | 0.76 | 0.11 | 1.33 | 0.67 | 0.31 | 0.78 |
| GR/Height, kg/m | 0.75 | 0.08 | 22.00 | 0.63 | 0.30 | 13.13 |
| GR/Height2, kg/m2 | 0.74 | 0.10 | 12.13 | 0.63 | 0.31 | 8.25 |
| GR/TBF, kg/kg | 0.71 | 0.11 | 1.66 | 0.64 | 0.31 | 0.82 |
| GR/Body Surface Area, kg/m2 | 0.75 | 0.11 | 18.70 | 0.65 | 0.30 | 12.82 |
| GR/LLM, kg/kg | 0.72 | 0.10 | 2.17 | 0.64 | 0.29 | 1.81 |
| GR/Percent fat, kg/% | 0.75 | 0.11 | 1.31 | 0.64 | 0.30 | 0.57 |
| GR/AFM, kg/kg | 0.72 | 0.12 | 3.98 | 0.64 | 0.33 | 1.44 |
| GR/ARMLM, kg/kg | 0.73 | 0.09 | 6.08 | 0.65 | 0.30 | 5.37 |
Full abbreviations are defined in supplemental table S3. Variable units are presented without cancellation for clarity. The Youden index is a global test accuracy performance— it ranges from 0 (poor diagnostic accuracy) to 1.0 (perfect accuracy) 24. The optimal cut-point represents the value that optimizes both sensitivity and specificity. Note: Youden’s index for body weight and height were not calculated due to their overlap with body mass index.
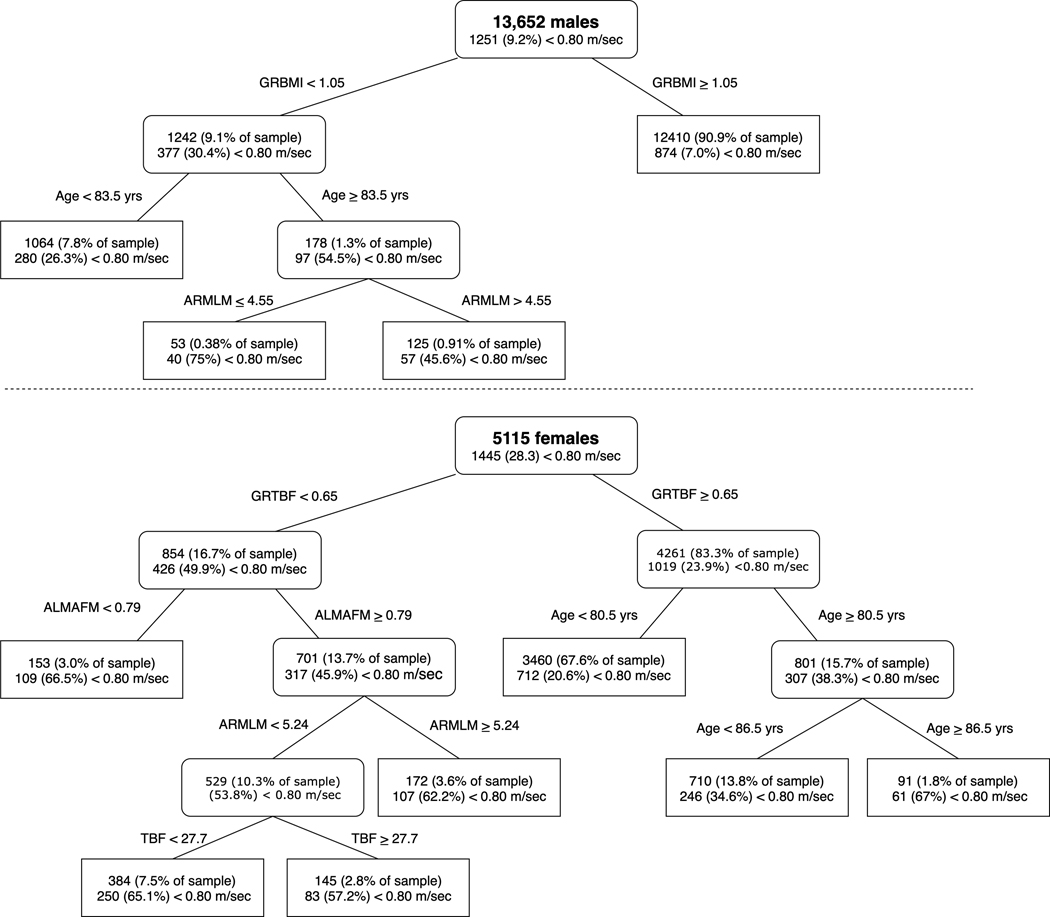
For slowness defined as walking speed <0.80 m/sec, CART analyses identified grip strength/body mass index (GRBMI<1.05) and grip strength/total body fat (GRTBF<0.65 kg/kg) as the primary nodes for men and women, respectively (Figures 1A and B for CART decision trees). Men with GRBMI≤1.05 kg/kg/m2 were about 4 times more likely to have slowness than those with GRBMI>1.05 kg/kg/m2. Women with a GRTBF<0.65 kg/kg were about twice as likely to have slowness than women with GRTBF≥0.65. In men, a secondary node of age≥83.5 yrs and a tertiary node of arm lean mass (ARMLM)<4.55 were split from GRBMI, although selection into these tertiary nodes was uncommon. There were only 53 men (0.38% of the sample) who had GRBMI<1.05, were ≥83.5 yrs old and had ARMLM≤4.55 kg. In women, a secondary node identified 153 women (3% of the sample) with low appendicular lean mass/appendicular fat mass (ALM/AFM<0.79) who had elevated risk of slowness. There were two additional nodes— ARMLM≥5.24 kg and TBF<27.7 kg— under ALM/AFM≥0.79 that represented a ~15% higher prevalence of slowness compared to the initial split at GRTBF<0.65. See Table 3 for summary of primary and secondary nodes for all walk speed outcomes.
Figure 1.
CART model for discriminating objective mobility limitation (gait speed <0.8 m/s) (men: Panel A, women: Panel B). Soft edge rectangles represent continuing nodes and hard edge rectangles represent terminal nodes
Table 3.
Summary of primary and secondary cut-points identified by CART models with various gait speed outcomes
| Slowness outcome | Node | Cutpoint | N (%) below cutpoint* | N (%) with slowness | |
|---|---|---|---|---|---|
| Men | 0.6 m/s | NA | No variables or cut-points chosen | NA | NA |
| 0.8 m/s | 1° | Grip/BMI < 1.05 | 1242 (9.1) | 377 (30.4%) | |
| 0.8 m/s | 2° | Age ≥ 83.5 | 178 (1.3) | 97 (54.5%) | |
| 1.0 m/s | 1° | Grip strength < 35.5 kg | 4203 (30.8%) | 1878 (44.7%) | |
| 1.0 m/s | 2° | Grip/BMI < 1.09 kg/kg/m2 | 1449 (10.6%) | 814 (56.2%) | |
| Continuous | 1° | Grip/BMI <1.46 | 6289 (46.1%) | NA | |
| Continuous | 2° | Grip (max) < 31.25 | 2150 (15.7%) | NA | |
| Women | 0.6 m/s | 1° | Grip/Arm lean mass < 3.26 | 231 (4.5%) | 82 (35.5%) |
| 0.6 m/s | 2° | Body Height <1.5 m | 22 (0.4%) | 16 (72.7%) | |
| 0.8 m/s | 1° | Grip/Total body fat < 0.65 | 854 (16.7%) | 426 (49.9%) | |
| 0.8 m/s | 2° | Appendicular lean mass/appendicular fat mass <0.79^ | 153 (3.0%) | 109 (66.5%) | |
| 1.0 m/s | 1° | Grip/BMI <0.79 kg/kg/m2 | 1983 (38.8%) | 1507 (76%) | |
| 1.0 m/s | 2° | No secondary node identified | NA | NA | |
| Continuous | 1° | Grip/body weight < 0.337 | 2142 (41.9%) | NA | |
| Continuous | 2° | Grip/arm lean mass < 4.1 | 659 (12.9%) | NA |
Values indicate the prevalence of those categorized below the cut-point identified. Prevalence was determined as function of the gender-specific total sample.
Not applicable (NA) indicates that no cut-points or nodes were identified and where the prevalence is unattainable for the continuous walk speed model. Variable units are presented without cancellation for clarity.
CART sensitivity analyses were conducted on alternative slowness definitions and continuous walking speed. CART decision trees are presented as supplemental figures for walking speed < 1.0 m/sec (supplemental Figure S1), walking speed<0.60 m/sec (supplemental Figure S2) and walking speed as a continuous variable (supplemental Figure S3). A summary of the CART primary and secondary nodes for all the models are listed in Table 3. For men, no variables were selected for walking speed < 0.60 m/sec, but the primary node of grip strength<35.5 kg was selected for walking speed<1.0 m/sec, and GRBMI<1.46 kg/kg/m2 was selected for continuous walk speed. In women, grip strength/arm lean mass<3.26 kg was identified for walk speed<0.60 m/sec, GRBMI<0.79 kg/kg/m2 was identified for walk speed<1.0 m/sec, and grip strength/body weight<0.337 was identified for walk speed as a continuous variable. Secondary nodes included GRBMI (<1.09 in men, walk speed<1.0 m/sec), grip strength (<31.25 in men, walk speed continuous), body height (<1.5 m in women for walk speed <0.60 m/sec) and grip strength/arm lean mass (<4.1 in women for walk speed continuous).
SDOC investigators examined the influence of race and ethnicity as well as accuracy of cross-validations. First, we evaluated the importance of having black race and Chinese as variables in the final CART models. These variables did not alter the CART variable selection and were not listed in the variable importance list. Second, the cross-validation predictive accuracy [(root node error*cross validation error)*100] for walking speed < 0.80 m/sec was 90.9% and 72.3% in men and women, respectively. For walking speed <1.0 m/sec it was 75% for men and 64.1% for women. For walking speed < 0.60 m/sec it was 90.9% in women (CART did not select variables for men). Lastly, for continuous walking speed CART models, cross-validation accuracy was high at 94.0% in men and 95.3% in women.
Discussion
This study demonstrated that maximal grip strength, with and without adjustment for body size or composition, consistently discriminated community-dwelling older men and women with slowness from those without. Although DXA-derived lean mass has traditionally been viewed as an important component of the sarcopenia definition, it did not emerge as potent discriminators of slowness.
The approach taken by the SDOC differs from work done by FNIH and EWGSOP groups, but resembles others who either verified or originated new grip and/or ALM cut points.26, 27 First, in both men and women, AUC ROC results demonstrate the accuracy of tests for leg lean mass and appendicular lean mass is fair-to-poor (< ~0.70) and is similar to accuracy of age alone. While we did not statistically compare all AUC measures with each other, it is clear that grip strength derivatives provided a slight numeric increase in accuracy with findings generally stronger in men than women. This is consistent with results from the FNIH Sarcopenia Project and when considered alone that muscle related factors are more relevant for walking speed in men than women.28 While the AUC ROC analyses provided a relatively simple approach for understanding accuracy, the approach is not sufficient for variable selection. CART approaches are useful for selecting variables and is robust to collinearity between variables. As such, the SDOC approach had the most commonly considered sarcopenia variables compete as discriminators of slowness. This yielded consistency in choosing the primary variable— namely grip strength, but there was some variability in choice of secondary and tertiary nodes. Overall, the work provides new knowledge regarding to how different expressions of sarcopenia-related variables are associated with slowness in a large and diverse pooled cohort.
When the primary outcome of slowness was defined by walking speed < 0.80 m/sec, approximately 30% of males with a GRBMI<1.05 kg/kg/m2 and 50% of women with GRTBF<0.65 were slow. In men with GRBMI<1.05 kg/kg/m2, an additional node that partitioned the data at age≥83.5 yrs identified a sub-sample with an even higher prevalence of slowness (55%). However, the sub-sample comprised only 1.3% of the total sample which questions its practical utility and potential overfitting of the model despite the internal cross-validation employed. For women, there was an additional node that identified women with low ALMAFM (<0.79) who had approximately 16% higher likelihood of slowness. Then, two additional nodes were identified in women with ALMAFM≥0.79 that ended with a chance of slowness of approximately 60%— which was similar to women with weakness and low ALMAFM. Similar findings were noted with other slowness outcomes. The practical utility of these nodes is dependent on both the increase in likelihood of slowness and the total sample. As such, nodes that reflect small sub-samples are not expected to yield significant benefit when considering the potential overall impact of additional time required for screening.
In men, the variables selected and cut-points identified in the current work have some similarities and differences with those reported elsewhere. The SDOC analyses consistently chose a form of grip strength as the CART primary node (GRBMI<1.05, grip strength<35.5 kg, GRBMI<1.46). Grip strength <35.5 kg is more consistent with cut-points derived from more representative data in the Health and Retirement Survey (White men <35 kg; Black men <40kg)26 than grip strength from the FNIH Sarcopenia Project (<26 kg), EWGSOP2 (<27 kg), or Woo et al. (<27 kg).2, 6, 29 Interestingly, CART selected GRBMI<1.05, which was very similar to the FNIH Sarcopenia Project alternate weakness measure of GRBMI <1.0. Previous research on this topic also recommended variables and cut-points for ALM (or ALM/BMI, ALM/HT2), which were not selected by the SDOC analyses as primary or secondary nodes in men. The forced selection of lean mass into models from previous efforts (but not the SDOC) constitutes a major difference between the SDOC analysis and others in the field.
In women, the SDOC analyses reliably chose grip strength as the primary node with different denominators (arm lean mass, total body fat, BMI and body weight). The variability in choice of denominators is the result of increased probability of slowness that a particular grip strength denominator provides in the CART analyses. For example, grip strength/total body fat <0.65 identified 17% of women with approximately double the likelihood of slowness (50% vs. 24% with slowness). Only 4.5% of women had grip strength/arm lean mass <3.26, but they had a 4-fold higher probability of walking slower than 0.6 m/sec than women >3.26 (35.5% vs. 7.9%). For the 1.0 m/sec outcome, there was a high prevalence of women (39%) with GRBMI <0.79 and the chance of slowness was only slightly elevated (76% vs. 55% of the sample with slowness). Lastly, with continuous walking, women with grip strength/body weight <0.34 represented a large proportion of the sample at 42%. The only direct comparison to the literature is from the FNIH Sarcopenia Project which chose GRBMI <0.56 as an alternative measure of muscle weakness. This value is significantly lower than the current analysis which chose a value of <0.79 to dichotomize women with slowness defined as walking <1.0 m/s. In women, ALM was only selected as secondary or tertiary nodes when components of fat mass were also included, suggesting that fatness or adiposity rather than lean mass may be an important factor to consider for women.
Measures of DXA-based lean mass were not selected as primary nodes in men nor women. While this might be unexpected, for the past decade, accumulating evidence suggests that DXA-based lean measures are poorly correlated with functional and medical outcomes.30, 31 Technically, it is important to note that DXA uses a 3-compartment model of body composition by directly measuring the differential absorption of two photon energies of fat and bone 32. Lean mass, which includes muscle tissue, water, organ weight, and all other non-bone (fibrous tissue) and non-fat soft tissue, is derived by subtraction from the total mass. As such, a lack of direct association may reflect inaccuracy in estimating muscle mass with DXA and not necessarily the true associations between muscle mass and outcomes as recently found by Cawthon et al.33 and argued by others.34, 35
There are several strengths and some limitations of the current work. The SDOC followed guidelines established by the TRIPOD Initiative that used analytic results to guide decisions for defining the sarcopenia phenotype. Additionally, the SDOC performed multiple sensitivity analyses to evaluate and compare output from four different slowness outcomes. Additional analyses reported in a manuscript in this issue of the Journal describes the relation of low grip strength and traditional DXA-based lean mass measures with health-related outcomes such as falls, hip fracture and mortality.13 Another strength is the geographic and racial diversity of the pooled cohorts that aids in generalizability of the selected variables and associated cut-points. As for limitations, the cohorts included only a small proportion of Hispanics. We note that other methodological approaches could have been used for decision tree definitions that have different optimization criteria than CART and that variable selection could differ. The comparison of other variable selection methodologies would be a topic for future sarcopenia research. Slowness was the only outcome evaluated for potential cut-points. CART models will choose variables even if they slightly outperform others in the pool and it will not take practicality into consideration. Lastly, grip strength measurement requires a high degree of volitional effort— it can be affected by mental status, arthritis of the hand, pain, and patient motivation and effort.
Using empirical results in a large and diverse sample of older adults, the SDOC analyses suggest that grip strength with and without adjustments for body composition and anthropometrics consistently identifies older adults with higher prevalence of slowness. These findings were presented to the SDOC Consensus Panel who used them and other information to develop the SDOC Position Statement that muscle weakness, as defined by low grip strength, should be included in the definition of sarcopenia. Additionally, lean mass and other body composition metrics by themselves were not selected for identifying slow walking older adults, but they may serve as adjustment factors for grip strength. Efforts to operationally define or create diagnostic guidelines for sarcopenia should reconsider the utility of lean mass by DXA as a hallmark measure for risk stratification. Overall, this work guided the formation of the SDOC position statements and supports the inclusion of grip strength measures alone to define sarcopenia.15
Supplementary Material
Supplemental Figure S1. CART model for discriminating objective mobility limitation (walk speed <1.0 m/sec). Soft edge rectangles represent continuing nodes and hard edge rectangles represent terminal nodes.
Supplemental Figure S2. CART model for discriminating objective mobility limitation (gait speed <0.6 m/s). Soft edge rectangles represent continuing nodes and hard edge rectangles represent terminal nodes.
Supplemental Figure S3. CART model for discriminating different levels of gait speed (continuous outcome) men Panel A, Women Panel B. Soft edge rectangles represent continuing nodes and hard edge rectangles represent terminal nodes.
Supplementary Table S1. Participant characteristics of included cohorts.
Supplementary Table S2. Grip strength and walk speed protocols between included cohorts.
Supplemental Table S3. Candidate sarcopenia predictors of slowness passed to classification of regression tree (CART) models.
Supplementary Table S4. Area under the receiver operating characteristic curve and Youden’s index for walk speed < 0.6 m/s according to measures of body anthropometry, DXA lean mass and grip strength in men and women
Supplementary Table S5. Area under the receiver operating characteristic curve and Youden’s index for walk speed < 1.0 m/s according to measures of body anthropometry, DXA lean mass and grip strength in men and women
Acknowledgements and conflicts of interest
Conflicts of Interest. Dr. Fielding reports grants from National Institutes of Health (National Institute on Aging) and the USDA, during the conduct of the study; grants, personal fees and other from Axcella Health, other from Inside Tracker, grants and personal fees from Biophytis, grants and personal fees from Astellas, personal fees from Cytokinetics, personal fees from Amazentis, grants and personal fees from Nestle’, personal fees from Glaxo Smith Kline, outside the submitted work.
Sponsor’s Role. The analysis of the pooled data in the Sarcopenia Definitions and Outcomes Consortium has been supported by the National Institute on Aging (NIA, grant number AG51421) and the Foundation for the National of Institutes of Health through grants CAWT16SARC2 and BHAS16SARC2. This research was supported in part by the intramural research program at the NIA.
Funding support for the individual studies that comprise the pooled data set is gratefully acknowledged: MrOS (US): The Osteoporotic Fractures in Men (MrOS) Study is supported by National Institutes of Health funding. The following institutes provide support: the National Institute on Aging (NIA), the National Institute of Arthritis and Musculoskeletal and Skin Diseases (NIAMS), the National Center for Advancing Translational Sciences (NCATS), and NIH Roadmap for Medical Research under the following grant numbers: U01 AG027810, U01 AG042124, U01 AG042139, U01 AG042140, U01 AG042143, U01 AG042145, U01 AG042168, U01 AR066160, and UL1 TR000128. MrOS data is available online: http://mrosdata.sfcc-cpmc.net.
MrOS Hong Kong: MrOS in Hong Kong was supported by a U.S. National Institute of Health R01 Grant AR049439-01A1, the Research Grants Council Earmarked Grant CUHK 4101/02M, and a direct grant for research of The Chinese University of Hong Kong (No. 2041657).
MrOS Sweden: Financial support was received from the Swedish Research Council (2006–3832), the Swedish Foundation for Strategic Research, the ALF/LUA research grant in Gothenburg, the Lundberg Foundation, the Torsten and Ragnar Söderberg’s Foundation, Petrus and Augusta Hedlunds Foundation, the Västra Götaland Foundation, the Göteborg Medical Society and the Novo Nordisk foundation.
SOF: The Study of Osteoporotic Fractures (SOF) is supported by National Institutes of Health funding. The National Institute on Aging (NIA) provides support under the following grant numbers: R01 AG005407, R01 AR35582, R01 AR35583, R01 AR35584, R01 AG005394, R01 AG027574, and R01 AG027576.’
Health Aging and Body Composition Study (Health ABC): This study was funded by the National Institutes of Aging. This research was supported by NIA contracts N01AG62101, N01AG62103, and N01AG62106.
Cardiovascular Health Study (CHS): This research was supported by contracts HHSN268201200036C, HHSN268200800007C, HHSN268201800001C, N01HC55222, N01HC85079, N01HC85080, N01HC85081, N01HC85082, N01HC85083, N01HC85086, and grants U01HL080295 and U01HL130114 from the National Heart, Lung, and Blood Institute (NHLBI), with additional contribution from the National Institute of Neurological Disorders and Stroke (NINDS). Additional support was provided by R01AG023629 from the National Institute on Aging (NIA). A full list of principal CHS investigators and institutions can be found at CHS-NHLBI.org.
Framingham Osteoporosis Study (FOS) / Framingham Heart Study (FHS): The study was funded by grants from the US National Institute for Arthritis, Musculoskeletal and Skin Diseases and National Institute on Aging (R01 AR 41398 and U24AG051129; DPK and R01AR057118. The Framingham Heart Study of the National Heart, Lung, and Blood Institute of the National Institutes of Health and Boston University School of Medicine were supported by the National Heart, Lung, and Blood Institute’s Framingham Heart Study (N01-HC-25195).
Johnson County Study: The Johnston County Osteoarthritis Project is supported in part by cooperative agreements S043, S1734, and S3486 from the Centers for Disease Control and Prevention/Association of Schools of Public Health; the NIAMS Multipurpose Arthritis and Musculoskeletal Disease Center grant 5-P60-AR30701; and the NIAMS Multidisciplinary Clinical Research Center grant 5 P60 AR49465–03.
Concord Health and Ageing in Men Project: CHAMP is funded by the National Health and Medical Research Council (project grant number 301916) and the Ageing and Alzheimer’s Institute.
LASA: The Longitudinal Aging Study Amsterdam (LASA) is largely supported by a grant from the Netherlands Ministry of Health, Welfare and Sports, Directorate of Long-Term Care. The data collection in 2012–2013 was financially supported by the Netherlands Organization for Scientific Research (NWO) in the framework of the project “New Cohorts of young old in the 21st century” (File Number 480–10-014).
Footnotes
The National Institute on Aging, Bethesda, MD. The participation of this individual or the materials should not be interpreted as representing the official viewpoint of the U.S. Department of Health and Human Services, the National Institutes of Health or the National Institute on Aging, except where noted.
References
- [1].Cruz-Jentoft AJ, Baeyens JP, Bauer JM, et al. Sarcopenia: European consensus on definition and diagnosis: Report of the European Working Group on Sarcopenia in Older People. Age Ageing. 2010;39: 412–423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Studenski SA, Peters KW, Alley DE, et al. The FNIH sarcopenia project: rationale, study description, conference recommendations, and final estimates. The journals of gerontology Series A, Biological sciences and medical sciences. 2014;69: 547–558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Fielding RA, Vellas B, Evans WJ, et al. Sarcopenia: an undiagnosed condition in older adults. Current consensus definition: prevalence, etiology, and consequences. International working group on sarcopenia. J Am Med Dir Assoc. 2011;12: 249–256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Kim TN, Choi KM. Sarcopenia: definition, epidemiology, and pathophysiology. Journal of bone metabolism. 2013;20: 1–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Morley JE, Anker SD, von Haehling S. Prevalence, incidence, and clinical impact of sarcopenia: facts, numbers, and epidemiology-update 2014. J Cachexia Sarcopenia Muscle. 2014;5: 253–259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Cruz-Jentoft AJ, Bahat G, Bauer J, et al. Sarcopenia: revised European consensus on definition and diagnosis. Age Ageing. 2019;48: 16–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Cawthon PM, Travison TG, Manini TM, et al. Establishing the Link Between Lean Mass and Grip Strength Cut-points With Mobility Disability and Other Health Outcomes: Proceedings of the Sarcopenia Definition and Outcomes Consortium Conference. The journals of gerontology Series A, Biological sciences and medical sciences. 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Manini TM, Clark BC. Dynapenia and Aging: An Update. The journals of gerontology Series A, Biological sciences and medical sciences. 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Manini TM, Hong SL, Clark BC. Aging and muscle: a neuron’s perspective. Curr Opin Clin Nutr Metab Care. 2013;16: 21–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Fritz S, Lusardi M. White paper:“walking speed: the sixth vital sign”. Journal of geriatric physical therapy. 2009;32: 2–5. [PubMed] [Google Scholar]
- [11].Middleton A, Fritz SL, Lusardi M. Walking speed: the functional vital sign. Journal of aging and physical activity. 2015;23: 314–322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Lusardi MM. Is walking speed a vital sign? Absolutely! Topics in Geriatric Rehabilitation. 2012;28: 67–76. [Google Scholar]
- [13].Cawthon PM, Manini TM, Patel SM, et al. Putative cut-points in sarcopenia components and their associations with incident adverse-health outcomes: A pooled data analysis from the Sarcopenia Definitions and Outcomes Consortium In Submission, J Am Geriatr Soc. 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Collins GS, Reitsma JB, Altman DG, Moons KG. Transparent Reporting of a multivariable prediction model for Individual Prognosis or Diagnosis (TRIPOD): the TRIPOD statement. Ann Intern Med. 2015;162: 55–63. [DOI] [PubMed] [Google Scholar]
- [15].Bhasin S, Travison TG, Manini TM, et al. The Position Statements of the Sarcopenia Definition and Outcomes Consortium In Submission, J Am Geriatr Soc. 2019. [DOI] [PubMed] [Google Scholar]
- [16].Guralnik JM, Ferrucci L, Pieper CF, et al. Lower extremity function and subsequent disability: consistency across studies, predictive models, and value of gait speed alone compared with the short physical performance battery. The journals of gerontology Series A, Biological sciences and medical sciences. 2000;55: M221–231. [DOI] [PubMed] [Google Scholar]
- [17].Studenski S, Perera S, Patel K, et al. Gait speed and survival in older adults. Jama. 2011;305: 50–58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Onder G, Penninx BW, Ferrucci L, Fried LP, Guralnik JM, Pahor M. Measures of physical performance and risk for progressive and catastrophic disability: results from the Women’s Health and Aging Study. The journals of gerontology Series A, Biological sciences and medical sciences. 2005;60: 74–79. [DOI] [PubMed] [Google Scholar]
- [19].Purser JL, Weinberger M, Cohen HJ, et al. Walking speed predicts health status and hospital costs for frail elderly male veterans. J Rehabil Res Dev. 2005;42: 535–546. [DOI] [PubMed] [Google Scholar]
- [20].Cesari M, Kritchevsky SB, Penninx BW, et al. Prognostic value of usual gait speed in well-functioning older people-results from the health, aging and body composition study. J Am Geriatr Soc. 2005;53: 1675–1680. [DOI] [PubMed] [Google Scholar]
- [21].Cummings SR, Studenski S, Ferrucci L. A diagnosis of dismobility--giving mobility clinical visibility: a Mobility Working Group recommendation. Jama. 2014;311: 2061–2062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Du Bois D, Du Bois EF. A formula to estimate the approximate surface area if height and weight be known. 1916. Nutrition (Burbank, Los Angeles County, Calif). 1989;5: 303–311; discussion 312–303. [PubMed] [Google Scholar]
- [23].Youden WJ. Index for rating diagnostic tests. Cancer. 1950;3: 32–35. [DOI] [PubMed] [Google Scholar]
- [24].Simundic AM. Measures of Diagnostic Accuracy: Basic Definitions. EJIFCC. 2009;19: 203–211. [PMC free article] [PubMed] [Google Scholar]
- [25].Breiman L, Friedman JH, Olshen R, Stone CJ. Classification and Regression Trees: Chapman & Hall, 1984. [Google Scholar]
- [26].Duchowny KA, Peterson MD, Clarke PJ. Cut Points for Clinical Muscle Weakness Among Older Americans. American journal of preventive medicine. 2017;53: 63–69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Woo J, Leung J, Morley JE. Defining sarcopenia in terms of incident adverse outcomes. J Am Med Dir Assoc. 2015;16: 247–252. [DOI] [PubMed] [Google Scholar]
- [28].Dam T-T, Peters KW, Fragala M, et al. An evidence-based comparison of operational criteria for the presence of sarcopenia. The journals of gerontology Series A, Biological sciences and medical sciences. 2014;69: 584–590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Woo J, Leung J. Anthropometric Cut Points for Definition of Sarcopenia Based on Incident Mobility and Physical Limitation in Older Chinese People. The journals of gerontology Series A, Biological sciences and medical sciences. 2016;71: 935–940. [DOI] [PubMed] [Google Scholar]
- [30].Manini TM, Clark BC. Dynapenia and aging: an update. The journals of gerontology Series A, Biological sciences and medical sciences. 2012;67: 28–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].Schaap LA, Koster A, Visser M. Adiposity, muscle mass, and muscle strength in relation to functional decline in older persons. Epidemiologic reviews. 2013;35: 51–65. [DOI] [PubMed] [Google Scholar]
- [32].Blake GM, Fogelman I. Technical principles of dual energy x-ray absorptiometry. Seminars in nuclear medicine. 1997;27: 210–228. [DOI] [PubMed] [Google Scholar]
- [33].Cawthon PM, Orwoll ES, Peters KE, et al. Strong Relation between Muscle Mass Determined by D3-creatine Dilution, Physical Performance and Incidence of Falls and Mobility Limitations in a Prospective Cohort of Older Men. The journals of gerontology Series A, Biological sciences and medical sciences. 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [34].Clark BC, Tavoian D, Goodpaster BH, Cawthon PM, Hansen RD, Manini TM. Comment on: “Pitfalls in the measurement of muscle mass: a need for a reference standard” by Buckinx et al et al. J Cachexia Sarcopenia Muscle. 2018;9: 1269–1271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [35].Evans WJ, Hellerstein M, Orwoll E, Cummings S, Cawthon PM. D3 -Creatine dilution and the importance of accuracy in the assessment of skeletal muscle mass. J Cachexia Sarcopenia Muscle. 2019;10: 14–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental Figure S1. CART model for discriminating objective mobility limitation (walk speed <1.0 m/sec). Soft edge rectangles represent continuing nodes and hard edge rectangles represent terminal nodes.
Supplemental Figure S2. CART model for discriminating objective mobility limitation (gait speed <0.6 m/s). Soft edge rectangles represent continuing nodes and hard edge rectangles represent terminal nodes.
Supplemental Figure S3. CART model for discriminating different levels of gait speed (continuous outcome) men Panel A, Women Panel B. Soft edge rectangles represent continuing nodes and hard edge rectangles represent terminal nodes.
Supplementary Table S1. Participant characteristics of included cohorts.
Supplementary Table S2. Grip strength and walk speed protocols between included cohorts.
Supplemental Table S3. Candidate sarcopenia predictors of slowness passed to classification of regression tree (CART) models.
Supplementary Table S4. Area under the receiver operating characteristic curve and Youden’s index for walk speed < 0.6 m/s according to measures of body anthropometry, DXA lean mass and grip strength in men and women
Supplementary Table S5. Area under the receiver operating characteristic curve and Youden’s index for walk speed < 1.0 m/s according to measures of body anthropometry, DXA lean mass and grip strength in men and women