Abstract
Background:
Atrial fibrillation (AF) is common in patients with transthyretin cardiac amyloidosis (ATTR-CA). The optimal strategy to prevent strokes in patients with ATTR-CA and AF is unknown.
Objectives:
To compare outcomes in patients with ATTR-CA and AF treated with warfarin versus novel oral anticoagulants (NOACs).
Methods:
This study was a retrospective analysis of patients with ATTR-CA stratified by presence or absence of AF and anticoagulation therapy. The primary outcome included a time to event analysis for the combined outcomes of stroke, transient ischemic attack (TIA), major bleed, or death.
Results:
Of 290 patients, 217 patients (74.8%) had AF. Of those with AF (n=217), 78 (35.9%) patients received warfarin compared with 116 (53.5%) patients who received NOACs. There were 17 thrombotic events, all in those diagnosed with AF compared with none in the patients without AF (p=0.01). Over a mean follow-up of 2.4 years (range 0.1–12) there was no difference in primary outcome between those with AF treated with warfarin compared with NOACs (p=0.35).
Conclusion:
Patient with ATTR-CA and AF are at increased risk for stroke compared to patients with ATTR-CA and without AF. Thrombotic events and major bleeds did not differ between those who received warfarin and NOACs.
Keywords: anticoagulation, atrial fibrillation, cardiac amyloidosis, transthyretin, stroke
Introduction
There are two main types of transthyretin cardiac (ATTR-CA): wild type transthyretin amyloidosis (ATTRwt), and hereditary transthyretin amyloidosis (ATTRv), [1,2]. Among subjects with ATTR-CA, atrial fibrillation (AF) is the most common sustained arrhythmia, affecting 20–70% of patients [3–8]. Anticoagulation is a key tenant of management in atrial fibrillation, but equivalence of warfarin and novel oral anticoagulants (NOACs) is unknown in this population.
Proposed mechanisms for the development of AF include deposition of fibrils in the atria leading to sinoatrial fibrosis, disruption of the conduction system, or focal fibrosis [9,10]. Alternatively, elevated left ventricular filling pressure from amyloid deposits in the ventricle may secondarily cause increased filling pressures and atrial wall dilatation and subsequently atrial fibrillation [11]. Moreover, while AF is independently associated with stroke, AF in cardiac amyloidosis is also associated with increased risk of cardiac thrombus [12]. Specifically, up to 18% of patients with ATTR-CA had an intra-atrial thrombus, while 8% of ATTR-CA patients died from thromboembolic events [12–14].
In patients with non-valvular AF and elevated congestive heart failure, hypertension, age above 75, diabetes, stroke, vascular disease, age above 65, sex (CHA2DS2-VASc) score, there is a Class I indication to treat patients preferably with NOACs, however warfarin is also acceptable therapy [15]. While a few studies have characterized the role of atrial fibrillation in ATTR-CA [3,8], there is limited data on the optimal anticoagulant strategy. Specifically, little is known about the safety of anticoagulation with NOACs in this population and whether there are differences in embolic events such as stroke or transient ischemic attack (TIA). Given the older age of the subjects, bleeding and thrombotic risk are higher. Moreover, compliance with a complex medical regimen and diet may be more difficult in this older adult population. Based on literature review and our experience, we hypothesized that patients with ATTR-CA and AF treated with NOACs would have superior outcomes in embolic events or major bleeds compared to patients with ATTR-CA and AF treated with warfarin.
Materials and methods
The subjects were adults (≥ 18 years of age) diagnosed with ATTR-CA and referred to the Cardiac Amyloidosis Program Columbia Irving Medical Center / New York- Presbyterian Hospital from December 2001 to February 2019. The Columbia Institutional Review Board approved this study.
Of all patients, 154 were diagnosed with ATTR-CA based on endomyocardial biopsy and 32 patients based on extra-cardiac biopsy along with a typical cardiac phenotype by echocardiography or cardiac magnetic resonance imaging. Biopsies were Congo red positive with confirmation that transthyretin was the precursor protein either by mass spectroscopy or immunohistochemistry. Ninety-eight patients were diagnosed by a Technetium Tc99m Pyrophosphate in the absence of a monoclonal protein, and the remaining six were diagnosed prior to the use of scintigraphy by a combination of negative serologic testing for monoclonal proteins, genetic testing revealing a TTR variant, and typical phenotype on echocardiograms [16]. Patients were labeled as having atrial fibrillation if they had atrial fibrillation or atrial flutter on 12 lead ECG or by clinical history, classified according to standard definitions for paroxysmal, persistent or long-term persistent [15].
The type of anticoagulation was dichotomized as either none, NOAC/DOAC or warfarin. For the rare patient (n=1) who switched anticoagulation, the type of anticoagulant was defined as the one they were taking at the time of the event. For patients receiving warfarin, they were defined as having a labile international normalized ratio (INR) if the documented INR was in range <60% of the time. Patients were diagnosed with strokes and TIAs by a combination of computerized tomography, magnetic resonance imaging, chart review, and patient reporting. Major bleeds were defined as requiring hospitalization, intracranial bleeding, or any drop in hemoglobin necessitating a transfusion.
The primary analysis was a time for first event for the combined endpoint of stroke, TIA, major bleed, or death among patients with ATTR-CA and AF receiving warfarin compared with NOACs. Patients who received a heart transplant were censored at time of transplant. Secondary endpoints included examination of stroke or TIA comparing patients with ATTR-CA without AF, patients with ATTR-CA and AF on warfarin, and patients with ATTR-CA and AF on NOACs.
Statistical Analysis
Categorical variables were reported as number of patients or percent of total sample, while continuous variables were expressed as mean +/− standard deviation. Analysis of categorical variables was performed with chi- squared, Fisher’s Exact Test, and event rate analysis, using a 2-sided unpaired Student’s t-test. All p values were 2-sided, and a cut-off value of <0.05 was considered significant. A Kaplan Meier curve with log-rank analysis was used to examine a combined primary outcome. Statistical analyses were conducted using R version 3.6.1.
Results
There were a total of 290 patients with ATTR-CA, for which baseline characteristics can be found in table 1. Of 290 patients, 217 (74.8%) had or developed a diagnosis of atrial fibrillation with 159/290 patients (54.5%) who presented with AF at baseline. Patients with AF were older compared to those without AF and were more often male and Caucasian. Wild-type ATTR (ATTRwt) was more prevalent in the AF group compared to patients without AF. The only significant difference between the patients treated with warfarin and NOACs was a higher hypertension, abnormal renal and liver function, stroke, bleeding, labile international normalized ratio (HAS-BLED) score in the warfarin group, which takes into account labile INR, only applicable to this group. The type of anticoagulation employed is shown in Table 2. NOACs were the most common form of anticoagulation, and apixaban was the most commonly used NOAC.
Table 1:
Clinical Characteristics of ATTR-CA with comparing patients without AF with patients with AF, along with comparing patients treated with warfarin with patients treated with NOACs
| Baseline Characteristics for Patients with ATTR-CA Comparing Atrial Fibrillation vs No Atrial Fibrillation and Warfarin vs. NOACs | ||||||
|---|---|---|---|---|---|---|
| Arrhythmias | Atrial Fibrillation Treatment | |||||
| Atrial Fibrillation | No Atrial Fibrillation | p Value | Warfarin | NOACs | p Value | |
| (n=217) | (n=73) | (n=78) | (n=116) | |||
| Age | 75.0 +/− 8.1 | 71.0 +/− 9.8 | < 0.01 | 75.2+/−8.3 | 75.3 +/− 7.6 | 0.97 |
| Gender (Male) | 194 (89.4%) | 54 (73.9%) | < 0.01 | 69 (88.5%) | 103 (88.8%) | 0.94 |
| Ethnicity | < 0.01 | 0.17 | ||||
| Caucasian | 165 | 42 | 58 | 92 | ||
| African | 42 | 30 | 18 | 17 | ||
| American | ||||||
| Genotype | < 0.01 | 0.17 | ||||
| Wildtype | 169 | 28 | 59 | 97 | ||
| Hereditary | 48 | 44 | 19 | 19 | ||
| A120S | 1 | 0 | 0 | 1 | ||
| Ala117Ser | 0 | 1 | 0 | 0 | ||
| Asp38Ala | 1 | 0 | 1 | 0 | ||
| Asp70Lys | 1 | 0 | 0 | 1 | ||
| Gln89 | 0 | 1 | 0 | 0 | ||
| Phe64Leu | 1 | 2 | 1 | 0 | ||
| Ser23Asn | 0 | 1 | 0 | 0 | ||
| Thr35 | 1 | 0 | 1 | 0 | ||
| Thr59Lys | 0 | 2 | 0 | 0 | ||
| Thr60Ala | 5 | 6 | 1 | 4 | ||
| Thr79Lys | 1 | 0 | 0 | 1 | ||
| V122I | 38 | 30 | 21 | 11 | ||
| Val30Met | 1 | 2 | 1 | 0 | ||
| BMI | 26.6 +/−3.8 | 25.6+/−3.9 | 0.05 | 26.1 +/−3.4 | 27.1+/−4.2 | 0.09 |
| Hypertension | 118 (54.3%) | 42 (57.5%) | 0.63 | 38 (48.7%) | 61 (52.6%) | 0.59 |
| Diabetes | 28 (12.9%) | 8 (10.9%) | 0.66 | 7 (9.0%) | 18 (15.5%) | 0.18 |
| Prior embolism | 35 (16.1%) | 7 (9.6%) | 0.17 | 16 (20.5%) | 14 (12.1%) | 0.11 |
| CKD Stage IV-V | 15 (6.9%) | 1 (1.4%) | 0.08 | 7 (9.0%) | 6 (5.2%) | 0.38 |
| Renally dosed NOACs | - | - | - | 27 (23.3%) | ||
| Aspirin | 73 (33.6%) | 37 (50.7%) | 0.01 | 29 (37.2%) | 36 (31.0%) | 0.44 |
| P2Y12 Inhibitors | 14 (6.5%) | 6 (8.2%) | 0.60 | 9 (11.5%) | 5 (4.3%) | 0.09 |
| Diflunisal | 31 (14.3%) | 19 (26.0%) | 0.03 | 10 (4.6%) | 17 (14.7%) | 0.83 |
| Tafamadis | 17 (7.8%) | 1 (1.4%) | 0.05 | 5 (6.4%) | 10 (8.6%) | 0.57 |
| CHA2DS2-VASc | 3.7 +/− 1.2 | 3.5 +/− 1.5 | 0.15 | 3.7+/− 1.2 | 3.8+/− 1.2 | 0.8 |
| HAS-BLED | 2.6 +/− 0.9 | 1.9 +/− 1.0 | <0.01 | 3.1 +/− 0.9 | 2.5 +/− 0.7 | < 0.01 |
AF= atrial fibrillation, NOACs = novel oral anticoagulants, BMI = body mass index, CKD = chronic kidney disease
Table 2.
Method of anticoagulation in patients with AF and ATTR-CA
| Method of anticoagulation | Number of patients (n=217) |
|---|---|
| Warfarin | 78 (35.9%) |
| NOAC | 116 (53.5%) |
| Dabigatran | 10 (4.6%) |
| Rivoraxaban | 45 (20.7%) |
| Apixaban | 60 (27.6%) |
| Warfarin switched to NOAC | 4 (1.8%) |
| No anticoagulation | 21 (9.7%) |
| Patient declined | 3 (1.4%) |
| Adverse event with anticoagulation | 3 (1.4%) |
| End of life / hospice | 2 (0.9%) |
| Peri transplant | 3 (1.4%) |
| Unknown | 10 (4.6%) |
The time to combined primary outcome of thromboembolism, major bleed, or death did not differ between patients on warfarin and patients on NOACs (p=0.35, Figure 1). Of primary events, 112 (38%) subjects experienced a stroke (n=9), TIA (n=8), major bleed (n = 21), and/or death (n=99). There were 17 embolic events in 15 patients; two patients had both a stroke and TIA. All thromboembolic events occurred in patients who had or developed AF compared with 0 patients in the group without AF (p = 0.01). Of patients treated with warfarin, six patients had an embolic event (five with stroke) for an event rate of 2.9 per 100 person years compared with eight patients treated with NOACs (three with stroke) for an event rate of 3.9 per 100 person years (p= 0.74). One patient with AF was not receiving anticoagulation at the time of the embolic event for an event rate of 1.9 per 100 person years.
Figure 1:
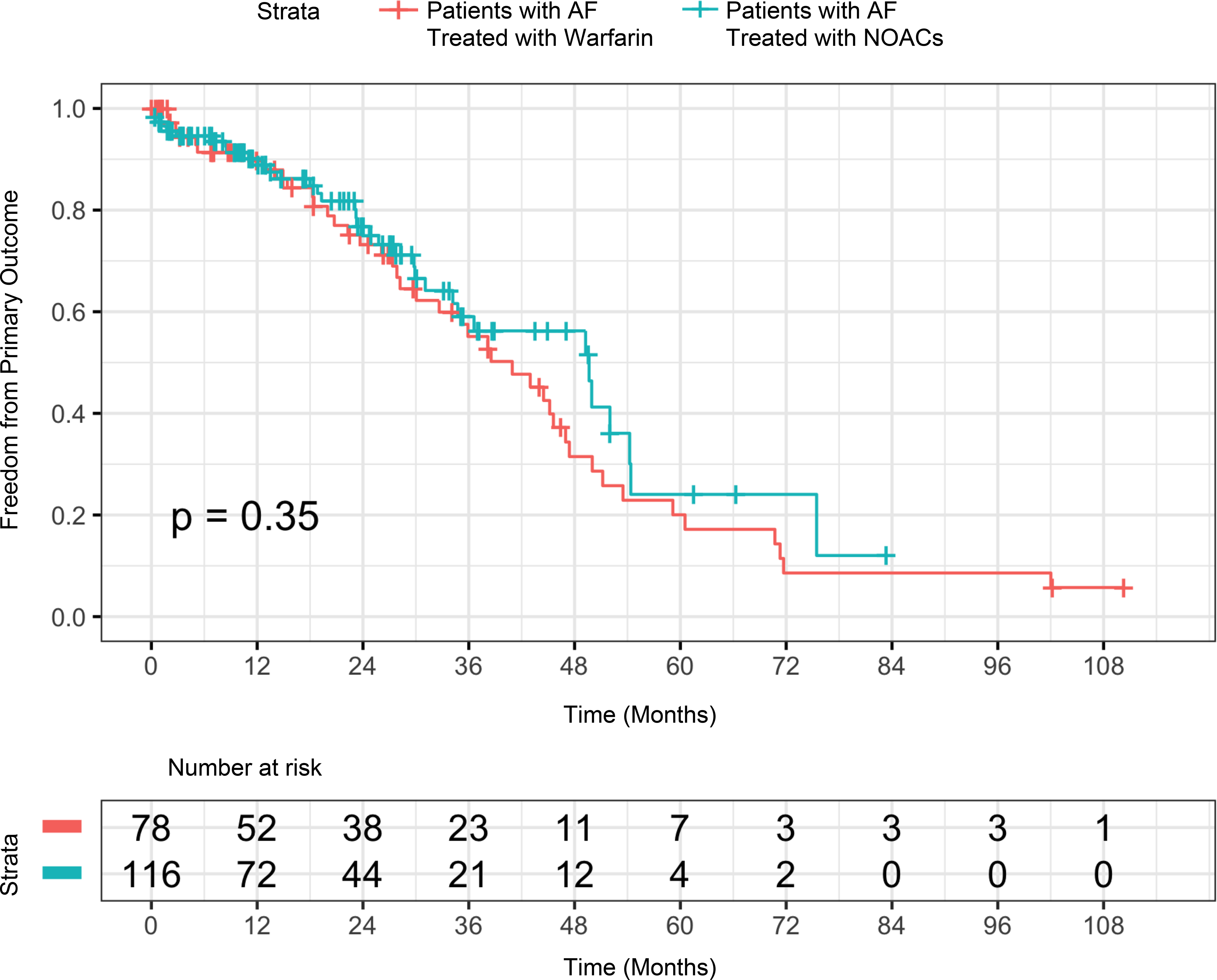
Time to event analysis of primary outcome including stroke, TIA, major bleed, or death in Patients with ATTR-CA and AF treated with warfarin compared with ATTR-CA and AF treated with NOACs. This Kaplan-Meier curve demonstrates the freedom from primary outcome comparing patients treated with warfarin with patients treated with NOACs (p=0.35).
There were 21 patients who had a major bleed while on anticoagulation. Of patients who were treated with warfarin, nine patients had a major bleed for an event rate of 3.74 per 100 person years compared with 12 patients treated with NOACs for an event rate of 5.21 per 100 person years (p= 0.45). Of the 78 patients treated with warfarin, 48 patients had data available for INRs and 87.5% of these patients had a labile INR. All patients on warfarin with a stroke or TIA (n=15) had labile INRs while no patients with consistent INRs in normal range had a stroke / TIA. Additionally, there were nine patients with a major bleed, and all nine had a labile INR compared to zero patients with a major bleed in the group with INRs in normal range.
Discussion
This study examined anticoagulation patterns and outcomes in patients with AF and ATTR-CA. ATTR-CA frequently presents with AF, and our study confirmed that 74% of patients in this group had or developed AF. Additionally, this study found no difference in combined outcome of stroke, TIA, major bleeding, or death in patients with ATTR-CA and AF treated with warfarin versus NOACs.
In our population, there was no difference in the rate of strokes and TIAs in warfarin-treated patients (2.9 per 100 person years) compared with patients treated with NOACs (3.9 per 100 person years). In the PREFER in AF study, 124 of 5310 patients who were treated with warfarin for AF had a thromboembolic event for an event rate of 2.1 per 100 person years compared with 53 out of 3156 patients who were treated with NOACs for an event rate of 1.7 per 100 person years [17]. For a heart failure population, the event rate of ischemic stroke in patients with AF was 2.5 – 3.4 per 100 person years [18]. Comparing AF and ATTR-CA population in this study, the event rate is similar though slightly higher, which may be concordant with previous literature showing cardiac amyloidosis is an independent risk for thromboembolism [12,13].
In terms of anticoagulation, while initial randomized trials focused on non-inferiority, many meta-analyses have shown NOACs to have a reduction in systemic embolism, major bleeding, hemorrhagic stroke, and all cause mortality compared with warfarin [19–21]. Patients with ATTR-CA deserve special consideration in terms of both increased risk of cardioembolic emboli and increased risk of bleeding, particularly intracranial hemorrhage [12,22]. In the general population, warfarin had an event rate of major bleeding in 2.2 – 3.9 per 100 patient years in various studies compared with event rate of 2.9 – 4.3 per 100 person years for NOACs [23,24]. In our population, the event rate of major bleeds was 3.7 and 5.2 per 100 person years in warfarin and NOACs respectively, which is on the higher end compared with reported values. Additionally, 87% patients had labile INR. Past studies have shown that patients treated with warfarin were in therapeutic range for warfarin around 55 to 68% [25]. While our study did not show superiority or significant differences of NOACs compared with warfarin, given that it is challenging to remain in a therapeutic INR range, especially in our older population, it would be important to consider NOACs as an alternative strategy for anticoagulation
This study is a retrospective study completed at a single center. The primary outcomes, namely, stroke, TIA, and major bleeding, are rare events limiting the statistical power in our population. Additionally, many patients had INRs checked at different institutions limiting the data available for this study. Lastly strokes and TIAs were diagnosed by patient report when imaging was not available.
In conclusion, we found that a majority of patients with ATTR-CA have or will develop AF over the course of their disease and those with AF and ATTR-CA are at increased risk of thromboembolic event compared to patients with ATTR-CA without AF. Importantly, there were no differences between warfarin and NOACs for thromboembolic events or major bleeds. Further studies with larger cohorts of ATTR-CA patients are needed to confirm these findings.
Acknowledgments
Funding: Dr. Maurer is funded by K24-AG036778 from the National Institute on Aging.
Disclosure statement
Dr. Maurer’s institution received research support for clinical studies from Pfizer and Alnylam. He has served on advisory boards or data safety and monitoring boards for Ackea, Ionis, Prothena, Pfizer, Alnyam, Eidos and GSK. The other authors report no conflict of interest.
Abbreviations:
- AF
atrial fibrillation
- ATTR-CA
transthyretin cardiac amyloidosis
- ATTRv
hereditary transthyretin amyloidosis
- ATTRwt
wild type transthyretin amyloidosis
- CHA2DS2-VASc
congestive heart failure, hypertension, age above 75, diabetes, stroke, vascular disease, age above 65, sex
- ECG
electrocardiogram
- HAS-BLED
hypertension, abnormal renal and liver function, stroke, bleeding, labile international normalized ratio, elderly, drugs or alcohol
- INR
international normalized ratio
- NOACs
novel oral anticoagulants
- TIA
transient ischemic attack
References:
- 1.Halatchev IG, Zheng J, Ou J. Wild-type transthyretin cardiac amyloidosis (ATTRwt-CA), previously known as senile cardiac amyloidosis: clinical presentation, diagnosis, management and emerging therapies. J Thorac Dis. 2018;10(3):2034–2045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Maurer MS, Elliott P, Comenzo R, et al. Addressing common questions encountered in the diagnosis and management of cardiac amyloidosis. Circulation. 2017;135(14):1357–1377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Longhi S, Quarta CC, Milandri A, et al. Atrial fibrillation in amyloidotic cardiomyopathy: prevalence, incidence, risk factors and prognostic role. Amyloid. 2015;22(3):147–155. [DOI] [PubMed] [Google Scholar]
- 4.Manuguerra R, Callegari S, Corradi D. Inherited structural heart diseases with potential atrial fibrillation occurrence. J Cardiovasc Electrophysiol. 2016;27(2):242–252. [DOI] [PubMed] [Google Scholar]
- 5.Murtagh B, Hammill SC, Gertz MA, et al. Electrocardiographic findings in primary systemic amyloidosis and biopsy-proven cardiac involvement. Am J Cardiol. 2005;95(4):535–537. [DOI] [PubMed] [Google Scholar]
- 6.Grogan M, Scott CG, Kyle RA, et al. Natural history of wild-type transthyretin cardiac amyloidosis and risk stratification using a novel staging system. J Am Coll Cardiol. 2016;68(10):1014–1020. [DOI] [PubMed] [Google Scholar]
- 7.Maurer MS, Hanna M, Grogan M, et al. Genotype and phenotype of transthyretin cardiac amyloidosis: THAOS (Transthyretin Amyloid Outcome Survey). J Am Coll Cardiol. 2016;68(2):161–172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Mints YY, Doros G, Berk JL, et al. Features of atrial fibrillation in wild-type transthyretin cardiac amyloidosis: a systematic review and clinical experience. ESC Heart Fail. 2018;5(5):772–779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ridolfi RL, Bulkley BH, Hutchins GM. The conduction system in cardiac amyloidosis. Clinical and pathologic features of 23 patients. Am J Med. 1977;62(5):677–686. [DOI] [PubMed] [Google Scholar]
- 10.Rocken C, Peters B, Juenemann G, et al. Atrial amyloidosis: an arrhythmogenic substrate for persistent atrial fibrillation. Circulation. 2002;106(16):2091–2097. [DOI] [PubMed] [Google Scholar]
- 11.Falk RH. Diagnosis and management of the cardiac amyloidoses. Circulation. 2005;112(13):2047–2060. [DOI] [PubMed] [Google Scholar]
- 12.Feng D, Syed IS, Martinez M, et al. Intracardiac thrombosis and anticoagulation therapy in cardiac amyloidosis. Circulation. 2009;119(18):2490–2497. [DOI] [PubMed] [Google Scholar]
- 13.Feng D, Edwards WD, Oh JK, et al. Intracardiac thrombosis and embolism in patients with cardiac amyloidosis. Circulation. 2007;116(21):2420–2426. [DOI] [PubMed] [Google Scholar]
- 14.Ruberg FL, Grogan M, Hanna M, et al. Transthyretin amyloid cardiomyopathy: JACC State-of-the-Art Review. J Am Coll Cardiol. 2019;73(22):2872–2891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.January CT, Wann LS, Calkins H, et al. 2019 AHA/ACC/HRS Focused Update of the 2014 AHA/ACC/HRS Guideline for the Management of Patients With Atrial Fibrillation. Circulation. 2019;140(2):e125–e151. [DOI] [PubMed] [Google Scholar]
- 16.Gillmore JD, Maurer MS, Falk RH, et al. Nonbiopsy diagnosis of cardiac transthyretin amyloidosis. Circulation. 2016;133(24):2404–2412. [DOI] [PubMed] [Google Scholar]
- 17.Rohla M, Weiss TW, Pecen L, et al. Risk factors for thromboembolic and bleeding events in anticoagulated patients with atrial fibrillation: the prospective, multicentre observational PREvention oF thromboembolic events - European Registry in Atrial Fibrillation (PREFER in AF). BMJ Open. 2019;9(3):e022478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.McManus DD, Hsu G, Sung SH, et al. Atrial fibrillation and outcomes in heart failure with preserved versus reduced left ventricular ejection fraction. J Am Heart Assoc. 2013;2(1):e005694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Dentali F, Riva N, Crowther M, et al. Efficacy and safety of the novel oral anticoagulants in atrial fibrillation: a systematic review and meta-analysis of the literature. Circulation. 2012;126(20):2381–2391. [DOI] [PubMed] [Google Scholar]
- 20.Adam SS, McDuffie JR, Ortel TL, et al. Comparative effectiveness of warfarin and new oral anticoagulants for the management of atrial fibrillation and venous thromboembolism: a systematic review. Ann Intern Med. 2012;157(11):796–807. [DOI] [PubMed] [Google Scholar]
- 21.Ntaios G, Papavasileiou V, Diener HC, et al. Nonvitamin-K-antagonist oral anticoagulants in patients with atrial fibrillation and previous stroke or transient ischemic attack: a systematic review and meta-analysis of randomized controlled trials. Stroke. 2012;43(12):3298–3304. [DOI] [PubMed] [Google Scholar]
- 22.Cannistraro RJ, Meschia JF. The clinical dilemma of anticoagulation use in patients with cerebral amyloid angiopathy and atrial fibrillation. Curr Cardiol Rep. 2018;20(11):106. [DOI] [PubMed] [Google Scholar]
- 23.van Walraven C, Hart RG, Singer DE, et al. Oral anticoagulants vs aspirin in nonvalvular atrial fibrillation: an individual patient meta-analysis. JAMA. 2002;288(19):2441–2448. [DOI] [PubMed] [Google Scholar]
- 24.Lamberts M, Staerk L, Olesen JB, et al. Major bleeding complications and persistence with oral anticoagulation in non-valvular atrial fibrillation: contemporary findings in real-life Danish patients. J Am Heart Assoc. 2017;6(2). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Agarwal S, Hachamovitch R, Menon V. Current trial-associated outcomes with warfarin in prevention of stroke in patients with nonvalvular atrial fibrillation: a meta-analysis. Arch Intern Med. 2012;172(8):623–631; discussion 631–633. [DOI] [PubMed] [Google Scholar]


