Abstract
Background
We validated the Data collection on Adverse events of anti-HIV Drugs (D:A:D) full- and short-risk score models for CKD in the Asian HIV cohorts.
Settings
A validation study among people living with HIV(PLHIV) aged ≥18 years among the cohorts in the Asia-Pacific region.
Methods
PLHIV with baseline eGFR>60 mL/min/1.73m2 were included for validation of the D:A:D CKD full version and the short version without cardiovascular risk factors. Those with <3 eGFR measurements from baseline or previous exposure to potentially nephrotoxic antiretrovirals were excluded. Kaplan-Meier methods were used to estimate the probability of CKD development. Area Under the Receiver Operating Characteristics (AUROC) was also used to validate the risk score.
Results
We included 5,701 participants in full model(median 8.1 [IQR 4.8-10.9] years follow-up) and 9,791 in short model validation(median 4.9 [IQR 2.5-7.3] years follow-up). The crude incidence rate of CKD was 8.1 (95%CI 7.3-8.9) per 1,000 person-years(PYS) in the full model cohort and 10.5 (95%CI 9.6-11.4) per 1,000 PYS in the short model cohort. The progression rates for CKD at 10 years in the full model cohort were 2.7%, 8.9% and 26.1% for low-, medium- and high-risk groups, and 3.5%, 11.7% and 32.4% in the short model cohort. The AUROC for the full and short risk score was 0.81 (95%CI 0.79-0.83) and 0.83 (95%CI 0.81-0.85), respectively.
Conclusion
The D:A:D CKD full- and short-risk score performed well in predicting CKD events among Asian PLHIV. These risk prediction models may be useful to assist clinicians in identifying individuals at high risk of developing CKD.
Keywords: chronic kidney disease, HIV/AIDS, people living with HIV, CKD risk score, the Asia-Pacific
Background
Chronic non-communicable diseases such as cardiovascular and renal diseases are the major challenges in HIV care as longevity of people living with HIV (PLHIV) continues to increase with advances in antiretroviral therapy (ART). Several studies have shown that chronic kidney disease (CKD) is more common in PLHIV than the general population and is also related to other comorbidities such as cardiovascular diseases and mortality1-4. There is also an increasing number of reports on CKD development among PLHIV globally and the prevalence varies across different regions, ranging from 6-15% among Asian PLHIV 5-8.
CKD poses an important challenge to HIV care services and health systems, especially within settings where treatments of end-stage kidney disease, such as renal replacement therapy, are less widely accessible 9. Risk score prediction models have been developed to predict the development of CKD in the general population 10,11 as well as in PLHIV 12,13. However, the application of these risk scores in clinical practice is still limited. A systematic review suggested that most of the renal prediction models being developed were generally poorly reported or lacked validation across different ethnicities 14.
Mocroft and colleagues developed a risk score model for CKD in PLHIV based on the Data Collection on Adverse Events of Anti-HIV Drugs (D:A:D) study 15. This CKD risk prediction score has integrated several HIV- and non-HIV-related factors including age, sex, HIV exposure, nadir CD4 cell count, hepatitis C co-infection, and cardiovascular risk factors. Moreover, the short version of the risk score model incorporates a limited number of variables, which can be particularly useful in settings with limited diagnostic tools or resources to routinely monitor cardiovascular risks among PLHIV. The model was externally validated in two different HIV cohorts (the Royal Free Hospital Clinic Cohort and the pooled samples from INSIGHT network trials) and the validation results showed similar predictive abilities of score models for CKD progression as the derivation cohort 15. More recently, the risk score was further validated in cohorts from Australia and U.S. and the findings suggested the D:A:D CKD risk model to be a useful tool in clinical settings 16,17.
Since the D:A:D CKD risk score model was developed within mainly European populations with the previous few validations done in other populations, the validity of these risk scores in Asian PLHIV population is uncertain. In this study, we aimed to validate both full and short versions of the D:A:D CKD risk prediction score models among two HIV cohorts from the Asia-Pacific.
Methods
Study population and design
The D:A:D CKD full- and short-risk score models were validated in two related Asian HIV cohorts. The TREAT Asia HIV Observational Database (TAHOD) is a collaborative observational cohort study established in 2003 that involves 21 sites in Cambodia, China and Hong Kong SAR, India, Indonesia, Japan, Malaysia, the Philippines, Singapore, South Korea, Taiwan, Thailand and Vietnam. Data transfers occur biannually and include data on demographics, weight, height, hepatitis infections, HIV characteristics such as CD4 counts, HIV-1 RNA levels and safety parameters such as creatinine, liver function tests, lipid profiles and fasting blood glucose levels, etc. The TAHOD Low Intensity Transfer (TAHOD-LITE) study is a sub-study of TAHOD which included retrospective data from 10 TAHOD sites in 8 countries. Contrary to TAHOD, TAHOD-LITE includes the entire adult clinical population at the participating sites, but collects a more limited data set (demographics, hepatitis serology, ART history, and HIV-related laboratory results). Not all TAHOD sites participated in TAHOD-LITE cohort. TAHOD selects patients who were most likely to remain in care. For the sites that participate in both TAHOD and TAHOD-LITE, all TAHOD patients should exist in TAHOD-LITE 18. Characteristics of TAHOD and TAHOD-LITE have been previously described 19-21. For the current study, we used the March 2019 TAHOD data transfer to validate of the CKD full risk score model and the 2017 TAHOD-LITE data transfer to validate the short risk score model. As TAHOD and TAHOD-LITE are entirely observational in nature, participation in the cohorts does not direct any clinic visits or follow-up schedules of the participants. Furthermore, all laboratory/imaging tests and clinical interventions including the choice of ART regimen are performed according to the site’s local practices. Participants in TAHOD were followed up from January 2003 to March 2019 and participants in TAHOD-LITE were followed-up from January 2003 to September 2017.
The eligible criteria used was adapted from the D:A:D CKD development study for the validation purposes. Adults participants (age ≥18 years at enrolment) were included in the analyses if they had ≥3 serum creatinine assessments which include the baseline creatinine assessment and more than 3 months of follow-up between the first and last creatinine assessment. Participants were excluded if they had previous exposure to potentially nephrotoxic antiretrovirals (ARVs): tenofovir disoproxil fumarate (TDF), atazanavir (ATV), ritonavir-boosted atazanavir (ATV/r), ritonavir-boosted lopinavir (LPV/r), or other boosted protease inhibitors before the baseline timepoint.
Data and definitions
The Chronic Kidney Disease Epidemiology Collaboration (CKD-EPI) formula was used to calculate the estimated glomerular filtration rate (eGFR) using serum creatinine levels 22. Baseline was defined as the first normal test result of eGFR (> 60 ml/min/1.73m2) occurred within the observed time period of each cohort. CKD was defined as having at least two consecutive eGFR measurements ≤60 ml/min/1.73 m2 taken at least 3 months apart.
Risk factors incorporated in the evaluation of full risk score model include age, sex, history of injecting drug use, nadir CD4 count, hepatitis C co-infection (defined as positive HCV antibody plus a detectable or unknown presence of HCV RNA level), baseline eGFR, and cardiovascular risk factors (hypertension, diabetes mellitus and prior cardiovascular diseases, CVD). Hypertension was defined as a blood pressure of >150/100 mmHg. Diabetes mellitus was defined as two fasting plasma glucose assessments ≥126 mg/dL and a prior CVD event was defined as an occurrence of myocardial infarction, invasive cardiovascular procedure, or stroke. Risk factors used in the short risk score model were the same as full model without including the CVD risk factors.
Validation of D:A:D full and short risk score models
Risk factors represented measurements taken simultaneously or prior to the baseline eGFR measurement. Individual risk scores were calculated by assigning the points provided in the D:A:D CKD risk score model 23 to the presence of each risk factor at baseline. Participants with missing cardiovascular risks were categorized as not having the risks. Each participant’s total risk score was calculated by counting the assigned risk points. Participants were categorized into low- (risk score < 0), medium- (risk score 0–4), and high-risk (risk score ≥ 5) groups based on their total risk score. The same categories were applicated for the D:A:D short version without having cardiovascular risk factors in the TAHOD-LITE cohort. For example, individuals who had a baseline eGFR of > 60 to ≤ 70 ml/min/1.73m2 scored were assigned 6 risk points and those who had nadir CD4 count >200 scored −1, to be used in the risk score calculations.
The crude incidence rates for CKD were calculated per 1,000 person-years of follow-up (PYFU). Crude CKD incidence rates and CKD progression rates as estimated by Kaplan-Meier methods were presented by risk group for the full and the short risk score model. Adjusted incidence risk ratios (aIRRs) per one-point increase in risk score (Poisson regression methods) were provided for both risk score models. Furthermore, Area Under the Receiver Operating Characteristics (AUROC) of CKD risk scores were estimated with logistic regression models to evaluate the performance of both full and short risk score models in the TAHOD and TAHOD-LITE cohorts. SAS version 9.4 and Stata version 14 (StataCorp LP, College Station, Texas, USA) were used for all data management and statistical analysis of the validation process.
Ethical considerations
Institutional Review Board approvals were obtained at all participating sites, the data management and analysis center (The Kirby Institute, University of New South Wales Sydney, Sydney, Australia) and the coordinating center (TREAT Asia/amfAR, Bangkok, Thailand).
Results
Participant characteristics
Of the 9,792 adults in TAHOD with at least one eGFR >60 mL/min/1.73 m2 after 2003 or the date of the cohort enrollment, 3,700 (37.8%) were excluded: 2,490 had <3 eGFR measurements (25.4%), 1,115 had been previously exposed to potentially nephrotoxic ARVs (11.4%), 94 had less than 3 months of follow-up between the first and last eGFR measurements (1%), and 1 had missing nadir CD4 measurement (<1%). Among the 47,253 enrolled in TAHOD-LITE with at least one eGFR >60 mL/min/1.73 m2, 37,536 (79.4%) were excluded: 34,646 had <3 eGFR measurements (73.3%), 2,547 had previous exposure to potentially nephrotoxic ARVs (4.6%), 330 had less than 3 months between first eGFR after baseline and last eGFR (5.4%), and 13 (<0.1%) had no nadir CD4 count measured. After excluding participants who did not meet the eligibility criteria, 6,092 TAHOD participants were included in the study to validate the D:A:D CKD full risk model and 9,717 TAHOD-LITE participants were included to validate the short version.
Baseline characteristics of participants are described in Table 1. Most of the participants were male from both TAHOD (68%) and TAHOD-LITE (74%). Sixty percent of TAHOD participants and 47% of TAHOD-LITE participants had nadir CD4 count ≤ 200 cells/mm3. Most participants had baseline eGFR >90 ml/min/1.73m2 in TAHOD (70%) and TAHOD-LITE (75%). There was low prevalence of hypertension (0.3%), diabetes mellitus (1.1%) and prior CVD events (0.8%) among TAHOD participants. Among the different potentially nephrotoxic ARVs evaluated, TDF was the most commonly used ARV after baseline, with 60% in TAHOD and 70% in TAHOD-LITE. Initiation of ATV/r after baseline was 9.6% in TAHOD and 1.5% in TAHOD-LITE (Table 1). The differences in the characteristics such as age, sex, nadir CD4 count, prevalence of hepatitis C co-infection and intravenous drugs use were not statistically significant between the participants excluded from both of the cohorts and the individuals included in the validation analyses.
Table 1.
Baseline characteristics of participants in TAHOD and TAHOD-LITE
| TAHOD (Full risk score) | TAHOD-LITE (Short risk score) | |||||
|---|---|---|---|---|---|---|
| Developed CKD | Did not develop CKD | All | Developed CKD | Did not develop CKD | All | |
| N (%) | N (%) | N (%) | N (%) | N (%) | N (%) | |
| Total | 391 (100) | 5701 (100) | 6092 (100) | 520 (100) | 9197 (100) | 9717 (100) |
| Sex | ||||||
| Female | 106 (27.1) | 1824 (32) | 1930 (31.7) | 117 (22.5) | 2410 (26.2) | 2527 (26) |
| Male | 285 (72.9) | 3877 (68) | 4162 (68.3) | 403 (77.5) | 6787 (73.8) | 7190 (74) |
| Age group | ||||||
| ≤35 | 66 (16.9) | 2993 (52.5) | 3059 (50.2) | 30 (5.8) | 4011 (43.6) | 4041 (41.6) |
| 36-50 | 176 (45) | 2328 (40.8) | 2504 (41.1) | 222 (42.7) | 4099 (44.6) | 4321 (44.5) |
| 51-60 | 102 (26.1) | 305 (5.3) | 407 (6.7) | 151 (29) | 830 (9) | 981 (10.1) |
| 61+ | 47 (12) | 75 (1.3) | 122 (2) | 117 (22.5) | 257 (2.8) | 374 (3.8) |
| HIV exposure | ||||||
| Not intravenous drug user | 379 (96.9) | 5342 (93.7) | 5721 (93.9) | 515 (99.0) | 8905 (96.8) | 9420 (96.9) |
| Intravenous drug user | 12 (3.1) | 359 (6.3) | 371 (6.1) | 5 (1) | 292 (3.2) | 297 (3.1) |
| Nadir CD4 count (cells/m3) | ||||||
| ≤200 | 290 (74.2) | 3381 (59.3) | 3671 (60.3) | 320 (61.5) | 4264 (46.4) | 4584 (47.2) |
| >200 | 101 (25.8) | 2320 (40.7) | 2421 (39.7) | 200 (38.5) | 4933 (53.6) | 5133 (52.8) |
| ART initiation year | ||||||
| ≤2002 | 103 (26.3) | 610 (10.7) | 713 (11.7) | 49 (9.4) | 390 (4.2) | 439 (4.5) |
| 2003-2005 | 79 (20.2) | 983 (17.2) | 1062 (17.4) | 56 (10.8) | 762 (8.3) | 818 (8.4) |
| 2006-2009 | 131 (33.5) | 1942 (34.1) | 2073 (34) | 206 (39.6) | 2917 (31.7) | 3123 (32.1) |
| ≥2010 | 78 (19.9) | 2111 (37) | 2189 (35.9) | 174 (42.6) | 192 (36.9) | 5062 (52.1) |
| not started | 0 (0) | 55 (1) | 55 (0.9) | 17 (4.2) | 17 (3.3) | 275 (2.8) |
| Hepatitis C coinfection | ||||||
| Negative | 362 (92.6) | 5069 (88.9) | 5431 (89.1) | 503 (96.7) | 8688 (94.5) | 9191 (94.6) |
| Positive | 29 (7.4) | 632 (11.1) | 661 (10.9) | 17 (3.3) | 509 (5.5) | 526 (5.4) |
| Hypertension | ||||||
| No | 218 (55.8) | 3141 (55.1) | 3359 (55.1) | |||
| Yes | 3 (0.8) | 13 (0.2) | 16 (0.3) | |||
| Unknown/missing | 170 (43.5) | 2547 (44.7) | 2717 (44.6) | |||
| Diabetes mellitus | ||||||
| No | 229 (58.6) | 3267 (57.3) | 3496 (57.4 | |||
| Yes | 23 (5.9) | 43 (0.8) | 66 (1.1) | |||
| Unknown/missing | 139 (35.6) | 2391 (41.9) | 2530 (41.5) | |||
| Prior CVD | ||||||
| No/not reported | 374 (95.7) | 5671 (99.5) | 6045 (99.2) | |||
| Yes | 17 (4.3) | 30 (0.5) | 47 (0.8) | |||
| Baseline eGFR | ||||||
| 61-70 | 106 (27.1) | 285 (5) | 391 (6.4) | 129 (24.8) | 347 (3.8) | 476 (4.9) |
| 71-90 | 167 (42.7) | 1276 (22.4) | 1443 (23.7) | 220 (42.3) | 1764 (19.2) | 1984 (20.4) |
| >90 | 118 (30.2) | 4140 (72.6) | 4258 (69.9) | 171 (32.9) | 7086 (77) | 7257 (74.7) |
| Starting potentially nephrotoxic ARVs after baseline | ||||||
| TDF | 271 (69.3) | 3441 (60.4) | 3712 (60.9) | 337 (64.8) | 6432 (69.9) | 6762 (69.6) |
| ATV | 27 (6.9) | 350 (6.1) | 377 (6.2) | 24 (4.6) | 434 (4.7) | 458 (4.7) |
| ATV/r | 42 (10.7) | 540 (9.5) | 582 (9.6) | 65 (1.2) | 1437 (1.6) | 1502 (1.5) |
| LPV/r | 76 (19.4) | 876 (15.4) | 952 (15.6) | 35 (6.7) | 914 (9.9) | 949 (9.8) |
| Other boosted PIs | 38 (9.7) | 498 (8.7) | 536 (8.8) | 24 (4.6) | 477 (5.2) | 501 (5.2) |
| Any of these | 298 (76.2) | 4041 (70.9) | 4339 (71.2) | 349 (6.7) | 6527 (7.1) | 6878 (70.8) |
The TREAT Asia HIV Observational Database (TAHOD) is a collaborative observational cohort study that involves 21 sites in the Asia and Pacific region. The participating countries are Cambodia, China and Hong Kong SAR, India, Indonesia, Japan, Malaysia, the Philippines, Singapore, South Korea, Taiwan, Thailand and Vietnam. TAHOD Low Intensity Transfer (TAHOD-LITE) study is a sub-study of TAHOD which collects demographics, hepatitis serology, ART history, and HIV-related laboratory results from all patients at 10 participating sites of 8 countries in TAHOD.
Abbreviations: CKD, chronic kidney disease; eGFR, estimated glomerular filtration rate; TDF, tenofovir disoproxil fumarate; ATV, atazanavir; ATV/r, ritonavir boosted atazanavir; LPV/r, lopinavir boosted ritonavir; PIs, protease inhibitors.
Validation of the D:A:D CKD Full Risk Score in TAHOD
Table 2 summarizes the incidence rates, Kaplan-Meir percentage and incidence rate ratio different risk groups for TAHOD and TAHOD-LITE. In TAHOD, 391 (6.4%) developed CKD over a median of 8.1 years (IQR 4.8, 10.9) of follow-up time (crude incidence rate 8.1 per 1,000 PYFU, 95% confidence interval [CI] 7.3, 8.9). The median CKD risk score at baseline was −2 (interquartile range, IQR −6, 1) overall, whereas it was 5 (IQR, 0, 10) for those who developed CKD. Incidence rates of CKD among low-, medium- and high-risk groups were 2.8 (95% CI 2.2, 3.4), 10.2 (95% CI 8.4, 12.4) and 33.2 (95% CI 28.9, 38.1) per 1,000 PYFU, respectively. At 5 years after baseline, 1.31% (95 % CI 0.99, 1.73), 5.53% (95% CI 4.33, 7.04) and 19.24% (95% CI 16.65, 22.17) from low-, medium- and high-risk groups were estimated to develop CKD. In addition, Kaplan-Meier estimates show that the probability of developing CKD at 10 years after baseline was 2.7% (95% CI 2.15, 3.4), 8.92% (95% CI 7.18, 11.05) and 26.1% (95% CI 22.99, 29.54) from low-, medium- and high-risk groups (Figure 1). The aIRR associated with one-point increase in the risk score for the development CKD was 1.21 (95% CI 1.19, 1.23) in TAHOD. The AUROC for the full risk score was 0.81 (95% CI 0.79, 0.83) for TAHOD (Figure 2).
Table 2.
Risk score model validated in TAHOD cohort
| Outcome | TAHOD - Full risk score | TAHOD-LITE - Short risk score | ||
|---|---|---|---|---|
| Number of participants included in the validation | 6,092 | 9,717 | ||
| Developed CKD, N (%) | 391 | 6.4% | 520 | 5.4% |
| Incidence of CKD/1,000 PYFU (95%CI) | 8.1 | (7.3, 8.9) | 10.5 | (9.6, 11.4) |
| Risk score model | ||||
| Baseline score, median (IQR) | −2 | (−6, 1) | −1 | (−6, 1) |
| Baseline score for those who developed CKD, median (IQR) | 5 | (0, 10) | 5 | (1, 10) |
| Events by score group: low/medium/high | 90/99/202 | 106/147/267 | ||
| Incidence of CKD/1,000 PYFU (95%CI) | ||||
| Low (risk score < 0) | 2.8 | (2.2, 3.4) | 3.1 | (2.5, 3.7) |
| Medium (risk score 0-4) | 10.2 | (8.4, 12.4) | 14.3 | (12.2, 16.8) |
| High (risk score ≥5) | 33.2 | (28.9, 38.1) | 54.6 | (48.4, 61.6) |
| Incidence rate ratio | ||||
| Low (risk score < 0) | 0.27 | (0.20, 0.36) | 0.21 | (0.17, 0.27) |
| Medium (risk score 0-4) | 1 | 1 | ||
| High (risk score ≥5) | 3.27 | (2.57, 4.15) | 3.81 | (3.12, 4.66) |
| Number at risk for CKD at 5 year: low/medium/high | 3027/916/563 | 3103/924/407 | ||
| Kaplan-Meier percent progressed at 5 yr (95%CI) | ||||
| Low (risk score < 0) | 1.31 | (0.99, 1.73) | 1.51 | (1.21, 1.89) |
| Medium (risk score 0-4) | 5.53 | (4.33, 7.04) | 7.01 | (5.86, 8.37) |
| High (risk score ≥5) | 19.24 | (16.65, 22.17) | 24.99 | (22.26, 27.98) |
| Number at risk for CKD at 10 year: low/medium/high | 1316/409/271 | 569/173/88 | ||
| Kaplan-Meier percent progressed at 10 yr (95%CI) | ||||
| Low (risk score < 0) | 2.7 | (2.15, 3.4) | 3.49 | (2.67, 4.56) |
| Medium (risk score 0-4) | 8.92 | (7.18, 11.05) | 11.68 | (9.59, 14.19) |
| High (risk score ≥5) | 26.10 | (22.99, 29.54) | 32.4 | (28.6, 27.98) |
| aIRR (95%CI) per unit increase in score | 1.21 | (1.19, 1.23) | 1.24 | (1.22, 1.25) |
Abbreviations: CKD, chronic kidney disease; PYFU, person-years of follow-up; CI, confidence interval; aIRR, adjusted risk ratio.
Figure 1.
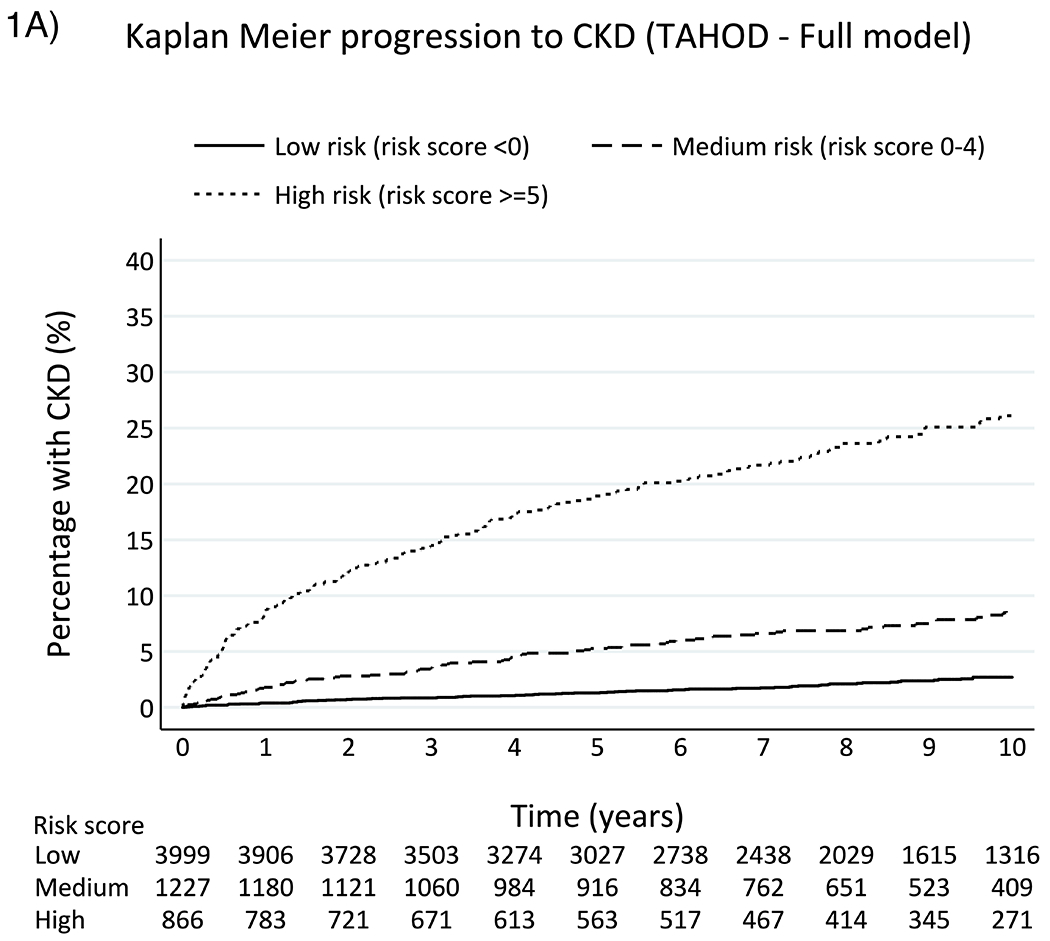
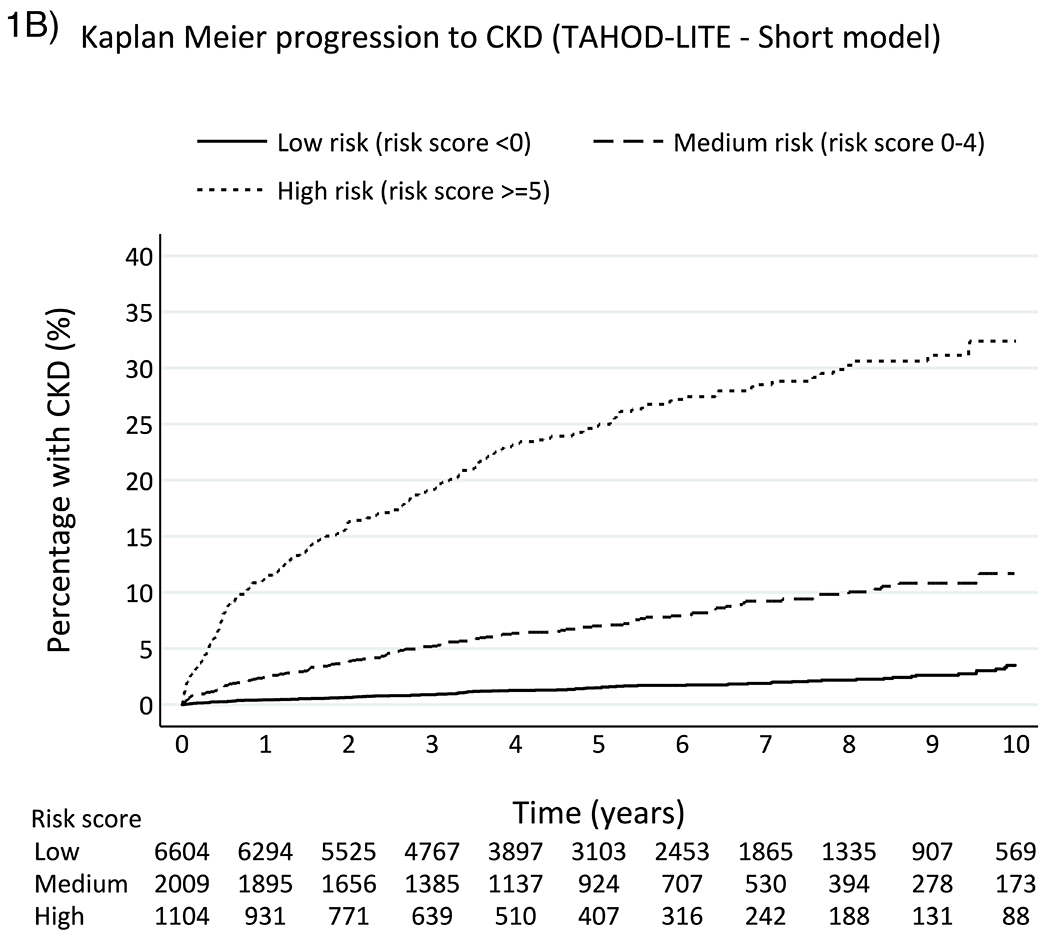
Kaplan-Meier progression to CKD among TAHOD (full risk score) and TAHOD-LITE (short risk score)
Figure 2.
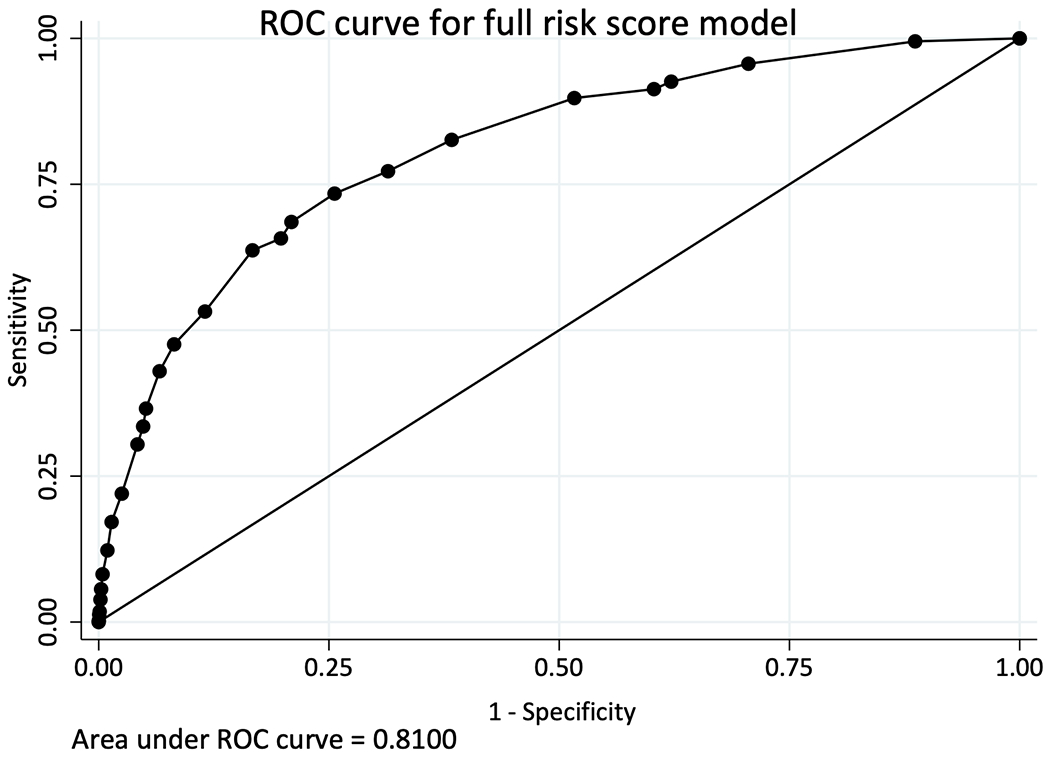
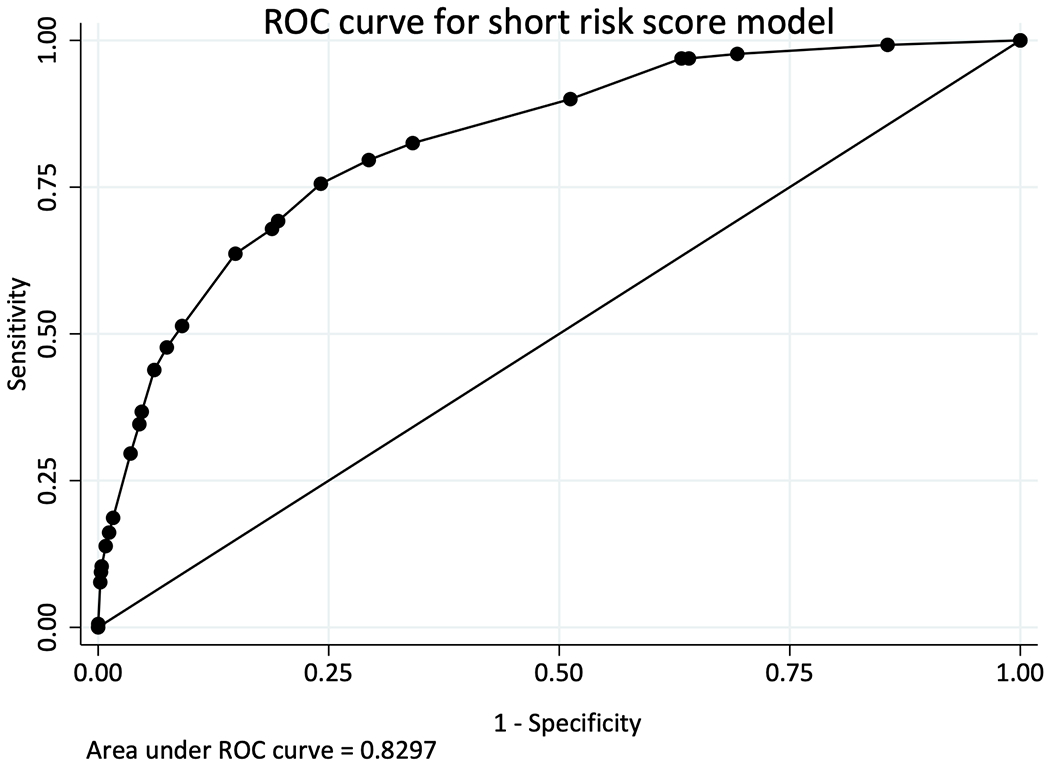
Calibration of full and short D:A:D CKD risk score models among TAHOD (full risk score) and TAHOD-LITE (short- risk score)
Validation of the D:A:D CKD Short Risk Score in TAHOD-LITE
In a median of 4.9 years (IQR 2.5-7.3) of follow-up time, 520 (5.35%) participants developed CKD in TAHOD-LITE. The crude incidence rate of CKD was 10.5 per 1,000 PYFU (95% CI 9.6, 11.4). The overall median baseline CKD score and score for those who developed CKD in TAHOD-LITE were similar as those in TAHOD (Table 2). The incidence rates of CKD were 3.1 per 1,000 PYFU (95% CI 2.5, 3.7) for low-risk, 14.3 per 1,000 PYFU (95% CI 12.2, 16.8) for medium-risk and 54.6 per 1,000 PYFU (95% CI 48.4, 61.6) for high-risk groups, respectively. The incidence rate ratio of developing CKD in TAHOD-LITE was 0.21 (95% CI 0.17, 0.27) for low-risk group and 3.81 (95% CI 3.12, 4.66) for high-risk group, when compared to the medium-risk group. The Kaplan-Meier progression rates for all three risk groups among TAHOD-LITE participants were higher than those rates in TAHOD at 5 and 10 years after baseline (Figure 1). The aIRR associated with one-point increase in the risk score for CKD development in TAHOD-LITE was 1.24 (95% CI 1.22, 1.25). The AUROC for the short version of the D:A:D CKD risk score was 0.83 (95% CI 0.81, 0.85) for TAHOD-LITE (Figure 2).
Discussions
The D:A:D CKD risk prediction score was developed from a population where the majority of participants were European. We extended the work and validated the D:A:D CKD risk prediction scores in Asian populations using CKD-EPI equation for eGFR measurements. The prevalence of CKD was approximately 6% in both TAHOD and TAHOD-LITE used in the validation analyses. The overall crude CKD incidence was 8.1 per 1,000 PYFU in TAHOD and 10.5 per 1,000 PYFU in TAHOD-LITE. The full and short scores were highly predictive of the risks for CKD progression in our cohorts. The performance of the risk scores measured by AUROC showed acceptable discrimination, with similar AUROC values between the two risk score models.
Incidence rates of CKD for low- and medium-risk groups were similar between the cohorts using both full and short versions of the D:A:D CKD risk score models. The short model without having cardiovascular risk factors in the scores predicted higher probability of CKD progression for high-risk group than the full model. The incidence of CKD in high-risk group was 33.2 per 1,000 PYFU for the full risk score and 54.6 per 1,000 PYFU for the short risk score. The short risk score also predicted higher probability of CKD development at 5 and 10 years after baseline than the full risk score without the CVD risk factors. One possible explanation for these discrepancies is that our full risk score grouped those with missing CVD risks factors as not having the risks. Therefore, those with a risk factor like diabetes who were not assessed (or had only one fasting plasma glucose test and thus not considered to have DM) may have been classified as having low risk, despite having this important risk factor. It is also noted that the prevalence of CVD risk factors in TAHOD was lower than D:A:D derivation cohort which was a large data collection from the European populations 15. However, the short risk score which included only basic HIV information and without CVD risk factors appeared to perform similarly well as the full risk score in our Asian cohorts. This could be particularly useful in the settings where the assessments of CVD risk factors are not feasible or less regularly monitored.
Moreover, despite the low prevalence of CVD risk factors in our cohorts compared to D:A:D cohort, the incidence rates of CKD among PLHIV of these Asian cohorts were moderately higher than the derivation D:A:D cohort (6.2 per 1,000 PYFU) 23 but similar to a recent study which validated the D:A:D CKD prediction scores using CKD-EPI equation (10.8 per 1,000 PYFU) from a U.S. cohort 17. Despite having similar age as the derivative study (median 43 years in TAHOD and 40 years in TAHOD-LITE vs. 40 years in D:A:D cohort), the participants in our cohorts had lower median nadir CD4 count (216 cells/mm3 in TAHOD and 140 cells/mm3 in TAHOD-LITE vs. 290 cells/mm3 in D:A:D cohort) and lower prevalence of intravenous drug users than the D:A:D study. Other characteristics such as baseline eGFR levels, and HCV co-infection prevalence were comparable between the cohorts.
The difference in CKD incidence is also likely due to the eGFR calculation method that was used in the validation process. Although the risk score from the original D:A:D cohort was developed from eGFR of Cockcroft-Gault equation (CGE), there were good discrimination with the validation cohorts (Royal Free Hospital cohort and participants from SMART/ESPRIT trials) that used eGFR calculated from CKD-EPI equation 23. Mills and colleagues also showed in their recent validation study that the CKD-EPI estimated higher CKD events than CGE (10.8 vs. 7.3 per 1,000 PYFU) 17. However, although there are different perspectives on the utility of different eGFR equations, some HIV treatment guidelines recommend the use of CKD-EPI equation to evaluate the renal function estimation among PLHIV 24,25.
It is noted that aIRR values with one-point increase in the score in both TAHOD using full risk score (1.21, 95% CI 1.19, 1.23) and TAHOD-LITE using short risk score (1.24, 95% CI 1.22, 1.25) were lower than the D:A:D original cohort (1.32 for full score and 1.33 for short score). However, the aIRR in TAHOD-LITE was similar to a study from the U.S. using CKD-EPI (1.25, 95% CI 1.24, 1.27) 17.
A recent meta-analysis, which estimated the global burden of CKD among PLHIV of different WHO regions, also suggested that the prevalence was higher in Asian than European PLHIV using different formulas for eGFR calculations 8. Another possible explanation of the higher CKD incidence in Asian PLHIV included in the study might be that our participants from both cohorts presented late and hence, late initiation of cART. Compared to the derivative cohort in which the D:A:D CKD risk score was developed 15, PLHIV from both of our Asian cohorts had lower nadir CD4 cell counts with nearly half of them had CD4 count <200 cells/mm3. Guidelines recommending HIV treatment regardless of CD4 counts in most of the Asia-Pacific region were introduced later than the early treatment initiation practices from the European countries. However, the effect of ethnicity on the CKD progression rates among PLHIV seemed to be diminished in the modern era when cART is initiated earlier with higher nadir CD4 counts 26.
Mocroft and colleagues reported in the D:A:D CKD risk score development study that the addition of different potentially nephrotoxic ARVs in the existing model increased the risk of CKD progression equivalent to higher scores compared to those who did not start these drugs in the calculation of CKD risk prediction in the model 15. For example, the study showed that starting of TDF or ATV/r or other boosted protease inhibitors except (LPV/r) increased CKD incidence for 74%. A previous TAHOD analysis also showed that PI use was associated with higher risks of renal dysfunction and those having an eGFR before starting TDF ≥60 mL/min/1.73m2 had lower risk to develop renal impairment 27. Despite the short-term effects of nephrotoxic ARVs that have been previously documented 28,29, the reports from several clinical trials also showed that switching to a different cART regimen could reverse the renal side effects, such as switching to tenofovir alafenamide, TAF, from TDF 30 or to a dual cART regimen sparing a nephrotoxic ARV 31,32. However, it is unclear whether an intervention like this could prevent the long-term development of CKD, especially among the individuals who have started nephrotoxic ARVs with low baseline eGFR.
Nearly 25% of TAHOD and 75% of TAHOD-LITE participants were excluded in our study analysis due to the lack of at least 3 eGFR measurements, which highlight the need of more frequent eGFR monitoring in the cohorts. There were 54 in TAHOD and 124 in TAHOD-LITE who had their first available eGFR ≤60 mL/min/1.73m2. Among those, 48 (89%) patients in TAHOD and 105 (85%) patients in TAHOD-LITE rebounded to eGFR > 60 mL/min/1.73 m2 at least once during follow-up and were included in the validation process.
With the increasing number of different non-communicable diseases and risks for kidney diseases reported in both PLHIV and the general population in the Asia-Pacific 33,34, monitoring of renal functions become more relevant, not only to monitor the side effects of ARVs but also to prevent the development of chronic renal insufficiency in the population. While a prediction score could be useful to evaluate the probability of CKD development, the numbers of eGFR measurements need to be adequate to identify the risk and include in the assessments. It was also suggested that CKD and CVD risk factors should be addressed together since both of the events share the complex relationships and common modifiable traditional risk factors 35. Therefore, routine evaluation of kidney functions along with the screening of other comorbidities should be implemented in HIV care.
To our knowledge, this is the first study to validate the D:A:D CKD risk score in the diverse PLHIV population among the Asia-Pacific region. However, several limitations of our study should be acknowledged. Although the previous study suggested that addition of urine analysis data such as proteinuria could increase the predictability of the risk score for CKD development 36, we were not able to include such data due to the limitations of data availability. Secondly, we did not calculate eGFR according to the modified equation for each specific ethnic group included in the cohorts 37,38, and hence, there may be differences in the estimation of actual GFR or the prevalence of CKD across our cohorts’ participants of different ethnicities. We also did not include the use of anti-hypertensive agents or anti-diabetes drugs in our case definition of DM and hypertension due to limited data availability from the cohorts. Lastly, since both cohorts used in the validation process are observational, the treatment decisions (e.g., initiation of TDF) were solely dependent on the practices and decisions of clinicians from the different participating cohort study sites. Nonetheless, our study confirmed the validity of D:A:D CKD prediction scores in Asian PLHIV population and these tools can be useful in the clinical settings where routine eGFR measurements are collected.
In conclusion, our analysis showed that the D:A:D risk scores were acceptable to identify those at high risks of CKD among Asian PLHIV. In addition, the short risk score performed similar in the validation process as the full risk score and it could be beneficial to the settings where CVD risk factors were not routinely collected. The D:A:D CKD risk score can be used to provide the guidance for the timely interventions of switching to renal friendly ARVs, and to implement targeted evaluation or frequent screening for those with high risk scores among HIV care services.
Acknowledgements
TAHOD study members
PS Ly*, V Khol, National Center for HIV/AIDS, Dermatology & STDs, Phnom Penh, Cambodia;
FJ Zhang*, HX Zhao, N Han, Beijing Ditan Hospital, Capital Medical University, Beijing, China;
MP Lee*, PCK Li, W Lam, YT Chan, Queen Elizabeth Hospital, Hong Kong SAR;
N Kumarasamy* †, C Ezhilarasi, Chennai Antiviral Research and Treatment Clinical Research Site (CART CRS), VHS-Infectious Diseases Medical Centre, VHS, Chennai, India;
S Pujari*, K Joshi, S Gaikwad, A Chitalikar, Institute of Infectious Diseases, Pune, India;
S Sangle*, V Mave, I Marbaniang, S Nimkar, BJ Government Medical College and Sassoon General Hospital, Pune, India;
TP Merati*, DN Wirawan, F Yuliana, Faculty of Medicine Udayana University & Sanglah Hospital, Bali, Indonesia;
E Yunihastuti*, A Widhani, S Maria, Karjadi TH, Faculty of Medicine Universitas Indonesia - Dr. Cipto Mangunkusumo General Hospital, Jakarta, Indonesia;
J Tanuma* ‡, S Oka, T Nishijima, National Center for Global Health and Medicine, Tokyo, Japan;
JY Choi*, Na S, JM Kim, Division of Infectious Diseases, Department of Internal Medicine, Yonsei University College of Medicine, Seoul, South Korea;
YM Gani*, NB Rudi, Hospital Sungai Buloh, Sungai Buloh, Malaysia;
I Azwa*, A Kamarulzaman, SF Syed Omar, S Ponnampalavanar, University Malaya Medical Centre, Kuala Lumpur, Malaysia;
R Ditangco*, MK Pasayan, ML Mationg, Research Institute for Tropical Medicine, Muntinlupa City, Philippines;
YJ Chan*, WW Ku, PC Wu, E Ke, Taipei Veterans General Hospital, Taipei, Taiwan;
OT Ng*, PL Lim, LS Lee, D Liang, Tan Tock Seng Hospital, Singapore (note: OT Ng was also supported by the NMRC Clinician Scientist Award (NMRC/CSA-INV/0002/2016), which had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.);
A Avihingsanon*, S Gatechompol, P Phanuphak, C Phadungphon, HIV-NAT/Thai Red Cross AIDS Research Centre, Bangkok, Thailand;
S Kiertiburanakul*, A Phuphuakrat, L Chumla, N Sanmeema, Faculty of Medicine Ramathibodi Hospital, Mahidol University, Bangkok, Thailand;
R Chaiwarith*, T Sirisanthana, J Praparattanapan, K Nuket, Research Institute for Health Sciences, Chiang Mai, Thailand;
S Khusuwan*, P Kantipong, P Kambua, Chiangrai Prachanukroh Hospital, Chiang Rai, Thailand;
KV Nguyen*, HV Bui, DTH Nguyen, DT Nguyen, National Hospital for Tropical Diseases, Hanoi, Vietnam;
CD Do*, AV Ngo, LT Nguyen, Bach Mai Hospital, Hanoi, Vietnam;
AH Sohn*, JL Ross*, B Petersen, TREAT Asia, amfAR - The Foundation for AIDS Research, Bangkok, Thailand;
MG Law*, A Jiamsakul*, R Bijker, D Rupasinghe, The Kirby Institute, UNSW Sydney, NSW, Australia.
* TAHOD Steering Committee member; † Steering Committee Chair; ‡ co-Chair
Funding
This work was supported by the U.S. National Institutes of Health’s National Institute of Allergy and Infectious Diseases, the Eunice Kennedy Shriver National Institute of Child Health and Human Development, the National Cancer Institute, the National Institute of Mental Health, the National Institute on Drug Abuse, the National Heart, Lung, and Blood Institute, the National Institute on Alcohol Abuse and Alcoholism, the National Institute of Diabetes and Digestive and Kidney Diseases, and the Fogarty International Center, as part of the International Epidemiology Databases to Evaluate AIDS [IeDEA; U01AI069907]. The Kirby Institute is funded by the Australian Government Department of Health and Ageing, and is affiliated with the Faculty of Medicine, UNSW Sydney. The content of this publication is solely the responsibility of the authors and does not necessarily represent the official views of any of the governments or institutions mentioned above.
Footnotes
Conflict of interest
The authors declare no conflict of interests related to this work.
References
- 1.Levey AS, Atkins R, Coresh J, et al. Chronic kidney disease as a global public health problem: approaches and initiatives - a position statement from Kidney Disease Improving Global Outcomes. Kidney Int. 2007;72(3):247–259. [DOI] [PubMed] [Google Scholar]
- 2.Go AS, Chertow GM, Fan D, McCulloch CE, Hsu CY. Chronic kidney disease and the risks of death, cardiovascular events, and hospitalization. N Engl J Med. 2004;351(13):1296–1305. [DOI] [PubMed] [Google Scholar]
- 3.Rasmussen LD, May MT, Kronborg G, et al. Time trends for risk of severe age-related diseases in individuals with and without HIV infection in Denmark: a nationwide population-based cohort study. Lancet HIV. 2015;2(7):e288–298. [DOI] [PubMed] [Google Scholar]
- 4.Islam FM, Wu J, Jansson J, Wilson DP. Relative risk of renal disease among people living with HIV: a systematic review and meta-analysis. BMC public health. 2012;12:234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Nishijima T, Kawasaki Y, Mutoh Y, et al. Prevalence and factors associated with chronic kidney disease and end-stage renal disease in HIV-1-infected Asian patients in Tokyo. Scientific reports. 2017;7(1):14565–14565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kim EJ, Ahn JY, Kim YJ, et al. The Prevalence and Risk Factors of Renal Insufficiency among Korean HIV-Infected Patients: The Korea HIV/AIDS Cohort Study. Infect Chemother. 2017;49(3):194–204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Tanuma J, Jiamsakul A, Makane A, et al. Renal Dysfunction during Tenofovir Use in a Regional Cohort of HIV-Infected Individuals in the Asia-Pacific. PLOS ONE. 2016;11(8):e0161562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ekrikpo UE, Kengne AP, Bello AK, et al. Chronic kidney disease in the global adult HIV-infected population: A systematic review and meta-analysis. PloS one. 2018;13(4):e0195443–e0195443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Liyanage T, Ninomiya T, Jha V, et al. Worldwide access to treatment for end-stage kidney disease: a systematic review. Lancet (London, England). 2015;385(9981):1975–1982. [DOI] [PubMed] [Google Scholar]
- 10.Hippisley-Cox J, Coupland C. Predicting the risk of chronic Kidney Disease in men and women in England and Wales: prospective derivation and external validation of the QKidney Scores. BMC Fam Pract. 2010;11:49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Halbesma N, Jansen DF, Heymans MW, et al. Development and validation of a general population renal risk score. Clin J Am Soc Nephrol. 2011;6(7):1731–1738. [DOI] [PubMed] [Google Scholar]
- 12.Mocroft A, Lundgren JD, Ross M, et al. Development and validation of a risk score for chronic kidney disease in HIV infection using prospective cohort data from the D:A:D study. PLoS Med. 2015;12(3):e1001809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Scherzer R, Gandhi M, Estrella MM, et al. A chronic kidney disease risk score to determine tenofovir safety in a prospective cohort of HIV-positive male veterans. AIDS. 2014;28(9):1289–1295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Collins GS, Omar O, Shanyinde M, Yu LM. A systematic review finds prediction models for chronic kidney disease were poorly reported and often developed using inappropriate methods. J Clin Epidemiol. 2013;66(3):268–277. [DOI] [PubMed] [Google Scholar]
- 15.Mocroft A, Lundgren JD, Ross M, et al. Development and validation of a risk score for chronic kidney disease in HIV infection using prospective cohort data from the D:A:D study. PLoS Med. 2015;12(3):e1001809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Woolnough EL, Hoy JF, Cheng AC, et al. Predictors of chronic kidney disease and utility of risk prediction scores in HIV-positive individuals. Aids. 2018;32(13):1829–1835. [DOI] [PubMed] [Google Scholar]
- 17.Mills AM, Schulman KL, Fusco JS, et al. Validation of the Data Collection on Adverse Events of Anti-HIV Drugs (D:A:D) chronic kidney disease risk score in HIV-infected patients in the USA. HIV Med. 2020;21(5):299–308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.De La Mata NL, Ahn M-Y, Kumarasamy N, et al. A pseudo-random patient sampling method evaluated. Journal of clinical epidemiology. 2017;81:129–139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Zhou J, Kumarasamy N, Ditangco R, et al. The TREAT Asia HIV Observational Database: baseline and retrospective data. J Acquir Immune Defic Syndr. 2005;38(2):174–179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.A Decade of Combination Antiretroviral Treatment in Asia: The TREAT Asia HIV Observational Database Cohort. AIDS Res Hum Retroviruses. 2016;32(8):772–781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Bijker R, Jiamsakul A, Uy E, et al. Cardiovascular disease-related mortality and factors associated with cardiovascular events in the TREAT Asia HIV Observational Database (TAHOD). HIV Med. 2019;20(3):183–191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Levey AS, Stevens LA, Schmid CH, et al. A new equation to estimate glomerular filtration rate. Ann Intern Med. 2009;150(9):604–612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Mocroft A, Lundgren JD, Ross M, et al. Development and validation of a risk score for chronic kidney disease in HIV infection using prospective cohort data from the D:A:D study. PLoS medicine. 2015;12(3):e1001809–e1001809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Cristelli MP, Cofan F, Rico N, et al. Estimation of renal function by CKD-EPI versus MDRD in a cohort of HIV-infected patients: a cross-sectional analysis. BMC Nephrol. 2017;18(1):58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lucas GM, Ross MJ, Stock PG, et al. Clinical practice guideline for the management of chronic kidney disease in patients infected with HIV: 2014 update by the HIV Medicine Association of the Infectious Diseases Society of America. Clinical infectious diseases : an official publication of the Infectious Diseases Society of America. 2014;59(9):e96–e138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Schoffelen AF, Smit C, van Lelyveld SFL, et al. Diminished Impact of Ethnicity as a Risk Factor for Chronic Kidney Disease in the Current HIV Treatment Era. The Journal of Infectious Diseases. 2015;212(2):264–274. [DOI] [PubMed] [Google Scholar]
- 27.Tanuma J, Jiamsakul A, Makane A, et al. Renal Dysfunction during Tenofovir Use in a Regional Cohort of HIV-Infected Individuals in the Asia-Pacific. PLoS One. 2016;11(8):e0161562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ryom L, Mocroft A, Kirk O, et al. Predictors of advanced chronic kidney disease and end-stage renal disease in HIV-positive persons. Aids. 2014;28(2):187–199. [DOI] [PubMed] [Google Scholar]
- 29.Mocroft A, Lundgren JD, Ross M, et al. Cumulative and current exposure to potentially nephrotoxic antiretrovirals and development of chronic kidney disease in HIV-positive individuals with a normal baseline estimated glomerular filtration rate: a prospective international cohort study. Lancet HIV. 2016;3(1):e23–32. [DOI] [PubMed] [Google Scholar]
- 30.Pozniak A, Arribas JR, Gathe J, et al. Switching to Tenofovir Alafenamide, Coformulated With Elvitegravir, Cobicistat, and Emtricitabine, in HIV-Infected Patients With Renal Impairment: 48-Week Results From a Single-Arm, Multicenter, Open-Label Phase 3 Study. Journal of acquired immune deficiency syndromes (1999). 2016;71(5):530–537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Baril JG, Angel JB, Gill MJ, et al. Dual Therapy Treatment Strategies for the Management of Patients Infected with HIV: A Systematic Review of Current Evidence in ARV-Naive or ARV-Experienced, Virologically Suppressed Patients. PLoS One. 2016;11(2):e0148231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Jabłonowska E, Siwak E, Bociąga-Jasik M, et al. Real-life study of dual therapy based on dolutegravir and ritonavir-boosted darunavir in HIV-1-infected treatment-experienced patients. PLOS ONE. 2019;14(1):e0210476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Low WY, Lee YK, Samy AL. Non-communicable diseases in the Asia-Pacific region: Prevalence, risk factors and community-based prevention. Int J Occup Med Environ Health. 2015;28(1):20–26. [DOI] [PubMed] [Google Scholar]
- 34.Patel P, Rose CE, Collins PY, et al. Noncommunicable diseases among HIV-infected persons in low-income and middle-income countries: a systematic review and meta-analysis. Aids. 2018;32 Suppl 1:S5–s20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Boyd MA, Mocroft A, Ryom L, et al. Cardiovascular disease (CVD) and chronic kidney disease (CKD) event rates in HIV-positive persons at high predicted CVD and CKD risk: A prospective analysis of the D:A:D observational study. PLoS Med. 2017;14(11):e1002424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Gupta SK, Mamlin BW, Johnson CS, Dollins MD, Topf JM, Dube MP. Prevalence of proteinuria and the development of chronic kidney disease in HIV-infected patients. Clin Nephrol. 2004;61(1):1–6. [DOI] [PubMed] [Google Scholar]
- 37.Horio M, Imai E, Yasuda Y, Watanabe T, Matsuo S. Modification of the CKD epidemiology collaboration (CKD-EPI) equation for Japanese: accuracy and use for population estimates. Am J Kidney Dis. 2010;56(1):32–38. [DOI] [PubMed] [Google Scholar]
- 38.Praditpornsilpa K, Townamchai N, Chaiwatanarat T, et al. The need for robust validation for MDRD-based glomerular filtration rate estimation in various CKD populations. Nephrol Dial Transplant. 2011;26(9):2780–2785. [DOI] [PubMed] [Google Scholar]


