Abstract
Purpose of review
TCRα+CD4−CD8− double negative T (DNT) cells, a principal subset of mature T lymphocytes, have been closely linked with autoimmune/inflammatory conditions. However, controversy persists regarding their ontogeny and function. Here we present an overview on DN T cells in different autoimmune diseases to advance a deeper understanding of the contribution of this population to disease pathogenesis.
Recent findings
DNT cells have been characterized in various chronic inflammatory diseases and they have been proposed to display pathogenic or regulatory function. The tissue location of DN T cells and the effector cytokines they produce bespeak to their active involvement in chronic inflammatory diseases.
Summary
By producing various cytokines, expanded DNT cells in inflamed tissues contribute to the pathogenesis of a variety of autoimmune inflammatory diseases. However, it is unclear whether this population represents a stable lineage consisting of different subsets similar to CD4 T helper cell subset. Better understanding of the possible heterogeneity and plasticity of DNT cells is needed to reveal interventional therapeutic opportunities.
Keywords: Double negative T cells, ontogeny, heterogeneity, autoimmune diseases
Introduction
The most important hallmark of immune disorders is the activation and accumulation of T lymphocytes, the majority of which express both alpha and beta chains of the T cell receptor (TCR) and are therefore referred as αβ T cells[1]. Among αβ T cells, CD4+ helper or CD8+ cytotoxic T cells are most prevalent subsets[2]. However, a small population of αβ T cells which do not express both CD4 and CD8, termed “double negative” T (DNT) cells[3,4], have been considered to contribute to the pathophysiology of a series of autoimmune diseases[4].
DN T cells were initially identified and characterized in lpr and gld mice (deficiency of either Fas or Fas ligand) in which lymphoproliferative syndrome developed due to impaired Fas-mediated apoptosis[5–9]. The massively expanded DNT cells results in the lymphadenopathy and splenomegaly which leads to the early hypothesis that DNT cells are immunopathogenic[5]. Later on, expanded DNT cells were observed in patients with different immune disorders including Autoimmune lymphoproliferative syndrome (ALPS)[10,11], systemic lupus erythematosus (SLE)[12,13] and sjogren’s syndrome[14,15]. Although DN T cells only represent a small portion of αβ T cells compared to either CD4 or CD8 T cells in normal subjects[16,5], the expansion of DN T cells in various autoimmune diseases and the presence of DNT cells at sites of injury in different inflammatory conditions strongly suggest their critical roles in inflammation[4]. However, our understanding of DNT cell ontogeny and function still remains limited[17,3–5].
We propose that the discrepancy on the differentiation and function of DNT cells could be explained by the heterogeneity and plasticity of this type of cells.
Ontogeny of DNT cells
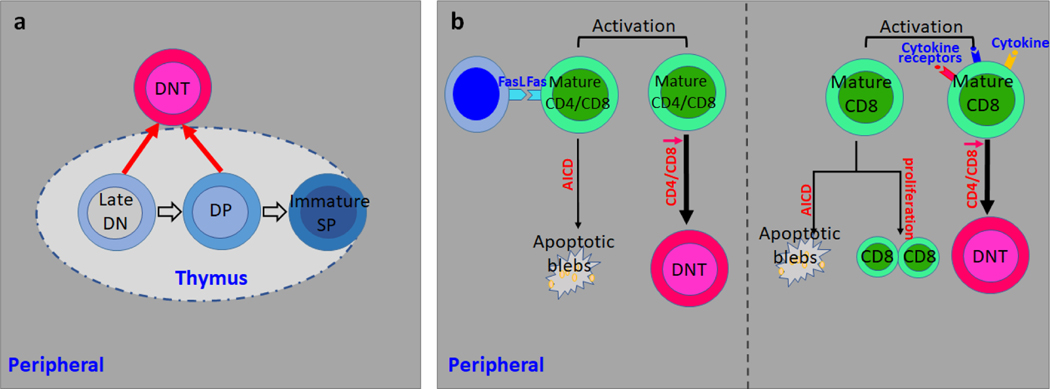
In healthy individuals, DNT cell only comprise a small portion of αβ T cells and are considered quiescent[5,4]. αβ T cells are derived from the developing progenitors within the thymus, the thymocytes. Developing thymocytes undergo a series of maturation steps before egressing from the thymus[18] and the earliest developing thymocytes lack the expression of the co-receptors CD4 and CD8 and are termed double negative (DN) population[18,19], which leads to the hypothesis that peripheral DNT cells may represent primitive αβ T cells which originate in the thymus but escape the late development followed by migration to the periphery (Fig. 1a)[20,3]. For late stage thymocyte development, TCR signal strength and duration determine the lineage commitment to either CD4+ or CD8+ T cells. Typically lower intensity TCR signals leads to full maturation of either CD4+ or CD8+ T cells while cells with high TCR strength are deleted during the development to avoid autoimmunity[21,22]. This process has be well recapitulated by in vitro cultured thymocytes in the presence of cortical epithelial cells[23]. Considering the fact that CD4 or CD8 expression is essential in augmenting TCR signaling by stabilizing interactions between TCR-MHC complex[24,25], it is reasonable to postulate that low or negative expression of CD4 and CD8 coreceptors protects thymocytes away from high intensity TCR signaling mediated depletion and promotes their thymic egress[26,27]. In contrast to low concentrations of ligands that induce maturation to single positive (SP) thymocyte, double positive (DP) thymocytes cocultured with cortical epithelial cells loaded with high concentrations of high affinity ligands acquire DNT phenotype with downregulation of both CD4 and CD8[20,28].
Fig. 1. Ontogeny of DN T cells.
a. Peripheral DNT cells derive directly from immature DN thymocytes or from DP thymocytes through the downregulation of both CD4 and CD8.
b. Left: Activated CD8 T cells without proper apoptotic signals escape AICD and give rise to DNT cells. Right: Cytokine signal inputs help the conversion from CD8 T cells to DNT cells.
However, there is sufficient evidence to suggest that DNT cells are generated in the periphery. For example, DNT cells can develop in thymectomized mice reconstituted with T cell-depleted bone marrow cells[29]. The fact that mice deficient in β2-microglobulin have reduced DNT cell lymphoproliferation[30] and polyclonal DNT cells regain CD8 expression in lymphopenic environment[31], indicates that DNT may derive from peripheral mature CD8 T cells[4]. Similar evidence was generated from human studies[32,33]. First, gene expression pattern analysis revealed that DNT display more similarities with CD8 rather than CD4 T cells[32]. Second, the analysis of Vα and β usage of TCR revealed the high similarity between CD8 T and DNT in patients with ALPS[34]. The dysregulated DNT cell homeostasis in lpr, gld mice and ALPS patients[35–37] has directed the attention to defective apoptosis mediated by Fas dependent pathway[38,39]. The loss-of-function mutations in the Fas pathway in T cells lead to impaired apoptosis after repeated TCR engagement[9,11]. Activation-induced cell death (AICD), a Fas/FasL dependent negative regulator of activated T cells upon repeated TCR stimulation[40,41], is important for the maintenance of T-cell homeostasis and abnormalities in this process may result in autoimmunity[42]. The evidence above depicts a possible model for the pathogenic DNT cell expansion in autoimmunity in which autoreactive CD8 T cells skip antigen induced AICD by losing CD8, and execute their pathogenic role in vivo[5,4]. Along this line, TCRαβ+ CD8 T cells lost their CD8 expression upon stimulation with high concentration of anti-CD3 in vitro[32,33]. Adoptively transferred CD8 T cells with transgenic TCR acquired DNT like phenotype after encountering exogenous or endogenous antigens in vivo[43,44,16]. Moreover, increased Ki67 expression, narrowed TCR Vβ repertoire usage and diluted T-cell receptor excision circles (TRECs) observed in DNT cells indicated the clonal proliferation and expansion possibly driven by endogenous self-antigens[44–49]. Of note, in vivo antigen administration to MHC class I-restricted TCR transgenic mice on lpr background resulted in expansion of DNT cells[33], which further supported the concept that the expanded DNT cells under chronic inflammation might derive from antigen activated CD8 T cells[4,17]. However, it remains a mystery whether the absence of proper apoptotic signals or addition of supportive signals such as cytokines help activated CD8 T cells escape AICD and acquire DNT cell phenotype (Fig. 1b).
To date, the controversy on the origin of DNT cells continues. There are several possible scenarios which are worth of attention: (1) DN T cells directly originate from those immature DN thymocytes which could not recognize MHC class I or MHC class II molecules but for some reason are not appropriately depleted in thymic positive selection. (2) DN T cells represent a unique lineage which is selected by recognition of neither class I nor MHC class II but certain unknown MHC like molecules. (3) There are different types of DNT cells with either intrathymic or extrathymic origin, a model which we favor most since it fits the best for the above augments [17,5].
DN T cells, the evil or the angel in inflammation
Under naïve status, DNT cells represent a minor population in total αβ T cells with unrecognized roles in immune system. However, the lupus like symptoms in lpr or gld mice and disease-associated expansion of DNT cells lead to the supposition that DNT cells are assigned a pro-inflammatory role[50,9,6–8]. The findings that DNT cells are also expanded in patients with various inflammatory rheumatic disorders including ALPS and SLE reinforce this concept[12,11]. Evidence has emerged which supports the pathogenic role of DNT cells[4]. Ex vivo analysis on the cytokine profiles of DNT cells from various murine models has shown the great ability to produce various inflammatory cytokines including IL-2, IL-4, TNFα and IL-17A[43,31,51]. Similar results were also achieved from studies in human subjects with diverse autoimmune diseases[4]. In addition, DNT cells provide help to B cells to enhance autoantibody production in vitro[12]. Immune cell infiltration is generally considered as a major contributor of tissue damages during chronic inflammation[52]. Along this line, DNT cells are present in inflamed kidney[13,44,48,47], skin[53], salivary gland[14], entheses[54] and ischemic brain[55], which suggests they present good therapeutic targets to control inflammation in various diseases.
The activation of T cells requires signaling through TCR and the coreceptors CD4 and CD8 are essential augmenting TCR signaling[1,24,56,25]. It has been argued that the cognate TCR-antigen interaction without proper augmentation by CD4 and CD8 molecules is sufficient to drive DNT cell activation in vivo. Mice with concomitant deficiency of both CD4 and CD8 developed inflammatory responses and immunopathology compared to wild type mice during acute Staphylococcal enterotoxin B infection (SEB)[57]. Of note, chronic exposure to SEB precipitated a lupus-like inflammatory disease characterized by lympho-monocytic infiltration in multiple tissues along with production of autoantibodies in these double gene deficient mice[57]. Interestingly, disease development was accompanied with the expansion of DNT cells[57]. In line with their response to SEB, in the lung of mice challenged with live vaccine strain (LSV) of Francisella tularensis, DNT cells represent the major responding T cell subset[58]. Also in HIV-infected patients, DNT cells represent a significant portion of the cellular viral load in T cells[59,60], which suggest in vivo they might function similar to CD4 T cells.
In contrast to above studies, evidence has been generated suggesting that at least subsets of DNT cells exert regulatory activity[17,46]. In skin or bone marrow allograft murine model, DNT cells were capable of suppressing syngeneic CD4 or CD8 T cells in both Fas-dependent and Fas-independent manners[17]. In addition, DNT cells were also capable of inhibiting NK cell-mediated rejection of allogenic bone marrow through perforin-dependent killing[61]. In agreement with their role in transplantation, a number of studies in autoimmune diabetes revealed that transferred DNT cells can efficiently prevent diabetes onset in non-obese diabetic (NOD) mice by producing IL-10[62,63,17]. The phenotypic counterparts of murine suppressive DNT cells have been identified also in humans[63,46]. Interestingly, in a small cohort of patients with allogeneic bone marrow transplantation, there was an inverse correlation between the frequency of circulating DN T cells and the severity of graft versus host diseases[64] although further mechanistic studies are needed.
Heterogeneity and possible plasticity of DNT cells
Variable phenotypes of DN T cells with diverse cytokine profiles have been reported[4,17], which indicates that DN T cells, similar as CD4 helper T cells[65,66], may be divided into different subsets. Five major CD4 helper T cell lineages, Th1, Th2, Th17, Tfh and Treg have been identified based on the expression of specific transcription factors and cytokine profile essential for fate determination and function[66,67]. DNT cells represent a relatively small population among total CD3+ T lymphocytes with polyclonal repertoire[44,34,45] but they are selectively expanded under various inflammatory conditions. Of note, expanded DNT cells display many terminal differentiation characteristics including Ki67 expression, a narrowed TCR Vβ repertoire and a low content of TRECs[44,47,48,46]. In different disease models, DNT cells exhibit completely divergent cytokine profiles. For instance, in lupus and chronic infection settings, DNT cells produce pro-inflammatory cytokine IL-17, which plays an essential role in the clearance of extracellular pathogens but also contributes heavily to inflammation mediated tissue damages. Moreover, in lupus prone mice and SLE patients, DNT cells can be sub-grouped based on PD1 expression[43]. Notably, PD1+ but not PD1− DNT cells contain a large portion subset with self-reactive TCRs and they are the main source of IL-17[43], which is the first solid evidence of heterogeneity among DNT cells. Similar as Th17s[68], IL-23 promotes but IL-2 attenuates IL-17 producing DNT cells[69,44,70].
In contrast, in allograft rejection and non-obese diabetes, DNT cells produce high amounts of immunosuppressive IL-10, which is essential for their regulatory capacity[17,62,46]. Successful identification of bonafide markers to separate functionally distinct DNT subsets will help reconcile the observed discrepancies. It is possible to postulate that under naïve status, the regulatory DNT cells are predominant and essential for self-tolerance. During chronic inflammation, the balance of regulatory DNT cells with proinflammatory DNT cells is disturbed and pathogenic DNT cells characterized by IL-17 or other proinflammatory cytokine production become prevalent instead[67]. Although evidence suggests that DNT cells display a terminal differentiation status and proliferate poorly upon anti-CD3 stimulation[4], the possibility can not be excluded that DNT cells are plastic. The de novo generation of DN T cells from CD8+ T lymphocytes[44,43,32] and the observation that DNT cells regain CD8 expression in lymphopenic environments pinpoint cell plasticity at least between DNT and CD8 T lineages[31]. Moreover, the key factors controlling the transition between different CD4 helper T subsets are various combinations of cytokines, which suppress or reinforce lineage specific transcription factors[67]. Considering the fact that reduction of TGFβ and increase of IL-23 create a milieu which favors the expansion of IL-17 producing DNT cells[44], the cytokine environment appears to tightly control the pathogenesis of DNT cells in chronic inflammation. It is highly possible that DNT cells, similar as their CD4 counterparts, are relatively unstable and reshaped cytokine environment may result in the fate plasticity with potential ability to switch between anti- and pro-inflammatory phenotypes, although more evidence is needed to support this postulate.
In addition, cell plasticity relies on cell heterogeneity. DNT cell pool might not represent a “pure” differentiating population. Some of them might be fully differentiated with limited plasticity, whereas others may retain the flexibility because of their partial differentiation state. Exploring the key factors controlling the redifferentiation holds the promise for future treatment of DNT cell involved inflammatory diseases.
DNT cells in autoimmune disorders
Autoimmune lymphoproliferative syndrome (ALPS) and ALPS-like diseases
ALPS is an autoimmune disorder with a progressive lymphoproliferation, massive lymphadenopathy and splenomegaly[71,50], phenotypically similar to the autoimmunity predisposed lpr and gld mice[6,8]. The massive accumulation of DN T cells in the blood and secondary lymphoid organs, the main manifestation of chronic nonmalignant lymphoproliferation, now is considered a key requirement for ALPS diagnosis[71–73], and this elevation results from a primary defect in Fas-mediated apoptosis[72,9,41,11]. In patients who develop some features of ALPS but do not fulfill the diagnostic criteria for ALPS, mutations in other components of pathways central to lymphocyte growth, activation and apoptosis have been identified including Caspase-8 and FADD[74,71]. These have been grouped into ALPS-like diseases and some patients in this category have increased DNT cells also[74,71].
Interestingly, CDR3 sequencing has revealed a significant overlap of TCR Vβ-Jβ transcripts between DNT cells and CD8 T cells from ALPS patients[34,49], which strongly suggest the at least partial CD8 origin of DNT cells in ALPS. The concept that DNT cells contribute to autoimmune symptoms in ALPS patients and autoimmunity predisposed lpr and gld mice comes from the following facts: (1) The progressive expansion of DNT cells is closely associated with disease development[75]. (2) The presence of autoantibodies in most ALPS patients correlates with the number of DN T cells[76,77]. (3) Effective treatment ameliorates autoimmune symptoms in ALPS with significant elimination of abnormal DNT cells[78–80]. Although the elevation of DNT cells in ALPS is not in dispute, further evidence is needed to validate their pathophysiological significance.
Systemic lupus erythematosus (SLE)
SLE is a clinically heterogeneous autoimmune disease with systemic inflammation and organ damage[81]. Various T cell abnormalities were reported and the expansion of DNT cells represents a prominent one[12,13,82]. The early observation that SLE patients have expanded numbers of DNT cells in the peripheral blood and this expansion correlates with disease activity leads to the supposition that expanded DNT cells contribute to the pathophysiology of SLE[83,13]. The first evidence was from in vitro co-culture assays which clearly demonstrated that DNT cells provide help to B cells to promote antibody production[12]. IL-17A, a pro-inflammatory cytokine, has been documented with crucial contribution for systemic inflammation and tissue damage in SLE[84–86,13]. The findings that DNT cells represent a major source of IL-17A in SLE patients reinforced the concept on DNT cell pathogenesis in SLE[13,4]. Moreover, DNT cell invasion in the kidneys of patients with lupus nephritis has also been recorded[37]. A series of experiments reported from our laboratory have demonstrated that a large portion of expanded DN T cells in SLE were derived from self-reactive CD8 T cells[44,32,16,31]. Self-antigens derived from apoptotic cells can activate self-reactive CD8 T cells, which give rise to DN T cells through the downregulation of CD8 expression on the cell surface. These cells displayed acquired proliferating or proliferated phenotype (Ki67 expression, diluted TREC, and narrowed TCR repertoire)[44]. CD8 Treg cells have been described as CD8 T cells specific for antigen delivered to immune-privileged sites and to control the effector T-cell responses by CD8 and perforin dependent killing[87–89]. The whole process of conversion from CD8 T cells into DNT cells contributes to the pathogenesis of lupus based on the loss of CD8-dependent immunosuppressive potentials and the acquisition of ability to produce different pro-inflammatory cytokines and especially IL-17[44]. In addition to TCR signaling and co-receptor signaling, cytokines provide the third signal for T cell activation and surviving[90]. The fact that skewed inflammatory cytokine environment in lupus favors the expansion of DNT cells suggests that cytokines compensate reduced TCR signaling strength due to the loss of CD8 for cell activation and survival[44].
Sjögren’s syndrome, Psoriasis, Axial spondylarthritis and other rheumatic diseases
Sjögren’s syndrome is a systemic autoimmune disease characterized by lymphocytic infiltration in salivary and tear glands[91]. Sjögren’s syndrome may occur as primary disease but most often occurs in the context of other autoimmune disorders[91], including SLE and rheumatoid arthritis. Similar as in SLE, DNT cells are expanded and become the main source of IL-17 in patients with primary Sjögren’s syndrome[92,14,15]. The expansion of DNT cells correlates well with disease activity and IL-17+ DNT cell infiltration was detected in inflamed salivary glands[14,15].
Psoriasis is a complex inflammatory skin disease characterized by immune cell infiltration to the skin[93]. IL-17-producing DNT cell infiltration was found in the epidermis of mice with induced psoriasis[94] and patients with plaque-type psoriasis[53]. Axial spondylarthritis is another chronic inflammatory disease which affects primarily the spine and the sacroiliac joints[95] but shares many genetic features with psoriasis[96]. Interestingly, in a widely accepted murine model of spondyloarthropathy, IL-23R+ DNT cells were detected in the inflamed entheses[54]. Again, these observations reinforce the perception that DNT cells contribute heavily to pathogenesis of many inflammatory rheumatoid disorders. Furthermore, DNT cells are expanded in a subset of pediatric patients with various autoimmune diseases including mixed connective tissue disease (MCTD), juvenile idiopathic arthritis (JIA) juvenile dermatomyositis[97] and Behcet’s disease[98] although additional investigations are required for their precise role in these patients.
Type 1 diabetes (T1D)
Type 1 diabetes (T1D) is an organ-specific autoimmune disease with severe loss of pancreatic β cells[99]. Both CD4 and CD8 T cells play distinct and highly pathogenic roles in β cell destruction[100]. In contrast to the pathogenesis of DNT cells in inflammatory rheumatoid disorders listed above, a number of studies have demonstrated the immunosuppressive ability of DNT cells and their ability to inhibit the development of autoimmune diabetes[17,3]. First, a progressive loss of DNT cells with age was observed in non-obese diabetic (NOD) mice[62]. Second, adoptive transfer of DNT cells efficiently inhibited the development of autoimmune diabetes in several different diabetic mouse models[63,101,102]. Third, transfer of NOD CD8 T cells resulted in diabetes but co-transfer of NOD CD8 T cells with DNT cells did not, which indicates that DNT cells act directly on pathogenic T cells to exercise theirs immunosuppressive function[62,17]. However, controversies remain on the nature of immunosuppression of DNT cells. Distinct mechanisms with different molecules involved have been proposed for DNT cell mediated suppression including elimination of effector T cells by either Fas/FasL-mediated apoptosis[103,104] or perforin mediated killing[102,105,106,46] and modulation on antigen presenting cells by producing IL-10[63,62] or IFNγ[107,46,108]. Of note, both IL-10 and IFNγ function as a double-edged sword in autoimmune diseases[109,110] and the immune environments determine whether they are beneficial or detrimental. Therefore, more mechanistic studies are needed.
Therapeutic interventions targeting DNT cells
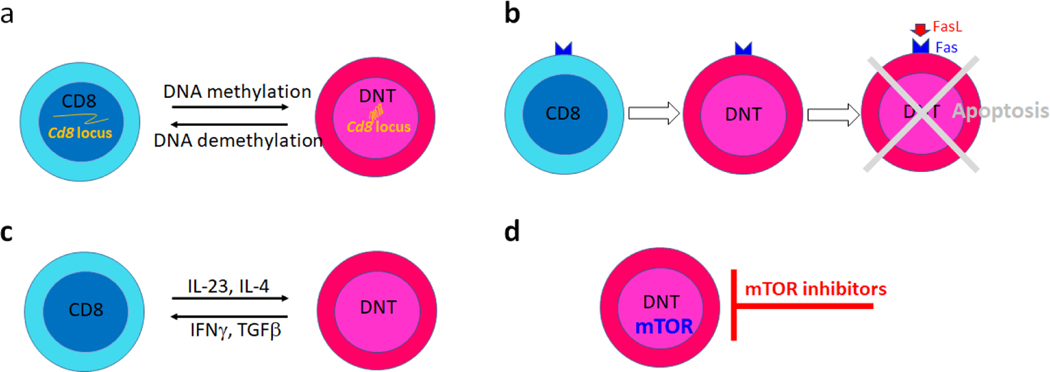
In autoimmune diseases where expanded DNT cells display distinct pathogenic capacity their selective ablation or specific modulation of the processes that render them less pathogenic should be considered for therapeutic purposes. More attention should be given to the design of specific drugs able to limit the expansion pathogenic DNT cells or if possible favor regulatory DNT activation. In light of understanding of DNT cell generation in lupus, more and more approaches directly or indirectly targeting DNT cells have been tested. In lupus prone mice and SLE patients, a large portion of DNT cells were derived from antigen stimulated CD8 T cells. The activation induced chromatin remodeling and epigenetic silencing on various promoters and enhancers of Cd8 locus might be responsible for the de novo generation of DNT cells from CD8 T cells. As expected, the methylome of DNT cells affirmed hypermethylation on regulatory elements of Cd8 locus[111]. In brief, the transcription factor cAMP responsive element modulator (CREM)α orchestrates DNA methyltransferase (DNMT)3a and histone methyltransferase G9a to directly enhance DNA and histone methylation on Cd8 locus[112,113], which results in stable epigenetic silencing[113,114]. Accordingly, genetic deficiency of Crem in lupus prone mice significantly ameliorates disease manifestations by reducing IL-17+ DNT cells[115]. DNA methylation patterns in SLE T cells are complex with both hypomethylated and hypermethylated cytosine-guanine sites[116,117]. Generalized DNA hypomethylation in CD4 T cells has been well linked to the disease manifestation[118,119]. Surprisingly, in contrast to systemic delivery of 5-azacytidine[120], a DNA methyltransferase inhibitor, which profoundly augments disease progression[121], its targeted delivery to CD8 T cells using a nanolipogel delivery system significantly ameliorated disease severity in lupus prone mice by restraining the expansion of pathogenic DNT cells[122]. This result is consistent with the proposition that CD8 T cells acquire pro-inflammatory DNT cell phenotype through enhanced DNA methylation mediated CD8 loss[4]. In line with these observations, well controlled CD8 expression on CD8 T cells and DNT cells by proper modulation of epigenetic modification on Cd8 locus should present a valuable therapeutic strategy for the treatment of autoimmune disease with involvement of DNT cells (Fig. 2a).
Fig. 2. Therapeutic interventions targeting DNT cells.
a. Regulate the conversion between DNT cells and CD8 T cells through epigenetic modulation.
b. Eliminate DNT cells by adding missing signals for apoptosis.
c. Regulate the conversion between DNT cells and CD8 T cells by reshaping the cytokine milieu.
d. Inhibit DNT cell activation and expansion by targeting DNT cell metabolism.
The expanded DNT cells in human and mice with defective Fas-mediated pathway depicted another picture, in which DN T cells were derived from mature T cells with failed apoptosis[5]. Along this line, DNT cells with resistance to AICD could be generated in vitro from Fas-sufficient T-cells with repeated anti-CD3 stimulation[33,32]. However, further studies are warranted to validate whether addition of FasL or other apoptosis inducing molecules could modulate the generation of DNT cells as expected in vitro and in vivo (Fig. 2b) since the controversies persist on the therapeutic values of FasL in autoimmune disease[123]. Fas and FasL play essential immunosuppressive roles in controlling T cell homeostasis, as recorded with the development of autoimmunity in lpr or gld mice[5]. Paradoxically, Fas also plays a proinflammatory role in certain settings since lpr or gld mice are resistant to induced rheumatoid arthritis[124] and type I diabetes[125]. The constitutive expression of Fas in many types of cells may explain the observed complexity of Fas-mediated immune response[126]. Therefore, further insights into Fas-dependent and Fas-independent DNT cell homeostasis are needed for better therapeutic strategies.
The requirements for signal 3 provided by cytokines to DNT cell activation and differentiation link the cytokine milieu to loss of CD8 expression in CD8+ T cells[90,44]. It has been reported that IL-4-induced STAT6 orchestrates GATA3 for transcriptional repression of Cd8[114]. Interestingly, IFN-γ partially recovered CD8 expression in a subset of DN T cells[114] which is consistent with the observation that DNT cells could re-attain CD8 expression in the proper cytokine milieu in lymphopenic hosts[31]. Furthermore, in vivo, elevated IL-23 along with reduced TGFβ facilitate self-reactive DN T-cell activation, expansion, and survival[44]. Targeting cytokines, specific intracellular kinases or transcription factors provides an alternative therapeutic choice (Fig. 2c) although caution has to be applied because of shared components between different pathways.
It has become clear that metabolic processes control the fate decision of T cell differentiation and further the function of T cells. In autoimmune diseases, the disturbed or skewed metabolic pathways in T cells have been frequently reported[127,128]. However, most studies focus on CD4 T cells and very little attention has been given to DNT cells. Observation made in a clinical trial of sirolimus in patients with active SLE showed dramatic reduction of IL-4+ and IL-17+ producing DNT cells 12 months after treatment[129], which strongly suggests that mTOR blockade corrects pro-inflammatory DNT cell differentiation and activation. Consistently, PP2A, a serine/threonine phosphatase, plays a key role in restraining the activation of the metabolic checkpoint kinase mTOR and the PP2A activating molecule FTY720 induced DNT cell apoptosis in lupus prone murine[130]. Thus, the development of novel therapies to control the activity of metabolic enzymes in DNT cells represents a promising exercise for treatment of autoimmune diseases (Fig. 2d).
Conclusion
DNT cells represent important component of the immune system[5]. Although the possibility can not be excluded that some DNT cells are direct thymic escapes, a great portion of DNT cells are generated from peripheral CD8 T cells which lose CD8 expression on cell surface following the stimulation with combination of various signals including TCR engagement and cytokine stimulation[32,43,44,17]. Distinct epigenetic processes are responsible for this process and more studies are wanted for more details[4]. The fact that DNT cells infiltrate various inflamed organs including the skin and the kidney in different diseases[4] along with their ability to help B cells to produce autoantibody[12] and various pro-inflammatory cytokines including IL-17[13] underwrites their important contribution to the pathogenesis of autoimmune diseases. It is highly possible that a subset of DNT cells may instead have regulatory capacity in certain disease settings like organ transplantation and non-obese diabetes[17]. A growing understanding of DNT cell origin and functional features has prompted the consideration of therapeutic approaches including targeted reopening of the CD8 locus, precise modulation of cell activation and survival, inhibition of proinflammatory metabolic pathways and blockade of the inflammatory milieu which enables their generation or enabling their demise.
A number of questions needs urgent attention. A clear characterization of DNT subsets is needed through novel spectral cytometry and single cell sequencing technologies. The factors which enable the expansion of proinflammatory or regulatory DNT cells in various diseases need to be defined. Using advanced protocols, including Slide-Seq[131], the exact interaction between DNT cells and other immune cells or tissue resident cells should be defined. Prospective clinical studies are needed to define their appearance during the evolution of the disease process. Such studies may reveal that certain characteristic of DNT cells in the periphery can serve as biomarkers of organ inflammation and disease activity.
Key bullet points.
DNT cells are expanded in various chronic inflammatory diseases and they display pathogenic or regulatory function.
DNT cells are present in inflamed tissues and produce effector cytokines through which they exercise their function.
It is unclear whether they represent a distinct lineage or they originate from single positive cells, whether they represent a homogenous group of cells and whether they display plasticity.
Acknowledgments
Financial support and sponsorship
This worked was supported by grants RO1 AI085567 to G.C.T. and T32 DK007199 to H.L.
Footnotes
Conflicts of interest
There are no conflicts of interest.
References and recommended reading
Papers of particular interest, published within the annual period of review, have been highlighted as:
* Of special interest. ** Of outstanding interest.
- 1.Davis MM, Boniface JJ, Reich Z, Lyons D, Hampl J, Arden B, Chien Y (1998) Ligand recognition by alpha beta T cell receptors. Annu Rev Immunol 16:523–544. doi: 10.1146/annurev.immunol.16.1.523 [DOI] [PubMed] [Google Scholar]
- 2.Miceli MC, Parnes JR (1991) The roles of CD4 and CD8 in T cell activation. Semin Immunol 3 (3):133–141 [PubMed] [Google Scholar]
- 3.D’Acquisto F, Crompton T (2011) CD3+CD4-CD8- (double negative) T cells: saviours or villains of the immune response? Biochem Pharmacol 82 (4):333–340. doi: 10.1016/j.bcp.2011.05.019 [DOI] [PubMed] [Google Scholar]
- 4.Brandt D, Hedrich CM (2018) TCRalphabeta(+)CD3(+)CD4(−)CD8(−) (double negative) T cells in autoimmunity. Autoimmun Rev 17 (4):422–430. doi: 10.1016/j.autrev.2018.02.001 [DOI] [PubMed] [Google Scholar]
- 5.Martina MN, Noel S, Saxena A, Rabb H, Hamad AR (2015) Double negative (DN) alphabeta T cells: misperception and overdue recognition. Immunol Cell Biol 93 (3):305–310. doi: 10.1038/icb.2014.99 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Morse HC 3rd, Davidson WF, Yetter RA, Murphy ED, Roths JB, Coffman RL (1982) Abnormalities induced by the mutant gene Ipr: expansion of a unique lymphocyte subset. J Immunol 129 (6):2612–2615 [PubMed] [Google Scholar]
- 7.Davidson WF, Dumont FJ, Bedigian HG, Fowlkes BJ, Morse HC, 3rd (1986) Phenotypic, functional, and molecular genetic comparisons of the abnormal lymphoid cells of C3H-lpr/lpr and C3H-gld/gld mice. J Immunol 136 (11):4075–4084 [PubMed] [Google Scholar]
- 8.Roths JB, Murphy ED, Eicher EM (1984) A new mutation, gld, that produces lymphoproliferation and autoimmunity in C3H/HeJ mice. J Exp Med 159 (1):1–20. doi: 10.1084/jem.159.1.1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Cheng J, Zhou T, Liu C, Shapiro JP, Brauer MJ, Kiefer MC, Barr PJ, Mountz JD (1994) Protection from Fas-mediated apoptosis by a soluble form of the Fas molecule. Science 263 (5154):1759–1762. doi: 10.1126/science.7510905 [DOI] [PubMed] [Google Scholar]
- 10.Sneller MC, Straus SE, Jaffe ES, Jaffe JS, Fleisher TA, Stetler-Stevenson M, Strober W (1992) A novel lymphoproliferative/autoimmune syndrome resembling murine lpr/gld disease. J Clin Invest 90 (2):334–341. doi: 10.1172/JCI115867 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Fisher GH, Rosenberg FJ, Straus SE, Dale JK, Middleton LA, Lin AY, Strober W, Lenardo MJ, Puck JM (1995) Dominant interfering Fas gene mutations impair apoptosis in a human autoimmune lymphoproliferative syndrome. Cell 81 (6):935–946. doi: 10.1016/0092-8674(95)90013-6 [DOI] [PubMed] [Google Scholar]
- 12.Shivakumar S, Tsokos GC, Datta SK (1989) T cell receptor alpha/beta expressing double-negative (CD4-/CD8-) and CD4+ T helper cells in humans augment the production of pathogenic anti-DNA autoantibodies associated with lupus nephritis. J Immunol 143 (1):103–112 [PubMed] [Google Scholar]
- 13.Crispin JC, Oukka M, Bayliss G, Cohen RA, Van Beek CA, Stillman IE, Kyttaris VC, Juang YT, Tsokos GC (2008) Expanded double negative T cells in patients with systemic lupus erythematosus produce IL-17 and infiltrate the kidneys. J Immunol 181 (12):8761–8766. doi: 10.4049/jimmunol.181.12.8761 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Alunno A, Bistoni O, Bartoloni E, Caterbi S, Bigerna B, Tabarrini A, Mannucci R, Falini B, Gerli R (2013) IL-17-producing CD4-CD8- T cells are expanded in the peripheral blood, infiltrate salivary glands and are resistant to corticosteroids in patients with primary Sjogren’s syndrome. Ann Rheum Dis 72 (2):286–292. doi: 10.1136/annrheumdis-2012-201511 [DOI] [PubMed] [Google Scholar]
- 15.Alunno A, Carubbi F, Bistoni O, Caterbi S, Bartoloni E, Bigerna B, Pacini R, Beghelli D, Cipriani P, Giacomelli R, Gerli R (2014) CD4(−)CD8(−) T-cells in primary Sjogren’s syndrome: association with the extent of glandular involvement. J Autoimmun 51:38–43. doi: 10.1016/j.jaut.2014.01.030 [DOI] [PubMed] [Google Scholar]
- 16.Rodriguez-Rodriguez N, Apostolidis SA, Fitzgerald L, Meehan BS, Corbett AJ, Martin-Villa JM, McCluskey J, Tsokos GC, Crispin JC (2016) Pro-inflammatory self-reactive T cells are found within murine TCR-alphabeta(+) CD4(−) CD8(−) PD-1(+) cells. Eur J Immunol 46 (6):1383–1391. doi: 10.1002/eji.201546056 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Juvet SC, Zhang L (2012) Double negative regulatory T cells in transplantation and autoimmunity: recent progress and future directions. J Mol Cell Biol 4 (1):48–58. doi: 10.1093/jmcb/mjr043 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Anderson G, Jenkinson EJ (2001) Lymphostromal interactions in thymic development and function. Nat Rev Immunol 1 (1):31–40. doi: 10.1038/35095500 [DOI] [PubMed] [Google Scholar]
- 19.Spits H (2002) Development of alphabeta T cells in the human thymus. Nat Rev Immunol 2 (10):760–772. doi: 10.1038/nri913 [DOI] [PubMed] [Google Scholar]
- 20.Mixter PF, Russell JQ, Morrissette GJ, Charland C, Aleman-Hoey D, Budd RC (1999) A model for the origin of TCR-alphabeta+ CD4-CD8- B220+ cells based on high affinity TCR signals. J Immunol 162 (10):5747–5756 [PubMed] [Google Scholar]
- 21.Kurd N, Robey EA (2016) T-cell selection in the thymus: a spatial and temporal perspective. Immunol Rev 271 (1):114–126. doi: 10.1111/imr.12398 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Klein L, Kyewski B, Allen PM, Hogquist KA (2014) Positive and negative selection of the T cell repertoire: what thymocytes see (and don’t see). Nat Rev Immunol 14 (6):377–391. doi: 10.1038/nri3667 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Takahama Y (2006) Journey through the thymus: stromal guides for T-cell development and selection. Nat Rev Immunol 6 (2):127–135. doi: 10.1038/nri1781 [DOI] [PubMed] [Google Scholar]
- 24.Li QJ, Dinner AR, Qi S, Irvine DJ, Huppa JB, Davis MM, Chakraborty AK (2004) CD4 enhances T cell sensitivity to antigen by coordinating Lck accumulation at the immunological synapse. Nat Immunol 5 (8):791–799. doi: 10.1038/ni1095 [DOI] [PubMed] [Google Scholar]
- 25.Holler PD, Kranz DM (2003) Quantitative analysis of the contribution of TCR/pepMHC affinity and CD8 to T cell activation. Immunity 18 (2):255–264. doi: 10.1016/s1074-7613(03)00019-0 [DOI] [PubMed] [Google Scholar]
- 26.Starr TK, Jameson SC, Hogquist KA (2003) Positive and negative selection of T cells. Annu Rev Immunol 21:139–176. doi: 10.1146/annurev.immunol.21.120601.141107 [DOI] [PubMed] [Google Scholar]
- 27.Kreslavsky T, Kim HJ, Koralov SB, Ghitza D, Buch T, Cantor H, Rajewsky K, von Boehmer H (2013) Negative selection, not receptor editing, is a physiological response of autoreactive thymocytes. J Exp Med 210 (10):1911–1918. doi: 10.1084/jem.20130876 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Wang R, Wang-Zhu Y, Grey H (2002) Interactions between double positive thymocytes and high affinity ligands presented by cortical epithelial cells generate double negative thymocytes with T cell regulatory activity. Proc Natl Acad Sci U S A 99 (4):2181–2186. doi: 10.1073/pnas.042692799 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ford MS, Zhang ZX, Chen W, Zhang L (2006) Double-negative T regulatory cells can develop outside the thymus and do not mature from CD8+ T cell precursors. J Immunol 177 (5):2803–2809. doi: 10.4049/jimmunol.177.5.2803 [DOI] [PubMed] [Google Scholar]
- 30.Zhang Y, Wang H, Li J, Dong L, Xu P, Chen W, Neve RL, Volpe JJ, Rosenberg PA (2006) Intracellular zinc release and ERK phosphorylation are required upstream of 12-lipoxygenase activation in peroxynitrite toxicity to mature rat oligodendrocytes. J Biol Chem 281 (14):9460–9470. doi: 10.1074/jbc.M510650200 [DOI] [PubMed] [Google Scholar]
- 31.Rodriguez-Rodriguez N, Flores-Mendoza G, Apostolidis SA, Rosetti F, Tsokos GC, Crispin JC (2020) TCR-alpha/beta CD4(−) CD8(−) double negative T cells arise from CD8(+) T cells. J Leukoc Biol. doi: 10.1002/JLB.1AB0120-548R [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Crispin JC, Tsokos GC (2009) Human TCR-alpha beta+ CD4- CD8- T cells can derive from CD8+ T cells and display an inflammatory effector phenotype. J Immunol 183 (7):4675–4681. doi: 10.4049/jimmunol.0901533 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Mehal WZ, Crispe IN (1998) TCR ligation on CD8+ T cells creates double-negative cells in vivo. J Immunol 161 (4):1686–1693 [PubMed] [Google Scholar]
- 34.Bristeau-Leprince A, Mateo V, Lim A, Magerus-Chatinet A, Solary E, Fischer A, Rieux-Laucat F, Gougeon ML (2008) Human TCR alpha/beta+ CD4-CD8- double-negative T cells in patients with autoimmune lymphoproliferative syndrome express restricted Vbeta TCR diversity and are clonally related to CD8+ T cells. J Immunol 181 (1):440–448. doi: 10.4049/jimmunol.181.1.440 [DOI] [PubMed] [Google Scholar]
- 35.Mohamood AS, Bargatze D, Xiao Z, Jie C, Yagita H, Ruben D, Watson J, Chakravarti S, Schneck JP, Hamad AR (2008) Fas-mediated apoptosis regulates the composition of peripheral alphabeta T cell repertoire by constitutively purging out double negative T cells. PLoS One 3 (10):e3465. doi: 10.1371/journal.pone.0003465 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Teachey DT, Manno CS, Axsom KM, Andrews T, Choi JK, Greenbaum BH, McMann JM, Sullivan KE, Travis SF, Grupp SA (2005) Unmasking Evans syndrome: T-cell phenotype and apoptotic response reveal autoimmune lymphoproliferative syndrome (ALPS). Blood 105 (6):2443–2448. doi: 10.1182/blood-2004-09-3542 [DOI] [PubMed] [Google Scholar]
- 37.Shirai T, Abe M, Yagita H, Okumura K, Morse HC 3rd, Davidson WF (1990) The expanded populations of CD4-CD8- T cell receptor alpha/beta+ T cells associated with the lpr and gld mutations are CD2. J Immunol 144 (10):3756–3761 [PubMed] [Google Scholar]
- 38.Suda T, Takahashi T, Golstein P, Nagata S (1993) Molecular cloning and expression of the Fas ligand, a novel member of the tumor necrosis factor family. Cell 75 (6):1169–1178. doi: 10.1016/0092-8674(93)90326-l [DOI] [PubMed] [Google Scholar]
- 39.Watanabe-Fukunaga R, Brannan CI, Copeland NG, Jenkins NA, Nagata S (1992) Lymphoproliferation disorder in mice explained by defects in Fas antigen that mediates apoptosis. Nature 356 (6367):314–317. doi: 10.1038/356314a0 [DOI] [PubMed] [Google Scholar]
- 40.Green DR, Droin N, Pinkoski M (2003) Activation-induced cell death in T cells. Immunol Rev 193:70–81. doi: 10.1034/j.1600-065x.2003.00051.x [DOI] [PubMed] [Google Scholar]
- 41.Nagata S (1997) Apoptosis by death factor. Cell 88 (3):355–365. doi: 10.1016/s0092-8674(00)81874-7 [DOI] [PubMed] [Google Scholar]
- 42.Zhang J, Xu X, Liu Y (2004) Activation-induced cell death in T cells and autoimmunity. Cell Mol Immunol 1 (3):186–192 [PubMed] [Google Scholar]
- 43.Rodriguez-Rodriguez N, Apostolidis SA, Penaloza-MacMaster P, Martin Villa JM, Barouch DH, Tsokos GC, Crispin JC (2015) Programmed cell death 1 and Helios distinguish TCR-alphabeta+ double-negative (CD4-CD8-) T cells that derive from self-reactive CD8 T cells. J Immunol 194 (9):4207–4214. doi: 10.4049/jimmunol.1402775 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Li H, Adamopoulos IE, Moulton VR, Stillman IE, Herbert Z, Moon JJ, Sharabi A, Krishfield S, Tsokos MG, Tsokos GC (2020) Systemic lupus erythematosus favors the generation of IL-17 producing double negative T cells. Nat Commun 11 (1):2859. doi: 10.1038/s41467-020-16636-4**An important update on the expansion of DNT cells in the inflamatory milieu of various autoimmune dseases.
- 45.Herron LR, Eisenberg RA, Roper E, Kakkanaiah VN, Cohen PL, Kotzin BL (1993) Selection of the T cell receptor repertoire in Lpr mice. J Immunol 151 (7):3450–3459 [PubMed] [Google Scholar]
- 46.Fischer K, Voelkl S, Heymann J, Przybylski GK, Mondal K, Laumer M, Kunz-Schughart L, Schmidt CA, Andreesen R, Mackensen A (2005) Isolation and characterization of human antigen-specific TCR alpha beta+ CD4(−)CD8- double-negative regulatory T cells. Blood 105 (7):2828–2835. doi: 10.1182/blood-2004-07-2583 [DOI] [PubMed] [Google Scholar]
- 47.Sadasivam M, Noel S, Lee SA, Gong J, Allaf ME, Pierorazio P, Rabb H, Hamad ARA (2019) Activation and Proliferation of PD-1(+) Kidney Double-Negative T Cells Is Dependent on Nonclassical MHC Proteins and IL-2. J Am Soc Nephrol 30 (2):277–292. doi: 10.1681/ASN.2018080815*This study provides further evidence on the pathogenesis of DNT cells in tissue injury.
- 48.Martina MN, Noel S, Saxena A, Bandapalle S, Majithia R, Jie C, Arend LJ, Allaf ME, Rabb H, Hamad AR (2016) Double-Negative alphabeta T Cells Are Early Responders to AKI and Are Found in Human Kidney. J Am Soc Nephrol 27 (4):1113–1123. doi: 10.1681/ASN.2014121214 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Rensing-Ehl A, Volkl S, Speckmann C, Lorenz MR, Ritter J, Janda A, Abinun M, Pircher H, Bengsch B, Thimme R, Fuchs I, Ammann S, Allgauer A, Kentouche K, Cant A, Hambleton S, Bettoni da Cunha C, Huetker S, Kuhnle I, Pekrun A, Seidel MG, Hummel M, Mackensen A, Schwarz K, Ehl S (2014) Abnormally differentiated CD4+ or CD8+ T cells with phenotypic and genetic features of double negative T cells in human Fas deficiency. Blood 124 (6):851–860. doi: 10.1182/blood-2014-03-564286 [DOI] [PubMed] [Google Scholar]
- 50.Fleisher TA (2008) The autoimmune lymphoproliferative syndrome: an experiment of nature involving lymphocyte apoptosis. Immunol Res 40 (1):87–92. doi: 10.1007/s12026-007-8001-1 [DOI] [PubMed] [Google Scholar]
- 51.Zlotnik A, Godfrey DI, Fischer M, Suda T (1992) Cytokine production by mature and immature CD4-CD8- T cells. Alpha beta-T cell receptor+ CD4-CD8- T cells produce IL-4. J Immunol 149 (4):1211–1215 [PubMed] [Google Scholar]
- 52.Nathan C, Ding A (2010) Nonresolving inflammation. Cell 140 (6):871–882. doi: 10.1016/j.cell.2010.02.029 [DOI] [PubMed] [Google Scholar]
- 53.Brandt D, Sergon M, Abraham S, Mabert K, Hedrich CM (2017) TCR(+)CD3(+)CD4(−)CD8(−) effector T cells in psoriasis. Clin Immunol 181:51–59. doi: 10.1016/j.clim.2017.06.002 [DOI] [PubMed] [Google Scholar]
- 54.Sherlock JP, Joyce-Shaikh B, Turner SP, Chao CC, Sathe M, Grein J, Gorman DM, Bowman EP, McClanahan TK, Yearley JH, Eberl G, Buckley CD, Kastelein RA, Pierce RH, Laface DM, Cua DJ (2012) IL-23 induces spondyloarthropathy by acting on ROR-gammat+ CD3+CD4-CD8- entheseal resident T cells. Nat Med 18 (7):1069–1076. doi: 10.1038/nm.2817 [DOI] [PubMed] [Google Scholar]
- 55.Meng H, Zhao H, Cao X, Hao J, Zhang H, Liu Y, Zhu MS, Fan L, Weng L, Qian L, Wang X, Xu Y (2019) Double-negative T cells remarkably promote neuroinflammation after ischemic stroke. Proc Natl Acad Sci U S A 116 (12):5558–5563. doi: 10.1073/pnas.1814394116*An important article demostrating the presence of DNT cells in neuroinflammation.
- 56.Artyomov MN, Lis M, Devadas S, Davis MM, Chakraborty AK (2010) CD4 and CD8 binding to MHC molecules primarily acts to enhance Lck delivery. Proc Natl Acad Sci U S A 107 (39):16916–16921. doi: 10.1073/pnas.1010568107 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Chowdhary VR, Krogman A, Tilahun AY, Alexander MP, David CS, Rajagopalan G (2017) Concomitant Disruption of CD4 and CD8 Genes Facilitates the Development of Double Negative alphabeta TCR(+) Peripheral T Cells That Respond Robustly to Staphylococcal Superantigen. J Immunol 198 (11):4413–4424. doi: 10.4049/jimmunol.1601991**An important article demonstrating that cognate TCR-antigen interaction without proper augmentation by CD4 or CD8 could be sufficient driving T cell activation in vivo.
- 58.Jia Q, Lee BY, Bowen R, Dillon BJ, Som SM, Horwitz MA (2010) A Francisella tularensis live vaccine strain (LVS) mutant with a deletion in capB, encoding a putative capsular biosynthesis protein, is significantly more attenuated than LVS yet induces potent protective immunity in mice against F. tularensis challenge. Infect Immun 78 (10):4341–4355. doi: 10.1128/IAI.00192-10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.DeMaster LK, Liu X, VanBelzen DJ, Trinite B, Zheng L, Agosto LM, Migueles SA, Connors M, Sambucetti L, Levy DN, Pasternak AO, O’Doherty U (2015) A Subset of CD4/CD8 Double-Negative T Cells Expresses HIV Proteins in Patients on Antiretroviral Therapy. J Virol 90 (5):2165–2179. doi: 10.1128/JVI.01913-15 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Meziane O, Salahuddin S, Pham TNQ, Farnos O, Pagliuzza A, Olivenstein R, Thomson E, Alexandrova Y, Orlova M, Schurr E, Ancuta P, Haddad E, Chomont N, Cohen EA, Jenabian MA, Costiniuk CT (2020) HIV infection and persistence in pulmonary mucosal double negative T-cells in vivo. J Virol. doi: 10.1128/JVI.01788-20 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Su Y, Huang X, Wang S, Min WP, Yin Z, Jevnikar AM, Zhang ZX (2012) Double negative Treg cells promote nonmyeloablative bone marrow chimerism by inducing T-cell clonal deletion and suppressing NK cell function. Eur J Immunol 42 (5):1216–1225. doi: 10.1002/eji.201141808 [DOI] [PubMed] [Google Scholar]
- 62.Duncan B, Nazarov-Stoica C, Surls J, Kehl M, Bona C, Casares S, Brumeanu TD (2010) Double negative (CD3+ 4– 8-) TCR alphabeta splenic cells from young NOD mice provide long-lasting protection against type 1 diabetes. PLoS One 5 (7):e11427. doi: 10.1371/journal.pone.0011427 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Hillhouse EE, Beauchamp C, Chabot-Roy G, Dugas V, Lesage S (2010) Interleukin-10 limits the expansion of immunoregulatory CD4-CD8- T cells in autoimmune-prone non-obese diabetic mice. Immunol Cell Biol 88 (8):771–780. doi: 10.1038/icb.2010.84 [DOI] [PubMed] [Google Scholar]
- 64.Hillhouse EE, Thiant S, Moutuou MM, Lombard-Vadnais F, Parat R, Delisle JS, Ahmad I, Roy DC, Guimond M, Roy J, Lesage S (2019) Double-Negative T Cell Levels Correlate with Chronic Graft-versus-Host Disease Severity. Biol Blood Marrow Transplant 25 (1):19–25. doi: 10.1016/j.bbmt.2018.09.008**This study suggests the immunoregulatory potential of DNT cells in vivo.
- 65.Bluestone JA, Mackay CR, O’Shea JJ, Stockinger B (2009) The functional plasticity of T cell subsets. Nat Rev Immunol 9 (11):811–816. doi: 10.1038/nri2654 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Zhou L, Chong MM, Littman DR (2009) Plasticity of CD4+ T cell lineage differentiation. Immunity 30 (5):646–655. doi: 10.1016/j.immuni.2009.05.001 [DOI] [PubMed] [Google Scholar]
- 67.Zhu J, Paul WE (2010) Heterogeneity and plasticity of T helper cells. Cell Res 20 (1):4–12. doi: 10.1038/cr.2009.138 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Teng MW, Bowman EP, McElwee JJ, Smyth MJ, Casanova JL, Cooper AM, Cua DJ (2015) IL-12 and IL-23 cytokines: from discovery to targeted therapies for immune-mediated inflammatory diseases. Nat Med 21 (7):719–729. doi: 10.1038/nm.3895 [DOI] [PubMed] [Google Scholar]
- 69.Mizui M, Koga T, Lieberman LA, Beltran J, Yoshida N, Johnson MC, Tisch R, Tsokos GC (2014) IL-2 protects lupus-prone mice from multiple end-organ damage by limiting CD4-CD8- IL-17-producing T cells. J Immunol 193 (5):2168–2177. doi: 10.4049/jimmunol.1400977 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Kyttaris VC, Zhang Z, Kuchroo VK, Oukka M, Tsokos GC (2010) Cutting edge: IL-23 receptor deficiency prevents the development of lupus nephritis in C57BL/6-lpr/lpr mice. J Immunol 184 (9):4605–4609. doi: 10.4049/jimmunol.0903595 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Teachey DT (2012) New advances in the diagnosis and treatment of autoimmune lymphoproliferative syndrome. Curr Opin Pediatr 24 (1):1–8. doi: 10.1097/MOP.0b013e32834ea739 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Price S, Shaw PA, Seitz A, Joshi G, Davis J, Niemela JE, Perkins K, Hornung RL, Folio L, Rosenberg PS, Puck JM, Hsu AP, Lo B, Pittaluga S, Jaffe ES, Fleisher TA, Rao VK, Lenardo MJ (2014) Natural history of autoimmune lymphoproliferative syndrome associated with FAS gene mutations. Blood 123 (13):1989–1999. doi: 10.1182/blood-2013-10-535393 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Neven B, Magerus-Chatinet A, Florkin B, Gobert D, Lambotte O, De Somer L, Lanzarotti N, Stolzenberg MC, Bader-Meunier B, Aladjidi N, Chantrain C, Bertrand Y, Jeziorski E, Leverger G, Michel G, Suarez F, Oksenhendler E, Hermine O, Blanche S, Picard C, Fischer A, Rieux-Laucat F (2011) A survey of 90 patients with autoimmune lymphoproliferative syndrome related to TNFRSF6 mutation. Blood 118 (18):4798–4807. doi: 10.1182/blood-2011-04-347641 [DOI] [PubMed] [Google Scholar]
- 74.Worth A, Thrasher AJ, Gaspar HB (2006) Autoimmune lymphoproliferative syndrome: molecular basis of disease and clinical phenotype. Br J Haematol 133 (2):124–140. doi: 10.1111/j.1365-2141.2006.05993.x [DOI] [PubMed] [Google Scholar]
- 75.Bleesing JJ, Brown MR, Novicio C, Guarraia D, Dale JK, Straus SE, Fleisher TA (2002) A composite picture of TcR alpha/beta(+) CD4(−)CD8(−) T Cells (alpha/beta-DNTCs) in humans with autoimmune lymphoproliferative syndrome. Clin Immunol 104 (1):21–30. doi: 10.1006/clim.2002.5225 [DOI] [PubMed] [Google Scholar]
- 76.Li P, Huang P, Yang Y, Hao M, Peng H, Li F (2016) Updated Understanding of Autoimmune Lymphoproliferative Syndrome (ALPS). Clin Rev Allergy Immunol 50 (1):55–63. doi: 10.1007/s12016-015-8466-y [DOI] [PubMed] [Google Scholar]
- 77.Lisco A, Wong CS, Price S, Ye P, Niemela J, Anderson M, Richards E, Manion M, Mystakelis H, Similuk M, Lo B, Stoddard J, Rosenzweig S, Vanpouille C, Rupert A, Maric I, Perez-Diez A, Parenti D, Burbelo PD, Rao VK, Sereti I (2019) Paradoxical CD4 Lymphopenia in Autoimmune Lymphoproliferative Syndrome (ALPS). Front Immunol 10:1193. doi: 10.3389/fimmu.2019.01193 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Teachey DT, Obzut DA, Axsom K, Choi JK, Goldsmith KC, Hall J, Hulitt J, Manno CS, Maris JM, Rhodin N, Sullivan KE, Brown VI, Grupp SA (2006) Rapamycin improves lymphoproliferative disease in murine autoimmune lymphoproliferative syndrome (ALPS). Blood 108 (6):1965–1971. doi: 10.1182/blood-2006-01-010124 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Teachey DT, Greiner R, Seif A, Attiyeh E, Bleesing J, Choi J, Manno C, Rappaport E, Schwabe D, Sheen C, Sullivan KE, Zhuang H, Wechsler DS, Grupp SA (2009) Treatment with sirolimus results in complete responses in patients with autoimmune lymphoproliferative syndrome. Br J Haematol 145 (1):101–106. doi: 10.1111/j.1365-2141.2009.07595.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Kossiva L, Theodoridou M, Mostrou G, Vrachnou E, Le Deist F, Rieux-Laucat F, Kanariou MG (2006) Mycophenolate mofetil as an alternate immunosuppressor for autoimmune lymphoproliferative syndrome. J Pediatr Hematol Oncol 28 (12):824–826. doi: 10.1097/MPH.0b013e31802d7503 [DOI] [PubMed] [Google Scholar]
- 81.Tsokos GC (2011) Systemic lupus erythematosus. N Engl J Med 365 (22):2110–2121. doi: 10.1056/NEJMra1100359 [DOI] [PubMed] [Google Scholar]
- 82.Masuda T, Ohteki T, Abo T, Seki S, Nose S, Nagura H, Kumagai K (1991) Expansion of the population of double negative CD4–8- T alpha beta-cells in the liver is a common feature of autoimmune mice. J Immunol 147 (9):2907–2912 [PubMed] [Google Scholar]
- 83.Dean GS, Anand A, Blofeld A, Isenberg DA, Lydyard PM (2002) Characterization of CD3+ CD4- CD8- (double negative) T cells in patients with systemic lupus erythematosus: production of IL-4. Lupus 11 (8):501–507. doi: 10.1191/0961203302lu234oa [DOI] [PubMed] [Google Scholar]
- 84.Hsu HC, Yang P, Wang J, Wu Q, Myers R, Chen J, Yi J, Guentert T, Tousson A, Stanus AL, Le TV, Lorenz RG, Xu H, Kolls JK, Carter RH, Chaplin DD, Williams RW, Mountz JD (2008) Interleukin 17-producing T helper cells and interleukin 17 orchestrate autoreactive germinal center development in autoimmune BXD2 mice. Nat Immunol 9 (2):166–175. doi: 10.1038/ni1552 [DOI] [PubMed] [Google Scholar]
- 85.Amarilyo G, Lourenco EV, Shi FD, La Cava A (2014) IL-17 promotes murine lupus. J Immunol 193 (2):540–543. doi: 10.4049/jimmunol.1400931 [DOI] [PubMed] [Google Scholar]
- 86.Zhang Z, Kyttaris VC, Tsokos GC (2009) The role of IL-23/IL-17 axis in lupus nephritis. J Immunol 183 (5):3160–3169. doi: 10.4049/jimmunol.0900385 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Sharabi A, Mozes E (2009) Harnessing regulatory T cells for the therapy of lupus and other autoimmune diseases. Immunotherapy 1 (3):385–401. doi: 10.2217/imt.09.2 [DOI] [PubMed] [Google Scholar]
- 88.Dinesh RK, Skaggs BJ, La Cava A, Hahn BH, Singh RP (2010) CD8+ Tregs in lupus, autoimmunity, and beyond. Autoimmun Rev 9 (8):560–568. doi: 10.1016/j.autrev.2010.03.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Mishra S, Liao W, Liu Y, Yang M, Ma C, Wu H, Zhao M, Zhang X, Qiu Y, Lu Q, Zhang N (2021) TGF-beta and Eomes control the homeostasis of CD8+ regulatory T cells. J Exp Med 218 (1). doi: 10.1084/jem.20200030 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Curtsinger JM, Schmidt CS, Mondino A, Lins DC, Kedl RM, Jenkins MK, Mescher MF (1999) Inflammatory cytokines provide a third signal for activation of naive CD4+ and CD8+ T cells. J Immunol 162 (6):3256–3262 [PubMed] [Google Scholar]
- 91.Voulgarelis M, Tzioufas AG (2010) Pathogenetic mechanisms in the initiation and perpetuation of Sjogren’s syndrome. Nat Rev Rheumatol 6 (9):529–537. doi: 10.1038/nrrheum.2010.118 [DOI] [PubMed] [Google Scholar]
- 92.Alunno A, Carubbi F, Bartoloni E, Bistoni O, Caterbi S, Cipriani P, Giacomelli R, Gerli R (2014) Unmasking the pathogenic role of IL-17 axis in primary Sjogren’s syndrome: a new era for therapeutic targeting? Autoimmun Rev 13 (12):1167–1173. doi: 10.1016/j.autrev.2014.08.022 [DOI] [PubMed] [Google Scholar]
- 93.Lowes MA, Suarez-Farinas M, Krueger JG (2014) Immunology of psoriasis. Annu Rev Immunol 32:227–255. doi: 10.1146/annurev-immunol-032713-120225 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Ueyama A, Imura C, Fusamae Y, Tsujii K, Furue Y, Aoki M, Suzuki M, Okuda T, Oshima I, Yasui K, Shichijo M, Yamamoto M (2017) Potential role of IL-17-producing CD4/CD8 double negative alphabeta T cells in psoriatic skin inflammation in a TPA-induced STAT3C transgenic mouse model. J Dermatol Sci 85 (1):27–35. doi: 10.1016/j.jdermsci.2016.10.007 [DOI] [PubMed] [Google Scholar]
- 95.Sieper J, Poddubnyy D (2017) Axial spondyloarthritis. Lancet 390 (10089):73–84. doi: 10.1016/S0140-6736(16)31591-4 [DOI] [PubMed] [Google Scholar]
- 96.Wellcome Trust Case Control C, Australo-Anglo-American Spondylitis C, Burton PR, Clayton DG, Cardon LR, Craddock N, Deloukas P, Duncanson A, Kwiatkowski DP, McCarthy MI, Ouwehand WH, Samani NJ, Todd JA, Donnelly P, Barrett JC, Davison D, Easton D, Evans DM, Leung HT, Marchini JL, Morris AP, Spencer CC, Tobin MD, Attwood AP, Boorman JP, Cant B, Everson U, Hussey JM, Jolley JD, Knight AS, Koch K, Meech E, Nutland S, Prowse CV, Stevens HE, Taylor NC, Walters GR, Walker NM, Watkins NA, Winzer T, Jones RW, McArdle WL, Ring SM, Strachan DP, Pembrey M, Breen G, St Clair D, Caesar S, Gordon-Smith K, Jones L, Fraser C, Green EK, Grozeva D, Hamshere ML, Holmans PA, Jones IR, Kirov G, Moskivina V, Nikolov I, O’Donovan MC, Owen MJ, Collier DA, Elkin A, Farmer A, Williamson R, McGuffin P, Young AH, Ferrier IN, Ball SG, Balmforth AJ, Barrett JH, Bishop TD, Iles MM, Maqbool A, Yuldasheva N, Hall AS, Braund PS, Dixon RJ, Mangino M, Stevens S, Thompson JR, Bredin F, Tremelling M, Parkes M, Drummond H, Lees CW, Nimmo ER, Satsangi J, Fisher SA, Forbes A, Lewis CM, Onnie CM, Prescott NJ, Sanderson J, Matthew CG, Barbour J, Mohiuddin MK, Todhunter CE, Mansfield JC, Ahmad T, Cummings FR, Jewell DP, Webster J, Brown MJ, Lathrop MG, Connell J, Dominiczak A, Marcano CA, Burke B, Dobson R, Gungadoo J, Lee KL, Munroe PB, Newhouse SJ, Onipinla A, Wallace C, Xue M, Caulfield M, Farrall M, Barton A, Biologics in RAG, Genomics Study Syndicate Steering C, Bruce IN, Donovan H, Eyre S, Gilbert PD, Hilder SL, Hinks AM, John SL, Potter C, Silman AJ, Symmons DP, Thomson W, Worthington J, Dunger DB, Widmer B, Frayling TM, Freathy RM, Lango H, Perry JR, Shields BM, Weedon MN, Hattersley AT, Hitman GA, Walker M, Elliott KS, Groves CJ, Lindgren CM, Rayner NW, Timpson NJ, Zeggini E, Newport M, Sirugo G, Lyons E, Vannberg F, Hill AV, Bradbury LA, Farrar C, Pointon JJ, Wordsworth P, Brown MA, Franklyn JA, Heward JM, Simmonds MJ, Gough SC, Seal S, Breast Cancer Susceptibility C, Stratton MR, Rahman N, Ban M, Goris A, Sawcer SJ, Compston A, Conway D, Jallow M, Newport M, Sirugo G, Rockett KA, Bumpstead SJ, Chaney A, Downes K, Ghori MJ, Gwilliam R, Hunt SE, Inouye M, Keniry A, King E, McGinnis R, Potter S, Ravindrarajah R, Whittaker P, Widden C, Withers D, Cardin NJ, Davison D, Ferreira T, Pereira-Gale J, Hallgrimsdo’ttir IB, Howie BN, Su Z, Teo YY, Vukcevic D, Bentley D, Brown MA, Compston A, Farrall M, Hall AS, Hattersley AT, Hill AV, Parkes M, Pembrey M, Stratton MR, Mitchell SL, Newby PR, Brand OJ, Carr-Smith J, Pearce SH, McGinnis R, Keniry A, Deloukas P, Reveille JD, Zhou X, Sims AM, Dowling A, Taylor J, Doan T, Davis JC, Savage L, Ward MM, Learch TL, Weisman MH, Brown M (2007) Association scan of 14,500 nonsynonymous SNPs in four diseases identifies autoimmunity variants. Nat Genet 39 (11):1329–1337. doi: 10.1038/ng.2007.17 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Tarbox JA, Keppel MP, Topcagic N, Mackin C, Ben Abdallah M, Baszis KW, White AJ, French AR, Cooper MA (2014) Elevated double negative T cells in pediatric autoimmunity. J Clin Immunol 34 (5):594–599. doi: 10.1007/s10875-014-0038-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Ling E, Shubinsky G, Press J (2007) Increased proportion of CD3+CD4-CD8- double-negative T cells in peripheral blood of children with Behcet’s disease. Autoimmun Rev 6 (4):237–240. doi: 10.1016/j.autrev.2006.08.008 [DOI] [PubMed] [Google Scholar]
- 99.Ilonen J, Lempainen J, Veijola R (2019) The heterogeneous pathogenesis of type 1 diabetes mellitus. Nat Rev Endocrinol 15 (11):635–650. doi: 10.1038/s41574-019-0254-y [DOI] [PubMed] [Google Scholar]
- 100.Pugliese A (2017) Autoreactive T cells in type 1 diabetes. J Clin Invest 127 (8):2881–2891. doi: 10.1172/JCI94549 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Liu T, Cong M, Sun G, Wang P, Tian Y, Shi W, Li X, You H, Zhang D (2016) Combination of double negative T cells and anti-thymocyte serum reverses type 1 diabetes in NOD mice. J Transl Med 14:57. doi: 10.1186/s12967-016-0815-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Dugas V, Beauchamp C, Chabot-Roy G, Hillhouse EE, Lesage S (2010) Implication of the CD47 pathway in autoimmune diabetes. J Autoimmun 35 (1):23–32. doi: 10.1016/j.jaut.2010.01.002 [DOI] [PubMed] [Google Scholar]
- 103.Zhang ZX, Ma Y, Wang H, Arp J, Jiang J, Huang X, He KM, Garcia B, Madrenas J, Zhong R (2006) Double-negative T cells, activated by xenoantigen, lyse autologous B and T cells using a perforin/granzyme-dependent, Fas-Fas ligand-independent pathway. J Immunol 177 (10):6920–6929. doi: 10.4049/jimmunol.177.10.6920 [DOI] [PubMed] [Google Scholar]
- 104.Young KJ, Yang L, Phillips MJ, Zhang L (2002) Donor-lymphocyte infusion induces transplantation tolerance by activating systemic and graft-infiltrating double-negative regulatory T cells. Blood 100 (9):3408–3414. doi: 10.1182/blood-2002-01-0235 [DOI] [PubMed] [Google Scholar]
- 105.Zhang D, Yang W, Degauque N, Tian Y, Mikita A, Zheng XX (2007) New differentiation pathway for double-negative regulatory T cells that regulates the magnitude of immune responses. Blood 109 (9):4071–4079. doi: 10.1182/blood-2006-10-050625 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Voelkl S, Gary R, Mackensen A (2011) Characterization of the immunoregulatory function of human TCR-alphabeta+ CD4- CD8- double-negative T cells. Eur J Immunol 41 (3):739–748. doi: 10.1002/eji.201040982 [DOI] [PubMed] [Google Scholar]
- 107.Zhang ZX, Yang L, Young KJ, DuTemple B, Zhang L (2000) Identification of a previously unknown antigen-specific regulatory T cell and its mechanism of suppression. Nat Med 6 (7):782–789. doi: 10.1038/77513 [DOI] [PubMed] [Google Scholar]
- 108.Zhang L, Thomson AW (2011) New partners for tolerogenic dendritic cells. Am J Transplant 11 (10):2003–2004. doi: 10.1111/j.1600-6143.2011.03654.x [DOI] [PubMed] [Google Scholar]
- 109.Kelchtermans H, Billiau A, Matthys P (2008) How interferon-gamma keeps autoimmune diseases in check. Trends Immunol 29 (10):479–486. doi: 10.1016/j.it.2008.07.002 [DOI] [PubMed] [Google Scholar]
- 110.Lalani I, Bhol K, Ahmed AR (1997) Interleukin-10: biology, role in inflammation and autoimmunity. Ann Allergy Asthma Immunol 79 (6):469–483. doi: 10.1016/S1081-1206(10)63052-9 [DOI] [PubMed] [Google Scholar]
- 111.Renauer PA, Coit P, Sawalha AH (2015) The DNA methylation signature of human TCRalphabeta+CD4-CD8- double negative T cells reveals CG demethylation and a unique epigenetic architecture permissive to a broad stimulatory immune response. Clin Immunol 156 (1):19–27. doi: 10.1016/j.clim.2014.10.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Hedrich CM, Crispin JC, Rauen T, Ioannidis C, Koga T, Rodriguez Rodriguez N, Apostolidis SA, Kyttaris VC, Tsokos GC (2014) cAMP responsive element modulator (CREM) alpha mediates chromatin remodeling of CD8 during the generation of CD3+ CD4- CD8- T cells. J Biol Chem 289 (4):2361–2370. doi: 10.1074/jbc.M113.523605 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Hedrich CM, Rauen T, Crispin JC, Koga T, Ioannidis C, Zajdel M, Kyttaris VC, Tsokos GC (2013) cAMP-responsive element modulator alpha (CREMalpha) trans-represses the transmembrane glycoprotein CD8 and contributes to the generation of CD3+CD4-CD8- T cells in health and disease. J Biol Chem 288 (44):31880–31887. doi: 10.1074/jbc.M113.508655 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Harland KL, Day EB, Apte SH, Russ BE, Doherty PC, Turner SJ, Kelso A (2014) Epigenetic plasticity of Cd8a locus during CD8(+) T-cell development and effector differentiation and reprogramming. Nat Commun 5:3547. doi: 10.1038/ncomms4547 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Yoshida N, Comte D, Mizui M, Otomo K, Rosetti F, Mayadas TN, Crispin JC, Bradley SJ, Koga T, Kono M, Karampetsou MP, Kyttaris VC, Tenbrock K, Tsokos GC (2016) ICER is requisite for Th17 differentiation. Nat Commun 7:12993. doi: 10.1038/ncomms12993 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Jeffries MA, Dozmorov M, Tang Y, Merrill JT, Wren JD, Sawalha AH (2011) Genome-wide DNA methylation patterns in CD4+ T cells from patients with systemic lupus erythematosus. Epigenetics 6 (5):593–601. doi: 10.4161/epi.6.5.15374 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Chung SA, Nititham J, Elboudwarej E, Quach HL, Taylor KE, Barcellos LF, Criswell LA (2015) Genome-Wide Assessment of Differential DNA Methylation Associated with Autoantibody Production in Systemic Lupus Erythematosus. PLoS One 10 (7):e0129813. doi: 10.1371/journal.pone.0129813 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Sawalha AH (2008) Epigenetics and T-cell immunity. Autoimmunity 41 (4):245–252. doi: 10.1080/08916930802024145 [DOI] [PubMed] [Google Scholar]
- 119.Hedrich CM, Tsokos GC (2011) Epigenetic mechanisms in systemic lupus erythematosus and other autoimmune diseases. Trends Mol Med 17 (12):714–724. doi: 10.1016/j.molmed.2011.07.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Christman JK (2002) 5-Azacytidine and 5-aza-2’-deoxycytidine as inhibitors of DNA methylation: mechanistic studies and their implications for cancer therapy. Oncogene 21 (35):5483–5495. doi: 10.1038/sj.onc.1205699 [DOI] [PubMed] [Google Scholar]
- 121.Yoshida H, Yoshida M, Merino R, Shibata T, Izui S (1990) 5-Azacytidine inhibits the lpr gene-induced lymphadenopathy and acceleration of lupus-like syndrome in MRL/MpJ-lpr/lpr mice. Eur J Immunol 20 (9):1989–1993. doi: 10.1002/eji.1830200917 [DOI] [PubMed] [Google Scholar]
- 122.Li H, Tsokos MG, Bickerton S, Sharabi A, Li Y, Moulton VR, Kong P, Fahmy TM, Tsokos GC (2018) Precision DNA demethylation ameliorates disease in lupus-prone mice. JCI Insight 3 (16). doi: 10.1172/jci.insight.120880 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Rossin A, Miloro G, Hueber AO (2019) TRAIL and FasL Functions in Cancer and Autoimmune Diseases: Towards an Increasing Complexity. Cancers (Basel) 11 (5). doi: 10.3390/cancers11050639 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Tu-Rapp H, Hammermuller A, Mix E, Kreutzer HJ, Goerlich R, Kohler H, Nizze H, Thiesen HJ, Ibrahim SM (2004) A proinflammatory role for Fas in joints of mice with collagen-induced arthritis. Arthritis Res Ther 6 (5):R404–414. doi: 10.1186/ar1205 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Itoh N, Imagawa A, Hanafusa T, Waguri M, Yamamoto K, Iwahashi H, Moriwaki M, Nakajima H, Miyagawa J, Namba M, Makino S, Nagata S, Kono N, Matsuzawa Y (1997) Requirement of Fas for the development of autoimmune diabetes in nonobese diabetic mice. J Exp Med 186 (4):613–618. doi: 10.1084/jem.186.4.613 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Strasser A, Jost PJ, Nagata S (2009) The many roles of FAS receptor signaling in the immune system. Immunity 30 (2):180–192. doi: 10.1016/j.immuni.2009.01.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Sharabi A, Tsokos GC (2020) T cell metabolism: new insights in systemic lupus erythematosus pathogenesis and therapy. Nat Rev Rheumatol 16 (2):100–112. doi: 10.1038/s41584-019-0356-x [DOI] [PubMed] [Google Scholar]
- 128.Yang Z, Matteson EL, Goronzy JJ, Weyand CM (2015) T-cell metabolism in autoimmune disease. Arthritis Res Ther 17:29. doi: 10.1186/s13075-015-0542-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Lai ZW, Kelly R, Winans T, Marchena I, Shadakshari A, Yu J, Dawood M, Garcia R, Tily H, Francis L, Faraone SV, Phillips PE, Perl A (2018) Sirolimus in patients with clinically active systemic lupus erythematosus resistant to, or intolerant of, conventional medications: a single-arm, open-label, phase 1/2 trial. Lancet 391 (10126):1186–1196. doi: 10.1016/S0140-6736(18)30485-9**An important article demostrating the metabolic processes control DNT cell homeostasis.
- 130.Okazaki H, Hirata D, Kamimura T, Sato H, Iwamoto M, Yoshio T, Masuyama J, Fujimura A, Kobayashi E, Kano S, Minota S (2002) Effects of FTY720 in MRL-lpr/lpr mice: therapeutic potential in systemic lupus erythematosus. J Rheumatol 29 (4):707–716 [PubMed] [Google Scholar]
- 131.Rodriques SG, Stickels RR, Goeva A, Martin CA, Murray E, Vanderburg CR, Welch J, Chen LM, Chen F, Macosko EZ (2019) Slide-seq: A scalable technology for measuring genome-wide expression at high spatial resolution. Science 363 (6434):1463–1467. doi: 10.1126/science.aaw1219 [DOI] [PMC free article] [PubMed] [Google Scholar]