Extended Data Fig. 10 |. The CALHM2 gap junction.
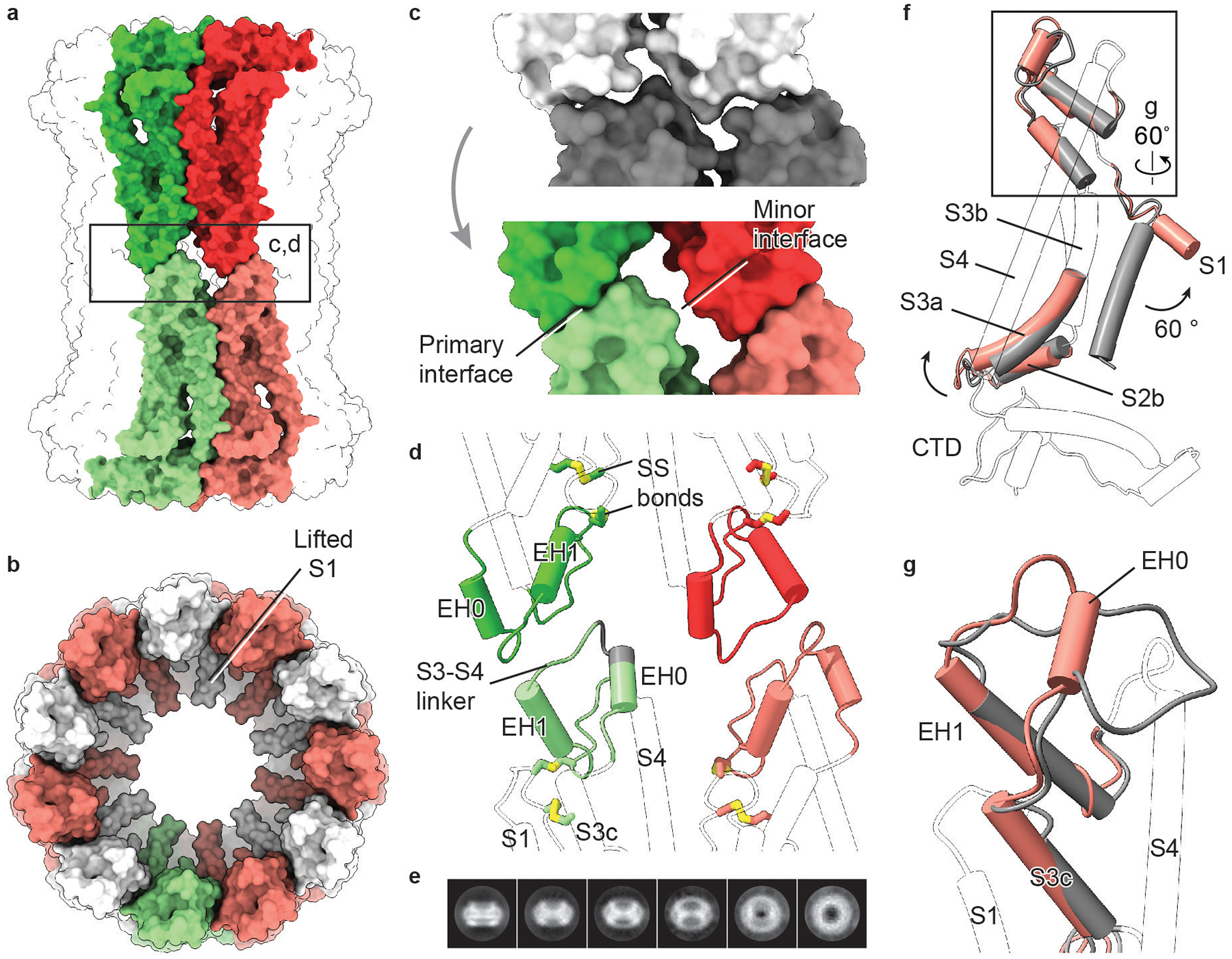
a, Surface representation of a gap junction viewed parallel to the membrane. Two paired subunits are highlighted. b, Surface representation of a hemichannel in the gap junction, viewed from extracellular side. c, Interface remodelling when docking two hemichannels (shown in grey; top) into a gap junction (shown in colour; bottom). d, Cartoon representation of the interface between two hemichannels. The two disulfide bonds are shown. The grey segment of S3–S4 linker represents deleted residues in a mutant (CALHM2(Δ143–146)). Only parts involved in the docking of hemichannels and disulfide bonds are highlighted in colour. e, Selected 2D class averages of CALHM2(Δ143–146). This mutant yielded only hemichannels, and not gap junctions. f, Superimposition of single subunits of EDTA–CALHM2hemi (grey) and EDTA–CALHM2gap (pink) using the CTD. Only parts with conformational changes are highlighted in colour. To understand the conformational changes upon docking, we compared single subunits of a hemichannel and a gap junction. In the hemichannel, the loop connecting segment S3c and EH1 in the S3–S4 linker is flat and lacks extensive contact with the rest of the protein, giving rise to a flexible area that is probably required for the initiation of docking. Indeed, the S3–S4 linker is defined better in RUR–CALHM2 than in EDTA–CALHM2hemi, by forming interactions with the adjacent subunit. We suggest that the restricted S3–S4 linker in the RUR–CALHM2 hinders the docking of the hemichannels. g, Enlargement of the box in f, showing the remodelling of the S3–S4 linker from EDTA–CALHM2hemi (grey) to EDTA–CALHM2gap (pink). Upon docking, the S3c–EH1 loop remodels into two short loops and a short α helix (EH0); the EH0–EH1 loop forms the primary interface and EH0 forms the minor interface in d. This motion accompanies an elevation of the S3–S4 linker and segment S3c that leads to an outward flexing of S3a, which breaks the loose interface between S3a and helix S1 in f. As a consequence, the S1 helix is detached from S3 and moves into a lifted conformation. The conformational changes of TMD upon docking in EDTA–CALHM2 are notably consistent with those induced by RUR. Moreover, the two docked hemichannels in the gap junction have similar conformations of their S1 helices.
