Abstract
The goal of this Review is to discuss the clinical approach to patients who do not respond to treatment for eosinophilic oesophagitis (EoE). Refractory EoE is challenging to manage as there are limited data to guide decision-making. In this Review, refractory EoE is defined as persistent eosinophilia in the setting of incomplete resolution of the primary presenting symptoms and incomplete resolution of endoscopic findings following a PPI trial, and after treatment with either topical steroids or dietary elimination. However, this definition is controversial. This Review will examine these controversies, explore how frequently non-response is observed, and highlight potential explanations and predictors of non-response. Non-response is common and affects a large proportion of patients with EoE. It is important to systematically assess multiple possible causes of non-response, as well as consider treatment complications and an incorrect diagnosis of EoE. If non-response is confirmed, second-line treatments are required. Although the overall response rate for second-line therapy is disappointing, with only half of patients eventually responding, there are several promising agents that are currently under investigation, and the future is bright for new treatment modalities for refractory EoE.
Eosinophilic oesophagitis (EoE) is a chronic allergic immune-mediated clinicopathological condition in which an oesophageal eosinophil infiltrate causes symptoms of oesophageal dysfunction1. EoE was first described in the late 1970s2,3, and the condition as it is recognized today was reported in a series of publications in the early 1990s4–6. Since the publication of these reports, there has been a rapid increase in both the incidence and prevalence of EoE, which has outpaced the increase in awareness of the condition7,8. Although EoE is a worldwide disease, its prevalence is highest (~1 in 2,000 people) in the USA, Western Europe and Australia, and its current incidence is estimated to be 10 cases per 100,000 individuals per year7,9,10. EoE represents a major cause of upper gastrointestinal morbidity in patients of any age, but is more common in children and young adults. Accordingly, it has become the second most common cause of oesophagitis7,11, the most common cause of food bolus impaction12,13 and is seen in up to 23% of patients who undergo upper endoscopy for symptoms of dysphagia14–17. Annual costs related to EoE in the USA alone are estimated to be ~US$1 billon per year18.
To diagnose EoE, patients must present with symptoms of oesophageal dysfunction and an oesophageal biopsy with at least 15 eosinophils per high-power microscopy field (eos/hpf)19–21. Symptoms of EoE include dysphagia, food impaction, heartburn and chest pain; abdominal pain, vomiting and poor growth can be manifestations in children. However, other local and systemic secondary causes of eosinophilia must be excluded, and eosinophilia must persist after a high-dose trial of a PPI20. Although current guidelines suggest that after a PPI trial treatment for EoE can comprise either topical corticosteroid or dietary elimination therapy, there are currently no FDA-approved treatments for EoE19,20,22. Topical corticosteroids that are asthma preparations, such as fluticasone or budesonide, can be swallowed rather than inhaled to coat the oesophagus and provide an anti-inflammatory effect. Dietary elimination removes potential food triggers, and can be directed either by allergy testing or empirically. In some cases, a hypoallergenic elemental formula composed of amino acid solids, simple carbohydrates and medium-chain triglycerides can also be used.
The goal of this Review is to discuss the clinical approach to patients with EoE who do not respond or are refractory to treatment. The definition of refractory EoE will be discussed first, as well as controversies related to this definition. Second, the frequency of non-response will be explored and potential explanations and predictors of non-response will be highlighted. Last, the evidence base for second-line treatments in EoE will be reviewed and emerging therapeutic options will be discussed.
What is refractory EoE?
From a conceptual standpoint, refractory EoE can be defined as ongoing symptoms, abnormal endoscopic findings (FIG. 1) and persistent oesophageal eosinophilia after a PPI trial and after treatment with either topical steroids or dietary elimination therapy. Notably, this definition does not perfectly parallel the diagnostic criteria, which do not require endoscopic findings19,20. In addition, for the purposes of this Review, PPIs remain within the diagnostic algorithm for EoE and are not considered as treatment modalities. However, it is important to acknowledge that the role of PPIs in oesophageal eosinophilia and EoE is an area of ongoing controversy and has a rapidly evolving evidence base. In patients who have an EoE clinical phenotype, there is increasing recognition that individuals who respond to PPI treatment (PPI-responsive oesophageal eosinophilia; PPI-REE) are similar in almost all aspects of the disease to those who do not respond (classically defined as EoE)14,23–27. A systematic review and meta-analysis that was published in 2016 showed that ~50% of such patients with oesophageal eosinophilia responded to PPI therapy28. Moreover, other studies have identified novel acid-independent anti-eosinophil properties of PPIs that can mechanistically explain the pharmacological response to this class of medications29,30. Consequently, the question remains as to whether PPIs should be considered for the treatment of EoE, rather than solely as a diagnostic test; however, guidelines have not yet changed to reflect this aspect31. Thus, it would be difficult to consider a patient who does not respond to a PPI trial to have refractory EoE without that patient also failing to respond to either topical steroids or dietary elimination. This nuance has been reflected in the earlier conceptual definition of refractory EoE.
Figure 1 |. Endoscopic appearance of refractory EoE.
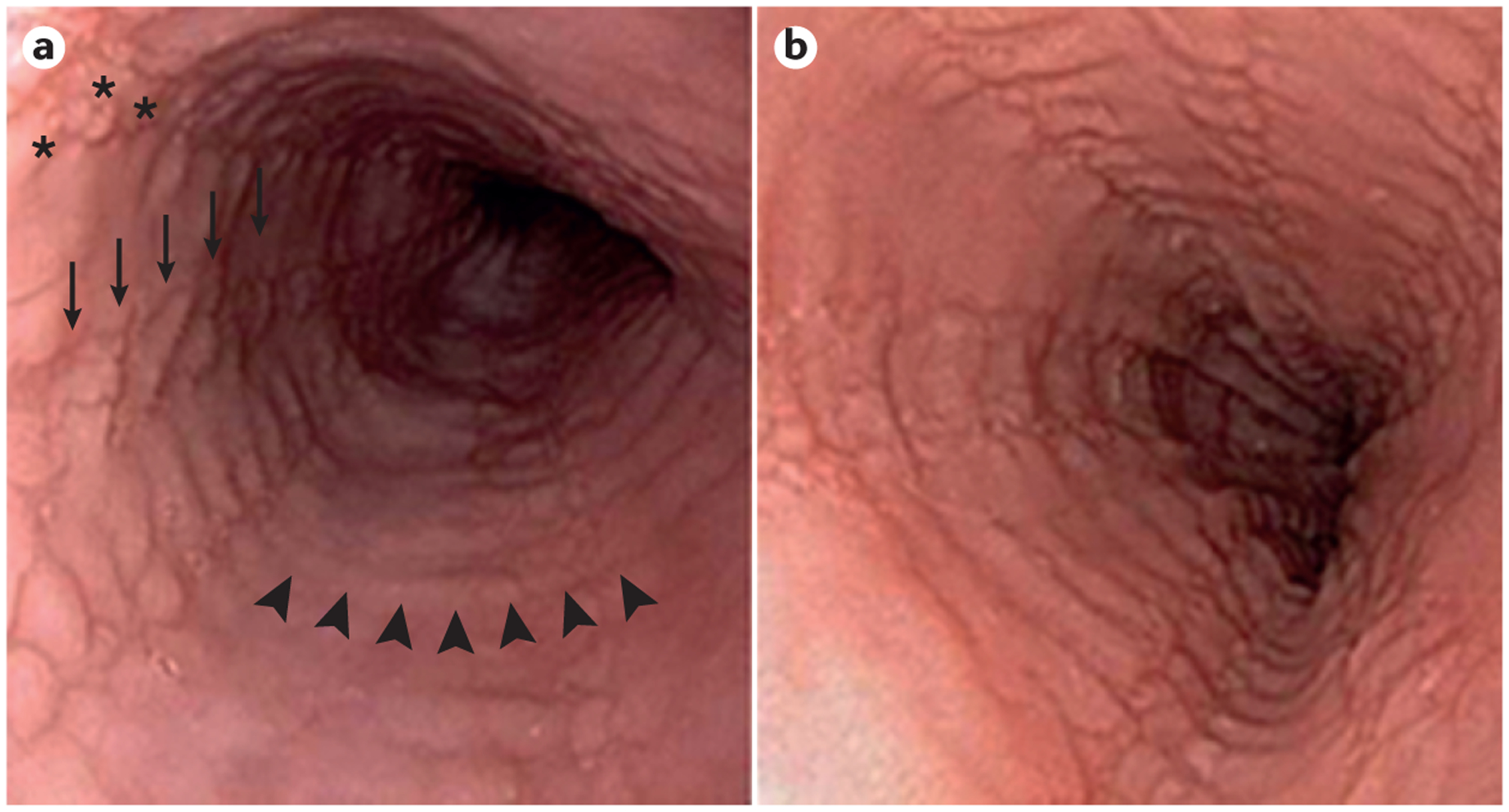
Paired endoscopic images of the oesophagus of a patient with eosinophilic oesophagitis (EoE) at diagnosis (part a) and after treatment with oral viscous budesonide at a dose of 1 mg twice daily (part b). At both time points, the patient remained symptomatic with dysphagia and there is no change in either the endoscopic findings (oedema (a diffuse loss of vascular markings), rings (arrowheads), subtle exudates (asterisks) and furrows (arrows) are seen at both time points) or histological findings (60 eos/hpf at diagnosis and 55 eos/hpf post-treatment). Eos/hpf, eosinophils per high-power field.
Application of the definition of refractory EoE in clinical practice can be challenging. Current guidelines do not discuss this issue in depth20 and there are limited studies published specifically on this topic32–35. In addition, each clinicopathological component of EoE has to be considered when assessing response36. For example, it is unclear as to what threshold constitutes true symptom non-response, and whether non-response should be solely defined as no change in symptoms, <50% decrease in symptoms, or some other threshold. Although there are now several validated symptom and quality of life measures for use in EoE37–42, these measures are not typically used in clinical practice as they can be somewhat time-consuming, and were initially developed as research tools and have an undefined response threshold. Similarly, it is unclear as to what constitutes histological non-response — potential criteria include failure to normalize a biopsy sample with any number of persistent eosinophils, failure to achieve a set percentage decrease in eosinophil count or failure to lower the eosinophil count past a pre-specified threshold. The same issue remains in regard to endoscopic findings. It is unclear whether endoscopic non-response requires the complete normalization of the appearance of the oesophagus, a pre-defined improvement in a validated endoscopic severity measure (such as the EoE Endoscopic Reference Score (EREFS))43, or any improvement in a measure such as this. As discussed later, there is also the possibility for response in one domain (that is, symptoms) and not in another (that is, histology or endoscopy).
Owing to the lack of data to guide this definition, and for the purpose of this Review, refractory EoE will be defined as persistent eosinophilia in ≥15 eos/hpf in the setting of incomplete resolution of the primary presenting symptoms and endoscopic findings (BOX 1).As discussed later in this Review, the histological response threshold for EoE is often empirically chosen and varies markedly between studies22. However, in the past few years there have been both retrospective and preliminary prospective data showing that a response threshold of 15 eos/hpf is associated with improved symptomatic and endoscopic responses, and that lowering this threshold to less than 15 eos/hpf does not result in a substantial additional symptom or endoscopy response44,45. Moreover, failure to reach this threshold has also been associated with an increased need for oesophageal dilation in patients who have EoE that is complicated by strictures or narrowing46. These emerging data lend some credibility to 15 eos/hpf being a reasonable response threshold, and this level provides symmetry with the diagnostic criteria. Nevertheless, the response threshold must be individualized in clinical practice. For example, a patient who has 200 eos/hpf at baseline and achieves a post-treatment eosinophil count of 16 eos/hpf, as well as symptomatic and endoscopic improvement, could be considered to be a responder. Conversely, a patient who progresses from 20 eos/hpf to 14 eos/hpf after treatment, with persistent symptoms and endoscopic findings, could be classified as a non-responder. Importantly, eosinophil counts (eos/hpf) will vary with the size of the high-power microscopy field used and, therefore, a more accurate method is to determine eosinophil density per unit area (eos/mm2)47. As this method is not yet frequently carried out in clinical practice, it is important to confirm that the size of the hpf used to assess eosinophil infiltrate post-treatment is identical to that used for the pre-treatment determination. For reference, a count of 15 eos/hpf is equivalent to ~60 eos/mm2 for common microscope field areas in the 0.24–0.3 mm2 range. Moreover, long-term outcome data are required concerning the clinical, endoscopic and histological consequences of persistent low-level oesophageal eosinophilia over time to more fully inform histological response thresholds. This information would also complement data showing that eosinophilia and diagnostic delay are associated with increased risk of strictures and fibrostenotic phenotypes48–50.
Box 1 |. Proposed definition of refractory EoE.
After a PPI trial, and following treatment with either topical corticosteroids or dietary elimination, refractory EoE can be defined as:
Persistent oesophageal eosinophilia (≥15 eos/hpf)
Incomplete resolution of the primary presenting symptoms
Incomplete resolution of endoscopic findings of EoE
EoE, eosinophilic oesophagitis; eos/hpf, eosinophils per high-power field.
How common is non-response?
Data concerning the frequency of non-response can be obtained from several sources, including case series, cohort studies, randomized controlled trials (RCTs) and meta-analyses. To date, there have been 12 RCTs that have examined response to topical corticosteroids in EoE51–62. With the exception of one study that evaluated a novel experimental budesonide formulation in which non-response occurred in 0–6% of patients59, the majority of studies showed histological non-response51–58,60–62. The frequency of non-response varied depending on the eos/hpf threshold used: non-response was reported in 6–85% of individuals using the most stringent histological threshold reported for that specific study, and in 22–69% of individuals using a response threshold of <15 eos/hpf (TABLE 1). In these studies, symptoms and endoscopic findings were not consistently evaluated, and validated measures have only been used in one new study60; therefore, it is difficult to determine exact symptomatic and endoscopic non-response rates (TABLE 1). Owing to the rigorous design and patient follow-up in RCTs, these results are probably the best that can be expected. Indeed, in the few large cohort studies that have been reported with real-world experience, non-response has been even more commonly reported. For example, Wolf and colleagues32 reported a 43% histological non-response rate using a threshold of <15 eos/hpf, Moawad and colleagues63 reported a 63% non-response rate (5 eos/hpf) in preliminary data and Leung and colleagues33 reported a 44% non-response rate (10 eos/hpf). However, these high non-response rates are not universal. Philpott and colleagues64 reported 8% non-response using a threshold of <5 eos/hpf, Boldorini and colleagues65 reported 24% non-response (≤6 eos/hpf) and Henderson and colleagues66 reported 19% (15 eos/hpf).
Table 1 |.
Frequency of non-response in RCTs of topical corticosteroids, by histological, symptom and endoscopic outcome measures
| Author, year | Medication and dose | Patient group | Most stringent histological response threshold reported | Non-response for most stringent level (%) | Non-response for <15 eos/hpf level (%) | Symptom response | Endoscopic response |
|---|---|---|---|---|---|---|---|
| Konikoff, 2006 (REF. 51) |
|
Children | ≤1 eos/hpf | 50 | 45* | Decreased vomiting | Decreased furrows distally |
| Schafer, 2008 (REF. 52) |
|
Children | <1 eos/hpf | 44* | 22* | Presenting symptom improved | – |
| Dohil, 2010 (REF. 53) |
|
Children | ≤6 eos/hpf | 13 | 23* | Improvement in Symptom Scoring Tool | Improvement in Endoscopy Scoring Tool |
| Straumann, 2010 (REF. 54) |
|
Adults | <5 eos/hpf | 28 | 11‡ | Improvement in the SDI | Improvement in exudates and furrows, not rings |
| Peterson, 2010 (REF. 62)§ |
|
Adults | ≤5 eos/hpf | 85 | 69 | No difference versus comparator arm with a 7-point dysphagia scale | – |
| Alexander, 2012 (REF. 56) |
|
Adults | >90% decrease in eosinophil counts | 48 | – | No difference versus comparator using the MDQ | 30% with resolution of pre-treatment findings |
| Dellon, 2012 (REF. 55) |
|
Adults | <1 eos/hpf | 36 | 27 | No difference versus comparator using the MDQ | Improvement in endoscopic findings except stricture |
| Moawad, 2013 (REF. 61)§ |
|
Adults | <7 eos/hpf | 81 | - | No difference in fluticasone arm using the MDQ | Decreased exudates |
| Butz, 2014 (REF. 57) |
|
Adolescents and adults | ≤1 eos/hpf | 35 | 23 | Decreased heartburn | – |
| Gupta, 2015 (REF. 58) |
|
Children | ≤1 eos/hpf | 23 | – | No difference versus comparator using the Clinical Symptom Score | – |
| Miehlke, 2015 (REF. 59) |
|
Adults | <16 eos/mm2 | 6 | – | No difference versus comparator using the SDI | Decrease in Endoscopic Severity Score versus comparator |
| Dellon, 2017 (REF. 60) |
|
Adolescents and adults | ≤1 eos/hpf | 69 | 53 | Improvement in the Dysphagia Symptom Questionnaire | Improvement in the EREFS score |
BET, budesonide effervescent tablet; BVS, budesonide viscous solution; EoE, eosinophilic oesophagitis; eos/hpf, eosinophils per high-power field; EREFS, EoE endoscopic reference score; MDQ, Mayo Dysphagia Questionnaire; OBS, oral budesonide suspension; OVB, oral viscous budesonide; RCTs, randomized controlled trials; SDI, Strumann Dysphagia Instrument.
Calculated from individual patient data presented in the manuscript.
For the reported threshold of <20 eos/hpf rather than <15 eos/hpf.
Patients in these studies had EoE, but not EoE as defined by consensus guidelines.
Although there are currently no published RCTs that evaluated dietary elimination therapy in EoE, there are several prospective and retrospective cohort studies that investigated this modality6,64,66–78, and summary data for non-response has been compiled in a meta-analysis79.This study found that elemental formula was the most effective treatment for EoE, with only a 9% non-response rate. The six-food elimination diet (SFED; typically with the elimination of dairy, wheat, egg, soy, nuts and seafood) had a non-response rate of 28% and targeted (allergy-directed) elimination had a non-response of 55%. However, there are studies of SFED in which non-response rates almost reach 50%64,74.
In summary, data for both pharmacological and dietary treatment modalities for EoE show that a large minority, and sometimes up to half, of patients with EoE will not respond to initial treatment. This aspect highlights a substantial unmet need in the therapeutic approach to these patients, and shows that non-response will commonly be encountered in clinical practice.
Evaluation and causes of non-response
Before deciding on a second-line treatment plan for patients with EoE, it is imperative to systematically consider the potential reasons for why a patient might have not responded to first-line therapy. As will be discussed below, there are multiple specific and identifiable causes of non-response, which if corrected could lead to response. Thus, it is important to not label a patient as non-responsive until these issues are investigated.
Symptom and histology discordance
First, the clinician must ask which component of the disease presentation has not improved. When a patient has persistent symptoms, endoscopic findings and oesophageal eosinophilia, there is probably true non-response. However, when there is discordance between some of these features, additional investigation is necessary. This discordant response can occur because of the separate inflammatory and fibrostenotic aspects of EoE49,80. The inflammatory component of EoE relates to local consequences of eosinophilia and manifests with tissue injury and endoscopic findings of oedema, linear furrows and white plaques. By contrast, the fibrostenotic component relates to oesophageal remodelling that results in oesophageal strictures or narrowing. An anti-inflammatory treatment, such as dietary elimination or topical steroids, can improve the histological findings, but symptoms of dysphagia can persist if there is an oesophageal stricture. Similarly, symptoms of heartburn, dysphagia or odynophagia can persist despite a histological response if there is a treatment complication, such as candidal oesophagitis or herpes oesophagitis. By contrast, symptoms can improve despite ongoing eosinophilia in several instances: if a stricture has been dilated, if a patient modifies his or her diet enough to mitigate symptoms (in the most extreme example, a person using a liquid-only diet would have no symptoms of dysphagia), or with regression to the mean. Furthermore, when assessing endoscopic findings of EoE, it is important to fully insufflate the oesophagus, take adequate time to assess multiple potential findings (including subtle findings, such as oedema or narrowing), and use a validated metric, such as the EoE EREFS, which also has an atlas of endoscopic images that can be used for reference43. The features of the EREFS that are considered inflammatory (oedema, exudates and furrows) might have a better response to anti-inflammatory or anti-eosinophil-directed therapy than the fibrostenotic features (rings and strictures). However, given the fact that fibrostenotic features can sometimes improve after treatment, a full assessment of all endoscopic signs is warranted81. As symptom and endoscopic or histological discordance seems to be a common feature of EoE36,82, all of these issues should be considered before proceeding with second-line treatments.
Non-response to topical steroids
Several factors underlie non-response to topical steroids34 (BOX 2). The first is adherence; not only must the patient take the medication but they must take the medication in the correct manner to maximize oesophageal deposition. For topical steroids, this adherence requires that they swallow the medication and refrain from eating or drinking for 30–60 min following ingestion. As there are no FDA-approved oesophageal-specific medications for EoE, clinicians currently rely on patients to correctly spray a multi-dose inhaler into their mouth or mix a viscous solution of budesonide correctly. If the oesophageal dwell time is too low, then the medication might be ineffective55,83. Given promising results of new oesophageal-specific formulations, a large proportion of non-response might eventually be attributed to poor oesophageal delivery58,59. In addition, as these medications are expensive and it can be difficult to obtain insurance or healthcare provider approval for them, some patients do not obtain their prescription medications owing to high cost. If non-adherence is noted, a discussion about the reason — cost, current adverse effects, concern about the long-term adverse effects, dosing frequency, preference for another treatment, lack of perceived effect on symptoms, and so on — should take place. Patients must also take the correct dose of the medication. The recommended dose of fluticasone is 440–880 μg per day in younger children and 880–1,760 μg per day in older children and adults, and the dose of budesonide is 1 mg per day in younger children and 2 mg per day in older children and adults20–22. However, for both medications it seems that doses at the higher end of these ranges are most effective52,56–59. Nevertheless, because these are unconventional medications, some patients and practitioners can be confused about the dosing and the route of administration; therefore, a carefully collated history regarding adherence is necessary to investigate this. As noted earlier, the topical use of steroids can be complicated by candidal or herpes oesophagitis53,55,56,84,85, which gives the impression that symptoms have not improved. Symptoms can also persist if there is an oesophageal stricture or narrowing that has not been recognized or dilated. Furthermore, there might be persistent food or environmental allergen exposure, associated allergic diseases that cause steroid non-response86 or it could be possible that patients might have a steroid-insensitive subtype of EoE.
Box 2 |. Potential explanations for non-response.
For topical corticosteroids:
Non-adherence
Dose too low
Inappropriate administration
Suboptimal formulation (low dwell time)
Persistent allergen exposure
Superimposed infection (for example, with Candida spp. or herpes simplex virus)
Stricture causing persistent symptoms
Incorrect diagnosis of EoE
For dietary elimination:
Non-adherence
Inadvertent contamination
Correct trigger, or triggers, not eliminated and/or persistent allergen exposure
Stricture causing persistent symptoms
Incorrect diagnosis of EoE
EoE, eosinophilic oesophagitis.
Non-response to dietary elimination
There are also causes of non-response to dietary elimination (BOX 2). For dietary elimination, adherence is particularly crucial, and given the complexities of some of the dietary elimination treatment options, it is understandable that patients might not be able to fully comply. Moreover, the increased costs that are associated with these speciality diets87 could also affect adherence. In addition, if a patient and their clinician are not working with a dietician or nutritionist who has EoE-specific knowledge, dietary treatment is much less likely to be effective88–90. Under these circumstances, lack of adherence would be directly related to a lack of EoE-specific knowledge; for example, in a patient who does not know that whey protein is derived from dairy and uses it as a supplemental protein source. In patients for whom non-adherence to dietary treatment is identified, it is important to have a detailed discussion with the patient about the reasons. Considerations can include that they are overwhelmed with the dietary restrictions, they need additional nutritional resources and support, they cannot afford speciality foods, they are unable to incorporate dietary restrictions into their current family or work lifestyle, they do not see any positive results on symptoms, or they feel reluctant to admit that it is not a desirable treatment for them. Once the underlying reasons are identified, they can potentially be addressed. A further consideration can be inadvertent cross-contamination, which can occur even with very careful patients who adhere to their dietary regi-men; however, there is limited data regarding the minimal amount of a food allergen that is required to trigger EoE. An additional cause of dietary non-response is that the true trigger for EoE in a patient is not included in the elimination diet. The existing allergy testing modalities of skin-prick testing and serum food-specific IgE testing do not provide high accuracy for the identification of food triggers in EoE91. Two separate prospective studies of SFED with subsequent food re-introduction in dietary responders found only a 13% concordance between skin-prick testing and the identifying trigger foods in EoE71,72, with an additional study published in 2016 confirming this discordance92. Atopy patch testing (APT) has shown some promise in being more accurate than skin-prick testing for identifying food triggers in EoE68, but has not been a reproducible technique66. Conceivably, a patient can be perfectly compliant with their prescribed diet, but might still not respond as there are unidentified food triggers that have not been eliminated.
Other reasons for non-response
If a patient does not respond to therapy as expected, it is important to reconsider the diagnosis of EoE. To this end, ensuring that the patient meets the consensus diagnostic guidelines for EoE is crucial, including non-response to a high-dose PPI trial19,20. In some cases, the original pathology slides must be re-examined to reaffirm that there were increased levels of oesophageal eosinophilia. If there are persistent symptoms, it is also important to determine whether these are due to EoE or whether there might be another cause. In particular, overlapping reflux disease, an oesophageal motility disorder, or a functional condition should be considered. Studies have shown that eosinophils can cause oesophageal hypersensitivity that can persist even with resolution of eosinophilia93 and can be associated with functional dyspepsia94.
Predictors of non-response
Limited data exist from studies that investigated predictors of non-response in EoE and most are related to topical steroids. In two separate retrospective cohort studies that used multivariate analyses of several clinical, endoscopic and histological features of EoE, only oesophageal dilation at the baseline endoscopy (before treatment) was associated with non-response, although the reasons for this association are unclear32,63. In one of these studies, increased levels of mast cells and eotaxin-3 in oesophageal tissues were associated with improved response rates32; however, this finding conflicts with another study that demonstrated that high levels of mast cells were associated with non-response65. Levels of phosphorylated extracellular signal-regulated kinase (ERK) were also shown to be increased in steroid-refractory patients95. In a RCT of fluticasone versus placebo, pre-treatment gene expression for histological non-responders compared with responders was assessed57. The results suggested that several genes might be differentially expressed before treatment in non-responders. Although these data hold promise for potentially individualizing treatment in the future, the finding must be independently replicated in a larger cohort of patients. Preliminary data regarding a polymorphism in the promoter of transforming growth factor β1 (TGFB1) have shown that a CC genotype is associated with favourable treatment response in children with EoE96.
A treatment-resistant EoE phenotype has been described97. Patients with extreme narrow-calibre oesophagus, defined by diffuse narrowing or severe stricturing that precluded passage of a standard adult upper endoscope (8–10 mm in diameter), were approximately one-third less likely to respond to topical steroids than patients without these fibrostenotic changes. This extreme narrow-calibre oesophagus phenotype was observed in 9% of cases of EoE, and the mechanisms of non-response in this group have yet to be elucidated. However, another study linked severe oesophageal rings in patients with EoE to decreased oesophageal compliance, as measured by an endoluminal functional luminal imaging probe98, which suggests that a transmural fibrotic process might occur99. Conceivably, the topical administration of medication would be insufficient in these cases, but this aspect remains to be examined. Despite data assessing predictors of response, there are studies in which no predictors have been identified54,100, which warrants substantially more work on this topic. In addition, given promising results and high response rates with new oesophageal-specific medication formulations58,59, it is possible that a strong predictor of non-response to topical steroids could be low oesophageal dwell time55.
Treatment options for non-responders
After excluding issues that are related to patient adherence, dosing, formulation or medication administration, ongoing exposure to allergens or food triggers, and knowledge related to dietary elimination, a patient who has EoE can be confirmed as a non-responder. At this stage, the clinician must then determine the next best option for treatment. Unfortunately, there are few data to guide this process. A suggested algorithm that is based on the existing data, clinical experience and current guidelines is presented in FIG. 2, the principle of which is to identify treatment options in a logical and stepwise manner. If there is continued non-response after treatment with standard first-line therapies for EoE, then there are several potential second-line agents that could be used, but each of these has limitations. However, data describing the success rates of second-line therapies are limited. One study that investigated this issue specifically found that only 48% of initial non-responders would proceed to have a response with second-line agents32. In another report, 54% of patients who were refractory to standard first-line EoE treatment responded to therapy with second-line agents33. Owing to this low response, patients who are refractory to standard EoE treatments should be considered for clinical trials, in which some novel compounds are under study. The next section will review the existing data for several potential second-line treatment options for EoE. Although these therapies are discussed individually and the algorithm suggests that these should be used separately, the clinical benefit of combination therapy for EoE is unknown. However, in certain patients, combination therapy might be worth considering if individual treatment options are ineffective.
Figure 2 |. Clinical approach to refractory EoE.
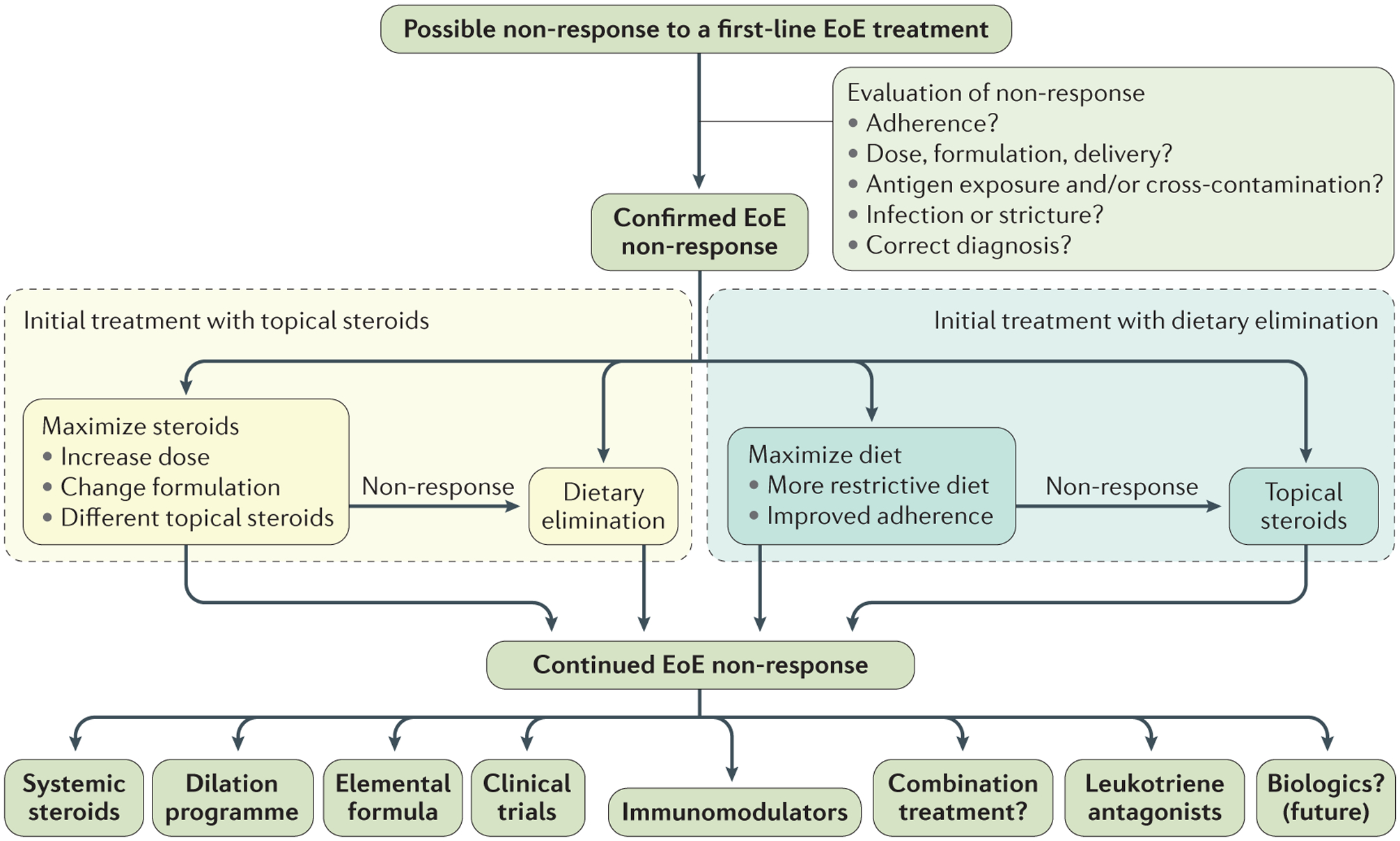
A suggested treatment algorithm for patients with eosinophilic oesophagitis (EoE) after a PPI trial who do not respond to a either topical steroid or dietary elimination treatment. When a patient might have non-response, the first step is to evaluate the potential causes of non-response and correct them. If non-response is confirmed, the next step will depend on the initial treatment strategy. If the original treatment was with topical steroids, then this modality could be optimized or the patient could be switched to dietary elimination. If the original treatment was dietary elimination, this could be maximized or the patient could be treated with topical steroids. If there is continued non-response, there are a wide range of options the choice of which will depend on the patient and disease characteristics. Often these patients are suitable candidates for participation in clinical trials of emerging treatment agents.
Topical steroids
For topical steroids, data suggest that a higher dose of oral viscous budesonide is associated with improved histological response. In an RCT of a new budesonide suspension in children with EoE, a high-dose arm (2.8–4 mg per day, depending on age) had a 94% histological response rate (≤6 eos/hpf) compared with the low-dose arm (0.35–0.5 mg per day, depending on age), for whom there was a 24% response58. In addition, RCTs that use fluticasone at high doses (1,760 μg per day52,57) tend to have better response rates than lower doses, such as 880 μg per day51, but there are no published trials that specifically investigate dose–response for fluticasone.
In addition to budesonide and fluticasone, other topical steroids, including beclomethasone, mometasone and ciclesonide, have been shown to be effective101–103. Ciclesonide is of particular interest, as this drug is a highly potent steroid that must be activated by an esterase, which is a hydrolase enzyme that is present in both the lungs and oesophagus103. A case series of four patients who had prior steroid failure showed that ciclesonide might be a promising agent103, but the results were not replicated in two other small series of patients32,104.
As oesophageal medication deposition and increased contact or dwell time have been associated with histological responses in EoE55, there is an active interest in developing oesophageal-specific formulations of topical steroids. A phase II study of a budesonide effervescent tablet showed rates of 95–100% histological response (<16 eos/mm2) in adults, as well as an improvement in endoscopic severity, but did not show a symptom improvement compared with placebo59. In addition, a phase II study of a mucoadherent budesonide oral solution had a 39% rate of histological response (≤6 eos/hpf), with associated improvements in endoscopic severity and the severity and frequency of dysphagia symptoms60. Both agents are currently in phase III studies. However, as there are no FDA-approved topical steroids for EoE, patients and physicians must adapt the medications that are currently available to maximize oesophageal deposition. Although the original formulation of oral viscous budesonide comprised aqueous budesonide respules with sucralose105,106, data now show that mixing aqueous budesonide with elemental formula, honey, powdered drink mixes, xanthan gum, or having it compounded in a speciality pharmacy, can also be effective83,107. For fluticasone, there is a preliminary report that showed that it is effective to open the diskus dry powder inhaler device and ingest the fluticasone powder directly108. Nevertheless, these unconventional approaches highlight the need for specifically developed oesophageal formulations for EoE that might increase the response rate to topical steroids.
Elemental formula
When empirical or allergy test-directed elimination diets are unsuccessful, the use of an elemental formula can be highly effective. Elemental formula was one of the first treatments to be used for EoE6, and the high rates of response in children (>90–95%) helped to confirm that EoE was an allergic disorder69,75,76. These hypoallergenic formulas are composed of amino acid solids, simple carbohydrates and medium-chain triglycerides, and rapidly lead to histological and symptomatic improvement if taken as the sole source of nutrition. The efficacy of these diets was initially demonstrated in children but has also been shown in adults77,78. Although effective, there can be several downsides to this treatment. First, a formula diet is not an issue in infants and young children, but is more difficult to maintain as a long-term treatment in older children, adolescents and adults. Second, historically, these formulas have not been palatable, and although that is changing, some patients cannot tolerate the flavour or the volume required and must use a feeding tube to maintain adequate nutrition. This feeding approach is a particular issue for adults who are accustomed to eating solid food; in one study, more than one-third of patients withdrew because they could not tolerate the formula77. Third, these formulas are expensive and might be difficult to secure through healthcare systems. Last, the goal of this therapy is not to remain on this treatment indefinitely, but to use the formula for nutrition while re-introducing foods to identify triggers of EoE. However, as all foods have been eliminated, the trigger identification process is prolonged and requires multiple repeat endoscopies, which is a large burden on patients and might be impractical. However, the excellent effect of this treatment in both children and adults makes this an option to consider for severe EoE when patients are non-responsive to other treatments.
Systemic steroids
Corticosteroids, such as prednisone or methylprednisolone, which work systemically, are effective for the treatment of EoE52,109. However, these agents are not ideal for routine use for several reasons. First, owing to the multiple adverse effects that are known to occur with long-term administration (weight gain, osteoporosis, diabetes mellitus, cataracts, adrenal insufficiency and complications from immunosuppression), this class of medication can only be used on a short-term basis. If required, these agents are best used as a bridge to a different treatment option, as EoE will recur rapidly once treatment with prednisone is stopped109. Second, in the sole comparative RCT of prednisone compared with swallowed fluticasone, response rates were similar, but more adverse effects were noted more frequently with prednisone52. Current guidelines suggest that the use of prednisone should be restricted to patients who have very severe symptoms; for example, malnutrition in children, for which a rapid treatment response is required20,21.
Leukotriene antagonists
As EoE is an allergic disease, the use of leukotriene antagonists might be appealing, as they are effective in other atopic conditions. Montelukast has been studied for the treatment of EoE, but the results are conflicting. In the initial case series reported, six of eight adults had a clinical response with montelukast at doses of 20–40 mg per day110. However, a subsequent study of eight children who used lower doses (4–10 mg per day) showed only a 38% response rate111, and a study of 11 adults who took 10 mg per day reported no responders112. In 2016, early reports from an RCT compared maintenance therapy (rather than first-line therapy) with montelukast (at 20 mg per day) versus placebo in patients who were topical steroid responders and reported no difference in rates of symptom recurrence between the two groups113. These data suggest that although montelukast might be effective in some patients, its overall clinical utility is probably limited. However, this medication has a favourable adverse effect profile and can be evaluated in patients who are refractory to initial steroid or dietary elimination treatments.
Mast cell stabilizers
As mast cells have a prominent role in the pathogenesis of EoE, are present at increased densities in the oesophageal epithelium of patients with EoE, and are involved in oesophageal fibrosis and dysmotility, targeting them for the treatment of EoE is a potentially appealing option80,114–119. Unfortunately, the available mast cell stabilizers, such as cromoglicic acid (also known as cromolyn sodium), have not been efficacious in EoE and are currently not recommended for use75. It is not known whether this lack of efficacy is due to the dose, the mechanism of action or the formulation, or whether they are targeting the mast cell populations too late during the pathogenesis of EoE.
Immunomodulators
As EoE is an immune-mediated disease, immunomodulatory drugs that might decrease the immune response, such as azathioprine and 6-mercaptopurine, could be effective treatment options22,120. However, these medications have been studied in only one case series of three patients with EoE who were refractory to steroids120. In this study, patients initially responded to these medications and then relapsed with recurrent eosinophilia when treatment was stopped. Owing to the limited amount of data and the potential adverse effects, these medications are not recommended for routine use for the treatment of EoE20. However, in selected patients with severe EoE symptoms who are refractory to other treatment options, they could be considered.
Emerging medications
In addition to the agents discussed previously, there are several emerging medications that have either been used to treat EoE or are under study for the treatment of EoE. These include biologic agents (typically antibodies that target inflammatory factors in EoE pathogenesis) or novel small molecules that target specific pathways in EoE. Many are novel treatment targets that are based on our expanding knowledge of EoE pathophysiology. A query on clinicaltrials.gov for the term ‘eosinophilic esophagitis’ in 2016 showed 104 EoE studies listed, 38 of which were actively recruiting and 20 of which were investigating new treatments.
Biologic agents.
Several biologic medications have been evaluated for EoE. In a pilot study of three patients with steroid-refractory EoE, the TNF inhibitor infliximab (5 mg/kg) was administered and no consistent treatment response was noted121. In addition, an RCT of the IgE-specific antibody omalizumab versus placebo in adults with EoE reported no change in eosinophil count and no difference in symptom improvement between the groups122. Owing to these results, neither of these agents has a current role for the treatment of refractory EoE.
The best-studied biologic agents for EoE are in the anti-interleukin 5 (anti-IL-5) class. The type 2 T helper (TH2) cytokine IL-5 is key in the pathogenesis of EoE owing to its involvement in eosinophil maturation and activation123. Three RCTs have evaluated these agents in EoE, two of which tested mepolizumab (one in adults and one in children)124,125, and one paediatric study tested reslizumab126. The latter remains the largest published RCT in EoE to date, with 226 children enrolled. The results of these trials were consistent, with modest to substantial decreases in eosinophil counts compared with placebo. However, these medications did not eliminate eosinophilic inflammation and few patients achieved a very low eosinophil count. Moreover, in the two paediatric studies, symptoms improved similarly in the active and placebo treatment arms, but it is important to note that the symptom measures used were not validated. Although these drugs are not currently being investigated for the treatment of EoE, both were recently approved by the FDA for the treatment of eosinophilic asthma. For patients who have severe eosinophilic asthma, which is a relatively uncommon asthma sub-phenotype, and coexisting refractory EoE, it would be possible to collaborate with a pulmonologist or allergist to treat the asthma and observe whether there is a concomitant improvement in the EoE symptoms and histology. However, the use of these medications specifically for the treatment of EoE is off-label.
IL-13-specific antibodies have also been evaluated. As with IL-5, IL-13 is also a key TH2 cytokine, the levels of which are increased in EoE, and it is involved in altering oesophageal gene expression and in stimulating the production of eotaxin-3, a potent chemokine that recruits eosinophils to the oesophageal epithelium123,127. QAX576, an IL-13-targeted antibody, was tested in a small pilot study that included 15 patients with EoE who received active medication and eight who received placebo128. This study showed a decrease in eosinophil counts following QAX576 treatment compared with placebo, as well as normalization of eosinophil gene expression. A similar IL-13-targeted antibody, RPC4046, was tested in a phase II RCT comprising 90 patients with EoE129. Preliminary data from this trial showed a significant (P <0.05) decrease in eosinophil counts and endoscopic severity, as well as a strong trend toward decreased symptoms of dysphagia, following RPC4046 treatment compared with placebo.
The IL-4-specific antibody dupilumab is currently being evaluated in a phase II study130. IL-4 is another TH2 cytokine that is involved in the pathogenesis of EoE, and dupilumab was shown to have efficacy for both asthma and atopic dermatitis131,132. However, no results are yet available for EoE.
Small molecules.
In addition to the biologic agents, there are also small molecules that are being studied. OC000459 is an antagonist of prostaglandin D2 receptor 2 (PTGDR2), which is present on TH2 cells and eosinophils, and therefore blocks the binding of prostaglandin D2 (REF. 133). In a pilot RCT of OC000459 in 26 adults with EoE, there was a modest but substantial decrease in eosinophil count and the treatment was well tolerated133. This class of medication remains under current clinical evaluation for the treatment of EoE. Losartan, an angiotensin II receptor antagonist, is also under early clinical investigation in EoE for its antagonistic effect on TGFβ, which is a major profibrotic factor in EoE115. Results using this agent in EoE are not yet available.
In the context of the controversy about the use of PPIs for EoE as noted earlier, a correspondence reported a response to vonoprazan, a novel potassium-competitive acid blocker, in four patients with EoE who had previously not responded to treatment with PPIs134. Although this report adds to the controversy that surrounds the overlap between EoE and GERD, given that a non-PPI medication for the treatment of GERD seems to be effective for EoE, it also highlights that some patients who have EoE that is difficult to treat might have overlapping GERD135. In these patients, the treatment of GERD should be maximized if there is a symptomatic response or if the PPI therapy results in some improvement in oesophageal eosinophil counts. Data have shown that patients who rapidly metabolize PPIs (owing to a specific cytochrome P450 2C19 (CYP2C19) genotype) might lose an initial response to PPI treatment if the dose is lowered136, and this issue could also be considered as a cause of poor responses in some patients.
Oesophageal dilation
The discussion thus far has centred on pharmacological and dietary treatments. However, there are patients who will not respond (or might not tolerate) any of the available options. For these patients, if they have evidence of fibrostenotic changes on endoscopy and symptoms of dysphagia, they could benefit from an oesophageal dilation programme to open oesophageal strictures and improve the calibre of the oesophagus. Importantly, endoscopic assessment of oesophageal calibre has fairly poor sensitivity, but reasonable options include assessing calibre with a barium esophagram, objectively measuring oesophageal calibre endoscopically with the so-called ‘balloon pull-through’ technique, or carrying out empirical dilation to assess for mucosal signs of dilation (rents or ‘dilation effect’)137–141. Although there were initial questions in regard to the safety of oesophageal dilation in EoE142,143, experience accumulated from multiple centres has now established it as a safe procedure, with a perforation risk of 0.3%, which is similar to the risk of oesophageal dilation for other causes of strictures140,144–149. Although dilation of the oesophagus does not affect the underlying inflammatory process146 and ongoing inflammation can lead to recurrent stricturing and the need for repeat dilation, this method is an effective way to improve symptoms and decrease the risk of food impaction in patients with fibrostenosis150. As previously described, this approach is also a good option for patients who have a histological response to anti-inflammatory treatment but have ongoing dysphagia symptoms due to a persistent oesophageal stricture.
Conclusions
Refractory EoE is a challenging condition to manage for many reasons. Although the condition is conceptually easy to describe, it is difficult to operationalize and define. Data to guide evidence-based decision-making are limited, and treatments that are currently available have suboptimal efficacies. Refractory EoE is also commonly encountered, which directly highlights the lack of medications that are targeted to the oesophagus and the difficulty of being able to reliably detect food triggers of EoE.
For the purposes of this Review, refractory EoE has been defined as persistent eosinophilia (≥15 eos/hpf) in the setting of incomplete resolution of the primary presenting symptoms and incomplete resolution of endoscopic findings following a PPI trial and after treatment with either topical steroids or dietary elimination. In this situation, it is important to assess the multiple possible causes for a lack of response, as well as treatment complications and an incorrect diagnosis of EoE. If non-response is confirmed, the approach is to first maximize the initial treatment, and then to switch from topical steroids to dietary elimination, or vice versa. If there is still no response, alternative treatments could be considered in selected patients, including custom topical steroid formulations, elemental formula, a short course of prednisone, immunomodulators or montelukast. Although there are no clinical data in regard to combination therapy, this modality could be considered as well. Patients with refractory EoE are also ideal candidates for clinical trials, as there are several promising agents that are under various phases of preclinical and clinical development, including biologic agents that target the mechanisms of EoE pathogenesis. If the safety and efficacy profiles of these novel agents are found to be acceptable, it is conceivable that biological agents will find a niche in the treatment of refractory EoE. There is also hope that oesophageal-specific formulations of topical steroids will decrease non-response rates in EoE. For patients with refractory EoE who have oesophageal strictures or narrowing and symptoms of dysphagia, an oesophageal dilation programme is effective for improving symptoms.
Despite the difficulties with the current management strategy for patients with refractory EoE, it is an exciting time for therapeutic development in EoE and the future is bright. Given our increasing knowledge of the pathogenesis of EoE, as well as the experimental agents that are already under development, there are important opportunities to improve patient care and treatment algorithms. Both patients and healthcare providers should be optimistic for new approaches for the treatment of refractory EoE in the future.
Key points.
Refractory eosinophilic oesophagitis (EoE) can be defined as persistent eosinophilia with incomplete symptom resolution, and persistent endoscopic findings after a PPI trial and after topical steroid treatment or dietary elimination
Depending on the treatment modality, non-response to topical steroid or dietary elimination in EoE can be in the range of 20–50%
Following non-response, it is important to systematically assess for explanations (for example, non-adherence; incorrect dosing, formulation or administration; ongoing allergen or food trigger exposure; inadequate dietary elimination; and validity of original diagnosis)
Few clinical predictors of non-response have been identified to date
Topical steroid or dietary elimination treatments should be maximized before switching between treatment modalities
Second-line treatment options include systemic corticosteroids, elemental formula, leukotriene antagonists, immunomodulators and experimental agents in clinical trials
Acknowledgements
E.S.D. is supported, in part, by the US National Institutes of Health (NIH; grant R01 DK101856), as well as the Consortium of Eosinophilic Gastrointestinal Disease Researchers (CEGIR; grant U54AI117804), which is part of the Rare Disease Clinical Research Network (RDCRN), an initiative of the Office of Rare Disease Research (ORDR), the US National Center for Advancing Translational Sciences (NCATS), and is funded through collaboration between the US National Institute of Allergy and Infectious Diseases (NIAID), the US National Institute of Diabetes and Digestive and Kidney Diseases (NIDDK) and NCATS.
Footnotes
Competing interests statement
E.S.D. is a consultant for Adare, Alivio, Banner, Glaxo-SmithKline, Receptos, Regeneron and Shire. He has received research funding from Meritage, Miraca Life Sciences, Nutricia, Receptos, Regeneron and Shire, and an educational grant from Banner.
References
- 1.Furuta GT et al. Eosinophilic esophagitis in children and adults: a systematic review and consensus recommendations for diagnosis and treatment. Gastroenterology 133, 1342–1363 (2007). [DOI] [PubMed] [Google Scholar]
- 2.Dobbins JW, Sheahan DG & Behar J Eosinophilic gastroenteritis with esophageal involvement. Gastroenterology 72, 1312–1316 (1977). [PubMed] [Google Scholar]
- 3.Landres RT, Kuster GG & Strum WB Eosinophilic esophagitis in a patient with vigorous achalasia. Gastroenterology 74, 1298–1301 (1978). [PubMed] [Google Scholar]
- 4.Attwood SE, Smyrk TC, Demeester TR & Jones JB Esophageal eosinophilia with dysphagia. A distinct clinicopathologic syndrome. Dig. Dis. Sci 38, 109–116 (1993). [DOI] [PubMed] [Google Scholar]
- 5.Straumann A, Spichtin HP, Bernoulli R, Loosli J & Vogtlin J Idiopathic eosinophilic esophagitis: a frequently overlooked disease with typical clinical aspects and discrete endoscopic findings. Schweiz. Med. Wochenschr 124, 1419–1429 (in German) (1994). [PubMed] [Google Scholar]
- 6.Kelly KJ et al. Eosinophilic esophagitis attributed to gastroesophageal reflux: improvement with an amino acid-based formula. Gastroenterology 109, 1503–1512 (1995). [DOI] [PubMed] [Google Scholar]
- 7.Dellon ES Epidemiology of eosinophilic esophagitis. Gastroenterol. Clin. North Am 43, 201–218 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Dellon ES et al. The increasing incidence and prevalence of eosinophilic oesophagitis outpaces changes in endoscopic and biopsy practice: national population-based estimates from Denmark. Aliment. Pharmacol. Ther 41, 662–670 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Dellon ES, Jensen ET, Martin CF, Shaheen NJ & Kappelman MD Prevalence of eosinophilic esophagitis in the United States. Clin. Gastroenterol. Hepatol 12, 589–596.e1 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Arias A, Perez-Martinez I, Tenias JM & Lucendo AJ Systematic review with meta-analysis: the incidence and prevalence of eosinophilic oesophagitis in children and adults in population-based studies. Aliment. Pharmacol. Ther 43, 3–15 (2016). [DOI] [PubMed] [Google Scholar]
- 11.Kidambi T, Toto E, Ho N, Taft T & Hirano I Temporal trends in the relative prevalence of dysphagia etiologies from 1999–2009. World J. Gastroenterol 18, 4335–4341 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Desai TK et al. Association of eosinophilic inflammation with esophageal food impaction in adults. Gastrointest. Endosc 61, 795–801 (2005). [DOI] [PubMed] [Google Scholar]
- 13.Sperry SL, Crockett SD, Miller CB, Shaheen NJ & Dellon ES Esophageal foreignbody impactions: epidemiology, time trends, and the impact of the increasing prevalence of eosinophilic esophagitis. Gastrointest. Endosc 74, 985–991 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Dellon ES et al. Clinical and endoscopic characteristics do not reliably differentiate PPI-responsive esophageal eosinophilia and eosinophilic esophagitis in patients undergoing upper endoscopy: a prospective cohort study. Am. J. Gastroenterol 108, 1854–1860 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Prasad GA et al. Prevalence and predictive factors of eosinophilic esophagitis in patients presenting with dysphagia: a prospective study. Am. J. Gastroenterol 102, 2627–2632 (2007). [DOI] [PubMed] [Google Scholar]
- 16.Mackenzie SH et al. Prospective analysis of eosinophilic esophagitis in patients presenting with dysphagia [abstract A18]. Am. J. Gastroenterol 101, S47 (2006). [Google Scholar]
- 17.Veerappan GR et al. Prevalence of eosinophilic esophagitis in an adult population undergoing upper endoscopy: a prospective study. Clin. Gastroenterol. Hepatol 7, 420–426 (2009). [DOI] [PubMed] [Google Scholar]
- 18.Jensen ET, Kappelman MD, Martin CF & Dellon ES Health-care utilization, costs, and the burden of disease related to eosinophilic esophagitis in the United States. Am. J. Gastroenterol 110, 626–632 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Liacouras CA et al. Eosinophilic esophagitis: updated consensus recommendations for children and adults. J. Allergy Clin. Immunol 128, 3–20.e6 (2011). [DOI] [PubMed] [Google Scholar]
- 20.Dellon ES et al. ACG Clinical Guideline: evidence based approach to the diagnosis and management of esophageal eosinophilia and eosinophilic esophagitis. Am. J. Gastroenterol 108, 679–692 (2013). [DOI] [PubMed] [Google Scholar]
- 21.Papadopoulou A et al. Management guidelines of eosinophilic esophagitis in childhood. J. Pediatr. Gastroenterol. Nutr 58, 107–118 (2014). [DOI] [PubMed] [Google Scholar]
- 22.Dellon ES & Liacouras CA Advances in clinical management of eosinophilic esophagitis. Gastroenterology 147, 1238–1254 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Molina-Infante J et al. Esophageal eosinophilic infiltration responds to proton pump inhibition in most adults. Clin. Gastroenterol. Hepatol 9, 110–117 (2011). [DOI] [PubMed] [Google Scholar]
- 24.Dranove JE, Horn DS, Davis MA, Kernek KM & Gupta SK Predictors of response to proton pump inhibitor therapy among children with significant esophageal eosinophilia. J. Pediatr 154, 96–100 (2009). [DOI] [PubMed] [Google Scholar]
- 25.Wen T et al. Transcriptome analysis of proton pump inhibitor-responsive esophageal eosinophilia reveals proton pump inhibitor-reversible allergic inflammation. J. Allergy Clin. Immunol 135, 187–197 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Sodikoff J & Hirano I Proton pump inhibitor-responsive esophageal eosinophilia does not preclude food-responsive eosinophilic esophagitis. J. Allergy Clin. Immunol 137, 631–633 (2016). [DOI] [PubMed] [Google Scholar]
- 27.Lucendo AJ, Arias A, Gonzalez-Cervera J, Olalla JM & Molina-Infante J Dual response to dietary/topical steroid and proton pump inhibitor therapy in adult patients with eosinophilic esophagitis. J. Allergy Clin. Immunol 137, 931–934.e2 (2016). [DOI] [PubMed] [Google Scholar]
- 28.Lucendo AJ, Arias A & Molina-Infante J Efficacy of proton pump inhibitor drugs for inducing clinical and histologic remission in patients with symptomatic esophageal eosinophilia: a systematic review and meta-analysis. Clin. Gastroenterol. Hepatol 14, 13–22.e1 (2016). [DOI] [PubMed] [Google Scholar]
- 29.Cheng E et al. Omeprazole blocks eotaxin-3 expression by oesophageal squamous cells from patients with eosinophilic oesophagitis and GORD. Gut 62, 824–832 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Zhang X et al. Omeprazole blocks STAT6 binding to the eotaxin-3 promoter in eosinophilic esophagitis cells. PLoS ONE 7, e50037 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Molina-Infante J et al. Proton pump inhibitor-responsive oesophageal eosinophilia: an entity challenging current diagnostic criteria for eosinophilic oesophagitis. Gut 65, 524–531 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Wolf WA et al. Predictors of response to steroid therapy for eosinophilic esophagitis and treatment of steroid-refractory patients. Clin. Gastroenterol. Hepatol 13, 452–458 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Leung J et al. Longitudinal perspective on managing refractory eosinophilic esophagitis. J. Allergy Clin. Immunol. Pract 3, 951–956 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Mukkada VA & Furuta GT Management of refractory eosinophilic esophagitis. Dig. Dis 32, 134–138 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Sodikoff J & Hirano I Therapeutic strategies in eosinophilic esophagitis: induction, maintenance and refractory disease. Best Pract. Res. Clin. Gastroenterol 29, 829–839 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Hirano I Therapeutic end points in eosinophilic esophagitis: is elimination of esophageal eosinophils enough? Clin. Gastroenterol. Hepatol 10, 750–752 (2012). [DOI] [PubMed] [Google Scholar]
- 37.Franciosi JP et al. Development of a validated patient-reported symptom metric for pediatric eosinophilic esophagitis: qualitative methods. BMC Gastroenterol 11, 126 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Martin LJ et al. Pediatric Eosinophilic Esophagitis Symptom Scores (PEESS v2.0) identify histologic and molecular correlates of the key clinical features of disease. J. Allergy Clin. Immunol 135, 1519–1528.e8 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Dellon ES, Irani AM, Hill MR & Hirano I Development and field testing of a novel patient-reported outcome measure of dysphagia in patients with eosinophilic esophagitis. Aliment. Pharmacol. Ther 38, 634–642 (2013). [DOI] [PubMed] [Google Scholar]
- 40.Schoepfer AM et al. Development and validation of a symptom-based activity index for adults with eosinophilic esophagitis. Gastroenterology 147, 1255–1266.e21 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Franciosi JP et al. PedsQL eosinophilic esophagitis module: feasibility, reliability, and validity. J. Pediatr. Gastroenterol. Nutr 57, 57–66 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Taft TH et al. The adult eosinophilic oesophagitis quality of life questionnaire: a new measure of health-related quality of life. Aliment. Pharmacol. Ther 34, 790–798 (2011). [DOI] [PubMed] [Google Scholar]
- 43.Hirano I et al. Endoscopic assessment of the oesophageal features of eosinophilic oesophagitis: validation of a novel classification and grading system. Gut 62, 489–495 (2013). [DOI] [PubMed] [Google Scholar]
- 44.Wolf WA et al. Evaluation of histologic cutpoints for treatment response in eosinophilic esophagitis. J. Gastroenterol. Hepatol. Res 4, 1780–1787 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Wolf WA et al. Evaluation of an optimal histologic threshold for treatment response in a prospective cohort of eosinophilic esophagitis patients [abstract Mo1181]. Gastroenterology 150 (Suppl. 1), S661 (2016). [Google Scholar]
- 46.Runge TM, Eluri S, Woosley JT, Shaheen NJ & Dellon ES Control of inflammation with topical steroids decreases subsequent esophageal dilation in patients with eosinophilic esophagitis [abstrasct Mo1187]. Gastroenterology 150 (Suppl. 1), S664 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Dellon ES, Aderoju A, Woosley JT, Sandler RS & Shaheen NJ Variability in diagnostic criteria for eosinophilic esophagitis: a systematic review. Am. J. Gastroenterol 102, 2300–2313 (2007). [DOI] [PubMed] [Google Scholar]
- 48.Schoepfer AM et al. Delay in diagnosis of eosinophilic esophagitis increases risk for stricture formation in a time-dependent manner. Gastroenterology 145, 1230–1236.e2 (2013). [DOI] [PubMed] [Google Scholar]
- 49.Dellon ES et al. A phenotypic analysis shows that eosinophilic esophagitis is a progressive fibrostenotic disease. Gastrointest. Endosc 79, 577–585.e4 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Lipka S, Kumar A & Richter JE Impact of diagnostic delay and other risk factors on eosinophilic esophagitis phenotype and esophageal diameter. J. Clin. Gastroenterol 50, 134–140 (2016). [DOI] [PubMed] [Google Scholar]
- 51.Konikoff MR et al. A randomized, double-blind, placebo-controlled trial of fluticasone propionate for pediatric eosinophilic esophagitis. Gastroenterology 131, 1381–1391 (2006). [DOI] [PubMed] [Google Scholar]
- 52.Schaefer ET et al. Comparison of oral prednisone and topical fluticasone in the treatment of eosinophilic esophagitis: a randomized trial in children. Clin. Gastroenterol. Hepatol 6, 165–173 (2008). [DOI] [PubMed] [Google Scholar]
- 53.Dohil R, Newbury R, Fox L, Bastian J & Aceves S Oral viscous budesonide is effective in children with eosinophilic esophagitis in a randomized, placebo-controlled trial. Gastroenterology 139, 418–429 (2010). [DOI] [PubMed] [Google Scholar]
- 54.Straumann A et al. Budesonide is effective in adolescent and adult patients with active eosinophilic esophagitis. Gastroenterology 139, 1526–1537.e1 (2010). [DOI] [PubMed] [Google Scholar]
- 55.Dellon ES et al. Viscous topical is more effective than nebulized steroid therapy for patients with eosinophilic esophagitis. Gastroenterology 143, |321–324.e1 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Alexander JA et al. Swallowed fluticasone improves histologic but not symptomatic responses of adults with eosinophilic esophagitis. Clin. Gastroenterol. Hepatol 10, 742–749.e1 (2012). [DOI] [PubMed] [Google Scholar]
- 57.Butz BK et al. Efficacy, dose reduction, and resistance to high-dose fluticasone in patients with eosinophilic esophagitis. Gastroenterology 147, 324–333.e5 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Gupta SK, Vitanza JM & Collins MH Efficacy and safety of oral budesonide suspension in pediatric patients with eosinophilic esophagitis. Clin. Gastroenterol. Hepatol 13, 66–76.e3 (2015). [DOI] [PubMed] [Google Scholar]
- 59.Miehlke S et al. A randomised, double-blind trial comparing budesonide formulations and dosages for short-term treatment of eosinophilic oesophagitis. Gut 65, 390–399 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Dellon ES et al. Budesonide oral suspension improves symptomatic, endoscopic, and histologic parameters compared with placebo in patients with eosinophilic esophagitis. Gastroenterology 152, 776–786.e5 (2017). [DOI] [PubMed] [Google Scholar]
- 61.Moawad FJ et al. Randomized controlled trial comparing aerosolized swallowed fluticasone to esomeprazole for esophageal eosinophilia. Am. J. Gastroenterol 108, 366–372 (2013). [DOI] [PubMed] [Google Scholar]
- 62.Peterson KA et al. Comparison of esomeprazole to aerosolized, swallowed fluticasone for eosinophilic esophagitis. Dig. Dis. Sci 55, 1313–1319 (2010). [DOI] [PubMed] [Google Scholar]
- 63.Moawad F et al. Predictors of non-response to topical steroids treatment in eosinophilic esophagitis [abstract 37]. Am. J. Gastroenterol 108 (Suppl. 1), S14 (2013). [Google Scholar]
- 64.Philpott H, Nandurkar S, Royce SG, Thien F & Gibson PR A prospective open clinical trial of a proton pump inhibitor, elimination diet and/or budesonide for eosinophilic oesophagitis. Aliment. Pharmacol. Ther 43, 985–993 (2016). [DOI] [PubMed] [Google Scholar]
- 65.Boldorini R, Mercalli F & Oderda G Eosinophilic oesophagitis in children: responders and non-responders to swallowed fluticasone. J. Clin. Pathol 66, 399–402 (2013). [DOI] [PubMed] [Google Scholar]
- 66.Henderson CJ et al. Comparative dietary therapy effectiveness in remission of pediatric eosinophilic esophagitis. J. Allergy Clin. Immunol 129, 1570–1578 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Spergel JM et al. 14 years of eosinophilic esophagitis: clinical features and prognosis. J. Pediatr. Gastroenterol. Nutr 48, 30–36 (2009). [DOI] [PubMed] [Google Scholar]
- 68.Spergel JM et al. Identification of causative foods in children with eosinophilic esophagitis treated with an elimination diet. J. Allergy Clin. Immunol 130, 461–467.e5 (2012). [DOI] [PubMed] [Google Scholar]
- 69.Kagalwalla AF et al. Effect of six-food elimination diet on clinical and histologic outcomes in eosinophilic esophagitis. Clin. Gastroenterol. Hepatol 4, 1097–1102 (2006). [DOI] [PubMed] [Google Scholar]
- 70.Kagalwalla AF et al. Identification of specific foods responsible for inflammation in children with eosinophilic esophagitis successfully treated with empiric elimination diet. J. Pediatr. Gastroenterol. Nutr 53, 145–149 (2011). [DOI] [PubMed] [Google Scholar]
- 71.Gonsalves N et al. Elimination diet effectively treats eosinophilic esophagitis in adults; food reintroduction identifies causative factors. Gastroenterology 142, 1451–1459.e1 (2012). [DOI] [PubMed] [Google Scholar]
- 72.Lucendo AJ et al. Empiric 6-food elimination diet induced and maintained prolonged remission in patients with adult eosinophilic esophagitis: a prospective study on the food cause of the disease. J. Allergy Clin. Immunol 131, 797–804 (2013). [DOI] [PubMed] [Google Scholar]
- 73.Molina-Infante J et al. Four-food group elimination diet for adult eosinophilic esophagitis: a prospective multicenter study. J. Allergy Clin. Immunol 134, 1093–1099.e1 (2014). [DOI] [PubMed] [Google Scholar]
- 74.Wolf WA, Jerath MR, Sperry SL, Shaheen NJ & Dellon ES Dietary elimination therapy is an effective option for adults with eosinophilic esophagitis. Clin. Gastroenterol. Hepatol 12, 1272–1279 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Liacouras CA et al. Eosinophilic esophagitis: a 10-year experience in 381 children. Clin. Gastroenterol. Hepatol 3, 1198–1206 (2005). [DOI] [PubMed] [Google Scholar]
- 76.Markowitz JE, Spergel JM, Ruchelli E & Liacouras CA Elemental diet is an effective treatment for eosinophilic esophagitis in children and adolescents. Am. J. Gastroenterol 98, 777–782 (2003). [DOI] [PubMed] [Google Scholar]
- 77.Peterson KA et al. Elemental diet induces histologic response in adult eosinophilic esophagitis. Am. J. Gastroenterol 108, 759–766 (2013). [DOI] [PubMed] [Google Scholar]
- 78.Warners MJ et al. Amino acid-based diet effectively decreases eosinophilic inflammation and improves symptoms in adult eosinophilic esophagitis patients [abstract 70]. Gastroenterology 150 (Suppl. 1), S18 (2016). [Google Scholar]
- 79.Arias A, Gonzalez-Cervera J, Tenias JM & Lucendo AJ Efficacy of dietary interventions for inducing histologic remission in patients with eosinophilic esophagitis: a systematic review and meta-analysis. Gastroenterology 146, 1639–1648 (2014). [DOI] [PubMed] [Google Scholar]
- 80.Hirano I & Aceves SS Clinical implications and pathogenesis of esophageal remodeling in eosinophilic esophagitis. Gastroenterol. Clin. North Am 43, 297–316 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Dellon ES et al. Accuracy of the Eosinophilic Esophagitis Endoscopic Reference Score in diagnosis and determining response to treatment. Clin. Gastroenterol. Hepatol 14, 31–39 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Safroneeva E et al. Symptoms have modest accuracy in detecting endoscopic and histologic remission in adults with eosinophilic esophagitis. Gastroenterology 150, 581–590.e4 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Hefner JN et al. A randomized controlled comparison of esophageal clearance times of oral budesonide preparations. Dig. Dis. Sci 61, 1582–1590 (2016). [DOI] [PubMed] [Google Scholar]
- 84.Lindberg GM, Van Eldik R & Saboorian MH A case of herpes esophagitis after fluticasone propionate for eosinophilic esophagitis. Nat. Clin. Pract. Gastroenterol. Hepatol 5, 527–530 (2008). [DOI] [PubMed] [Google Scholar]
- 85.Zimmermann D et al. Acute herpes simplex viral esophagitis occurring in 5 immunocompetent individuals with eosinophilic esophagitis. ACG Case Rep. J 3, 165–168 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Noel RJ et al. Clinical and immunopathologic effects of swallowed fluticasone for eosinophilic esophagitis. Clin. Gastroenterol. Hepatol 2, 568–575 (2004). [DOI] [PubMed] [Google Scholar]
- 87.Wolf WA et al. The six-food elimination diet for eosinophilic esophagitis increases grocery shopping cost and complexity. Dysphagia 31, 765–770 (2016). [DOI] [PubMed] [Google Scholar]
- 88.Doerfler B, Bryce P, Hirano I & Gonsalves N Practical approach to implementing dietary therapy in adults with eosinophilic esophagitis: the Chicago experience. Dis. Esophagus 28, 42–58 (2015). [DOI] [PubMed] [Google Scholar]
- 89.Vashi R & Hirano I Diet therapy for eosinophilic esophagitis: when, why and how. Curr. Opin. Gastroenterol 29, 407–415 (2013). [DOI] [PubMed] [Google Scholar]
- 90.Venter C & Fleischer DM Diets for diagnosis and management of food allergy: the role of the dietitian in eosinophilic esophagitis in adults and children. Ann. Allergy Asthma Immunol 117, 468–471 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Aceves SS Food allergy testing in eosinophilic esophagitis: what the gastroenterologist needs to know. Clin. Gastroenterol. Hepatol 12, 1216–1223 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Philpott H, Nandurkar S, Royce SG, Thien F & Gibson PR Allergy tests do not predict food triggers in adult patients with eosinophilic oesophagitis. A comprehensive prospective study using five modalities. Aliment. Pharmacol. Ther 44, 223–233 (2016). [DOI] [PubMed] [Google Scholar]
- 93.Hu Y et al. Increased acid responsiveness in vagal sensory neurons in a guinea pig model of eosinophilic esophagitis. Am. J. Physiol. Gastrointest. Liver Physiol 307, G149–G157 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Walker MM et al. Duodenal eosinophilia and early satiety in functional dyspepsia: confirmation of a positive association in an Australian cohort. J. Gastroenterol. Hepatol 29, 474–479 (2014). [DOI] [PubMed] [Google Scholar]
- 95.Dellon ES et al. Markers of tyrosine kinase activity in eosinophilic esophagitis: a pilot study of the FIP1L1–PDGFRα fusion gene, pERK 1/2, and pSTAT5. Dis. Esophagus 25, 166–174 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Aceves SS et al. Resolution of remodeling in eosinophilic esophagitis correlates with epithelial response to topical corticosteroids. Allergy 65, 109–116 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Eluri S et al. The extremely narrow-caliber esophagus is a treatment-resistant subphenotype of eosinophilic esophagitis. Gastrointest. Endosc 83, 1142–1148 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Chen JW et al. Severity of endoscopically identified esophageal rings correlates with reduced esophageal distensibility in eosinophilic esophagitis. Endoscopy 48, 794–801 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Rieder F et al. T-Helper 2 cytokines, transforming growth factor β1, and eosinophil products induce fibrogenesis and alter muscle motility in patients with eosinophilic esophagitis. Gastroenterology 146, 1266–1277.e9 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Albert D et al. Comparisons of fluticasone to budesonide in the treatment of eosinophilic esophagitis. Dig. Dis. Sci 61, 1996–2001 (2016). [DOI] [PubMed] [Google Scholar]
- 101.Faubion WA Jr et al. Treatment of eosinophilic esophagitis with inhaled corticosteroids. J. Pediatr. Gastroenterol. Nutr 27, 90–93 (1998). [DOI] [PubMed] [Google Scholar]
- 102.Bergquist H, Larsson H, Johansson L & Bove M Dysphagia and quality of life may improve with mometasone treatment in patients with eosinophilic esophagitis: a pilot study. Otolaryngol. Head Neck Surg 145, 551–556 (2011). [DOI] [PubMed] [Google Scholar]
- 103.Schroeder S et al. Successful treatment of eosinophilic esophagitis with ciclesonide. J. Allergy Clin. Immunol 129, 1419–1421 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Lee JJ et al. Topical inhaled ciclesonide for treatment of eosinophilic esophagitis. J. Allergy Clin. Immunol 130, 1011 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Aceves SS, Dohil R, Newbury RO & Bastian JF Topical viscous budesonide suspension for treatment of eosinophilic esophagitis. J. Allergy Clin. Immunol 116, 705–706 (2005). [DOI] [PubMed] [Google Scholar]
- 106.Aceves SS, Bastian JF, Newbury RO & Dohil R Oral viscous budesonide: a potential new therapy for eosinophilic esophagitis in children. Am. J. Gastroenterol 102, 2271–2279 (2007). [DOI] [PubMed] [Google Scholar]
- 107.Lee J et al. Oral viscous budesonide can be successfully delivered through a variety of vehicles to treat eosinophilic esophagitis in children. J. Allergy Clin. Immunol. Pract 4, 767–768 (2016). [DOI] [PubMed] [Google Scholar]
- 108.Kia L et al. Oral fluticasone powder improves histopathology in adults with eosinophilic esophagitis [abstract 1706]. Am. J. Gastroenterol 110 (Suppl. 1), S724–S725 (2015). [Google Scholar]
- 109.Liacouras CA, Wenner WJ, Brown K & Ruchelli E Primary eosinophilic esophagitis in children: successful treatment with oral corticosteroids. J. Pediatr. Gastroenterol. Nutr 26, 380–385 (1998). [DOI] [PubMed] [Google Scholar]
- 110.Attwood SE et al. Eosinophilic oesophagitis: a novel treatment using Montelukast. Gut 52, 181–185 (2003). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Stumphy J, Al-Zubeidi D, Guerin L, Mitros F & Rahhal R Observations on use of montelukast in pediatric eosinophilic esophagitis: insights for the future. Dis. Esophagus 24, 229–234 (2011). [DOI] [PubMed] [Google Scholar]
- 112.Lucendo AJ et al. Montelukast was inefficient in maintaining steroid-induced remission in adult eosinophilic esophagitis. Dig. Dis. Sci 56, 3551–3558 (2011). [DOI] [PubMed] [Google Scholar]
- 113.Alexander JA et al. Montelukast does not maintain symptom remission after topical steroid therapy for eosinophilic esophagitis. Clin. Gastroenterol. Hepatol 15, 214–221.e2 (2017). [DOI] [PubMed] [Google Scholar]
- 114.Abonia JP et al. Involvement of mast cells in eosinophilic esophagitis. J. Allergy Clin. Immunol 126, 140–149 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Aceves SS et al. Mast cells infiltrate the esophageal smooth muscle in patients with eosinophilic esophagitis, express TGF-β1, and increase esophageal smooth muscle contraction. J. Allergy Clin. Immunol 126, 1198–1204.e4 (2010). [DOI] [PubMed] [Google Scholar]
- 116.Lucendo AJ et al. Immunophenotypic characterization and quantification of the epithelial inflammatory infiltrate in eosinophilic esophagitis through stereology: an analysis of the cellular mechanisms of the disease and the immunologic capacity of the esophagus. Am. J. Surg. Pathol 31, 598–606 (2007). [DOI] [PubMed] [Google Scholar]
- 117.Straumann A, Bauer M, Fischer B, Blaser K & Simon HU Idiopathic eosinophilic esophagitis is associated with a TH2-type allergic inflammatory response. J. Allergy Clin. Immunol 108, 954–961 (2001). [DOI] [PubMed] [Google Scholar]
- 118.Dellon ES et al. Tryptase staining of mast cells may differentiate eosinophilic esophagitis from gastroesophageal reflux disease. Am. J. Gastroenterol 106, 264–271 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Dellon ES et al. Markers of eosinophilic inflammation for diagnosis of eosinophilic esophagitis and proton pump inhibitor-responsive esophageal eosinophilia: a prospective study. Clin. Gastroenterol. Hepatol 12, 2015–2022 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Netzer P et al. Corticosteroid-dependent eosinophilic oesophagitis: azathioprine and 6-mercaptopurine can induce and maintain long-term remission. Eur. J. Gastroenterol. Hepatol 19, 865–869 (2007). [DOI] [PubMed] [Google Scholar]
- 121.Straumann A, Bussmann C, Conus S, Beglinger C & Simon HU Anti-TNF-α (infliximab) therapy for severe adult eosinophilic esophagitis. J. Allergy Clin. Immunol 122, 425–427 (2008). [DOI] [PubMed] [Google Scholar]
- 122.Clayton F et al. Eosinophilic esophagitis in adults is associated with IgG4 and not mediated by IgE. Gastroenterology 147, 602–609 (2014). [DOI] [PubMed] [Google Scholar]
- 123.Rothenberg ME Molecular, genetic, and cellular bases for treating eosinophilic esophagitis. Gastroenterology 148, 1143–1157 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Straumann A et al. Anti-interleukin-5 antibody treatment (mepolizumab) in active eosinophilic oesophagitis: a randomised, placebo-controlled, double-blind trial. Gut 59, 21–30 (2010). [DOI] [PubMed] [Google Scholar]
- 125.Assa’ad AH et al. An antibody against IL-5 reduces numbers of esophageal intraepithelial eosinophils in children with eosinophilic esophagitis. Gastroenterology 141, 1593–1604 (2011). [DOI] [PubMed] [Google Scholar]
- 126.Spergel JM et al. Reslizumab in children and adolescents with eosinophilic esophagitis: results of a double-blind, randomized, placebo-controlled trial. J. Allergy Clin. Immunol 129, 456–463.e3 (2012). [DOI] [PubMed] [Google Scholar]
- 127.Blanchard C et al. IL-13 involvement in eosinophilic esophagitis: transcriptome analysis and reversibility with glucocorticoids. J. Allergy Clin. Immunol 120, 1292–1300 (2007). [DOI] [PubMed] [Google Scholar]
- 128.Rothenberg ME et al. Intravenous anti-IL-13 mAb QAX576 for the treatment of eosinophilic esophagitis. J. Allergy Clin. Immunol 135, 500–507 (2015). [DOI] [PubMed] [Google Scholar]
- 129.Hirano I et al. A randomized, double-blind, placebo-controlled trial of a novel recombinant, humanized, anti-interleukin-13 monoclonal antibody (RPC4046) in patients with active eosinophilic esophagitis: results of the HEROES study [abstract]. United European Gastroenterol. J 4 (Suppl.5), OP325 (2016). [Google Scholar]
- 130.US National Library of Medicine. ClinicalTrials.gov https://clinicaltrials.gov/ct2/show/NCT02379052 (2017). [DOI] [PubMed]
- 131.Simpson EL et al. Dupilumab therapy provides clinically meaningful improvement in patient-reported outcomes (PROs): a phase IIb, randomized, placebo-controlled, clinical trial in adult patients with moderate to severe atopic dermatitis (AD). J. Am. Acad. Dermatol 75, 506–515 (2016). [DOI] [PubMed] [Google Scholar]
- 132.Wenzel S et al. Dupilumab efficacy and safety in adults with uncontrolled persistent asthma despite use of medium-to-high-dose inhaled corticosteroids plus a long-acting β2 agonist: a randomised double-blind placebo-controlled pivotal phase 2b dose-ranging trial. Lancet 388, 31–44 (2016). [DOI] [PubMed] [Google Scholar]
- 133.Straumann A et al. Anti-eosinophil activity and clinical efficacy of the CRTH2 antagonist OC000459 in eosinophilic esophagitis. Allergy 68, 375–385 (2013). [DOI] [PubMed] [Google Scholar]
- 134.Ishimura N, Ishihara S & Kinoshita Y Sustained acid suppression by potassium-competitive acid blocker (P-CAB) may be an attractive treatment candidate for patients with eosinophilic esophagitis. Am. J. Gastroenterol 111, 1203–1204 (2016). [DOI] [PubMed] [Google Scholar]
- 135.Spechler SJ, Genta RM & Souza RF Thoughts on the complex relationship between gastroesophageal reflux disease and eosinophilic esophagitis. Am. J. Gastroenterol 102, 1301–1306 (2007). [DOI] [PubMed] [Google Scholar]
- 136.Molina-Infante J et al. Long-term loss of response in proton pump inhibitor-responsive esophageal eosinophilia is uncommon and influenced by CYP2C19 genotype and rhinoconjunctivitis. Am. J. Gastroenterol 110, 1567–1575 (2015). [DOI] [PubMed] [Google Scholar]
- 137.Gentile N et al. Oesophageal narrowing is common and frequently under-appreciated at endoscopy in patients with oesophageal eosinophilia. Aliment. Pharmacol. Ther 40, 1333–1340 (2014). [DOI] [PubMed] [Google Scholar]
- 138.Menard-Katcher C, Swerdlow MP, Mehta P, Furuta GT & Fenton LZ Contribution of esophagram to the evaluation of complicated pediatric eosinophilic esophagitis. J. Pediatr. Gastroenterol. Nutr 61, 541–546 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Madanick RD, Shaheen NJ & Dellon ES A novel balloon pull-through technique for esophageal dilation in eosinophilic esophagitis (with video). Gastrointest. Endosc 73, 138–142 (2011). [DOI] [PubMed] [Google Scholar]
- 140.Bohm ME & Richter JE Review article: oesophageal dilation in adults with eosinophilic oesophagitis. Aliment. Pharmacol. Ther 33, 748–757 (2011). [DOI] [PubMed] [Google Scholar]
- 141.Saligram S & McGrath K The safety of a strict wire-guided dilation protocol for eosinophilic esophagitis. Eur. J. Gastroenterol. Hepatol 26, 699–703 (2014). [DOI] [PubMed] [Google Scholar]
- 142.Cohen MS et al. An audit of endoscopic complications in adult eosinophilic esophagitis. Clin. Gastroenterol. Hepatol 5, 1149–1153 (2007). [DOI] [PubMed] [Google Scholar]
- 143.Kaplan M et al. Endoscopy in eosinophilic esophagitis: “feline” esophagus and perforation risk. Clin. Gastroenterol. Hepatol 1, 433–437 (2003). [DOI] [PubMed] [Google Scholar]
- 144.Straumann A et al. Eosinophilic esophagitis: analysis of food impaction and perforation in 251 adolescent and adult patients. Clin. Gastroenterol. Hepatol 6, 598–600 (2008). [DOI] [PubMed] [Google Scholar]
- 145.Dellon ES et al. Esophageal dilation in eosinophilic esophagitis: safety and predictors of clinical response and complications. Gastrointest. Endosc 71, 706–712 (2010). [DOI] [PubMed] [Google Scholar]
- 146.Schoepfer AM et al. Esophageal dilation in eosinophilic esophagitis: effectiveness, safety, and impact on the underlying inflammation. Am. J. Gastroenterol 105, 1062–1070 (2010). [DOI] [PubMed] [Google Scholar]
- 147.Jung KW et al. Occurrence of and risk factors for complications after endoscopic dilation in eosinophilic esophagitis. Gastrointest. Endosc 73, 15–21 (2011). [DOI] [PubMed] [Google Scholar]
- 148.Jacobs JW Jr & Spechler, S. J. A systematic review of the risk of perforation during esophageal dilation for patients with eosinophilic esophagitis. Dig. Dis. Sci 55, 1512–1515 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Runge TM et al. Outcomes of esophageal dilation in eosinophilic esophagitis: safety, efficacy, and persistence of the fibrostenotic phenotype. Am. J. Gastroenterol 111, 206–213 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Richter JE Eosinophilic esophagitis dilation in the community — try it — you will like it — but start low and go slow. Am. J. Gastroenterol 111, 214–216 (2016). [DOI] [PubMed] [Google Scholar]


